Abstract
BK virus (BKV) is widely accepted to be the causative agent of polyomavirus nephropathy. In immunocompromised individuals, especially kidney transplant recipients, BKV can replicate in kidney epithelial cells, causing loss of renal function and eventual destruction of the graft. Advances in immunosuppressive therapies may be partially responsible for the increasing incidence of polyomavirus nephropathy among transplant recipients by more effectively eliminating components of the immune system, such as gamma interferon (IFN-γ)-producing lymphocytes, that keep BKV infections at a subclinical level. In this study, we investigated the role of IFN-γ in regulating lytic infection by BKV. Treatment with IFN-γ inhibited the expression of the viral early protein large tumor antigen (TAg) and the late protein VP1 in a dose-dependent manner. We detected 1.6- and 12-fold reductions in TAg transcripts at 48 and 96 h postinfection, respectively, with 250 U/ml IFN-γ, suggesting that IFN-γ-mediated inhibition occurs at the level of transcription. Furthermore, IFN-γ inhibited the level of viral progeny production as much as 50-fold at a multiplicity of infection (MOI) of 0.5 and 80-fold at an MOI of 0.1. The inhibitory effects of IFN-γ were similar for three different strains of BKV examined. These results indicate an important role for IFN-γ in regulating BKV lytic infection.
Polyomavirus nephropathy (PVN) results in renal dysfunction and graft loss in up to 10% of all kidney transplant recipients (18). It is widely accepted that BK virus (BKV) is the etiological agent responsible for the majority of cases of PVN, which are typically diagnosed within the first year after transplantation (19, 20). PVN is characterized by the lytic, destructive replication of BKV in proximal tubule epithelial cells in the transplanted kidney and is normally diagnosed by renal biopsy to assess histological effects of infection, PCR to determine viral presence and loads in the urine and blood, and the detection of decoy cells, which are cells with distinct intranuclear inclusion bodies that are shed during active BKV replication, in the urine (reviewed in references 10, 17, and 30). Since there are currently no effective antiviral treatments for BKV infection, the most common approach used to control PVN is to decrease the patient's immunosuppressive regimen. However, such an approach increases the risk of graft rejection and thus is not an appealing strategy. The prevalence of PVN is increasing with the advent of new, more powerful immunosuppressive therapies, making it a growing concern for the transplant community.
The human polyomavirus BKV was first isolated in 1971 in the urine of a renal transplant recipient (11). BKV virions are small (40 to 45 nm in diameter), nonenveloped, and icosahedral and contain a circular, double-stranded DNA genome of approximately 5.2 kb (reviewed in reference 29) which is associated with cellular histones to form a chromatin-like structure (28). The genome encodes only six known proteins: the early proteins, large tumor antigen (TAg) and small tumor antigen, and the late proteins, VP1, VP2, VP3, and agnoprotein. BKV infects nearly the entire population, with seroprevalence reaching 60 to 80% by the age of 10 (reviewed in reference 23). BKV is thought to be contracted by respiratory transmission, and the primary infection is typically subclinical. Following the initial infection, BKV spreads to other cells of the body, most notably peripheral blood mononuclear cells (9) and cells of the kidney and urinary tract (4, 16, 39), in which the virus establishes a persistent, subclinical infection. It is at these sites in immunocompromised patients that BKV reactivates to a lytic infection, resulting in BKV-associated diseases, such as PVN.
Previously, we described an in vitro system that allows the study of BKV lytic infection of primary human proximal tubule epithelial cells (27). The functions of proximal tubule cells in the kidney include facilitation of the recovery of blood products, maintenance of blood pressure and volume, and production and release of cytokines and chemokines to communicate with the host immune system (2, 6). Proximal tubule cells remain in a differentiated state for up to six passages in tissue culture (21) and thus provide an environment similar to that which BKV encounters in an immunocompromised host. By introducing individual elements of the immune system to this model, we can begin to determine which components regulate BKV replication. In part because proximal tubule epithelial cells are known to interact both with neighboring cells and with the immune system through the production of cytokines and chemokines, namely, interleukin-6 (IL-6), IL-8, IL-15, tumor necrosis factor alpha (TNF-α), monocyte chemoattractant protein 1 (MCP-1), RANTES, and transforming growth factor β (6), we began to investigate the role of cytokines in mediating the regulation of BKV replication.
Our interest in the effects of cytokines on BKV replication was initiated by clinical reports and observations that argued for the importance of the cell-mediated immune response, as opposed to other arms of the immune system, in controlling BKV persistent infection. First, it has been demonstrated that up to 90% of the adult population has BKV-specific antibodies (23) and that patients with levels of anti-BKV antibodies similar to those of healthy individuals still develop PVN (12, 19). Several reports detail the activation of the humoral immune response in patients with PVN, although antibody levels are shown to increase only after the stabilization of renal function and a decrease in viral load (3, 5, 12). Furthermore, high titers of BKV-specific antibodies in the donor correlate well with the incidence of BKV reactivation in the recipient (1). These findings suggest that the presence of BKV-specific antibodies does not prevent the reactivation and development of PVN, although they may be effective at controlling viremia (3). In addition, the failure of the cytotoxic T-lymphocyte response to eliminate all cells harboring BKV, as demonstrated by the well-documented periodic shedding of virus in immunocompetent individuals, indicates that another facet of the immune system plays a role in controlling persistent infection. There are studies demonstrating the correlation of low levels of BKV-specific gamma interferon (IFN-γ)-producing cells and the development of viremia and PVN (3, 5). Furthermore, others have reported the inhibitory effect of IFN-γ on the promoters of simian virus 40, a related polyomavirus, and human cytomegalovirus, which lacks structural relatedness to BKV but has similar epidemiological features, such as latency in the kidney and reactivation upon immunosuppression (15, 34, 35). These findings prompted us to investigate the potential regulation of BKV replication by IFN-γ.
IFN-γ is the sole member of the type II family of interferons and is a secreted glycoprotein of ∼25 kDa produced primarily by natural killer cells during the innate immune response and specific antigen-activated T lymphocytes during the adaptive immune response (reviewed in references 31 and 37). Activation of the IFN-γ cascade within a cell is initiated by the binding of IFN-γ to the cell surface receptor and subsequent activation of the Jak-Stat signaling pathway. Jak1 and Jak2 kinases, which are associated with the two chains of the IFN-γ receptor, IFNGR1 and IFNGR2, respectively, become phosphorylated upon IFN-γ binding. As a result, Stat1 homodimers are phosphorylated and subsequently translocated to the nucleus, where they act as transcription factors to mediate the regulation of IFN-γ-responsive genes (reviewed in references 31 and 37). IFN-γ was first discovered by its antiviral activity (42) and remains widely known as a potent antiviral cytokine. Among the genes up-regulated by the IFN-γ signaling cascade are protein kinase R, which inhibits cellular translation by phosphorylating and inactivating eukaryotic initiation factor 2, 2′-5′-oligoadenylate synthetase, which activates RNase L to nonspecifically degrade mRNA transcripts and inhibit gene expression, and interferon regulatory factor 1 (IRF-1), which up-regulates the production of caspase 1 and promotes apoptosis of the cell. IFN-γ also plays a major role in the activation and recruitment of immune cells and up-regulates the expression of major histocompatibility complex class I and II molecules on the cell surface. More generally, IFN-γ significantly (at least twofold) affects the expression of more than 100 genes in mammalian cells (7). In addition, other genes may be regulated indirectly by a group of IFN-γ-responsive transcription factors that perpetuate the signaling cascade. These transcription factors may also act on viral promoters to inhibit viral gene expression and replication.
