Abstract
Oxidative-stress resistance in Staphylococcus aureus is linked to metal ion homeostasis via several interacting regulators. In particular, PerR controls the expression of a regulon of genes, many of which encode antioxidants. Two PerR regulon members, ahpC (alkylhydroperoxide reductase) and katA (catalase), show compensatory regulation, with independent and linked functions. An ahpC mutation leads to increased H2O2 resistance due to greater katA expression via relief of PerR repression. Moreover, AhpC provides residual catalase activity present in a katA mutant. Mutation of both katA and ahpC leads to a severe growth defect under aerobic conditions in defined media (attributable to lack of catalase activity). This results in the inability to scavenge exogenous or endogenously produced H2O2, resulting in accumulation of H2O2 in the medium. This leads to DNA damage, the likely cause of the growth defect. Surprisingly, the katA ahpC mutant is not attenuated in two independent models of infection, which implies reduced oxygen availability during infection. In contrast, both AhpC and KatA are required for environmental persistence (desiccation) and nasal colonization. Thus, oxidative-stress resistance is an important factor in the ability of S. aureus to persist in the hospital environment and so contribute to the spread of human disease.
Reactive oxygen species, such as H2O2, are produced as a by-product of aerobic bacterial growth (22). Also, during bacterial infection, the host produces H2O2 and other reactive oxygen species inside neutrophils, as part of an oxidative killing mechanism (15). H2O2 can oxidize cysteine and methionine residues, which may lead to protein inactivation. Furthermore, H2O2 can disrupt the iron-sulfur clusters of aconitase and fumarase. However, toxicity is mainly attributed to the reduction of H2O2 by Fe(II) and the subsequent formation of hydroxyl radicals. This Fenton reaction primarily leads to DNA damage, due to the association of Fe(II) with nucleic acids (22). Previous in vivo studies have shown H2O2 to cause DNA damage, which is reduced by the presence of iron chelators (21, 48). Thus, scavenging of H2O2 is important for aerobic survival. Catalase (KatA) is known to detoxify H2O2 and was proposed to be a major virulence determinant in Staphylococcus aureus (34), where strains producing low levels of KatA were more sensitive to killing by neutrophils. However, a katA mutant of S. aureus was not attenuated in a murine-abscess model of infection (17). Also, all catalase-negative strains of S. aureus retain virulence (37).
In contrast to Escherichia coli and Bacillus subtilis, KatA is the sole catalase protein in S. aureus (2, 18, 33). In S. aureus, the expression of katA is positively regulated by the ferric uptake regulator (Fur) in an Fe(II)-dependent manner (17). Thus, under high Fe(II) conditions, the increase in catalase reduces the level of H2O2 and prevents the formation of toxic hydroxyl radicals. In many bacteria, including S. aureus, the role of Fur is primarily in iron homeostasis (10, 17, 58). Fur binds to a consensus sequence, designated the Fur box, and represses the transcription of genes involved in iron uptake. In S. aureus, a Fur box is not present in the promoter region of katA (17). Regulation may occur indirectly, as observed for the sodB gene of Escherichia coli, which is positively regulated by Fur via the RyhB small regulatory RNA (35).
S. aureus contains two additional Fur homologues: the peroxide response regulator (PerR) and the zinc uptake regulator (Zur) (18, 32). The PerR regulon in S. aureus comprises genes encoding antioxidant proteins (for example, KatA, AhpCF, Bcp, and TrxB), iron storage proteins (Ftn and MrgA), Fur, and PerR. In Bacillus subtilis, PerR is an iron- and manganese-responsive transcriptional repressor (16). Binding of Fe(II) renders the B. subtilis PerR protein more susceptible to oxidation by peroxides, which disrupts DNA binding and relieves repression (31). The S. aureus PerR is a Mn(II)-dependent repressor, with repression being relieved in the presence of Fe(II) and/or peroxides (18). Mn(II) ions have antioxidant properties and can scavenge both superoxide and H2O2 (3, 4, 54). The presence of Mn(II) may therefore reduce the requirement for the PerR-controlled antioxidant proteins. In S. aureus, Mn(II) uptake is mediated by the MntABC and MntH transporters, which are regulated by MntR (19). MntR is an Mn(II)-dependent regulator of transcription. Diminished Mn(II) uptake results in reduced growth and increased sensitivity to oxidative stress, which indicates the importance of Mn(II) homeostasis. However, PerR-repressing Mn(II) concentrations increased the sensitivity of wild-type S. aureus cells to H2O2 stress (18). Thus, Mn(II) cannot fully compensate for the antioxidant proteins regulated by PerR, as occurs in B. subtilis (9).
Previous work has shown that catalase mutants of E. coli did not display any growth defects or an increased mutation rate (33). Further studies revealed that H2O2-scavenging activity was retained in the catalase mutants due to the presence of alkyl hydroperoxide reductase (AhpC) (50). AhpC is a member of the peroxiredoxin family of enzymes, which have activity against H2O2, organic peroxides, and peroxynitrite (44). E. coli AhpC was a more efficient scavenger of low-levels H2O2 than catalase and was denoted the primary scavenger of endogenously produced H2O2. In comparison, catalase was described as the primary scavenger of H2O2 at high levels (50). Strains lacking catalase and AhpC, Hpx− cells, displayed an aerobic growth defect, attributed to accumulation of endogenously produced H2O2 and subsequent Fenton reaction-derived DNA damage. Supplementation of the media with an iron chelator or catalase enhanced the growth of Hpx− cells due to a reduction in the Fenton reaction. Furthermore, overproduction of Dps, an iron sequestration protein, complemented the growth defect of the Hpx− strain, probably due to its iron-binding activity (42).
In S. aureus, the expression of ahpC is regulated by PerR, and a putative PerR box is present within the promoter region. Proteins of the PerR regulon were overexpressed in perR and katA mutant strains (18). It is likely that the lack of catalase increases the level of intracellular H2O2, which is sensed by PerR and results in derepression of the PerR regulon. Therefore, the lack of attenuation during pathogenesis for an S. aureus katA mutant may be due to the PerR-dependent increase in ahpC and its compensatory activity. In this study, we analyzed the roles and regulation of katA and ahpC in S. aureus. Mutually compensatory expression exists for both katA and ahpC. This leads to a dual function in oxidative-stress resistance, environmental persistence, and host-pathogen interaction.
MATERIALS AND METHODS
Media and growth conditions.
S. aureus and Escherichia coli strains and plasmids are listed in Table 1. E. coli was grown in Luria-Bertani (LB) medium at 37°C unless otherwise stated. S. aureus was grown in BHI (Oxoid), Chelex-treated BHI (CT-BHI), or CLR minimal medium at 37°C with shaking at 250 r.p.m. CLR and CT-BHI were prepared as described by Horsburgh et al. (17, 19). Experimental 25-ml cultures in acid-washed 250-ml flasks were incubated at a starting optical density at 600 nm (OD600) of 0.01, unless otherwise stated, before growth at 37°C. When required, antibiotics were added at the following concentrations: ampicillin, 100 mg liter−1; chloramphenicol, 12.5 mg liter−1; tetracycline, 5 mg liter−1; erythromycin, 5 mg liter−1; lincomycin, 25 mg liter−1; kanamycin, 50 mg liter−1; neomycin, 50 mg liter−1; and rifampin, 1 mg liter−1.
TABLE 1.
