Abstract
Chronic exposure of pancreatic islets to supraphysiologic concentrations of glucose causes adverse alterations in β cell function, a phenomenon termed glucose toxicity and one that may play a secondary pathogenic role in type 2 diabetes. However, no mechanism of action has been definitively identified for glucose toxicity in β cells. To ascertain whether chronic oxidative stress might play a role, we chronically cultured the β cell line, HIT-T15, in medium containing 11.1 mM glucose with and without the antioxidants, N-acetyl-l-cysteine (NAC) or aminoguanidine (AG). Addition of NAC or AG to the culture medium at least partially prevented decreases in insulin mRNA, insulin gene promoter activity, DNA binding of two important insulin promoter transcription factors (PDX-1/STF-1 and RIPE-3b1 activator), insulin content, and glucose-induced insulin secretion. These findings suggested that one mechanism of glucose toxicity in the β cell may be chronic exposure to reactive oxygen species, i.e., chronic oxidative stress. To ascertain the effects of these drugs on diabetes, NAC or AG was given to Zucker diabetic fatty rats, a laboratory model of type 2 diabetes, from 6 through 12 weeks of age. Both drugs prevented a rise in blood oxidative stress markers (8-hydroxy-2′-deoxyguanosine and malondialdehyde + 4-hydroxy-2-nonenal), and partially prevented hyperglycemia, glucose intolerance, defective insulin secretion as well as decrements in β cell insulin content, insulin gene expression, and PDX-1 (STF-1) binding to the insulin gene promoter. We conclude that chronic oxidative stress may play a role in glucose toxicity, which in turn may worsen the severity of type 2 diabetes.
Keywords: diabetes, insulin gene, oxidative stress, glucotoxicity
Glucose is the primary fuel and regulator for pancreatic islet β cell function. The primary function of insulin is to maintain blood glucose levels in the normal range. However, this homeostatic relationship is disrupted when glucose remains at supraphysiologic levels for protracted periods of time, a consequence referred to as glucose toxicity (1–4). Resultant defects in insulin content and secretion have been ascribed to adverse effects of glucose on insulin gene expression as evidenced by loss of insulin mRNA, insulin gene promoter activity, and DNA binding of two important transcription factors, PDX-1/STF-1 and RIPE-3b1 activator (5–14).
Although associations between the toxic effects of glucose on β cell function and changes in key constituents of insulin gene expression and insulin synthesis have been established, no mechanism of action has yet been demonstrated. Among potential mechanisms is chronic oxidative stress because glucose can generate reactive oxygen species (ROS) (15–18), which have adverse effects on islet function (19–24). This possibility is supported by experiments reported by Matsuoka et al. (25) demonstrating that decrements in insulin mRNA in HIT-T15 cells can be induced by short-term exposure to d-ribose and prevented by the antioxidant N-acetyl-l-cysteine (NAC). Consequently, we designed experiments using HIT-T15 cells to ascertain whether the antioxidants NAC or aminoguanidine (AG) would prevent chronic glucotoxic effects on insulin gene expression and promoter activity, DNA binding of PDX-1/STF-1 and RIPE-3b1 activator, insulin content, and glucose-induced insulin secretion. To assess whether use of antioxidants in vivo would prevent glucose toxicity and ameliorate the progression of diabetes mellitus, we determined whether NAC or AG would alter the development of this disease in Zucker diabetic fatty (ZDF) rats, a laboratory model of type 2 diabetes (26, 27). Animals were treated beginning at 6 weeks of age; pretreatment and 6-week posttreatment measures included oral glucose tolerance testing, glucose-induced insulin secretion, pancreatic islet insulin content, insulin mRNA, and insulin gene promoter binding of PDX-1 (STF-1).
MATERIALS AND METHODS
HIT-T15 Cells.
HIT-T15 cells were grown as described (28). Beginning at passage 74, cells were split weekly and continuously cultured in RPMI medium 1640 containing 11.1 mM glucose with and without 0.1, 0.5, or 1.0 mM of NAC (Sigma) or 0.01 mM of AG (Sigma). Cell doubling times were determined as described (5).
Animals.
Male Zucker lean control (ZLC/Gmi - +/fa or +/+) rats and ZDF (ZDF/Gmi - fa/fa) rats were obtained from Genetic Models (Indianapolis) at 5 weeks of age and fed Purina 5008 chow. At 6 weeks of age, the rats were divided into four experimental groups (n = 10–15 per group) and treated with NAC (200 mg/kg per day i.p. or 1 g/liter in drinking water), AG (400 mg/kg per day i.p.), a nitric oxide synthesis inhibitor S-methylisothiourea (SMT; Sigma; 50 mg/liter in drinking water), or saline for 6 weeks. Plasma 8-hydroxy-2′-deoxyguanosine (8OHdG) levels by kit (Genox, Baltimore) and malondialdehyde plus 4-hydroxy-2-nonenal (4HNE) levels by kit (Calbiochem) were measured. Animals (n = 4–6) were randomly chosen from each group for oral (2 g/kg) glucose tolerance tests at 6, 8, 10, and 12 weeks of age.
Isolated Islet Insulin Secretion and Content.
