Abstract
The cyanobacteria Synechococcus elongatus strain PCC7942 and Synechococcus sp. strain UTEX625 decomposed exogenously supplied cyanate (NCO−) to CO2 and NH3 through the action of a cytosolic cyanase which required HCO3− as a second substrate. The ability to metabolize NCO− relied on three essential elements: proteins encoded by the cynABDS operon, the biophysical activity of the CO2-concentrating mechanism (CCM), and light. Inactivation of cynS, encoding cyanase, and cynA yielded mutants unable to decompose cyanate. Furthermore, loss of CynA, the periplasmic binding protein of a multicomponent ABC-type transporter, resulted in loss of active cyanate transport. Competition experiments revealed that native transport systems for CO2, HCO3−, NO3−, NO2−, Cl−, PO42−, and SO42− did not contribute to the cellular flux of NCO− and that CynABD did not contribute to the flux of these nutrients, implicating CynABD as a novel primary active NCO− transporter. In the S. elongatus strain PCC7942 ΔchpX ΔchpY mutant that is defective in the full expression of the CCM, mass spectrometry revealed that the cellular rate of cyanate decomposition depended upon the size of the internal inorganic carbon (Ci) (HCO3− + CO2) pool. Unlike wild-type cells, the rate of NCO− decomposition by the ΔchpX ΔchpY mutant was severely depressed at low external Ci concentrations, indicating that the CCM was essential in providing HCO3− for cyanase under typical growth conditions. Light was required to activate and/or energize the active transport of both NCO− and Ci. Putative cynABDS operons were identified in the genomes of diverse Proteobacteria, suggesting that CynABDS-mediated cyanate metabolism is not restricted to cyanobacteria.
Cyanate (NCO−) metabolism has been studied extensively in Escherichia coli and to various degrees in a range of heterotrophic and autotrophic bacteria (references 4, 23, 41, and 50 and references therein). Key to this process is the enzyme cyanase (EC 4.2.1.104) which catalyzes the bicarbonate-dependent decomposition of cyanate to CO2 and NH3 (2, 15, 21, 47) according to the following reaction: NCO− + HCO3− + 2H+ → 2CO2 + NH3. The net formation of CO2 also means that cyanase cocatalyzes the irreversible dehydration of HCO3− (16).
Kinetic, isotopic, and X-ray crystallographic studies show that cyanase binds both NCO− and HCO3− in the active site forming a dianion intermediate that enzymatically decarboxylates to CO2 and carbamate (3, 28, 49). Spontaneous decarboxylation of the carbamate subsequently yields a second CO2 and NH3. Assimilation of cyanate-derived NH3 and CO2 then proceeds through conventional metabolic pathways providing a unique source of nitrogen (N) for growth in a variety of bacteria and a source of carbon (C) for autotrophic metabolism (7, 15, 30, 41, 50, 53).
In E. coli, the coexpression of carbonic anhydrase (CA) is vital to maintain ongoing cyanate metabolism (17, 23). Mutants lacking CA activity do not readily catalyze cyanate decomposition, are unable to grow with NCO− as the sole N source, and are far more susceptible than wild-type cells to the toxic effects of cyanate itself on growth (18, 22-24). CA involvement is related to the absolute requirement by cyanase for HCO3− (rather than CO2) as a substrate in the reaction. Physiological studies (17, 18, 22, 23) indicate that in the absence of CA, the CO2 generated from cyanate decomposition diffuses out of cells faster than it can be hydrated nonenzymatically to HCO3−. This leads to a cellular depletion of HCO3− and cessation of cyanate metabolism through substrate deprivation. CA prevents this cellular depletion by trapping CO2 and catalytically regenerating HCO3− within cells at a rate that is not limiting for cyanase.
Carbonic anhydrase, cyanase, and a hydrophobic protein designated as CynX are encoded by cynT, cynS, and cynX (4, 44), respectively, which are arranged in an operon in E. coli, ensuring the coordinated expression of the two enzymes required for cyanate decomposition. Expression of the cynTSX operon is induced by exogenous cyanate and positively regulated by CynR (45), a member of the LysR family of regulatory proteins. The cynR gene is located immediately upstream of the cynTSX operon but is transcribed in the opposite direction.
The photoautotrophic cyanobacterium Synechococcus sp. strain UTEX 625 also converts exogenous cyanate to CO2 and NH3 as described in the reaction above (30). Inhibitor studies (30) have shown that cyanate-derived NH3 is rapidly incorporated by this cyanobacterium via the central nitrogen assimilation pathway, and it has recently been suggested that NCO− can serve as the sole source of N for growth of the globally important marine cyanobacterium Synechococcus sp. strain WH8102 (35, 43). CO2 arising from cyanate decomposition is also rapidly assimilated by Synechococcus sp. strain UTEX 625 through the photosynthetic carbon reduction (Calvin) cycle. Consequently, NCO− supports photosynthetic oxygen evolution (30). Biochemical and molecular studies have demonstrated cyanase activity in whole-cell extracts of Synechococcus sp. strain UTEX 625, Synechococcus elongatus strain PCC7942 and Synechocystis sp. strain PCC6803, which is absent from derived strains carrying engineered mutations within cynS homologs (19, 20, 30).
Unlike E. coli, the decomposition of exogenous cyanate by Synechococcus sp. strain UTEX625 is light dependent (30). The expression of cynS is not induced by exogenous cyanate, but it is negatively regulated by NH3 and controlled by the global nitrogen regulator NtcA (19, 43). Sequence analysis also indicates that an operon similar to E. coli cynTSX is absent from the genomes of cyanobacteria examined to date. Although monocistronic cynT homologs have been identified and characterized as part of the CO2-concentrating mechanism (CCM) of cyanobacteria (5, 39, 40), a corequirement for CA activity in cyanobacterial NCO− decomposition has not been demonstrated. Instead, it has been proposed that the active HCO3− transport systems that normally provide inorganic carbon (Ci) ([CO2] + [HCO3−] + [CO32−]) for photosynthesis may fulfill the role that is played by CA in E. coli (30).
The ability of heterotrophs and autotrophs to utilize exogenous NCO− as a source of N and C presumably relies upon the transport of this anion into cells. Transport studies in E. coli indicate that N14CO− uptake involves an energy-dependent, saturable transporter with a Km of 400 μM and a Vmax of 4.4 nmol min−1 109 cells−1. CynT was initially implicated as the cyanate permease (46). But as it is now known that CynT is a soluble, cytoplasmic CA, its role in the observed intracellular accumulation of N14CO− likely reflects the trapping of cyanate-derived 14CO2, as H14CO3−, within the cells (17). Since these studies were carried out, CynX has been recognized through amino acid sequence similarity to be a member of the major facilitator superfamily of transport proteins (36). However, an E. coli mutant defective in cynX was found to metabolize cyanate in a manner similar to the wild-type strain (18). Consequently, if CynX is a cyanate permease, there may be multiple pathways for NCO− uptake in E. coli. Similarly, the absence of cynX homologs in cyanobacteria suggests the occurrence of an alternate path for cyanate transport in these organisms. Thus, there appear to be fundamental differences in the molecular components required to support cyanate metabolism in Synechococcus sp. strain UTEX625 and E. coli.
In the present study, we have used radiochemical and mass spectrometric techniques to investigate the transport of cyanate by cyanobacteria and to follow the fate of cyanate-derived CO2 during metabolism. To determine the specificity of cyanate transport, we examined the role played by the major nutrient anion transporters in the acquisition of cyanate. We also investigated the role of an ABC-type (ATP-binding cassette) transporter that is encoded upstream of cynS and forms a putative operon (cynABDS) in S. elongatus strain PCC7942 and Synechococcus sp. strain PCC6301 but is absent in Synechocystis sp. strain PCC6803 (19, 20). Classification of this ABC transporter as a cyanate permease is based primarily on the proximity of cynABD to cynS (19, 20), but it is annotated as an NO3− or HCO3− transporter due to the high degree of amino acid sequence similarity between CynA and the respective periplasmic binding proteins NrtA and CmpA (29). The hypothesis that the Ci transport systems of cyanobacteria are essential to support ongoing cyanate metabolism was tested in a mutant lacking CO2-HCO3− transport capability.
MATERIALS AND METHODS
Strains and growth conditions.
The unicellular cyanobacteria Synechococcus sp. strain UTEX 625 (University of Texas Culture Collection, Austin TX; also known as Synechococcus sp. strain PCC6301), S. elongatus strain PCC7942, and Synechocystis sp. strain PCC6803 (Pasteur Culture Collection, Paris, France) were grown photoautotrophically in BG11 medium containing 17.5 mM NaNO3. The presence of NO3− ensured that cyanase was fully induced (19). Cells were grown either under Ci limitation in standing culture, where diffusion of atmospheric CO2 was the sole means of CO2 delivery, or under high-Ci growth conditions where CO2 was supplied by gassing buffered {25 mM 1,3-bis[Tris(hydroxymethyl)-methylamino[propane [BTP], pH 8 } cell suspensions with sterile air containing 5% (vol/vol) CO2 at 200 ml min−1 (8). The cynA and cynS mutants of S. elongatus strain PCC7942 were grown in the presence of kanamycin (10 μg ml−1), spectinomycin (40 μg ml−1), or ampicillin (100 μg ml−1). The ΔchpX ΔchpY mutant of S. elongatus strain PCC7942 (52) was kindly provided by G. D. Price (Australian National University) and was grown in the presence of kanamycin (12 μg ml−1) and chloramphenicol (5 μg ml−1). E. coli DH5α (Gibco-BRL) was grown on Luria-Bertani medium (38) and supplemented with ampicillin (100 μg ml−1) or spectinomycin (50 μg ml−1), when appropriate.
Insertional inactivation of cynA and cynS.
Targeted mutants of S. elongatus strain PCC7942 were constructed by homologous recombination using antibiotic resistance cassettes to disrupt the coding sequences of the cynA and cynS genes.
