Abstract
Bacillus subtilis trpG encodes a glutamine amidotransferase subunit that participates in the biosynthesis of both tryptophan and folic acid. TRAP inhibits translation of trpG in response to tryptophan by binding to a site that overlaps the trpG Shine-Dalgarno sequence, thereby blocking ribosome binding. Similar mechanisms regulate trpP and ycbK translation. The equilibrium binding constants of tryptophan-activated TRAP for the trpG, ycbK, and trpP transcripts were determined to be 8, 3, and 50 nM, respectively. Despite TRAP having a higher affinity for the trpG transcript, TRAP exhibited the least control of trpG expression. The trpG Shine-Dalgarno sequence overlaps the stop codon of the upstream pabB gene, while six of nine triplet repeats within the TRAP binding site are located upstream of the pabB stop codon. Thus, ribosomes translating the upstream pabB cistron could be capable of reducing TRAP-dependent control of TrpG synthesis by displacing bound TRAP. Expression studies using pabB-trpG′-′lacZ fusions in the presence or absence of an engineered stop codon within pabB suggest that translation-mediated displacement of bound TRAP reduces TRAP-dependent inhibition of TrpG synthesis from transcripts originating from the folate operon promoter (PpabB). A new trpG promoter (PtrpG) was identified in the pabB coding sequence that makes a larger contribution to trpG expression than does PpabB. We found that TRAP-dependent regulation of trpG expression is more extensive for a transcript originating from PtrpG and that transcripts originating from PtrpG are not subject to translation-mediated displacement of bound TRAP.
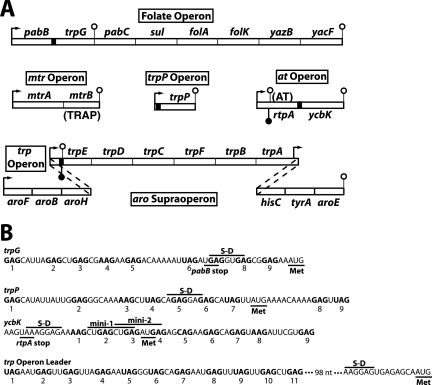
The trpEDCFBA operon of Bacillus subtilis carries six of the seven tryptophan biosynthetic genes and is contained within a larger aromatic amino acid supraoperon (Fig. 1A). The seventh tryptophan biosynthetic gene, trpG (pabA), is present in a folic acid biosynthesis operon. Expression of the tryptophan biosynthetic genes is regulated in response to tryptophan by trp RNA-binding attenuation protein (TRAP) via transcription attenuation and translation control mechanisms (reviewed in references 3 and 13). TRAP also regulates translation of trpP, a gene that encodes an apparent tryptophan transporter (22, 29), and ycbK, a gene that shares sequence homology to known efflux protein genes (23, 28). TRAP consists of 11 identical subunits arranged in a single ring (2). When activated by tryptophan, 11 KKR motifs on the perimeter of TRAP interact with multiple NAG trinucleotide repeats in target transcripts, with a preference for N of G ≈ U > A > C, thereby wrapping the RNA around TRAP's perimeter (1, 6, 32). Optimal spacing between triplet repeats is 2 nucleotides (nt) (5, 7), although 1-nt spacers and spacers as large as 14 nt have been observed in natural TRAP targets (Fig. 1B).
FIG. 1.
Organization of B. subtilis tryptophan metabolism genes and sequence comparison of the four known TRAP binding sites. (A) The six genes of the trp operon, which is part of the aromatic amino acid supraoperon, and trpG encode the tryptophan biosynthetic enzymes. trpP encodes a putative tryptophan transporter, while the ycbK gene product is similar to known efflux proteins. mtrA and the folate operon encode proteins involved in folic acid metabolism. mtrB encodes TRAP. TRAP regulates the expression of tryptophan metabolism genes by transcription attenuation (trpEDCFBA operon) and translation control (trpG, trpP, ycbK, and trpE) mechanisms. rtpA encodes anti-TRAP (AT), a protein that binds to and inactivates tryptophan-activated TRAP (25). Filled and open lollipop structures represent antiterminator and terminator structures, respectively. The four known TRAP binding sites are represented by black boxes, and bent arrows represent promoters. (B) The triplet repeats of the four known TRAP binding sites are shown in bold and are numbered. The S-D sequences and translation start codons (Met) are shown for trpG, trpP, ycbK, and trpE (first gene in the trp operon). There is a 98-nt gap in the sequence between the last triplet repeat in the trp operon leader and the trpE S-D sequence. The positions of the two dipeptide-encoding minigenes in the ycbK translation initiation region are marked. Expression of minigene 1 has a small inhibitory effect on translation of ycbK (28). The pabB and rtpA stop codons overlap the S-D sequences of trpG and ycbK, respectively.
The trpEDCFBA operon leader transcript is capable of folding into mutually exclusive antiterminator and terminator structures that participate in the transcription attenuation mechanism. When activated by tryptophan, TRAP can bind to 11 triplet repeats present in the nascent trp leader transcript (Fig. 1B). Bound TRAP prevents formation of the antiterminator structure because six of the triplet repeats are present within its stem. As a consequence, formation of the overlapping terminator hairpin causes RNA polymerase (RNAP) to terminate transcription in the leader region. In the absence of TRAP binding, formation of the antiterminator allows transcriptional readthrough into the trp operon structural genes (4, 6, 19). TRAP also regulates translation initiation of trpE (Fig. 1A). TRAP binding to trp operon readthrough transcripts promotes formation of a trpE Shine-Dalgarno (S-D) sequence-sequestering hairpin. Formation of this RNA structure inhibits translation initiation by preventing ribosome binding (11, 18).
A third TRAP-dependent regulatory mechanism is responsible for controlling translation initiation of trpG, ycbK, and trpP, with bound TRAP directly blocking ribosome binding (12, 18, 22, 23, 28, 29, 31). TrpG functions as a common glutamine amidotransferase subunit in the biosynthesis of both tryptophan and folic acid. TRAP binds to nine triplet repeats that overlap the trpG S-D sequence (Fig. 1B). The TRAP binding site in the trpP transcript also contains nine triplet repeats that overlap its S-D sequence (Fig. 1B). One distinction between trpP and trpG translation control is that the TRAP binding site in trpP mRNA extends into the trpP coding sequence, whereas the TRAP binding site in trpG ends just prior to the coding sequence. TRAP also inhibits translation initiation of ycbK by a similar mechanism; however, in this case, all nine triplet repeats are downstream from the S-D sequence and extend further into the ycbK coding sequence than is the case for trpP (Fig. 1B). In addition, expression of a dipeptide-encoding minigene that utilizes the ycbK S-D sequence has a small inhibitory effect on YcbK synthesis (Fig. 1B) (28).