In this report, we characterize the specific inhibitory effect of IFN-γ on BKV gene expression and replication during lytic infection of proximal tubule cells. We were interested in determining the points at which the viral life cycle is affected and the conditions under which IFN-γ has the strongest inhibitory effect on viral replication. IFN-γ inhibited BKV gene expression, both at the level of transcription and at the level of translation, and reduced the level of viral progeny production during lytic infection. These results are important for understanding the host immune response to BKV and, more specifically, the role of cytokines in regulating BKV replication and infection.
MATERIALS AND METHODS
Cell culture.
Primary human renal proximal tubule epithelial (RPTE) cells (Cambrex) were maintained for up to six passages in renal epithelial cell basal medium (Cambrex) supplemented with human epidermal growth factor, fetal bovine serum, hydrocortisone, epinephrine, insulin, triiodothyronine, transferrin, and GA-1000 as indicated for renal epithelial cell growth medium (REGM) (supplements obtained as REGM SingleQuots; Cambrex). RPTE cells were grown at 37°C with 5% CO2 in a humidified incubator.
Viruses.
The genome of the TU strain of BKV was cloned into the EcoRI site of pGEM-7Zf(−). Genomes of the Dunlop and Proto-2 strains of BKV were cloned into the BamHI site of pBR322 (gift of P. M. Howley). BKV stocks were prepared from these genomic clones: 4 μg plasmid DNA was digested with restriction enzymes [EcoRI for BKV(TU) and BamHI for BKV(Dunlop, Proto-2)], recircularized with T4 DNA ligase, and phenol-chloroform extracted, and the resulting DNA was transfected into one T75 flask of 60% confluent RPTE cells (∼4 × 106 cells) using Effectene (QIAGEN). After 3 weeks, cells and supernatants were collected and viral lysates were prepared by three freeze (−80°C)-thaw (37°C) cycles. The resulting lysates were used to infect four T75 flasks of 70% confluent RPTE cells, and after 3 weeks, viral lysates were prepared as described above. The resulting viral stocks were titrated by a fluorescent focus assay, and the integrity of the noncoding control region (NCCR) was confirmed by sequencing of PCR products.
Cytokines and chemokines.
Recombinant human IFN-γ, IL-6, IL-8, MCP-1, RANTES, and TNF-α were purchased from PeproTech, Inc. and reconstituted according to the manufacturer's recommendations. Recombinant human IFN-α was purchased from Sigma and supplied as a solution in phosphate-buffered saline (PBS). The doses used to treat BKV-infected RPTE cells were as follows: IL-6, IL-8, MCP-1, and TNF-α were used at 100 ng/ml, RANTES was used at 300 ng/ml, IFN-α was used at 50 or 250 U/ml, and IFN-γ was used primarily at 50 or 250 U/ml, with the exception of the dose-response experiment in which six fivefold dilutions were used, starting from 1,250 U/ml.
Infections.
Unless otherwise stated, 70% confluent RPTE cells were infected with the TU strain of BKV at a multiplicity of infection (MOI) of 0.5 and incubated for 1 hour at 37°C. The viral lysate used for the infection was replaced with fresh medium (REGM), and cytokines were added at 3 to 6 hours postinfection.
Western blotting.
Unless otherwise stated, total cell protein was harvested at 4 days postinfection using E1A lysis buffer (14) supplemented with 5 μg/ml phenylmethylsulfonyl fluoride, 5 μg/ml aprotinin, 5 μg/ml leupeptin, 0.05 M sodium fluoride, and 0.2 mM sodium orthovanadate. The Bio-Rad protein assay was used to determine the protein concentration of each lysate, and 8 μg of protein was electrophoresed on an 8% sodium dodecyl sulfate-polyacrylamide gel. Proteins were transferred to a nitrocellulose membrane in 1× Towbin buffer (25 mM Tris, 192 mM glycine, 20% methanol) for 12 to 14 h at 60 V (constant) and 4°C. The following primary antibodies were diluted in PBS containing 0.1% Tween and 5% nonfat dry milk: pAb416 (13) for the detection of TAg expression, P5G6 (gift of D. Galloway) for the detection of VP1 expression, and Ab8245 (Abcam) for the detection of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression as a loading control. Blots were washed in PBS containing 0.1% Tween, probed with horseradish peroxidase-conjugated anti-mouse immunoglobulin G secondary antibody (Sigma), and developed using ECL Plus reagent (Amersham) and exposure to film.
Fluorescent focus assay.
Viral lysates were harvested at time zero (after 1 hour of adsorption at 37°C) and at 4 days postinfection (or at time zero and 1, 2, 3, 4, and 6 days postinfection for the viral growth curve) by collecting and preparing infected cells and supernatants by three freeze-thaw cycles, as described above. Seventy percent confluent RPTE cells in 24-well plates were infected with 10-fold dilutions of viral lysates for 4 days at 37°C. Cells were fixed with 50% methanol-50% acetone for 10 min at room temperature, air dried for 15 min, wrapped in parafilm, and stored overnight at −20°C as an antigen retrieval step. Plates were thawed briefly at room temperature, rehydrated with PBS, and incubated for 1 hour at 37°C with pAb416 in PBS, followed by fluorescein isothiocyanate-conjugated anti-mouse immunoglobulin G secondary antibody (Sigma) in PBS with 0.005% Evans blue staining. The titer was determined by counting five random fields in at least three replicate wells and is expressed as infectious units per ml (IU/ml). Statistical significance was determined using a two-tailed Student t test assuming unequal variance, and P values of <0.05 were considered significant.
RNA extraction and cDNA synthesis.
Total cell RNA was harvested at 2 or 4 days postinfection using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. RNA samples were treated with DNase I (Promega) to reduce contaminating DNA, and the integrity of the RNA was confirmed by electrophoresis on an agarose gel. To generate cDNA, a reverse transcription (RT) reaction was performed using 1 μg RNA as the template and an iScript cDNA synthesis kit (Bio-Rad) according to the manufacturer's instructions.