Insulin secretion was measured during static incubations of 10 isolated islets/well in Krebs–Ringer buffer (118.5 mM NaCl, 2.54 mM CaCl2·2H2O, 1.19 mM KHP2O4, 4.74 mM KCl, 25 mM NaHCO3, 1.19 mM Mg SO4, 10 mM HEPES, 0.1% BSA, pH 7.4) containing increasing glucose concentrations as described (22). Samples were collected and assayed for insulin by using the Sensitive Rat Insulin and Rat Insulin RIA Kit (Linco Research Immunoassay, St. Charles, MO).
Electromobility Shift Assays.
Nuclear extracts from HIT-T15 cells were prepared, and the binding of PDX-1 (STF-1) and RIPE-3b1-activator proteins to the insulin promoter was analyzed by electrophoretic mobility shift assay as described (6). The oligodeoxynucleotide probes consisted of the human insulin CT2 (−230 to −210) and rat insulin II RIPE-3b1 (−126 to −101).
Northern Blot Analysis of Insulin mRNA. HIT-T15 cells that had been chronically treated with various concentrations of NAC or AG were subcultured for 48 h in RPMI medium 1640 containing 11.1 mM glucose without drugs. After electroblotting RNA, the nylon membrane was prehybridized then hybridized for 16 h with 32P-labeled Syrian hamster preproinsulin cDNA (29) and human β-actin cDNA (30) probe. Under the hybridization conditions used, the probe hybridized to a single 0.5-kb band consistent with HIT-T15 cell preproinsulin mRNA (31). Insulin and β-actin mRNA were quantitated by scanning densitometry of autoradiograms, and data are expressed as the density ratio of insulin mRNA to β-actin mRNA.
Because of limited quantities of islet tissue, one-step reverse transcription–PCR using 100 hand-picked islets/sample was carried out by using the Gold RT-PCR kit from Perkin–Elmer. Fluorescence detection of amplication products over a range of wavelengths (500–650 nm) was performed by an ABI Prism 770 sequence detector equipped with a thermocycler (Taqman R technology) and a cooled charge-coupled device camera (Perkin–Elmer). Probe and primer sequences were: rat insulin probe, 6FAM-AGCTTCCACCAAGTGAGAACCACAAAGGT-TAMRA; rat insulin forward primer, GCCCAGGCTTTTGTCAAACA; rat insulin reverse primer, CTCCCCACACACCAGGTAGAG; rat β-actin probe, 6FAM-AGGCATCCTGACCCTGAAGTACCCCA-TAMRA; rat β-actin forward primer, ACGAGGCCCAGAGCAAGA; and rat β-actin reverse primer, TTGGTTACAATGCCGTGTTCA (FAM, reporter dye; TAMRA, quencher dye).
Cell Transfections, Chloramphenicol Acetyl Transferase (CAT) Assay, and Luciferase Assay.
The plasmid INSCAT containing the human insulin gene sequences −326 to +30 linked to the CAT reporter gene (6) was transfected by using Lipofectin (GIBCO/BRL) to HIT-T15 cells chronically treated with NAC or AG after subculturing in 6-well culture plates at densities of 1.3 × 106 cells/well for 48 h without drugs. CAT assay was performed by using a kit (United States Biochemical). To control for transfection efficiency, 0.1 μg of pGL3LUC, a plasmid containing pGL3 promoter (Promega), was cotransfected and luciferase activity was measured by using a luminometer (LUMAT LB 9507; EG&G Berthold, Bad Wildbad, Germany).
Expression of Data and Statistics.
Data are presented as mean ± SEM and were analyzed by Student’s t test and ANOVA where appropriate.
RESULTS
HIT-T15 Cells.
Cell growth. Beginning with passage 74, HIT-T15 cell counts were performed weekly. Although there was a general trend for rates to increase in the older passages, no significant difference was observed in cell doubling times between treated and nontreated cells (data not shown).
Insulin mRNA.
Cells from passages 105, 107, and 108 showed the expected dramatic decline (14.8 ± 1.6% control) in insulin mRNA (Fig. 1). However, this disappearance of insulin mRNA was partially (56.5 ± 18.8 and 53.3 ± 13.4%, P < 0.01) prevented when NAC in 0.5 and 1.0 mM concentrations, respectively, was included in the medium. In other experiments (data not shown), slot-blots showed that the protective effect of NAC was first visible by passage 94 when insulin mRNA was clearly disappearing. Similarly, addition of 0.01 mM AG in culture medium partially (61% control) prevented disappearance of insulin mRNA (Fig. 1).
Figure 1.
Northern analysis of insulin mRNA in HIT-T15 cells cultured in various concentrations of NAC and AG for 35 weeks. The autoradiogram depicts representative experiments from passage 105 chronically cultured with NAC and from passage 104 chronically cultured with AG. The relative density of insulin mRNA to β-actin is normalized to that of control data from HIT-T15 cells at passage 75. NAC 0, 0.15 ± 0.2; NAC 0.1, 0.16 ± 0.01; NAC 0.5, 0.57 ± 0.19*; NAC 1, 0.53 ± 0.13*; AG 0, 0.16; AG 0.01, 0.61). Data are presented as mean ± SEM for three independent experiments at passages 105, 107, and 108 in the NAC study, and are presented as mean of two independent experiments at passages 97 and 104 in the AG study (*, P < 0.01 vs. NAC 0).
Insulin gene promoter activity.