Strains, plasmids and primers used in this study
| Strain, plasmid, or primer | Genotype or description | Reference or source |
|---|---|---|
| Strains | ||
| E. coli EL250 | F−mcr Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 recA1 deoR araD139 Δ(ara-leu)7697 galU galK rpsL (Strr) endA1 nupG [λcl857 araC-PBADflpe] | 30 |
| S. aureus | ||
| RN4220 | Restriction-deficient transformation recipient | Laboratory stock |
| SH1000 | Wild-type strain cured of prophages; rsbU+ | 20 |
| KC055 | katA::pAZ106 katA+ | This study |
| KC056 | perR::kan katA::pAZ106 katA+ | This study |
| KC067 | ahpC::tet katA::pAZ106 katA+ | This study |
| KC070 | ahpC::tet perR::kan katA::pAZ106 katA+ | This study |
| KC041 | ahpC::tet | This study |
| KS100 | katA::Tn917 (ery) | 53 |
| KC043 | ahpC::tet katA::Tn917 (ery) | This study |
| MHKA | sodA::Tn917 (ery) | 26 |
| KC075 | ahpC::tet sodA::Tn917 (ery) | This study |
| KC072 | ahpC::tet katA::Tn917 (ery) perR::kan | This study |
| MHK2 | fur::tet | Laboratory stock |
| KC044 | ahpC::tet fur::kan | This study |
| KC073 | katA::Tn917 (ery) fur::kan | This study |
| KC072 | ahpC::tet fur::kan katA::Tn917 (ery) | This study |
| GC3 | ahpC::tet katA::Tn917 (ery) pSK5630 | This study |
| GC2 | ahpC::tet katA::Tn917 (ery) pSK5630 (ahpC+) | This study |
| Plasmids | ||
| SABAC1 | S. aureus bacterial artificial chromosome containing genomic DNA from 457727 to 401407 (COL coordinates) | J. Iandolo, http://www.genome.ou.edu/staph.html |
| pGL400 | tet cassette-containing vector | J. Garcia-Lara, personal communication |
| pSK5630 | E. coli-S. aureus shuttle vector | 12 |
| pKC2 | AhpC + 1-AhpC + 2 ahpC gene and promoter region in pSK5630 | This study |
| Primers | ||
| AhpCtet1 | AACAAAGAAATCTTACCATTTACAGCGCAAGCTTTCGATCCACGGATTTTATGACCGATGAGAAG | This study |
| AhpCtet2 | AATCTAAACCAGGTTGCAATGTTTTAGCGCCTTCTTCCCAAGAAATCCCTTTGAGAATGTTT | This study |
| AhpC + 1 | AATGTTGGATCCTGTCAGTGAAATGAATG | This study |
| AhpC + 2 | TCACTCGGATCCTTCATCCATCTCGAAC | This study |
| Acn1 | TAAGCGTTTTCTAAGCATGATG | This study |
| Acn3 | ACGTGATGACATATAGTTTTTAG | This study |
Construction of ahpC mutant strains.
SABAC1 containing 56.4 kb of S. aureus genomic DNA, including the ahpC gene, was obtained from J. Iandolo (http://www.genome.ou.edu/staph.html) and used to transform electrocompetent E. coli EL250. The tetracycline antibiotic resistance cassette was PCR amplified from pGL400 (J. Garcia-Lara, personal communication), using λred primers AhpCtet1 and AhpCtet2 and purified by gel extraction. Electrocompetent EL250 SABAC1 cells were prepared, λred genes were induced, and the cells were electroporated with ∼80 ng of purified tet DNA (30, 52), which recombined with SABAC1 to produce SABAC(tet). Large-scale SABAC(tet) purification was performed using the Clontech maxi BAC purification kit. S. aureus RN4220 competent cells were prepared and electroporated with ∼1 μg of SABAC1(tet) DNA (52). The unresolved locus was transferred into recipient strains SH1000 and KS100 (katA) by phage transduction (40) using φ11 as the transducing phage. Southern blotting was used to verify the mutant strains.
Complementation analysis.
Plasmid pKC2, for complementation of the ahpC mutation, was generated by amplification of ahpC, ahpF, and the promoter region using the primer pair AhpC + 1 and AhpC + 2 (Table 1). The purified DNA, digested with BamHI, was cloned into appropriately digested shuttle vector pSK5630 (14). The pSK5630 and pKC2 plasmids were used to transform electrocompetent S. aureus RN4220 and then transferred into the SH1000 and KC043 (ahpC katA) strains by phage transduction using φ11. Complementation of the ahpC mutation was analyzed by assaying paraquat sensitivity using disk diffusion.
β-Galactosidase assays.
Levels of β-galactosidase activity were measured using 4-methyl umbelliferone-β-d-galactoside (Sigma) as a substrate, as described previously (17). One unit of β-galactosidase activity was defined as the amount of enzyme that catalyzed the production of 1 pmol methylumbelliferyl min−1 OD600 unit−1. Assays were performed on duplicate samples, and the values were averaged. The results presented here are representative of two independent experiments, which showed less than 20% variability.
Catalase assays and sensitivity testing.
Catalase activity of washed cells, lysed with lysostaphin (100 μg ml−1), was detected after electrophoresis on a 12% (wt/vol) native polyacrylamide gel, pH 7.5 (56). The Wayne and Diaz (56) staining method was used to visualize bands of activity. Catalase activity was also assayed spectrophotometrically at 240 nm, as described by Beers and Sizer, using 0.1 M potassium phosphate buffer (pH 7.0) and 10 mM H2O2 (6). The protein concentration was measured using the method of Bradford, with bovine serum albumin as the standard (7). Screening for sensitivity to paraquat, sodium nitroprusside (SNP), SNP plus paraquat, diamide, and tellurite was performed by disk diffusion assay, as described previously (19), using CT-BHI agar without MnSO4. The following amounts of each stress compound were used: 50 μl of 3 M paraquat, 50 μl of 1 M SNP, 50 μl of a 50:50 mixture of 3 M paraquat and 1 M SNP, 25 μl of 1 M diamide, and 25 μl of 1 M potassium tellurite. H2O2 resistance assays were performed on cells grown to exponential phase in CT-BHI, as described previously (17). A similar method was employed to test cumene hydroperoxide sensitivity, except the cells were diluted in phosphate-buffered saline (PBS). For testing sensitivity to heat, strains were grown in 50 ml BHI (inoculated at an OD600 of 0.005) to an OD600 of 0.1. The culture was split in two, and one flask was heat shocked at 54°C for 10 min while the other was kept static at 37°C for 10 min. A similar method was used to test salt shock sensitivity, except the starting OD600 was 0.05, and the cells were grown to an OD600 of 0.5 and shocked with 2 M NaCl. Growth inhibition was measured after heat and salt shocks.
Measuring H2O2 scavenging and production.
H2O2 was detected using the amplex red/horseradish peroxidase assay as described by Seaver and Imlay, measuring absorbance at 568 nm (50). Scavenging of H2O2 was measured using exponential-phase cells grown in CT-BHI (CLR was used for measuring 2 μM H2O2 scavenging), washed, and resuspended in PBS to an OD600 of 0.1. H2O2 was added at either 20 or 2 μM concentrations, and at intervals, 450-μl aliquots were removed and assayed immediately for H2O2 content. Production of H2O2 was measured by assaying samples taken during growth in CLR.
Measuring DNA damage.
Strains were grown in CLR for 24 h to stationary phase, and genomic DNA was extracted from 3 ml of culture (25 ml for KC043 [ahpC katA]) using a DNeasy Tissue kit (QIAGEN). The extracted DNA was quantified at A260. Primers Acn1 and Acn2 were used to amplify a 10-kb region surrounding the aconitase gene. PCR was performed using the Reddy extensor mix system (ABgene). The 25-μl PCR mixture contained 1 μg of genomic DNA as a template, a 4-μM concentration (each) of the two primers, 350 μM (each) of the four deoxynucleoside triphosphates, PCR buffer with 2.25 mM MgCl2, and 1.25 U of Taq DNA polymerase. The PCR was carried out using the innermost wells of a Techgene PCR machine (Techne). Genomic DNA was initially denatured for 1 min at 94°C and then subjected to 25 cycles of 94°C for 15 seconds, 50°C for 30 seconds, and 72°C for 10 min, with a final extension time of 12 min at 72°C. PCR products were separated by 1% (wt/vol) agarose gel electrophoresis, stained with ethidium bromide, and photographed using a UV transilluminator at 260 nm, a UVi Tec Digital camera, and a Uvi Doc Gel documentation system. The density of each band was analyzed using ImageJ software (http://rsb.info.nih.gov/ij/). To measure the rate of spontaneous mutagenesis, strains were grown in CLR for 24 h to stationary phase. Cultures were diluted to an OD600 of 1.0, and 500 μl was mixed with CT-BHI top agar containing rifampin. This was used to overlay a CT-BHI agar plate, also containing rifampin, and incubated at 37°C overnight. The mutagenesis rate was calculated by dividing the number of rifampin-resistant colonies by the number of viable colonies.