Real-time PCR: Taqman assay.
Primers and probes were designed using Primer3 software (36) to amplify 90- and 105-base-pair fragments of the TAg and GAPDH genes, respectively; sequences are shown in Table 1. Primers were synthesized by Invitrogen, and probes, tagged with 6-carboxyfluorescein as the reporter dye at the 5′ end and 6-carboxytetramethylrhodamine-Sp as the quencher dye at the 3′ end, were synthesized by Integrated DNA Technologies, Inc. Reactions were performed in a total volume of 25 μl using 2× TaqMan Universal PCR master mix (Applied Biosystems), 2.5 μl cDNA template, 500 nM of each primer, and 200 nM probe. Amplification was performed in 96-well PCR plates (Bio-Rad) using the iCycler iQ5 real-time detection system (Bio-Rad) with the following PCR program: 2 min at 50°C; 10 min at 95°C; 40 cycles of denaturation at 95°C for 15 s, and annealing and extension at 56°C for 1 min. Results are presented as the changes (n-fold) in TAg transcript levels, with the levels in samples treated with 250 U/ml IFN-γ arbitrarily set to 1. Results were normalized to the levels of GAPDH transcripts present using the 2−ΔΔC(T) (Livak) method (25). Statistical significance was determined using a two-tailed Student t test assuming unequal variance, and P values of <0.05 were considered significant.
TABLE 1.
Sequences of primers and probes used in this study
| Gene | Primer or probe | Sequence (5′ to 3′) |
|---|---|---|
| TAg | Forward primer | AAAAATGGAGCAGGATGTAAAGGT |
| Reverse primer | TCTTCTGTTCCATAGGTTGGCA | |
| Probe | AGCTACTCCAGGTTCCAAAATCAGGCTGA | |
| GAPDH | Forward primer | GCCTCAAGATCATCAGCAAT |
| Reverse primer | CTGTGGTCATGAGTCCTTCC | |
| Probe | AAGGTCATCCATGACAACTTTGGTATCG |
RESULTS
IFN-γ inhibits the expression of viral genes during infection with BKV.
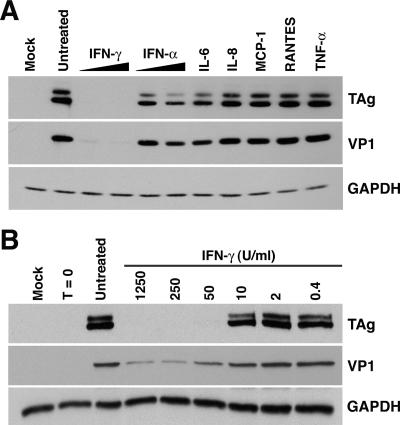
To begin our investigation of the role of cytokines and chemokines in regulating BKV infection, we examined the effect of several molecules on viral gene expression during infection of RPTE cells. The cytokines and chemokines used in this experiment were chosen for their well-known antiviral effects (IFN-α and IFN-γ) or because they are produced by proximal tubule epithelial cells following injury or stimulation (6). RPTE cells were infected with BKV, treated with IFN-γ, IFN-α, IL-6, IL-8, MCP-1, RANTES, or TNF-α, and total cell protein was harvested at 4 days postinfection. As a control, the ability of each cytokine to stimulate RPTE cells was analyzed by Western blotting: phosphorylation of Stat1 was observed for IFN-γ and IFN-α, while phosphorylation of extracellular signal-regulated kinases 1 and 2 was observed for IL-6, IL-8, MCP-1, and TNF-α; results for RANTES were inconclusive (data not shown). Western blot analysis of lysates probing for the viral proteins TAg, representing early gene expression, and VP1, representing late gene expression, revealed that only IFN-γ had a significant effect on viral gene expression (Fig. 1A). Similar levels of GAPDH, a cellular housekeeping gene, were detected in all samples, showing that the inhibitory effect on viral gene expression was specific and could not be attributed to the known nonspecific cellular effects of IFN-γ, namely, the induction of antiproliferative and proapoptotic pathways (Fig. 1A). Comparable results were obtained when samples were normalized to the total number of cells per lysate (data not shown). Although both IFN-α and IL-6 appeared to have a slight inhibitory effect on TAg and VP1, the potential roles of these cytokines in regulating BKV replication were not pursued at this time. The dramatic inhibition of viral protein expression with IFN-γ treatment is the subject of further investigation in this study.
FIG. 1.
Dose-dependent IFN-γ inhibition of BKV gene expression. RPTE cells were infected with the TU strain of BKV at an MOI of 0.5 and treated with cytokines at 3 to 6 hours postinfection, and total cell protein was harvested at 4 days postinfection. Samples were analyzed by Western blotting, probing for TAg, VP1, and GAPDH. (A) Infected cells were treated with 50 or 250 U/ml IFN-γ or IFN-α, 100 ng/ml IL-6, IL-8, MCP-1, or TNF-α, or 300 ng/ml RANTES. (B) Infected cells were treated with the indicated concentrations of IFN-γ. Mock, mock-infected samples with no cytokine treatment; untreated, BKV-infected samples with no cytokine treatment; T = 0, samples harvested directly after 1 hour of adsorption with BKV.
To determine whether IFN-γ exerts an inhibitory effect on BKV gene expression in a dose-dependent manner, RPTE cells were infected with BKV and treated with fivefold dilutions of IFN-γ. Total cell protein was harvested at 4 days postinfection and analyzed for TAg, VP1, and GAPDH expression by Western blotting (Fig. 1B). At the three higher doses (1,250, 250, and 50 U/ml IFN-γ), levels of TAg were undetectable, while at the three lower doses (10, 2, and 0.4 U/ml IFN-γ), TAg expression approached the level of the untreated sample. At higher doses, VP1 expression was detectable but greatly reduced compared to that of the untreated sample, and levels of VP1 increased as the dose of IFN-γ decreased. Samples harvested directly after the initial infection (1 hour of incubation with viral lysates), denoted time zero, were analyzed to determine the amount of detectable VP1 from input virions, which was negligible (Fig. 1B). Thus, levels of VP1 in all samples corresponded to de novo protein expression during infection. Levels of GAPDH were similar for all samples, indicating equal protein loading and accounting for the general cellular effects of IFN-γ.
IFN-γ does not affect the kinetics of BKV replication.
