Abstract
The Mycobacterium tuberculosis acyl-coenzyme A (CoA) carboxylases provide the building blocks for de novo fatty acid biosynthesis by fatty acid synthase I (FAS I) and for the elongation of FAS I end products by the FAS II complex to produce meromycolic acids. The M. tuberculosis genome contains three biotin carboxylase subunits (AccA1 to -3) and six carboxyltransferase subunits (AccD1 to -6), with accD6 located in a genetic locus that contains members of the FAS II complex. We found by quantitative real-time PCR analysis that the transcripts of accA3, accD4, accD5, and accD6 are expressed at high levels during the exponential growth phases of M. tuberculosis in vitro. Microarray analysis of M. tuberculosis transcripts indicated that the transcripts for accA3, accD4, accD5, accD6, and accE were repressed during later growth stages. AccD4 and AccD5 have been previously studied, but there are no reports on the function of AccD6. We expressed AccA3 (α3) and AccD6 (β6) in E. coli and purified them by affinity chromatography. We report here that reconstitution of the α3-β6 complex yielded an active acyl-CoA carboxylase. Kinetic characterization of this carboxylase showed that it preferentially carboxylated acetyl-CoA (1.1 nmol/mg/min) over propionyl-CoA (0.36 nmol/mg/min). The activity of the α3-β6 complex was inhibited by the ɛ subunit. The α3-β6 carboxylase was inhibited significantly by dimethyl itaconate, C75, haloxyfop, cerulenin, and 1,2-cyclohexanedione. Our results suggest that the β6 subunit could play an important role in mycolic acid biosynthesis by providing malonyl-CoA to the FAS II complex.
Tuberculosis causes 2 million deaths each year, according to the World Health Organization. Mycobacterium tuberculosis, the pathogen that causes the disease, infects 8 million people each year and is one of the world's deadliest pathogens (9). The ongoing AIDS pandemic has developed a deadly synergy with tuberculosis, which is the leading cause of death among AIDS patients (2). Multidrug-resistant M. tuberculosis strains have been emerging rapidly (9), and the need for identifying novel drug targets in this pathogen has become urgent. The cell wall of M. tuberculosis is lipid enriched and acts as an impermeable barrier to many common broad-spectrum antibiotics (14).
The first committed step of fatty-acid biosynthesis, which is the biotin-dependent carboxylation of acyl-coenzyme A (CoA) to produce malonyl-CoA and methylmalonyl-CoA, is catalyzed by the acyl-CoA carboxylase. The reaction consists of two catalytic steps, which involve the biotin carboxylase and the carboxyltransferase (8). In M. tuberculosis, the biotin carboxylation step is catalyzed by the α subunit; there are three open reading frames (ORFs) that can encode the α subunit (accA1 to -A3) in the genome. Carboxyl transfer is catalyzed by the β subunit, and there are six β subunits (accD1 to -D6) in the genome of the pathogen (6).
Previously, the catalytic activities of the α3, β4, and β5 subunits were studied (10, 11, 22, 24). However, the levels of expression of the various subunits have not been examined. Our analysis of transcripts from M. tuberculosis cells indicate that the α3, β4, β5, β6, and ɛ ORFs are the main subunits regulated during cell growth. To determine whether the highly expressed β6 subunit possessed enzymatic activity and to assess its substrate specificity, we expressed and purified the β6 subunit and reconstituted it with the purified α3 subunit. We report that an active acyl-CoA carboxylase was reconstituted with the purified β6 and α3 subunits and that it preferentially carboxylated acetyl-CoA over propionyl-CoA. This is the first report showing that β6, which is a member of a fatty acid synthase II (FAS II) gene locus, is a functional carboxyltransferase of the acyl-CoA carboxylase in M. tuberculosis, and these results suggest that β6 might make a significant contribution to mycolate biosynthesis.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
M. tuberculosis H37Rv was grown in Middlebrook 7H9 (supplemented with 0.05% Tween 80, 10% oleic acid-albumin-dextrose-catalase enrichment, and 0.2% glycerol). All subcloning procedures were carried out in Escherichia coli DH5α according to the method of Sambrook et al. Recombinant-protein expression was performed in E. coli BL21 Star (DE3) (Invitrogen). Luria-Bertani broth was used for all E. coli cultures, and when required, antibiotics were added to the culture at the following concentrations: kanamycin, 50 μg/ml; carbenicillin, 50 μg/ml; chloramphenicol, 34 μg/ml.
Chemicals and reagents.
[14C]sodium bicarbonate (50 Ci · mol−1) was purchased from American Radiolabeled Chemicals, Inc. All other chemicals were purchased from Sigma and Fisher Scientific. All media were purchased from Difco. Nucleotide primers were synthesized by Integrated DNA Technologies, Inc.
qPCR analysis.
Total RNA (5 μg) was treated with 2.5 units of DNase I (Ambion) according to the manufacturer's instructions. First-strand cDNA was synthesized using SuperScript III and 6 μg of random primers (Invitrogen). The cDNA was purified with a QIAquick PCR purification kit (QIAGEN). The primers used are listed in Table 1. The primers corresponding to each ORF were designed to amplify less than 100 bp of product. Quantitative real-time PCR (qPCR) was performed in triplicate with three different concentrations of the diluted cDNAs (0.5 ng, 1 ng, and 5 ng) and 25 μl of iQ SyBr Green Supermix (2× Premixture; Bio-Rad) in a total volume of 50 μl. The qPCR was carried out at 95°C for 20 s and 60°C for 20 s (40 cycles). Calculated thresholds were determined using the maximum-curvature approach after determination of per-well baseline cycles (iCycler; Bio-Rad). The amplified DNA samples were further subjected to melting-curve analysis and 1.2% agarose gel electrophoresis to verify the amplification product. Relative quantities of target ORF transcripts were calculated using the cycle threshold (CT) values of the housekeeping gene, sigA (Rv2703), as an internal standard as described previously (12, 23).