Nonreplicating, pUC19-based plasmids containing either cynA or cynS (20) were cut with a restriction endonuclease at a unique ClaI or unique BglII site present within the midportion of the respective genes. The nptII gene, conferring kanamycin resistance, was ligated into the restricted site within cynA while the aadA gene, conferring spectinomycin resistance, was ligated into the restricted site within cynS. The resulting plasmids pP12::ORF440 (Ampr Kanr::cynA) and pXH::ORF146 (Ampr Sptr::cynS) were propagated in E. coli DH5α, purified, and transformed into S. elongatus strain PCC7942 using the method described by Laudenbach and Grossman (26). Transformants recovered from BG11 plates were maintained initially in the dark and then at low light (10 μmol photons m−2 s−1; 400 to 700 nm) on 5% (vol/vol) CO2 in air at 30°C and supplemented with 5 mM sodium thiosulfate along with the appropriate selective antibiotic (10 μg ml−1 kanamycin or 40 μg ml−1 spectinomycin). Transformants were serially streaked on fresh plates three times to encourage complete segregation. Mutants arising from homologous recombination between plasmid and cynA or cynS were recovered by replica plating and identified as colonies that were ampicillin sensitive. Genomic DNA was subsequently isolated (25) from putative, homozygous mutants and analyzed by Southern hybridization (38) to confirm the presence and location of the insert.
Database queries.
Homologs of the S. elongatus strain PCC7942 CynA, CynS, CmpA, and NrtA proteins were identified by searching the NCBI database (National Center for Biotechnology Information, http://www.ncbi.nlm.nih.gov/) with the resident BLASTp program (1) using proteins AAB58736, AAB02940, YP_400505, and YP_400256, respectively, as individual queries. Phylogenetic trees were constructed using the TreeView program from multiple protein sequence alignments generated by Clustal X (48).
Preparation of cells.
Cells (4 to 6 μg of chlorophyll [Chl] ml−1) used for transport and physiological studies were harvested in mid-log phase, washed three times by centrifugation, and suspended in Ci- and Na+-free 25 mM BTP-23.5 mM HCl buffer to a final density of 7 to 18 μg of Chl ml−1. All experiments were conducted at 30°C and pH 8 to 8.3. The [Chl] of cell suspensions was measured spectrophotometrically at 665 nm after extraction in methanol (8).
O2 evolution and chlorophyll a fluorescence yield.
Photosynthetic O2 evolution was measured in a Clarke-type electrode (Hansatech, Norfolk, United Kingdom) (8). Chl a fluorescence quenching was measured with a pulse amplitude modulation fluorometer (PAM 101; H. Walz, Effeltrich, Germany) as described previously (6). In both of the Synechococcus strains used, the extent of Chl a fluorescence quenching is directly proportional to the magnitude of the intracellular Ci pool (6). Fluorescence was, therefore, used as a surrogate to continuously monitor the formation and dissipation of the Ci pool in real time. Fluorescence quenching was expressed as a percentage of the variable fluorescence, FV*, such that FV* = FM* − FO, where FM is the maximum fluorescence yield and FO is the instantaneous low-fluorescence yield (6).
Mass spectrometry (MS) and measurement of dissolved gas fluxes.
The concentrations of 16O2, 12CO2, 13CO2, and 14CO2 (m/z values of 32, 44, 45, and 46, respectively) dissolved in reaction media or cell suspensions or arising from the metabolism of cyanate were measured using a magnetic sector MS (model MM 14-80 SC; VG Gas Analysis, Middlewich, United Kingdom,) (30). The membrane-covered inlet to the MS was inserted into a port in the side of a transparent, temperature-controlled reaction vessel to allow direct measurements of dissolved gas concentrations over time. The response of the instrument was calibrated by the addition of known concentrations of 16O2, K212CO3, K213CO3, or NaH14CO3. The content of the reaction vessel was isolated from the atmosphere using a Plexiglass stopper fitted with a dual O-ring. A capillary bore in the stopper facilitated the addition of reagents to the cell suspension.
Cell suspensions in the reaction vessel were illuminated with a tungsten-halogen projector lamp (300 μmol of photons m−2 s−1; 400 to 700 nm). Simultaneous measurements of CO2 and O2 fluxes and Chl a fluorescence yield were obtained by placing the convergent end of the four-armed fiber optic bundle of the fluorometer at the surface of the MS reaction vessel at a 90° angle to the white light source.
Radiochemical analysis of NCO−, HCO3−, and CO2 transport.
Transport and intracellular accumulation were assayed using the silicone fluid filtering centrifugation technique with N14CO−, H14CO3−, or 14CO2 as substrate, essentially as described by Miller et al. (32). To determine the level of acid-stable products of photosynthesis and the acid-labile intracellular pool of NCO− or Ci, the cell samples were subsequently processed as described by Espie et al. (10). Intracellular concentrations of NCO− or Ci were calculated using an internal cellular water volume of 49 μl mg−1 of Chl (9).
Four variations on the transport assay protocol were used. With method A, cells were incubated in the light (300 μmol of photons m−2 s−1; 400 to 700 nm) in the O2-electrode chamber and allowed to deplete the medium of Ci, as indicated by the cessation of O2 evolution. A cell sample (100 μl) was then removed and placed into a 400-μl microcentrifuge tube containing 100 μl of silicone fluid (middle layer, silicone oils AR20:AR200 at 1.75:1 (vol/vol); Wacker Chemie, Munich, Germany) and 100 μl of terminating solution (bottom layer, 2 M KOH in 10% [vol/vol] methanol). The tube was placed in a Beckman microcentrifuge E, illuminated from above (300 μmol of photons m−2 s−1) for a further 1 min, and the experiment was started by the addition of KO14CN to the cells using a 10-μl Hamilton syringe. Uptake was stopped at timed intervals by centrifuging (1 min at 12,500 × g) the cells through the silicone fluid and into the terminating solution. In method B, when N14CO− accumulation was measured in mutant cells or when long-term uptake assays were conducted, KO14CN was introduced directly into the O2-electrode chamber. Then, under constant illumination, 100-μl samples were removed and layered on top of the silicone fluid/termination solution contained in microcentrifuge tubes. The experiment was terminated as above. For method C, another approach was used in experiments to evaluate the effect of HCO3− on NCO− transport and the effect of NCO− on HCO3− transport. In these experiments, the silicone fluid was overlaid with 50 μl of buffer containing KO14CN + NaHCO3 or KOCN + NaH14CO3. The headspace was flushed with N2. Cells (50 μl) depleted of Ci were then introduced into the microcentrifuge tubes in such a way as to create an N2-filled gap between the cell suspension and the reaction buffer. The sealed tubes (six at once) were then placed in the microcentrifuge and illuminated for 1 min. To start the experiments, the cell suspensions and reaction buffers were rapidly mixed by briefly (for less than 1 s) engaging the drive of the microcentrifuge. The uptake experiments were terminated as above. For method D, when the effect of NCO− on 14CO2 transport was determined, NCO− and 14CO2 were added in rapid sequence to cells already in a microcentrifuge tube. Uptake was terminated after 10 s as described above. The 14CO2 was created by adding NaH14CO3 to 10 mM phthalic acid buffer, pH 4.
KOCN and Ci contamination.
Reagent grade KOCN was obtained from BDH Chemicals (Toronto, Canada) and was crystallized three times from a water-ethanol mixture prior to use (21). KO14CN was obtained from Dupont Canada (Mississauga), and KO13CN was from MSD Isotopes (Montreal). Stock solutions were made by dissolving crystallized KOCN in Ci-free water and by dissolving KO14CN or KO13CN in Ci-free 25 mM BTP buffer, pH 8. At pH 8, virtually all the KOCN existed in the form of the NCO− anion (pKa of HOCN is 3.7 at 27°C) (27) and slowly decomposed (0.01% h−1) forming CO2 and NH3. The level of contaminant 12CO2, 13CO2, or 14CO2 present in KOCN solutions, “Ci-free” buffers, and other solutions was routinely determined by MS. Equilibrium Ci concentrations were subsequently calculated for the experimental temperature and pH as described by Stumm and Morgan (42). Typically, freshly crystallized and freshly prepared 1 mM KOCN stock solutions contained 7.1 ± 2 μM 12Ci (n = 7) while 1 mM KO14CN contained 6.5 ± 1.8 μM 14Ci (n = 7), and 1 mM KO13CN contained 86.5 ± 9.7 μM 13Ci (n = 7). These values increased over time. The KO14CN stock solutions contained less than 2% acid-stable material, and its presence was corrected for in calculations. In some experiments, the level of Ci contamination in solutions could be reduced to near-zero by first allowing cells to photosynthetically deplete the reaction medium. But in all cases, appropriate corrections were made to the specific activities of radiochemicals and to concentrations of reactants to account for the presence of contaminant Ci.
Cyanate is relatively stable in alkaline solution (hydrolysis rate of 0.01% h−1) but rapidly decomposes to CO2 and NH3 below about pH 4.5 (27). Consequently, unreacted N14CO− was removed from samples by acidification and driving off the resulting 14CO2 by evaporation.
Nucleotide sequence accession numbers.
The nucleotide sequence of the cynABDS region determined in this study has been deposited in the GenBank database at NCBI under accession numbers AF001333 and U59481.
RESULTS
Cyanate uptake and the fate of cyanate-derived 14C.
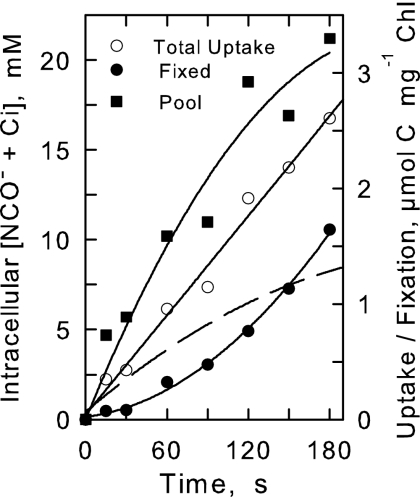
Figure 1 shows a typical time course of 14C uptake by Synechococcus sp. strain UTEX 625 (PCC6301) cells provided with 1 mM KO14CN. The intracellular 14C was found in two major fractions: an acid-stable fraction composed of metabolic intermediates and an acid-labile fraction consisting of unreacted N14CO− and 14Ci. Over the course of the experiment (180 s), the intracellular acid-labile pool of 14C rose to about 21 mM.