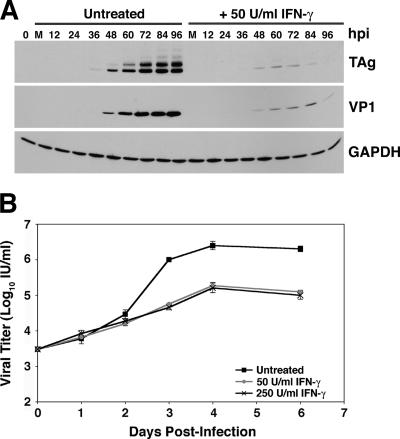
To determine whether IFN-γ-mediated inhibition of viral gene expression was the result of a delay in the progression of infection or a reduction in the level of gene expression, we infected RPTE cells with BKV, treated with 50 or 250 U/ml IFN-γ, and harvested total cell protein at 12-h intervals over 4 days (Fig. 2A). Samples were assayed for TAg, VP1, and GAPDH expression by Western blotting. In both untreated samples and samples treated with 50 U/ml IFN-γ, TAg expression was first detected at 36 h postinfection, while VP1 expression was first detected at 48 h postinfection, indicating that there was no delay in the initiation of viral gene expression. Similar results were obtained from the analysis of samples treated with 250 U/ml IFN-γ (data not shown). An interesting pattern of viral gene expression was apparent in treated samples: the levels of TAg and VP1 peaked at 72 and 84 h postinfection, respectively, and then decreased at 96 h postinfection (Fig. 2A). This level of inhibition of viral gene expression was maintained up to 13 days postinfection (data not shown).
FIG. 2.
BKV replication kinetics in the presence of IFN-γ. RPTE cells were infected with the TU strain of BKV at an MOI of 0.5 and treated with 50 U/ml (A and B) or 250 U/ml (B) IFN-γ at 3 to 6 hours postinfection. (A) Total cell protein was harvested every 12 h for 4 days. Samples were analyzed by Western blotting, probing for TAg, VP1, and GAPDH. Untreated, BKV-infected samples with no IFN-γ treatment; M, mock-infected samples; hpi, hours postinfection; 0 hpi, samples harvested directly after 1 hour of adsorption with BKV. (B) Viral lysates were harvested at 0, 1, 2, 3, 4, and 6 days postinfection, and progeny production was determined by a fluorescent focus assay. Data are represented as the log of the viral titer in IU/ml, and samples were assayed in triplicate. Error bars are too small to be seen for some samples.
To examine the effect of IFN-γ on the timing and level of viral progeny production, viral lysates were harvested at time zero and at 1, 2, 3, 4, and 6 days postinfection from BKV-infected RPTE cells either untreated or treated with 50 or 250 U/ml IFN-γ. The titer of each lysate was determined by a fluorescent focus assay, which detects the expression of TAg in newly infected cells (Fig. 2B). Increases in viral titers of untreated and IFN-γ-treated samples were similar during the first 48 h of infection, after which the untreated samples abruptly increased in viral titer whereas the IFN-γ-treated samples maintained the lower rate of increase seen in the first 48 h. Interestingly, the viral titers of both untreated and IFN-γ-treated samples reached a plateau at 4 days postinfection; however, the titers of the IFN-γ-treated samples were approximately 16- and 20-fold lower (for 50 and 250 U/ml IFN-γ, respectively) than those of the untreated samples at 6 days postinfection. The timing of viral protein expression and that of progeny production were similar for untreated samples and samples treated with 50 or 250 U/ml IFN-γ, and comparable results were obtained when cells were treated with IFN-γ prior to infection (data not shown). These data suggest that IFN-γ inhibits the level of viral gene expression and does not delay the progression of infection. This finding rules out a block at entry or trafficking to the nucleus as the mechanism of IFN-γ inhibition.
Treatment with IFN-γ results in a reduction in the level of early region transcripts.
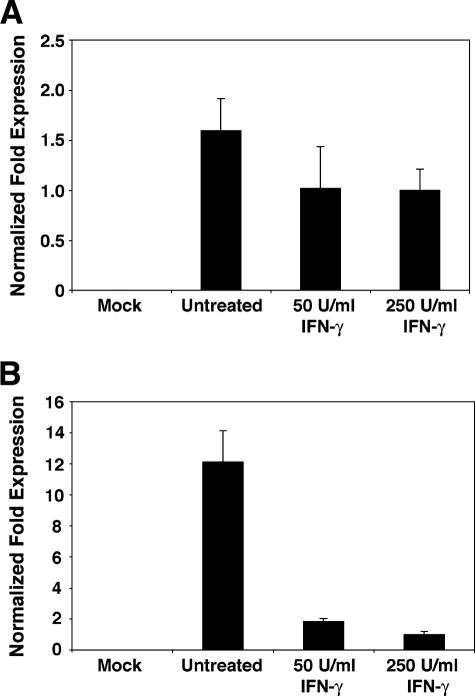
The previous experiments demonstrated the inhibitory effect of IFN-γ on the level of viral protein expression and progeny production. To determine whether IFN-γ-mediated inhibition occurs at the level of transcription or translation, we examined the effect of IFN-γ on viral transcript production. Total cell RNA was harvested at 48 or 96 h postinfection from RPTE cells infected with BKV and treated with 50 or 250 U/ml IFN-γ. RNA samples were analyzed by real-time RT-PCR to detect the levels of TAg transcripts. By designing the TAg primers and probe set to amplify a 90-base-pair amplicon across the splice site of the early region, amplification was limited to the amplicon produced from the TAg cDNA template, as opposed to that from the small tumor antigen or unspliced cDNA template. Results were normalized to the levels of GAPDH transcripts to account for the nonspecific cellular effects of IFN-γ, using the 2−ΔΔC(T) method, and are presented as the changes (n-fold) in TAg transcript levels, with the levels in samples treated with 250 U/ml IFN-γ arbitrarily set to 1.
At 48 h postinfection, there was a modest 1.6-fold decrease in TAg transcript levels with 50 and 250 U/ml IFN-γ (Fig. 3A). These results were confirmed by Northern blot analysis, using a probe specific for the entire BKV early region to detect levels of all species of early region transcripts (data not shown). A similar level of inhibition was observed at 48 h postinfection at the level of protein expression (Fig. 2A). At 96 h postinfection, there was a more dramatic and highly significant inhibitory effect with IFN-γ treatment, such that treatments with 50 and 250 U/ml IFN-γ resulted in 6.6-fold (P < 0.005) and 12.1-fold (P < 0.004) reductions in TAg transcript levels, respectively (Fig. 3B). These findings are consistent with our previous observations of the protein levels at 96 h postinfection (Fig. 2A). The relative correlation of real-time RT-PCR data (measuring the effect of IFN-γ on the steady-state level of TAg mRNA) and observations made from Western blot analysis (showing the effect of IFN-γ on the level of viral protein expression) suggest predominately IFN-γ-mediated effects on transcription. However, IFN-γ may also mediate an inhibitory effect at the level of translation. Future studies on the mechanism of inhibition will provide more insight on this subject.