In the present study, we compared the extents of TRAP-mediated translation inhibition of trpG, ycbK, and trpP. Our results led to the hypothesis that the pabB and trpG gene arrangement might contribute to the low level of TRAP-dependent regulation of trpG. Our data suggest that ribosomes that translate pabB are capable of displacing bound TRAP from the trpG S-D sequence. A new trpG promoter was also identified that is not subject to translation-mediated displacement of bound TRAP.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The B. subtilis strains used in this study are listed in Table 1. The cloning vectors pTZ18U and pTZ19R (U.S. Biochemical) contain a T7 RNA polymerase promoter upstream from their polylinkers. Plasmids pPB77, containing the B. subtilis trp operon leader (4), pPB31, containing trpG (6), and pHZB6, containing trpP (29), and the B. subtilis integration vector ptrpBGI-PLK (18) were described previously.
TABLE 1.
B. subtilis strains used in this study
| Strain | Genotypea | Reference |
|---|---|---|
| BG4233 | ΔmtrB | 14 |
| PLBS338 | Prototroph | 29 |
| PLBS339 | amyE::PtrpPtrpP′-′lacZ Cmr | 29 |
| PLBS341 | ΔmtrB amyE::PtrpPtrpP′-′lacZ Cmr | 29 |
| PLBS343 | rho::neo (Kmr) amyE::PtrpPtrpP′-′lacZ Cmr | 29 |
| PLBS418 | amyE::PatycbK′-′lacZ Cmr | 28 |
| PLBS420 | ΔmtrB amyE::PatycbK′-′lacZ Cmr | 28 |
| PLBS421 | rho::neo (Kmr) amyE::PatycbK′-′lacZ Cmr | 28 |
| PLBS442 | amyE::PpabB (−92 to +138 and +1336 to +1498) trpG′-′lacZbCmr | This study |
| PLBS443 | amyE::PpabB (−92 to +138 and +1336 to +1498) trpG′-′lacZ(pabB stop codon)b,c Cmr | This study |
| PLBS486 | amyE::PpabB (−92 to +1498) trpG′-′lacZd Cmr | This study |
| PLBS487 | amyE::PpabB (−92 to +1498) trpG′-′lacZ(pabB stop codon)c,d Cmr | This study |
| PLBS496 | rho::neo (Kmr) amyE::PpabB (−92 to +1498) trpG′-′lacZd Cmr | This study |
| PLBS497 | rho::neo (Kmr) amyE::PpabB (−92 to +1498) trpG′-′lacZ(pabB stop codon)c,d Cmr | This study |
| PLBS498 | ΔmtrB amyE::Ppab (−92 to +1498) trpG′-′lacZd Cmr | This study |
| PLBS499 | ΔmtrB amyE::PpabB (−92 to +1498) trpG′-′lacZ(pabB stop codon)c,d Cmr | This study |
| PLBS503 | amyE::PtrpG (+1040 to +1498) trpG′-′lacZe Cmr | This study |
| PLBS504 | amyE::PtrpG (+1040 to +1498) trpG′-′lacZ(pabB stop codon)c,e Cmr | This study |
| PLBS508 | ΔmtrB amyE::PtrpG (+1040 to +1498) trpG′-′lacZe Cmr | This study |
| PLBS509 | ΔmtrB amyE::PtrpG (+1040 to +1498) trpG′-′lacZ(pabB stop codon)c,e Cmr | This study |
| PLBS510 | ΔmtrB amyE::PpabB (−92 to +138 and +1336 to +1498) trpG′-′lacZb Cmr | This study |
| PLBS511 | ΔmtrB amyE::PpabB (−92 to +138 and +1336 to +1498) trpG′-′lacZ(pabB stop codon)b,c Cmr | This study |
| PLBS512 | rho::neo (Kmr) amyE::PtrpG (+1040 to +1498) trpG′-′lacZeCmr | This study |
| PLBS513 | rho::neo (Kmr) amyE::PtrpG (+1040 to +1498) trpG′-′lacZ(pabB stop codon)c,e Cmr | This study |
| PLBS514 | rho::neo (Kmr) amyE::PpabB (−92 to +138 and +1336 to +1498) trpG′-′lacZb Cmr | This study |
| PLBS515 | rho::neo (Kmr) amyE::PpabB (−92 to +138 and +1336 to +1498) trpG′-′lacZ(pabB stop codon)b,c Cmr | This study |
PpabB and PtrpG denote the pabB and trpG promoters, respectively. A prime symbol indicates truncation of the gene.
This trpG′-′lacZ translational fusion is driven exclusively by the PpabB promoter.
This trpG′-′lacZ translational fusion contains an engineered stop codon that replaces the GGA glycine codon at nt +1336 to +1338 relative to the PpabB promoter transcription start site.
This trpG′-′lacZ translational fusion is driven by the PpabB and PtrpG promoters.
This trpG′-′lacZ translational fusion is driven exclusively by the PtrpG promoter.
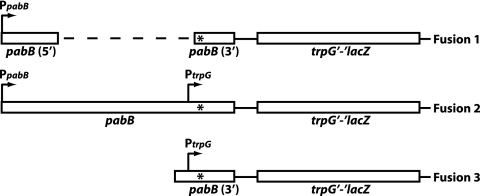
Plasmid pYH53 contains a partial in-frame deletion of pabB cloned into pTZ18U. The resulting fragment (nt −92 to +138 and +1336 to +1498 relative to the pabB transcriptional start site) was subcloned into ptrpBGI-PLK, thereby producing a trpG′-′lacZ translational fusion (pYH55) driven exclusively by PpabB (prime symbols indicate truncations of the genes) (Fig. 2, fusion 1). Plasmid pYH54 contains a TAA stop codon in place of the GGA glycine codon (nt +1336 to +1338 relative to the pabB transcriptional start site) that is present in pYH53. Plasmid pYH56 is identical to pYH55 except that it contains the mutation of the GGA glycine codon to a TAA stop codon. Plasmids pYH55 and pYH56 were linearized and integrated into the chromosomal amyE locus of B. subtilis strain PLBS338 (prototrophic), resulting in strains PLBS442 and PLBS443, respectively.
FIG. 2.
