Abstract
The papillomavirus E1 protein is essential for the initiation of viral replication. We previously showed that the bovine papillomavirus E1 protein is unstable and becomes resistant to ubiquitin-mediated degradation when tightly bound to cyclin E-cyclin-dependent kinase 2 (Cdk2) before the start of DNA synthesis. However, neither the protection nor the targeted degradation of E1 appears to depend on its phosphorylation by Cdk. Here, we report that Cdk phosphorylation of E1 is also not a prerequisite for the initiation of viral DNA replication either in vitro or in vivo. Nevertheless, we found that phosphorylation of one Cdk site, Ser283, abrogates E1 replicative activity only in a cellular context. We show that this site-specific phosphorylation of E1 drives its export from the nucleus and promotes its continuous nucleocytoplasmic shuttling. In addition, we find that E1 shuttling occurs in S phase, when cyclin A-Cdk2 is activated. E1 interacts with the active cyclin A-Cdk2 complex and is phosphorylated on Ser283 by this kinase. These data suggest that the phosphorylation of E1 on Ser283 is a negative regulatory event that is involved in preventing the amplification of viral DNA during S phase. This finding reveals a novel facet of E1 regulation that could account for the variations of the viral replication capacity during different cell cycle phases, as well as in different stages of the viral cycle.
Papillomaviruses are small DNA tumor viruses that induce persistent epithelial lesions in higher vertebrates, including humans. The viral genome of papillomaviruses is initially replicated and maintained as extrachromosomal plasmids in the basal cells of the infected host epithelium. In these proliferating cells, replication of the viral genomes is tightly regulated in order to occur only in S phase within the host cell nuclei. Furthermore, the maintenance of a fairly constant low copy number of viral genomes indicates that the viral DNA synthesis also must be controlled to prevent amplification, as an increasing viral copy number is likely to be incompatible with viral persistence in the basal epithelial layer. However, in differentiated, suprabasal cells, the viral genome can be amplified, suggesting that replication controls are then abrogated to allow the accumulation of viral genomes associated with the production of new virions (5, 12, 16, 17).
Like other small DNA viruses, papillomaviruses do not encode all the proteins required for their replication and so need to use the replication machinery of the host cell. Two viral proteins, E1 and E2, are needed for replication and stable plasmid maintenance (6, 34-36). However, by using the bovine papillomavirus (BPV) as a model system, we found that E1 is the only viral protein required for the initiation of viral DNA synthesis in vitro (3, 9). E1 possesses all the functions required for the initiation of replication. It is an ATP-dependent helicase that specifically binds to and unwinds the virus replication origin, and it recruits the cellular replication machinery through a direct, specific interaction with the DNA polymerase α (4, 7, 14, 26, 31, 32, 37). Although the viral system seems highly regulated in an infected cell, it is capable of uncontrolled replication in vitro, indicating that the host cell imposes the major restrictions on viral DNA replication (3).
Eukaryotic cells have developed multiple levels of control to regulate the initiation of DNA replication to ensure that genomic DNA is replicated exactly once within each cell cycle (reviewed in reference 2). In contrast to the viral initiation process, the initiation of DNA replication on the host cellular chromatin depends on the sequential association of more than 16 factors: the six proteins of the origin recognition complex (ORC); the proteins Cdc6 and Cdt1; the proteins that form the minichromosome maintenance (MCM) complex, which presents the helicase activity; and the proteins Cdc45 and MCM10 that recruit the DNA polymerases. The ordered assembly of these proteins at the origins of replication is cell cycle regulated and involves phosphorylation events controlled by cyclin-dependent kinases (Cdks). Cdk activity has a dual role in the regulation of DNA replication. First, Cdk activity is required for the activation of origins of replication as cells enter into S phase. Second, Cdk activity is also required to prevent reinitiation during S, G2, and M phases by inhibiting the formation of new preinitiation complexes. This leads to inactivation by degradation, inhibition of chromatin binding, or nuclear exclusion. Although ORC, Cdc6, Cdt1, and MCM proteins are all targeted by Cdks, their mutant forms, which cannot be modified by Cdks, continue to function during the initiation process. Thus, while it is clear that Cdks are required for the initiation of replication, the proteins that must be phosphorylated to promote replication remain unknown (reviewed in reference 2).
While the viral initiator E1 is functionally equivalent to the ORC and MCM complexes during initiation, our recent findings have revealed that BPV E1 is a direct target of the control mechanisms that regulate the Cdc6 protein (22, 29). We have shown that E1 is a substrate of the ubiquitin-dependent proteolytic pathway and have identified the anaphase-promoting complex (APC) as the ubiquitin ligase that controls E1 levels (23, 25). Studies in a cell-free system derived from Xenopus egg extracts also indicated that E1 is protected from degradation by its tight association with cyclin E-Cdk2 (23). We determined that the viral initiator interacts functionally with cyclin E-Cdk2. No E1-dependent replication was observed in cyclin E-Cdk2-depleted extracts, while it was restored by the addition of E1-cyclin E-Cdk2 complexes (9). Although E1 is phosphorylated by cyclin E-Cdk2 in vitro and is also targeted by Cdk2 in interphase egg extracts, neither the protection nor targeted degradation of E1 appeared to depend on its phosphorylation by Cdk in the Xenopus cell-free system (23). While we do not know whether the Cdk phosphorylation of BPV E1 is involved in the regulation of its replication functions, a previous study on human papillomavirus type 11 (HPV-11) E1 showed that its phosphorylation by Cdks induces dramatic defects in HPV-11 DNA replication both in vitro and in vivo (20, 21). It was subsequently reported that HPV-11 E1 phosphorylation by cyclin-Cdk is required for its retention in the nucleus by masking a nuclear export signal (NES) (10).
This led us to test the replicative functions of the different versions of the BPV E1 protein, generated by mutating its Cdk phosphorylation sites to alanine or glutamic acid. Our results from in vitro replication assays established that in contrast to HPV-11 E1, Cdk-mediated phosphorylation of BPV E1 has no influence on its replication functions. However, we found that in transfected cells, phosphorylation of only one putative Cdk site, Ser283, abrogates E1 replicative activity. We show that, although BPV-E1 is confined to the nucleus, it can shuttle continuously between the nucleus and the cytoplasm and that phosphorylation of Ser283 is required for this shuttling. Furthermore, we show that the nucleocytoplasmic shuttling of E1 occurs only during S phase. These data suggest that this site-specific phosphorylation of BPV E1 is a critical event involved in the cell cycle regulation of E1 functions and that it is likely to be important in order to negatively control viral DNA replication in the dividing cells of the infected host epithelium. However, even though this new property represents an additional similarity between BPV E1 and Cdc6, it also reveals the first difference in the strategies adopted by two different papillomaviruses to control their DNA replication in mammalian cells.
MATERIALS AND METHODS
Plasmids and mutagenesis.
