Abstract
Epstein-Barr virus (EBV) is associated with a number of human cancers, and latent EBV gene expression has been reported to interfere with cell cycle checkpoints and cell death pathways. Here we show that latent EBV can compromise the mitotic spindle assembly checkpoint and rescue Burkitt's lymphoma (BL)-derived cells from caspase-dependent cell death initiated in aberrant mitosis. This leads to unscheduled mitotic progression, resulting in polyploidy and multi- and/or micronucleation. The EBV latent genes responsible for this phenotype are expressed from the P3HR1 strain of virus and several viruses with similar genomic deletions that remove the EBNA2 gene. Although EBNA2 and the latent membrane proteins are not expressed, the EBNA3 proteins are present in these BL cells. Survival of the EBV-positive cells is not consistently associated with EBV lytic gene expression or with the genes that are expressed in EBV latency I BL cells (i.e., EBNA1, EBERs, and BARTs) but correlates with reduced expression of the cellular proapoptotic BH3-only protein Bim. These data suggest that a subset of latent EBV gene products may increase the likelihood of damaged DNA being inherited because of the impaired checkpoint and enhanced survival capacity. This could lead to greater genetic diversity in progeny cells and contribute to tumorigenesis. Furthermore, since it appears that this restricted latent EBV expression interferes with the responses of Burkitt's lymphoma-derived cells to cytotoxic drugs, the results of this study may have important therapeutic implications in the treatment of some BL.
Epstein-Barr virus (EBV) is a gammaherpesvirus that produces an asymptomatic infection in the majority of the human population. However, EBV is also associated with a number of human tumors of B-cell, T-cell, and epithelial origin. These include Burkitt's lymphoma (BL), some types of Hodgkin's lymphoma, immunoblastic B lymphoma in the immunosuppressed, nasopharyngeal carcinoma, and gastric carcinoma. Although the precise contribution EBV makes to the development of these diseases is not yet known, it has been suggested that interference with cell cycle checkpoints and cell death pathways by EBV may play an important role in B lymphomagenesis (reviewed in reference 20).
In vitro, EBV has the ability to infect and transform primary B cells into continuously proliferating lymphoblastoid cell lines (LCLs) that have a pattern of viral gene expression known as latency III. This is characterized by the expression of nine viral proteins: six Epstein-Barr nuclear antigens (EBNAs) (EBNA1, -2, -3A, -3B, -3C, and -LP) and three latent membrane proteins (LMPs) (LMP1, -2A, and -2B). Additional RNA species (the EBV-encoded RNAs [EBERs] and the BamHIA rightward transcripts [BARTs]) are also expressed during latency III, but their significance is not fully understood (3). It has been reported from studies with recombinant EBV that only six of the latency III proteins (EBNA1, -2, -3A, -3C, -LP, and -LMP1) are essential for efficient transformation of B cells into LCLs (3).
Current data on the persistence of EBV in humans are consistent with the viral genome residing long-term in a resting memory B-cell population. However, to establish persistence, EBV is thought to infect naïve B cells and drive these to proliferate as activated LCL-like B blasts. This expansion of an infected B-blast population in vivo is accompanied by differentiation into centroblasts and centrocytes and finally resting memory B cells. The precise series of events that the EBV-positive B cells undergo to reach the memory compartment is not yet known; however, it appears to involve regulated shutdown of latent EBV gene expression from latency type III in the B blasts, via a state called latency II (EBNA1, LMP1 and -2, and the RNAs), until in quiescent memory B cells no EBV proteins can be detected in a state termed latency 0 (30).
Generally, oncogenic viruses are able to disrupt cell cycle checkpoints that are induced by genotoxic stress and by other forms of cellular damage, such as microtubule disruption (reviewed in reference 20). Although the tumor suppressor p53 is one of the major targets of oncogenic viruses, several studies have shown that EBV does not specifically target p53 during the transformation of normal B cells into LCLs (1, 2). Nevertheless, recent studies demonstrated that although not able to directly target p53, EBV may still interfere with cell cycle checkpoints at both G1/S and G2/M phases (15, 21, 31).
Following genotoxic stress induced by cisplatin, EBV suppresses a checkpoint downstream of p53 by preventing the inactivation of cdk2 by p21WAF1/CIP1, and this appears to involve the modulation of p21WAF1/CIP1 stability (21). In addition to deregulating a G1 checkpoint, EBV has been shown to interfere with checkpoints acting at the G2/M transition. Treatment of EBV-negative Burkitt's lymphoma-derived cell lines with cisplatin, etoposide, or doxorubicin activates a p53- and p73-independent checkpoint, leading to G2/M arrest and default apoptosis. In contrast, similar Burkitt's lymphoma-derived cell lines infected with the B95.8 strain of EBV (or a deletion mutant virus, P3HR1) fail to arrest or to undergo apoptosis after treatment with these genotoxins (31). It has also been reported previously that latent EBV can disrupt the regulation of a checkpoint activated in G2 by a histone deacetylase inhibitor, azelaic bishydroxamine, and this appears to be associated with expression of the EBNA3 family of proteins (15, 25). Another recent report showed that BL41 cells infected with B95.8 virus exhibited significantly higher levels of micronucleus formation than EBV-negative BL41 cells. This is a further indication that the latency III pattern of gene expression found in these cells is associated with corrupted cell cycle checkpoints and genomic instability (10).
Here, we show that expression of a subset of EBV genes in Burkitt's lymphoma-derived cell lines treated with microtubule-disrupting drugs nocodazole and taxol produced abnormal mitotic progression, the formation of aberrant nuclei, and polyploidy. This involves suppression of the mitotic spindle assembly checkpoint and evasion of caspase-dependent cell death associated with mitotic slippage.
MATERIALS AND METHODS
Cell culture.
Burkitt's lymphoma cell lines (BL41, BL41/B95.8, Ramos, Ramos/AW, BL31, BL41-E2KO, BL31-E2KO, Ava-BL, Oku-BL, Sal-BL, Mutu-I [clone 179], Mutu-III, and Dante) were cultured in RPMI 1640 medium supplemented with 10% Serum Supreme (Biowhittaker, Wycombe, United Kingdom) or 10% bovine fetal calf serum, penicillin-streptomycin, and glutamine at 37°C in an incubator with 10% CO2. Hygromycin B (Roche) was added at a concentration of 100 μg/ml to BL41+E2KO and BL31+E2KO cells. For routine passage, cells were split 1:4 every 3 or 4 days. For experiments, all cells were resuspended in fresh medium at a density of 3 × 105 cells/ml 24 h prior to manipulation.
Cell treatments.
Cells were exposed to gamma-irradiation (850 rads) using a Gammacell 1000 Elite irradiator with a 137Cs source. Nocodazole (Sigma) and taxol (Bristol-Myers Squibb) were added to the cell medium to give a final concentration of 50 ng/ml. Viable cell counts were performed using a hemocytometer and based on the exclusion of trypan blue dye.
Microscopy.
Cells were harvested and resuspended at 1 × 106 cells/ml in phosphate-buffered saline (PBS). Approximately 8 × 104 cells were then transferred to cytospin chambers (Shandon), spun onto glass slides at 500 × g for 1 min, and air dried before being fixed in methanol:acetone (1:1) for 20 min at −20°C. After air drying, the slides were stored at −20°C until staining. For DAPI (4′,6′-diamidino-2-phenylindole) staining, slides were rehydrated in 1× PBS for 10 min and excess PBS was removed from the slide. Cells were covered with one drop of DAPI solution (0.001% in PBS-0.6% NP-40) and incubated at room temperature in the dark for 10 min. Slides were thoroughly washed in 1× PBS, mounted in Citifluor (London, United Kingdom), and stored at 4°C. DAPI-stained images were captured using an Axiophot fluorescence microscope (Carl Zeiss, Germany) with 40× and 63× lens objectives and Axio Vision 4 software.