Schematic representation of translational fusions used in this study. The PpabB and PtrpG promoters are shown. The dashed line in fusion 1 represents a 1,197-nt partial in-frame deletion of the pabB coding sequence. This deletion removes PtrpG. Each fusion was engineered with and without a TAA stop codon in the pabB coding sequence (*).
Plasmid pYH66 contains nt −92 to +1498 relative to the pabB transcriptional start site cloned into pTZ19R. Plasmid pYH67 is identical to pYH66 except that it contains the mutation of the GGA glycine codon to a TAA stop codon. The folate operon-specific DNAs from pYH66 and pYH67 were ligated into pTrpBGI-PLK. The resulting circular plasmids were linearized and subsequently integrated into the amyE locus by transforming strain PLBS338, thereby generating strains PLBS486 and PLBS487, respectively. The resulting trpG′-′lacZ translational fusions were driven by both PpabB and PtrpG (Fig. 2, fusion 2). Proper fusions were confirmed by sequencing PCR products from both strains.
Plasmids pYH68 and pYH69 were constructed by subcloning a DNA fragment containing nt +1040 to +1498 relative to the pabB transcription start site from pYH66 and pYH67 into ptrpBGI-PLK, respectively. In both cases, the resulting trpG′-′lacZ translational fusions were driven exclusively by the PtrpG promoter (Fig. 2, fusion 3). Plasmids pYH68 and pYH69 were linearized and subsequently integrated into the chromosomal amyE locus of strain PLBS338 as described above, giving rise to strains PLBS503 and PLBS504, respectively.
Transformation of B. subtilis strains PLBS442, PLBS443, PLBS486, PLBS487, PLBS503, and PLBS504 with chromosomal DNA from strain BG4233 (ΔmtrB TRAP−) (14) resulted in strains PLBS510, PLBS511, PLBS498, PLBS499, PLBS508, and PLBS509, respectively. The null rho::neo allele from linearized plasmid pYH14 (30) was used to replace the wild-type (WT) rho gene in strains PLBS442, PLBS443, PLBS486, PLBS487, PLBS503, and PLBS504 to yield strains PLBS514, PLBS515, PLBS496, PLBS497, PLBS512, and PLBS513, respectively.
β-Galactosidase assay.
B. subtilis cultures were grown at 37°C in minimal acid casein hydrolysate (0.2%) medium containing 5 μg of chloramphenicol/ml in the absence or presence of 200 μM tryptophan. Growth medium for rho null strains also contained 10 μg of kanamycin/ml. Cells were harvested in late exponential phase and assayed for β-galactosidase activity as described previously (11, 20).
Gel mobility shift assay.
Quantitative gel mobility shift assays used to examine TRAP-RNA interactions were performed by following published procedures (27, 29). TRAP was purified as described previously (27). trpG, trpP, ycbK, and trp operon leader RNAs were synthesized in vitro and 5′ end labeled with [γ-32P]ATP. Gel-purified transcripts were renatured by heating to 80°C for 1 min, followed by slow cooling. Binding reactions contained 40 mM Tris-HCl (pH 8.0), 4 mM MgCl2, 30 mM KCl, 1 mM dithiothreitol (DTT), 10% glycerol, 0.2 mg Escherichia coli tRNA/ml, 400 U of RNasin/ml, 5′-end-labeled RNA, 1.2 mM l-tryptophan, TRAP, and 0.1 mg of xylene cyanol/ml. The concentrations of labeled trpG, trpP, ycbK, and trp operon leader RNAs were 50 pM, 400 pM, 50 pM, and 10 pM, respectively. TRAP-RNA complexes were allowed to equilibrate at 37°C for 30 min. Samples were fractionated in native polyacrylamide gels. Radioactive bands were visualized and quantified, and Kd values were calculated as described previously (27).
Filter binding assay.
Filter binding reactions were performed following a published procedure (24). Purified TRAP and labeled RNAs were identical to those described for the gel mobility shift assay. Binding reactions contained 40 mM Tris-HCl (pH 8.0), 4 mM MgCl2, 250 mM KCl, 1 mM DTT, 0.2 mg of E. coli tRNA/ml, 5′-end-labeled RNA, 1.2 mM l-tryptophan, and TRAP. TRAP-RNA complexes were allowed to equilibrate at 37°C for 30 min. Samples were filtered and washed twice with 0.5 ml of 40 mM Tris-HCl (pH 8.0) and 250 mM KCl. Radioactive spots were visualized and quantified as described for the gel mobility shift assay.
Primer extension reaction.
Total RNA was isolated from B. subtilis strain PLBS338. Twenty micrograms of RNA was hybridized to a 32P-end-labeled DNA oligonucleotide complementary to nt +1325 to +1355 relative to the pabB transcriptional start site. Reaction mixtures (10 μl) containing 3 μl of hybridization mixture, a 375 μM concentration of each deoxynucleoside triphosphate, 10 mM DTT, 100 μg/ml of bovine serum albumin, 1× Moloney murine leukemia virus (MMLV) reaction buffer, and 20 U/μl MMLV reverse transcriptase were incubated for 15 min at 42°C. Reactions were terminated by the addition of stop solution (29), and samples were fractionated through 6% sequencing gels. Sequencing reactions were performed using pYH66 as the template and the same end-labeled DNA oligonucleotide as a primer.
In vitro transcription.
Single-round in vitro transcription reactions and data analysis were performed as described previously (26). B. subtilis σA RNAP was purified as described previously (21), with an additional HiTrap Heparin HP (Amersham) chromatography step. Stable transcription elongation complexes were formed in a reaction mix containing 10 μM ATP, 10 μM UTP, 2 μM GTP, 2 μCi [α-32P]UTP, and trpG DNA templates. Transcription elongation was halted after incorporation of the 21st nt due to the absence of CTP. Elongation of halted transcription complexes was resumed by the addition of all four nucleoside triphosphates together with heparin. The final concentrations were 150 μM CTP, 150 μM GTP, 150 μM UTP, 10 μM ATP, and 100 μg/ml of heparin. Elongation reactions were stopped after 10 min by the addition of 2× loading buffer. Samples were fractionated through 6% polyacrylamide sequencing gels. DNA templates used in this analysis included a 350-bp EcoRI-HindIII fragment of pYH66 (positions −200 to +150 relative to the start of PtrpG transcription) and a 188-bp PCR product (positions −75 to +113 relative to the start of PtrpG transcription). A DNA template giving rise to a 139-nt transcript was used as a size marker.
