Abstract
In Corynebacterium glutamicum, the transcriptional regulator RamB negatively controls the expression of genes involved in acetate metabolism. Here we show that RamB represses its own expression by direct interaction with a 13-bp motif in the ramB promoter region. Additionally, ramB expression is subject to carbon source-dependent positive control by RamA.
Corynebacterium glutamicum is an aerobic gram-positive soil bacterium well known for its use in large-scale biotechnological production of l-glutamate and lysine (10-14). We are interested in acetate metabolism and its regulation in this organism (7), and we recently identified and characterized two regulatory proteins, designated regulator of acetate metabolism A and B (RamA and RamB, respectively) from C. glutamicum ATCC 13032 (4, 6). RamA binds to single or tandem stretches of A/C/TG4-6T/C or AC4-5A/G/T and thereby acts as an activator of the pta, ack, aceA, and aceB genes, encoding phosphotransacetylase, acetate kinase, isocitrate lyase, and malate synthase, all involved in acetate metabolism of C. glutamicum. RamA was also shown to activate transcription of the surface layer protein gene (cspB) in C. glutamicum ATCC 14067 (9). Furthermore, RamA was shown to bind to the promoter region of its own gene and to act as a repressor there (3). The RamB protein specifically binds to a highly conserved 13-bp motif (AA/GAACTTTGCAAA) and represents a repressor of the pta, ack, aceA, and aceB genes when C. glutamicum is grown on glucose as a carbon and energy source (6). Inspection of the ramB promoter region revealed the presence of putative RamA and RamB binding sites, and this observation prompted us to study expression of ramB in cells growing in media containing glucose and/or acetate and to test for a regulatory function of RamA and RamB in ramB expression.
The bacterial strains and the plasmids, their relevant characteristics and sources, and the oligonucleotides used in this study are given in Table 1. The media used and the methods not outlined explicitly (DNA preparation, promoter binding assays with hexahistidyl-tagged RamA and RamB fusion proteins, enzyme assays, RNA preparation and identification of the transcriptional start site by the RACE [rapid amplification of cDNA ends] method, generation of polyclonal antibodies, and Western blotting) were described previously (3, 4, 6).
TABLE 1.
Bacterial strains, plasmids, and oligonucleotides used in this study
| Strain, plasmid, or oligonucleotide | Relevant characteristics or sequencea | Source, reference, or purpose |
|---|---|---|
| Strains | ||
| E. coli DH5 | supE44 hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | 8 |
| E. coli BL21 (DE) | ompT hsdSB (rB− mB−) gal dcm (DE3) | 5 |
| C. glutamicum WT | Wild-type strain ATCC 13032 | American Type Culture Collection |
| C. glutamicum RG1 | WT C. glutamicum with truncated ramB gene, shortened by 775 bp | 6 |
| C. glutamicum RG2 | WT C. glutamicum with truncated ramA gene, shortened by 364 bp | 4 |
| Plasmids | ||
| pET2 | Multicopy promoter probe vector carrying the promoterless cat gene; Kmr | 17 |
| pRIM2 | Integrative promoter probe vector carrying the promoterless cat gene; Kmr | 17 |
| pET28-RamAx6His | pET28 containing the ramA structural gene | 4 |
| pET29-RamBx6His | pET29 containing the ramB structural gene | 6 |
| pramBp1 | pET2 containing the 593-bp ramB promoter fragment | This work |
| pramBp3 | pET2 containing the 370-bp ramB promoter fragment | This work |
| pramBp3b | pET2 containing the 211-bp ramB promoter fragment | This work |
| pramBp3c | pET2 containing the 135-bp ramB promoter fragment | This work |
| pRIM-ramBp1 | pRIM containing the 593-bp ramB promoter fragment | This work |
| pRIM-ramBp3c | pRIM containing the 135-bp ramB promoter fragment | This work |
| pDrive | Cloning vector; Kmr AmprlacZα orif1 ori-pUC | QIAGEN GmbH, Hilden, Germany |