Flow cytometry.
All flow cytometry was performed using a Becton-Dickinson FACSort flow cytometer and analyzed with CellQuest software. Doublets (two cells registering as a single event) were electronically gated out of all analyses. For propidium iodide (PI) staining, 2 × 106 cells were pelleted at 700 × g in a Beckman-Coulter Allegra 6R centrifuge, washed twice in PBS, and transferred to 1.5-ml Eppendorf tubes for fixation in 70% ethanol for at least 1 h. After excess ethanol was washed away, cells were resuspended in 1 ml of propidium iodide solution (18 μg/ml PI with 8 μg/ml RNase A in PBS) and incubated at 4°C for at least 1 h before flow cytometric analysis.
Western immunoblot analysis.
Briefly, protein extracts were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to Protran nitrocellulose membranes (Schleicher & Schuell Bioscience) and immunoblot analysis was performed as described previously (31) using an ECL kit (Amersham Biosciences) for visualization. Primary antibodies (unless otherwise stated, used as directed by the manufacturer) were rabbit anti-poly(ADP-ribose) polymerase (PARP) polyclonal (Roche), mouse anti-γ-tubulin monoclonal (GTU-88; Sigma), mouse anti-BZLF1 monoclonal (BZ1 [34]), mouse anti-BHRF1 monoclonal (also known as EA-R-p17; Bcl2 homologue [5B11; Chemicon]), and rabbit anti-Bim polyclonal (Stressgen). Human serum EE was from a patient with chronic infectious mononucleosis and has a very high titer of antibodies recognizing EBV early lytic antigens (12). The human serum was used at a concentration of 1/10,000.
RESULTS
Some BL-derived cell lines, despite having a mutant p53, were previously shown to undergo cell cycle arrest and apoptosis after DNA damage by different genotoxic agents (31). For this reason, these cells are excellent tools for the study of B cells lacking wild-type p53 function and the effects EBV has on cell cycle and cell death regulation, since the virus does not appear to target p53 (1, 2, 22). A series of experiments were performed to characterize further the effect of latent EBV on the p53-independent responses of BL-derived cell lines to damage induced by irradiation and to the antimicrotubule poisons nocodazole and taxol, which specifically target mitosis.
EBV protects BL41/B95.8 cells from dying after aberrant mitosis following recovery from gamma-irradiation.
In order to investigate the effect of EBV on the response of BL41 cells to gamma-irradiation, both BL41 and BL41/B95.8 cells grown to similar densities were irradiated (850 rads) and then examined periodically by microscopy and their DNA content was analyzed by flow cytometry after staining with propidium iodide (Fig. 1a and b). These analyses revealed that BL41 cells accumulated with 4N DNA content and apparently normal interphase nuclei approximately 24 h after irradiation, consistent with the cells being blocked in G2/M. Similar responses in p53-negative B cells have been reported previously (19). EBV-positive BL41/B95.8 cells also accumulated with apparently normal nuclei and 4N DNA content. This indicated that EBV is unable to overcome the G2/M arrest after irradiation and is consistent with the results from previous experiments using EBV-immortalized LCLs (5, 21).
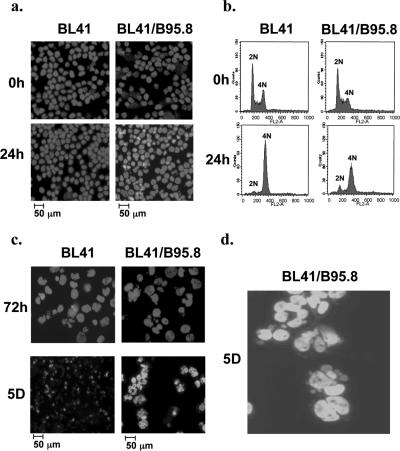
FIG. 1.
EBV does not prevent radiation-induced G2/M arrest but protects cells from dying as they subsequently enter mitosis with damaged DNA. BL41 and BL41/B95.8 cells were exposed to gamma-irradiation (850 rads) and harvested after treatment. Nuclear morphology was observed by microscopy, and flow cytometric analysis of cellular DNA content was performed at the times indicated. (a and b) After 24 h, both BL41 and BL41/B95.8 cells have normal nuclear morphology and are largely arrested, with 4N DNA content. (c) After 72 h, both populations recovered from the G2/M arrest and completed mitosis, and abnormal nuclei appeared in both populations. EBV-negative BL41 cells then died between 3 and 5 days (5D); in contrast, EBV-infected BL41/B95.8 cells survived with increasing multi- and micronucleation. (d) Enlarged view of aberrant nuclei 5 days after irradiation.
Three days after irradiation, both BL41 and BL41/B95.8 populations adapted, recovered from the G2/M-associated arrest, and progressed through mitosis. In both cases, this produced many morphologically aberrant nuclei (Fig. 1c, upper panels). The EBV-negative BL41 cells subsequently died rapidly. In contrast, the EBV-infected BL41/B95.8 cells survived with grossly abnormal nuclei; cells with multiple nuclei and micronuclei were both abundant (Fig. 1c, lower panels, and d). Similar experiments were performed using the EBV-negative BL line Ramos and its EBV-positive pair Ramos/AW. Ramos cells behaved in a manner very similar to BL41 cells and were dead within 5 days. In contrast, Ramos/AW cells survived, generally with morphologically abnormal nuclei (data not shown). Since the Ramos/AW cell line carries an EBV genome with a deletion that removes the EBNA2 gene and part of the EBNA-LP coding sequence, this suggested that a restricted subset of EBV gene expression was necessary for survival.
To summarize, these results showed that EBV appears not to overcome the p53-independent transient accumulation of cells at G2/M following irradiation. However, after cells adapt and recover from this arrest it appears that a restricted pattern of EBV expression may suppress a mitotic checkpoint and rescue cells from death during aberrant mitosis.
EBV overcomes a metaphase arrest in cells exposed to the microtubule-disrupting agent nocodazole.
The preceding series of experiments suggested that latent EBV has activities that interfere with mitotic checkpoint function. We therefore wanted to explore further the effect of latent EBV on other events associated with arrest in mitosis, in particular, the spindle assembly checkpoint that is activated in prometaphase by microtubule poisons.
Microtubule-depolymerizing agents such as nocodazole lead to disruption of mitotic spindle assembly. Because the kinetochores of condensed chromosomes cannot attach to a functional spindle, this activates a checkpoint that results in a metaphase arrest. In normal mammalian cells, this M-phase arrest can be transient and there is slippage from metaphase and abnormal progression through the remainder of the cell cycle.
Both BL41 and BL41/B95.8 cells were grown to similar densities, treated with 50 ng/ml nocodazole for 24 h, stained with PI, and analyzed by flow cytometry. As expected, nocodazole was able to induce an accumulation of BL41 cells with a 4N DNA content. Many BL41/B95.8 cells treated in a similar manner also accumulated with 4N DNA (Fig. 2a). Although it was evident that most cells in both populations accumulate with 4N DNA, subpopulations containing more than 4N DNA were also revealed. This indicated that cells were either completing abnormal mitosis but not cytokinesis or endoreduplicating DNA from a pseudo-G1. Interestingly, this >4N population was significantly greater in BL41/B95.8 cells (approximately 24%, compared with <10% in BL41 cells), which indicates that EBV interferes with the mitotic checkpoint activated by nocodazole.
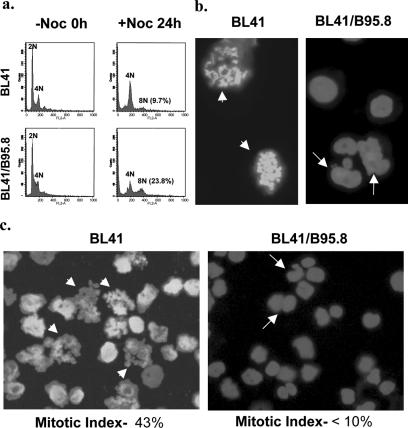
FIG. 2.