RESULTS
TRAP-mediated inhibition of trpG expression is modest despite high TRAP-trpG RNA affinity.
The affinities of tryptophan-activated TRAP for the four known TRAP binding sites in B. subtilis (Fig. 1B) were compared by gel mobility shift and filter binding assays (Table 2). TRAP exhibited the highest affinity for the trp operon leader RNA, followed by the ycbK, trpG, and trpP transcripts. With the exception of the twofold difference for trpP, the Kd values using the two methods were virtually identical. The affinities of TRAP for the four transcripts partially reflect the number of repeats as well as the number of repeats separated by optimal 2-nt spacers (Fig. 1B). For example, TRAP exhibited the highest affinity for the trp leader transcript, which contains 11 triplet repeats and 7 optimal spacers, whereas TRAP had the lowest affinity for trpP, a transcript that contains 9 repeats and only 4 optimal spacers. TRAP had an intermediate affinity for the trpG and ycbK transcripts, both of which contain nine repeats and six optimal spacers.
TABLE 2.
Affinities of TRAP for trpG, trpP, ycbK, and trp leader RNA
| Transcript |
Kd (nM)a
|
|
|---|---|---|
| Gel shift assay | Filter binding assay | |
| trpG | 8.4 ± 1.7 | 8.0 ± 3.0 |
| trpP | 34 ± 10 | 78 ± 5 |
| ycbK | 2.9 ± 0.9 | 3.4 ± 1.4 |
| trpL | 0.6 ± 0.3 | 0.7 ± 0.3 |
Values are averages for at least three independent experiments ± standard deviations.
TRAP is responsible for regulating expression of the tryptophan metabolism genes in response to tryptophan. The trpEDCFBA operon is regulated by TRAP-dependent transcription attenuation and translation control mechanisms, while trpG, trpP, and ycbK are regulated by TRAP at the level of translation only (Fig. 1). The extents of TRAP-dependent translation control of trpG, trpP, and ycbK were compared by examining the expression of translational fusions with lacZ (Table 3). The effect of exogenous tryptophan was assessed by the ratio of expression when cells were grown in the absence and presence of added tryptophan (−Trp/+Trp ratio). TRAP exhibited the tightest control of trpP expression (150-fold inhibition), whereas there was considerably less regulation of ycbK (9-fold) and trpG (5-fold) expression. Because TRAP is responsible for regulating the translation of trpG, trpP, and ycbK in response to tryptophan, expression of the three fusions did not respond to tryptophan in a ΔmtrB (TRAP-deficient) genetic background. The observation that expression levels of all three fusions under Trp-negative conditions were lower in WT strains than in the corresponding mtrB mutant strains indicates that the level of endogenously synthesized tryptophan is sufficient to partially activate TRAP. The extent of TRAP-mediated regulation in vivo was determined by comparing expression in the ΔmtrB strains with that in the WT strains grown in the presence of tryptophan (ΔmtrB/WT ratio). TRAP-dependent inhibition was highest for trpP (>900-fold), followed by ycbK (21-fold) and trpG (12-fold).
TABLE 3.
TRAP-mediated regulation of trpG, trpP, and ycbK expression
| Relevant genotype | Fusion | β-Galactosidase activitya
|
Inhibition (ΔmtrB/WT)b | ||
|---|---|---|---|---|---|
| −Trp | +Trp | −Trp/+Trp | |||
| WT | trpG′-′lacZ | 110 ± 20 | 21 ± 4 | 5.2 | 12 |
| ΔmtrB | trpG′-′lacZ | 260 ± 35 | 260 ± 20 | 1.0 | |
| WT | trpP′-′lacZ | 200 ± 30 | 1.3 ± 0.3 | 150 | 920 |
| ΔmtrB | trpP′-′lacZ | 1,200 ± 160 | 1,200 ± 220 | 1.0 | |
| WT | ycbK′-′lacZ | 12 ± 2 | 1.3 ± 0.2 | 9.2 | 21 |
| ΔmtrB | ycbK′-′lacZ | 30 ± 6 | 27 ± 4 | 1.1 | |
β-Galactosidase activities are given in Miller units (20). Values are averages for at least three independent experiments ± standard deviations.
The extent of TRAP-dependent inhibition was determined by dividing the value for the mtrB mutant by the value for the corresponding WT strain in the presence of tryptophan.
Translation-mediated displacement of bound TRAP reduces TRAP-dependent translation inhibition of trpG.
TRAP-dependent regulation is far more extensive for trpP than for trpG and ycbK, despite TRAP having 5- and 10-fold higher affinities for the trpG and ycbK transcripts, respectively (Tables 2 and 3). What could account for this apparent discrepancy? A possible explanation for the large difference in TRAP-dependent control of trpP and trpG translation could be related to the gene arrangement of the two operons. Interestingly, the pabB stop codon lies within the trpG S-D sequence (Fig. 1B). Since seven of the triplet repeats in the trpG TRAP binding site lie upstream or within the pabB stop codon, we hypothesized that translation of pabB could result in translation-mediated displacement of bound TRAP, resulting in a trpG S-D sequence that was transiently free of bound TRAP. Thus, translation-mediated displacement of bound TRAP might allow translation initiation of trpG, thereby reducing the inhibitory influence of TRAP. Since trpP is a single-gene operon, translation-mediated displacement of bound TRAP would not be a factor.
To determine whether translation-mediated displacement of bound TRAP influences the expression of trpG, a stop codon was introduced into the pabB coding sequence upstream from the TRAP binding site that overlaps the trpG translation initiation region. Thus, translation of pabB would terminate 36 codons upstream from the natural pabB stop codon, thereby eliminating any possibility of translation-mediated displacement of bound TRAP. To simplify integration of the trpG′-′lacZ translational fusion into the chromosomal amyE locus, the stop codon was combined with a large in-frame deletion of the pabB coding sequence (Fig. 2, fusion 1). β-Galactosidase activity was determined for WT and TRAP-deficient (ΔmtrB) strains grown in the absence and presence of tryptophan. If translation-mediated displacement of bound TRAP participated in regulating the expression of trpG, then reduced expression of fusions containing the stop codon mutation would be expected for WT strains grown in the presence of tryptophan but not for ΔmtrB strains. To test for the possible influence of transcriptional polarity caused by the engineered stop codon, experiments were also carried out in rho::neo (Rho−) strains. Expression from the trpG′-′lacZ translational fusion containing the partial in-frame deletion of pabB (fusion 1) was 8 Miller units in the absence of exogenously added tryptophan and was reduced threefold by the addition of tryptophan to the growth medium (Table 4, row 1). Expression from this fusion was not regulated in response to tryptophan in the ΔmtrB strain (Table 4, row 2), nor was expression influenced by the rho mutation (compare row 1 with row 3). The extent of TRAP-mediated regulation was determined by comparing expression in the ΔmtrB and WT strains grown in the presence of tryptophan. TRAP-dependent inhibition of fusion 1 was about fourfold (10/2.8). Importantly, when cells were grown in the presence of tryptophan, introduction of the stop codon resulted in a five- to sevenfold reduction in expression of fusion 1 in the WT (Table 4, compare row 1 with row 4) and rho mutant (compare row 3 with row 6) strains but not in the ΔmtrB strain (compare row 2 with row 5). These results are consistent with translation-mediated displacement of bound TRAP participating in the TRAP-dependent trpG translation control mechanism.
