Abstract
Phosphoenolpyruvate inhibited Escherichia coli NADP-isocitrate dehydrogenase allosterically (Ki of 0.31 mM) and isocitrate lyase uncompetitively (Ki′ of 0.893 mM). Phosphoenolpyruvate enhances the uncompetitive inhibition of isocitrate lyase by increasing isocitrate, which protects isocitrate dehydrogenase from the inhibition, and contributes to the control through the tricarboxylic acid cycle and glyoxylate shunt.
Phosphoenolpyruvate (PEP) is a critical metabolite in Escherichia coli, because it is essential for the efficient uptake of glucose and other carbohydrates (11) and the final intermediate of glycolysis. PEP acts as an effector of phosphofructokinase (1), and changes in the PEP concentration are related to the flux of the tricarboxylic acid (TCA) cycle and glyoxylate shunt (10, 13). The branch point at the TCA cycle and glyoxylate bypass is controlled by enzyme expression and depends on the growth conditions (16) and the NADP-isocitrate dehydrogenase activity (12), which is subject to inhibition by various metal ions (7, 14, 15) and phosphorylation/dephosphorylation of the protein (2, 9, 12). Here we report the inhibitory effects of PEP on the NADP-isocitrate dehydrogenase and isocitrate lyase purified from the archived clones of the icd- and aceA-overexpressing E. coli mutants (8), which were constructed with plasmid pCA24N from the E. coli strain K-12 W3110 (4).
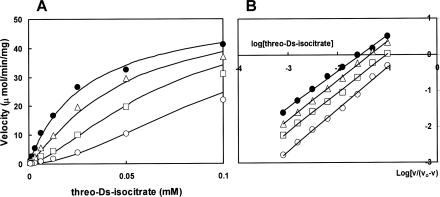
The effect of PEP on the activity of NADP-isocitrate dehydrogenase was analyzed. The 1-ml reaction mixture contained 100 mM morpholinepropanesulfonic acid (MOPS) buffer (pH 7.1), 0.5 mM NADP, 0.5 mM MgCl2, and various concentrations of threo-dS-isocitrate in the absence and presence of phosphoenolpyruvate, and the activity was determined by monitoring the change in the absorbance at 340 nm (7, 8). Plots of reaction velocity as a function of the substrate isocitrate concentration gave a hyperbolic curve, which became sigmoid in shape with the addition of PEP (Fig. 1A). The substrate concentration required for half-maximal velocity, S0.5 of 0.029 mM, increased to a value over 0.1 mM, and the Hill's interaction coefficient nH of 1.0 increased to 1.2 in the presence of 5 mM PEP (Fig. 1B). It was concluded that PEP acts as an allosteric inhibitor.
FIG. 1.
Effect of phosphoenolpyruvate on the activity of E. coli NADP-isocitrate dehydrogenase with respect to the threo-dS-isocitrate concentration. (A) threo-dS-Isocitrate saturation curves in the absence and presence of different concentrations of phosphoenolpyruvate. (B) Hill's plot of the data shown in panel A. Points represent experimental data, and lines are theoretically drawn by using the following equation: v = V · [S]n/([S]n + Kmn) where [S] is the concentration of isocitrate, Km is the concentration required for half-maximal velocity, and n is the Hill's interaction coefficient. Symbols: closed circles, no addition (Km = 0.029 mM and nH = 1.0); triangles, 0.2 mM PEP added (Km = 0.046 mM and nH = 1.1); squares, 1 mM PEP added (Km = 0.08 mM and nH = 1.1); open circles, 5 mM PEP added (Km = 0.16 mM and nH = 1.2).