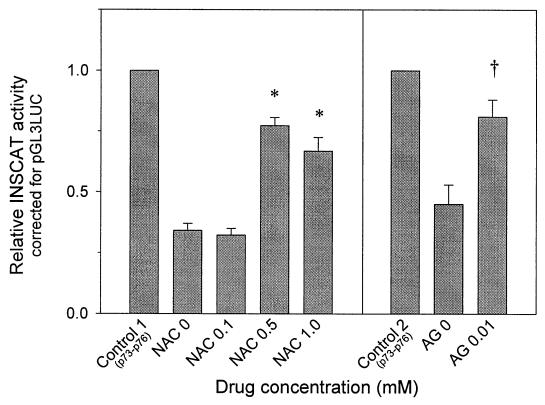
INSCAT reporter technique revealed a decrease (34.2 ± 2.9% control) in promoter activity by passages 107, 108, 111, and 112 (Fig. 2). This decrease was partially prevented by NAC (0.5 mM NAC, 77.3 ± 3.4% control; 1.0 mM NAC, 66.8 ± 5.7%; P < 0.01) and AG (81 ± 7% control) (Fig. 2).
Figure 2.
Expression of insulin gene promoter activity in HIT-T15 cells chronically cultured with various concentrations of NAC and AG. The relative expression of INSCAT to pGL3LUC is normalized to the expression found in the early control passage cells (passages 73–76). Data are presented as mean ± SEM for four independent experiments with triplicate observations at passages 107, 108, 111, and 112 in NAC study and at passages 106, 107, and 108 in AG study (*, P < 0.01 vs. NAC 0; †, P < 0.01 vs. AG 0).
PDX-1/STF-1 and RIPE-3b1 activator binding to DNA.
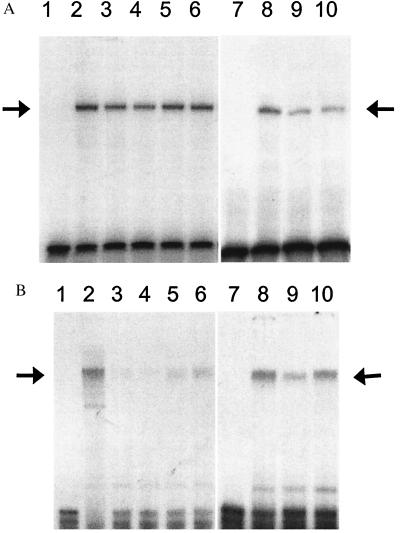
Binding to the CT2 sequence was decreased (61.1 ± 5.4% control) by passage 110, an effect partially prevented (78.9 ± 3.8% control and 78.5 ± 4.8%, P < 0.05) when NAC in 0.5 and 1.0 mM concentrations, respectively, was included in the culture medium (Fig. 3A). Binding to the RIPE-3b1 DNA probe by RIPE-3b1 activator was even more dramatically reduced (29.3 ± 4.2% control) by passage 110; binding to the RIPE-3b1 probe was partially restored (61.2 ± 9.6% control and 78.6 ± 9.7%, P < 0.01) when NAC in 0.5 and 1.0 mM concentrations, respectively, was included in the medium (Fig. 3B). In other experiments, cells were chronically cultured with or without AG. CT2 (Fig. 3A) and RIPE-3b1 (Fig. 3B) binding were decreased (57% and 56% control, respectively) by passage 104; AG partially (65% and 84% control, respectively) prevented these decreases. In two separate studies, H2O2 (200–500 μm) caused a concentration-dependent decrease in DNA binding by both transcription factors; this effect was prevented by 0.1 mM NAC (data not shown).
Figure 3.
Protein binding to the CT2 (A) and RIPE-3b1 (B) sites of the insulin gene promoter. Nuclear extracts from HIT-T15 cells chronically treated with NAC or AG were analyzed by EMSA, using a 32P-labeled human insulin promoter sequence −230 to −201 (A) and rat insulin gene II promoter sequence −126 to −101 (B). Lane 1, free probe. Lane 2, nuclear extract from HIT-T15 cells at p75. Lanes 3–6, nuclear extract from HIT-T15 cells at passage 107 chronically treated without (lane 3) or with 0.1 mM (lane 4), 0.5 mM (lane 5), and 1.0 mM (lane 6) of NAC. Lane 7, free probe. Lane 8, nuclear extract from HIT-T15 cells at p75. Lanes 9 and 10, nuclear extract from HIT-T15 cells at passage 104 chronically treated without (lane 9) or with 0.01 mM AG (lane 10). Arrows indicate the binding site of PDX-1/STF-1 (A) and RIPE-3b1 activator (B). The autoradiograms were quantitated by densitometry. The relative protein binding to the CT2 and RIPE-3b1 are normalized to the binding of control sample from HIT-T15 cells at passage 75. Relative CT2 binding: NAC 0, 0.61 ± 0.05; NAC 0.1, 0.58 ± 0.05; NAC 0.5, 0.79 ± 0.04*; NAC 1.0, 0.79 ± 0.05*; AG 0, 0.57; AG 0.01, 0.65. Relative RIPE-3b1 binding: NAC 0, 0.29 ± 0.04; NAC 0.1, 0.24 ± 0.61; NAC 0.5, 0.61 ± 0.10†; NAC 1.0, 0.79 ± 0.10†; AG 0, 0.56; AG 0.01, 0.85. Data are presented as mean ± SEM for four independent experiments at passage 105, 107, 108, and 109 in NAC study and are presented as mean of two independent experiments at passages 104 and 106 in AG study (*, P < 0.05 vs. NAC 0; †, P < 0.01 vs. NAC 0).