Impaired metaphase arrest in EBV-positive BL41/B95.8 cells treated with nocodazole (Noc). Cells were left untreated (−Noc) or treated with nocodazole (+Noc) (at a concentration of 50 ng/ml) for 24 h. (a) Cells were harvested for flow cytometry. An accumulation of cells with 4N DNA content was seen for both EBV-negative BL41 and EBV-positive BL41/B95.8 cells. In the EBV-positive population, there was a significantly greater increase in the proportion of cells with >4N DNA content (23.8%, compared with 9.7% in BL41 cells). (b and c) DAPI staining 24 h after treatment with nocodazole showed that while EBV-negative BL41 cells are arrested at metaphase with condensed chromosomes (metaphase spreads, arrowheads), EBV-positive BL41/B95.8 cells generally showed few chromosome spreads, suggesting that the EBV-positive cells have an impaired checkpoint. BL41/B95.8 cells with multiple or fractured nuclei were observed (arrows). Quantification of both metaphase and interphase cells 24 h after treatment with nocodazole showed a high percentage of BL41 cells arrested in metaphase (43%) and only a very small percentage of interphase cells. In contrast, the majority of BL41/B95.8 cells had interphase nuclei and almost no cells were obviously arrested at metaphase (<10%).
Analysis of nuclear morphology in DAPI-stained cells 24 h after nocodazole treatment was consistent with this interpretation. Nocodazole disrupts assembly of the mitotic spindle; therefore, the response of most cells is to arrest during prometaphase when condensed chromosomes line up to form the metaphase plate. The percentage of metaphase-arrested cells in a population is known as the mitotic index. Low-speed cytospins were prepared from both BL41 and BL41/B95.8 cells treated for 24 h with nocodazole and stained with DAPI. Figures 2b and c show BL41 cells in a typical experiment with metaphase-arrested morphology (condensed chromosomes can be seen as spreads). The mitotic index of this population of cells was determined to be about 43%. In contrast, staining BL41/B95.8 cells after similar treatment revealed very few cells with condensed chromosomes, indicating that the majority were not arrested in metaphase. The mitotic index of this population was judged to be about 10%, and it was noted that many of the cells were multinucleated or had developed morphologically abnormal nuclei (Fig. 2b and c, right panels). These cells with grossly abnormal nuclei probably represent the population with 4N or greater DNA content observed in the cell cycle profile of BL41/B95.8 cells after 24 h (Fig. 2a). Together, these results suggest that EBV latent gene expression in BL cells interferes with the activation or execution of the spindle assembly checkpoint and thus prevents metaphase arrest.
EBV-negative BL41 cells die after the mitotic arrest induced by nocodazole or taxol, while EBV-positive BL41/B95.8 cells become polyploid and develop aberrant nuclei.
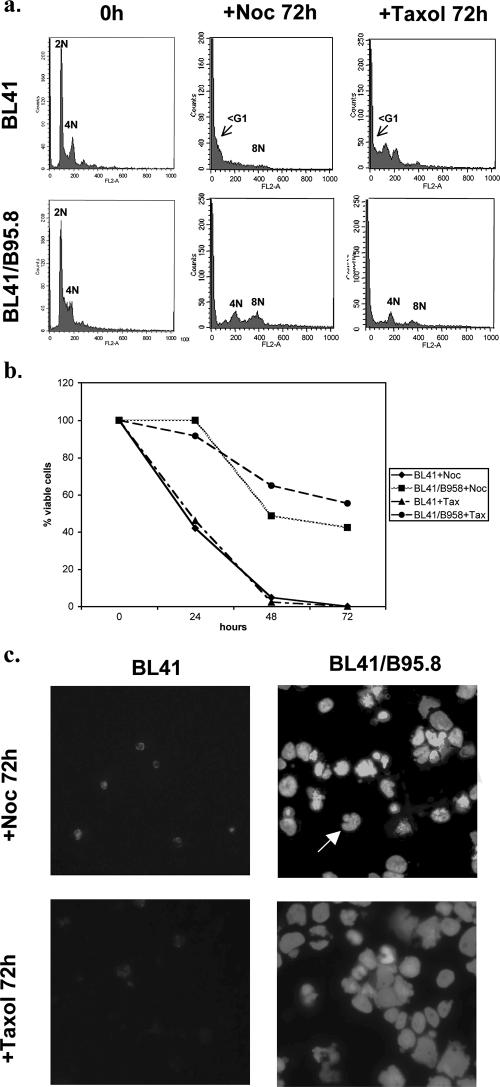
To further characterize the response of BL cells to nocodazole, BL41 and BL41/B95.8 cells were analyzed 72 h after treatment. Microscopy and flow cytometry were performed after DAPI or trypan blue staining and propidium iodide staining, respectively. Figure 3a shows that after 72 h there was a significant increase in the sub-G1 population of BL41 cells, indicating that most of these cells were undergoing an apoptosis-like cell death. Cellular morphology, as revealed by DAPI and counts of trypan blue-excluding viable cells, also indicated that most of the BL41 cells had died after 72 h, since only debris could be seen at this time (Fig. 3b and c). The cell cycle profile of BL41/B95.8 cells 72 h after treatment with nocodazole was markedly different from the profile of BL41 cells. In contrast to the BL41 cells, although the sub-G1 population in BL41/B95.8 cells had slightly increased by 72 h, most of the cells had a 4N or greater DNA content. Consistent with this, 72 h after the addition of nocodazole, microscopy showed that viable BL41/B95.8 cells were abundant (50% or more survived) and many had grossly abnormal or fractured nuclei (Fig. 3b and c). This indicated that these cells continued to synthesize DNA in the presence of nocodazole and became polyploid or aneuploid. Similar experiments were performed using taxol, which causes aberrant microtubule polymerization, prevents spindle assembly and kinetochore attachment, and so also activates the checkpoint. Taxol induced a metaphase arrest in the BL41 cells but (like nocodazole) failed to trigger a similar arrest in the EBV-positive BL41/B95.8 cells (data not shown). As a result, after 72 h, numerous 4N and >4N cells with abnormal nuclear morphology were seen in the EBV-positive population, whereas mass cell death was seen in the EBV-negative BL41 population (Fig. 3b and c).
FIG. 3.
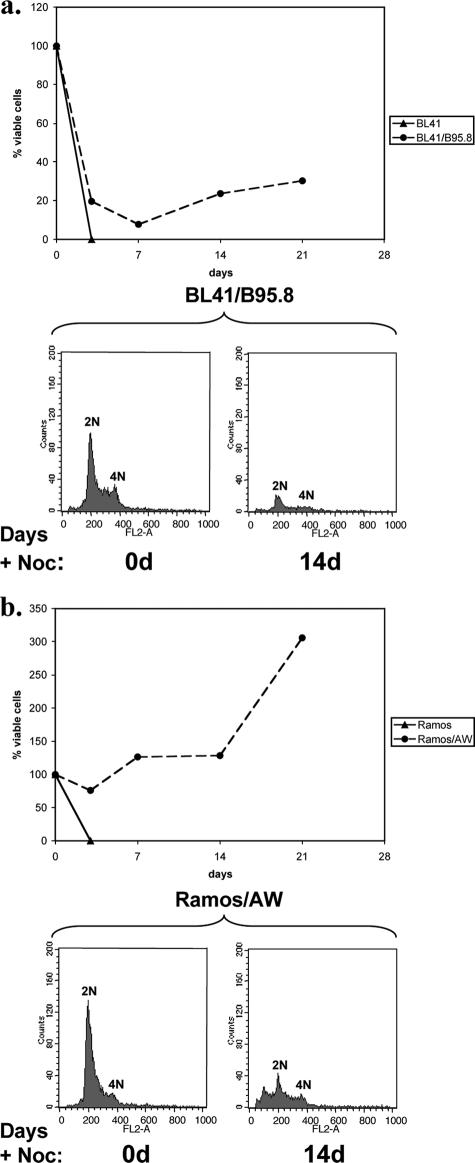
EBV-positive BL cells survive treatment with nocodazole or taxol. Cells were treated with either nocodazole (+Noc) or taxol (+Tax) (both at a concentration of 50 ng/ml) and harvested at the times indicated. (a) The cell cycle profiles of the BL41 EBV-negative cells and BL41/B95.8 EBV-positive cells were very different 72 h after treatment with nocodazole or taxol. A significant increase in the sub-G1 population indicated apoptosis or mitotic catastrophe in the EBV-negative BL41 cells. In contrast, EBV-positive BL41/B95.8 cells showed only modest increases in the sub-G1 population and most of the cells had a 4N or >4N DNA content after treatment. (b) The percentage of viable cells surviving after treatment with nocodazole or taxol was determined. Viable cells that excluded trypan blue were expressed as a percentage of the starting population. (c) DAPI staining confirmed that EBV-negative BL41 cells died, since after 72 h only cell debris was seen. In contrast, the EBV-infected BL41/B95.8 cells survived treatment with nocodazole or taxol and many cells became multinucleated or developed micronuclei (arrow).