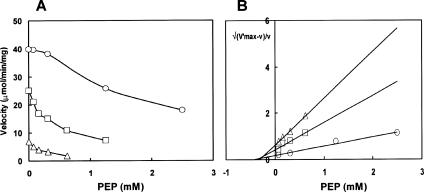
Plots of the reaction velocity against PEP concentration gave a sigmoid curve in the presence of higher substrate concentration, suggesting the cooperative binding of PEP to the enzyme (Fig. 2A). This allosteric system lends itself to the convenient method of analysis of Blangy et al. (1) using a function called the quotient function (Q), which is the ratio of the amount of the enzyme in the R state to that in the T state. We may write Q = R/(1 − R) = v/(V′ − v) = C/(1 + β)n where R represents the fraction of the enzyme molecules in the R (active) conformation and v is the velocity obtained in the presence of the inhibitor. V′ is the maximum velocity that can be reached in the presence of a given concentration of the substrate if the protein is entirely in the R conformation, and β is the normalized concentration of the inhibitor, that is, [I]/KT where KT is the microscopic dissociation constant of the inhibitor for the T (inactive) state of the enzyme. C is the constant including the normalized Km and the allosteric constant, which is the ratio of the R and T states in the absence of any ligand. It is, therefore, convenient to plot n√(V′ − v)/v against PEP concentration, assuming that there are two independent sites for PEP per enzyme molecule in the T conformation. As shown in Fig. 2B, all the inhibition curves were converted to the straight lines converging on the abscissa at the same point, −KT. This implies that there are indeed two independent sites for PEP per molecule, and the KT for PEP, that is, the Ki value, was calculated to be 0.31 mM. If a number of sites other than two is assumed for PEP, the functions deviate from linearity and do not meet on the abscissa at the same point. Thus, the most probable number of sites for PEP is two.
FIG. 2.
Inhibition of E. coli NADP-isocitrate dehydrogenase by phosphoenolpyruvate. Reaction conditions were similar to those in the legend to Fig. 1, except the following modifications. The PEP concentrations were varied, and the threo-dS-isocitrate concentrations were fixed at 0.1 mM (circles), 0.02 mM (squares), and 0.004 mM (triangles). (A) Inhibition by phosphoenolpyruvate. (B) Variation of the quotient function Q with respect to the inhibition by PEP, assuming two binding sites for this inhibitor. The values of V′ taken in constructing this plot were determined from the velocity without PEP.
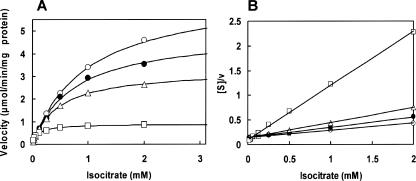
We examined the effect of PEP on isocitrate lyase activity by the phenylhydrazine method by measuring at 324 nm (5): the 1-ml reaction mixture contained 100 mM potassium phosphate buffer (pH 7.1), 10 mM MgCl2, 12 mM cysteine, various concentrations of threo-dS-isocitrate, and 4 mM phenylhydrazine-HCl in the absence and presence of PEP. PEP inhibited isocitrate lyase (Fig. 3A), and the Hofstee plot revealed that the inhibition was of the uncompetitive type with the Ki′ value for PEP of 0.893 ± 0.097 mM (Fig. 3B). The inhibition constants of isocitrate dehydrogenase and isocitrate lyase for PEP are within the intracellular PEP concentration range from 0.2 to 1.0 mM, depending on carbon sources (6), suggesting that inhibition by PEP is physiologically relevant.
FIG. 3.
Effect of phosphoenolpyruvate on the activity of E. coli isocitrate lyase with respect to the isocitrate concentration. (A) threo-dS-Isocitrate saturation curves in the presence of different concentrations of phosphoenolpyruvate. (B) Hofstee plot of the data in panel A. The Hofstee equation for uncompetitive inhibition is as follows: [S]/v = (1/Vmax) · (1 + [I]/Ki′) · [S] + Km/Vmax. The relationship between [S]/v and [S], the substrate concentrations in the presence of various concentrations of PEP, the inhibitor, [I] gave straight lines converging on the ordinate at Km/Vmax. Points represent experimental data, and lines are theoretically drawn as demonstrated in Fig. 1 except that the nH value was kept at 1. Symbols: open circles, no PEP (Km = 0.89 mM and Vmax = 6.72 μmol/min per mg protein); closed circles, 0.2 mM PEP added (Km = 0.72 mM and Vmax = 4.92 μmol/min per mg); triangles, 1 mM PEP added (Km = 0.47 mM and Vmax = 3.29 μmol/min per mg); squares, 5 mM PEP added (Km = 0.10 mM and Vmax = 0.894 μmol/min per mg).