Plasmids used for in vitro synthesis and in vivo expression of wild-type (wt) E1, green fluorescent protein (GFP)-E1 fusion protein, and E2 have been described previously (9, 23, 25). Site-directed mutagenesis was performed with a QuickChange site-directed mutagenesis kit (Stratagene) according to the manufacturer's instructions and was confirmed by DNA sequencing.
In vitro DNA replication assay.
Xenopus membrane-free egg extracts arrested in interphase were prepared as described previously (9). A GFP-E1 protein translated in reticulocyte lysate was used as a source of E1. Replication assays were carried out by first mixing 16 μl of egg extract supplemented with an ATP-regenerating system and with 2 to 4 μl of in vitro-translated GFP-E1 protein for 30 min at 25°C before the addition of 200 ng of pSKori+ and 2 μCi of [32P]dCTP. After a 30-min incubation, DNA products were purified, subjected to agarose gel electrophoresis, and detected by the incorporation of [32P]dCTP.
Transient BPV DNA replication assay.
Transient BPV DNA replication was performed with human 293 cells cotransfected with plasmids encoding E1 or GFP-E1 and E2 and with pSKO, which contains the minimal BPV origin of DNA replication. A total of 3 × 106 cells were seeded onto 100-mm dishes 24 h prior to transfection. A total of 3.3 μg of DNA consisting of 1.5 μg of either pCGEag or pEGFP-E1, 0.9 μg of pCGE2, and 0.9 μg of pSKori+ was transfected into cells using Lipofectamine Plus reagent according to the manufacturer's instructions (Invitrogen). Cells were harvested on days 2 and 3 following transfection. Low-molecular-weight DNA was isolated from cells and treated with restriction enzymes EcoRI, to linearize the BPV origin-containing plasmid, and DpnI, to cleave only the DNA that had not been replicated in eukaryotic cells (35). Digested DNA was separated on a 1% agarose gel and blotted onto nitrocellulose membranes under standard conditions for Southern blot analysis. The blot was probed with randomly primed pSKori DNA to analyze pSKO replication.
In vitro sumoylation assay.
The various GFP-E1 proteins were generated in the TNT rabbit reticulocyte lysate system in the presence of [35S]methionine (Promega). The recombinant SUMO-1-activating enzyme (SAE) glutathione S-transferase (GST)-SAE1-SAE2 and GST-SUMO1-97-1 were expressed in the Escherichia coli strain BL21 and purified by affinity chromatography on glutathione agarose. Recombinant His6-Ubc9 was purified over a cationic-exchange column (fast protein liquid chromatography Mono S) equilibrated with 20 mM MES (morpholineethanesulfonic acid; pH 5.8), 1 mM dithiothreitol (DTT) and eluted with a 0 to 0.5 M NaCl gradient. SUMO conjugation assays were performed with reaction mixtures containing 2 μl of radiolabeled GFP-E1, 0.2 μg purified recombinant GST-SAE1-SAE2, 1 μg purified Ubc9, 10 μg purified SUMO-1 in 50 mM Tris-HCl (pH 7.6), 1 mM DTT, and an ATP-regenerating system (2 mM ATP, 5 mM MgCl2, 10 mM creatine phosphate, and 7.5 μg/ml of creatine phosphokinase). After 2 h at 37°C, the reactions were terminated by boiling the samples in sodium dodecyl sulfate (SDS) sample buffer. The reaction mixtures were separated on SDS-polyacrylamide gel electrophoresis (PAGE) gels, and radioactively labeled bands were visualized with a PhosphorImager (Molecular Dynamics).
Protein interactions and in vitro kinase assays.
Purified cyclin A-Cdk2 active complexes (gift of G. Divita) were obtained by the production in E. coli of GST-Cdk2 which has been phosphorylated at Thr160 by coexpressing the Saccharomyces cerevisiae GST-Cak1 and human cyclin A (residues 173 to 432) as an untagged protein. Cyclin A lacking the first 173 amino acids (Δ173) is soluble and conserved its capacity to interact with its substrates. GST-E1 proteins were incubated either with cell lysate (lysis buffer consisting of 50 mM HEPES [pH 7.5], 200 mM NaCl, 1 mM EDTA, 50 mM sucrose, 5 mM DTT, and 0.1% Triton supplemented with protease inhibitors) or with the purified cyclin A-Cdk2 complexes diluted in lysis buffer. The complexes, washed three times in lysis buffer supplemented with 0.4% Triton and twice in kinase buffer (25 mM HEPES [pH 7.5], 10 mM MgCl2, 5 mM DTT), were used for kinase assays and subjected to SDS-PAGE and immunoblotting. To detect the different complexes, we used the following primary antibodies: anti-cyclin A (C 4710; Sigma), anti-cyclin E (HE12; Santa Cruz), and anti-Cdk2 (sc-163; Santa Cruz). As secondary antibodies, we used a peroxidase-conjugated anti-rabbit IgG (NA934; Amersham, Biosciences).
Immunofluorescence microscopy and heterokaryon analysis.
To analyze the localization of E1 or GFP-E1 proteins, 5 × 105 HeLa cells were seeded onto coverslips in 35-mm plates and transfected with 1 μg of pCGEag or pEGFP-E1 wild type or mutants using JetPEI transfection reagent (Qbiogene). Transfected cells were fixed on coverslips with 4% paraformaldehyde for 30 min. After washes in phosphate-buffered saline (PBS), cells were permeabilized with 0.1% Triton X-100 for 30 min. Cells were then incubated with a rabbit polyclonal antibody against the C-terminal region of E1 (anti-E1C; 1:500 dilution) for 1 h, rinsed three times with PBS for 5 min, and incubated with a secondary goat anti-rabbit immunoglobulin G antibody conjugated either to Texas Red or fluorescein isothiocyanate for 1 h. Finally, cells were counterstained with Hoechst 33342 (Molecular Probes) and mounted in Permaflour (Immunon; Thermo Shandon). For protein quantification, transfected cells were directly lysed with SDS sample buffer and were analyzed by Western blotting using an anti-GFP antibody (Torrey Pines Biolabs), as described previously. For binding and kinase assays, HeLa cells were synchronized by incubation in media containing thymidine (2 mM; incubation for 16 h plus 16 h with 12-h release in between) and released for 4 h in S phase.
For heterokaryon analysis, 5 × 105 HeLa cells, grown on coverslips, were transfected as described above. Eighteen hours posttransfection, 106 BALB/c mouse cells were plated on the coverslips in culture medium containing 20 μg/ml cycloheximide for 3 h. After a PBS wash, HeLa cells were fused with BALBc mouse cells by incubation in 50% (wt/vol) polyethylene glycol for 3 min to form heterokaryons. After the cells were rinsed three times with PBS, the fused cells were incubated for 2 h in medium containing 20 μg/ml cycloheximide before fixation. Cells were processed for immunofluorescence as described above. For bromodeoxyuridine (BrdU) incorporation, fused cells were incubated with BrdU (20 μM) for 1 h. BrdU incorporation into DNA was detected by incubating the fixed cells with a mouse monoclonal anti-BrdU antibody in the presence of 10 U of DNase I/ml for 1 h at 37°C.