Latent infection with P3HR1 also increases cell survival after treatment with spindle poisons.
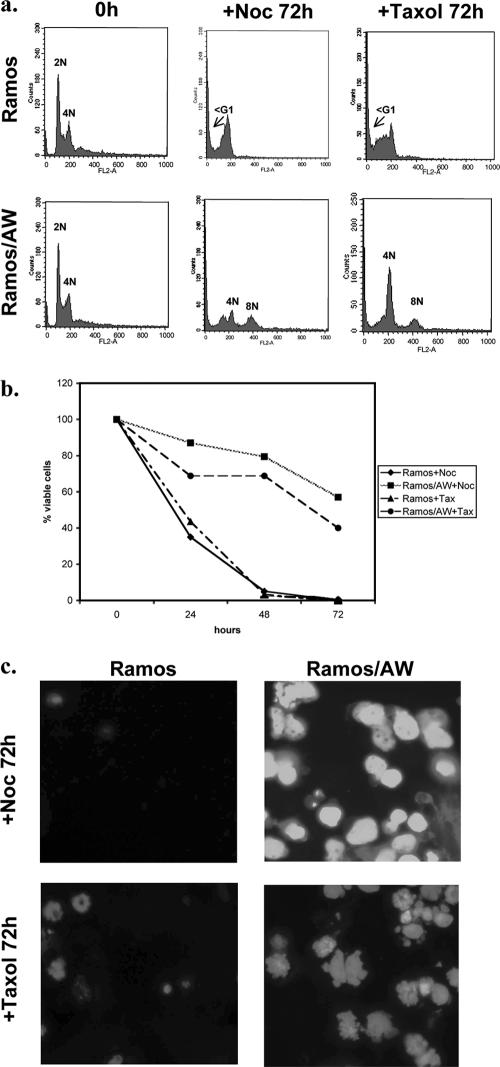
In order to test whether the responses associated with the spindle assembly checkpoint described above were restricted to B95.8-infected cells, similar experiments were performed on Ramos BL-derived cells latently infected with P3HR1 virus. The responses of P3HR1-infected BL cells to both nocodazole and taxol were very similar to those of cells carrying latent B95.8 virus. Ramos/AW cells that carry latent P3HR1 (and express only EBNA1, truncated EBNA-LP, EBNA3A, -3B, and -3C proteins, and the EBER and BART RNAs) neither arrest in metaphase nor die; rather, they progress through mitosis and develop gross nuclear abnormalities. In contrast, the parental EBV-negative Ramos cells initially arrested in metaphase and then died in a manner similar to BL41 cells (Fig. 4 and data not shown).
FIG. 4.
P3HR1-positive BL cells also survive treatment with nocodazole (+Noc) or taxol (+Tax). (a) Cell cycle profiles of the EBV-negative Ramos cells and P3HR1 EBV-positive Ramos/AW cells are very different 72 h after treatment with either nocodazole or taxol. A significant increase in the sub-G1 population was observed for EBV-negative Ramos cells, indicating apoptosis had occurred. In contrast, EBV-positive Ramos/AW cells showed only a slight increase in the sub-G1 population and most of the cells had developed 4N or >4N DNA content. (b) The percentage of viable cells surviving after treatment with nocodazole or taxol was determined. Viable cells that excluded trypan blue were expressed as a percentage of the starting population. (c) DAPI staining showed that EBV-negative Ramos cells died, and 72 h after the treatment with either nocodazole or taxol only cell debris was seen. In contrast, the EBV-infected Ramos/AW cells survived treatment and many cells became multinucleated or developed micronuclei.
A subpopulation of EBV-positive BL41 and Ramos cells survive for up to 3 weeks and proliferate.
In order to determine the long-term fate of cells that were still viable after 72 h of exposure to nocodazole, the lines were treated with nocodazole, washed, and resuspended in freshly conditioned medium. By this time, all of the EBV-negative BL41 and Ramos cells were dead. However, depending on the cell line and experiment, 20 to 70% of the EBV-positive converted cells were viable after being washed and resuspended in fresh medium. On further incubation (with feeding every 4 to 5 days), it was revealed that in both BL41/B95.8 and Ramos/AW cultures viable cells survived for at least 3 weeks and some, presumably those containing a less severely damaged diploid genome, were able to resume proliferating; hence, the number of viable cells increased (Fig. 5).
FIG. 5.
EBV-positive cells can recover from exposure to nocodazole. BL41 and BL41/B95.8 cells (a) and Ramos and Ramos/AW cells (b) were treated with nocodazole (+Noc) (50 ng/ml) for 72 h. The cells were then washed and resuspended in conditioned medium (without nocodazole), and viable cells were counted. The cells were then cultured for a further 18 days, and samples were removed, stained with trypan blue, and counted at weekly intervals. Samples were also taken at the beginning of the experiment and, after 14 days (14d), stained with PI and analyzed by flow cytometry.
Latent infection with P3HR1-like viruses increases BL survival after treatment with spindle poisons.