TABLE 4.
Ribosome-mediated displacement of bound TRAP influences trpG′-′lacZ expression
| Row | Relevant genotype | Fusiona | Stop codonb | β-Galactosidase activityc
|
Inhibition (ΔmtrB/WT)d | ||
|---|---|---|---|---|---|---|---|
| −Trp | +Trp | −Trp/+Trp | |||||
| 1 | WT | 1 | No | 8 ± 2 | 2.8 ± 0.2 | 2.9 | 3.6 |
| 2 | ΔmtrB | 1 | No | 10 ± 1 | 10 ± 2 | 1.0 | |
| 3 | Δrho | 1 | No | 7 ± 2 | 3.8 ± 1.6 | 1.8 | |
| 4 | WT | 1 | Yes | 7.4 ± 0.6 | 0.5 ± 0.1 | 15 | 28 |
| 5 | ΔmtrB | 1 | Yes | 14 ± 2 | 14 ± 1 | 1.0 | |
| 6 | Δrho | 1 | Yes | 5 ± 1 | 0.5 ± 0.1 | 10 | |
| 7 | WT | 2 | No | 110 ± 20 | 21 ± 2 | 5.2 | 12 |
| 8 | ΔmtrB | 2 | No | 260 ± 40 | 260 ± 20 | 1.0 | |
| 9 | Δrho | 2 | No | 75 ± 15 | 16 ± 3 | 4.7 | |
| 10 | WT | 2 | Yes | 100 ± 20 | 11 ± 2 | 9.1 | 25 |
| 11 | ΔmtrB | 2 | Yes | 290 ± 10 | 270 ± 15 | 1.1 | |
| 12 | Δrho | 2 | Yes | 70 ± 10 | 8 ± 1 | 8.8 | |
| 13 | WT | 3 | No | 60 ± 10 | 5 ± 1 | 12 | 50 |
| 14 | ΔmtrB | 3 | No | 230 ± 40 | 250 ± 50 | 0.9 | |
| 15 | Δrho | 3 | No | 50 ± 5 | 7 ± 1 | 7.1 | |
| 16 | WT | 3 | Yes | 80 ± 5 | 6 ± 1 | 13 | 37 |
| 17 | ΔmtrB | 3 | Yes | 270 ± 30 | 220 ± 30 | 1.2 | |
| 18 | Δrho | 3 | Yes | 55 ± 10 | 10 ± 2 | 5.5 | |
The three fusions are (1) PpabB-pabB′-Δ-′pabB-trpG′-′lacZ, (2) PpabB-pabB-PtrpG-trpG′-′lacZ, and (3) PtrpG-trpG′-′lacZ, where P denotes a promoter and Δ indicates a partial in-frame deletion of pabB.
Presence (yes) or absence (no) of an engineered stop codon within the coding sequence of pabB.
β-Galactosidase activities are given in Miller units (20). Values are averages for at least three independent experiments ± standard deviations.
The extent of TRAP-dependent inhibition was determined by dividing the value for the mtrB mutant by the value for the corresponding WT strain in the presence of tryptophan.
Identification of a new trpG promoter.
As described above, expression from fusion 1 was only 8 and 3 Miller units in the absence and presence of added tryptophan, respectively (Table 4, row 1). For comparison, expression from a trpG′-′lacZ translational fusion containing the complete pabB coding sequence was previously reported to be 56 Miller units in the absence of tryptophan and 8 Miller units in its presence (31). Accordingly, we examined the expression of trpG′-′lacZ translational fusions containing the entire pabB coding sequence in the absence or presence of the engineered stop codon described above (Fig. 2, fusion 2). Expression from fusion 2 was about 10-fold higher than that from fusion 1 (Table 4, compare row 1 with row 7). Fusion 2 expression was regulated fivefold in response to tryptophan (Table 4, row 7). As previously observed for fusion 1, expression from fusion 2 was not regulated in response to tryptophan in the ΔmtrB strain (Table 4, row 8), nor was expression influenced by the rho mutation (compare row 7 with row 9). TRAP-dependent inhibition of fusion 2 was 12-fold (260/21). As previously observed for fusion 1, the stop codon did not influence expression of the ΔmtrB strain (Table 4, compare row 8 with row 11). Notably, fusion 2 expression was reduced only twofold by the engineered stop codon when cells were grown in the presence of excess tryptophan for the WT (Table 4, compare row 7 with row 10) and rho mutant (compare row 9 with row 12) strains. One likely explanation for these results is that the in-frame pabB deletion associated with fusion 1 removed a previously unidentified promoter that was not subject to translation-mediated displacement of bound TRAP.
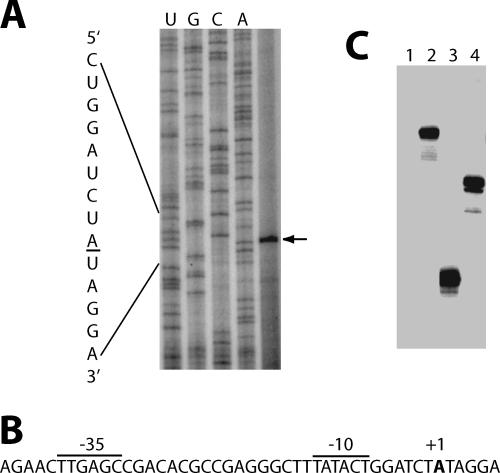
Primer extension experiments were carried out using total cellular RNA to map the 5′ ends of transcripts within the region delineated by the deletion endpoints from fusion 1. A single primer extension product was identified (Fig. 3A). A likely σA-dependent promoter (PtrpG) was identified just upstream that matched the −35 and −10 consensus sequences in four of six and five of six positions, respectively. The spacing between the −35 and −10 sequences matched the 17-nt consensus (Fig. 3B). In vitro transcription reactions using purified B. subtilis RNAP confirmed that this was a σA-dependent promoter (Fig. 3C).