FIG. 1.
Time course (180 s) of NCO− uptake in the light for Synechococcus sp. strain UTEX 625 standing culture cells provided with 1 mM KO14CN, as determined by method A. Shown are total uptake (○), the acid-stable products of C-fixation (•), and the acid-labile intracellular pool of NCO− + Ci (▪). Data are the average of three replicates ±10.4% (maximum). Also shown (dashed line) is the calculated intracellular pool of NCO− corrected for intracellular Ci.
Interpretation of cyanate uptake experiments (Fig. 1 and 2) is complicated by the presence of 14Ci which invariably contaminated the KO14CN (7 to 25 μM/1 mM). This 14Ci was undoubtedly transported rapidly into the cells by the high-affinity Ci transport systems and fixed by the Calvin cycle enzyme ribulose-1,5-bisphospahate carboxylase, thereby contributing to both the acid-labile and acid-stable fractions (9, 12, 31, 32). In fact, nearly all the 14C fixed during the uptake experiments (Fig. 1) entered the acid-stable fraction via the photosynthetic carbon reduction cycle as evidenced by the fact that darkness, DCMU (3[3,4-dichlorophenyl]-1,1-dimethylurea), and the Calvin cycle inhibitor glycolaldehyde reduced 14C incorporation by 98%, 98%, and 94%, respectively (Table 1). Over the course of 25-min experiments (Fig. 2), 18 to 26% of the total added C (as KOCN) was found in the acid-stable fraction. This level of incorporation exceeded the 14Ci available in KO14CN solutions by at least 36-fold. During the short-term experiments (Fig. 1), 14C incorporation exceeded the available 14Ci by fourfold, indicating that most of the 14C found in the acid-stable fraction was derived from the 14C originally present in KO14CN.
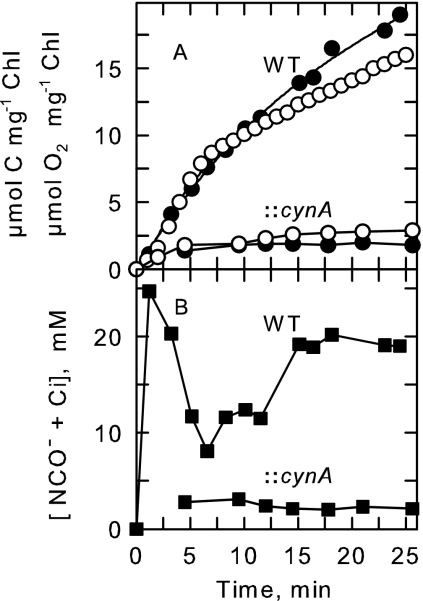
FIG. 2.
Typical time course (25 min) of NCO− uptake and utilization by S. elongatus PCC7942 wild type (WT) and the cynA mutant (::cynA). Cells grown in standing culture with low Ci concentrations were provided with 1 mM KO14CN in the light. Uptake and accumulation of 14C was determined as described in method B. (A) Simultaneous measurements of cyanate-dependent CO2 fixation (•) and O2 evolution (○) for the wild type and the cynA mutant. (B) Intracellular accumulation of acid-labile NCO− + Ci (▪) for the wild type and the cynA mutant (::cynA).
TABLE 1.
Characterization of NCO− uptake and metabolism in Synechococcus sp. strain UTEX 625
| Experimental conditionsa | N14CO− uptake (% of control) | 14C fixation (% of control) |
|---|---|---|
| Light (control) | 100 | 100 |
| Dark | 6.3 | 2.4 |
| Light + 25 μM DCMU | 7.9 | 2.2 |
| Light + 15 mM glycolaldhyde | 59.1 | 5.6 |
| Light + 15 mM oxalate | 88 | 99.7 |
| Light + 1 mM ethoxyzolamide | 39 | 32.3 |
NCO− uptake and C fixation were measured by the silicone fluid filtering centrifugation technique using method A. Cells grown in standing culture were incubated with 1 mM KO14CN for 2 min in BTP/HCl buffer, pH 8.0, containing 25 mM NaCl at 30°C and illuminated at 300 μmol m−2 s−1. Data are the average of 5 experiments in triplicate ±6.5%.
The incorporation of 14C into acid-stable products initially lagged behind the accumulation of the acid-labile internal pool of 14C (Fig. 1). But as this pool increased in size so, too, did the rate of C incorporation, approaching a steady-state rate as the internal pool approached a maximum. The dynamics of these two fractions were indicative of a precursor-product relationship in which the acid-labile C (NCO− and Ci) was first transported and accumulated within the cells and, subsequently, incorporated into acid-stable products of photosynthesis.
Cyanate supported light-dependent O2 evolution at a linear rate for about 10 min, after which the rate declined to a lower steady-state rate (Fig. 2). The decline in the rate of O2 evolution was paralleled by a decline in C-fixation and coincided in time with a decrease in the internal NCO− + Ci pool (Fig. 2). With time, the internal pool stabilized at a new steady-state level leading to a lower steady-state rate of cyanate-dependent O2 evolution and C-fixation. Biphasic time courses of O2 evolution were commonly observed when cells were provided with KOCN exceeding about 100 μM. A near 1:1 stoichiometry was found between added KOCN, O2 evolution, and CO2 fixation (Fig. 2) (30), consistent with the complete decomposition of cyanate to NH3 and CO2 and subsequent fixation of CO2 into acid-stable products of photosynthesis.
The ability of Synechococcus sp. UTEX 625 to utilize cyanate-derived CO2 to support photosynthesis was affected by the level of Ci experienced during growth. For cells grown in standing culture (low Ci concentration, approximately 20 μM), with air bubbling (intermediate Ci concentration, approximately 180 μM), or bubbling with 5% CO2 (high Ci concentration), analysis of cyanate response curves indicated K0.5 (the substrate concentration that yields one-half the maximum rate) (NCO−) values for O2 evolution of 0.34 ± 0.04, 0.43 ± 0.13, and 0.70 ± 0.05 mM and maximum rates of 138 ± 10, 187 ± 34, and 233 ± 43 μmol O2 mg−1 of Chl h−1 (± standard deviation; n = 3), respectively. Since growth on Ci also affects the level of induction of the CCM (5), the data suggest a possible role for the CCM in modulating cyanate utilization.
Active transport and accumulation of NCO−.
Cyanate uptake was stimulated by light and inhibited by DCMU, suggesting that photosynthetic electron transport may be involved in energizing or activating NCO− transport (Table 1). An acid-labile pool of C was also formed in the presence of glycolaldehyde, indicating that uptake was not strictly tied to the photosynthetic metabolism of cyanate. Oxalate, a membrane-impermeant inhibitor of both E. coli and cyanobacterial cyanase (3, 30), had little effect on either the acid-labile or acid-stable fractions (Table 1), indicating that cyanase was absent from the periplasm. This finding is consistent with previous biochemical studies which show cyanase activity in the soluble, cytoplasmic fraction of cell lysates (19, 30). Consequently, NCO− and/or HOCN must permeate the inner membrane prior to decomposition.
In order to distinguish between N14CO− uptake and accumulation and 14Ci uptake and accumulation (Fig. 1), we conducted a series of experiments in the presence of unlabeled 12Ci (Table 2). Through dilution with 12Ci, the specific activity of the 14Ci present as a contaminant in KO14CN solutions was reduced by up to 1,000-fold, effectively eliminating the contribution of 14Ci uptake to the intracellular acid-labile pool. Table 2 shows that the initial rate (20 s) of 14C uptake by cells presented with 1 mM KO14CN was 48% or 49% of the control value in the presence of 2 or 5 mM 12Ci, respectively. Similarly, the internal pool was reduced to 45% or 46% of the control value, while 14C-fixation declined to 47% to 42% of the control. Slightly larger decreases in the rate of uptake and intracellular accumulation were observed over 120-s time intervals while 14C-fixation declined by as much as 75% (Table 2). Consequently, we estimated that 40% to 45% of the total acid-labile intracellular pool of 14C was comprised of N14CO−. Based on this estimate, the intracellular concentration of cyanate would be expected to be about 2.7 mM at 20 s, 6.3 mM at 120 s (Table 2), and 8.4 mM at 180 s (Fig. 1). This latter value is 8.4-fold higher than the initial concentration of KOCN provided to the cells. Assuming a membrane potential of −0.12 V (37), the electrochemical potential for NCO− was calculated to be 16.6 kJ mol−1 (−0.63 V), indicating that a component of NCO− uptake involved carrier-mediated, active transport.
TABLE 2.
Effect of [12Ci] on N14 CO− transport by Synechococcus sp. strain UTEX 625 grown in standing culturea
| Experimental condition (with 1 mM KO14CN) | % of control value at 20 s
|
% of control value at 2 min
|
||||
|---|---|---|---|---|---|---|
| N14CO− uptake | Pool | 14C fixation | N14CO− uptake | Pool | 14C fixation | |
| 0 mM KHCO3 | 100 | 100b | 100c | 100 | 100d | 100e |
| 2 mM KHCO3 | 48.2 ± 9.3 | 45.4 ± 16.2 | 47.5 ± 21.6 | 38.4 ± 15.2 | 41.0 ± 12.9 | 24.9 ± 10.6 |
| 5 mM KHCO3 | 49.4 ± 7.3 | 46.4 ± 19.3 | 42.9 ± 12.2 | 39.6 ± 14.6 | 41.5 ± 18.7 | 30.4 ± 19.7 |
Values were determined using method C in BTP-HCl buffer, pH 8, containing 25 mM NaCl. Light was supplied at 300 μmol of photons m−2 s−1. Data are the average of seven experiments ± standard deviations.
Control value, 6.0 ± 2.0 mM.
Control value, 0.06 μmol of C mg−1 Chl.
Control value, 11.0 ± 7.0 mM.
Control value, 0.23 μmol of C mg−1 Chl.
Transport specificity.