| pDrive ramBpRACE | pDrive derivative plasmid containing the ramB PCR-amplified fragment from the RACE assay | This work |
| Oligonucleotidesb | ||
| ramBpLF forw | 5′-ACGCGTCGACCTAACAGTCATGGCACCTCCAGTGTGG-3′ | ramBp1and ramBp5 |
| ramBpLF rev | 5′-CGCGGATCCCAAGGGTTGCTGCTAAGGATGCCTG-3′ | ramBp1, ramBp3, ramBp3a, ramBp3b, ramBp3c |
| ramBp forw | 5′-AGCGAAAATCAACAAGTTTGCAACACCTCAGT-3′ | ramBp 13-bp motif |
| ramBp rev | 5′-ACTGAGGTGTTGCAAACTTGTTGATTTTCGCT-3′ | ramBp 13-bp motif |
| ramBpKF forw | 5′-ACGCGTCGACGCTTCCTCACAGGATACCGA-3′ | ramBp3 |
| ramBp3a forw | 5′-CAGGGAGCAACTTTGCGCAG-3′ | ramBp3a |
| ramBp3b forw | 5′-ACGCGTCGACGATGTGGCCCGACCACGCCG-3′ | ramBp3b |
| ramBp3c forw | 5′-ACGCGTCGACCTCAGTGCCAAGAGTGGTTA-3′ | ramBp3c |
| ramBp5 rev | 5′-CGCAGGTAGAGCACACTCAAT-3′ | ramBp5 |
| CM4 | 5′-GAAAATCTCGTCGAAGCTCG-3′ | cDNA synthesis; 5′-RACE ramA and ramB transcriptional start site determination |
| Oligonucleotide dT anchor primer | 5′-GACCACGCGTATCGATGTCGACTTTTTTTTTTTTTTTTV-3′ | Amplification of dA-tailed cDNA (Roche, 5′/3′ RACE kit) |
Restriction sites in the oligonucleotides are underlined. V represents an A, C, or G.
forw., forward; rev, reverse.
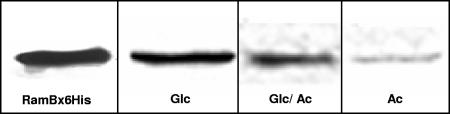
Western blot experiments with RamB-specific antibodies and crude extracts from C. glutamicum wild-type (WT) cells grown in minimal medium containing glucose, glucose plus acetate, and acetate showed that RamB is present in cells grown on either carbon source (Fig. 1). In agreement with the results of DNA affinity chromatography experiments (4), the largest amounts of RamB were observed in the cells grown on glucose alone.
FIG. 1.
Western blot using specific antibodies against RamB and cell extracts of C. glutamicum WT (100 μg total protein) grown in minimal medium containing glucose (Glc), glucose and acetate (Glc/Ac), or acetate (Ac). The presence of RamB in the extracts was tested by incubation with specific mouse antibodies followed by incubation with peroxidase-conjugated goat anti-mouse immunoglobulin G Fab fragments and visualization by chemiluminescence. Purified RamB protein (RamBx6His; 0.2 μg) was used as a control.
To test for ramB promoter activities in C. glutamicum cells grown in minimal media with glucose and/or acetate, we constructed reporter gene fusions by cloning a 593-bp ramB promoter fragment (covering the region 521 bp upstream to 72 bp downstream of the ramB start codon) into the promoter test vector pET2, resulting in plasmid pramBp1. This vector was transformed into C. glutamicum WT, and the plasmid-bound ramB promoter activity of ramB was then tested in C. glutamicum (pramBp1) by measuring the specific activities of the reporter gene product chloramphenicol acetyltransferase (CAT). As shown in Table 2, C. glutamicum (pramBp1) showed about fourfold-lower specific CAT activity when grown in medium containing acetate or a mixture of both carbon sources instead of glucose. To exclude copy number or titration effects, we also tested the ramBp1-cat fusion after monocopy integration into the chromosome of WT C. glutamicum. For this purpose, the PCR-generated fragment ramBp1 (Fig. 2A) was ligated into plasmid pRIM2, and the resulting plasmid was integrated into the chromosome as described elsewhere (17). As in the case of the multicopy ramBp1-cat fusion, the specific CAT activity was about fourfold lower when the cells were grown on glucose instead of acetate (0.004 and 0.001 U/mg protein, respectively). These result show the presence of a promoter on the ramBp1 fragment and indicates transcriptional regulation of ramB, i.e., induction or derepression when cells are grown on glucose as the sole carbon and energy source and/or repression when acetate is present in the growth medium. Taken together, the results indicate that the amount of RamB protein in C. glutamicum is dependent on the carbon source in the growth medium and that the respective control is due to transcriptional regulation of the ramB gene.