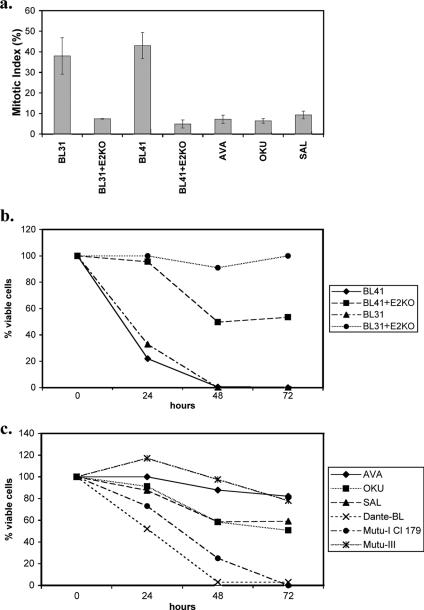
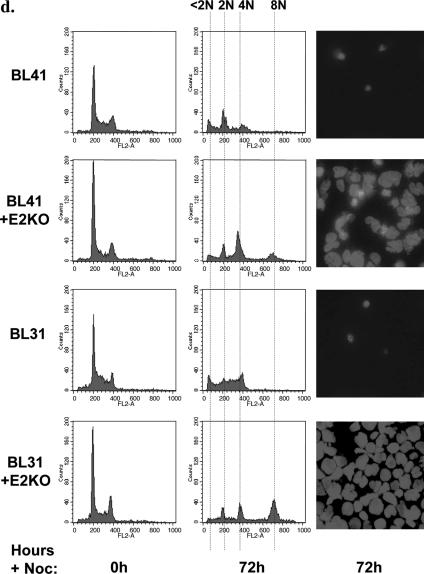
Since BL41, BL41/B95.8, Ramos, and Ramos/AW cells have all been grown in culture for many years, they may have developed clonal idiosyncrasies. In order to reinforce the hypothesis that EBV gene expression is responsible for the phenotypes described above and to confirm that the limited pattern of viral proteins associated with P3HR1 is sufficient, newly established lines produced with a recombinant virus were investigated. The EBV-negative BL lines BL41 and BL31 were infected with a recombinant hygromycin-resistant strain of EBV from which the EBNA2 open reading frame had been deleted to produce a P3HR1-like virus (14). EBV-positive converts of BL41 and BL31 were produced by selection in hygromycin, and like BL cells latently infected with P3HRI virus, each convert expresses EBNA1 and the EBNA3 proteins but not EBNA2 or LMP1 (14). Unlike P3HR1-infected cells, the EBNA2 knockout (KO) converts express full-length EBNA-LP. The parental lines (BL41 and BL31) and the converts (BL41+E2KO and BL31+E2KO) were treated with nocodazole and analyzed as described above. After 24 h of exposure to nocodazole, metaphase-arrested cells were counted to establish the mitotic index of each cell line. While the mitotic index of the parental cells was consistently about 40%, that of the E2KO converts was consistently about 10% (Fig. 6a). Seventy-two hours after initial exposure to nocodazole, the parental BL41 and BL31 cells were all dead and only cell fragments remained. In contrast, the EBNA2-KO converts were viable, with 4N or 8N DNA content (Fig. 6b and d). As anticipated, the phenotype of these cells was similar to that of BL41/B95.8 and Ramos/AW cells and therefore consistent with the restricted pattern of EBV latent gene expression associated with P3HR1 impairing the spindle checkpoint and rescuing the cells from programmed cell death in mitosis. Similar experiments performed with BL cell lines (Dante and Mutu-I) that exhibit the type I pattern of latency (EBNA1, EBERs, and BARTS) showed their response to nocodazole to be similar to that of EBV-negative BL cells. EBNA1, EBERs, and BARTs do not reduce the mitotic index or rescue cells from death in mitosis (Fig. 6c and e and data not shown). It is important to note that Mutu cells that had drifted in culture to produce the full latency III pattern of EBV gene expression (Mutu-III cells) were fully protected from the lethal effects of nocodazole (Fig. 6c and e). This shows that Mutu cells per se are not intrinsically more susceptible for reasons unrelated to EBV gene expression.
FIG. 6.
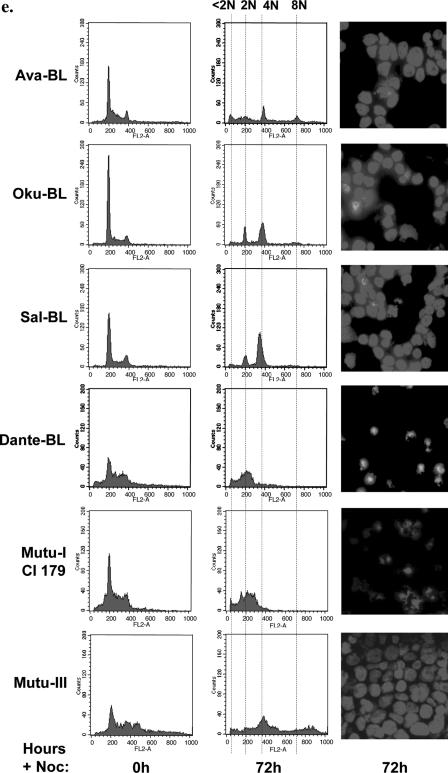
BL cells carrying P3HR1-like EBV have an impaired spindle checkpoint and are rescued from cell death induced by nocodazole. (a) Cells were treated with nocodazole (50 ng/ml) for 24 h, and the mitotic index was determined. Spreads of condensed metaphase chromosomes were scored as arrested cells and expressed as a percentage of the total number of DAPI-stained cells counted in three separate fields (a minimum of 300 cells was counted in each experiment). The results of a typical experiment are shown graphically, with the means and standard deviations of three fields given. (b and c) The percentages of viable cells surviving after treatment with nocodazole were determined. Viable cells that excluded trypan blue were expressed as a percentage of the starting population. (d and e) Seventy-two hours after the addition of nocodazole (+Noc), cells were analyzed by flow cytometry and microscopy. EBV-negative BL41 and BL31 and EBV-positive, latency I Dante and Mutu-I (clone [Cl] 179) cells were all dead, and only cell debris was visible. The EBV-positive EBNA2-KO converts and Ava-BL, Oku-BL, Sal-BL, and Mutu-III cells appeared largely viable, with a significant 4N or greater DNA content.
Recently, Rickinson and colleagues described Burkitt's lymphoma biopsy material and newly established cell lines (Ava-BL, Oku-BL, and Sal-BL) that carry EBV genomes including similar deletions to P3HR1 and consequently express a P3HR1-like pattern of EBV proteins (13, 14). The responses of low-passage Ava-BL, Oku-BL, and Sal-BL cells to nocodazole were determined and found to be very similar to those obtained with cells infected with B95.8 or P3HR1, that is, the cells exhibited a relatively low mitotic index, survived the treatment, and developed aberrant nuclei (Fig. 6a, c, and e). It would appear that, as with latency III, the restricted pattern of EBV gene expression found in Ramos/AW cells, E2-KO converts, and Ava-BL, Oku-BL, and Sal-BL cells is sufficient to impair the spindle assembly checkpoint and rescue cells from programmed cell death during aberrant mitosis.
Proteolytic cleavage of PARP indicates caspase-dependent cell death.
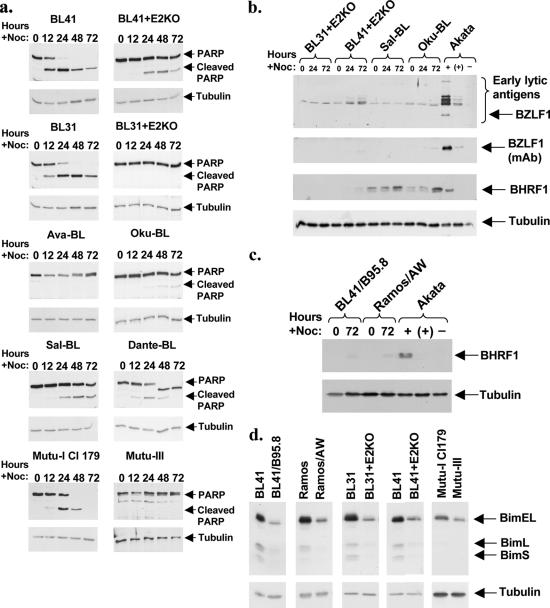
To further characterize the fate of the BL cells responding to microtubule poisons, we asked whether the cell death seen with BL41 and BL31 cells was associated with caspase activity and whether this was suppressed by latent EBV. Cells were treated with nocodazole for up to 72 h, and samples were taken periodically for Western blot analysis. Blots were probed to show caspase-specific cleavage of PARP and also for tubulin as a protein loading control. Consistent with the death of these metaphase-arrested cells involving activation of caspases, full-length PARP was completely cleaved in EBV-negative BL41, BL31, and also latency I Dante and Mutu-I cells but remained largely or completely unaffected in the EBNA2-KO converts and Mutu-III cells (Fig. 7a). Similarly, the PARP in the cells from the newly isolated BL lines (Ava, Oku, and Sal) that carry P3HR1-like virus remained largely or entirely intact after the cells were exposed to nocodazole.
FIG. 7.