In vitro survival assays.
Starvation survival experiments were carried out in glucose-limiting CDM medium, with shaking at 250 rpm at 37°C (55). Desiccation tolerance testing was performed on stationary-phase cells grown in CT-BHI, washed, and resuspended in PBS to an OD600 of 0.05. The cells were desiccated at 37°C in the dark on 1-cm by 1-cm pieces of acetate sheet for 24 h. Viability was determined by vortexing the acetate sheet in 2 ml of PBS with serial dilution before plating the cells on BHI agar. Comparison of strains was evaluated on the recovery of bacteria using Student's t test.
Pathogenicity tests.
Tests of survival inside human neutrophils were performed as described previously (43) on exponential-phase cells grown in CT-BHI and diluted to an OD600 of 0.0065 in Hanks balanced salt solution containing 4% (vol/vol) human serum. The cells were incubated at 37°C for 10 min to allow opsonization. Purified human neutrophils and the opsonized bacteria were mixed in a 50:1 ratio in Hanks balanced salt solution and incubated at 37°C. At intervals, samples were taken, the neutrophils were lysed with 0.05% saponin, and bacterial counts were performed using serial dilutions. The virulence of S. aureus strains was determined using a murine septic-arthritis model of infection as described by Jonsson et al. (25) and a novel atopic-dermatitis model. The latter involved inducing a delayed-type hypersensitivity reaction by applying 3% (wt/vol) oxazolone to the belly of the mouse. After 5 to 7 days, 1% (wt/vol) oxazolone was applied to the ears of each mouse every 2 days in order to elicit dermatitis. Two weeks later, the mice were anesthetized, and a 1 × 106 inoculum of S. aureus cells was applied to each ear. After 2 days, the mice were sacrificed, and the ears were surgically removed and homogenized. Serial dilutions were plated onto blood agar plates in order to determine the number of CFU ml−1 of S. aureus cells. Comparison of strains was evaluated on the recovery of bacteria using Student's t test.
Nasal colonization assay.
Nasal colonization by S. aureus strains was determined using a cotton rat model as described previously (29). Comparison of strains was evaluated on the recovery of bacteria using Student's t test.
RESULTS
Inactivation of ahpC.
To determine the function of AhpC, a tetracycline resistance cassette was introduced into the gene by direct recombination using the λred system (30). After transformation of RN4220, a transductional outcross into S. aureus SH1000 resulted in strain KC041 (ahpC), which was verified by PCR amplification and Southern blotting (results not shown).
Regulatory interplay between ahpC and katA.
We propose that as KatA and AhpC are thought to play linked roles in S. aureus, a possible regulatory interplay between the two genes exists. Using a katA::lacZ fusion, the regulation of katA by AhpC and PerR was examined. Maximal levels of katA expression were observed in postexponential phase (Fig. 1A), with increased transcription in MHK1 (perR), KC041 (ahpC), and KC042 (ahpC perR). The absence of both AhpC and PerR had no additive effect on katA transcription (over and above perR), and therefore, regulation by AhpC is likely to occur via PerR. Catalase activity also increased in an ahpC-dependent manner (Fig. 1B and C). Catalase activity was consistently higher, throughout growth, in KC041 (ahpC) compared to the wild type (SH1000), with a threefold difference at stationary phase (Fig. 1C). Previous studies have shown an increase in AhpC production in ST16 (in the 8325-4 strain background, katA), although at a lower level than that observed in MJH001 (perR) (18).
FIG. 1.
Regulatory interplay between ahpC and katA. (A) β-Galactosidase expression (closed symbols) and growth (open symbols) were measured in SH1000 (wild type) (squares), ahpC (triangles), perR (diamonds), and ahpC perR (circles) backgrounds using the fusion strains KC055 (katA-lacZ), KC067 (ahpC katA-lacZ), KC056 (perR katA-lacZ), and KC070 (ahpC perR katA-lacZ) grown in CT-BHI. The perR and ahpC perR β-galactosidase activity data overlie each other. (B) The catalase activity of lysed exponential-phase cells (OD600, 0.5) grown in CT-BHI was determined using a native polyacrylamide gel electrophoresis gel (20-μl sample, equivalent to 0.4 OD600 units). (C) Growth (open symbols) and catalase activity (closed symbols) of SH1000 (squares), KC041 (ahpC) (triangles), KS100 (katA) (diamonds), and KC043 (ahpC katA) (circles).
The roles of AhpC and KatA in growth of S. aureus.
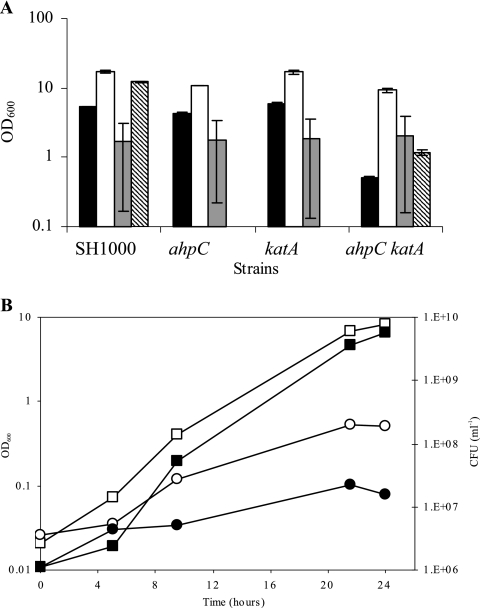
The above-described analyses identified compensatory regulation of ahpC and katA. To analyze the functional interplay between AhpC and KatA, a mutant strain lacking both proteins was created by phage transduction. No growth defect was observed for any of the strains in BHI. KC043 (ahpC katA) had a reduced growth yield in CLR (17) compared to the wild type and the single-mutant strains (Fig. 2A). CLR supplemented with catalase restored wild-type growth levels to KC043 (ahpC katA), which was only partial with boiled catalase. This confirmed that complementation was due to the enzymatic activity of catalase. When grown under conditions of low aeration, KC043 (ahpC katA) showed no growth defect (Fig. 2A). Moreover, when grown under strictly anaerobic conditions on CLR agar plates, all strains had similar levels of growth (results not shown). Thus, AhpC and KatA are most important under aerobic conditions. The 16-fold difference in growth (OD600) yield between the wild type and KC043 (ahpC katA), grown in CLR aerobically, equates to a 350-fold difference in viability (CFU ml−1) (Fig. 2B). The reduced plating efficiency in KC043 (ahpC katA) suggested that during aerobic growth, both AhpC and KatA are required to prevent cell damage.
FIG. 2.
Roles of ahpC and katA in aerobic growth. (A) Final growth yield (24 h) of strains SH1000 (wild type), KC041 (ahpC), KS100 (katA), and KC043 (ahpC katA) grown in CLR (black bars), CLR supplemented with 1 mg ml−1 catalase (white bars), and under low-aeration conditions (100 ml CLR in a 125-ml conical flask; 41 h) (gray bars). A boiled-catalase control was carried out only for SH1000 and KC043 (ahpC katA) (hatched bars). The error bars indicate standard errors of the means. (B) Optical density (open symbols) and viability (closed symbols) of SH1000 (squares) and KC043 (ahpC katA) (circles) grown in CLR.
Roles of KatA and AhpC in hydrogen peroxide resistance.
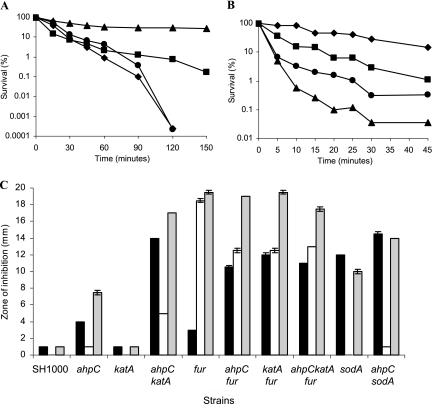
Previous studies showed that KatA is required for H2O2 stress resistance in S. aureus (18). Furthermore, AhpC was shown to have H2O2-scavenging activity in E. coli (50). Using an H2O2 death assay, KC041 (ahpC), KS100 (katA), and KC043 (ahpC katA) were compared with SH1000 (wild type) (Fig. 3A). KC041 (ahpC) was more resistant than the wild type to H2O2, probably due to increased katA expression and activity (Fig. 1). In contrast, KS100 (katA) was hypersensitive to H2O2, as expected. However, there was no additional effect on H2O2 stress resistance in KC043 (ahpC katA). Thus, the major component involved in resistance to externally applied H2O2 is KatA.