FIG. 3.
Effect of IFN-γ on viral early region transcript levels. RPTE cells were infected with the TU strain of BKV at an MOI of 0.5 and treated with 50 or 250 U/ml IFN-γ at 3 to 6 hours postinfection, and total cell RNA was prepared at (A) 48 or (B) 96 h postinfection. The level of TAg transcripts in each sample was determined using real-time RT-PCR, with normalization to the level of GAPDH transcripts; the levels of samples treated with 250 U/ml IFN-γ were arbitrarily set to 1. Each bar represents the average from two (A) or three (B) independent experiments analyzed in triplicate in the same assay. Mock, mock-infected samples with no IFN-γ treatment; untreated, BKV-infected samples with no IFN-γ treatment.
Effect of IFN-γ during infections with different MOIs.
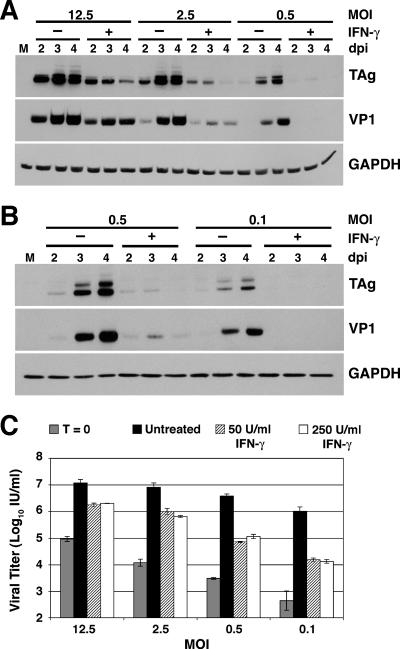
To investigate whether the inhibitory effect of IFN-γ is dependent on levels of input virus, RPTE cells were infected with fivefold dilutions of BKV. These infections of various MOIs may represent different stages of BKV infection in the host. For example, an infection at an MOI of 0.1 might be similar to the subclinical state of persistence seen in immunocompetent hosts, while an infection at an MOI of 12.5 might be more similar to the reactivation and lytic infection of BKV preceding the development of PVN in immunosuppressed hosts. Infected cells were treated with 50 or 250 U/ml IFN-γ, and total cell protein was harvested at 2, 3, and 4 days postinfection for analysis of viral protein expression by Western blotting (Fig. 4A and B). Regardless of the MOI used during the infection, TAg and VP1 expression levels were strongly inhibited with IFN-γ treatment and peak protein levels occurred at 3 days postinfection, similar to the pattern seen in previous experiments. Not surprisingly, levels of TAg and VP1 expression were higher in samples from infections with more input virus, but IFN-γ-treatment still mediated a strong reduction in viral gene expression compared to untreated samples under the same infection conditions. These data suggest that IFN-γ-mediated inhibition may be important for controlling BKV replication in both immunocompetent and immunosuppressed hosts.
FIG. 4.
Effect of IFN-γ during infections with different MOIs. RPTE cells were infected with the TU strain of BKV at an MOI of 12.5, 2.5, 0.5, or 0.1 and treated with 50 U/ml (A to C) or 250 U/ml (C) IFN-γ at 3 to 6 hours postinfection. (A and B) Total cell protein was harvested at 2, 3, and 4 days postinfection and analyzed by Western blotting, probing for TAg, VP1, and GAPDH. The analysis of total cell protein harvested from infection at an MOI of 0.5 was repeated for panel B for direct comparison to samples from infection at an MOI of 0.1. M, mock-infected samples with no IFN-γ treatment; dpi, days postinfection. (C) Viral lysates were harvested at time zero and at 4 days postinfection, and viral progeny production was determined by a fluorescent focus assay. Data are represented as the log of the viral titer in IU/ml, and samples were assayed in triplicate. Untreated, BKV-infected samples with no IFN-γ treatment.
Viral lysates were harvested at 4 days postinfection from cells infected under these same conditions and analyzed for viral progeny production using a fluorescent focus assay. While significant inhibitory effects mediated by IFN-γ were seen in all treated samples (for MOIs of 12.5, 2.5, and 0.5, P < 0.05; for an MOI of 0.1, P = 0.05), the effect was greater when less input virus was used during the infection (Fig. 4C). Cells treated with IFN-γ and infected at an MOI of 0.1 showed an almost 80-fold reduction in viral progeny production, while cells infected at MOIs of 12.5 (7-fold reduction), 2.5 (13-fold reduction), and 0.5 (53-fold reduction) were affected less severely by IFN-γ treatment. A comparison of viral titers of IFN-γ-treated samples with those of input virus (time zero) showed that BKV progeny was produced in the presence of IFN-γ. This suggests that, despite the inhibitory effects observed, IFN-γ is not driving BKV infection into a state of latency, in which the complete absence of late gene expression and progeny production would be expected.
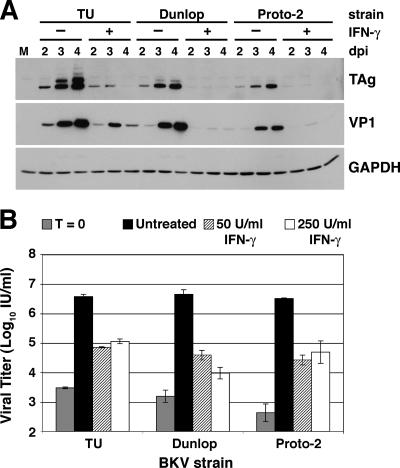
Response of various BKV strains to IFN-γ treatment.
To determine whether the inhibitory effect of IFN-γ on viral replication and gene expression is consistent for different strains of BKV, we infected RPTE cells with the TU strain, which was used throughout the above-described experiments, and two additional strains, Dunlop and Proto-2. Cells were treated with 50 or 250 U/ml IFN-γ, and total cell protein was harvested at 2, 3, and 4 days postinfection for analysis of viral protein expression by Western blotting (Fig. 5A). For each strain, TAg and VP1 expression levels were strongly inhibited by treatment with 50 U/ml IFN-γ, and as described before, viral protein levels in treated samples peaked at 3 days postinfection (Fig. 5A). Results were similar for samples treated with 250 U/ml IFN-γ (data not shown). These data suggest that all strains of BKV may respond similarly to IFN-γ. In addition, viral lysates were harvested at the initiation of infection (time zero) and at 4 days postinfection from RPTE cells infected with the three BKV strains and treated with 50 or 250 U/ml IFN-γ (Fig. 5B). IFN-γ treatment significantly inhibited progeny production for each of the strains (P < 0.05): viral titer was reduced in the presence of IFN-γ as much as 456-fold for infection with the Dunlop strain, 114-fold for infection with the Proto-2 strain, and 53-fold for infection with the TU strain (Fig. 5B).