The reduction in 14C accumulation in the presence of 12Ci (Table 2) may also indicate a competition between Ci and NCO− during transport. Consequently, the possibility that NCO− transport was gratuitously mediated via one of the Ci transport systems or one of the major nutrient anion transport systems was tested in a series of competition experiments in the presence of CO2, HCO3−, NO3−, NO2−, Cl−, PO42−, or SO42− (Fig. 3 and 4).
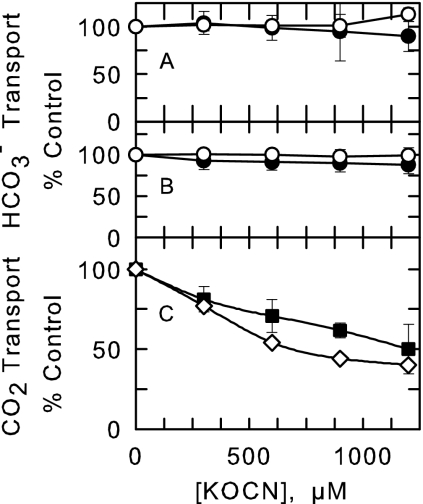
FIG. 3.
Effect of KOCN on HCO3− and CO2 transport in Synechococcus sp. strain UTEX 625 cells grown in standing culture. The initial rate (20 s) of HCO3− transport was measured (method C) at substrate concentrations of 50 μM (•) and 250 μM (○) in pH 8 buffer to determine the effect of increasing [KOCN] on Na+-independent HCO3− transport in the presence of 100 μM NaCl (A) or Na+-dependent HCO3− transport in the presence 30 mM NaCl (B). (C) The initial rate (10 s) of CO2 transport (▪) was measured at 10 μM in the presence of 100 μM NaCl (method D). Also shown is the effect of KOCN on CO2 transport in cells grown under high-Ci conditions(⋄). The data are the average of six determinations ± standard deviations.
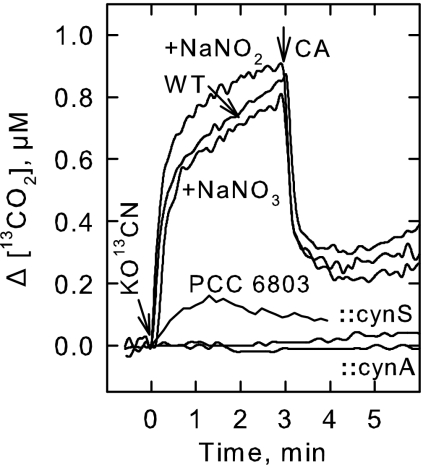
FIG. 4.
KOCN-dependent CO2 efflux by cells grown under high-CO2 conditions. Illuminated wild-type (WT) S. elongatus PCC7942 cells were provided with 1 mM KO13CN, and the efflux of 13CO2 was followed by MS over time in the absence or presence of 25 mM NaNO3 or NaNO2. Bovine CA was added to the suspension to illustrate that CO2 was the Ci species arising in the cell suspensions. Also shown are the CO2 fluxes arising from the cynA and cynS mutants and from Synechocystis sp. strain PCC6803 grown in high-CO2 concentrations.
Physiologically, Ci transport in Synechococcus sp. strain UTEX 625 (PCC6301) and S. elongatus strain PCC7942 is comprised of an active, Na+-dependent HCO3− uptake activity, an active Na+-independent HCO3− uptake activity, and high- and low-affinity active CO2 uptake activities (6, 9, 12, 31). These transport activities are thought to involve the membrane proteins SbtA, CmpABCD, NdhF3/NdhD3/ChpY, and NdhF4/NdhD4/ChpX, respectively (reviewed in reference 5). If one or more of these transporters is involved in NCO− transport, then NCO− must also be an inhibitor of Ci transport activity. Figure 3A and B show that neither Na+-independent HCO3− transport nor Na+-dependent HCO3− transport was inhibited by up to a 24-fold excess of KOCN. However, in cells grown with low Ci concentrations in standing culture and with high Ci concentrations, CO2 transport was reduced by up to 50% with 1 mM KOCN (Fig. 3C). Thus, NCO− transport may occur through the CO2 transport system. The fact that NCO− is an isoelectronic structural analog of CO2 supports this view. However, although 14CO2 transport assays were conducted rapidly, the 12CO2 generated from the decomposition of cyanate (Fig. 4) may also have contributed to the apparent inhibition of 14CO2 transport. Thus, the involvement of the CO2 transport systems in NCO− transport requires further analysis (see below).
The involvement of nitrate/nitrite transport systems in NCO− transport was tested in a different way. The Synechococcus strains grown under high-Ci conditions have a greatly reduced capacity for HCO3− transport and rely primarily on the low affinity CO2 uptake system for Ci acquisition (5). Consequently, the contribution (if any) of the CO2 transport systems to NCO− transport can be minimized. When cells grown with high Ci concentrations were presented with N13CO−, a large and sustained rise in the extracellular 13CO2 concentration was observed that was light dependent (Fig. 4). The addition of bovine CA to cell suspensions reduced the [13CO2], indicating that it was CO2 and not HCO3− that exited the cells and that the CO2 was present at a concentration well above its chemical equilibrium with HCO3−. The use of KO13CN as substrate confirmed that the 13CO2 arising in the medium was derived directly from cyanate uptake and decomposition rather than from respiration or any other process (30). Consequently, in vivo cyanate uptake and decomposition can be followed in real time by measuring KOCN-dependent CO2 efflux via MS (Fig. 4).
When S. elongatus strain PCC7942 was presented with KO13CN in the presence of either 25 mM NaNO3 or 25 mM NaNO2, both the qualitative and quantitative patterns of 13CO2 formation were very similar to the control pattern observed with cells in buffer alone (Fig. 4) or in the presence of 25 mM NaCl (not shown). Thus, neither NO3− nor NO2− interfered with the process of cyanate uptake or its decomposition to CO2. Since the nitrate/nitrite transport systems were saturated at these levels, we conclude that these transporters were not involved in the transport of NCO−. Similarly, neither phosphate nor sulfate affected the patterns of KOCN-dependent CO2 efflux (not shown).
In cells grown under low-Ci conditions, the steady-state rate of KOCN-dependent O2 evolution in the light was also not substantially reduced by the presence of nutrient anions. Measured rates (100 μM KOCN; n = 3) in the presence of 25 mM NO3−, NO2−, Cl−, PO42−, and SO42 were 92.0%, 97.1%, 104%, 94.1%, and 89.2% of the control value (25 mM BTP, pH 8). Collectively, the data indicated that none of the major inorganic anion transport systems played a significant role in the active transport of NCO−.
cynS is required but not sufficient to promote the metabolism of exogenous NCO−.
Southern hybridization analysis of ClaI-digested DNA from S. elongatus strain PCC7942 wild-type and the cynS mutant was carried out using a 187-bp EcoR1-BglII restriction fragment from cynS as the probe. A single hybridization signal of approximately 7.0 kb was obtained from DNA from the cynS mutant compared to a 5.0-kb band from wild-type DNA (not shown). The 2-kb difference corresponded well to the expected insertion of the Sptr cassette into cynS and indicates that only a single disrupted copy of cynS was present in the genome of the S. elongatus strain PCC7942 cynS mutant.
Previous studies have shown that targeted interruption of cynS in S. elongatus strain PCC7942 results in the loss of intracellular cyanase activity (19). Consistent with this is the observation that our cynS mutant was unable to utilize exogenously supplied KOCN to support photosynthetic O2 evolution (Fig. 5A). The inability of the cynS mutant to utilize NCO− was not related to a defect in the photosynthetic utilization of cyanate-derived CO2 since it grew photoautotrophically in a normal fashion under low-Ci conditions and responded in a comparable manner to wild-type cells when supplied with 25 μM Ci. The cynS mutant was also able to form and retain a large internal pool of Ci when provided with exogenous 25 μM Ci, as judged by an increase in Chl a fluorescence quenching (Fig. 5B). The addition of KOCN also elicited substantial fluorescence quenching (43% of FV* after 5 min) in wild-type cells which corresponded to an internal Ci pool of about 28 to 30 mM (6). KOCN-dependent fluorescence quenching preceded the onset of steady-state photosynthesis, consistent with an initial uptake and subsequent decomposition of cyanate to form an internal pool of CO2. These responses to KOCN were completely absent in the cynS mutant (Fig. 5B), as was the rise in CO2 concentration following KOCN addition to cells grown under high-Ci conditions (Fig. 4). The disruption of cynS thus led to the loss of intracellular cyanase activity (19) and to an inability to metabolize exogenous cyanate in vivo.
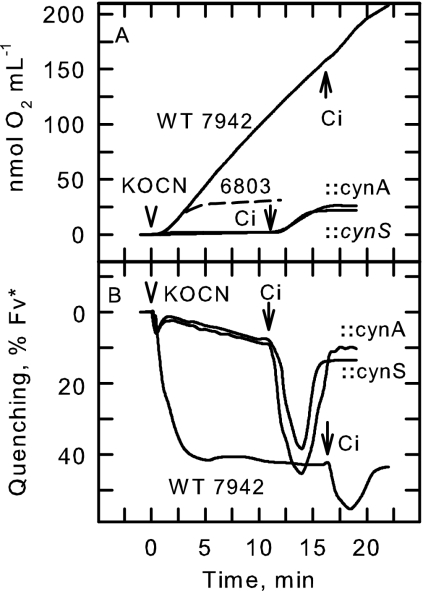
FIG. 5.
Measurements of KOCN-dependent O2 evolution (A) and Chl a fluorescence quenching (B) in S. elongatus PCC7942 wild-type (WT) cells and the cynA and cynS mutants. Cells grown under low-Ci conditions were allowed to deplete the medium of Ci in the light. Cells were then provided with 1 mM KOCN, and O2 evolution and Chl a fluorescence quenching were measured simultaneously over time. Once a steady state was achieved, Ci-dependent O2 evolution and Chl a fluorescence quenching were also measured following the addition of 25 μM Ci to the cell suspensions. Also shown in panel A is the response of Synechocystis sp. strain PCC6803 to the addition of 1 mM KOCN.