TABLE 2.
Specific CAT activities of C. glutamicum grown in minimal medium (MM) containing glucose and/or acetate as the carbon and energy source
| C. glutamicum strain | CAT sp act (U/mg protein)a on MM with:
|
||
|---|---|---|---|
| Glucose | Glucose + acetate | Acetate | |
| WT(pramBp1) | 0.28 ± 0.02 | 0.07 ± 0.01 | 0.06 ± 0.01 |
| RG1(pramBp1) (ΔramB) | 0.45 ± 0.03 | 0.21 ± 0.01 | 0.15 ± 0.02 |
| RG2(pramBp1) (ΔramA) | 0.08 ± 0.01 | 0.08 ± 0.01 | NG |
| WT(pramBp3) | 0.28 ± 0.03 | 0.09 ± 0.01 | 0.10 ± 0.01 |
| WT(pramBp3b) | 0.14 ± 0.01 | 0.05 ± 0.01 | 0.05 ± 0.01 |
| WT(pramBp3c) | 0.45 ± 0.02 | 0.22 ± 0.01 | 0.16 ± 0.02 |
Values are means ± standarsd deviations obtained from at least three independent cultivations and two determinations per experiment. NG, no growth.
FIG. 2.
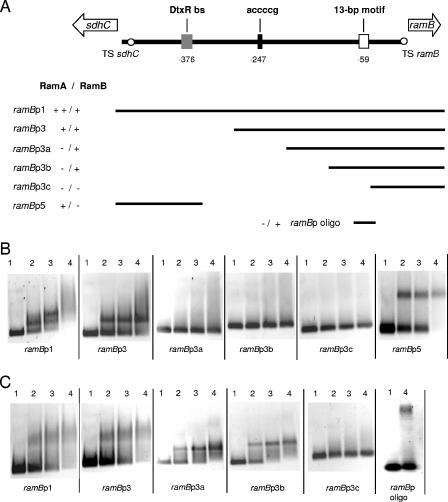
Genomic locus of the ramB promoter region and DNA fragments used for mapping the relevant RamA and RamB binding loci (A) and representative EMSAs using various DNA fragments and RamA protein (B) and RamB protein (C). sdhC codes for a protein annotated as a membrane anchor protein of the succinate dehydrogenase. The transcriptional start sites for the ramB gene and the sdhC gene are designated TS ramB and TS sdhC, respectively. (A) The RamB binding site is designated as a 13-bp motif. The nucleotide sequence of the putative RamA binding site is given above the black box. The putative DtxR binding site (DtxR bs) is indicated by a dark gray box. The numbers indicate the positions of the centers of these sites relative to the ramB TS site. The fragments used for the binding assays are shown as narrow bars. Also indicated are binding (+) and lack of binding (−) of RamA and RamB. Lanes 1 to 4 (B and C) show EMSAs using 0, 0.25, 0.5, and 1.0 μg of RamA or RamB, respectively.
To identify the ramB transcriptional initiation site (TS) and to localize the respective promoter, total RNA from C. glutamicum (pramBp1) was isolated, and cDNA of the 5′ end of the ramB transcript was generated with reverse transcriptase. Subsequently, this cDNA was amplified and subcloned into the vector pDrive, and the resulting plasmid, pDrive ramBpRACE, was sequenced, leading to the identification of the TS for ramB. In four independently obtained pDrive ramBpRACE clones, the TS of ramB was found to be an A residue which is identical to the translational start site proposed (11) (NCBI accession number NC_006958). Such leaderless transcripts are not uncommon in C. glutamicum and other actinomycetes (16). Inspection of the upstream region of the TS led to the identification of a potential −10 box (TATAGT) which is conserved in five of six nucleotides relative to the −10 consensus sequence described for corynebacteria (TA[C/T]AAT) (15). No apparent −35 region (TTGCCA) could be recognized, which also is a common feature of C. glutamicum promoters (15, 16).