FIG. 3.
Oxidative-stress resistance of S. aureus. Strains grown in CT-BHI were analyzed using a death assay in response to 7.5 mM H2O2 (A) or 7.5 mM cumene hydroperoxide (B). Exponential-phase cells of SH1000 (▪), KC041 (ahpC) (▴), KS100 (katA) (⧫), and KC043 (ahpC katA) (•) were used. (C) Stress resistance of S. aureus strains analyzed using disk diffusion assays. Exponential-phase cells were added to CT-BHI agar and overlaid onto CT-BHI agar plates. A 1.3-cm-diameter disk spotted with 50 μl of 3 M paraquat (black bars), 50 μl of 1 M SNP (white bars), or 50 μl of a 50:50 mixture of 3 M paraquat and 1 M SNP (gray bars) was placed on each plate and incubated at 37°C overnight. The data are the mean of two plates for each strain from two independent experiments; the standard error of the mean is indicated.
Roles of KatA and AhpC in organic-hydroperoxide resistance.
In B. subtilis, AhpC has been identified as an organic-peroxide resistance protein (2). Studies of organic-peroxide sensitivity commonly use cumene or tert-butyl hydroperoxide. The sensitivity of S. aureus to both organic peroxides was tested using death (Fig. 3B) and disk diffusion assays (results not shown). KC041 (ahpC) had a reduced ability to survive cumene hydroperoxide stress compared to the wild type, whereas KS100 (katA) showed increased survival (Fig. 3B). Thus, AhpC is a major component involved in cumene hydroperoxide resistance. The increased resistance observed in KS100 (katA) is probably due to an increase in ahpC expression via PerR. KS043 (ahpC katA) was more resistant to cumene hydroperoxide than KC041 (ahpC), possibly because lack of both peroxidases caused the induction of other unknown resistance proteins. Both AhpC and KatA have roles in resistance to tert-butyl hydroperoxide (50 μl, 14% [vol/vol]), as shown in a disk diffusion assay of bacteria grown on LB agar. KC043 (ahpC katA) showed an increased zone of inhibition (24.5 mm) compared to SH1000 (19.5 mm), KC041 (ahpC) (19 mm), and KS100 (katA) (19 mm) (results not shown).
Roles of KatA and AhpC in paraquat resistance.
Paraquat is a generator of internal superoxide, which is converted by superoxide dismutase (SOD) enzymes to H2O2 (26). In S. aureus, inactivation of the major SOD enzyme, encoded by sodA, results in reduced ability to survive paraquat stress (Fig. 3C) (26). The sensitivities of KC041 (ahpC), KS100 (katA), and KC043 (ahpC katA) to paraquat were tested using disk diffusion assays and compared to SH1000 (wild type) (Fig. 3C). Zones of growth inhibition for KC041 (ahpC) and KS100 (katA) were comparable to that of the wild type. Also, inactivation of ahpC did not increase the paraquat sensitivity of MHKA (sodA). However, KC043 (ahpC katA) showed a significant increase (P = 0.01) in sensitivity to paraquat compared to the wild type, probably due to increased sensitivity to the H2O2 produced by enzymatic dismutation. The addition of a perR mutant did not decrease the zone of inhibition for KC043 (ahpC katA), suggesting that relief of PerR repression cannot compensate for the lack of AhpC and KatA by other mechanisms (results not shown).
Sensitivity to H2O2 increases in the presence of excess iron due to the Fenton reaction (22). We tested whether inactivation of fur affected the sensitivity of S. aureus to paraquat. MHK2 (fur) showed no difference in paraquat sensitivity compared to the wild type. However, inactivation of fur in KC041 (ahpC) or KS100 (katA) resulted in increased sensitivity to paraquat (P = 0.014) compared to MHK2 (fur). Thus, AhpC and KatA have roles to play in paraquat resistance in the absence of Fur and subsequent iron excess.
Roles of KatA and AhpC in peroxynitrite resistance.
Peroxynitrite is formed by the reaction of superoxide with nitric oxide (NO). SNP is an NO-releasing compound that was used in a 50:50 mixture with paraquat to generate peroxynitrite stress. Disk diffusion assays were utilized to test the sensitivities of ahpC and katA mutants to SNP and SNP-plus-paraquat stress; the results were then compared to those for the wild type (Fig. 3C). No zone of growth inhibition was observed for the wild type, KS100 (katA), or MHKA (sodA) when challenged with SNP. MHK2 (fur) displayed increased sensitivity to SNP compared to the wild type, probably due to the formation of toxic Fe-nitrosyl complexes. The inactivation of ahpC and/or katA decreased the zone of growth inhibition in MHK2 (fur). Absence of AhpC or KatA is likely to cause increased oxidative stress and derepression of the PerR regulon, which may protect against NO stress. An increased sensitivity to SNP plus paraquat was observed in all fur mutant strains compared to the wild type, probably due to increased Fe(II) levels. There was a significant increase in the zone of growth inhibition for KC043 (ahpC katA) (P = 0.002) when challenged with SNP plus paraquat compared to paraquat alone. However, KS100 (katA) displayed similar sensitivities to SNP plus paraquat and to paraquat, whereas KC041 (ahpC) showed a significant increase in sensitivity compared to SNP (P = 0.01) or paraquat (P = 0.03) alone. Thus, AhpC maybe a predominant peroxynitrite resistance protein present in S. aureus.
An increase in AhpC production was observed in S. aureus following growth in 2.5 M NaCl (4a). Furthermore, heat and salt shocks induced the expression of ahpC in B. subtilis (2). However, neither AhpC nor KatA has a role in heat or salt stress, and they do not provide tellurite or diamide resistance, as determined by growth inhibition and disk diffusion assays (results not shown).
Complementation of ahpC.
The paraquat sensitivity of KC043 (ahpC katA) (10 mm) was partially restored by complementation of the ahpC mutation with a plasmid containing the cloned ahpC gene in strain GC2 (ahpC katA [pSK5630 ahpC+]) (7.5 mm) compared to strain GC3 (ahpC katA pSK5630) (10 mm) (results not shown). The plasmid carrying strains in the ahpC katA background were relatively unstable, possibly due to the accumulation of DNA damage (see below).
Catalase activities of AhpC and KatA.
The hypersensitivity of KC043 (ahpC katA) to paraquat stress suggests that AhpC and KatA are responsible for resistance to internally generated H2O2 stress. Catalase activity is higher in KC041 (ahpC) than in the wild type due to the PerR-dependent increase in katA expression in the absence of AhpC (Fig. 1B and 1C). The low catalase activity observed in KS100 (katA) may represent the activity of AhpC and is supported by the loss of activity in KC043 (ahpC katA) (Fig. 1C).
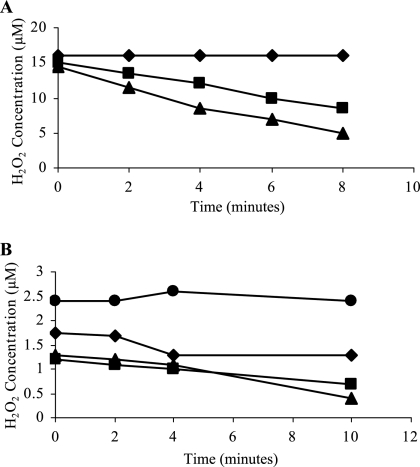
In E. coli, AhpC is proposed to scavenge low levels of H2O2, becoming saturated at high levels due to the dependence on NADPH for enzymatic recycling. In contrast, KatA has greater H2O2-scavenging activity and is not saturated (50). We tested the effect of inactivating ahpC and katA on the ability of S. aureus to scavenge low and high levels of H2O2. Only KC041 (ahpC) and the wild type were able to scavenge 20 μM H2O2 (Fig. 4A). Thus, in S. aureus, KatA is the primary scavenger of high levels of H2O2. In order to analyze low-level H2O2-scavenging abilities, cells were grown in CLR freshly made to reduce endogenous peroxide levels (50) and challenged with 2.5 μM H2O2 (Fig. 4B). KC041 (ahpC) and KS100 (katA) were equally able to scavenge 2.5 μM H2O2, and KS100 (katA) to a lesser extent. KC043 (ahpC katA) was unable to scavenge either high or low levels of H2O2, indicating that AhpC and KatA are the enzymes responsible for scavenging H2O2 in S. aureus.