Coverslips were examined under a DMRA fluorescence microscope (Leica) with a Leica PL APO 40×/1.25 oil lens. Images were captured with a Photometrics Coolsnap Fx digital camera operated via the Metamorph software (Universal Imaging Corp.).
RESULTS
Cdk-mediated E1 phosphorylation is not required for BPV replication in vitro.
The dipeptide Ser/Thr-Pro is the sole absolutely conserved phosphorylated sequence in the Cdk substrates. It has been previously shown that Thr102 of BPV E1 is a site for phosphorylation by a mitotic Cdk, Cdk1, in vitro. However, mutation of this site has no detectable effects on viral DNA replication (19). In contrast, mutation of the four putative Cdk sites in HPV-11 E1 severely compromises DNA replication both in vitro and in vivo (21). In addition to Thr102, the BPV E1 protein contains two other putative Cdk sites, Thr126 and Ser283 (Fig. 1A). Our previous results showed that BPV E1 treated with cyclin E-Cdk2, immunopurified from Xenopus egg extracts, is phosphorylated on both threonine and serine residues (9). In order to determine whether the BPV E1 activity is also regulated by Cdk phosphorylation, we first tested the replicative activity of wild-type and mutated forms of E1 in which the Cdk phosphorylation sites were individually changed to alanine. E1 activity was determined using an in vitro replication assay carried out with Xenopus egg extracts. The levels of replicative intermediates (late RI), radiolabeled by incorporation of [α-32P]dCTP and corresponding to newly replicated DNA, were similar for each reaction (Fig. 1B, left panel), indicating that Cdk phosphorylation of any of the three sites is required for the replication function of BPV E1. To further examine the consequences of E1 phosphorylation at these three Cdk sites, we next analyzed the replicative activity of a nonphosphorylatable mutant in which all three Cdk sites were mutated to alanine (E1-AAA) and that of a mutant in which the three sites were mutated to glutamic acid (E1-EEE) to mimic a constitutively Cdk-phosphorylated form of E1. Both mutants were able to trigger the replication of the BPV origin plasmid to the same extent as wild-type E1 (E1wt) (Fig. 1B, right panel). Therefore, in contrast to HPV-11 E1, BPV E1 does not require Cdk phosphorylation for its function in the initiation of viral replication, and obviously, this phosphorylation does not have evident effects on the replication activity of the viral initiator in vitro.
FIG. 1.
Mutation of the Cdk phosphorylation sites in E1 has no influence on its replication activity in vitro. (A) Schematic drawing of BPV E1 (E1wt) showing several functional domains: a nuclear localization sequence (NLS) at amino acids 84 to 112; a DNA binding domain (DBD) located between amino acids 140 and 300; an ATPase helicase domain; and the positions of the three putative Cdk phosphorylation sites, Thr102, Thr126, and Ser283. (B) The in vitro replication assay was conducted as described in Materials and Methods, and DNA products were detected by the incorporation of [32P]dCTP. FI, early RI, and late RI denote the positions of supercoiled monomer circles and early and late replicative intermediates, respectively.
Cdk-mediated E1 phosphorylation regulates BPV replication in living cells.
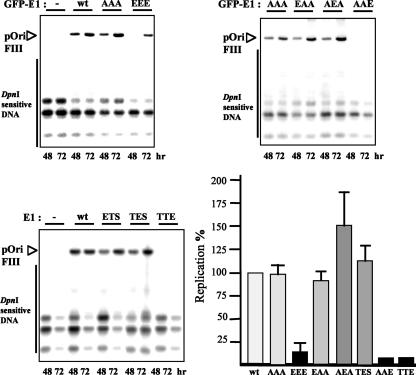
We then tested the E1 phosphorylation site mutants in human 293 cells for their capacity to orchestrate viral replication in the presence of BPV E2, as previously described (23, 25). Unlike the results from the in vitro experiments, the mutants clearly had effects on replication in the transfected cells. The GFP-E1-AAA mutant was able to trigger viral replication in a fashion similar to that of the GFP-E1wt protein, whereas the GFP-E1-EEE mutant displayed reduced activity (Fig. 2). To examine how the charge affects E1 functions in transiently transfected cells, each single site was separately mutated into a glutamic acid (E1-EAA, E1-AEA, and E1-AAE). Among these mutants, the GFP-E1-EAA mutant was found to behave like GFP-E1-AAA, the GFP-E1-AEA mutant displayed an increased replicative activity, and the GFP-E1-AAE mutant had a greatly reduced replication activity (Fig. 2) in comparison to that of the wild-type E1. Thus, the reduced activity of the GFP-E1-EEE mutant is related to the negative charge that mimics a constitutively phosphorylated serine at the Cdk site 283. To substantiate this conclusion, we analyzed the replication activity of native E1 proteins after the incorporation of a single threonine/serine-to-glutamic acid mutation at the three Cdk phosphorylation sites. The negative effect on replication of a glutamic acid substitution at Ser 283 was confirmed by the E1-TTE mutant, while the positive effect of this substitution at threonine 126 (E1-TES) was not as pronounced as with the E1-AEA mutant.
FIG. 2.
Effect of mutations of the Cdk phosphorylation sites in E1 on transient BPV DNA replication. 293 cells were transfected with expression plasmids for the indicated wild-type or mutant E1 protein, E2, and a BPV origin-containing plasmid (pSKori). Low-molecular-weight DNA was extracted at 48 h and 72 h following transfection, digested with EcoRI and DpnI, and analyzed by Southern blotting. The blots were probed with the radiolabeled pSKori+ plasmid to detect the presence of DpnI-resistant DNA products. The bar graph indicates the relative level of pSKori replication measured for the indicated E1 protein at the 72-h time point. The data shown are representative of six independent experiments carried out with GFP-E1-AAA or GFP-E1-EEE; four independent experiments with GFP-E1-AEA or GFP-E1-AAE; and three independent experiments with the untagged E1wt, TES (E1-T126E), and TTE (E1-S283E). The level of viral replication detected in cells transfected with wild-type E1 was set at 100%. Lane − contained an equal amount of GFP parental expression vector.
Taken together, these in vivo data support the conclusion that Cdk phosphorylation of E1 is not essential for its replication function. In contrast, they indicate that a negative charge at the Cdk Ser283 site can downregulate E1 replicative activity in living cells.
Cdk phosphorylation has no influence on E1 nuclear localization and E1 sumoylation.