Although the cyanobacterium Synechocystis sp. strain PCC6803 also expresses a high level of cyanase activity when grown with nitrate as its N source (19), the wild-type cells were unable to utilize exogenous cyanate to support O2 evolution in the light (Fig. 5A). In addition, Synechocystis sp. strain PCC6803 cells grown under high-Ci conditions failed to create the sustained rise in extracellular CO2 concentration in the light that was characteristic of cyanate decomposition (Fig. 4), in spite of the presence of abundant and functional cyanase (19). These data suggest that molecular components beyond cynS are required to facilitate the metabolism of exogenous cyanate in the two Synechococcus strains and that some or all of these components are lacking naturally or are not expressed in Synechocystis sp. strain PCC6803.
cynA is required for cyanate transport.
Immediately upstream of cynS in S. elongatus strain PCC7942 and Synechococcus sp. strain PCC6301, but not Synechocystis sp. strain PCC6803, is a three-gene cluster (GenBank accession numbers AF001333 and U59481) initially designated as orf440, orf263, and orf289 and later renamed cynA, cynB, and cynD, respectively (19, 20). The cyn genes are all transcribed in the same direction (cynA to cynS) and are separated by 9, 3, and 10 nucleotides, respectively. Putative ribosome binding sites are located 7 to 10 bp upstream of start codons in all but cynD (16). A potential transcription termination sequence is located downstream of cynS (20) while an NtcA-dependent promoter is located upstream of cynA between −334 and −293 (19) (GenBank accession number AB005890). Sequence analysis, therefore, predicts that cynA, cynB, cynD, and cynS form an operon. This view is supported by Northern hybridization analysis which indicates that both cynA (20) and cynS (19, 20) are present on a transcript approximately 4.3 kb in size.
The deduced amino acid sequences of CynA, CynB, and CynD show significant sequence identity to proteins that are members of the ABC-type superfamily of membrane transport proteins (19, 20). The protein encoded by cynB has characteristics typical of the integral membrane permease component of the transport system while CynD contains ATP-binding motifs characteristic of the peripheral membrane component involved in the energization of transport (19, 20). CynA, a putative periplasmic binding protein, has a high degree of amino acid similarity to its paralogs NtrA (46%) and CmpA (45%). The latter two periplasmic proteins are known to bind NO3−/NO2− and HCO3−, respectively (29, 34). Thus, CynA may be a monovalent anion binding protein.
To determine if the cynABDS operon is involved in cyanate transport, we characterized a S. elongatus strain PCC7942 cynA mutant, which was created by interrupting the cynA gene with a Kanr cassette. Southern hybridization analysis of PstI-digested DNA from S. elongatus strain PCC7942 wild type and the cynA mutant strain, using a 687-bp PstI-ClaI restriction fragment from cynA as the probe, revealed the presence of a single disrupted copy of cynA in the genome of the mutant (not shown). The cynA mutant was unable to carry out KOCN-dependent O2 evolution or KOCN-dependent Chl a fluorescence quenching, although the mutant was capable of typical wild-type Ci-dependent O2 evolution and Ci-dependent Chl a fluorescence quenching (Fig. 5). Similarly, cynA mutant cells grown with high Ci concentrations did not evolve CO2 when presented with KOCN (Fig. 4), indicating that the lesion in cynA had disrupted their ability to utilize exogenous cyanate. Transport assays (Fig. 2) revealed a marked decline in intracellular N14CO− accumulation and the absence of sustained 14C fixation in the light compared to wild-type cells. In these experiments, sampling of the cynA mutant was begun 5 min after the addition of 2 mM KO14CN. This allowed the mutant sufficient time to fix contaminating 14Ci into acid-stable products, as judged by O2 evolution and fluorescence measurements. Consequently, direct measurements of the acid-labile intracellular N14CO− pool were possible (Fig. 2B). The average intracellular concentration of NCO− in the cynA mutant was 2.1 ± 1.1 mM (n = 6) after a 5-min exposure and 3.1 ± 1.9 mM (n = 6) after a 10-min exposure. In comparison, wild-type S. elongatus strain PCC7942 accumulated a total pool of 27.1 ± 8.1 mM (n = 8) after 5 min and 37.1 ± 11.1 mM (n = 8) after 10 min. From these data, internal concentrations of NCO− of 12.2 and 16.7 mM, respectively, were calculated assuming that 45% of the measured pool in wild-type cells was free NCO−. Consequently, the level of NCO− accumulation in the cynA mutant is at least fivefold lower than in wild-type cells. Calculations using the Henderson-Hasselbach equation indicate that about one-half of the observed accumulation in the cynA mutant was accounted for by the diffusion of HOCN into the cells and its passive equilibration with NCO−. Thus, the ability of the cynA mutant to transport and concentrate NCO− is severely impaired.
Inhibition of CO2 transport by NCO−.
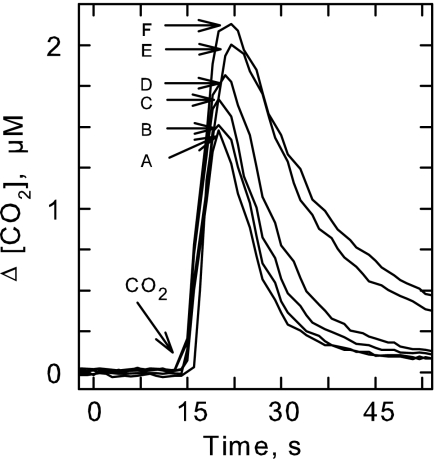
Since the cynA mutant does not generate CO2 from NCO−, this mutant was used to further examine KOCN inhibition of CO2 transport (Fig. 3) using an MS assay (11). Pure CO2 was introduced into the reaction cuvette, and its disappearance was followed over time (Fig. 6). The differences between the curves obtained in the light and the dark were a consequence of cellular uptake and a measure of CO2 transport (11). In contrast to wild-type cells (Fig. 3C), 1 mM KOCN had little effect on CO2 transport in the mutant (Fig. 6). However, at 10 mM KOCN, CO2 transport was reduced by nearly 90%. Although high concentrations of KOCN inhibited CO2 transport in the mutant, the lack of an effect at 1 mM indicated that the inhibition by 1 mM KOCN on 14CO2 transport in wild-type cells (Fig. 3C) was due mainly to competition with 12CO2 generated from the decomposition of cyanate. NCO−, therefore, is an inhibitor of the CO2 uptake system but is not a transported substrate.
FIG. 6.
Effect of KOCN on CO2 transport. S. elongatus PCC7942 cynA mutant cells grown under low-Ci conditions were placed in the reaction chamber attached to the MS and allowed to equilibrate (25 mM BTP-HCl buffer, pH 8, at 30°C) in the light (300 μmol of photons m−2 s−1) in the presence of KOCN. CO2 transport was initiated by adding 3.5 μM CO2 to the reaction vessel, and its concentration was followed over time. The difference between the curves obtained in the light (A, 0 mM KOCN, maximum uptake) and the dark (F, no uptake) was taken as a measure of CO2 transport. The effect of 1, 3, 5, or 10 mM KOCN (B, C, D, and E) on CO2 transport are also shown.
The role of the CCM and the Ci transporters in cyanate metabolism.
HCO3− is also required by cyanase as a substrate, and it seems reasonable to assume that the CCM of S. elongatus strain PCC7942 plays a direct role in its provision in the light, particularly given that the net rate of cyanate decomposition may reach 230 μmol mg−1 of Chl h−1. The role of the internal Ci pool in promoting cyanate metabolism was tested using the ΔchpX ΔchpY mutant of S. elongatus strain PCC7942 grown on 5% CO2. As this mutant lacks CO2 transport capabilities and HCO3− transport is repressed by growth under high-Ciconditions, it has little or no ability to concentrate Ci internally (52). Consequently, diffusion of Ci largely controls the size of the internal Ci pool in the ΔchpX ΔchpY mutant both in the light and dark. In these experiments (Fig. 7), we controlled the size of the Ci pool by imposing diffusion gradients between Ci-free cells and the medium through the provision of various concentrations of exogenous 13Ci. Following equilibration between the medium and the cells, we measured the average rate of decomposition of 1 mM KOCN as 12Ci efflux over a 6- to 8-min period in the light and dark. A typical time course is shown for the ΔchpX ΔchpY mutant in the presence and absence of 10 mM NaH13CO3 (Fig. 7A). The data confirm that cyanate transport was light dependent and demonstrated that the rate of cyanate decomposition was dependent upon and limited by the size of the internal Ci pool, approaching a maximum rate beyond 20 mM Ci (Fig. 7B). In wild-type cells grown under low-Ci conditions, a 20 mM Ci pool is readily formed in the light when the external Ci concentration is about 20 μM (6). Consequently, the Ci that contaminated buffers and KOCN in our experiments was a sufficient source of substrate for the CCM to provide an internal HCO3− concentration that was able to support a near-maximum rate of KOCN decomposition without supplementing the buffer with Ci (Fig. 7B). Supplementing the buffer with 7.5 or 15 mM NaH13CO3 resulted in only a modest increase in the rate of cyanate decomposition by wild-type cells grown under low-Ci conditions. This result is consistent with the internal HCO3− concentration being at a near-saturating level for cyanase when the external Ci concentration is extremely low.
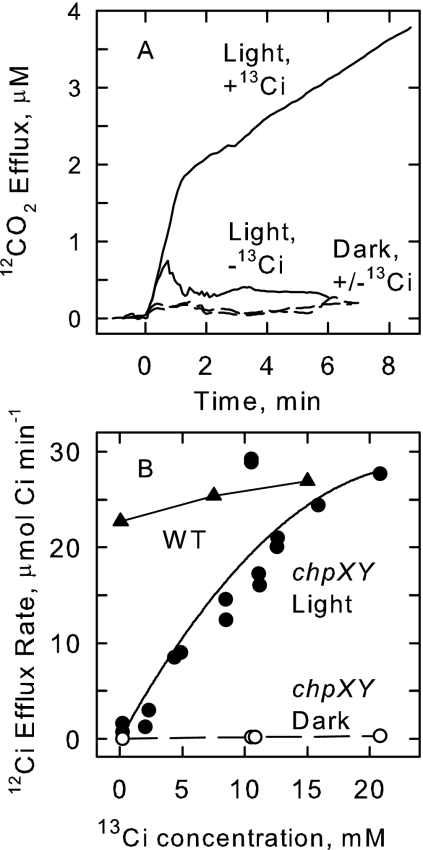
FIG. 7.