FIG. 4.
Scavenging of H2O2 by whole S. aureus cells was measured at 568 nm using the reagents Ultra Amplex Red and horseradish peroxidase. Strains SH1000 (▪), KC041 (ahpC) (▴), KS100 (katA) (⧫), and KC043 (ahpC katA) (•) were grown to exponential phase in CT-BHI and challenged with ∼20 μM H2O2 (the KS100 [katA] and KC043 [ahpC katA] results entirely overlap) (A) and grown in CLR and challenged with ∼2.5 μM H2O2 (B).
Production of hydrogen peroxide in ahpC katA.
The production of H2O2 in S. aureus during growth was measured, and minimal levels of H2O2 throughout growth in the wild type and KC041 (ahpC) were determined (Fig. 5). A slight accumulation of H2O2 was observed in KS100 (katA) at stationary phase (2.3 μM). However, in KC043 (ahpC katA), H2O2 rose to 24.3 μM at stationary phase. Thus, in the absence of AhpC and KatA, the accumulation of H2O2 was greatly increased compared to the wild type, and both proteins are responsible for scavenging endogenously produced H2O2.
FIG. 5.
S. aureus produces H2O2. Growth (open symbols) and H2O2 accumulation (closed symbols) were measured in SH1000 (▪), KS100 (katA) (⧫), and KC043 (ahpC katA) (•) during growth in CLR. The SH1000 (▪) and KC041 (ahpC) (▴) results entirely overlap.
Detection of DNA damage in ahpC katA.
The accumulation of H2O2 in KC043 (ahpC katA) may cause the observed aerobic growth defect due to Fenton-derived DNA damage. The presence of nicked DNA was investigated using PCR analysis with genomic DNA from stationary-phase cells. KC041 (ahpC) and KS100 (katA) showed increased density of an identical 10-kb PCR product compared to the wild type (Fig. 6A). However, the density of the PCR product from KC043 (ahpC katA) was 13-fold lower than that of the wild type (P < 0.001), indicative of increased DNA damage.
FIG. 6.
Roles of AhpC and KatA in prevention of DNA damage. (A) Identical 10-kb PCR products were amplified from genomic DNA extracted from stationary-phase cells grown in CLR, and the percentage density compared to that of the wild type (100%) was measured using ImageJ software. (B) Rate of spontaneous rifampin-resistant mutants in S. aureus strains. The error bars indicate standard deviations.
Quantitative analysis of DNA damage was performed by calculating the frequency of spontaneous rifampin-resistant mutants (Fig. 6B). Similar rates were observed in the wild type, KC041 (ahpC), and KS100 (katA), whereas KC043 (ahpC katA) displayed a 10-fold increase in the mutagenesis rate (P = 0.03), thus confirming that AhpC and KatA have roles to play in preventing DNA damage, most likely caused by H2O2 produced endogenously during aerobic growth.
Survival of ahpC and katA under glucose starvation and desiccation conditions.
KatA has been identified as important for survival during glucose starvation (18). In order to analyze the role of AhpC, KC041 (ahpC) and KC043 (ahpC katA) were grown in glucose-limiting minimal medium for 12 days, and the percentage survival was compared to those of KS100 (katA) and the wild type (Fig. 7A). The ability of KC041 (ahpC) to survive glucose starvation was comparable to that of the wild type, whereas KS100 (katA) and KC043 (ahpC katA) showed decreased viability. The inactivation of ahpC had no additional effect on starvation survival in KS100 (katA). Thus, AhpC does not have a major role in glucose starvation survival.
FIG. 7.
(A) Survival of strains SH1000 (▪), KC041 (ahpC) (▴), KS100 (katA) (⧫), and KC043 (ahpC katA) (•) under glucose starvation. The strains were grown in glucose-limiting CDM at 37°C with shaking at 250 rpm. (B) Desiccation tolerances of S. aureus strains. The viability of stationary-phase cells grown in CT-BHI and desiccated in PBS for 24 h at 37°C was determined. The error bars indicate standard deviations.
The roles of AhpC and KatA in desiccation tolerance were investigated using a survival assay. The numbers of bacteria recovered after desiccation at 37°C for 24 h were significantly reduced for KC041 (ahpC) (P < 0.001), KS100 (katA) (P = 0.009), and KC043 (ahpC katA) (P < 0.001) compared to the wild type (Fig. 7B). Thus, AhpC and KatA both have roles to play in desiccation tolerance.
AhpC and KatA are not required for virulence.
KatA was described as a major virulence determinant in S. aureus, but studies with a catalase mutant revealed no difference in virulence in a murine abscess model of infection (34, 18). We have shown compensatory expression and activity of AhpC in the absence of KatA. However, using the murine models of septic arthritis and atopic dermatitis (25) KC043 (ahpC katA) was not attenuated (results not shown). Furthermore, the survival rates of KC041 (ahpC), KS100 (katA), and KC043 (ahpC katA) inside human neutrophils were comparable to that of the wild type (results not shown).
AhpC and KatA are important for nasal colonization.
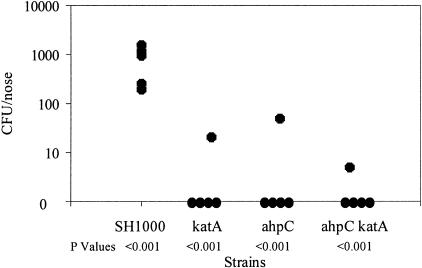
There is a significant link between S. aureus nasal colonization and development of S. aureus infections (29). The cotton rat (Sigmodon hispidus) has been used to study human respiratory pathogens, due to its susceptibility to human diseases and similarity in disease progression and its epithelial tissue, which is comparable to that of humans (29). Nasal colonization by ahpC and katA mutants and the wild type was tested as described previously (10). The numbers of bacteria recovered from KC041 (ahpC) (P < 0.001), KS100 (katA) (P < 0.001), and KC043 (ahpC katA) (P < 0.001) were significantly reduced compared to the wild type (Fig. 8).
FIG. 8.
Nasal colonization by S. aureus strains in a cotton rat model. Approximately 109 CFU of each strain was inoculated intranasally into 6-week old female cotton rats (n = 5). Twenty days after inoculation, the rats were euthanized, the noses were removed and homogenized, and viable bacteria were counted after dilution and growth on tryptic soy agar plates.
DISCUSSION
The PerR-regulated proteins KatA and AhpC have compensatory roles in cellular physiology; they are both responsible for oxidative-stress resistance. KatA is highly specific and efficient at removing H2O2, whereas AhpC has broad-spectrum activity against H2O2, organic peroxides, and peroxynitrite. In vivo, KatA and AhpC are not important for pathogenicity but have roles in bacterial survival and persistence, specifically in nasal colonization.
Compensatory regulation of ahpC and katA.
In S. aureus, both katA and ahpC are mutually compensatory. The katG and ahpC genes of E. coli are also mutually compensatory, with a 10-fold increase in katG expression and increased catalase activity in the ahpC mutant background (50).