TABLE 1.
qPCR primers used in this study
| Gene (ORF no.) | Primer sequencea | Product size (bp) | Reference |
|---|---|---|---|
| accA1 (Rv2501c) | F: 5′-GCACGGTGGTCGGCAGTGATTA-3′ | 54 | This study |
| R: 5′-CGTGCGCAATCACCTTGGTGA-3′ | |||
| accA2 (Rv0973c) | F: 5′-TCGCACACGGCTTCACGGTT-3′ | 86 | This study |
| R: 5′-TGACAGTGCCACCAGGTGAACG-3′ | |||
| accA3 (Rv3285) | F: 5′-CTGGATCGAGACCGAGTGGAATA-3′ | 57 | This study |
| R: 5′-AGAGGTTCGCCGTCGGTAAA-3′ | |||
| accD1 (Rv2502c) | F: 5′-AAGGGAGCGCATTTCATCGAGC-3′ | 59 | This study |
| R: 5′-TGCAGGAACAGCAGCGGAATC-3′ | |||
| accD2 (Rv0974c) | F: 5′-TTCTTACACAACACCACCGGCTACA-3′ | 67 | This study |
| R: 5′-CATGCTTGATCATCCCGCCTT-3′ | |||
| accD3 (Rv0904c) | F: 5′-CGCGATCGTGTTCCGAGACA-3′ | 92 | This study |
| R: 5′-CGACAATCCCCGACTTCAGTAGGT-3′ | |||
| accD4 (Rv3799c) | F: 5′-CCGGAAGAGCTTCGTCGAGAATT-3′ | 85 | This study |
| R: 5′-GATGACGGCATCGATGAACCC-3′ | |||
| accD5 (Rv3280) | F: 5′-CGGCTCCTCGACGACGAATT-3′ | 67 | This study |
| R: 5′-CGAACCCCACCACGATGTTTT-3′ | |||
| accD6 (Rv2247) | F: 5′-TTCGACGAGTTCCAGGCCAATT-3′ | 95 | This study |
| R: 5′-AGCGGGTTGTTGGCCAGTACAC-3′ | |||
| accE (Rv3281) | F: 5′-AGCCCCACATCGAGATACTGCG-3′ | 76 | This study |
| R: 5′-ATACTGCCCAGCACCGCGAT-3′ | |||
| sigA (Rv2703) | F: 5′-CCGATGACGACGAGGAGATC-3′ | 51 | 12 |
| R: 5′-CGGAGGCCTTGTCCTTTTC-3′ | 51 |
F, forward primer; R, reverse primer.
Microarray hybridization.
The Institute for Genomic Research (Pathogen Functional Genomics Resource Center [http://www.tigr.org]) provided the Mycobacterium tuberculosis genome microarray for this study. The genome microarray consisted of 70-mer oligonucleotides representing 4,127 ORFs from the M. tuberculosis reference strain H37Rv and 623 unique ORFs from strain CDC1551 that are not present in the H37Rv strain's annotated gene complement (98% of H37Rv ORFs). The full 70-mer complement was printed four times on the surface of the microarray. Total M. tuberculosis RNA was isolated using a TRIzol (Invitrogen) extraction and RNeasy (QIAGEN) purification as described previously (32). Two-color (Cy3 and Cy5) hybridization was used for the microarray analysis. Generally, RNA extracted from cells growing exponentially at an optical density (A600) of approximately 0.5 in Middlebrook 7H9 medium (pH 7.0) was used to create fluorescent Cy3-labeled reference cDNA for each experiment (mid-log phase). The reference Cy3-labeled cDNA was hybridized together with the Cy5-labeled cDNA synthesized from RNA extracted from cells when the optical density was approximately doubled (late-log phase). Labeled cDNA was prepared as follows. Ten micrograms of total RNA and 2 μg of random oligonucleotide hexamers (Invitrogen, CA) were incubated for 15 min at 65°C, cooled on ice, combined with Stratascript reverse transcriptase buffer, 0.5 mM dATP, 0.5 mM dGTP, 0.5 mM dCTP, 0.02 mM dUTP, 1.5 nmol aminoallyl-dUTP (Amersham, NJ), and 2 μl Stratascript reverse transcriptase II (Invitrogen, CA) in a total volume of 30 μl. This mixture was incubated for 10 min at room temperature and at 42°C overnight. Synthesized cDNA was purified with a QIAquick PCR purification kit (QIAGEN, CA) according to the manufacturer's recommendations. The aminoallyl-dUTP-conjugated cDNA probes were spin dried (SpeedVac; Savant) and resuspended in 50 mM sodium carbonate buffer (pH 9.3) and cyanine monofunctional dyes (Amersham) for 1 h at room temperature, followed by quenching with 4 M hydroxylamine. The cyanine-labeled probes were purified again with a QIAquick PCR purification kit. All hybridizations were performed with dye reversal replicates. QuantArray (version 3.0; Perkin-Elmer) was used for 16-bit TIFF image quantification and initial data visualization. The hybridization signal was subjected to normalization and clustering by using an open-source R (version 2.1.1) package (http://www.bioconductor.org/).