FIG. 5.
Response of various BKV strains to IFN-γ treatment. RPTE cells were infected with the TU, Dunlop, or Proto-2 strain of BKV at an MOI of 0.5 and treated with 50 U/ml (A and B) or 250 U/ml (B) IFN-γ at 3 to 6 hours postinfection. (A) Total cell protein was harvested at 2, 3, and 4 days postinfection and analyzed by Western blotting, probing for TAg, VP1, and GAPDH. M, mock-infected samples with no IFN-γ treatment; dpi, days postinfection. (B) Viral lysates were harvested at time zero and at 4 days postinfection, and viral progeny production was determined by a fluorescent focus assay. Data are represented as the log of the viral titer in IU/ml, and samples were assayed in triplicate. Untreated, BKV-infected samples with no IFN-γ treatment.
DISCUSSION
Polyomavirus nephropathy is a complication associated with kidney transplantation, resulting from the reactivation of BKV. Our knowledge about the immune response to BKV is limited, making the management of PVN difficult. The experiments described in this paper begin to characterize the inhibitory effect of IFN-γ on the lytic infection of proximal tubule epithelial cells by BKV. We have shown that IFN-γ specifically inhibits viral gene expression and progeny production in a dose-dependent manner. In addition, infected cells exposed to IFN-γ have lower levels of TAg transcripts than untreated cells, suggesting that IFN-γ-mediated inhibition occurs at the level of transcription. Cells infected with different MOIs of BKV responded similarly to treatment with IFN-γ; however, the more virus present, the weaker the inhibitory effect. In addition, IFN-γ had a significant inhibitory effect on viral gene expression and progeny production during infections with three different strains of BKV. These findings suggest that IFN-γ plays an important role in regulating BKV infection.
It is important to note that samples treated with IFN-γ maintained detectable de novo viral gene expression and progeny production. It is unclear whether this is a result of a low level of viral replication in all cells due to incomplete inhibition by IFN-γ or a normal level of replication in a fraction of cells that were unresponsive to IFN-γ. In the latter scenario, IFN-γ may force BKV into a state of latency in responsive cells. However, the finding that viral replication continued to some extent in the presence of IFN-γ correlates well with the observation that healthy immunocompetent individuals shed BKV in their urine periodically throughout their lives. A reduction of IFN-γ production by antigen-specific T lymphocytes in immunosuppressed patients may be, in part, responsible for the reactivation and subsequent development of BKV-associated disease.
An interesting pattern of viral gene expression was noted in samples exposed to IFN-γ: protein levels, though severely reduced compared to those of untreated samples, peaked at 3 days postinfection and then declined until a steady, low level of expression was reached. We hypothesize that treatment with IFN-γ may either shorten the duration of TAg expression or force the virus into a persistent or latent state. Since we observed progeny production at times when TAg expression was either very low or undetectable by Western blotting, a minimal threshold level of TAg seems to be sufficient to facilitate the progression of a productive BKV infection.
Activation of the IFN-γ signaling cascade as a result of IFN-γ binding to the surface receptor has a multitude of effects on the cell (reviewed in references 37 and 38). IFN-γ has overall antiproliferative and proapoptotic effects, mediated primarily by protein kinase R and IRF-1, respectively. In our studies, we have accounted for these nonspecific cellular effects by normalizing each sample to the levels of a housekeeping gene, GAPDH, or to cell number. IFN-γ signaling also plays a major role in activating and recruiting cells of the immune system, for example, by up-regulating the surface expression of major histocompatibility complex class I and II molecules and inducing expression of inflammatory cytokines. However, the system we used to study the lytic infection of BKV does not incorporate cells of the immune system and thus eliminates immune activation from the potential mechanisms of IFN-γ-specific inhibition of BKV replication. In addition, IFN-γ signaling activates the type I IFN cascade, which establishes a general antiviral environment within the cell. However, the direct treatment of infected cells with IFN-α has little effect on viral gene expression (Fig. 1A). The last major category of IFN-γ signaling effects involves the activation or repression of transcription factors that regulate the expression of genes. We speculate that the mechanism by which IFN-γ inhibits BKV replication employs regulatory transcription factors that act on the early and late viral promoters. However, regulation of the early promoter to inhibit TAg expression alone would be sufficient to explain a decrease in levels of TAg, VP1, and viral progeny production, since TAg is a key mediator of the progression of the BKV life cycle. IFN-γ-activated transcription factors include members of the Stat and IRF families, IFN-stimulated gene factor 3, c-Myc, and c-Jun, as well as other factors affected by downstream elements of the IFN-γ signaling cascade and subsequently regulated at a later time (7, 8). We are currently investigating candidate IFN-γ-regulated factors that may be responsible for the inhibitory effects on BKV.
The NCCR is the region of greatest variability between different strains of BKV. Rearrangements of the transcription factor binding blocks (designated O-P-Q-R-S in the archetypal BKV strain WW) occur frequently in tissue culture systems and seem to arise in individuals with high viral loads or reactivation. We examined the responses of three different strains of BKV to IFN-γ treatment during lytic infection. BKV TU (NCCR structure, O-P-Q-R1-12-P16-68-Q1-35-R52-63-S) and BKV Dunlop (O-P-P1-7;26-68-P1-64-S) are strains commonly found in immunocompetent individuals, while BKV Proto-2 (O-P-P1-7;26-68-P-Q1-28-S7-63) was isolated from the urine of human immunodeficiency virus-infected patients (9, 29, 40, 41). The observation that IFN-γ is able to inhibit viral gene expression and progeny production of three different strains of BKV with dissimilar NCCR structures suggests that our findings may be applicable to all strains of BKV. Therefore, we speculate that transcription factor binding sites common to all three NCCRs (and potentially to most BKV strains) mediate the inhibitory effect of IFN-γ on the viral promoters.
It is still not understood what factors cause certain kidney transplant patients to undergo reactivation of BKV and develop PVN while others on the same immunosuppressive regimen do not. One possibility involves differences in host genetics. For example, genetic polymorphisms of the IFNG gene may render some patients more susceptible to BKV reactivation than others. Polymorphisms are frequent in the promoter region of the IFNG gene and affect the level of IFN-γ normally produced in the body (32, 33). Recent reports attempt to establish a correlation between certain IFNG polymorphisms and susceptibility to various diseases, including human papillomavirus-induced cervical cancer (24), tuberculosis (26), and parvovirus B19 infections (22). It is possible that kidney transplant patients with IFNG polymorphisms resulting in lower levels of IFN-γ production are naturally more susceptible to reactivation of BKV under immunosuppressive therapies. Screening of patients for such polymorphisms may help to determine the appropriate level of immunosuppression required to prevent graft rejection but still maintain the ability of the immune system to repress BKV reactivation. In addition, treatment with IFN-γ may prove to be beneficial as an alternative to reducing immunosuppressive therapies upon detection of active BKV replication. There are precedents for successful interferon therapy: IFN-α has been used to treat a wide range of cancers, hepatitis B virus, and hepatitis C virus, while IFN-γ has proven to be effective against chronic granulomatous disease (reviewed in reference 31).