The compensatory expression probably occurs due to an accumulation of reactive oxygen species, which is sensed by PerR and leads to derepression of the PerR regulon. There are a number of proposed mechanisms for peroxide sensing by PerR. In B. subtilis, the sensing mechanism involves metal-catalyzed oxidation, with the PerR::Fe form being >104-fold more sensitive to H2O2 inactivation than PerR::Mn (31). The metal-catalyzed reduction of H2O2 to hydroxyl radicals results in oxidation of histidine residues, which act as ligands for the regulatory metal ion. The metal-catalyzed oxidation of the PerR protein by H2O2 has been demonstrated in vivo and results in inactivation of the protein, which is probably degraded and not repaired. Hence, while the PerR::Mn form is more resistant to H2O2, the addition of Mn(II) cannot restore DNA binding to oxidized PerR (31). In S. aureus, PerR responds to Fe(II) and Mn(II), with PerR-dependent repression occurring only in the presence of Mn(II). The roles of these metal ions in peroxide sensing are unknown; perhaps iron may act as a PerR corepressor but is sensitive to endogenous peroxide oxidants. Alternatively, as PerR contains the conserved CXXC motif, oxidative-stress sensing may occur via cysteine oxidation.
Roles of AhpC and KatA in oxidative-stress resistance.
Previous studies have shown that KatA has a role in H2O2 stress resistance in S. aureus (18). An ahpC mutant was more resistant than the wild type when challenged with 7.5 mM H2O2, probably due to an increase in katA expression and catalase activity. This phenomenon was also observed in B. subtilis and the plant pathogen Xanthomonas campestris (2, 38). In S. aureus, the ahpC katA mutant was no more sensitive to H2O2 stress than the katA mutant when analyzed in a death assay. Therefore, KatA must be more important in external H2O2 stress than AhpC. The study of AhpC in gram-negative and gram-positive bacteria has shown a primary role in organic hydroperoxide stress resistance (2, 23). The ahpC mutant of S. aureus was more sensitive to cumene hydroperoxide than the wild type, indicating a role for AhpC in organic-peroxide stress resistance. The katA mutant was more resistant than the wild type, possibly due to a compensatory increase in the expression of ahpC. In B. subtilis, the absence of both AhpC and KatA resulted in increased expression of ohrA, an organic-peroxide resistance gene, in response to H2O2 stress (13). Putative ohr genes are present in the S. aureus genome and may have a role in organic-peroxide resistance, induced in the absence of AhpC and KatA during oxidative stress.
KC043 (ahpC katA) displayed increased paraquat sensitivity, which is probably an H2O2 effect, as sufficient SOD enzymes are present within the cell. Similarly, in the catalase-deficient bacterium Streptococcus pyogenes, the ahpC mutant strain has a growth defect when grown aerobically in the presence of 10 mM paraquat, which can be complemented by supplementing the medium with catalase (27). As the ahpC katA perR mutant of S. aureus was no more resistant to paraquat than ahpC katA, derepression of the PerR regulon is not effective in protecting against superoxide stress. Thus, AhpC and KatA are probably the primary enzymes responsible for H2O2 stress resistance in S. aureus.
AhpC is a member of the peroxiredoxin family, whose members are known to detoxify H2O2, organic peroxides, and peroxynitrite (44). Peroxynitrite is one of a number of reactive nitrogen species that can damage thiol and amine groups. All strains lacking Fur were more sensitive to NO stress than the wild type. In B. subtilis, the fur mutant has a growth defect in the presence of SNP (39). Moore et al. (39) suggested that this is due to the formation of Fe-nitrosyl complexes, which leads to the release of nitroxyl anion and formation of nitrous oxide (45). The ahpC katA mutant of S. aureus was more sensitive to SNP than the wild type but less than the fur mutant. Thus, AhpC and KatA may have roles in NO stress resistance, but they are less important than Fur. The ahpC and ahpC katA mutants had increased sensitivity (compared to the wild type) to SNP with paraquat, which was significantly greater than that to SNP or paraquat alone. AhpC is likely to function in removing peroxynitrite, but the role of KatA in NO stress is unknown. A similar role for AhpC was observed in Mycobacterium smegmatis and Mycobacterium tuberculosis, as the ahpC mutants had decreased survival after the addition of peroxynitrite to the growth medium (36).
Compensatory roles protect the cell from oxidative damage.
A severe growth defect and reduction in plating efficiency were observed for KC043 (ahpC katA) when grown aerobically in minimal medium. A similar growth defect was also observed in minimal medium for the katG ahpC mutant of E. coli (50). The growth defect in E. coli was restored by addition of catalase or growth under anaerobic conditions. Similar results were obtained with KC043 (ahpC katA) of S. aureus. Therefore, AhpC and KatA have roles that are important in an aerobic environment, where reactive oxygen species are disadvantageously produced during respiration (22).
The catalase activity of KS100 (katA) may represent that of AhpC, as none was found for KC043 (ahpC katA). The low level of catalase activity suggests that, despite the abundance of AhpC, its affinity for H2O2 is lower than that of KatA. Seaver and Imlay have postulated that KatA is responsible for detoxifying high levels of H2O2, whereas AhpC is responsible for the removal of low levels of H2O2 (50). The H2O2-scavenging experiment with S. aureus revealed that AhpC is less effective at detoxifying H2O2 than KatA. In S. aureus, the removal of H2O2 appears to occur predominately via KatA, with AhpC acting as an alternative.
Samples from ahpC katA mutant cells assayed during growth revealed that substantial amounts of H2O2 were being produced. A previous study using nonisogenic strains showed that there was a possible increase in H2O2 production as a result of the lack of catalase (37). The production of H2O2 by ahpC katA mutant cells in S. aureus was 24.3 μM after 24 h of growth. An internal concentration of 2 μM H2O2 is proposed to inhibit growth of E. coli cells (51). Thus, the growth defect observed for S. aureus in the absence of AhpC and KatA is most likely due to the accumulation of a toxic level of H2O2.
H2O2 is likely to exert a growth-inhibiting effect on ahpC katA mutant cells through the formation of hydroxyl radicals. Hydroxyl radicals are produced by the Fenton reaction and can cause DNA damage due to the binding of Fe(II) to nucleic acids (22). Nucleic acid damage was observed in the ahpC katA strain via loss of PCR product and an increase in spontaneous rifampin-resistant mutants. Park et al. also reported an increase in the number of DNA lesions in the ahpC katG mutant of E. coli (42).
Antioxidants are not required for virulence but for survival, persistence, and nasal colonization.
Reactive oxygen species are proposed to have a role in host defense systems; they are produced by NADPH oxidase present in the phagolysosomal membranes of neutrophils (15). These oxidative processes were thought to mediate the majority of neutrophil-dependent killing of bacteria (24). We have found that neither AhpC nor KatA is important for resistance to neutrophil-dependent killing or virulence. Studies by Reeves et al. have shown that the production of reactive oxygen species by neutrophils leads to an influx of K+, possibly through an H+ transporter (47). This activates the release of granule proteins and increases the pH to an optimum level (pH 8) for protease activity (47). Thus, survival inside the host may rely on granule protein resistance mechanisms. Moreover, we suggest that many niches within the host constitute relatively anaerobic environments, where AhpC and KatA are not important.
The expression of ahpC and katA occurs in late exponential phase, possibly to protect the cell as it enters stationary phase (18). Stationary-phase survival is important in persistence of the bacterium in the environment. Previous work has elucidated a role for KatA in glucose starvation survival (18). This investigation showed no role for AhpC under the same conditions and no additive effect in the absence of both KatA and AhpC.
Desiccation tolerance is important for the survival of bacterial communities in soil and dust and on the skin of mammals (46). Hence, desiccation in the natural environment of aerobic bacteria involves air drying, which results in the formation of reactive oxygen species and subsequent damage to proteins, membranes, and DNA. Electron spin resonance data has shown the presence of free radicals in desiccated cells of cyanobacteria (46). Previous studies have shown that some S. aureus strains remain viable 9 weeks after desiccation (5). There was also a correlation with epidemiology, as some methicillin-resistant S. aureus strains remained viable longer than methicillin-susceptible S. aureus strains, suggesting that desiccation tolerance provides a selective advantage, leading to increased persistence (5, 49). We have shown that both AhpC and KatA have roles in desiccation tolerance and thus may be important for the spread of S. aureus in the hospital environment. The nature of the oxidative stress during desiccation that requires both AhpC and KatA is unknown, and in fact, the stress may occur during recovery on rich medium. This has been shown for ctaA, where the mutant survives long-term starvation but has a recovery defect that is alleviated by catalase (11).