Recombinant-protein expression and preparation of cell extracts.
The ORF for β6 (accD6; Rv2247) was cloned by PCR using Pfu Turbo HotStart DNA polymerase (Stratagene) from the genomic DNA of M. tuberculosis H37Rv. The expression construct was prepared in pET 200 directional-TOPO expression vector (Invitrogen), and sequence integrity was confirmed by DNA sequencing. BL21 Star (DE3) host cells were transformed with the expression construct, and the overnight culture was diluted 1:50 in fresh medium. IPTG (isopropyl-β-d-thiogalactopyranoside) was added to a final concentration of 1 mM when the culture reached an optical density at 600 nm (OD600) of 0.7, and the induction was carried out for 4 h in a 37°C shaker. The α3 subunit was expressed and biotinylated with the E. coli biotin ligase as described previously (22). Following induction, the cells were washed and resuspended in lysis buffer (50 mM sodium phosphate, 300 mM NaCl, 1 mM phenylmethylsulfonyl fluoride). The cells were disrupted by sonication using a Branson Sonifier 450 (Branson Ultrasonics Corp.). The cell lysates were clarified by centrifugation at 12,000 × g, and the supernatants were used for purification of the expressed proteins. The β5 (Rv3280) and ɛ (Rv3281) subunits were expressed in E. coli as described previously (22).
Purification, SDS-PAGE analysis, and dialysis of expressed proteins.
The clear supernatants obtained from the above-mentioned lysates were applied to TALON cobalt affinity resin (BD Biosciences), and the His-tagged β6 and α3 (after biotinylation) were purified by the batch/gravity procedure of the manufacturer with the following modifications: the bound proteins were washed with 10 mM imidazole and eluted from the affinity resin with 100 mM imidazole and 500 imidazole elution steps. The eluted fractions were analyzed by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and fractions containing pure protein were pooled and dialyzed against 100 mM potassium phosphate (pH 8.0) containing 10% glycerol at 4°C. The β5 and ɛ subunits were purified as described previously (22).
Reconstitution of the carboxylase complex.
Following dialysis, the purified α3 subunit (100 μg/ml) was mixed with the purified β6 subunit (100 μg/ml), and the purified α3, β6, and ɛ subunits were mixed together in a 1:1:2 molar ratio and incubated on ice for 6 h. Following this, the solutions were concentrated four- to sixfold by ultrafiltration in Centriprep YM-10 filters (Millipore) at 700 × g for 4 h at 4°C and used in the carboxylase assay.
Acyl-CoA carboxylase assay.
The carboxylase activity of the reconstituted α3-β6 complex was measured by following the incorporation of the radiolabel from NaH14CO3 into acid-stable reaction product using a modified procedure of Hunaiti and Kolattukudy (15). The reaction was carried out in a 100-μl volume containing 100 mM potassium phosphate, pH 8.0, 300 μg bovine serum albumin, 3 mM ATP, 5 mM acetyl-CoA or propionyl-CoA, 5 mM NaH14CO3, 5 mM MgCl2, and 20 to 30 μg of reconstituted enzyme. Following incubation at 30°C for 2 h, the reaction was stopped with 150 μl of 6 M HCl, and the entire solution was evaporated to dryness in a heating block at 100°C. The residue was resuspended in 100 μl water, and the radioactivity was measured by liquid scintillation counting. The effects of inhibitors were determined by preincubating the reconstituted α3-β6 complex with the inhibitor for 10 min at 24°C, after which the percent inhibition above the respective solvent control was determined. The kinetic parameters for the α3-β6 carboxylase were calculated using nonlinear regression analysis with the Michaelis-Menten equation, and the 50% inhibitory concentrations (IC50s) were determined from the sigmoidal dose-response equation (GraphPad Prism version 3.02; GraphPad Software).
Microarray accession number.
The data discussed in this publication have been deposited in NCBI's Gene Expression Omnibus (GEO [http://www.ncbi.nlm.nih.gov/geo/]) and are accessible through GEO Series accession number GSE5977.
RESULTS
Analysis of carboxylase subunit transcript levels.
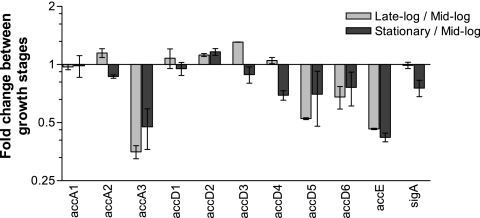
To determine which of the multiple carboxylase subunit genes are actually expressed in M. tuberculosis, we analyzed the expression levels of all of the ORFs encoding the carboxylase subunits by qPCR and microarray analyses. RNA isolated from cells at exponential growth phase was subjected to qPCR, and each CT value was normalized to the level of the internal control sigA qPCR CT value as described previously (12, 23). By comparison with the normalized CT values of sigA, we determined the relative levels of expression of the ACC subunits during exponential growth of M. tuberculosis. The results showed that the levels of expression of the β4, β5, β6, and ɛ subunits were highest among the carboxylase subunit ORFs when the mycobacterial cells were in exponential growth phase (Fig. 1). α3 was the most highly expressed gene among the acyl-CoA carboxylase α-subunit ORFs. To monitor gene expression changes during cell growth, independent batches of cells were subjected to microarray analysis. Comparison of transcription profiles obtained at various growth stages indicated that the α3, β4, β5, β6, and ɛ ORFs were the most significantly repressed subunits among all the acyl-CoA carboxylase ORFs as the mycobacterial cells transitioned from mid-log to late-log and stationary phases (Fig. 2), while the levels of transcripts for other ACC subunits did not change much.