Since the E1-EEE mutant displayed a reduced replicative activity in living cells but was as active as E1wt in a soluble cell-free system, we considered whether the glutamic acid mutants presented an appropriate cellular compartmentalization. The subcellular localization of the various E1 mutants was analyzed by immunofluorescence after transfection of the GFP-E1 expression vectors in HeLa cells. Since lower-molecular-weight forms of the fusion protein are generated in the cells, we analyzed the intracellular location of the wild-type GFP-E1 protein by indirect immunofluorescence using E1 antibodies directed against the C-terminal part of the protein (anti-E1C) to specifically detect the GFP-E1 full-length protein. In all cells which expressed E1, either wild-type or mutant forms, the full-length E1 protein was exclusively located in the cell nucleus (Fig. 3A). Furthermore, similar levels of wild-type and mutant E1 proteins were expressed, as demonstrated by Western blot analysis (Fig. 3B). Thus, mutation of Ser283 to glutamic acid did not prevent nuclear accumulation of E1. In addition, mutations of the Cdk phosphorylation sites near the nuclear localization signal of E1 had no detectable effect on E1 localization, suggesting that BPV E1 nuclear import is not regulated by Cdk phosphorylation.
FIG. 3.
Cdk phosphorylation of E1 does not affect its cellular localization and stability or its sumoylation in vitro. (A) HeLa cells were transiently transfected with an expression vector for GFP-E1wt or mutant E1 proteins, as indicated, and visualized at 24 h posttransfection. HeLa cells were stained to reveal the distribution of full-length GFP-E1 detected with the anti-E1C antibody, and nuclei were stained with the dye Hoechst. (B) HeLa cells were transfected with the wild-type or mutant GFP-E1 expression vectors. Cells were harvested 24 h after transfection, and total cell lysates were assayed by Western blotting for GFP-E1 using an antibody against GFP and actin as a loading control. The lane labeled with a minus contained an equal amount of the GFP parental expression vector. (C) In vitro-translated and 35S-radiolabeled GFP-E1 wt or mutant proteins were incubated for 2 h in the presence (+) or absence (−) of the assay components GST-SAE1-SAE2, Ubc9, and SUMO-1. After incubation, reaction products were fractionated by SDS-PAGE and the dried gel was analyzed by phosphorimaging. SUMO-1-conjugated forms of E1 (bottom, +1 SUMO-1; top, +2 SUMO-1) are indicated by asterisks.
We also examined whether the E1-AAE mutant which showed the highest reduction in replicative activity still had the two activities that are required for replication in cells but dispensable in the soluble cell-free system. First, E1 interacts with the transcriptional regulatory protein E2, and it was shown that E2 has the capacity to target E1 to nuclear replication foci (33). The glutamic acid mutants E1-AEA and E1-AAE were able to bind as well to E2 as E1wt was (data not shown). Second, since sumoylation has been reported to be required for the E1 replication function in cells (30), we also tested the capacity of the E1-AAE mutant to be sumoylated in vitro. Mutation of either Thr126 or Ser283 did not affect the degree of sumoylation of E1 in vitro (Fig. 3C). Taken together, these data strongly suggest that the E1-AAE mutant presents a defect in an unknown property of E1.
E1wt shuttles between the nucleus and the cytoplasm.
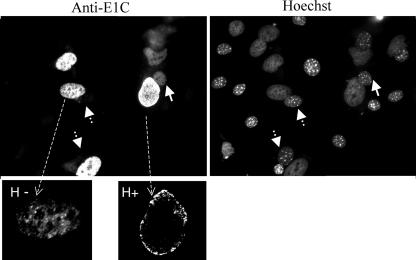
BPV E1 appears to be confined to the nucleus; however, its detection in the cytoplasm might be masked if E1 is exported from the nucleus and rapidly reimported. We set out to explore this possibility, using a heterokaryon assay to detect the putative movement of E1 from the original cell nucleus to the nucleus of the fusion cell partner. HeLa cells were transfected with E1wt expression vector and subsequently fused to BALB/c mouse fibroblasts to form heterokaryons. Before fusion, the cells were treated with cycloheximide to block any further protein synthesis in the heterokaryons. At 2 h postfusion, the cells were fixed and the distribution of E1 was monitored. E1wt, which was at first expressed in HeLa cells, was then detected within the mouse nuclei in 22% of the heterokaryons examined. In most of the heterokaryons, in which E1wt was shuttling and was, therefore, exported from the HeLa nucleus, a strong perinuclear E1 staining was detected in the HeLa cell nuclei (Fig. 4). This specific distribution was detected mainly in cells expressing high E1 levels, suggesting that the mechanism triggering E1 nuclear export might be saturated. These results demonstrate that E1 is not always confined to the nucleus and that it can be exported and imported continuously, resulting in its entry into the mouse nuclei. Thus, despite its apparent steady-state nuclear localization, E1 can actually dynamically shuttle between the nucleus and the cytoplasm.
FIG. 4.
Shuttling of E1wt in HeLa cell-BALB/c heterokaryons. HeLa cells transfected with the E1wt expression vector and pretreated with cycloheximide were fused to BALB/c mouse cells. The cells were then incubated in the presence of cycloheximide for 2 h to allow protein shuttling and stained using the anti-E1C antibody and a secondary antibody conjugated to Texas Red. Mouse nuclei exhibit a mottled appearance when stained with the dye Hoechst, while the human nuclei stain evenly, enabling the two to be distinguished easily. The arrows indicate mouse nuclei fused to HeLa cell nuclei in heterokaryons. Detection of E1, expressed originally in the human cells, within the mouse nuclei is indicative of shuttling and, therefore, of nuclear export. The perinuclear staining of E1wt in HeLa cell nuclei detected in heterokaryons in which E1 shuttled (H+) was strong compared to that in heterokaryons in which E1 did not shuttle (H−).
A negative charge at the Cdk Ser283 site promotes the nuclear export of E1.
On the basis of the previous results, we then tested whether the phosphorylation status of E1 might affect its nuclear export by using the heterokaryon assay. The GFP-E1wt protein was again found to shuttle in 20 to 25% of heterokaryons examined. In contrast, the GFP-E1-AAA mutant was never detected in the murine nuclei, indicating that its nuclear export was compromised. Murine nuclei were also not stained in heterokaryons formed with GFP-E1-AEA-positive HeLa cell nuclei, indicating that the phosphorylation of Thr126 is not involved in the nuclear export of E1. However, the mutants GFP-E1-EEE and GFP-E1-AAE could be detected within the mouse nuclei in all heterokaryons (Fig. 5). These results demonstrate that the export of E1 from the nucleus requires a negative charge at Ser283. As shown in Fig. 6A, the E1-TTE (S283E) mutant was also detected in all mouse nuclei fused to E1-positive HeLa cell nuclei. However, while no shuttling was observed in E1-AEA heterokaryons, of the 44 E1-TES heterokaryons examined, 4 were found in which E1 was shuttling, indicating that if phosphorylation at Ser283 is not compromised, a negative charge at Thr126 is not sufficient to block E1 nuclear export. In summary, E1 shuttling was observed only within heterokaryons in which E1 mutants present a negative charge at Ser283 or when this site is available for phosphorylation (Table 1).