FIG. 3.
Identification of a new trpG promoter. (A) Primer extension mapping of the 5′ end of the trpG transcript. Total RNA was isolated from a WT B. subtilis strain, hybridized to an end-labeled DNA primer, and subsequently extended with MMLV reverse transcriptase. Sequencing reactions were performed using the same end-labeled DNA primer. An arrow marks the single reverse transcriptase product corresponding to the 5′ end of the transcript originating from PtrpG. (B) σA trpG promoter sequence. The trpG promoter sequences (−35 and −10) and the transcription start site (+1) are marked. (C) In vitro transcription analysis of the trpG promoter, using σA-containing B. subtilis RNAP. Lane 1, no-template control; lane 2, DNA restriction fragment template giving rise to a 150-nt trpG transcript; lane 3, PCR-derived template giving rise to a 113-nt trpG transcript; lane 4, PCR-derived template giving rise to a 139-nt control transcript.
Expression studies similar to those performed with fusions 1 and 2 were carried out with trpG′-′lacZ translational fusions driven exclusively by PtrpG (Fig. 2 and Table 4, fusion 3). Expression from PtrpG (fusion 3) was considerably higher than expression from the corresponding fusion containing only PpabB (fusion 1) (Table 4, compare rows 1 and 13). Fusion 3 expression was regulated 12-fold in response to tryptophan (Table 4, row 13). As previously observed for fusions 1 and 2, expression from fusion 3 was not regulated in response to tryptophan in the ΔmtrB strain (Table 4, row 14), nor was expression influenced by the rho mutation (compare row 13 with row 15). In this case, TRAP-dependent inhibition was 50-fold (250/5). Expression from fusion 3 was not subject to translation-mediated displacement of bound TRAP; expression levels were not altered by the engineered stop codon in the WT or the ΔmtrB or rho mutant strain (Table 4, compare row 13 with row 16, row 14 with row 17, and row 15 with row 18). Taken together, our results establish that trpG transcription initiates from two promoters (PpabB and PtrpG), that transcription levels are considerably higher from PtrpG than from PpabB, and that only transcripts originating from PpabB are subject to translation-mediated displacement of bound TRAP.
One would expect that the expression levels of fusion 1 (PpabB) and fusion 3 (PtrpG) would add up to the expression level of fusion 2, containing both promoters. In the absence of added tryptophan (−Trp), the combined expression levels of fusions 1 and 3 were similar to the expression level of fusion 2 in all strains (Table 4). However, in the presence of added tryptophan (+Trp), the combined expression levels of fusions 1 and 3 in the WT strains (with or without the stop codon) were two- to threefold lower than those from fusion 2. Note that this discrepancy was not observed for the ΔmtrB or rho mutant strain. While we do not have an explanation for this observation for the WT strains, we have ruled out mRNA stability as a source of this apparent discrepancy (data not shown).
DISCUSSION
TRAP regulates translation initiation of trpG, trpP, and ycbK by directly blocking ribosome access to their cognate S-D sequences (12, 18, 22, 23, 28, 29, 31). The TRAP binding sites in trpG, trpP, and ycbK each contain nine triplet repeats (Fig. 1B). While trpG is present in an operon primarily concerned with folic acid biosynthesis (Fig. 1A), the TrpG polypeptide functions as the common glutamine amidotransferase subunit of anthranilate synthase (TrpE-TrpG) and para-aminobenzoate synthase (PabB-TrpG) in tryptophan and folic acid biosynthesis, respectively (13). The finding that trpG is regulated less tightly than trpP, despite having a higher affinity for TRAP (Tables 2 and 3), combined with the arrangement of the pabB and trpG coding sequences with respect to the TRAP binding site (Fig. 1B), led to the hypothesis that translation-mediated displacement of bound TRAP could play a role in the trpG translation control mechanism. Genetic results from this study suggest that ribosomes translating the pabB coding sequence are capable of displacing tryptophan-activated TRAP from its binding site in the trpG transcript (Table 4). Since seven of the nine triplet repeats in the trpG TRAP binding site lie upstream or within the pabB stop codon, and because the pabB stop codon overlaps the trpG S-D sequence (Fig. 1B), translation of pabB would displace bound TRAP, resulting in a trpG S-D sequence that is transiently free of TRAP. The same or a different ribosome can then bind and initiate translation before another tryptophan-activated TRAP molecule binds to the trpG transcript. Since trpP is a single-gene operon, translation-mediated displacement of bound TRAP cannot be a factor. It is also interesting that the stop codon for rtpA overlaps the ycbK S-D sequence, although all nine of the triplet repeats are downstream from the rtpA stop codon (Fig. 1B). Like the case for trpG, we engineered a stop codon to test whether ribosome-mediated displacement of bound TRAP affected expression of ycbK. However, in this case, our results indicate that ribosome-mediated displacement of bound TRAP, if it occurs, does not influence the expression of ycbK (data not shown).
Two promoters control the transcription of trpG. The PpabB promoter is located just upstream from pabB (Fig. 1A), while PtrpG is located ∼250 bp upstream from the trpG initiation codon within the 3′ end of the pabB coding sequence (Fig. 2 and 3). Expression from PtrpG is considerably higher than expression from PpabB (Table 4). Furthermore, our results indicate that translation-mediated displacement of bound TRAP can only occur for transcripts originating from PpabB. One physiological role for this translation displacement mechanism would be to ensure a sufficient level of TrpG synthesis to maintain folic acid biosynthesis in the presence of excess tryptophan; expression from fusion 1, which is only driven by PpabB, is low when cells are grown in the presence of excess tryptophan (Table 4). However, the presence of PtrpG would appear to be sufficient to maintain folic acid biosynthesis under excess tryptophan growth conditions (Table 4, fusion 3). While our initial hypothesis was that translation-mediated displacement of bound TRAP would provide an answer for how translation of trpG can be regulated less tightly than that of trpP, despite TRAP having a higher affinity for the trpG message, it is apparent that this mechanism can only partially explain this dichotomy (Tables 2 and 3). Perhaps PtrpG evolved relatively recently and at one time PpabB was the only available promoter for trpG expression. Thus, it is possible that the translation-mediated displacement mechanism was necessary for survival at some point during the evolution of B. subtilis. It is also possible that transcription initiation from these promoters is differentially regulated. While it was previously shown that folate operon transcript levels increased markedly when stationary-phase cells were diluted in fresh medium (9), it is unclear whether PpabB, PtrpG, or both were responsible for the increased expression.