(a) Nocodazole induces proteolytic cleavage of PARP in EBV-negative and latency I BL cells but not in cells infected with P3HR1-like EBV. Cells were treated with nocodazole (+Noc), and at the times indicated, samples were taken and protein extracts prepared. Proteins were resolved by SDS-PAGE (7.5%), and Western immunoblotting was performed with rabbit anti-PARP and a mouse anti-γ-tubulin MAb. Full-length (113-kDa) PARP and the 89-kDa product of caspase-mediated cleavage are both indicated by arrows. (b) Rescue from cell death is not dependent on EBV lytic gene expression. Protein extracts from the nocodazole-treated cells were separated by SDS-PAGE (12.5%), and Western immunoblotting was performed with mouse anti-BZLF1 and anti-BHRF1 MAbs or the human serum EE. Akata BL cells were left untreated (−) or treated with anti-Ig [+ and (+)] to stimulate the expression of EBV lytic proteins. The induced population was approximately 15%, as judged by anti-BZLF1 immunofluorescence staining (Carol McDonald, personal communication), and the track labeled (+) shows anti-Ig-induced Akata extracts diluted to 1/10 with EBV-negative BL31 extract. (c) BHRF1 is neither expressed nor induced by nocodazole in BL41/B95.8 or Ramos/AW cells. (d) Bim levels correlate with sensitivity to nocodazole. Protein extracts from untreated BL cells were separated by SDS-PAGE (12.5%), and Western immunoblotting was performed using a rabbit anti-Bim polyclonal antibody. The three Bim isoforms (BimEL, BimL, and BimS) are indicated. γ-Tubulin was used as a control for equal loading in all of these Western blots.
Rescue from nocodazole-induced cell death is not dependent on EBV lytic gene expression.
We showed previously that sometimes activators of the EBV lytic program are able to induce caspase activity and apoptosis but that EBV lytic gene expression protects the cells containing replicating virus from cell death (12). It has also been reported previously that some cytotoxic agents (for instance, irradiation) can induce the EBV lytic cycle in some cells (33). In order to determine whether lytic gene products were involved in the rescue from mitotic death seen here, various lines judged to be resistant were treated with nocodazole for up to 72 h and Western blotting was performed with protein extracts for evidence of EBV lytic activity. Figure 7b shows that nocodazole failed to consistently induce the expression of the lytic switch protein BZLF1 or various EBV early antigens recognized by human serum EE in a selection of EBV-positive BL cells used in this study. Extracts were also probed with a monoclonal antibody (MAb) raised against the EBV Bcl2 homologue BHRF1 (Fig. 7b and c). In the BL41, BL31, and Ramos EBV-positive lines, this protein was neither constitutively expressed nor induced by nocodazole. However, it can be seen that, in Sal- and Oku-BL cells, BHRF1 was constitutively expressed at a low level and that this was slightly induced after 72 h. This was surprising because by using the very sensitive serum EE and the BZLF1 MAb we calculated that <1.5% of the Sal and Oku cells were in the lytic cycle (see the legend for Fig. 7). This suggests that in these two lines BHRF1 may be expressed as a latent gene. We conclude that EBV lytic activity is not generally required to rescue cells from death in aberrant mitosis but that BHRF1 could make a minor contribution to survival in the unusual BL lines Sal and Oku.
Bim could be a determinant of sensitivity to nocodazole.
It has been shown previously that the BH3-only proapoptotic protein Bim may be a major determinant of sensitivity to spindle-disrupting drugs in transformed epithelial cells (27). Bim is also particularly important in the regulation of apoptosis during B-cell development, and its regulation by cMyc can play an important role in the pathogenesis of BL (8, 11, 26). Furthermore, a recent report indicated that EBV-converted BL41 and Ramos cells express lower levels of Bim than the parental lines (7). Protein extracts from the matched pairs of EBV−ve and EBV+ve cells previously screened for caspase activity were analyzed by Western blotting and probed for expression of the Bim proteins (Fig. 7d). Consistent with Bim playing a role in the mitotic death of the EBV-negative cells and EBV causing Bim down-regulation, levels of all three isoforms of Bim were significantly higher in BL31 and BL41 cells than in the EBV-converted partners. Mutu-III cells also expressed significantly lower levels of Bim than the EBNA1, EBER, and BART-only Mutu-I cells. These data extend the report that latent EBV infection can lead to a down-regulation of Bim in B lymphocytes and confirm that the reduction of expression does not require EBNA2 or the latent membrane proteins (7).
DISCUSSION
After experiments with ionizing radiation suggested that EBV might interfere with the regulation of mitosis, a study in which the spindle assembly checkpoint was specifically triggered by exposing cells to microtubule-disrupting agents was performed. This has very clearly demonstrated that EBV-negative lines BL41, Ramos, and BL31 and EBV-positive BL lines Dante and Mutu-I, which express only EBNA1, EBERs, and BARTs, are all very sensitive to the lethal actions of nocodazole and taxol. In each case, a transient arrest in metaphase was followed by high levels of caspase-associated cell death that probably occurs during aberrant mitosis following adaptation and slippage from metaphase (24, 29, 32). Since the precise distinction between apoptosis and mitotic catastrophe remains unclear and somewhat controversial (4, 6, 17), here this process is described as caspase-dependent mitotic cell death.
When the EBV-negative BL lines are latently infected with EBV, in established cell lines such as BL41/B95.8 or Ramos/AW or in newly converted lines produced using EBNA2 knockout recombinant EBV, the cells exhibit an impaired metaphase arrest and are rescued from cell death. Newly isolated BL lines (Ava, Sal, and Oku) carrying P3HR1-like EBV showed a similar response. The consistency of these responses suggested that clonal variations that are independent of EBV infection are very unlikely to play a significant role in the observed phenotypes.
Which EBV gene product is responsible for this mitotic phenotype? EBNA2 and the LMPs can be excluded as candidates because these are not expressed in any of the P3HR1-converted cells, the EBNA2 knockout lines, or the newly established Ava-, Oku-, and Sal-BL cells (14, 31). This is because EBNA2 is required as a transactivator of the LMP genes. Since EBNA2, LMP1, and LMP2 have all been shown to promote cell survival, this is a rather surprising observation (9, 16, 23). EBNA-LP is truncated in the P3HR1-converted cells and is expressed at a very low level in the nocodazole-resistant Oku-BL cells, arguing against EBNA-LP playing a significant role (14). BL cells with a latency I pattern of EBV gene expression (Mutu-I and Dante cells) are very sensitive to nocodazole relative to a latency III-expressing derivative, so it is unlikely that EBNA1, the EBERs, or the BARTs play any role in this resistance. Nocodazole failed to consistently induce lytic gene expression, so the various antiapoptotic EBV lytic proteins cannot be involved in most cases. We did note that Sal-BL and Oku-BL cells expressed low levels of BHRF1. Here it is possible that BHRF1 may contribute to survival. The only full-length EBV-encoded factors that are common to all of the nocodazole-resistant cells are EBNA3A, EBNA3B, and EBNA3C. Thus, in principle one or more of this family of nuclear proteins are likely to be responsible for this striking phenotype. Nevertheless, we cannot formally exclude the role of EBV latent gene products that have yet to be identified or the possibility that entry of a small number of cells into the lytic cycle may exert paracrine effects on the main population.
There appear to be two components of the resistance to spindle poisons seen in these EBV-carrying cells: suppression of metaphase arrest leading to a reduced mitotic index and escape from caspase-associated cell death leading to nuclear abnormalities (summarized in Fig. 8). It is presently unclear to what extent these phenomena are linked and precisely which EBV function(s) is involved. Recent reports of cells with a weakened or completely ablated spindle assembly checkpoint incurring much less mitotic catastrophe/apoptosis than their checkpoint-proficient counterparts are consistent with the hypothesis that EBV need only target the checkpoint (18, 29, 32), and our previous observation that EBNA3C can suppress the spindle checkpoint in U2OS cells is also consistent with this hypothesis (22).