FIG. 5.
Mutation of Ser283 to glutamic acid is sufficient to drive the export of E1. The HeLa cell-BALB/c heterokaryon assay was performed as described in the Fig. 4 legend, and HeLa cells were transfected with expression vectors for GFP-E1wt, GFP-E1-AAA, GFP-E1-EEE, GFP-E1-AEA, or GFP-E1-AAE, as indicated. The arrows indicate mouse nuclei fused to HeLa cell nuclei in heterokaryons. H+, heterokaryons in which E1 shuttled; H−, heterokaryons in which E1 shuttling was not detected.
FIG. 6.
Nucleocytoplasmic shuttling of E1 is inhibited upon leptomycin B treatment. (A) The HeLa cell-BALB/c heterokaryon assay was performed as described in the Fig. 4 legend, with HeLa cells transfected with an expression vector for E1-TES (T126E) or E1-TTE (S283E). (B) The heterokaryon assay was performed with HeLa cells transfected with E1-TTE, and cells were treated with LMB (50 ng/ml) or left untreated. Cells were stained using antibodies against E1 and counterstained with Hoechst dye. The arrows indicate mouse nuclei fused to HeLa cell nuclei in heterokaryons in which E1-S283E has shuttled (H+) or mouse nuclei in heterokaryons in which E1-S283E shuttling was inhibited following LMB treatment.
TABLE 1.
Quantification of shuttling of wt E1 and E1 mutants in HeLa cell/BALB/c heterokaryonsa
| Protein | Total no. of heterokaryons examined | No. of heterokaryons in which E1 shuttled | % Heterokaryons in which E1 shuttled (mean ± SD) |
|---|---|---|---|
| E1wt | 55 | 12 | 22 ± 1.5 |
| GFP-E1wt | 70 | 14 | 21.6 ± 3.4 |
| GFP-E1-AAA | 50 | 0 | 0 |
| GFP-E1-EEE | 50 | 50 | 100 |
| GFP-E1-AEA | 50 | 0 | 0 |
| GFP-E1-AAE | 50 | 50 | 100 |
| E1-T126E | 44 | 4 | 8.7 ± 1.45 |
| E1-S283E | 45 | 45 | 100 |
Heterokaryons that contained one transfected HeLa cell nucleus and at least one BALB/c nucleus were randomly chosen and examined for E1 shuttling. The percentage of positive BALB/c nuclei in the heterokaryons relative to the number of fused cells examined is shown. The values are representative of three independent experiments.
Recent studies have shown that export of HPV-11 E1 or human Cdc6 from the nucleus to the cytoplasm is mediated by an export receptor, exportin 1 (Crm1). The cytotoxin leptomycin B (LMB) specifically inhibits Crm1-dependent protein export. To determine whether nucleocytoplasmic shuttling of BPV E1 is also dependent on Crm1, heterokaryon assays were carried out with the E1-S283E mutant in the presence of LMB. Murine nuclei did not show any E1 staining in heterokaryons formed with E1-S283E-positive HeLa cell nuclei treated with LMB, indicating that BPV E1 nuclear export is also mediated by Crm1 (Fig. 6B).
Since E1wt, as well as the mutant forms, is never detected in the cytoplasm, it was also important to know whether the E1 mutants which are exported into the cytoplasm present an accelerated turnover that could explain their reduced activities in cells. However, no significant changes in the levels of the different mutants were detected following a cycloheximide treatment (data not shown). Thus, following its export from the nucleus into the cytoplasm, E1 must be rapidly reimported into the nucleus. All together, these data led us to conclude that phosphorylation at the Cdk Ser283 site triggers a continuous nucleocytoplasmic shuttling of E1 that interferes with its replication functions.
E1 shuttling occurs in S phase.
Since phosphorylation at the Cdk Ser283 site of E1 appears to negatively regulate viral DNA replication, it was important to determine at which phase of the cell cycle it occurs. Heterokaryon assays were performed with HeLa cells expressing either E1wt or E1-S283E. We first conducted an analysis of the relative DNA content of more than 150 HeLa cell nuclei randomly chosen using the computer program MetaMorph, in order to obtain an estimate of the cell distribution during the different cell cycle phases. Next, the DNA-staining intensity was quantified in 16 HeLa cell nuclei presenting E1-S283E shuttling and 10 HeLa cell nuclei positive for E1wt. The E1-S283E shuttling occurred regardless of the DNA content of the HeLa cell nuclei and, therefore, of the cell cycle stage. Interestingly, in the case of E1wt, the shuttling was observed only in cells with a nuclear DNA content typical of S phase (Fig. 7A). In order to confirm this observation, heterokaryon assays were carried out with E1wt to which BrdU was added for 1 hour following fusion to identify the cells that had entered S phase. In two independent assays, 85 to 90% of heterokaryons in which E1wt shuttled presented a clear BrdU-positive HeLa cell nucleus, indicating that E1 shuttling was mainly triggered during S phase (Fig. 7B).
FIG. 7.
Nucleocytoplasmic shuttling of E1wt occurs in S phase. (A) Quantification of DNA content of HeLa cell nuclei in E1wt and E1-S283E heterokaryons that contain E1-positive BALB/c nuclei. The HeLa cell-BALB/c heterokaryon assay was performed with HeLa cells transfected with expression vectors for E1wt or E1-S283E. The cells were stained to reveal the distribution of E1 using the anti-E1C antibody, and the nuclei were stained with Hoechst stain. The Hoechst stain signal in each nucleus was quantified from digital images using the MetaMorph software. The relative DNA content was measured for 150 HeLa cell nuclei, randomly chosen (all); 16 HeLa cell nuclei expressing E1-S283E in heterokaryons presenting E1-positive BALB/c nuclei (E1-S283E sh+); and 10 HeLa cell nuclei expressing E1wt in heterokaryons presenting E1-positive BALB/c nuclei (E1wt sh+). The number of HeLa cell nuclei was plotted against the DNA content. (B) HeLa cells were transfected with an expression vector for E1wt. Twenty-four hours after transfection, the heterokaryon assay, in which cells were treated with BrdU for 1 h after cell fusion to identify cells in S phase, was performed. Cells were stained using antibodies against E1 and BrdU. The green signal represents the E1wt protein and the red signal the sites of BrdU incorporation which mark replication foci. HeLa cell nuclei (h) and BALB/c nuclei (m) are indicated. (C) Active cyclin A-Cdk2 complexes reconstituted with cyclin A(Δ173) were mixed with glutathione-Sepharose beads coated with 1 μg of either GST, GST-E1wt, or GST-E1-S283A. Complexes were assayed for E1 kinase activity in the presence of [γ-32P]ATP and analyzed by SDS-PAGE and immunoblotting. The resulting membrane, stained with Ponceau S as a loading control (left panel), was then used for phosphorylated E1 quantification by autoradiography (right panel) and immunoblot analysis with anti-cyclin A antibodies (lower panel). (D) GST (1 μg), GST-E1wt (1 μg), or GST-E1-S283E (1 μg) was incubated with 100 μg of HeLa cell extracts prepared from cells synchronized by a double thymidine block and released for 4 h in S phase. Associated proteins were collected with glutathione-agarose beads and subjected to SDS-PAGE, and the presence of cyclin A, cyclin E, and Cdk2 was detected by Western blotting. Cell extracts (20 μg) were loaded as a control (left panel). GST-E1wt or GST-E1-S283E-associated proteins were assayed for E1 kinase activity in the presence of [γ-32P]ATP. Proteins were resolved by SDS-PAGE and visualized by autoradiography or Coomassie blue staining. When indicated, the reaction mixture was supplemented with 0.5 mM olomoucine (right panel).