Of the various Bacillus species for which sequence information is available for the trp and folate operons, B. subtilis, Bacillus licheniformis, Bacillus halodurans, and Bacillus clausii contain TRAP. In each of these organisms, a single trpG (pabA) gene is located immediately downstream from pabB in the folate operon. It appears that the internal PtrpG promoter is conserved in all four organisms (see Fig. S1 in the supplemental material). Examination of the pabB-trpG intercistronic region reveals the presence of appropriately spaced triplet repeats such that TRAP would be capable of inhibiting translation of trpG. Moreover, the potential of ribosome-mediated displacement of bound TRAP from transcripts originating from PpabB is conserved as well; in each case, the majority of the triplet repeats that constitute the putative TRAP binding site lie upstream of or within the pabB stop codon (see Fig. S2 in the supplemental material). Interestingly, the Bacillus species that do not contain TRAP have separate trpG and pabA genes. In these instances, trpG is the second gene of the trp operon, while pabA is immediately downstream of pabB in the folate operon. It appears that the internal PtrpG (PpabA) promoter is conserved in these species as well (see Fig. S1 in the supplemental material). The trp operons in the Bacillus species that do not contain TRAP are regulated by the T box antitermination mechanism, in which uncharged tryptophanyl tRNA binds to the untranslated leader and promotes transcription readthrough (15). Surprisingly, despite the absence of TRAP in these organisms, five appropriately spaced triplet repeats are present in the analogous pabB-pabA intercistronic region, like the case for the TRAP-regulated trpG (pabA) genes (see Fig. S2 in the supplemental material).
We are not aware of any previous studies demonstrating the role of translation-mediated displacement of a specific RNA binding protein in regulating gene expression. However, translation-mediated displacement of RNA binding proteins has been implicated in the nonsense-mediated decay mechanism in eukaryotes. Nonsense-mediated decay rids eukaryotic cells of aberrant mRNAs containing premature termination codons. Premature stop codons are distinguished from true termination codons by downstream exon-exon junctions (17). It was shown that hUpf3 remains bound at exon-exon junctions following splicing. It was proposed that during the first round of translation, the progressing ribosome displaces hUpf3 and associated hUpf2 when bound upstream from the termination codon. If these proteins are not displaced, subsequent binding of hUpf1 triggers mRNA degradation (17). Using an in vitro system, it was shown that ribosome-mediated displacement of Y14, an RNA binding protein that is part of the exon-exon junction complex, occurs from translationally active mRNAs (10). More recently, it was suggested that movement of the ribosome along the mRNA might displace AUF1, a protein that promotes the rapid decay of AU-rich element-containing RNAs. This last mechanism would be distinct from those described above because these AU-rich elements are present in the 3′-untranslated regions of certain eukaryotic messages (16).
The precise mechanism or energy required for protein displacement has not been addressed in any of the studies just described. However, footprinting data indicate that the 5′- and 3′-most triplet repeats were least protected by bound TRAP (6, 28). A weak interaction between TRAP and the 3′ triplet plays a critical role in the degradation of terminated trp operon leader RNA so that TRAP can be recycled (8). Thus, it is conceivable that a relatively weak interaction between TRAP and the 5′-most triplet repeat in the trpG transcript is important for translation-mediated displacement of bound TRAP. Disruption of the TRAP interaction with the first repeat by the ribosome would generate a new 5′-most TRAP-bound triplet (i.e., the second repeat). Sequential disruption of TRAP interaction with each repeat by the translating ribosome may ultimately lead to TRAP dissociation and, hence, translation of trpG.
Acknowledgments
This work was supported by grant GM52840 from the National Institutes of Health.
Footnotes
Published ahead of print on 17 November 2006.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Antson, A. A., E. J. Dodson, G. Dodson, R. B. Greaves, X.-P. Chen, and P. Gollnick. 1999. Structure of the trp RNA-binding attenuation protein, TRAP, bound to RNA. Nature 401:235-242. [DOI] [PubMed] [Google Scholar]
- 2.Antson, A. A., J. B. Otridge, A. M. Brzozowski, E. J. Dodson, G. G. Dodson, K. S. Wilson, T. M. Smith, M. Yang, T. Kurecki, and P. Gollnick. 1995. The three dimensional structure of trp RNA-binding attenuation protein. Nature 374:693-700. [DOI] [PubMed] [Google Scholar]
- 3.Babitzke, P. 2004. Regulation of transcription attenuation and translation initiation by allosteric control of an RNA binding protein: the Bacillus subtilis TRAP protein. Curr. Opin. Microbiol. 7:132-139. [DOI] [PubMed] [Google Scholar]
- 4.Babitzke, P., and C. Yanofsky. 1993. Reconstitution of Bacillus subtilis trp attenuation in vitro with TRAP, the trp RNA-binding attenuation protein. Proc. Natl. Acad. Sci. USA 90:133-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Babitzke, P., D. G. Bear, and C. Yanofsky. 1995. TRAP, the trp RNA-binding attenuation protein of Bacillus subtilis, is a toroid-shaped molecule that binds transcripts containing GAG or UAG repeats separated by two nucleotides. Proc. Natl. Acad. Sci. USA 92:7916-7920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Babitzke, P., J. T. Stults, S. J. Shire, and C. Yanofsky. 1994. TRAP, the trp RNA-binding attenuation protein of Bacillus subtilis, is a multisubunit complex that appears to recognize G/UAG repeats in the trpEDCFBA and trpG transcripts. J. Biol. Chem. 269:16597-16604. [PubMed] [Google Scholar]
- 7.Baumann, C., S. Xirsagar, and P. Gollnick. 1997. The trp RNA-binding attenuation protein (TRAP) from Bacillus subtilis binds to unstacked trp leader RNA. J. Biol. Chem. 272:19863-19869. [DOI] [PubMed] [Google Scholar]