FIG. 8.
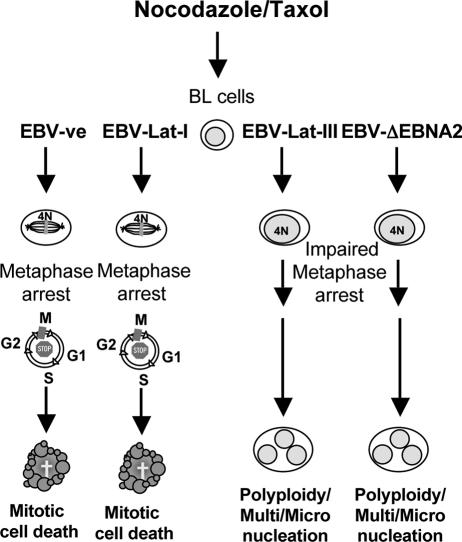
Summary of the responses to activators of the spindle assembly checkpoint in BL cells. We present a schematic diagram showing the responses of EBV-positive and EBV-negative BL-derived cells to mitotic spindle damaging agents nocodazole and taxol. EBV-negative BL cells (EBV-ve) and those expressing the latency I pattern of EBV genes (EBV-Lat-I) arrest largely in prometaphase and subsequently die by caspase-dependent mitotic catastrophe or apoptosis. In contrast, EBV-positive cells expressing either the full spectrum of latent EBV genes (EBV-Lat-III) or the P3HR1-like pattern (EBV-ΔEBNA2) may fail to arrest in prometaphase, survive aberrant mitosis, develop abnormal nuclei, and sometimes become polyploid. EBV-positive cells sometimes recover sufficiently to resume proliferation.
One or more of the EBNA3 genes, whether expressed from a recombinant EBV or the Ava, Oku, and Sal strains of EBV, mediate survival of cells exposed to ionomycin or anti-IgM (14); however, it should be noted that even complete latent EBV gene expression does not always enhance cell survival. For instance, LCLs are induced to undergo apoptosis by various DNA-damaging agents (1, 2, 21, 31) and LCLs and DG75 BL cells expressing the EBNA3 proteins are induced to die by a histone deacetylase inhibitor (15, 25). It is evident that the EBNA3 proteins do not suppress apoptosis per se. Nevertheless, since the level of the proapoptotic factor Bim is a determinant of sensitivity to taxol in breast epithelial cells and can influence the pathogenesis of BL (8, 11, 27), it is probable that the down-regulation of Bim levels by EBV plays a role in the development of resistance to spindle poisons in the BL cells studied here. The data shown are consistent with one or more of the EBNA3 proteins reducing Bim expression and increasing cell survival by this and perhaps other means. Systematic deletion of each of the EBNA3 genes in recombinant EBV and the conversion of nocodazole-sensitive BL lines should cast further light on these issues and may distinguish between cell cycle and cell survival activities.
A weakened or compromised spindle assembly checkpoint is seen frequently with cancer cells, and because it can result in the passage of a damaged genome to progeny cells it may directly contribute to tumorigenesis (reviewed in references 24, 29, and 32). Since even after exposure to 50 ng/ml nocodazole for 72 h, some EBV-carrying Ramos and BL41 cells survive in culture for up to 3 weeks and resume proliferating (Fig. 5), we suggest that this could be the case when lymphomas carry latent EBV and express the EBNA3 proteins. It is of practical significance that the presence of these proteins appears to dramatically modulate the sensitivity of BL cells to agents used in anticancer therapy. Two reports have led to suggestions that microtubule-disrupting agents may be highly suitable for the clinical management of a subset of lymphomas, including BL. Burns and colleagues showed that silencing the polo-like kinase Snk/Plk2 gene leads to a significant increase in mitotic catastrophe or apoptosis in cells exposed to taxol-like drugs. They therefore suggested that this knowledge could be of therapeutic value when using taxanes or other microtubule-disrupting agents on tumors (4). More recently, Syed and colleagues reported that Snk/Plk2 is often silenced by promoter methylation in several different B-cell malignancies. More specifically, they showed that this methylation-dependent silencing occurs with very high frequency in BL (n = 24/25). On this basis, they suggest that BL may be a particularly good target for taxane-like drugs (28).
The data we have presented here support this speculation. The EBV-negative BL and type I latency BL cells examined were indeed very sensitive to nocodazole and taxol. However, it is clear from recent studies (13, 14) that a minor but significant subpopulation of BL cells may carry deletion mutant EBV expressing the EBNA2 knockout pattern of genes that includes the EBNA3 family of proteins. It is not known how frequently such tumors arise, but when they occur it is likely that they will have a significantly reduced sensitivity to cytotoxic therapies based on the disruption of microtubules or agents that damage DNA.
Acknowledgments
We are very grateful to Gemma Kelly (Birmingham, United Kingdom), for the low-passage Ava-BL, Oku-BL, Sal-BL, BL41-E2KO, and BL31-E2KO cells and to Carol McDonald and Paul Farrell for the anti-BZLF1 antibodies and Akata protein samples. We thank Jenny O'Nions and Paul Farrell for helpful comments on the manuscript.
This work was partly supported by grants from the Wellcome Trust and the Portuguese Foundation for Science and Technology (grant reference no. SRFR/BD/2730/2000) and MRC Ph.D. studentships.
Footnotes
Published ahead of print on 11 October 2006.
REFERENCES
- 1.Allday, M. J., G. J. Inman, D. H. Crawford, and P. J. Farrell. 1995. DNA damage in human B cells can induce apoptosis, proceeding from G1/S when p53 is transactivation competent and G2/M when it is transactivation defective. EMBO J. 14:4994-5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allday, M. J., A. Sinclair, G. Parker, D. H. Crawford, and P. J. Farrell. 1995. Epstein-Barr virus efficiently immortalizes human B cells without neutralizing the function of p53. EMBO J. 14:1382-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bornkamm, G. W., and W. Hammerschmidt. 2001. Molecular virology of Epstein-Barr virus. Philos. Trans. R. Soc. Lond. B 356:437-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burns, T. F., P. Fei, K. A. Scata, D. T. Dicker, and W. S. El-Deiry. 2003. Silencing of the novel p53 target gene Snk/Plk2 leads to mitotic catastrophe in paclitaxel (Taxol)-exposed cells. Mol. Cell. Biol. 23:5556-5571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cannell, E. J., P. J. Farrell, and A. J. Sinclair. 1998. Cell cycle arrest following exposure of EBV-immortalised B-cells to gamma irradiation correlates with inhibition of cdk2 activity. FEBS Lett. 439:297-301. [DOI] [PubMed] [Google Scholar]
- 6.Castedo, M., J. L. Perfettini, T. Roumier, A. Valent, H. Raslova, K. Yakushijin, D. Horne, J. Feunteun, G. Lenoir, R. Medema, W. Vainchenker, and G. Kroemer. 2004. Mitotic catastrophe constitutes a special case of apoptosis whose suppression entails aneuploidy. Oncogene 23:4362-4370. [DOI] [PubMed] [Google Scholar]
- 7.Clybouw, C., B. McHichi, S. Mouhamad, M. T. Auffredou, M. F. Bourgeade, S. Sharma, G. Leca, and A. Vazquez. 2005. EBV infection of human B lymphocytes leads to down-regulation of Bim expression: relationship to resistance to apoptosis. J. Immunol. 175:2968-2973. [DOI] [PubMed] [Google Scholar]