In conclusion, IFN-γ has potent inhibitory effects on BKV gene expression, both at the level of transcription and at the level of translation, and viral progeny production in proximal tubule cells. These effects are similar for the three different strains of BKV examined and are more effective at inhibiting gene expression and progeny production in the presence of less virus. The exact mechanism of IFN-γ-mediated inhibition is not yet known, but we speculate that the activity of the BKV promoters is regulated by IFN-γ-responsive transcription factors. These findings expand the characterization of the host immune response to BKV and may lead to new approaches for the prevention of BKV reactivation in kidney transplant recipients.
Acknowledgments
We thank members of the Imperiale lab for help with this work and Steve King and Alice Telesnitsky for assistance with real-time RT-PCR assays. We thank Kathy Spindler and Keith Bishop for critical reviews of the manuscript.
This work was supported by AI060584 awarded to M.J.I. from the NIH and in part by CA 46592 awarded to the University of Michigan Cancer Center from the NIH. J.R.A. was supported by the NIH National Research Service Award T32-GM07544 from the National Institute of General Medical Sciences.
Footnotes
Published ahead of print on 11 October 2006.
REFERENCES
- 1.Bohl, D. L., G. A. Storch, C. Ryschkewitsch, M. Gaudreault-Keener, M. A. Schnitzler, E. O. Major, and D. C. Brennan. 2005. Donor origin of BK virus in renal transplantation and role of HLA C7 in susceptibility to sustained BK viremia. Am. J. Transplant. 5:2213-2221. [DOI] [PubMed] [Google Scholar]
- 2.Briggs, J. P., W. Kriz, and J. B. Schnermann. 2001. Overview of renal function structure, p. 3-19. In A. Greenberg et al. (ed.), Primer on kidney diseases, 3rd ed. Academic Press, New York, NY.
- 3.Chen, Y., J. Trofe, J. Gordon, R. A. Du Pasquier, P. Roy-Chaudhury, M. J. Kuroda, E. S. Woodle, K. Khalili, and I. J. Koralnik. 2006. Interplay of cellular and humoral immune responses against BK virus in kidney transplant recipients with polyomavirus nephropathy. J. Virol. 80:3495-3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chesters, P. M., J. Heritage, and D. J. McCance. 1983. Persistence of DNA sequences of BK virus and JC virus in normal human tissues and in diseased tissues. J. Infect. Dis. 147:676-684. [DOI] [PubMed] [Google Scholar]
- 5.Comoli, P., A. Azzi, R. Maccario, S. Basso, G. Botti, G. Basile, I. Fontana, M. Labirio, A. Cometa, F. Poli, F. Perfumo, F. Locatelli, and F. Ginevri. 2004. Polyomavirus BK-specific immunity after kidney transplantation. Transplantation 78:1229-1232. [DOI] [PubMed] [Google Scholar]
- 6.Daha, M. R., and C. van Kooten. 2000. Is the proximal tubular cell a proinflammatory cell? Nephrol. Dial. Transplant. 15(Suppl. 6):41-43. [DOI] [PubMed] [Google Scholar]
- 7.Der, S. D., A. Zhou, B. R. Williams, and R. H. Silverman. 1998. Identification of genes differentially regulated by interferon alpha, beta, or gamma using oligonucleotide arrays. Proc. Natl. Acad. Sci. USA 95:15623-15628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Veer, M. J., M. Holko, M. Frevel, E. Walker, S. Der, J. M. Paranjape, R. H. Silverman, and B. R. Williams. 2001. Functional classification of interferon-stimulated genes identified using microarrays. J. Leukoc. Biol. 69:912-920. [PubMed] [Google Scholar]
- 9.Dörries, K., E. Vogel, S. Gunther, and S. Czub. 1994. Infection of human polyomaviruses JC and BK in peripheral blood leukocytes from immunocompetent individuals. Virology 198:59-70. [DOI] [PubMed] [Google Scholar]
- 10.Drachenberg, C. B., H. H. Hirsch, E. Ramos, and J. C. Papadimitriou. 2005. Polyomavirus disease in renal transplantation: review of pathological findings and diagnostic methods. Hum. Pathol. 36:1245-1255. [DOI] [PubMed] [Google Scholar]
- 11.Gardner, S. D., A. M. Field, D. V. Coleman, and B. Humle. 1971. New human papovavirus (B.K.) isolated from urine after renal transplantation. Lancet i:1253-1257. [DOI] [PubMed] [Google Scholar]
- 12.Hariharan, S., E. P. Cohen, B. Vasudev, R. Orentas, R. P. Viscidi, J. Kakela, and B. DuChateau. 2005. BK virus-specific antibodies and BKV DNA in renal transplant recipients with BKV nephritis. Am. J. Transplant. 5:2719-2724. [DOI] [PubMed] [Google Scholar]
- 13.Harlow, E., L. V. Crawford, D. C. Pim, and N. M. Williamson. 1981. Monoclonal antibodies specific for simian virus 40 tumor antigens. J. Virol. 39:861-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harlow, E., P. Whyte, B. R. Franza, Jr., and C. Schley. 1986. Association of adenovirus early-region 1A proteins with cellular polypeptides. Mol. Cell. Biol. 6:1579-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harms, J. S., and G. A. Splitter. 1995. Interferon-gamma inhibits transgene expression driven by SV40 or CMV promoters but augments expression driven by the mammalian MHC I promoter. Hum. Gene Ther. 6:1291-1297. [DOI] [PubMed] [Google Scholar]
- 16.Heritage, J., P. M. Chesters, and D. J. McCance. 1981. The persistence of papovavirus BK DNA sequences in normal human renal tissue. J. Med. Virol. 8:143-150. [DOI] [PubMed] [Google Scholar]
- 17.Hirsch, H. H. 2005. BK virus: opportunity makes a pathogen. Clin. Infect. Dis. 41:354-360. [DOI] [PubMed] [Google Scholar]
- 18.Hirsch, H. H., D. C. Brennan, C. B. Drachenberg, F. Ginevri, J. Gordon, A. P. Limaye, M. J. Mihatsch, V. Nickeleit, E. Ramos, P. Randhawa, R. Shapiro, J. Steiger, M. Suthanthiran, and J. Trofe. 2005. Polyomavirus-associated nephropathy in renal transplantation: interdisciplinary analyses and recommendations. Transplantation 79:1277-1286. [DOI] [PubMed] [Google Scholar]
- 19.Hirsch, H. H., W. Knowles, M. Dickenmann, J. Passweg, T. Klimkait, M. J. Mihatsch, and J. Steiger. 2002. Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. N. Engl. J. Med. 347:488-496. [DOI] [PubMed] [Google Scholar]
- 20.Hirsch, H. H., and J. Steiger. 2003. Polyomavirus BK. Lancet Infect. Dis. 3:611-623. [DOI] [PubMed] [Google Scholar]
- 21.Humes, H. D., W. H. Fissell, W. F. Weitzel, D. A. Buffington, A. J. Westover, S. M. MacKay, and J. M. Gutierrez. 2002. Metabolic replacement of kidney function in uremic animals with a bioartificial kidney containing human cells. Am. J. Kidney Dis. 39:1078-1087. [DOI] [PubMed] [Google Scholar]
- 22.Kerr, J. R., M. McCoy, B. Burke, D. L. Mattey, V. Pravica, and I. V. Hutchinson. 2003. Cytokine gene polymorphisms associated with symptomatic parvovirus B19 infection. J. Clin. Pathol. 56:725-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knowles, W. A. 2001. The epidemiology of BK virus and the occurrence of antigenic and genomic subtypes, p. 527-559. In K. Khalili and G. L. Stoner (ed.), Human polyomaviruses: molecular and clinical perspectives. Wiley-Liss Inc., New York, NY.