Inspection of the ramB promoter region revealed the presence of a typical 13-bp RamB binding motif (ACAAGTTTGCAAC; mismatches underlined), centered 59 bp upstream of the ramB TS. Furthermore, a nucleotide sequence resembling the RamA binding site was also found, centered 247 bp upstream of the ramB TS (Fig. 2A). Further possible RamA binding sites are located in the region near the sdhC transcriptional start site (not shown in Fig. 2A). To test whether expression of ramB in C. glutamicum in fact is subject to transcriptional control by RamA and/or RamB, the ramB promoter activities were determined in the ramB and ramA deletion mutants C. glutamicum RG1 and RG2. For this purpose, plasmid pramBp1 (see above) was transformed into both mutants, and after growth in minimal medium containing glucose and/or acetate, the ramB promoter activities were tested by measuring the specific CAT activity and compared to that of C. glutamicum WT (pramBp1). The C. glutamicum RG2 derivative did not grow on minimal medium containing acetate as the sole carbon source, a phenotype which was previously found for the parental strain RG2 (4).
C. glutamicum RG1 (pramBp1) cells showed about twofold-higher specific CAT activities than C. glutamicum WT (pramBp1) cells on glucose and about threefold-higher activities in the presence of acetate (Table 2). On the other hand, only very low CAT activity was observed in C. glutamicum RG2 (pramBp1) cells independent of the presence or absence of glucose. These results indicate that (i) ramB transcription is negatively autoregulated by RamB under all conditions tested and (ii) ramB expression is positively regulated by RamA when glucose is the sole carbon and energy source. Since the ramB mutant RG1 (pramBp1), in spite of a deregulation, showed different CAT activities when grown in medium with glucose alone, glucose plus acetate, or acetate alone (Table 2), it must be concluded that either RamA is responsible for the induction (or derepression) of the ramB gene in the presence of glucose or another regulatory factor is involved.
Wennerhold and Bott (18) and Brune et al. (2) identified a binding site for the iron regulator DtxR centered 376 bp upstream of the ramB TS. However, although this binding site is closer (105 bp) to the TS of the neighboring succinate dehydrogenase subunit C gene sdhC (Fig. 2A), we speculated about a function of this motif for control of ramB or sdhC (18). To test for involvement of DtxR in the regulation of ramB expression, we determined CAT activities in WT C. glutamicum(pramBp1) and in C. glutamicum ΔdtxR (17) transformed with plasmid pramBp1. Both strains showed identical CAT activities in glucose minimal medium under iron limitation (1 μM) and iron excess (100 μM) conditions (data not shown). These results suggest that DtxR is not involved as a transcriptional regulator in the control of ramB expression.
Electrophoretic mobility shift assays (EMSAs) with hexahistidyl-tagged RamA and RamB fusion proteins and a series of ramB promoter fragments (Fig. 2A) were performed to assay for direct binding of RamA and/or RamB to the putative RamA and RamB binding sites observed within the ramB promoter region. Different amounts of purified RamA or RamB protein were incubated with the ramB promoter fragments and separated on an agarose gel. The relevant results of these EMSAs with the ramB fragments and RamA and RamB protein are shown in Fig. 2B and C, respectively.
The promoter fragments ramBp1, ramBp3, and ramBp5 were retarded effectively by RamA (Fig. 2B). Retardation in all three cases was complete with 1 μg of RamA protein, corresponding to molar excesses (protein/DNA) of about 50. The fragment ramBp1 formed two RamA/DNA complexes, while the fragments ramBp3 and ramBp5 formed only one RamA/DNA complex. No retardation was observed with fragments lacking the putative RamA binding sites (i.e., ramBp3a, ramBp3b, and ramBp3c). These results show relatively tight binding of RamA to the ramB promoter region.
As shown in Fig. 2C, the ramB promoter fragments containing the putative 13-bp motif (i.e., ramBp1, ramBp3, ramBp3a, and ramBp3b) were retarded by RamB, while the fragment ramBp3c (without this motif) showed no retardation. A 32-bp oligonucleotide covering the 13-bp motif also revealed retardation, corroborating our conclusion that this motif in fact is responsible for the specific binding of RamB to the ramB promoter.
The fragments ramBp3, ramBp3b, and ramBp3c were also tested for ramB promoter activity. For this purpose, we constructed respective reporter gene fusions in the promoter test vector pET2, resulting in plasmids pramBp3, pramBp3b, and pramBp3c. The promoter activities of these fragments were tested in C. glutamicum WT by measuring specific CAT activity. As shown in Table 2, pramBp3 conferred the same promoter activity to C. glutamicum as pramBp1, on all media tested. This result shows that the RamA bindings site located near the sdhC TS site has no influence on ramB expression. Plasmid pramBp3b, lacking the RamA binding site 247 bp upstream of the ramB TS, conferred about twofold-lower promoter activity in glucose medium than pramBp1 and pramBp3, and the activities of WT C. glutamicum carrying pramBp3b were comparable to those of the RamA-negative mutant RG2(pramBp1). These results indicate that RamA activates ramB expression in C. glutamicum by direct binding to the ramB promoter region when cells are grown in glucose medium.