- 8.Dang, C. V., K. A. O'Donnell, and T. Juopperi. 2005. The great MYC escape in tumorigenesis. Cancer Cell 8:177-178. [DOI] [PubMed] [Google Scholar]
- 9.Dirmeier, U., R. Hoffmann, E. Kilger, U. Schultheiss, C. Briseno, O. Gires, A. Kieser, D. Eick, B. Sugden, and W. Hammerschmidt. 2005. Latent membrane protein 1 of Epstein-Barr virus coordinately regulates proliferation with control of apoptosis. Oncogene 24:1711-1717. [DOI] [PubMed] [Google Scholar]
- 10.Gualandi, G., L. Giselico, M. Carloni, F. Palitti, P. Mosesso, and A. M. Alfonsi. 2001. Enhancement of genetic instability in human B cells by Epstein-Barr virus latent infection. Mutagenesis 16:203-208. [DOI] [PubMed] [Google Scholar]
- 11.Hemann, M. T., A. Bric, J. Teruya-Feldstein, A. Herbst, J. A. Nilsson, C. Cordon-Cardo, J. L. Cleveland, W. P. Tansey, and S. W. Lowe. 2005. Evasion of the p53 tumour surveillance network by tumour-derived MYC mutants. Nature 436:807-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inman, G. J., U. K. Binne, G. A. Parker, P. J. Farrell, and M. J. Allday. 2001. Activators of the Epstein-Barr virus lytic program concomitantly induce apoptosis, but lytic gene expression protects from cell death. J. Virol. 75:2400-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelly, G., A. Bell, and A. Rickinson. 2002. Epstein-Barr virus-associated Burkitt lymphomagenesis selects for downregulation of the nuclear antigen EBNA2. Nat. Med. 8:1098-1104. [DOI] [PubMed] [Google Scholar]
- 14.Kelly, G. L., A. E. Milner, R. J. Tierney, D. S. Croom-Carter, M. Altmann, W. Hammerschmidt, A. I. Bell, and A. B. Rickinson. 2005. Epstein-Barr virus nuclear antigen 2 (EBNA2) gene deletion is consistently linked with EBNA3A, -3B, and -3C expression in Burkitt's lymphoma cells and with increased resistance to apoptosis. J. Virol. 79:10709-10717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krauer, K. G., A. Burgess, M. Buck, J. Flanagan, T. B. Sculley, and B. Gabrielli. 2004. The EBNA-3 gene family proteins disrupt the G2/M checkpoint. Oncogene 23:1342-1353. [DOI] [PubMed] [Google Scholar]
- 16.Lee, J. M., K. H. Lee, C. J. Farrell, P. D. Ling, B. Kempkes, J. H. Park, and S. D. Hayward. 2004. EBNA2 is required for protection of latently Epstein-Barr virus-infected B cells against specific apoptotic stimuli. J. Virol. 78:12694-12697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mansilla, S., W. Priebe, and J. Portugal. 2006. Mitotic catastrophe results in cell death by caspase-dependent and caspase-independent mechanisms. Cell Cycle 5:53-60. [DOI] [PubMed] [Google Scholar]
- 18.Nitta, M., O. Kobayashi, S. Honda, T. Hirota, S. Kuninaka, T. Marumoto, Y. Ushio, and H. Saya. 2004. Spindle checkpoint function is required for mitotic catastrophe induced by DNA-damaging agents. Oncogene 23:6548-6558. [DOI] [PubMed] [Google Scholar]
- 19.O'Connor, P. M., J. Jackman, D. Jondle, K. Bhatia, I. Magrath, and K. W. Kohn. 1993. Role of the p53 tumor suppressor gene in cell cycle arrest and radiosensitivity of Burkitt's lymphoma cell lines. Cancer Res. 53:4776-4780. [PubMed] [Google Scholar]
- 20.O'Nions, J., and M. J. Allday. 2004. Deregulation of the cell cycle by the Epstein-Barr virus. Adv. Cancer Res. 92:119-186. [DOI] [PubMed] [Google Scholar]
- 21.O'Nions, J., and M. J. Allday. 2003. Epstein-Barr virus can inhibit genotoxin-induced G1 arrest downstream of p53 by preventing the inactivation of CDK2. Oncogene 22:7181-7191. [DOI] [PubMed] [Google Scholar]
- 22.Parker, G. A., R. Touitou, and M. J. Allday. 2000. Epstein-Barr virus EBNA3C can disrupt multiple cell cycle checkpoints and induce nuclear division divorced from cytokinesis. Oncogene 19:700-709. [DOI] [PubMed] [Google Scholar]
- 23.Portis, T., and R. Longnecker. 2004. Epstein-Barr virus (EBV) LMP2A mediates B-lymphocyte survival through constitutive activation of the Ras/PI3K/Akt pathway. Oncogene 23:8619-8628. [DOI] [PubMed] [Google Scholar]
- 24.Rieder, C. L., and H. Maiato. 2004. Stuck in division or passing through: what happens when cells cannot satisfy the spindle assembly checkpoint. Dev. Cell 7:637-651. [DOI] [PubMed] [Google Scholar]
- 25.Sculley, T. B., M. Buck, B. Gabrielli, P. G. Parsons, and K. G. Krauer. 2002. A histone deacetylase inhibitor, azelaic bishydroxamic acid, shows cytotoxicity on Epstein-Barr virus transformed B-cell lines: a potential therapy for posttransplant lymphoproliferative disease. Transplantation 73:271-279. [DOI] [PubMed] [Google Scholar]
- 26.Strasser, A. 2005. The role of BH3-only proteins in the immune system. Nat. Rev. Immunol. 5:189-200. [DOI] [PubMed] [Google Scholar]
- 27.Sunters, A., S. Fernandez de Mattos, M. Stahl, J. J. Brosens, G. Zoumpoulidou, C. A. Saunders, P. J. Coffer, R. H. Medema, R. C. Coombes, and E. W. Lam. 2003. FoxO3a transcriptional regulation of Bim controls apoptosis in paclitaxel-treated breast cancer cell lines. J. Biol. Chem. 278:49795-49805. [DOI] [PubMed] [Google Scholar]
- 28.Syed, N., P. Smith, A. Sullivan, L. C. Spender, M. Dyer, L. Karran, J. O'Nions, M. Allday, I. Hoffmann, D. Crawford, B. Griffin, P. J. Farrell, and T. Crook. 2006. Transcriptional silencing of Polo-like kinase 2 (SNK/PLK2) is a frequent event in B-cell malignancies. Blood 107:250-256. [DOI] [PubMed] [Google Scholar]
- 29.Tao, W. 2005. The mitotic checkpoint in cancer therapy. Cell Cycle 4:1495-1499. [DOI] [PubMed] [Google Scholar]
- 30.Thorley-Lawson, D. A., and A. Gross. 2004. Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N. Engl. J. Med. 350:1328-1337. [DOI] [PubMed] [Google Scholar]
- 31.Wade, M., and M. J. Allday. 2000. Epstein-Barr virus suppresses a G2/M checkpoint activated by genotoxins. Mol. Cell. Biol. 20:1344-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weaver, B. A., and D. W. Cleveland. 2005. Decoding the links between mitosis, cancer, and chemotherapy: the mitotic checkpoint, adaptation, and cell death. Cancer Cell 8:7-12. [DOI] [PubMed] [Google Scholar]
- 33.Westphal, E. M., W. Blackstock, W. Feng, B. Israel, and S. C. Kenney. 2000. Activation of lytic Epstein-Barr virus (EBV) infection by radiation and sodium butyrate in vitro and in vivo: a potential method for treating EBV-positive malignancies. Cancer Res. 60:5781-5788. [PubMed] [Google Scholar]
- 34.Young, L. S., R. Lau, M. Rowe, G. Niedobitek, G. Packham, F. Shanahan, D. T. Rowe, D. Greenspan, J. S. Greenspan, A. B. Rickinson, et al. 1991. Differentiation-associated expression of the Epstein-Barr virus BZLF1 transactivator protein in oral hairy leukoplakia. J. Virol. 65:2868-2874. [DOI] [PMC free article] [PubMed] [Google Scholar]