- 24.Lai, H. C., C. C. Chang, Y. W. Lin, S. F. Chen, M. H. Yu, S. Nieh, T. W. Chu, and T. Y. Chu. 2005. Genetic polymorphisms of the interferon-gamma gene in cervical carcinogenesis. Int. J. Cancer 113:712-718. [DOI] [PubMed] [Google Scholar]
- 25.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402-408. [DOI] [PubMed] [Google Scholar]
- 26.López-Maderuelo, D., F. Arnalich, R. Serantes, A. Gonzalez, R. Codoceo, R. Madero, J. J. Vazquez, and C. Montiel. 2003. Interferon-gamma and interleukin-10 polymorphisms in pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 167:970-975. [DOI] [PubMed] [Google Scholar]
- 27.Low, J., H. D. Humes, M. Szczypka, and M. Imperiale. 2004. BKV and SV40 infection of human kidney tubular epithelial cells in vitro. Virology 323:182-188. [DOI] [PubMed] [Google Scholar]
- 28.Meneguzzi, G., P. F. Pignatti, G. Barbanti-Brodano, and G. Milanesi. 1978. Minichromosome from BK virus as a template for transcription in vitro. Proc. Natl. Acad. Sci. USA 75:1126-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moens, U., and O. P. Rekvig. 2001. Molecular biology of BK virus and clinical and basic aspects of BK virus renal infection, p. 359-408. In K. Khalili and G. L. Stoner (ed.), Human polyomaviruses: molecular and clinical perspectives. Wiley-Liss Inc., New York, NY.
- 30.Nickeleit, V., H. K. Singh, and M. J. Mihatsch. 2003. Polyomavirus nephropathy: morphology, pathophysiology, and clinical management. Curr. Opin. Nephrol. Hypertens. 12:599-605. [DOI] [PubMed] [Google Scholar]
- 31.Pestka, S., C. D. Krause, and M. R. Walter. 2004. Interferons, interferon-like cytokines, and their receptors. Immunol. Rev. 202:8-32. [DOI] [PubMed] [Google Scholar]
- 32.Pravica, V., A. Asderakis, C. Perrey, A. Hajeer, P. J. Sinnott, and I. V. Hutchinson. 1999. In vitro production of IFN-gamma correlates with CA repeat polymorphism in the human IFN-gamma gene. Eur. J. Immunogenet. 26:1-3. [DOI] [PubMed] [Google Scholar]
- 33.Pravica, V., C. Perrey, A. Stevens, J. H. Lee, and I. V. Hutchinson. 2000. A single nucleotide polymorphism in the first intron of the human IFN-gamma gene: absolute correlation with a polymorphic CA microsatellite marker of high IFN-gamma production. Hum. Immunol. 61:863-866. [DOI] [PubMed] [Google Scholar]
- 34.Qin, L., Y. Ding, D. R. Pahud, E. Chang, M. J. Imperiale, and J. S. Bromberg. 1997. Promoter attenuation in gene therapy: interferon-gamma and tumor necrosis factor-alpha inhibit transgene expression. Hum. Gene Ther. 8:2019-2029. [DOI] [PubMed] [Google Scholar]
- 35.Ritter, T., C. Brandt, S. Prosch, A. Vergopoulos, K. Vogt, J. Kolls, and H. D. Volk. 2000. Stimulatory and inhibitory action of cytokines on the regulation of hCMV-IE promoter activity in human endothelial cells. Cytokine 12:1163-1170. [DOI] [PubMed] [Google Scholar]
- 36.Rozen, S., and H. J. Skaletsky. 2000. Primer3 on the WWW for general users and for biologist programmers, p. 365-386. In S. Krawetz and S. Misener (ed.), Bioinformatics methods and protocols: methods in molecular biology. Humana Press, Totowa, NJ. [DOI] [PubMed]
- 37.Schroder, K., P. J. Hertzog, T. Ravasi, and D. A. Hume. 2004. Interferon-gamma: an overview of signals, mechanisms and functions. J. Leukoc. Biol. 75:163-189. [DOI] [PubMed] [Google Scholar]
- 38.Sen, G. C. 2001. Viruses and interferons. Annu. Rev. Microbiol. 55:255-281. [DOI] [PubMed] [Google Scholar]
- 39.Shinohara, T., M. Matsuda, S. H. Cheng, J. Marshall, M. Fujita, and K. Nagashima. 1993. BK virus infection of the human urinary tract. J. Med. Virol. 41:301-305. [DOI] [PubMed] [Google Scholar]
- 40.Sundsfjord, A., T. Flaegstad, R. Flo, A. R. Spein, M. Pedersen, H. Permin, J. Julsrud, and T. Traavik. 1994. BK and JC viruses in human immunodeficiency virus type 1-infected persons: prevalence, excretion, viremia, and viral regulatory regions. J. Infect. Dis. 169:485-490. [DOI] [PubMed] [Google Scholar]
- 41.Sundsfjord, A., T. Johansen, T. Flaegstad, U. Moens, P. Villand, S. Subramani, and T. Traavik. 1990. At least two types of control regions can be found among naturally occurring BK virus strains. J. Virol. 64:3864-3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wheelock, E. F. 1965. Interferon-like virus-inhibitor induced in human leukocytes by phytohemagglutinin. Science 149:310-311. [PubMed] [Google Scholar]