The lack of the RamB binding site in plasmid pramBp3c [WT C. glutamicum(pramBp3c)] resulted in high ramB promoter activities similar to those observed in the ramB mutant C. glutamicum RG1(pramBp1). In accordance, the specific CAT activity of a ramBp3c-cat fusion after monocopy integration into the chromosome of C. glutamicum WT (tested by ligating fragment ramBp3c [Fig. 2A] into plasmid pRIM2 and integration) was significantly higher than that of the ramBp1-cat fusion, independent of the carbon source used (0.014 and 0.004 U/mg protein on glucose and 0.007 and 0.001 U/mg protein on acetate as the carbon sources). These results show that RamB negatively autoregulates its expression by direct binding to the 13-bp motif located 59 bp upstream of the ramB TS.
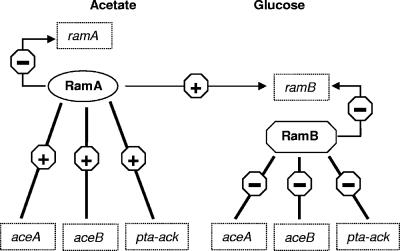
In conclusion, we provide evidence that expression of the ramB gene as well as the amount of RamB protein is significantly higher in glucose-grown cells than in acetate-grown cells, that both RamA and RamB bind to the ramB promoter region, and that ramB expression is subject to negative control by RamB and to carbon source-dependent positive control by RamA. Since RamA negatively controls the expression of its own gene (3) and additionally positively influences the expression of ramB, it can be concluded that RamA is a master regulator of acetate metabolism in C. glutamicum. A model summarizing the present knowledge on the regulation network involving RamA and RamB in C. glutamicum is shown in Fig. 3. It is interesting that genes encoding orthologs of ramA and ramB have been found in other corynebacteria, such as C. efficiens, C. diphtheriae, and C. jeikeium (1). In the former two species, the ramB ortholog is preceded by a motif resembling the 13-bp motif (centered 59 and 275 bp, respectively, upstream of the postulated translational start codons) and by several motifs similar to the C. glutamicum RamA binding sites. These observations may indicate similar regulation of ramB expression by RamB itself and by RamA in C. efficiens and C. diphtheriae.
FIG. 3.
Model of the regulatory network involving RamA and RamB in C. glutamicum grown in medium containing either glucose or acetate as the carbon source. The model is based on previous data (3, 4, 6, 7) and on data obtained here. Activation and repression are indicated by plus and minus signs, respectively. The thickness of the lines give a rough indication of the strength of activation/repression.
Acknowledgments
We thank J. Kalinowski (University of Bielefeld) for helpful comments on our manuscript.
The support of the BMBF (grants 031U213D and 0313105 “Genome research on bacteria relevant for agriculture, environment and biotechnology”) and of the Degussa AG is gratefully acknowledged.
Footnotes
Published ahead of print on 17 November 2006.