FIG. 1.
Quantitative real-time PCR analysis of acyl-CoA carboxylase gene expression in exponentially growing cultures of M. tuberculosis. Total RNA was prepared from mid-log-phase cultures of M. tuberculosis H37Rv (OD600 = 0.5). The transcript level of each acyl-CoA carboxylase subunit is indicated relative to the expression level of sigA as an internal control. Values represented are means and standard errors.
FIG. 2.
Expression levels of carboxylase subunits during normal M. tuberculosis growth. Wild-type M. tuberculosis was grown in 7H9 complete medium (pH 7.0; 37°C) under normal aeration in a rolling culture tube. The cells were harvested at OD600s of 0.53 (after 6 days of inoculation; “Mid-log”), 1.17 (after 8 days; “Late-log”), and 1.42 (after 12 days; “Stationary”). The total RNA was isolated and hybridized on DNA oligonucleotide microarrays as described in Materials and Methods. The hybridization signals were subjected to scale print tip median absolute deviation and quantile normalization (34). The relative expression level change for each acyl-CoA carboxylase subunit was obtained by comparing mid-log growth phase as a reference signal at late-log and stationary growth phases. The error bars indicate standard deviations.
Purification of α3 and β6 subunits.
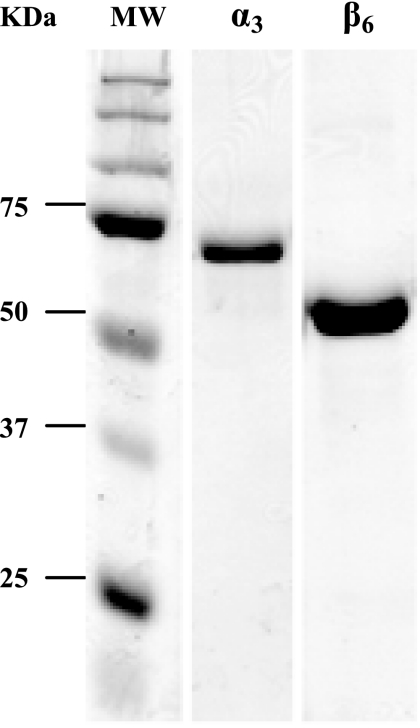
Although the β6 (accD6) ORF is located within a cluster of genes encoding FAS II components (Fig. 3) which have been shown to be involved in mycolic acid biosynthesis (28), the enzymatic activity of β6 has never been investigated. Since β6 was reported to be essential for the viability of M. tuberculosis, along with α3, β4, and ɛ subunits (26), and there is no α subunit in the genetic neighborhood of β6, we deduced that the α3-β6 complex might constitute a functional acyl-CoA carboxylase. To investigate the carboxyltransferase activity of β6, we expressed β6 and α3 as His-tagged fusion proteins in E. coli and purified them by cobalt affinity chromatography. The α3 subunit was biotinylated using the E. coli biotin ligase prior to purification as described previously (22). The fractions eluted from the TALON resin were analyzed on SDS-PAGE (Fig. 4), and the fractions containing pure protein were used for reconstitution of the acyl-CoA carboxylase. The ɛ and β5 subunits were purified as described previously (22).
FIG. 3.
Genetic locus of AccD6 (β6) in pathogenic mycobacteria. The gene numbers and the amino acid identities of the respective AccD6 subunits are indicated. AccA3, acetyl/propionyl-CoA carboxylase (α3 subunit); AccD6, acetyl/propionyl-CoA carboxylase (β6 subunit); FabD, malonyl-ACP transacylase.
FIG. 4.
Purification of M. tuberculosis α3 and β6 subunits expressed in E. coli. Fractions eluted from the cobalt affinity resin were analyzed by 10% SDS-PAGE, followed by Coomassie staining. Representative fractions containing pure subunits used in assays are shown. Prior to purification, α3 was biotinylated using E. coli biotin ligase. MW, molecular weight standards.
Reconstitution and characterization of the enzymatic activity of α3-β6 carboxylase.
Following dialysis, the α3 and β6 subunits were mixed together in a 1:1 molar ratio, and the reconstituted α3-β6 complex was assayed for acyl-CoA carboxylase activity. The α3-β6 complex carboxylated acetyl-CoA with high specific activity (1.1 nmol/mg/min) and propionyl-CoA at lower levels (0.36 nmol/mg/min). The activity increased linearly with time and protein concentration (Fig. 5A and B) and displayed typical Michaelis-Menten kinetics for bicarbonate (Fig. 5C), acetyl-CoA and propionyl-CoA (Fig. 5D), and ATP (Fig. 5E). Under the assay conditions, saturation was reached with 2 mM bicarbonate, 5 mM acetyl-CoA or 2.5 mM propionyl-CoA, and 0.5 mM ATP. The optimal MgCl2 concentration was 10 mM for acetyl-CoA carboxylase (ACC) activity and 5 mM for propionyl-CoA carboxylase (PCC) activity. The kinetic parameters for the α3-β6 complex are tabulated in Table 2.
FIG. 5.
Kinetic characterization of the reconstituted α3-β6 acyl-CoA carboxylase of M. tuberculosis. The time course (A) and protein dependence (B) of ACC (▪) and PCC (□) activities are shown. The dependence of carboxylase activity on the concentrations of bicarbonate (C), acyl-CoA (D), ATP (E), and MgCl2 (F) was determined by measuring acid-stable radioactivity following carboxylation with NaH14CO3. Values represented are means and standard errors.
