Abstract
Streptococcus mutans is considered one of the primary etiologic agents of dental caries. Previously, we characterized the VicRK two-component signal transduction system, which regulates multiple virulence factors of S. mutans. In this study, we focused on the vicX gene of the vicRKX tricistronic operon. To characterize vicX, we constructed a nonpolar deletion mutation in the vicX coding region in S. mutans UA159. The growth kinetics of the mutant (designated SmuvicX) showed that the doubling time was longer and that there was considerable sensitivity to paraquat-induced oxidative stress. Supplementing a culture of the wild-type UA159 strain with paraquat significantly increased the expression of vicX (P < 0.05, as determined by analysis of variance [ANOVA]), confirming the role of this gene in oxidative stress tolerance in S. mutans. Examination of mutant biofilms revealed architecturally altered cell clusters that were seemingly denser than the wild-type cell clusters. Interestingly, vicX-deficient cells grown in a glucose-supplemented medium exhibited significantly increased glucosyltransferase B/C (gtfB/C) expression compared with the expression in the wild type (P < 0.05, as determined by ANOVA). Moreover, a sucrose-dependent adhesion assay performed using an S. mutans GS5-derived vicX null mutant demonstrated that the adhesiveness of this mutant was enhanced compared with that of the parent strain and isogenic mutants of the parent strain lacking gtfB and/or gtfC. Also, disruption of vicX reduced the genetic transformability of the mutant approximately 10-fold compared with that of the parent strain (P < 0.05, as determined by ANOVA). Collectively, these findings provide insight into important phenotypes controlled by the vicX gene product that can impact S. mutans pathogenicity.
The oral cavity contains distinct habitats that support a diverse bacterial flora. Dental biofilms harbor more than 700 bacterial species, and most of the bacteria are nonpathogenic streptococci (15, 23, 24, 30). Oral infections, such as dental caries, are promoted by environmental changes (e.g., changes in pH) that cause ecological shifts among plaque residents that favor the proliferation of aciduric bacteria. One of the oral inhabitants, Streptococcus mutans, has long been considered the primary bacterium involved in the initiation and progression of dental caries, one of the most prevalent human infectious diseases. Dissolution of the tooth enamel marks the onset of tooth decay due to repeated exposure to lactic acid generated by S. mutans and other oral bacteria as a metabolic end product of carbohydrate metabolism. In addition to producing acid, when dietary sucrose is available, S. mutans uses this sugar to produce aggressively sticky glucan polymers via glucosyltransferases (encoded by the gtfB and gtfC genes) that facilitate the attachment of cells to the tooth pellicle, as well as to other microbes, thereby promoting biofilm formation (3, 28, 29). Previous studies (33) have indicated that these enzymes, as well as a third glucosyltransferase encoded by the gtfD gene, are regulated at the transcriptional level by the vicR/K genes, which comprise part of the vicRKX operon in the S. mutans chromosome (33). Each glucosyltransferase makes glucan products that can be distinguished by their glucosidic linkages. For example, GtfB makes primarily water-insoluble α-1,3-linked glucosidic polymers, whereas GtfD makes water-soluble α-1,6-linked glucosidic polymers. On the other hand, GtfC appears to synthesize both types of glucan products, with the water-insoluble glucans predominating. The water-insoluble glucans produced by the gtfB/C-encoded enzymes are critical for sucrose-dependent colonization of smooth surfaces by the mutans streptococci (12, 20). In fact, rats infected with S. mutans strains deficient in either of these genes had significantly reduced levels of dental caries (4, 21, 32, 40), which emphasized the conclusion that the glucosyltransferases have an important role in caries etiology.
Previously, we examined the VicRK two-component signal transduction system (TCSTS), which is one of 13 such systems found in S. mutans UA159 (33). Based on sequence homology, the vicRK genes encode a surface-associated histidine kinase (VicK) and an intracellular response regulator (VicR). Typically, these TCSTS components act in concert to sense and adapt to transient environmental signals. Using quantitative real-time PCR (rtPCR), we previously demonstrated that the vicRK genes regulate the expression of gtfB/C/D, the fructosyltransferase gene (ftf), and gbpB encoding glucan-binding protein B (33). In addition, mutagenesis of the vicR and vicK coding regions affected S. mutans growth, sucrose-dependent adhesion, biofilm formation, and development of genetic competence (33). The latter phenotype, which enables natural genetic transformation, helps the bacteria to take up and incorporate heterologous DNA. In the oral cavity, the plaque biofilm likely provides a “gene pool” from which oral microbes can acquire DNA and develop new heritable phenotypes (5, 6). It is well established that transformation mediates horizontal gene transfer that can lead to the emergence of new phenotypes with increased virulence potential, including antibiotic resistance (7-9).