Islet insulin content and secretion.
A decrease (7.5 ± 5.0% control, P < 0.01) in insulin stores was evident by passages 105, 109, and 110 (Table 1). NAC (0.5 and 1.0 mM) partially prevented this reduction (34.6 ± 4.7% control and 27.1 ± 8.1% control, respectively, P < 0.01). In the AG experiments, insulin content was decreased (28.3 ± 0.8% control, P < 0.01) by passages 97, 106, and 108 and AG partially prevented (61.2 ± 12.7% control, P < 0.05) this decrease. Similarly, decreased glucose-induced insulin secretion was partially prevented by NAC and AG (Table 1).
Table 1.
Insulin content and glucose-induced insulin release from HIT-T15 cells chronically cultured in media containing 11.1 mM glucose with or without NAC or AG and then incubated in Krebs buffer containing 4 mM glucose for 2 h
| Experimental condition | Insulin content (microunits/mg⋅protein) | Glucose-induced insulin secretion (fold) |
|---|---|---|
| Control 1 | 20,800 ± 1,200 | 5.33 ± 0.81 |
| NAC 0 | 1,550 ± 1,030* | 1.31 ± 0.05* |
| NAC 0.1 | 413 ± 218* | 1.44 ± 0.07* |
| NAC 0.5 | 7,190 ± 970*‡ | 2.55 ± 0.16*§ |
| NAC 1.0 | 5,630 ± 1,690*§ | 2.52 ± 0.24*§ |
| Control 2 | 20,300 ± 1,300 | 6.70 ± 0.95 |
| AG 0 | 5,760 ± 160* | 1.61 ± 0.19* |
| AG 0.01 | 12,500 ± 2,600†¶ | 3.46 ± 0.12†¶ |
Numbers after NAC and AG refer to mM drug concentration.∗, P < 0.01 vs. control;
, P < 0.05 vs. control;
, P < 0.01 vs. NAC 0;
, P < 0.05 vs. NAC 0;
, P < 0.05 vs. AG 0. Controls, passage 73–78; drug conditions, passage 105–110.
ZDF Rats.
From 6 to 12 weeks of age, both plasma 8OHdG and malondialdehyde + 4HNE levels significantly rose in vehicle-treated ZDF rats (Table 2; P < 0.01). These levels were significantly reduced (P < 0.05) to the range of 6-week-old animals by NAC or AG treatment. No statistical difference was observed in blood glucose level between ZLC and ZDF rats at 6 weeks of age. Overt hyperglycemia was observed in nontreated ZDF rats after 9 weeks of age (Table 2); AG or NAC treatment partially prevented development of hyperglycemia. A selective iNOS inhibitor, SMT, did not influence the development of diabetes in ZDF rats. There was no difference in glucose level between treated and nontreated ZLC rats (data not shown). Although there was no significant difference in nonfasting glucose levels between ZDF and ZLC rats at 6 weeks of age, the areas under the curve (AUC) for glucose during glucose tolerance tests were significantly higher in ZDF rats. AUC-glucose was significantly elevated (P < 0.001) after the 6-week experimental period in nontreated ZDF rats; AG or NAC treatment prevented development of glucose intolerance but SMT did not. Insulin responses were increased at 8 weeks of age in treated and nontreated ZDF rats. These values gradually decreased in all groups, but these decreases were partially prevented by AG or NAC treatment at 12 weeks of age (Table 2). Insulin responses were uniformly lower in ZLC rats (data not shown).
Table 2.
Biochemical measures in ZDF rats at 6 and 12 weeks that received daily treatment with NAC, AG, SMT, or saline
| ZDF 6w | ZDF 12w
|
||||
|---|---|---|---|---|---|
| Vehicle | NAC | AG | SMT | ||
| Number | 10 | 15 | 15 | 15 | 15 |
| Body weight (g) | 166.8 ± 6.9 | 347.8 ± 4.5* | 365.0 ± 6.0* | 364.9 ± 6.8* | 352.8 ± 6.4* |
| Nonfasted glucose (mg/ml) | 137.4 ± 3.7 | 489.3 ± 44.3* | 284.3 ± 18.7‡§ | 243.2 ± 24.8†§ | 423.7 ± 25.3* |
| 8OHdG (ng/ml) | 70.5 ± 11.2 | 118.4 ± 9.0† | 78.6 ± 4.9¶ | 72.8 ± 3.2¶ | |
| MDA + 4HNE (μM) | 1.26 ± 0.10 | 2.26 ± 0.35‡ | 1.61 ± 0.08‖ | 1.38 ± 0.17‖ | |
| OGTT | |||||
| AUC-glucose (g/ml⋅min) | 23.3 ± 2.3 | 47.1 ± 5.6* | 29.9 ± 2.7¶ | 25.3 ± 1.7§ | 42.1 ± 4.4* |
| Insulin response (microunits/ml) | 47.3 ± 9.3 | 55.9 ± 9.4 | 134.8 ± 29.9‖ | 147.0 ± 34.0‖ | 83.6 ± 20.1 |
| Urinary glucose | 2.74 ± 0.77 | 0.75 ± 0.11§ | 0.55 ± 0.14§ | 2.44 ± 0.32 | |
| excretion (g/24 h) | |||||
| Insulin content is islets | 20.3 ± 3.6 | 30.9 ± 6.0 | 92.5 ± 20.5‖ | 183.2 ± 34.7§ | 42.3 ± 6.7 |
| (microunits/ml per 50 islets) | |||||
, P < 0.001;
, P < 0.01;
, P < 0.05 vs. 6-week data;
, P < 0.001;
, P < 0.01,
, P < 0.05 vs. vehicle treatment.