TABLE 2.
Kinetic parameters for the acyl-CoA carboxylase α3-β6 of M. tuberculosisa
| Substrate | ACC
|
PCC
|
||||
|---|---|---|---|---|---|---|
| Vmax | Km | SC | Vmax | Km | SC | |
| Acetyl-CoA | 1.1 | 4.9 | 0.22 | NA | NA | NA |
| Propionyl-CoA | NA | NA | NA | 0.36 | 0.97 | 0.37 |
| NaHCO3 | 0.61 | 0.68 | 0.9 | 0.43 | 0.66 | 0.65 |
| ATP | 0.67 | 0.12 | 5.6 | 0.34 | 0.06 | 5.67 |
Vmax, nmol/mg/min; Km, mM; SC, specificity constant (Vmax/Km); NA, not applicable.
Effects of inhibitors on α3-β6.
The carboxylase activities of α3-β6 were inhibited by avidin and several other inhibitors. As shown in Table 3, at 100 μM, dimethyl itaconate and C75 inhibited the ACC activity of α3-β6 by 96% and 87%, respectively, and the PCC activity by 53% and 54%, respectively. Haloxyfop, cerulenin, and 1,2-cyclohexanedione inhibited the enzymatic activities significantly. The dose dependence of inhibition by dimethyl itaconate and C75 of the ACC and PCC activities of α3-β6 was investigated, and the results are shown in Fig. 6. From this sigmoidal dose-response analysis, the IC50s for dimethyl itaconate were calculated to be 8.2 μM for ACC and 10.8 μM for PCC. For C75, the IC50s were 50.2 μM for ACC and 101.5 μM for PCC.
TABLE 3.
Effects of inhibitors on the carboxylase activities of the reconstituted M. tuberculosis α3-β6 complex
| Inhibitor | % Inhibitiona
|
|
|---|---|---|
| ACC | PCC | |
| Avidin | 93 ± 0.1 | 94 ± 0.7 |
| Dimethyl itaconate | 96 ± 5 | 53 ± 4 |
| C75 | 87 ± 16 | 54 ± 12 |
| Haloxyfop | 60 ± 4 | 53 ± 8 |
| Cerulenin | 54 ± 20 | 31 ± 10 |
| 1,2-Cyclohexanedione | 40 ± 23 | 45 ± 11 |
| Diclofop | 13 ± 3 | 9 ± 6 |
| Alloxydim | 11 ± 3 | 20 ± 9 |
| 5-(Tetradecyloxy)-2-furoic acid | 8 ± 0.7 | 0 |
| 5-Iodotubericidin | 0 | 28 ± 5 |
The α3-β6 complex was preincubated with 1 unit avidin (binds 1 μg d-biotin) or 100 μM inhibitor and used in the assay. Percent inhibition above the respective solvent control is given as mean ± standard deviation from three independent assays of the carboxylase with inhibitor.
FIG. 6.
Inhibition of acyl-CoA carboxylase activities of α3-β6 by dimethyl itaconate and C75. Inhibitions by dimethyl itaconate of ACC (▪) and PCC (□) activities (A) and by C75 of ACC (▴) and PCC (▵) activities (B) were measured after preincubation of the respective inhibitor at the indicated concentrations with reconstituted α3-β6 for 10 min at 24°C prior to the carboxylase assay. Percent inhibition above the solvent (dimethyl sulfoxide) control was plotted as the mean with standard deviation.
Effect of the epsilon subunit on the α3-β6 complex and stoichiometry of the α3-β6 carboxylase.
We examined the effect of the epsilon (ɛ) subunit on the activity of the α3-β6 complex. In our previous report on the effect of the ɛ subunit on the carboxylase activities of the α3-β5 complex, we determined that the optimal molar ratio of α3 to β5 to ɛ was 1:1:2 (22). Therefore, we incubated the α3, β6, and ɛ subunits together at the same molar ratio of 1:1:2 prior to assay. As shown in Fig. 7, the ɛ subunit inhibited both the ACC and PCC activities of the α3-β6 complex. However, the ɛ subunit stimulated the ACC and PCC activities of the α3-β5 complex, as reported previously (reference 22 and data not shown). We analyzed the stoichiometry of the reconstituted α3-β6 carboxylase by gel filtration chromatography on Sepharose CL-6B (a 1,100-mm by 5-mm column equilibrated with 50 mM potassium phosphate buffer, pH 7.0, and 50 mM NaCl). The reconstituted α3-β6 complex eluted in a peak with the apparent molecular mass of a dodecamer comprising six molecules each of the α3 and β6 subunits (data not shown).
FIG. 7.
Effect of the epsilon subunit on the α3-β6 carboxylase. The purified α3 and β6 subunits were preincubated together in a 1:1 molar ratio, and the α3, β6, and ɛ subunits were preincubated together in a 1:1:2 molar ratio on ice for 6 h prior to a carboxylation assay with acetyl-CoA or propionyl-CoA. Values represented are means and standard errors.