The Km value of NADP-isocitrate dehydrogenase for isocitrate (Km = 0.029 mM) is much lower than that of isocitrate lyase (Km = 0.89 mM). Isocitrate dehydrogenase is, thus, less sensitive to the availability of isocitrate, as the enzyme operates largely in the zero-order region (i.e., the concentration of isocitrate is greater than the Km), but the glyoxylate pathway operates at low flux in E. coli cells, in which isocitrate concentration is below the Km of isocitrate lyase. Flux of the glyoxylate shunt largely depends on the inactivation/phosphorylation of isocitrate dehydrogenase catalyzed by isocitrate dehydrogenase kinase/phosphatase (12) and the stimulation of isocitrate lyase (3). Inactivation of isocitrate dehydrogenase causes the possible enhancement of isocitrate lyase by increasing isocitrate concentrations. For example, when the pck gene encoding phosphoenolpyruvate carboxykinase is deleted, isocitrate lyase increased the activity of isocitrate dehydrogenase twofold, which decreases the activity to 16 to 17% of the wild-type cells (13), and thus, a larger portion of isocitrate is expected to be metabolized through the glyoxylate shunt. However, the glyoxylate shunt activity is only one-third of the flux of the isocitrate dehydrogenase pathway in the pck deletion mutant (13).
PEP may participate in the control of glyoxylate bypass and the TCA cycle. Increased PEP concentrations in pck knockout cells cause inhibition of NADP-isocitrate dehydrogenase, but the resulting accumulation of isocitrate can relieve the enzyme from allosteric inhibition by PEP. On the other hand, isocitrate lyase is subject to uncompetitive inhibition by PEP, and an increase in the concentration of the substrate, isocitrate, will further enhance the inhibition by PEP, because the inhibitor binds only to the enzyme-substrate complex. Lower flux of glyoxylate shunt, in spite of increased induction of isocitrate lyase in cells with higher PEP concentrations, can be explained by the differential inhibitory effects of PEP on isocitrate dehydrogenase and isocitrate lyase, as well as the inhibition of isocitrate dehydrogenase kinase that protects isocitrate dehydrogenase from inactivation (9).
Acknowledgments
This research was carried out as a part of The Project for Development of a Technological Infrastructure for Industrial Bioprocesses on R&D of New Industrial Science and Technology Frontiers by the Ministry of Economy, Trade and Industry (METI) and entrusted by New Energy and Industrial Technology Development Organization (NEDO). This work was also supported in part by a grant for The 21st Century COE Program “Understanding and Control of Life's Function via Systems Biology” from The Ministry of Education, Culture, Sports, Science and Technology (MEXT).
Footnotes
Published ahead of print on 1 December 2006.