MDA, malondialdehyde.
Insulin mRNA.
Insulin gene expression was dramatically diminished (33.0%) by 12 weeks of age in nontreated ZDF rats. Treatment with NAC (64.5%) and AG (100%) partially preserved insulin gene expression in these animals (Fig. 4A). Treatments had no effect on the mRNA levels in the ZLC rats.
Figure 4.
(A) Measurement of insulin mRNA in islets isolated from ZDF rats. Insulin mRNA was measured by reverse transcription–PCR at 6 and 12 weeks of age in ZDF rats. All data (x̄ ± SD) were corrected for β-actin and normalized to the value of the 6-week-old ZDF group. Each value is presented as mean of two independent experiments with triplicate measurements. (B) Protein binding to the CT2 site of insulin gene promoter in islets isolated from Zucker rats. Nuclear extracts from isolated islets from 6- and 12-week-old animals analyzed by electrophoretic mobility shift assay. Lane 1, free probe. Lane 2, nuclear extract from 6-week-old ZDF rats. Lane 3, nuclear extract from 12-week-old vehicle-treated ZDF rats. Lane 4, nuclear extract from 12-week-old NAC-treated rats. Lane 5, nuclear extract from 12-week-old AG-treated ZDF rats.
PDX-1/STF-1 binding to the insulin gene promoter.
Binding to the CT2 motif was decreased (16.6%) in the islets isolated from nontreated ZDF rats at 12 weeks of age; NAC and AG partially preserved binding (60.1% and 85.0%, respectively; Fig. 4B). No effect was observed on binding in ZLC animals (data not shown).
Glucose-stimulated insulin secretion and insulin content in isolated islets.
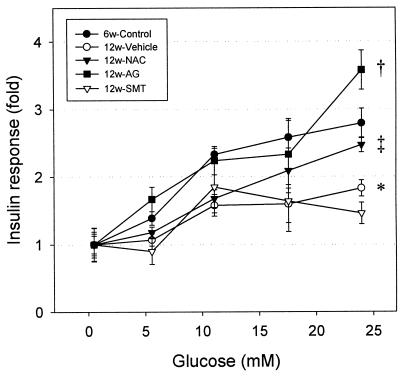
Glucose-stimulated insulin secretion was decreased in islets isolated from 12-week-old ZDF rats (1.83 ± 0.12-fold, P < 0.01) compared with those isolated from 6-week-old ZDF rats (2.79 ± 0.22-fold; Fig. 5). Treatment of ZDF rats with NAC (2.47 ± 0.11-fold) or AG (3.58 ± 0.29-fold) fully restored glucose-stimulated insulin secretion. No effects of the drugs were observed in ZLC rats (data not shown). AG or NAC treatment significantly increased insulin content in islets isolated from 12-week-old ZDF rats (Table 2), but had no effect on the insulin content in ZLC rat islets (data not shown).
Figure 5.
Glucose-stimulated insulin secretion during 1-h static incubations of isolated islets from ZDF rats. Each value represents mean ± SEM of triplicates, each consisting of 10 islets/well and corrected by subtraction of basal insulin level (*, P < 0.01 vs. ZLC 6w group; †, P < 0.001 vs. ZDF vehicle-treated group; ‡, P < 0.05 vs. ZDF vehicle-treated group). Insulin content is shown in Table 2.
DISCUSSION
We designed in vitro experiments using HIT-T15 cells to assess whether oxidative stress might be a mechanism of action for the toxic effects on β cell function caused by prolonged exposure to supraphysiologic glucose concentrations. The results confirm our previous observations that glucose toxicity leads to defective insulin gene expression, as well as decreased DNA binding by STF-1/PDX-1 and RIPE-3b1 activator, diminished insulin gene promoter activity, decreased insulin content, and defective glucose-induced insulin secretion (5–10). The current study demonstrates that all of these adverse consequences can be at least partially prevented by including NAC or AG in the culture medium. Because both agents are antioxidants, these data indicate that chronic oxidative stress may be one mechanism of action for glucose toxicity in the pancreatic β cell.
These observations prompted us to design in vivo studies to ascertain whether these antioxidants can ameliorate the development of type 2 diabetes mellitus in ZDF rats. We found that blood levels of 8OHdG and malondialdehyde + 4HNE, two markers of chronic oxidative stress, rose significantly when the animals developed hyperglycemia. As expected, placebo-treated animals developed progressive obesity, hyperglycemia, abnormal oral glucose tolerance tests, defective glucose-stimulated insulin secretion, as well as decreased islet insulin content, insulin gene expression, and PDX-1 (STF-1) binding to the insulin gene promoter. We previously have reported that glucose toxicity in HIT-T15 cells is associated with a marked decrease in PDX-1 gene expression and PDX-1 protein (7) and have observed decreased PDX-1 mRNA in 12-week-old ZDF rats (32). Treatment with NAC or AG through 6 to 12 weeks of age prevented the rise in oxidative stress markers and partially prevented the deleterious changes in β cell function, whereas treatment with SMT had no effect. In control experiments with ZLC animals, none of the four treatment regimes altered any of the measures. These findings indicate that intervention with antioxidants during the development of hyperglycemia in this laboratory model of type 2 diabetes can partially prevent worsening of hyperglycemia, an effect that is associated with partial preservation of insulin gene expression, insulin content, and insulin secretion. That these beneficial antioxidant effects are partial rather than complete is consistent with our hypothesis, i.e., that chronic oxidative stress caused by prolonged hyperglycemia secondarily worsens the diabetic state whereas the primary cause of diabetes is related to other mechanisms.