The anterior nares are the primary ecological niche for S. aureus, and colonization occurs via adherence to nasal mucosal cells (1). Adherence is proposed to occur either through nonspecific hydrophobic interactions or via specific interactions between components of host cell membranes and surface proteins of S. aureus (28). S. aureus surface components, such as IsdA, ClfB, and wall teichoic acids, have roles in adherence to nasal epithelial cells and nasal colonization (10, 41, 57). Interestingly, both AhpC and KatA were required for nasal colonization by S. aureus in a cotton rat model of infection. This is the first demonstration of the role of oxidative-stress resistance components in nasal colonization. It is unlikely that AhpC and KatA have roles in protecting against nasal secretions, as similar antimicrobial peptides are present inside neutrophils, but neutrophil-dependent killing was not increased in the ahpC and katA mutant strains. Instead, the attenuated nasal colonization may represent a reduced ability to survive and multiply in an aerobic environment, as observed in the growth, starvation survival, and, in particular, desiccation studies. Transient desiccation may be a factor in the harsh anterior-nares environment. A reduction in nasal colonization has been implicated in a lessened ability of S. aureus to cause infections. Therefore, despite the lack of attenuation in pathogenicity models presented in this study, AhpC and KatA have important roles in host-pathogen interaction.
The study of bacterial components required for nasal colonization not only reveals important S. aureus adherence and survival mechanisms, but by correlating this with in vitro data, begins to elucidate clues as to the prevailing environment within a crucial host niche.
Acknowledgments
We thank John Iandolo (University of Oklahoma) for the gift of plasmid SABAC1 and Lynne Prince (Hallamshire Hospital) for preparing purified human neutrophils. Statistical analysis of cotton rat nasal colonization was carried out by Bonnie LaFleur (Vanderbilt University).
This work was funded by the BBSRC (K.C. and G.C.). We acknowledge the S. aureus Genome Sequencing Project (8325) and B. A. Roe, Y. Qian, A. Dorman, F. Z. Najar, S. Clifton, and J. Iandolo, funded by NIH and the Merck Genome Research Institute.
Footnotes
Published ahead of print on 17 November 2006.
REFERENCES
- 1.Aly, R., H. I. Shinefield, W. G. Strauss, and H. I. Maibach. 1977. Bacterial adherence to nasal mucosal cells. Infect. Immun. 17:546-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antelmann, H., S. Engelmann, R. Schmid, and M. Hecker. 1996. General and oxidative stress responses in Bacillus subtilis: cloning, expression, and mutation of the alkyl hydroperoxide reductase operon. J. Bacteriol. 178:6571-6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Archibald, F. S., and I. Fridovich. 1981. Manganese and defenses against oxygen toxicity in Lactobacillus plantarum. J. Bacteriol. 145:442-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Archibald, F. S., and I. Fridovich. 1981. Manganese, superoxide dismutase, and oxygen tolerance in some lactic acid bacteria. J. Bacteriol. 146:928-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4a.Armstrong-Buisseret, L., M. B. Cole, and G. S. A. B. Stewart. 1995. A homologue to the Escherichia coli alkyl hydroperoxide reductase AhpC is induced by osmotic upshock in Staphylococcus aureus. Microbiology 141:1655-1661. [DOI] [PubMed] [Google Scholar]
- 5.Beard-Pegler, M. A., E. Stubbs, and A. M. Vickery. 1988. Observations on the resistance to drying of staphylococcal strains. J. Med. Microbiol. 26:251-255. [DOI] [PubMed] [Google Scholar]
- 6.Beers, R. F., Jr., and I. W. Sizer. 1952. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 195:133-140. [PubMed] [Google Scholar]
- 7.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 8.Bsat, N., L. Chen, and J. D. Helmann. 1996. Mutation of the Bacillus subtilis alkyl hydroperoxide reductase (ahpCF) operon reveals compensatory interactions among hydrogen peroxide stress genes. J. Bacteriol. 178:6579-6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, L., L. Keramati, and J. D. Helman. 1995. Coordinate regulation of Bacillus subtilis peroxide stress genes by hydrogen peroxide and metal ions. Proc. Natl. Acad. Sci. USA 92:8190-8194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clarke, S. R., K. J. Brummell, M. J. Horsburgh, P. W. McDowell, S. A. Mohamad, M. R. Stapleton, J. Acevedo, R. C. Read, N. P. Day, S. J. Peacock, J. J. Mond, J. F. Kokai-Kun, and S. J. Foster. 2006. Identification of in vivo-expressed antigens of Staphylococcus aureus and their use in vaccinations for protection against nasal carriage. J. Infect. Dis. 193:1098-1108. [DOI] [PubMed] [Google Scholar]
- 11.Clements, M. O., and S. J. Foster. 1998. Starvation recovery of Staphylococcus aureus 8325-4. Microbiology 144:1755-1763. [DOI] [PubMed] [Google Scholar]
- 12.Dubrac, S., and D. Touati. 2000. Fur positive regulation of iron superoxide dismutase in Escherichia coli: functional analysis of the sodB promoter. J. Bacteriol. 182:3802-3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuangthong, M., and J. D. Helmann. 2002. The OhrR repressor senses organic hydroperoxides by reversible formation of a cysteine-sulfenic acid derivative. Proc. Natl. Acad. Sci. USA 99:6690-6695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grkovic, S., M. H. Brown, K. M. Hardie, N. Firth, and R. A. Skurray. 2003. Stable low-copy-number Staphylococcus aureus shuttle vectors. Microbiology 149:785-794. [DOI] [PubMed] [Google Scholar]
- 15.Hampton, M. B., A. J. Kettle, and C. C. Winterbourn. 1996. Involvement of superoxide and myeloperoxidase in oxygen-dependent killing of Staphylococcus aureus by neutrophils. Infect. Immun. 64:3512-3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herbig, A. F., and J. D. Helmann. 2001. Roles of metal ions and hydrogen peroxide in modulating the interaction of the Bacillus subtilis PerR peroxide regulon repressor with operator DNA. Mol. Microbiol. 41:849-859. [DOI] [PubMed] [Google Scholar]
- 17.Horsburgh, M. J., E. Ingham, and S. J. Foster. 2001. In Staphylococcus aureus, fur is an interactive regulator with PerR, contributes to virulence, and is necessary for oxidative stress resistance through positive regulation of catalase and iron homeostasis. J. Bacteriol. 183:468-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horsburgh, M. J., M. O. Clements, H. Crossley, E. Ingham, and S. J. Foster. 2001. PerR controls oxidative stress resistance and iron storage proteins and is required for virulence in Staphylococcus aureus. Infect. Immun. 69:3744-3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horsburgh, M. J., S. J. Wharton, A. G. Cox, E. Ingham, S. Peacock, and S. J. Foster. 2002. MntR modulates expression of the PerR regulon and superoxide resistance in Staphylococcus aureus through control of manganese uptake. Mol. Microbiol. 44:1269-1286. [DOI] [PubMed] [Google Scholar]
- 20.Horsburgh, M. J., J. L. Aish, I. J. White, L. Shaw, J. K. Lithgow, and S. J. Foster. 2002. σB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325-4. J. Bacteriol. 184:5457-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imlay, J. A., S. M. Chin, and S. Linn. 1988. Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science. 240:640-642. [DOI] [PubMed] [Google Scholar]
- 22.Imlay, J. A. 2003. Pathways of oxidative damage. Annu. Rev. Microbiol. 57:395-418. [DOI] [PubMed] [Google Scholar]
- 23.Jacobson, F. S., R. W. Morgan, M. F. Christman, and B. N. Ames. 1989. An alkyl hydroperoxide reductase from Salmonella typhimurium involved in the defense of DNA against oxidative damage. Purification and properties. J. Biol. Chem. 264:1488-1496. [PubMed] [Google Scholar]