Involvement of the cyanobacterial CCM in cyanate metabolism. (A) Time course of KOCN-dependent 12CO2 efflux in the light (solid lines) and dark (dashed lines). S. elongatus strain PCC7942 ΔchpX ΔchpY cells grown on 5% CO2 were suspended in 100 mM EPPS (N-[2-hydroxyethyl]piperazine-N′-[3-propanesulfonic acid]) buffer, pH 8, and incubated in the light or dark for 5 min in the reaction cuvette, in the absence and presence of 10 mM NaH13CO3 (13Ci). The experiment was started by the addition of 1 mM KOCN, and 12CO2 efflux was measured over time by MS. (B) Dependence of 12Ci efflux rate on 13Ci concentration. The average rate of 12Ci efflux, at a constant cell density (7.5 μg of Chl a ml−1), was calculated from 12CO2 efflux measurements over an 8-min interval as a function of 13Ci concentration for ΔchpX ΔchpY (chpXY) cells in the light (•) and dark (○) provided with 1 mM KOCN. Also shown (dashed line and ▴) is the dependence on 13Ci concentration of 12CO2 efflux in S. elongatus PCC7942 wild-type (WT) cells grown under low-Ci conditions.
There was no specific requirement for either the Na+-dependent or Na+-independent HCO3− transport systems to form the Ci pool as growth under high-Ci conditions, which repressed these activities (5, 12), did not prevent cyanate uptake, decomposition (Fig. 4), or KOCN-dependent O2 evolution. Similarly, ethoxyzolamide, a CO2 transport inhibitor, did not completely prevent KO14CN accumulation and 14C- fixation in cells grown under low-Ci conditions (Table 1) or KOCN-dependent CO2 efflux (not shown). We conclude that an internal Ci pool in the millimolar range (Fig. 7), independent of the actual means by which it is formed, is a corequisite for KOCN metabolism in S. elongatus strain PCC7942. However, under normal physiological conditions (0.001 to 5 mM Ci), one or more of the Ci transport systems is required to actively create an internal Ci pool if cyanate metabolism is to proceed at a significant rate (Fig. 7B).
Occurrence of cynABDS among Bacteria.
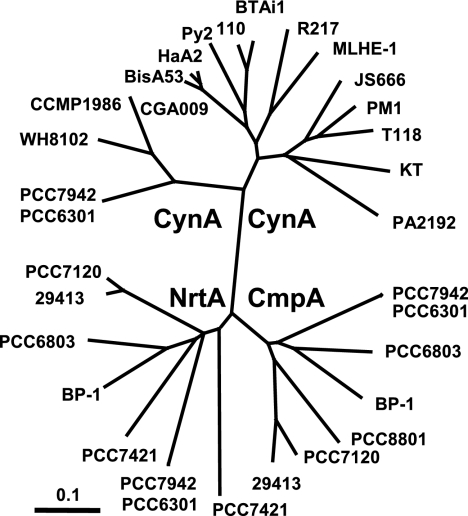
A search of the NCBI database using the BLASTp program identified 98 bacterial strains (out of 598 as of 22 July 2006) bearing a protein with amino acid sequence similarity to CynS of S. elongatus strain PCC7942. These included 12 of 24 cyanobacterial strains. Gene clusters encoding putative ABC-type transporters were also found adjacent to cynS in 17 strains, including four cyanobacteria, S. elongatus strain PCC7942, Synechococcus sp. strain PCC6301, Prochlorococcus marinus strain CCMP1986, and Synechococus sp. strain WH8102 (Fig. 8) (14, 43). Additional BLASTp searches using S. elongatus strain PCC7942 CynA as the query revealed that all 17 of the putative periplasmic substrate-binding proteins found encoded near cynS were highly related (E values less than 10−85) and more closely related to one another than to NrtA or CmpA (Fig. 8). Similarly, when CynB from S. elongatus strain PCC7942 was the query, the permease components most similar to it were those that were encoded by permease components adjacent to cynS (E values less than 10−59) (data not shown). However, this type of association broke down in the case of CynD, the ATP-binding protein that energizes transport. Since the substrate specificity of individual ABC-type transporters is determined by the binding protein and the permease subunits, while energization is a more generic function, it is likely that the newly identified cynABDS-like operons encode components for cyanate transport and degradation. Most of these ABC-transporters have previously been annotated as nitrate transporters and/or as simply possessing a twin-arginine signal sequence. In addition to the four cyanobacteria, cynABDS-like operons were found in seven strains of the Alphaproteobacteria, four strains of Betaproteobacteria, and two strains of Gammaproteobacteria (Fig. 8). Most of these strains (11/17) were obligate or facultative chemo- or photoautotrophs. The remaining strains consisted of heterotrophs that oxidized a range of organic compounds and a single obligate methylotroph.
FIG. 8.
Unrooted phylogenetic tree illustrating the relationship between CynA proteins identified from bacterial sources and NrtA and CmpA from cyanobacteria. Cyanobacterial species are as follows: S. elongatus PCC7942, Synechococcus sp. strain PCC6301, Synechocystis sp. strain PCC6803, Thermosynechococcus elongatus BP-1, Cyanothece sp. strain PCC8801, Nostoc sp. strain PCC7120, Anabaena variabilis ATCC 29413, Gloeobacter violaceus PCC7421, Synechococcus sp. strain WH8102, and P. marinus CCMP1986. Other organisms are Rhodopseudomonas palustris strains CGA009, BisA53, and HaA2; Xanthobacter autotrophicus Py2; Bradyrhizobium japonicum USDA 110; Bradyrhizobium sp. strain BTAi1; Roseovarius sp. strain R217; Alkalilimnicola ehrlichei MLHE-1; Polaromonas sp. strain JS666; Rubrivivax gelatinosus PM1; Rhodoferax ferrireducens T118; Methylobacillus flagellatus KT; and Pseudomonas aeruginosa PA2192. Organisms are represented on the figure generally by the strain designation or culture collection number.
DISCUSSION
A model for cyanate metabolism.
The results of our investigation indicate that the transport and decomposition of exogenously supplied cyanate by strains of the freshwater cyanobacterium Synechococcus depend upon the products of the cynABDS operon and the biophysical activity of the CCM in a light-dependent manner. With the exception of cynS, the molecular components required to support the transport and cellular decomposition of cyanate are distinct from the components encoded by the cynTSX operon of E. coli and thus constitute a novel mechanism that facilitates the assimilation of C and N from cyanate. Indeed, it is likely that an E. coli-like system for cyanate metabolism would be deleterious in cyanobacteria because the cytosolic CA component would circumvent the operation of parts of the CCM.
Inactivation of cynA, which encodes the periplasmic binding protein of a putative multicomponent ABC-type transporter, results in the loss of active cyanate transport and the ability to decompose NCO− to CO2 and NH3. Similarly, inactivation of cynS, which codes for a cytosolic cyanase, yields a mutant that is also unable to decompose cyanate. As a result, both mutants lack cyanate-dependent O2 evolution and CO2 efflux. An additional novel aspect of cyanate metabolism in cyanobacteria is its dependence on light and photosynthetic electron transport. This dependence was multifaceted and traced to the light dependence of both cyanate transport and Ci transport. Presumably, ATP derived from photophosphorylation is used to energize cyanate transport through the membrane-bound ATP-binding protein CynD. Although oxidative phosphorylation also yields ATP, there is little active cyanate transport in the dark. These results suggest that cyanate transport activity is regulated by an additional step beyond energy supply or simple kinetic control mechanisms associated with substrate concentration. The codependence of cyanate and Ci transport on light further suggests that the transporters may be controlled by a common activation/deactivation mechanism. Such a mechanism ensures that the transport of a potential toxin into the cells is coupled with the means to rapidly degrade it. However, ongoing Ci transport is not a prerequisite for the activation of NCO− transport as evidenced by the fact that the ΔchpX ΔchpY mutant of S. elongatus strain PCC7942 was able to decompose exogenous NCO− when provided with an adequate supply of internal HCO3−, which was acquired through CO2 diffusion (Fig. 7).
The role of the CCM in cyanate metabolism.
The rate of cyanate decomposition depends on the size of the internal HCO3− pool, which in turn is controlled by the activity of the CCM in the light (Fig. 7). In the absence of a functional CCM, cyanate decomposition was severely retarded at external Ci concentrations usually experienced during growth and points to the fact that CO2 diffusion is normally inadequate to supply intracellular HCO3− for cyanase. Coupling of cyanate decomposition to the CCM is a unique strategy for HCO3− acquisition that distinguishes S. elongatus strain PCC7942 from E. coli and, potentially, autotrophic cyanate metabolism from heterotrophic cyanate metabolism, which relies on the combined action of cyanase and CA. CA, which is crucial for the provision of HCO3− in the cytosol of E. coli, is confined to carboxysomes in the Synechococcus strains and functions in the opposite direction to supply CO2 for photosynthetic fixation (5, 39). A direct role for cyanobacterial CA in the trapping of HCO3− in the cytosol for cyanase is not indicated by virtue of the massive efflux of cyanate-derived CO2 that occurs in cells grown under high-Ci conditions. The high capacity of the cyanobacterial CCM to concentrate HCO3− internally over a wide range of external Ci concentrations is an important factor which circumvents the limitation imposed by external Ci concentration and CO2 diffusion on the cellular capacity for cyanate decomposition and is a key reason why Synechococcus strains are able to have a 20-fold higher capacity for cyanate decomposition compared to E. coli (30).