Several maneuvers had been reported previously to preserve or restore insulin gene expression in late passage glucotoxic HIT-T15 cells. Robertson et al. (5) cultured cells in 0.8 mM rather than 11.1 mM glucose to avoid exposure to supraphysiologic glucose concentrations and thereby preserved insulin mRNA and glucose-induced insulin secretion. Moran et al. (33) attempted to reverse glucotoxic effects in HIT-T15 cells at passage 100 by placing cells that had been chronically cultured in 11.1 mM glucose in medium containing 0.8 mM glucose and observed approximately 17% recovery of insulin mRNA that had been lost. Harmon et al. (10) performed PDX-1/STF-1 transient transfection using glucotoxic cells with diminished insulin gene promoter activity and observed a partial reconstitution of promoter activity. Matsuoka et al. (25) reported that NAC protected HIT-T15 cells against inhibition of insulin gene expression caused by exposing them for 72 h to the strong reducing sugar d-ribose. However, we know of no previously reported experiments that have examined the preventive ability of antioxidants to prevent the toxic effects on insulin gene expression of long-term exposure to supraphysiologic concentrations of glucose.
NAC is used frequently in therapeutic and experimental settings as an antioxidant because it is known to directly reduce ⋅OH and H2O2 although not ⋅O-2 (34). Another major pharmacologic effect of NAC is to support glutathione synthesis. Therefore, because glutathione itself serves as an intracellular antioxidant, the antioxidant effect of NAC may be both direct and indirect. None of the other known pharmacologic actions of NAC (34) seem likely to explain its protective effects against decreases in insulin gene expression. AG usually is thought of as an inhibitor of the formation of advanced glycosylation end products. Recently, Giardino et al. (35) reported that AG also acts as an antioxidant in vivo, quenching hydroxyl radicals and lipid peroxidation in cells.
Many investigators have studied the proposal that chronic exposure to elevated glucose concentrations can cause damage to tissues through mechanisms involving oxidative stress (15–25, 35–46). One putative mechanism by which ROS can be formed by chronic exposure to hyperglycemia involves nonenzymatic glycation of proteins and the formation of products that in turn lead to the production of ROS (15, 16). The pancreatic islets themselves have been reported to have low expression of antioxidant enzymes (20, 22) and therefore might be especially susceptible to ROS. In support of the hypothesis that chronic oxidative stress might play a secondary pathogenic role in diabetes mellitus, Leinonen et al. (45) and Dandona et al. (46) have reported that levels of 8OHdG are elevated in the urine and blood of type 2 diabetic patients. Kaneto et al. (47) and Tajiri et al. (39) reported that the pancreatic islet β cell undergoes oxidative stress when it is exposed to supraphysiologic concentrations of glucose, and Tajiri et al. (39) demonstrated that AG can ameliorate this process. Although the exact site and mechanism of action for ROS-induced damage to the β cell is not known, there are at least two possibilities. ROS might cause a modification of DNA to form the product 8OHdG (42), which itself is a mutagen. ROS also might attack membrane lipids, causing lipid peroxidation and breakdown of lipid hydroperoxides to form aldehydes such as 4HNE (41). The formation of HNE involves peroxidation of unsaturated fatty acids such as arachidonic acid, which is of particular interest because arachidonic acid metabolites have been postulated to play a role in the dysregulation of β cell function in diabetes mellitus (48, 49). Most recently, Ihara et al. (24) reported that levels of 8OHdG and HNE-modified proteins are present in the pancreatic β cells of Goto-Kakizaki (GK) rats, another model of type 2 diabetes. They observed that dietary sucrose increases levels of OHdG and HNE and suggested that chronic hyperglycemia might be responsible for the oxidative stress-related changes observed in the pancreatic β cells of GK rats. Our results with the ZDF rat attempt to associate chronic oxidative stress with deteriorating β cell function in this animal model and demonstrate that in vivo treatment of a type 2 diabetes laboratory model with structurally unrelated antioxidants can partially prevent the development of hyperglycemia and loss of insulin gene expression and β cell function. Although NAC and AG have other pharmacologic effects, these structurally unrelated drugs have in common that they are antioxidants. On the other hand, SMT, an inhibitor of nitric oxide synthase, had no beneficial effects on the development of hyperglycemia in this animal model.
The clinical implications of our findings and the findings of other investigators interested in the interaction between chronic oxidative stress and the β cell relate to the continued worsening of hyperglycemia in type 2 diabetes mellitus in humans. The findings reported in this manuscript strengthen the contention that chronic oxidative stress may play an important role in progressive β cell dysfunction and demonstrate that treatment with antioxidants can partially prevent this deterioration.
Acknowledgments
We gratefully acknowledge the superb technical assistance of Ms. Elizabeth Oseid and Kimberly Berger and the excellent manuscript preparation by Shannon Greer. This work was supported by National Institutes of Health Grant DK 38325 (R.P.R.) and the American Diabetes Association Mentor-Based Postdoctoral Fellowship Program (Y.T.).