REFERENCES
- 1.Blangy, D., H. Buc, and J. Monod. 1968. Kinetics of the allosteric interactions of phosphofructokinase from Escherichia coli. J. Mol. Biol. 31:13-35. [DOI] [PubMed] [Google Scholar]
- 2.Holms, W. H. 1986. The control metabolic pathways in Escherichia coli: relationship between flux and control at a branchpoint, efficiency of conversion to biomass, and excretion of acetate. Curr. Top. Cell Regul. 28:69-105. [DOI] [PubMed] [Google Scholar]
- 3.Hoyt, J. C., and H. C. Reeves. 1988. In vivo phosphorylation of isocitrate lyase from Escherichia coli D5H3G7. Biochem. Biophys. Res. Commun. 153:875-880. [DOI] [PubMed] [Google Scholar]
- 4.Kitagawa, M., T. Ara, M. Arifuzzaman, T. Ioka-Nakamichi, E. Inamoto, H. Toyonaga, and H. Mori. 2005. Complete set of ORF clones of Escherichia coli ASKA library (a complete set of E. coli K-12 ORF archive): unique resources for biological research. DNA Res. 12:291-299. [DOI] [PubMed] [Google Scholar]
- 5.McFadden, B. A. 1969. Isocitrate lyase. Methods Enzymol. 13:163-170. [Google Scholar]
- 6.Morikawa, M., K. Izui, M. Taguchi, and H. Katsuki. 1980. Regulation of Escherichia coli phosphoenolpyruvate carboxylase by multiple effectors in vivo. Estimation of the activities in the cells grown on various compounds. J. Biochem. 87:441-449. [DOI] [PubMed] [Google Scholar]
- 7.Murakami, K., M. Haneda, S. Iwata, and M. Yoshino. 1997. Role of metal cations in the regulation of NADP-linked isocitrate dehydrogenase from porcine heart. Biometals 10:169-174. [DOI] [PubMed] [Google Scholar]
- 8.Murakami, K., R. Tsubouchi, M. Fukayama, T. Ogawa, and M. Yoshino. 2006. Oxidative inactivation of reduced NADP-generating enzymes in E. coli: iron-dependent inactivation with affinity cleavage of NADP-isocitrate dehydrogenase. Arch. Microbiol. 186:385-392. [DOI] [PubMed] [Google Scholar]
- 9.Nimmo, G. A., and H. G. Nimmo. 1984. The regulatory properties of isocitrate dehydrogenase kinase and isocitrate dehydrogenase phosphatase from Escherichia coli ML308 and the roles of these activities in the control of isocitrate dehydrogenase. Eur. J. Biochem. 141:409-414. [DOI] [PubMed] [Google Scholar]
- 10.Peng, L., M. J. Arauzo-Bravo, and K. Shimizu. 2004. Metabolic flux analysis for a ppc mutant Escherichia coli based on 13C-labelling experiments together with enzyme activity assays and intracellular metabolite measurements. FEMS Microbiol. Lett. 235:17-23. [DOI] [PubMed] [Google Scholar]
- 11.Postma, P. W., J. W. Lengeler, and G. R. Jacobson. 1996. Phosphoenolpyruvate:carbohydrate phosphotransferase systems, p. 1149-1174. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, DC.
- 12.Walsh, K., and D. E. Koshland, Jr. 1985. Branch point control by the phosphorylation state of isocitrate dehydrogenase. J. Biol. Chem. 260:8430-8437. [PubMed] [Google Scholar]
- 13.Yang, C., Q. Hua, T. Baba, H. Mori, and K. Shimizu. 2003. Analysis of Escherichia coli anaplerotic metabolism and its regulation mechanisms from the metabolic responses to altered dilution rates and phosphoenolpyruvate carboxykinase knockout. Biotechnol. Bioeng. 84:129-144. [DOI] [PubMed] [Google Scholar]
- 14.Yoshino, M., Y. Yamada, and K. Murakami. 1992. Inhibition by aluminum ion of NAD- and NADP-dependent isocitrate dehydrogenases from yeast. Int. J. Biochem. 24:1615-1618. [DOI] [PubMed] [Google Scholar]
- 15.Yoshino, M., and K. Murakami. 1992. Aluminum: a pH-dependent inhibitor of NADP-isocitrate dehydrogenase from porcine heart. Biometals 5:217-221. [DOI] [PubMed] [Google Scholar]
- 16.Zhao, J., and K. Shimizu. 2003. Metabolic flux analysis of Escherichia coli K12 grown on 13C-labeled acetate and glucose using GC-MS and powerful flux calculation method. J. Biotechnol. 101:101-117. [DOI] [PubMed] [Google Scholar]