The dependence of cyanate decomposition on the activity of the CCM also facilitates the assimilation of cyanate-derived CO2 through the photosynthetic carbon reduction cycle. This is most evident in cells grown in low Ci concentrations where a high-affinity CCM is fully induced. In these cells, CO2 efflux is substantially diminished (30), and this is reflected in a K0.5 (NCO−) value for photosynthetic cyanate utilization that is twofold lower compared to cells grown in high Ci concentrations. The superior retention of cyanate-derived CO2 by cells grown in low Ci concentrations coincides with the up-regulation of the high-affinity CO2 uptake system (51) that is predominantly localized to the thylakoid membranes. Presumably, CO2 loss from the cytoplasm is curtailed through its conversion to HCO3− by the light-driven action of the NdhF3/NdhD3/ChpY complex rather than carboxysome-localized CA. The trapped HCO3− then contributes to the overall internal pool available for photosynthetic fixation. Cyanate-derived CO2 that is lost from cells will undoubtedly be recovered by the Ci transport systems and eventually fixed, as evidenced by the 1:1 stoichiometry between added NCO− and CO2 fixation (O2 evolution). In the short term, the proportion of CO2 that is lost from the cell to that which is retained will depend on the level of induction of the CCM and the capacity of the cell for CO2 fixation.
Cyanate transport.
The high degree of amino acid sequence similarity between CynA and its paralogs NrtA and CmpA suggests that CynA may also perform similar transport functions in cellular metabolism. Consequently, the notion that CynABD acts as an NO3−/NO2− transporter or HCO3− transporter in S. elongatus strain PCC7942 was investigated along with the hypothesis that native NO3−/NO2− transporters or HCO3− transporters accept NCO− as an alternative substrate. In competition assays (Fig. 4), however, neither excess NO3− nor NO2− (as well as Cl−, PO42−, or SO42−) impaired the release of cyanate-derived CO2 from whole cells, indicating that these anions did not interfere with the initial uptake of NCO−, as would be expected for alternative substrates of a common transporter. It seems unlikely, therefore, that CynABD is an NO3−/NO2− transporter that gratuitously transports NCO−. For the same reason, it is also unlikely that NCO− transport in S. elongatus strain PCC7942 is mediated by the bona fide NO3−/NO2− transporter, NrtABCD, as suggested for Azotobacter chroococcum (33).
The potential role of the HCO3− and CO2 transport systems in NCO− transport proved to be more difficult to unravel due to the nonenzymatic interconversion between HCO3− and CO2, the presence of multiple Ci transporters, the contamination of labeled KOCN solutions with labeled Ci, the slow but spontaneous hydrolysis of cyanate-forming Ci, and the rapid enzymatic decomposition of NCO− that yielded a large quantity of CO2 within seconds of addition. 14C-bicarbonate transport assays (Fig. 3) revealed that NCO− did not compete with H14CO3− for uptake, consistent with a lack of involvement of CmpABCD or SbtA in the transport of NCO− by S. elongatus strain PCC7942. The roughly 50 to 60% reduction in 14C accumulation observed in N14CO− transport assays (Table 2) when cells were supplied with excess 12Ci can be ascribed to the competitive elimination by H12CO3− of the transport of H14CO3− that contaminates KO14CN solutions rather than a direct inhibition of NCO− transport by HCO3−, since a reciprocal inhibition of HCO3− transport by NCO− did not occur. The fact that the effect of “cold” HCO3− on N14CO− transport saturated at 2 mM suggests that CynABD is not an HCO3− transporter since high HCO3− concentrations should eliminate NCO− transport.
Inhibitor studies have provided evidence both for and against a role for the CO2 transport system in NCO− transport (Fig. 3 and 6). While 1 mM NCO− appeared to inhibit 14CO2 transport in wild-type S. elongatus strain PCC7942 cells, 1 mM NCO− had no effect on CO2 transport in the cynA mutant, as judged by MS. The most likely explanation for this apparent discrepancy is that cyanate-derived 12CO2 released from wild-type cells during transport experiments competed with 14CO2 for uptake, thereby reducing the internal concentration of 14C. Since the cynA mutant does not decompose cyanate, cyanate-derived 12CO2 competition is not a complicating factor in determining whether or not cyanate inhibits CO2 transport. Thus, these results lead us to conclude that cyanate is not a substrate of the CO2 transport systems. This conclusion is also consistent with the observed inability of Synechocystis sp. strain PCC6803 to decompose externally supplied cyanate to CO2 and NH3 (Fig. 4), in spite of possessing a functional CO2 transport system to potentially deliver NCO− to the cytosol for cyanase.
The inability of Synechocystis sp. strain PCC6803 to decompose externally supplied cyanate in the light as well as the inability of the S. elongatus strain PCC7942 ΔchpX ΔchpY mutant to decompose cyanate in the dark at high internal HCO3− concentrations (Fig. 7) indicates that the diffusive entry of HOCN contributes minimally to the gross uptake of cyanate. Thus, the collective data demonstrate that the Synechococcus strains are able to actively transport and concentrate NCO− through a specific pathway that involves CynA and, therefore, CynB and CynD. We are now in the process of determining if CynA is an NCO− binding protein. The detailed kinetics of cyanate transport have yet to be resolved, due to the technical difficulties of separating the initial flux of NCO− from the flux and reflux of CO2 and HCO3−. It is clear, however, that a transient net flux of up to 230 μmol mg−1 of Chl h−1 is possible as this is the maximum steady-state rate of cyanate-dependent O2 evolution. In our experiments, the ratio of [NCO−]in/[NCO−]out was in the range of 8 to 10 at 1 mM NCO−. These estimates were complicated by the need to apply a substantial correction for 14Ci accumulation and are subject to future refinement. Nevertheless, the data indicate that NCO− was concentrated above the level expected from diffusion alone.
cynABDS-like operons are present in a diverse range of cyanobacteria and proteobacteria, including the globally important oceanic cyanobacteria Synechococcus sp. strain WH8102 and P. marinus strain CCMP1986 (Fig. 8). It seems likely that the HCO3− required for NCO− decomposition is provided by the CCM of these two organisms. A CCM has yet to be identified in autotrophic proteobacteria, such as Rhodopseudomonas palustris and Bradyrhizobium sp. strain BTAi1, which possess cynABDS-like operons. Interestingly, these organisms lack carboxysomes, a hallmark of the cyanobacterial CCM. Thus, it is possible that they may employ their own unique strategy for HCO3− acquisition or utilize a cytosolic CA. Alternatively, NCO− degradation may be restricted to naturally occurring high Ci environments where CO2 diffusion could play a role. This latter route for HCO3− acquisition may also be of particular importance for the heterotrophs which possess cynABDS-like operons.
Physiological role.
One experimentally verified role for CynABDS in cellular metabolism is to provide a vehicle for the transport and decomposition of exogenous cyanate. Since the Synechococcus strains readily assimilate cyanate-derived CO2 (Fig. 1 and 2) and NH3 (30), CynABDS permits the utilization of cyanate as a niche source of C and N for growth. CynS alone cannot fulfill this metabolic role. Thus, CynABDS may be legitimately included as an input branch to the major C and N assimilatory pathways in cyanobacteria. The relatively high capacity for NCO− transport and decomposition may reflect a metabolic strategy for rapid utilization from environments where NCO− is only sporadically available and has a short half-life. Utilization of NCO− broadens the range of available C and N sources and may provide a small advantage over organisms that can only assimilate NH3, particularly in oligotrophic environments where Ci and N sources limit growth (13, 14, 43). In other environments such as industrial waste waters, relatively high concentrations of NCO− are available, produced by bacterial consortia (heterotrophs and autotrophs) that are actively degrading thiocyanate (41, 50). Here, the opportunistic utilization of cyanate by cyanobacteria may reflect their participation in thiocyanate metabolism. We are currently investigating the possibility that cyanobacteria may also directly metabolize thiocyanate.
Acknowledgments
This work was supported by grants from the Natural Sciences and Engineering Research Council of Canada to G.S.E. Part of this work was conducted by G.S.E. at the Research School of Biological Sciences, The Australian National University.
We sincerely thank G. D. Price and M. R. Badger for the provision of research facilities and for access to the S. elongatus strain PCC7942 ΔchpX ΔchpY mutant. We also thank Ben Long, The Australian National University, and A. G. Miller, St. Francis Xavier University, for valuable discussions.