ABBREVIATIONS
- NAC
N-acetyl-l-cysteine
- AG
aminoguanidine
- ZDF
Zucker diabetic fatty
- ZLC
Zucker lean control
- SMT
S-methylisothiourea
- 8OHdG
8-hydroxy-2′-deoxyguanosine
- CAT
chloramphenicol acetyl transferase
- ROS
reactive oxygen species
- 4HNE
4-hydroxy-2-nonenal
References
- 1.Unger R H, Grundy S. Diabetologia. 1985;28:119–121. doi: 10.1007/BF00273856. [DOI] [PubMed] [Google Scholar]
- 2.Rossetti L, Giaccari A, DeFronzo R A. Diabetes Care. 1990;13:610–630. doi: 10.2337/diacare.13.6.610. [DOI] [PubMed] [Google Scholar]
- 3.Robertson R P, Olson L K, Zhang H J. Diabetes. 1994;43:1085–1089. doi: 10.2337/diab.43.9.1085. [DOI] [PubMed] [Google Scholar]
- 4.Robertson R P. Diabetes. 1989;38:1501–1505. doi: 10.2337/diab.38.12.1501. [DOI] [PubMed] [Google Scholar]
- 5.Robertson R P, Zhang H J, Pyzdrowski K L, Walseth T F. J Clin Invest. 1992;90:320–325. doi: 10.1172/JCI115865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olson L K, Redmon J B, Towle H C, Robertson R P. J Clin Invest. 1993;92:514–519. doi: 10.1172/JCI116596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olson L K, Sharma A, Peshavaria M, Wright C V, Towle H C, Rodertson R P, Stein R. Proc Natl Acad Sci USA. 1995;92:9127–9131. doi: 10.1073/pnas.92.20.9127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma A, Olson L K, Robertson R P, Stein R. Mol Endocrinol. 1995;9:1127–1134. doi: 10.1210/mend.9.9.7491105. [DOI] [PubMed] [Google Scholar]
- 9.Poitout V, Olson L K, Robertson R P. J Clin Invest. 1996;97:1041–1046. doi: 10.1172/JCI118496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harmon J S, Tanaka Y, Olson L K, Robertson R P. Diabetes. 1998;47:900–904. doi: 10.2337/diabetes.47.6.900. [DOI] [PubMed] [Google Scholar]
- 11.Briaud I, Rouault C, Reach G, Poitout V. Metabolism. 1999;48:319–323. doi: 10.1016/s0026-0495(99)90079-3. [DOI] [PubMed] [Google Scholar]
- 12.Zangen D H, Bonner-Weir S, Lee C H, Latimer J B, Miller C P, Habener J F, Weir G C. Diabetes. 1997;46:258–264. doi: 10.2337/diab.46.2.258. [DOI] [PubMed] [Google Scholar]
- 13.Lu M, Seufert J, Habener J F. J Biol Chem. 1997;272:28349–28359. doi: 10.1074/jbc.272.45.28349. [DOI] [PubMed] [Google Scholar]
- 14.Seufert J, Weir G C, Habener J F. J Clin Invest. 1998;101:2528–2539. doi: 10.1172/JCI2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hunt J V, Dean R T, Wolff S P. Biochem J. 1988;256:205–212. doi: 10.1042/bj2560205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakurai T, Tsuchiya S. FEBS Lett. 1988;236:406–410. doi: 10.1016/0014-5793(88)80066-8. [DOI] [PubMed] [Google Scholar]
- 17.Williamson J R, Chang K, Frangos M, Hasan K S, Ido Y, Kawamura T, Nyengaard J R, van den Enden M, Kilo C, Tilton R G. Diabetes. 1993;42:801–813. doi: 10.2337/diab.42.6.801. [DOI] [PubMed] [Google Scholar]
- 18.Baynes J W. Diabetes. 1991;40:405–412. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 19.Burkart V, Koike T, Brenner H H, Kolb H. Diabetologia. 1992;35:1028–1034. doi: 10.1007/BF02221677. [DOI] [PubMed] [Google Scholar]
- 20.Lenzen S, Drinkgern J, Tiedge M. Free Radical Biol Med. 1996;20:463–466. doi: 10.1016/0891-5849(96)02051-5. [DOI] [PubMed] [Google Scholar]
- 21.Kubisch H M, Wang J, Bray T M, Phillips J P. Diabetes. 1997;46:1563–1566. doi: 10.2337/diabetes.46.10.1563. [DOI] [PubMed] [Google Scholar]
- 22.Tiedge M, Lortz S, Drinkgern J, Lenzen S. Diabetes. 1997;46:1733–1742. doi: 10.2337/diab.46.11.1733. [DOI] [PubMed] [Google Scholar]
- 23.Hohmeier H E, Thigpen A, Tran V V, Davis R, Newgard C B. J Clin Invest. 1998;101:1811–1820. doi: 10.1172/JCI1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ihara Y, Toyokuni S, Uchida K, Odaka H, Tanaka T, Ikeda H, Hiai H, Seino Y, Yamada Y. Diabetes. 1999;48:927–932. doi: 10.2337/diabetes.48.4.927. [DOI] [PubMed] [Google Scholar]