Since cyclin A-Cdk2 complexes account for almost all of the S-phase Cdk activity, we tested whether E1 interacts directly with cyclin A and can serve as a substrate for cyclin A-Cdk2 in vitro. To test this, GST-E1wt or E1-S283A fusion proteins and purified active cyclin A-Cdk2 complexes were mixed in a GST-binding assay. As shown in Fig. 7C, cyclin A did associate both with GST-E1wt and with GST-E1-S283A but not with GST. When the associated complexes were analyzed for E1 kinase activity, the phosphorylation level of E1-Ser283A was reduced to 10% of that of wild-type E1, indicating that Ser283 serves as a major phosphorylation site for cyclin A-Cdk2 (Fig. 7C). GST-E1wt or GST-E1-S283E was incubated with extracts from HeLa cells synchronized in S phase after release from a double thymidine block. While the negative control (GST alone) did not show any interaction with the cyclins and Cdk2, GST-E1wt, as well as GST-E1-S283E, did bind cyclin A and a faster-migrating form of Cdk2, indicating that both E1 proteins interact specifically with active cyclin A-Cdk2 complexes, which are present in S-phase cell extracts (Fig. 7D). Furthermore, in kinase assays performed with the associated complexes, the phosphorylation level of the Ser283 E1 mutant was again greatly reduced compared to that of E1wt. Next, the Cdk inhibitor olomoucine was added to the E1wt reaction mixture. As shown in Fig. 7D, the associated kinase activity was effectively inhibited in the presence of the Cdk inhibitor. Based on these data, we suggest that cyclin A-Cdk2 is a likely candidate for E1 phosphorylation on Ser283 during S phase.
DISCUSSION
In this study, we report that Cdk phosphorylation of the BPV replicative helicase E1 is not required for direct regulation of its replicative functions either in vitro or in transfected cells. Nevertheless, we discovered an alternative function for one of the putative Cdk phosphorylation sites of E1, which is to regulate its nucleocytoplasmic shuttling. Our results unequivocally demonstrate that a negative charge at the Cdk Ser283 site is required to drive the export of E1 from the nucleus, promoting its continuous nucleocytoplasmic shuttling. We also show that a negative charge on the same site downregulates E1 activity only in a cellular context. These data support the conclusion that phosphorylation at this Cdk site must regulate E1 activity, albeit indirectly, by decreasing its availability in the nucleus. Importantly, we observed that E1 can shuttle only in cells that have entered S phase, indicating that this site-specific phosphorylation event is cell cycle regulated and contributes to the prevention of reinitiation events during S phase.
This new property represents an additional similarity between the BPV E1 replicative helicase and the Cdc6 protein, which is required for initiating DNA replication at chromosomal origins of replication. Cdc6 is nuclear in G1, but its cyclin A-Cdk2-dependent phosphorylation in S phase induces its relocalization from the nucleus to the cytoplasm (15, 27, 28). However, if the cell cycle-dependent export of Cdc6 results in the accumulation of Cdc6 in the cytoplasm, a redistribution of E1 with a resultant cytoplasmic accumulation is never observed. Instead, BPV E1 is exclusively nuclear under steady-state conditions. This suggests that E1 must remain only transiently in the cytoplasm and is rapidly reimported into the nucleus.
Several lines of evidence suggest that cyclin A-Cdk2 might be responsible for phosphorylation of Ser283. The capacity of E1 to shuttle occurs only in HeLa cells that have entered S phase, coinciding with the presence of cyclin A-Cdk2 kinase activity. In vitro data show that cyclin A-Cdk2 binds to E1 and that Ser283 is a major phosphorylation site for this Cdk. Our previous data showed that BPV E1 interacts with an unusually high affinity with cyclin E from Xenopus as well as from a mammalian origin and is phosphorylated in vitro by cyclin E-Cdk2 (9; unpublished data). The levels of cyclin E in S-phase cell extracts are, however, too low to allow detection of an interaction between E1 and cyclin E. Given the temporal pattern of cyclin activation, however, cyclin E being expressed and activated before cyclin A (8), our data do not exclude that phosphorylation of E1 by cyclin E-Cdk2 might also be acting to drive the nuclear export of E1 molecules in cells that have entered S phase. Furthermore, it also cannot be excluded that other kinases beside Cdk might target Ser283 in vivo.
According to our results, unphosphorylatable E1 is functional both in vitro and in transfected cells. While E1-AAA is confined in the nuclei, it does not replicate more efficiently than wild-type E1. This observation shows that while nucleocytoplasmic shuttling of E1 contributes to the mechanism that prevents overreplication in S phase, additional events must be acting in a combinatorial fashion to regulate E1 activity within the cell cycle. If Ser283 is a negative regulatory site in S phase, phosphorylation at the two other putative Cdk sites should also vary with the cell cycle. Our data show that a glutamic acid substitution for Thr102 has no influence on the E1 activity, while a negative charge at Thr126 stimulates E1 activity in transient replication assays, suggesting that phosphorylation at this residue has a positive effect on E1 function.
Although Thr102 and Thr126 are located near the nuclear localization signal of E1, their mutations have, however, no detectable effects on E1 nuclear localization, suggesting that their phosphorylation may not be involved in the regulation of E1 nuclear import. The role of phosphorylation at these residues in E1 function remains to be determined. Regulation of E1 activity by several other kinases, including casein kinase II, protein kinase A (PKA), and PKC, has previously been reported. BPV E1 is a substrate for casein kinase II at Ser48 and Ser584 in vitro (18, 24). Mutation of either Ser48 or Ser584 to alanine abrogates replication in transient-replication assays. In contrast, an E1 mutant in which Ser109, a site for PKA and PKC, is mutated to alanine replicates more efficiently than wild-type E1 in vivo, suggesting that phosphorylation of S109 downregulates E1 activity (38). While the specific roles of these different phosphorylation sites have not been determined, these data indicate that BPV E1 may be regulated by a complex pattern of phosphorylation events with opposing effects, which could be required for regulating both cell cycle- and cell differentiation-specific changes in viral DNA replication.