Footnotes
Published ahead of print on 22 November 2006.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, P. M. 1980. Purification and properties of the inducible enzyme cyanase. Biochemistry 19:2882-2888. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, P. M., W. V. Johnson, J. A. Endrizzi, R. M. Little, and J. J. Korte. 1987. Interaction of mono- and dianions with cyanase: evidence for apparent half-site binding. Biochemistry 26:3938-3943. [DOI] [PubMed] [Google Scholar]
- 4.Anderson, P. M., Y. C. Sung, and J. A. Fuchs. 1990. The cyanase operon and cyanate metabolism. FEMS Microbiol. Rev. 87:247-252. [DOI] [PubMed] [Google Scholar]
- 5.Badger, M. R., G. D. Price, B. M. Long, and F. J. Woodger. 2006. The environmental plasticity and ecological genomics of the cyanobacterial CO2 concentrating mechanism. J. Exp. Bot. 57:249-265. [DOI] [PubMed] [Google Scholar]
- 6.Crotty, C. M., P. N. Tyrrell, and G. S. Espie. 1994. Quenching of chlorophyll a fluorescence in response to Na+-dependent HCO3− transport-mediated accumulation of inorganic carbon in the cyanobacterium Synechococcus UTEX 625. Plant Physiol. 104:785-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorr, P. K., and C. J. Knowles. 1989. Cyanide oxygenase and cyanase activities of Pseudomonas fluorescens NCIMB 11764. FEMS Microbiol. Rev. 60:289-294. [Google Scholar]
- 8.Espie, G. S., and D. T. Canvin. 1987. Evidence for Na+-independent HCO3− uptake by the cyanobacterium Synechococcus leopoliensis. Plant Physiol. 84:125-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Espie, G. S., and R. A. Kandasamy. 1992. Na+-independent HCO3− transport and accumulation in the cyanobacterium Synechococcus UTEX 625. Plant Physiol. 98:560-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Espie, G. S., A. G. Miller, and D. T. Canvin. 1988. Characterization of the Na+ requirement in cyanobacterial photosynthesis. Plant Physiol. 88:757-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Espie, G. S., A. G. Miller, and D. T. Canvin. 1989. Selective and reversible inhibition of active CO2 transport by hydrogen sulfide in a cyanobacterium. Plant Physiol. 91:387-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Espie, G. S., A. G. Miller, R. A. Kandasamy, and D. T. Canvin. 1991. Active HCO3− transport in cyanobacteria. Can. J. Bot. 69:936-944. [Google Scholar]
- 13.Flores, E., and A. Herrero. 2005. Nitrogen assimilation and nitrogen control in cyanobacteria. Biochem. Soc. Trans. 33:164-167. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Fernandez, J. M., N. Tandeau de Marsac, and J. Diez. 2004. Streamlined regulation and gene loss as adaptive mechanisms in Prochlorococcus for optimized nitrogen utilization in oligotrophic environments. Microbiol. Mol. Biol. Rev. 68:630-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guilloton, M., and F. Karst. 1987. Isolation and characterization of Escherichia coli mutants lacking inducible cyanase. J. Gen. Microbiol. 133:645-653. [DOI] [PubMed] [Google Scholar]
- 16.Guilloton, M. B., G. S. Espie, and P. M. Anderson. 2002. What is the role of cyanase in plants? Rev. Plant Biochem. Biotechnol. 1:57-79. [Google Scholar]
- 17.Guilloton, M. B., J. J. Korte, A. F. Lamblin, J. A. Fuchs, and P. M. Anderson. 1992. Carbonic anhydrase in Escherichia coli: a product of the cyn operon. J. Biol. Chem. 267:3731-3734. [PubMed] [Google Scholar]
- 18.Guilloton, M. B., A. F. Lamblin, E. I. Kozliak, M. Gerami-Nejad, C. Tu, D. Silverman, and J. A. Fuchs. 1993. A physiological role for cyanate-induced carbonic anhydrase in Escherichia coli. J. Bacteriol. 175:1443-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harano, Y., I. Suzuki, S. I. Maeda, T. Kaneko, S. Tabata, and T. Omata. 1997. Identification and nitrogen regulation of the cyanase gene from the cyanobacteria Synechocystis sp. strain PCC 6803 and Synechococcus sp. strain PCC 7942. J. Bacteriol. 179:5744-5750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jalali, F. 1997. Molecular cloning, insertional inactivation and characterization of the cyanate lyase gene from the cyanobacterium Synechococcus PCC 7942. M.S. thesis. University of Toronto, Mississauga, Ontario, Canada.
- 21.Johnson, W. V., and P. M. Anderson. 1987. Bicarbonate is a recycling substrate for cyanase. J. Biol. Chem. 262:9021-9025. [PubMed] [Google Scholar]
- 22.Kozliak, E. I., J. A. Fuchs, M. B. Guilloton, and P. M. Anderson. 1995. Role of bicarbonate/CO2 in the inhibition of Escherichia coli growth by cyanate. J. Bacteriol. 177:3213-3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kozliak, E. I., M. B. Guilloton, J. A. Fuchs, and P. M. Anderson. 2000. Bacterial carbonic anhydrases, p. 547-565. In W. R. Chegwidden, N. D. Carter, and Y. H. Edwards (ed.), The carbonic anhydrases: new horizons. Birkhauser Verlag, Basil, Switzerland. [DOI] [PubMed]
- 24.Kozliak, E. I., M. B. Guilloton, M. Gerami-Nejad, J. A. Fuchs, and P. M. Anderson. 1994. Expression of proteins encoded by the Escherichia coli cyn operon: carbon dioxide-enhanced degradation of carbonic anhydrase. J. Bacteriol. 176:5711-5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuhlemeier, C. J., and G. A. van Arkel. 1987. Host-vector systems for gene cloning in cyanobacteria. Methods Enzymol. 153:199-215. [DOI] [PubMed] [Google Scholar]
- 26.Laudenbach, D. E., and A. R. Grossman. 1991. Characterization and mutagenesis of sulfur-regulated genes in a cyanobacterium: evidence for function in sulfate transport. J. Bacteriol. 73:2739-2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lister, M. W. 1955. Some observations on cyanic acid and cyanates. Can. J. Biochem. 33:426-440. [Google Scholar]
- 28.Little, R. M., and P. M. Anderson. 1987. Structural properties of cyanase: denaturation, renaturation, and role of sulfhydryls and oligomeric structure in catalytic activity. J. Biol. Chem. 262:10120-10126. [PubMed] [Google Scholar]
- 29.Maeda, N., G. D. Price, M. R. Badger, C. Enomoto, and T. Omata. 2000. Bicarbonate binding activity of the CmpA protein of the cyanobacterium Synechococcus sp. strain PCC 7942 involved in active transport of bicarbonate. J. Biol. Chem. 275:20551-20555. [DOI] [PubMed] [Google Scholar]
- 30.Miller, A. G., and G. S. Espie. 1994. Photosynthetic metabolism of cyanate by the cyanobacterium Synechococcus UTEX 625. Arch. Microbiol. 162:151-157. [Google Scholar]
- 31.Miller, A. G., G. S. Espie, and D. T. Canvin. 1991. Active CO2 transport in cyanobacteria. Can. J. Bot. 69:925-935. [Google Scholar]
- 32.Miller, A. G., G. S. Espie, and D. T. Canvin. 1988. Active transport of CO2 by the cyanobacterium Synechococcus UTEX 625: measurement by mass spectrometry. Plant Physiol. 86:677-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muñoz-Centeno, M. C., A. Paneque, and F. J. Cejudo. 1996. Cyanate is transported by the nitrate permease in Azotobacter chroococcum. FEMS Microbiol. Lett. 137:91-94. [Google Scholar]
- 34.Omata, T., Y. Takahashi, O. Yamaguchi, and T. Nishimura. 2002. Structure, function and regulation of the cyanobacterial high-affinity bicarbonate transporter, BTC1. Funct. Plant Biol. 29:151-159. [DOI] [PubMed] [Google Scholar]
- 35.Palenik, B., B. Brahamsha, F. Larimer, M. Land, L. Hauser, P. Chain, J. Lamerdin, W. Regals, E. Allen, J. McCarren, I. Paulsen, A. Dufresne, F. Partensky, E. Webb, and J. Waterbury. 2003. The genome of a motile marine Synechococcus. Nature 424:1037-1042. [DOI] [PubMed] [Google Scholar]
- 36.Pao, S. S., I. Paulsen, and M. H. Saier. 1998. Major facilitator superfamily. Microbiol. Mol. Biol. Rev. 62:1-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ritchie, R. J. 1991. Membrane potential and pH control in the cyanobacterium Synechococcus R-2 (Anacystis nidulans) PCC 7942. Plant Physiol. 137:409-418. [Google Scholar]
- 38.Sambrook, J., E. F. Fritch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 39.So, A. K. C., and G. S. Espie. 2005. Cyanobacterial carbonic anhydrases. Can. J. Bot. 83:721-734. [Google Scholar]
- 40.So, A. K. C., M. E. John-McKay, and G. S. Espie. 2002. Characterization of a mutant lacking carboxysomal carbonic anhydrase from the cyanobacterium Synechocystis PCC6803. Planta 214:456-467. [DOI] [PubMed] [Google Scholar]
- 41.Sorokin, D. Y., T. P. Tourova, A. M. Lysenko, and J. G. Kuenen. 2001. Microbial thiocyanate utilization under highly alkaline conditions. Appl. Environ. Microbiol. 67:528-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stumm, W., and J. J. Morgan. 1981. Aquatic chemistry, 2nd ed. John Wiley & Sons, Toronto, Canada.
- 43.Su, Z., F. Mao, P. Dam, H. Wu, V. Olman, I. T. Paulsen, B. Palenik, and Y. Xu. 2006. Computational inference and experimental validation of the nitrogen assimilation network in cyanobacterium Synechococcus sp. WH 8102. Nucleic Acids Res. 34:1050-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sung, Y. C., and J. A. Fuchs. 1988. Characterization of the cyn operon in Escherichia coli K12. J. Biol. Chem. 263:14769-14775. [PubMed] [Google Scholar]
- 45.Sung, Y. C., and J. A. Fuchs. 1992. The Escherichia coli K-12 cyn operon is positively regulated by a member of the lysR family. J. Bacteriol. 174:3645-3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sung, Y. C., and J. A. Fuchs. 1989. Identification and characterization of a cyanate permease in Escherichia coli K-12. J. Bacteriol. 171:4674-4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taussig, A. 1960. The synthesis of the enzyme cyanase in E. coli. Biochim. Biophys. Acta 44:510-519. [DOI] [PubMed] [Google Scholar]
- 48.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. J. Higgins. 1997. The Clustal X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walsh, M. A., Z. Otwinowski, A. Perrakis, P. M. Anderson, and A. Joachimiak. 2000. Structure of cyanase reveals that a novel dimeric and decameric arrangement of subunits is required for formation of the enzyme active site. Struct. Fold Des. 8:505-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wood, A. P., D. P. Kelly, I. R. McDonald, S. L. Jordan, T. D. Morgan, S. Khan, J. C. Murrell, and E. Borodina. 1998. A novel, pink-pigmented facultative methylotroph, Methylbacterium thiocyanatum sp. nov., capable of growth on thiocyanate or cyanate as sole nitrogen source. Arch. Microbiol. 169:148-158. [DOI] [PubMed] [Google Scholar]
- 51.Woodger, F. J., M. R. Badger, and G. D. Price. 2003. Inorganic carbon limitation induces transcripts encoding components of the CO2-concentrating mechanism in Synechococcus sp. PCC7942 through a redox-independent pathway. Plant Physiol. 133:2069-2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Woodger, F. J., M. R. Badger, and G. D. Price. 2005. Sensing of inorganic carbon limitation in Synechococcus PCC7942 is correlated with the size of the internal inorganic carbon pool and involves oxygen. Plant Physiol. 139:1959-1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Youatt, J. B. 1954. Studies on the metabolism Thiobacillus thiocyanooxidans. J. Gen. Microbiol. 11:139-140. [DOI] [PubMed] [Google Scholar]