- 25.Matsuoka T, Kajimoto Y, Watada H, Kaneto H, Kishimoto M, Umayahara Y, Fujitani Y, Kamada T, Kawamori R, Yamasaki Y. J Clin Invest. 1997;99:144–150. doi: 10.1172/JCI119126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tokuyama Y, Sturis J, DePaoli A M, Takeda J, Stoffel M, Tang J, Sun X, Polonsky K S, Bell G I. Diabetes. 1995;44:1447–1457. doi: 10.2337/diab.44.12.1447. [DOI] [PubMed] [Google Scholar]
- 27.Peterson R G, Shaw W N, Neel M-A, Little L A, Eichberg J. ILAR News. 1990;32:16–19. doi: 10.1093/ilar.32.3.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang H J, Walseth T F, Robertson R P. Diabetes. 1989;38:44–48. doi: 10.2337/diab.38.1.44. [DOI] [PubMed] [Google Scholar]
- 29.Bell G I, Sanchez-Pescador R. Diabetes. 1984;33:297–300. doi: 10.2337/diab.33.3.297. [DOI] [PubMed] [Google Scholar]
- 30.Gunning P, Ponte P, Okayama H, Engel J, Blau H, Kedes L. Mol Cell Biol. 1983;3:787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hammonds P, Schofield P N, Ashcroft S J. FEBS Lett. 1987;213:149–154. doi: 10.1016/0014-5793(87)81481-3. [DOI] [PubMed] [Google Scholar]
- 32.Harmon, J. S., Gleason, C. E., Tanaka, Y., Oseid, E. A., Hunter-Berger, K. K. & Robertson, R. P. (1999) Diabetes, in press. [DOI] [PubMed]
- 33.Moran A, Zhang H J, Olson L K, Harmon J S, Poitout V, Robertson R P. J Clin Invest. 1997;99:534–539. doi: 10.1172/JCI119190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cotgreave I A. Adv Pharmacol. 1997;38:205–227. [PubMed] [Google Scholar]
- 35.Giardino I, Fard A K, Hatchell D L, Brownlee M. Diabetes. 1998;47:1114–1120. doi: 10.2337/diabetes.47.7.1114. [DOI] [PubMed] [Google Scholar]
- 36.Monnier V M, Sell D R, Nagaraj R H, Miyata S, Grandhee S, Odetti P, Ibrahim S A. Diabetes. 1992;41, Suppl. 2:36–41. doi: 10.2337/diab.41.2.s36. [DOI] [PubMed] [Google Scholar]
- 37.Bucala R, Model P, Cerami A. Proc Natl Acad Sci USA. 1984;81:105–109. doi: 10.1073/pnas.81.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pieper G M, Siebeneich W. J Cardiovasc Pharmacol. 1998;32:101–105. doi: 10.1097/00005344-199807000-00016. [DOI] [PubMed] [Google Scholar]
- 39.Tajiri Y, Moller C, Grill V. Endocrinology. 1997;138:273–280. doi: 10.1210/endo.138.1.4851. [DOI] [PubMed] [Google Scholar]
- 40.Toyokuni S, Tanaka T, Hattori Y, Nishiyama Y, Yoshida A, Uchida K, Hiai H, Ochi H, Osawa T. Lab Invest. 1997;76:365–374. [PubMed] [Google Scholar]
- 41.Uchida K, Szweda L I, Chae H Z, Stadtman E R. Proc Natl Acad Sci USA. 1993;90:8742–8746. doi: 10.1073/pnas.90.18.8742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kasai H. Mutat Res. 1997;387:147–163. doi: 10.1016/s1383-5742(97)00035-5. [DOI] [PubMed] [Google Scholar]
- 43.Traverso N, Menini S, Cosso L, Odetti P, Albano E, Pronzato M A, Marinari U M. Diabetologia. 1998;41:265–270. doi: 10.1007/s001250050902. [DOI] [PubMed] [Google Scholar]
- 44.Sano T, Umeda F, Hashimoto T, Nawata H, Utsumi H. Diabetologia. 1998;41:1355–1360. doi: 10.1007/s001250051076. [DOI] [PubMed] [Google Scholar]
- 45.Leinonen J, Lehtimaki T, Toyokuni S, Okada K, Tanaka T, Hiai H, Ochi H, Laippala P, Rantalaiho V, Wirta O, et al. FEBS Lett. 1997;417:150–152. doi: 10.1016/s0014-5793(97)01273-8. [DOI] [PubMed] [Google Scholar]
- 46.Dandona P, Thusu K, Cook S, Snyder B, Makowski J, Armstrong D, Nicotera T. Lancet. 1996;347:444–445. doi: 10.1016/s0140-6736(96)90013-6. [DOI] [PubMed] [Google Scholar]
- 47.Kaneto H, Fujii J, Myint T, Miyazawa N, Islam K N, Kawasaki Y, Suzuki K, Nakamura M, Tatsumi H, Yamasaki Y, Taniguchi N. Biochem J. 1996;320:855–863. doi: 10.1042/bj3200855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robertson R P. Diabetes Metab Rev. 1986;2:261–296. doi: 10.1002/dmr.5610020304. [DOI] [PubMed] [Google Scholar]
- 49.Robertson R P, Tsai P, Little S A, Zhang H J, Walseth T F. Diabetes. 1987;36:1047–1053. doi: 10.2337/diab.36.9.1047. [DOI] [PubMed] [Google Scholar]