REFERENCES
- 1.Brune, I., K. Brinkrolf, J. Kalinowski, A. Pühler, and A. Tauch. 2005. The individual and common repertoire of DNA-binding transcriptional regulators of Corynebacterium glutamicum, Corynebacterium efficiens, Corynebacterium diphtheriae, and Corynebacterium jeikeium deduced from the complete genome sequence. BMC Genomics 6:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brune, I., H. Werner, A. T. Hüser, J. Kalinowski, A. Pühler, and A. Tauch. 2006. The DtxR protein acting as dual transcriptional regulator directs a global regulatory network involved in iron metabolism of Corynebacterium glutamicum. BMC Genomics 7:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cramer, A., and B. J. Eikmanns. 2007. RamA, the transcriptional regulator of acetate metabolism in Corynebacterium glutamicum is subject to negative autoregulation. J. Mol. Microbiol. Biotechnol. 12:49-57. [DOI] [PubMed] [Google Scholar]
- 4.Cramer, A., R. Gerstmeir, S. Schaffer, M. Bott, and B. J. Eikmanns. 2006. Identification of RamA, a novel LuxR-type transcriptional regulator of genes involved in acetate metabolism of Corynebacterium glutamicum. J. Bacteriol. 188:2554-2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dubendorff, J. W., and F. W. Studier. 1991. Controlling basal expression in an inducible T7 expression system by blocking the target T7 promoter with lac repressor. J. Mol. Biol. 219:45-59. [DOI] [PubMed] [Google Scholar]
- 6.Gerstmeir, R., A. Cramer, P. Dangel, S. Schaffer, and B. J. Eikmanns. 2004. RamB, a novel transcriptional regulator of genes involved in acetate metabolism of Corynebacterium glutamicum. J. Bacteriol. 186:2798-2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerstmeir, R., V. F. Wendisch, S. Schnicke, H. Ruan, M. Farwick, D. Reinscheid, and B. J. Eikmanns. 2003. Acetate metabolism and its regulation in Corynebacterium glutamicum. J. Biotechnol. 104:99-122. [DOI] [PubMed] [Google Scholar]
- 8.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 9.Hansmeier, N., A. Albersmeier, A. Tauch, T. Damberg, R. Ros, D. Anselmetti, A. Pühler, and J. Kalinowski. 2006. The surface (S)-layer gene cspB of Corynebacterium glutamicum is transcriptionally activated by a LuxR-type regulator and located on a 6 kb genomic island absent from the type strain ATCC 13032. Microbiology 152:923-935. [DOI] [PubMed] [Google Scholar]
- 10.Hermann, T. 2003. Industrial production of amino acids by coryneform bacteria. J. Biotechnol. 104:155-172. [DOI] [PubMed] [Google Scholar]
- 11.Kalinowski, J., B. Bathe, D. Bartels, M. Bischoff, M. Bott, A. Burkovski, N. Dusch, L. Eggeling, B. J. Eikmanns, L. Gaigalat, A. Goesmann, M. Hartmann, K. Huthmacher, R. Krämer, B. Linke, A. C. McHardy, F. Meyer, B. Möckel, W. Pfefferle, A. Pühler, D. Rey, C. Rückert, O. Rupp, H. Sahm, V. F. Wendisch, I. Wiegräbe, and A. Tauch. 2003. The complete Corynebacterium glutamicum ATCC 13032 genome sequence and its impact on the production of l-aspartate-derived amino acids and vitamins. J. Biotechnol. 104:5-25. [DOI] [PubMed] [Google Scholar]
- 12.Kelle, R., T. Hermann, and B. Bathe. 2005. l-Lysine production, p. 465-488. In L. Eggeling and M. Bott (ed.), Handbook of Corynebacterium glutamicum. CRC Press, Boca Raton, FL.
- 13.Kimura, E. 2005. l-Glutamate production, p. 439-463. In L. Eggeling and M. Bott (ed.), Handbook of Corynebacterium glutamicum. CRC Press, Boca Raton, FL.
- 14.Liebl, W. 2005. Corynebacterium taxonomy, p. 9-34. In L. Eggeling and M. Bott (ed.), Handbook of Corynebacterium glutamicum. CRC Press, Boca Raton, FL.
- 15.Patek, M., B. J. Eikmanns, J. Patek, and H. Sahm. 1996. Promoters from Corynebacterium glutamicum: cloning, molecular analysis and search for a consensus motif. Microbiology 142:1297-1309. [DOI] [PubMed] [Google Scholar]
- 16.Patek, M., J. Nesvera, A. Guyonvarch, O. Reyes, and G. Leblon. 2003. Promoters of Corynebacterium glutamicum. J. Biotechnol. 104:311-323. [DOI] [PubMed] [Google Scholar]
- 17.Vasicova, P., Z. Abrhamova, J. Nesvera, M. Patek, H. Sahm, and B. Eikmanns. 1998. Integrative and autonomously replicating vectors for analysis of promoters in Corynebacterium glutamicum. Biotechnol. Techniques 12:743-746. [Google Scholar]
- 18.Wennerhold, J., and M. Bott. 2006. The DtxR regulon of Corynebacterium glutamicum. J. Bacteriol. 188:2907-2918. [DOI] [PMC free article] [PubMed] [Google Scholar]