DISCUSSION
Mycolic acid biosynthesis in M. tuberculosis involves FAS I, which carries out the de novo synthesis of C16-C26 fatty acids (16), and the FAS II multisubunit complex, which is incapable of de novo fatty-acid biosynthesis but elongates C14 and C16 primers to produce meromycolates (28). The FAS II complex in M. tuberculosis is located in a genetic locus that also contains a lone, uncharacterized acyl-CoA carboxylase carboxyltransferase subunit, accD6 (β6) (6), which could provide malonyl-CoA to the β-ketoacyl-ACP synthases and to FAS I. Malonyl-CoA is converted to malonyl-ACP by the malonyl-CoA-ACP transacylase (FabD) (19) and is utilized by the β-ketoacyl-ACP synthases (KasA/KasB) (5, 18, 27) in successive reactions with the other enzymes of the FAS II complex to produce the meromycolic acids. Protein-protein interaction analyses have shown that FabD is a structural component of the FAS II complex (30) and that KasA, KasB, InhA, MabA (FabG1), and FabH (β-ketoacyl-ACP synthase III) interact with each other (31).
The reconstituted α3-β6 acyl-CoA carboxylase, reported here, preferred acetyl-CoA over propionyl-CoA, in contrast to the α3-β5 complex, which preferred propionyl-CoA over acetyl-CoA (10, 22). This observation leads us to suggest that M. tuberculosis may utilize the β6 subunit with α3 to provide malonyl-CoA to FAS I and to the FAS II complex for de novo fatty-acid biosynthesis and mycolic acid biosynthesis, respectively, and the β5 subunit with α3 for providing methylmalonyl-CoA for branched-fatty-acid biosynthesis. The rate of acetyl-CoA carboxylation by the α3-β5 complex increased when the ɛ subunit was bound to it (10, 22), and thus, the α3-β5-ɛ complex could also provide malonyl-CoA for fatty-acid biosynthesis in mycobacteria. The α3 subunit also interacts with the β4 (AccD4) subunit, which is possibly involved in the carboxylation of fatty acids that may then be incorporated into mycolic acids (11, 22, 24).
Isoniazid was shown to inhibit the biosynthesis of mycolic acids (29), and the reductases involved in fatty-acid elongation isolated from Mycobacterium avium were shown to be selectively inhibited by the drug (17). More recent work has shown that the reductases are the targets of isoniazid inhibition in M. tuberculosis (1, 25). In isoniazid-treated M. tuberculosis cultures, the accD6 ORF was upregulated, along with the other FAS II complex members in its neighborhood, which have been shown to be involved in mycolic acid biosynthesis (33).
The β6 subunits of other pathogenic mycobacteria, Mycobacterium leprae and Mycobacterium bovis, show a high degree of identity (93% and 100%, respectively) with the M. tuberculosis β6 subunit (6, 7, 13). Since β6 is predicted to be an essential gene (26), it may not be possible to disrupt the gene to show a direct biochemical consequence. Our current and previous observations showed that the α3, β4, β5, and β6 ORFs are highly expressed in M. tuberculosis and that their products have catalytic activities (22). Interestingly, M. leprae, which has lost a significant portion of its genome, has retained only α3, β4, β5, and β6 as functional ORFs among the ACC-encoding ORFs in its genome (7).
Our microarray results indicated that the transcripts for the α3, β4, β5, β6, and ɛ subunits were repressed as mycobacterial cells entered late log phase, when they possibly begin to encounter unfavorable growth conditions, like nutrient starvation, suggesting that these carboxylase subunits may be less important for the mycobacterium during the late-log and stationary growth phases. The inhibition of the carboxylase activities of the α3-β6 complex when reconstituted with the ɛ subunit indicated that the ɛ subunit hindered interaction between the α3 and β6 subunits. Analysis of the stoichiometry of the α3-β6 complex indicated that it is reconstituted as a dodecamer containing six molecules each of the α3 and β6 subunits, similar to the α3-β5 complex, which was reported to exist as a dodecamer (10).
Inhibitors that were previously shown to target a particular enzyme have subsequently been demonstrated to inhibit novel targets. C75 is a synthetic inhibitor of fatty acid synthase and is a potential antiobesity and antitumor drug (20). C75-CoA was recently shown to inhibit carnitine palmitoyl transferase I in a novel mode of action (3). In another example, itaconate, which is a potent inhibitor of isocitrate lyase, was shown to inhibit propionyl-CoA carboxylase in cell extracts of Rhodospirillum rubrum (4). Isocitrate lyase is a glyoxylate shunt pathway enzyme that is used by M. tuberculosis to utilize fatty acids as a carbon source (21). Therefore, we investigated the effects of several inhibitors that have been shown to inhibit fatty-acid metabolism but had not been shown to inhibit ACC activity. Interestingly, we found that C75 and dimethyl itaconate severely inhibited the carboxylase activities of α3-β6 in our assays (Fig. 6). Further work on the mechanism of action of these inhibitors is needed, and the characterization of such inhibitors, which target multiple biochemical pathways, may lead to the identification of potent drug candidates against M. tuberculosis.
Acknowledgments
This work was supported by grants AI46582 and AI35272 from the National Institutes of Health.
Footnotes
Published ahead of print on 17 November 2006.