The nucleocytoplasmic shuttling of E1 in S phase was detected in the absence of other viral elements. The specific phosphorylation event required for shuttling is, therefore, not coupled directly to viral replication and targets free E1 molecules. Assessment of the location of Ser283 in the crystal structure of the DNA binding domain of BPV E1 (residues 159 to 303) indicates that this residue is located on the surface of the E1 DNA binding domain and is fully accessible for phosphorylation, even when E1 is bound to the viral origin of replication as a dimer (11).
The other important finding from our results is that Cdk phosphorylation appears to have opposite effects on the cellular localization of two papillomavirus E1 proteins. In striking contrast with a role of Cdk in promoting nuclear retention of HPV-11 E1, Cdk phosphorylation of BPV E1 drives its nuclear export. The Cdk phosphorylation sites are not conserved between BPV E1 and HPV-11 E1. It was shown that unphosphorylatable HPV-11 E1 (the triple mutant S89A, S93A, S107A) accumulates in the cytoplasm. A Crm1-regulated NES was identified in HPV-11 E1. Given that Ser107 is located within this NES and that the S107A mutation in HPV-11 E1 was the most defective in both nuclear retention and replication activity, it was proposed that phosphorylation at this site is required to inactivate the NES (10). However, mutation of the NES did not restore the activity of the S107A mutant, which must present another defect, since it is also unable to replicate in vitro (21). In another study, deletion of the N-terminal 166 amino acids of HPV-11 E1, which encompass the three Cdk sites and the cyclin recognition motif, does not affect E1-dependent DNA replication in 293 cell extracts, indicating that, similarly to BPV E1, unphosphorylatable HPV-11 E1 is functional in vitro (1).
We have shown in the present study that the nuclear export of BPV E1 is also mediated by CRM1, but the sequence which mediates the CRM1 interaction has not yet been defined. The consensus NES sequence identified in HPV-11 E1, which is highly conserved among HPV E1 proteins, is not found in BPV E1. Thus, while previous studies showed that E1 proteins from BPV and HPV-11 can replicate both genomes and, thus, present significant conservation of structure and functions, they are differently regulated. In support of these observations, the degradation motifs such as the KEN-box and D-box required for APC-mediated degradation of BPV E1 are not present in HPV E1 proteins. We nevertheless found that like BPV E1, HPV-11 and HPV-18 E1 proteins are both targeted by the ubiquitin/proteasome pathway (C. Bonne-Andrea, unpublished results).
This study provides additional insight on how BPV can use the cell cycle machinery to maintain its genome copies at an approximately stable number in proliferating cells of the basal layer of infected epithelia. Our previous data have shown that APC-mediated E1 degradation controls the copy number of BPV origin plasmids both in vitro and in transfected cells (25). The ubiquitin ligase APC is activated at specific stages of the cell cycle, namely from mitosis until the G1/S transition. Thus, the continuous shuttling of E1 between the nucleus and the cytoplasm during S phase might be a way to control E1 activity until the subsequent reactivation of the APC (13). Cdc6, which is exported from nuclei in S phase, is then degraded by the APC during mitosis (29). In both cases, however, the Cdk phosphorylation in S phase appears to be a mechanism to reduce the availability of these initiation factors, either by nuclear exclusion or continuous nucleocytoplasmic shuttling, thereby preventing potential new initiation events.
Acknowledgments
We thank Elisabetta Andermarcher and Andrew Burgess for critical reading of the manuscript, Ronald T. Hay for supplying vectors encoding recombinant GST-SAE1-SAE2 and GST-SUMO-1, Gilles Divita for the gift of cyclin A-Cdk2, Isabelle Jariel-Encontre and Veronique Gire for advice and assistance to set up the heterokaryon assay and the BrdU detection, and Pierre Travo for assistance with fluorescence microscopy. We are also grateful to Marie-Helene Malcles for performing some of the Cdk site mutations.
This work was supported by a grant from the French Ligue Nationale Contre le Cancer (Equipe labelisée).
Footnotes
Published ahead of print on 11 October 2006.
REFERENCES
- 1.Amin, A. A., S. Titolo, A. Pelletier, D. Fink, M. G. Cordingley, and J. Archambault. 2000. Identification of domains of the HPV11 E1 protein required for DNA replication in vitro. Virology 272:137-150. [DOI] [PubMed] [Google Scholar]
- 2.Bell, S. P., and A. Dutta. 2002. DNA replication in eukaryotic cells. Annu. Rev. Biochem. 71:333-374. [DOI] [PubMed] [Google Scholar]
- 3.Bonne-Andrea, C., S. Santucci, and P. Clertant. 1995. Bovine papillomavirus E1 protein can, by itself, efficiently drive multiple rounds of DNA synthesis in vitro. J. Virol. 69:3201-3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonne-Andrea, C., S. Santucci, P. Clertant, and F. Tillier. 1995. Bovine papilloma virus E1 protein binds specifically DNA polymerase α but not replication protein A. J. Virol. 69:2341-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broker, T. R., and M. Botchan. 1986. Papillomaviruses: retrospectives and prospectives. Cancer Cells 4:17-36. [Google Scholar]
- 6.Chow, L. T., and T. R. Broker. 1994. Papillomavirus DNA replication. Intervirology 37:150-158. [DOI] [PubMed] [Google Scholar]
- 7.Conger, K. L., J. S. Liu, S. R. Kuo, L. T. Chow, and T. S. Wang. 1999. Human papillomavirus DNA replication. Interactions between the viral E1 protein and two subunits of human DNA polymerase alpha/primase. J. Biol. Chem. 274:2696-2705. [DOI] [PubMed] [Google Scholar]
- 8.Coverley, D., H. Laman, and R. A. Laskey. 2002. Distinct roles for cyclins E and A during DNA replication complex assembly and activation. Nat. Cell Biol. 4:523-528. [DOI] [PubMed] [Google Scholar]
- 9.Cueille, N., R. Nougarede, F. Mechali, M. Philippe, and C. Bonne-Andrea. 1998. Functional interaction between the bovine papillomavirus type 1 replicative helicase E1 and cyclin E-Cdk2. J. Virol. 72:7255-7262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng, W., B. Y. Lin, G. Jin, C. G. Wheeler, T. Ma, J. W. Harper, T. R. Broker, and L. T. Chow. 2004. Cyclin/CDK regulates the nucleocytoplasmic localization of the human papillomavirus E1 DNA helicase. J. Virol. 78:13954-13965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enemark, E. J., A. Stenlund, and L. Joshua-Tor. 2002. Crystal structures of two intermediates in the assembly of the papillomavirus replication initiation complex. EMBO J. 21:1487-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilbert, D. M., and S. M. Cohen. 1987. Bovine papilloma virus plasmids replicate randomly in mouse fibroblasts throughout S phase of the cell cycle. Cell 50:59-68. [DOI] [PubMed] [Google Scholar]