- 24.Johnston, R. B., Jr., B. B. Keele, Jr., H. P. Misra, J. E. Lehmeyer, L. S. Webb, R. L. Baehner, and K. V. RaJagopalan. 1975. The role of superoxide anion generation in phagocytic bactericidal activity. Studies with normal and chronic granulomatous disease leukocytes. J. Clin. Investig. 55:1357-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jonsson, I. M., S. Arvidson, S. J. Foster, and A. Tarkowski. 2004. Sigma factor B and RsbU are required for virulence in Staphylococcus aureus-induced arthritis and sepsis. Infect. Immun. 72:6106-6111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karavolos, M. H., M. J. Horsburgh, E. Ingham, and S. J. Foster. 2003. Role and regulation of the superoxide dismutases of Staphylococcus aureus. Microbiology 149:2749-2758. [DOI] [PubMed] [Google Scholar]
- 27.King, K. Y., J. A. Horenstein, and M. G. Caparon. 2000. Aerotolerance and peroxide resistance in peroxidase and PerR mutants of Streptococcus pyogenes. J. Bacteriol. 182:5290-5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kluytmans, J., A. van Belkum, and H. Verbrugh. 1997. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin. Microbiol. Rev. 10:505-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kokai-Kun, J. F., S. M. Walsh, T. Chanturiya, and J. J. Mond. 2003. Lysostaphin cream eradicates Staphylococcus aureus nasal colonization in a cotton rat model. Antimicrob. Agents Chemother. 47:1589-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee, E. C., D. Yu, J. Martinez de Velasco, L. Tessarollo, D. A. Swing, D. L. Court, N. A. Jenkins, and N. G. Copeland. 2001. A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics 73:56-65. [DOI] [PubMed] [Google Scholar]
- 31.Lee, J.-W., and J. D. Helmann. 2006. The PerR transcription factor senses H2O2 by metal-catalysed histidine oxidation. Nature 440:363-367. [DOI] [PubMed] [Google Scholar]
- 32.Lindsay, J. A., and S. J. Foster. 2001. zur: a Zn(II)-responsive regulatory element of Staphylococcus aureus. Microbiology 147:1259-1266. [DOI] [PubMed] [Google Scholar]
- 33.Loewen, P. C. 1984. Isolation of catalase-deficient Escherichia coli mutants and genetic mapping of katE, a locus that affects catalase activity. J. Bacteriol. 116:693-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mandell, G. L. 1975. Catalase, superoxide dismutase, and virulence of Staphylococcus aureus. In vitro and in vivo studies with emphasis on staphylococcal-leukocyte interaction. J. Clin. Investig. 55:561-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masse, E., and S. Gottesman. 2002. A small RNA regulates the expression of genes involved in iron metabolism in Escherichia coli. Proc. Natl. Acad. Sci. USA 99:4620-4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Master, S. S., B. Springer, P. Sander, E. C. Boettger, V. Deretic, and G. S. Timmins. 2002. Oxidative stress response genes in Mycobacterium tuberculosis: role of ahpC in resistance to peroxynitrite and stage-specific survival in macrophages. Microbiology 148:3139-3144. [DOI] [PubMed] [Google Scholar]
- 37.Messina, C. G., E. P. Reeves, J. Roes, and A. W. Segal. 2002. Catalase negative Staphylococcus aureus retain virulence in mouse model of chronic granulomatous disease. FEBS Lett. 518:107-110. [DOI] [PubMed] [Google Scholar]
- 38.Mongkolsuk, S., W. Whangsuk, P. Vattanaviboon, S. Loprasert, and M. Fuangthong. 2000. A Xanthomonas alkyl hydroperoxide reductase subunit C (ahpC) mutant showed an altered peroxide stress response and complex regulation of the compensatory response of peroxide detoxification enzymes. J. Bacteriol. 182:6845-6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moore, C. M., M. M. Nakano, T. Wang, R. W. Ye, and J. D. Helmann. 2004. Response of Bacillus subtilis to nitric oxide and the nitrosating agent sodium nitroprusside. J. Bacteriol. 186:4655-4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Novick, R. P. 1991. Genetic systems in staphylococci. Methods Enzymol. 204:587-636. [DOI] [PubMed] [Google Scholar]
- 41.O'Brien, L. M., E. J. Walsh, R. C. Massey, S. J. Peacock, and T. J. Foster. 2002. Staphylococcus aureus clumping factor B (ClfB) promotes adherence to human type I cytokeratin 10: implications for nasal colonisation. Cell Microbiol. 4:759-770. [DOI] [PubMed] [Google Scholar]
- 42.Park, S., X. You, and J. A. Imlay. 2005. Substantial DNA damage from submicromolar intracellular hydrogen peroxide detected in hpx-mutants of Escherichia coli. Proc. Natl. Acad. Sci. USA 102:9317-9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peschel, A., R. W. Jack, M. Otto, L. V. Collins, P. Staubitz, G. Nicholson, H. Kalbacher, W. F. Nieuwenhuizen, G. Jung, A. Tarkowski, K. P. van Kessel, and J. A. van Strijp. 2001. Staphylococcus aureus resistance to human defensins and evasion of neutrophil killing via the novel virulence factor MprF is based on modification of membrane lipids with l-lysine. J. Exp. Med. 193:1067-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poole, L. B. 2005. Bacterial defenses against oxidants: mechanistic features of cysteine-based peroxidases and their flavoprotein reductases. Arch. Biochem. Biophys. 433:240-254. [DOI] [PubMed] [Google Scholar]
- 45.Poole, R. K., and M. N. Hughes. 2000. New functions for the ancient globin family: bacterial responses to nitric oxide and nitrosative stress. Mol. Microbiol. 36:775-783. [DOI] [PubMed] [Google Scholar]
- 46.Potts, M. 1994. Desiccation tolerance of prokaryotes. Microbiol. Rev. 58:755-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reeves, E. P., H. Lu, H. L. Jacobs, C. G. Messina, S. Bolsover, G. Gabella, E. O. Potma, A. Warley, J. Roes, and A. W. Segal. 2002. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature 416:291-297. [DOI] [PubMed] [Google Scholar]
- 48.Repine, J. E., R. B. Fox, and E. M. Berger. 1981. Hydrogen peroxide kills Staphylococcus aureus by reacting with staphylococcal iron to form hydroxyl radical. J. Biol. Chem. 256:7094-7096. [PubMed] [Google Scholar]
- 49.Rountree, P. M. 1963. The effect of desiccation on the viability of Staphylococcus aureus. J. Hyg. 61:265-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seaver, L. C., and J. A. Imlay. 2001. Alkyl hydroperoxide reductase is the primary scavenger of endogenous hydrogen peroxide in Escherichia coli. J. Bacteriol. 183:7173-7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seaver, L. C., and J. A. Imlay. 2001. Hydrogen peroxide fluxes and compartmentalization inside growing Escherichia coli. J. Bacteriol. 183:7182-7189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schenk, S., and R. A. Ladagga. 1992. Improved method for electroporation of Staphylococcus aureus. FEMS Microbiol. Lett. 94:133-138. [DOI] [PubMed] [Google Scholar]
- 53.Simmonite, K. J. 2004. Ph.D. thesis. University of Sheffield, Sheffield, United Kingdom.
- 54.Stadtman, E. R., B. S. Berlett, and P. B. Chock. 1990. Manganese-dependent disproportionation of hydrogen peroxide in bicarbonate buffer. Proc. Natl. Acad. Sci. USA 87:384-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Watson, S. P., M. Antonio, and S. J. Foster. 1998. Isolation and characterization of Staphylococcus aureus starvation-induced, stationary-phase mutants defective in survival or recovery. Microbiology 144:3159-3169. [DOI] [PubMed] [Google Scholar]
- 56.Wayne, L. G., and G. A. Diaz. 1986. A double staining method for differentiating between two classes of mycobacterial catalase in polyacrylamide electrophoresis gels. Anal. Biochem. 157:89-92. [DOI] [PubMed] [Google Scholar]
- 57.Weidenmaier, C., J. F. Kokai-Kun, S. A. Kristian, T. Chanturiya, H. Kalbacher, M. Gross, G. Nicholson, B. Neumeister, J. J. Mond, and A. Peschel. 2004. Role of teichoic acids in Staphylococcus aureus nasal colonization, a major risk factor in nosocomial infections. Nat. Med. 10:243-245. [DOI] [PubMed] [Google Scholar]
- 58.Xiong, A., V. K. Singh, G. Cabrera, and R. K. Jayaswal. 2000. Molecular characterization of the ferric-uptake regulator, fur, from Staphylococcus aureus. Microbiology 146:659-668. [DOI] [PubMed] [Google Scholar]