REFERENCES
- 1.Banerjee, A., E. Dubnau, A. Quemard, V. Balasubramanian, K. S. Um, T. Wilson, D. Collins, G. de Lisle, and W. R. Jacobs, Jr. 1994. InhA, a gene encoding a target for isoniazid and ethionamide in Mycobacterium tuberculosis. Science 263:227-230. [DOI] [PubMed] [Google Scholar]
- 2.Bates, I., C. Fenton, J. Gruber, D. Lalloo, A. Medina Lara, S. B. Squire, S. Theobald, R. Thomson, and R. Tolhurst. 2004. Vulnerability to malaria, tuberculosis, and HIV/AIDS infection and disease. Part 1: determinants operating at individual and household level. Lancet Infect. Dis. 4:267-277. [DOI] [PubMed] [Google Scholar]
- 3.Bentebibel, A., D. Sebastian, L. Herrero, E. Lopez-Vinas, D. Serra, G. Asins, P. Gomez-Puertas, and F. G. Hegardt. 2006. Novel effect of C75 on carnitine palmitoyltransferase I activity and palmitate oxidation. Biochemistry 45:4339-4350. [DOI] [PubMed] [Google Scholar]
- 4.Berg, I. A., L. V. Filatova, and R. N. Ivanovsky. 2002. Inhibition of acetate and propionate assimilation by itaconate via propionyl-CoA carboxylase in isocitrate lyase-negative purple bacterium Rhodospirillum rubrum. FEMS Microbiol. Lett. 216:49-54. [DOI] [PubMed] [Google Scholar]
- 5.Bhatt, A., L. Kremer, A. Z. Dai, J. C. Sacchettini, and W. R. Jacobs, Jr. 2005. Conditional depletion of KasA, a key enzyme of mycolic acid biosynthesis, leads to mycobacterial cell lysis. J. Bacteriol. 187:7596-7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry III, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, A. Krogh, J. McLean, S. Moule, L. Murphy, K. Oliver, J. Osborne, M. A. Quail, M. A. Rajandream, J. Rogers, S. Rutter, K. Seeger, J. Skelton, R. Squares, S. Squares, J. E. Sulston, K. Taylor, S. Whitehead, and B. G. Barrell. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. (Erratum, 396:190.) [DOI] [PubMed] [Google Scholar]
- 7.Cole, S. T., K. Eiglmeier, J. Parkhill, K. D. James, N. R. Thomson, P. R. Wheeler, N. Honore, T. Garnier, C. Churcher, D. Harris, K. Mungall, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. M. Davies, K. Devlin, S. Duthoy, T. Feltwell, A. Fraser, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, C. Lacroix, J. Maclean, S. Moule, L. Murphy, K. Oliver, M. A. Quail, M. A. Rajandream, K. M. Rutherford, S. Rutter, K. Seeger, S. Simon, M. Simmonds, J. Skelton, R. Squares, S. Squares, K. Stevens, K. Taylor, S. Whitehead, J. R. Woodward, and B. G. Barrell. 2001. Massive gene decay in the leprosy bacillus. Nature 409:1007-1011. [DOI] [PubMed] [Google Scholar]
- 8.Cronan, J. E. Jr., and G. L. Waldrop. 2002. Multi-subunit acetyl-CoA carboxylases. Prog. Lipid Res. 41:407-435. [DOI] [PubMed] [Google Scholar]
- 9.Dye, C., B. G. Williams, M. A. Espinal, and M. C. Raviglione. 2002. Erasing the world's slow stain: strategies to beat multidrug-resistant tuberculosis. Science 295:2042-2046. [DOI] [PubMed] [Google Scholar]
- 10.Gago, G., D. Kurth, L. Diacovich, S. C. Tsai, and H. Gramajo. 2006. Biochemical and structural characterization of an essential acyl coenzyme A carboxylase from Mycobacterium tuberculosis. J. Bacteriol. 188:477-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gande, R., J. K. Gibson, A. K. Brown, K. Krumbach, L. G. Dover, H. Sahm, S. Shioyama, T. Oikawa, G. S. Besra, and L. Eggeling. 2004. Acyl-CoA carboxylases (accD2 and accD3), together with a unique polyketide synthase (Cg-pks), are key to mycolic acid biosynthesis in Corynebacterianeae such as Corynebacterium glutamicum and Mycobacterium tuberculosis. J. Biol. Chem. 279:44847-44857. [DOI] [PubMed] [Google Scholar]
- 12.Gao, Q., K. Kripke, Z. Arinc, M. Voskuil, and P. Small. 2004. Comparative expression studies of a complex phenotype: cord formation in Mycobacterium tuberculosis. Tuberculosis 84:188-196. [DOI] [PubMed] [Google Scholar]
- 13.Garnier, T., K. Eiglmeier, J. C. Camus, N. Medina, H. Mansoor, M. Pryor, S. Duthoy, S. Grondin, C. Lacroix, C. Monsempe, S. Simon, B. Harris, R. Atkin, J. Doggett, R. Mayes, L. Keating, P. R. Wheeler, J. Parkhill, B. G. Barrell, S. T. Cole, S. V. Gordon, and R. G. Hewinson. 2003. The complete genome sequence of Mycobacterium bovis. Proc. Natl. Acad. Sci. USA 100:7877-7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong, X., and A. J. Hopfinger. 2004. Molecular modeling and simulation of Mycobacterium tuberculosis cell wall permeability. Biomacromolecules 5:1066-1077. [DOI] [PubMed] [Google Scholar]
- 15.Hunaiti, A. R., and P. E. Kolattukudy. 1982. Isolation and characterization of an acyl-coenzyme A carboxylase from an erythromycin-producing Streptomyces erythreus. Arch. Biochem. Biophys. 216:362-371. [DOI] [PubMed] [Google Scholar]
- 16.Kikuchi, S., D. L. Rainwater, and P. E. Kolattukudy. 1992. Purification and characterization of an unusually large fatty acid synthase from Mycobacterium tuberculosis var. bovis BCG. Arch. Biochem. Biophys. 295:318-326. [DOI] [PubMed] [Google Scholar]