- 13.Harper, J. W., J. L. Burton, and M. J. Solomon. 2002. The anaphase-promoting complex: it's not just for mitosis any more. Genes Dev. 16:2179-2206. [DOI] [PubMed] [Google Scholar]
- 14.Holt, S. E., G. Schuller, and V. G. Wilson. 1994. DNA binding specificity of the bovine papillomavirus E1 protein is determined by sequences contained within an 18-base-pair inverted repeat element at the origin of replication. J. Virol. 68:1094-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang, W., N. J. Wells, and T. Hunter. 1999. Multistep regulation of DNA replication by Cdk phosphorylation of HsCdc6. Proc. Natl. Acad. Sci. USA 96:6193-6198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lambert, P. F., C. Baker, and P. M. Howley. 1988. The genetics of bovine papillomavirus type 1. Annu. Rev. Genet. 22:235-258. [DOI] [PubMed] [Google Scholar]
- 17.Law, M. F., D. R. Lowy, I. Dvoretzky, and P. M. Howley. 1981. Mouse cells transformed by bovine papillomavirus contain only extrachromosomal viral DNA sequences. Proc. Natl. Acad. Sci. USA 78:2727-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lentz, M., T. Zanardi, R. Filzen, J. Carter, and M. Hella. 2002. Functional analysis of a carboxyl-terminal phosphorylation mutant of the bovine papillomavirus E1 protein. J. Mol. Biol. 316:599-609. [DOI] [PubMed] [Google Scholar]
- 19.Lentz, M. R., D. Pak, I. Mohr, and M. R. Botchan. 1993. The E1 replication protein of bovine papillomavirus type 1 contains an extended nuclear localization signal that includes a p34cdc2 phosphorylation site. J. Virol. 67:1414-1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin, B. Y., T. Ma, J. S. Liu, S. R. Kuo, G. Jin, T. R. Broker, J. W. Harper, and L. T. Chow. 2000. HeLa cells are phenotypically limiting in cyclin E/CDK2 for efficient human papillomavirus DNA replication. J. Biol. Chem. 275:6167-6174. [DOI] [PubMed] [Google Scholar]
- 21.Ma, T., N. Zou, B. Y. Lin, L. T. Chow, and J. W. Harper. 1999. Interaction between cyclin-dependent kinases and human papillomavirus replication-initiation protein E1 is required for efficient viral replication. Proc. Natl. Acad. Sci. USA 96:382-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mailand, N., and J. F. Diffley. 2005. CDKs promote DNA replication origin licensing in human cells by protecting Cdc6 from APC/C-dependent proteolysis. Cell 122:915-926. [DOI] [PubMed] [Google Scholar]
- 23.Malcles, M.-H., N. Cueille, F. Mechali, O. Coux, and C. Bonne-Andrea. 2002. Regulation of bovine papillomavirus replicative helicase E1 by the ubiquitin-proteasome pathway. J. Virol. 76:11350-11358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McShan, G. D., and V. G. Wilson. 2000. Contribution of bovine papillomavirus type 1 E1 protein residue 48 to replication function. J. Gen. Virol. 81:1995-2004. [DOI] [PubMed] [Google Scholar]
- 25.Mechali, F., C.-Y. Hsu, A. Castro, T. Lorca, and C. Bonne-Andrea. 2004. Bovine papillomavirus replicative helicase E1 is a target of the ubiquitin ligase APC. J. Virol. 78:2615-2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park, P., W. Copeland, L. Yang, T. Wang, M. R. Botchan, and I. J. Mohr. 1994. The cellular DNA polymerase α-primase is required for papillomavirus DNA replication and associates with the viral E1 helicase. Proc. Natl. Acad. Sci. USA 91:8700-8704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pelizon, C., M. A. Madine, P. Romanowski, and R. A. Laskey. 2000. Unphosphorylatable mutants of Cdc6 disrupt its nuclear export but still support DNA replication once per cell cycle. Genes Dev. 14:2526-2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petersen, B. O., J. Lukas, C. S. Sorensen, J. Bartek, and K. Helin. 1999. Phosphorylation of mammalian CDC6 by cyclin A/CDK2 regulates its subcellular localization. EMBO J. 18:396-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petersen, B. O., C. Wagener, F. Marinoni, E. R. Kramer, M. Melixetian, E. L. Denchi, C. Gieffers, C. Matteucci, J. M. Peters, and K. Helin. 2000. Cell cycle- and cell growth-regulated proteolysis of mammalian CDC6 is dependent on APC-CDH1. Genes Dev. 14:2330-2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rangasamy, D., K. Woytek, S. A. Khan, and V. G. Wilson. 2000. SUMO-1 modification of bovine papillomavirus E1 protein is required for intranuclear accumulation. J. Biol. Chem. 275:37999-38004. [DOI] [PubMed] [Google Scholar]
- 31.Sedman, J., and A. Stenlund. 1998. The papillomavirus E1 protein forms a DNA-dependent hexameric complex with ATPase and DNA helicase activities. J. Virol. 72:6893-6897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seo, Y. S., F. Müller, M. Lusky, and J. Hurwitz. 1993. Bovine papillomavirus (BPV)-encoded E1 protein contains multiple activities required for BPV DNA replication. Proc. Natl. Acad. Sci. USA 90:702-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swindle, C. S., N. Zou, B. A. Van Tine, G. M. Shaw, J. A. Engler, and L. T. Chow. 1999. Human papillomavirus DNA replication compartments in a transient DNA replication system. J. Virol. 73:1001-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ustav, E., M. Ustav, P. Szymanski, and A. Stenlund. 1993. The bovine papillomavirus origin of replication requires a binding site for the E2 transcriptional activator. Proc. Natl. Acad. Sci. USA 90:898-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ustav, M., and A. Stenlund. 1991. Transient replication of BPV-1 requires two viral polypeptides encoded by the E1 and E2 open reading frames. EMBO J. 10:449-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ustav, M., E. Ustav, P. Szymanski, and A. Stenlund. 1991. Identification of the origin of replication of bovine papillomavirus and characterization of the viral origin recognition factor E1. EMBO J. 10:4321-4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang, L., I. Mohr, E. Fouts, D. A. Lim, M. Nohaile, and M. Botchan. 1993. The E1 protein of bovine papilloma virus 1 is an ATP-dependent DNA helicase. Proc. Natl. Acad. Sci. USA 90:5086-5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zanardi, T. A., C. M. Stanley, B. M. Saville, S. M. Spacek, and M. R. Lentz. 1997. Modulation of bovine papillomavirus DNA replication by phosphorylation of the viral E1 protein. Virology 228:1-10. [DOI] [PubMed] [Google Scholar]