- 8.Deikus, G., P. Babitzke, and D. H. Bechhofer. 2004. Recycling of an RNA-binding protein by ribonuclease digestion. Proc. Natl. Acad. Sci. USA 101:2747-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Saizieu, A., P. Vankan, C. Vockler, and A. P. G. M. van Loon. 1997. The trp RNA-binding attenuation protein (TRAP) regulates the steady-state levels of transcripts of the Bacillus subtilis folate operon. Microbiology 143:979-989. [DOI] [PubMed] [Google Scholar]
- 10.Dostie, J., and G. Dreyfuss. 2002. Translation is required to remove Y14 from mRNAs in the cytoplasm. Curr. Biol. 12:1060-1067. [DOI] [PubMed] [Google Scholar]
- 11.Du, H., and P. Babitzke. 1998. trp RNA-binding attenuation protein-mediated long distance RNA refolding regulates translation of trpE in Bacillus subtilis. J. Biol. Chem. 273:20494-20503. [DOI] [PubMed] [Google Scholar]
- 12.Du, H., R. Tarpey, and P. Babitzke. 1997. The trp RNA-binding attenuation protein regulates TrpG synthesis by binding to the trpG ribosome binding site of Bacillus subtilis. J. Bacteriol. 179:2582-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gollnick, P., P. Babitzke, A. Antson, and C. Yanofsky. 2005. Complexity in regulation of tryptophan biosynthesis in Bacillus subtilis. Annu. Rev. Genet. 39:47-68. [DOI] [PubMed] [Google Scholar]
- 14.Gollnick, P., S. Ishino, M. I. Kuroda, D. J. Henner, and C. Yanofsky. 1990. The mtr locus is a two gene operon required for transcription attenuation in the trp operon of Bacillus subtilis. Proc. Natl. Acad. Sci. USA 87:8726-8730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gutierrez-Preciado, A., R. A. Jansen, C. Yanofsky, and E. Merino. 2005. New insights into regulation of the tryptophan biosynthetic operon in gram-positive bacteria. Trends Genet. 21:432-436. [DOI] [PubMed] [Google Scholar]
- 16.Lu, J.-Y., N. Bergman, N. Sadri, and R. J. Schneider. 2006. Assembly of AUF1 with eIF4G-poly(A) binding protein complex suggests a translation function in AU-rich mRNA decay. RNA 12:883-893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lykke-Andersen, J., M.-D. Shu, and J. A. Steitz. 2000. Human Upf proteins target an mRNA for nonsense-mediated decay when bound downstream of a termination codon. Cell 103:1121-1131. [DOI] [PubMed] [Google Scholar]
- 18.Merino, E., P. Babitzke, and C. Yanofsky. 1995. trp RNA-binding attenuation protein (TRAP)-trp leader RNA interactions mediate translational as well as transcriptional regulation of the Bacillus subtilis trp operon. J. Bacteriol. 177:6362-6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Otridge, J., and P. Gollnick. 1993. MtrB from Bacillus subtilis binds specifically to trp leader RNA in a tryptophan dependent manner. Proc. Natl. Acad. Sci. USA 90:128-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Platt, T., B. Müller-Hill, and J. H. Miller. 1972. Assay of β-galactosidase. In J. H. Miller (ed.), Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 21.Qi, Y., and F. M. Hulett. 1998. Role of Pho-P in transcriptional regulation of genes involved in cell wall anionic polymer biosynthesis in Bacillus subtilis. Mol. Microbiol. 28:1187-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarsero, J. P., E. Merino, and C. Yanofsky. 2000. A Bacillus subtilis gene of previously unknown function, yhaG, is translationally regulated by tryptophan-activated TRAP and appears to be concerned with tryptophan transport. J. Bacteriol. 182:2329-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarsero, J. P., E. Merino, and C. Yanofsky. 2000. A Bacillus subtilis operon containing genes of unknown function senses tRNAtrp charging and regulates expression of the genes for tryptophan biosynthesis. Proc. Natl. Acad. Sci. USA 97:2656-2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schaak, J., H. Yakhnin, P. C. Bevilacqua, and P. Babitzke. 2003. A Mg2+-dependent RNA tertiary structure forms in the Bacillus subtilis trp operon leader transcript and appears to interfere with trpE translation control by inhibiting TRAP binding. J. Mol. Biol. 332:555-574. [DOI] [PubMed] [Google Scholar]
- 25.Valbuzzi, A., P. Gollnick, P. Babitzke, and C. Yanofsky. 2002. The anti-trp RNA-binding attenuation protein (anti-TRAP), AT, recognizes the tryptophan-activated RNA binding domain of the TRAP regulatory protein. J. Biol. Chem. 277:10608-10613. [DOI] [PubMed] [Google Scholar]
- 26.Yakhnin, A. V., and P. Babitzke. 2002. NusA-stimulated RNA polymerase pausing and termination participates in the Bacillus subtilis trp operon attenuation mechanism in vitro. Proc. Natl. Acad. Sci. USA 99:11067-11072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yakhnin, A. V., J. J. Trimble, C. R. Chiaro, and P. Babitzke. 2000. Effects of mutations in the l-tryptophan binding pocket of the trp RNA-binding attenuation protein of Bacillus subtilis. J. Biol. Chem. 275:4519-4524. [DOI] [PubMed] [Google Scholar]
- 28.Yakhnin, H., A. V. Yakhnin, and P. Babitzke. 2006. The trp RNA-binding attenuation protein (TRAP) of Bacillus subtilis regulates translation initiation of ycbK, a gene encoding a putative efflux protein, by blocking ribosome binding. Mol. Microbiol. 61:1252-1266. [DOI] [PubMed] [Google Scholar]
- 29.Yakhnin, H., H. Zhang, A. V. Yakhnin, and P. Babitzke. 2004. The trp RNA-binding attenuation protein (TRAP) of Bacillus subtilis regulates translation of the tryptophan transport gene, trpP (yhaG), by blocking ribosome binding. J. Bacteriol. 186:278-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yakhnin, H., J. E. Babiarz, A. V. Yakhnin, and P. Babitzke. 2001. Expression of the Bacillus subtilis trpEDCFBA operon is influenced by translational coupling and Rho termination factor. J. Bacteriol. 183:5918-5926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang, M., A. de Saizieu, A. P. G. M. Van Loon, and P. Gollnick. 1995. Translation of trpG in Bacillus subtilis is regulated by the trp RNA-binding attenuation protein (TRAP). J. Bacteriol. 177:4272-4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang, M., X.-P. Chen, K. Militello, R. Hoffman, B. Fernandez, C. Baumann, and P. Gollnick. 1997. Alanine-scanning mutagenesis of Bacillus subtilis trp RNA-binding attenuation protein (TRAP) reveals residues involved in tryptophan binding and RNA binding. J. Mol. Biol. 270:696-710. [DOI] [PubMed] [Google Scholar]