- 17.Kikuchi, S., T. Takeuchi, M. Yasui, T. Kusaka, and P. E. Kolattukudy. 1989. A very long-chain fatty acid elongation system in Mycobacterium avium and a possible mode of action of isoniazid on the system. Agric. Biol. Chem. 53:1689-1698. [Google Scholar]
- 18.Kremer, L., L. G. Dover, S. Carrere, K. M. Nampoothiri, S. Lesjean, A. K. Brown, P. J. Brennan, D. E. Minnikin, C. Locht, and G. S. Besra. 2002. Mycolic acid biosynthesis and enzymic characterization of the beta-ketoacyl-ACP synthase A-condensing enzyme from Mycobacterium tuberculosis. Biochem. J. 364:423-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kremer, L., K. M. Nampoothiri, S. Lesjean, L. G. Dover, S. Graham, J. Betts, P. J. Brennan, D. E. Minnikin, C. Locht, and G. S. Besra. 2001. Biochemical characterization of acyl carrier protein (AcpM) and malonyl-CoA:AcpM transacylase (mtFabD), two major components of Mycobacterium tuberculosis fatty acid synthase II. J. Biol. Chem. 276:27967-27974. [DOI] [PubMed] [Google Scholar]
- 20.Kuhajda, F. P., E. S. Pizer, J. N. Li, N. S. Mani, G. L. Frehywot, and C. A. Townsend. 2000. Synthesis and antitumor activity of an inhibitor of fatty acid synthase. Proc. Natl. Acad. Sci. USA 97:3450-3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munoz-Elias, E. J., and J. D. McKinney. 2005. Mycobacterium tuberculosis isocitrate lyases 1 and 2 are jointly required for in vivo growth and virulence. Nat. Med. 11:638-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oh, T. J., J. Daniel, H. J. Kim, T. D. Sirakova, and P. E. Kolattukudy. 2006. Identification and characterization of Rv3281 as a novel subunit of a biotin-dependent acyl-CoA carboxylase in Mycobacterium tuberculosis H37Rv. J. Biol. Chem. 281:3899-3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfaffl, M. W. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Portevin, D., C. de Sousa-D'Auria, H. Montrozier, C. Houssin, A. Stella, M. A. Laneelle, F. Bardou, C. Guilhot, and M. Daffe. 2005. The acyl-AMP ligase FadD32 and AccD4-containing acyl-CoA carboxylase are required for the synthesis of mycolic acids and essential for mycobacterial growth: identification of the carboxylation product and determination of the acyl-CoA carboxylase components. J. Biol. Chem. 280:8862-8874. [DOI] [PubMed] [Google Scholar]
- 25.Quemard, A., J. C. Sacchettini, A. Dessen, C. Vilcheze, R. Bittman, W. R. Jacobs, Jr., and J. S. Blanchard. 1995. Enzymatic characterization of the target for isoniazid in Mycobacterium tuberculosis. Biochemistry 34:8235-8241. [DOI] [PubMed] [Google Scholar]
- 26.Sassetti, C. M., D. H. Boyd, and E. J. Rubin. 2003. Genes required for mycobacterial growth defined by high density mutagenesis. Mol. Microbiol. 48:77-84. [DOI] [PubMed] [Google Scholar]
- 27.Schaeffer, M. L., G. Agnihotri, C. Volker, H. Kallender, P. J. Brennan, and J. T. Lonsdale. 2001. Purification and biochemical characterization of the Mycobacterium tuberculosis beta-ketoacyl-acyl carrier protein synthases KasA and KasB. J. Biol. Chem. 276:47029-47037. [DOI] [PubMed] [Google Scholar]
- 28.Takayama, K., C. Wang, and G. S. Besra. 2005. Pathway to synthesis and processing of mycolic acids in Mycobacterium tuberculosis. Clin. Microbiol. Rev. 18:81-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takayama, K., L. Wang, and H. L. David. 1972. Effect of isoniazid on the in vivo mycolic acid synthesis, cell growth, and viability of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2:29-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Veyron-Churlet, R., S. Bigot, O. Guerrini, S. Verdoux, W. Malaga, M. Daffe, and D. Zerbib. 2005. The biosynthesis of mycolic acids in Mycobacterium tuberculosis relies on multiple specialized elongation complexes interconnected by specific protein-protein interactions. J. Mol. Biol. 353:847-858. [DOI] [PubMed] [Google Scholar]
- 31.Veyron-Churlet, R., O. Guerrini, L. Mourey, M. Daffe, and D. Zerbib. 2004. Protein-protein interactions within the Fatty Acid Synthase-II system of Mycobacterium tuberculosis are essential for mycobacterial viability. Mol. Microbiol. 54:1161-1172. [DOI] [PubMed] [Google Scholar]
- 32.Voskuil, M. I., K. C. Visconti, and G. K. Schoolnik. 2004. Mycobacterium tuberculosis gene expression during adaptation to stationary phase and low-oxygen dormancy. Tuberculosis 84:218-227. [DOI] [PubMed] [Google Scholar]
- 33.Wilson, M., J. DeRisi, H. H. Kristensen, P. Imboden, S. Rane, P. O. Brown, and G. K. Schoolnik. 1999. Exploring drug-induced alterations in gene expression in Mycobacterium tuberculosis by microarray hybridization. Proc. Natl. Acad. Sci. USA 96:12833-12838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang, Y. H., S. Dudoit, P. Luu, D. M. Lin, V. Peng, J. Ngai, and T. P. Speed. 2002. Normalization for cDNA microarray data: a robust composite method addressing single and multiple slide systematic variation. Nucleic Acids Res. 30:e15. [DOI] [PMC free article] [PubMed] [Google Scholar]