Despite our knowledge of the various physiological properties that are subject to the control of vicRK, a putative role for the third gene (vicX) in the tricistronic operon has not been investigated previously. In the present study we focused on the S. mutans vicX gene and its impact on several important phenotypes. A blastP search of the VicX deduced amino acid sequence revealed >85% similarity with VicX orthologs in Streptococcus pneumoniae and Streptococcus pyogenes. The S. pneumoniae vicX gene product has been shown to control virulence in a mouse model, whereas in vitro experiments have demonstrated that VicX has a role in modulating genetic competence in this organism (39). In the present study, we found that S. mutans VicX not only is involved in the regulation of gtfB/C expression but also controls several other physiological properties important for growth, adherence, biofilm formation, genetic transformation, and oxidative stress tolerance. While these results enhance our understanding of how S. mutans can regulate various phenotypes that can contribute to its pathogenicity, they also highlight the importance of the vic locus in modulating virulence attributes in S. mutans.
MATERIALS AND METHODS
Strains, plasmids, and growth media.
Information for the bacterial strains used in this study is summarized in Table 1. Todd-Hewitt yeast extract medium (THYE) (BBL Becton Dickinson, Cockeysville, MD) was used for growth and routine maintenance of the S. mutans UA159 wild-type strain (provided by J. Ferretti, University of Oklahoma) and its vicX-deficient derivative (SmuvicX). Todd-Hewitt broth (THB) (Sigma) was used for growth and maintenance of S. mutans strain GS5 and its derivatives, including the isogenic vicX mutant (SG440) and the gtfB-, gtfC- and gtfD-deficient strains, which were utilized for the adhesion assays. During experiments, overnight cultures of each strain were diluted 20-fold using fresh medium before they were grown to the desired optical density. The mutants were grown in the presence of erythromycin (10 μg/ml) unless indicated otherwise. All S. mutans strains were grown as standing cultures with 5% CO2 at 37°C. The pDL277 (Specr) Escherichia coli-streptococcus shuttle plasmid was used to assess transformation frequencies.
TABLE 1.
Bacterial strains used for ligation-PCR mutagenesis
| S. mutans strain | Relevant characteristics | Source or reference |
|---|---|---|
| UA159 | Wild type, Erms | J. Ferretti, University of Oklahoma |
| GS5 | Wild type, Erms | Howard K. Kuramitsu, SUNY Buffalo |
| SmuvicX | UA159 vicX::ermAM, Ermr | This study |
| SmuvicK | UA159 vicK::ermAM, Ermr | 33 |
| Smuvic+ | UA159 vicRKX overexpressor, Ermr | 33 |
| SG440 | GS5 vicX::ermAM, Ermr | This study |
| GtfB− | GtfB-deficient GS5, Ermr | 1 |
| GtfC− | GtfC-deficient GS5, Ermr | 13 |
| GtfB/C− | GtfB/C-deficient GS5, Ermr | 14 |
Construction of S. mutans vicX-deficient mutants.
An erythromycin resistance cassette was used to disrupt the vicX coding region in the S. mutans UA159 wild-type chromosome by PCR ligation mutagenesis as previously described (17). The primers utilized for construction of the mutagenic construct are shown in Table 2. To confirm that integration of the erythromycin gene into the vicX coding region was successful, chromosomal DNA was isolated from erythromycin-resistant transformants and PCR amplified using combinations of primers (VicX-P1 and VicX-P4, VicX-P1 and Erm cst-F, and VicX-P4 and Erm cst-B) (Table 2). The resulting vicX null mutant was designated SmuvicX. The null mutation was confirmed using a combination of primers (Table 2) as previously described (17).
TABLE 2.
Primers used for PCR
| Primer | Nucleotide sequencea | Annealing temp (°C) | Amplicon size (bp) |
|---|---|---|---|
| VicX-P1 | 5′ CCAGATTTTTCTTCACCCTTAC 3′ | 50.5 | 478 |
| VicX-P2 | 5′ GGCGCGCCTGATACCTCGCCAGATACTG 3′ | ||
| VicX-P3 | 5′ GGCCGGCCCACTTTTCGGTCTATTTCTGC 3′ | 50.8 | 401 |
| VicX-P4 | 5′ AGGCTTGGGTATTCCTAAG 3′ | ||
| Erm cst- F | 5′ GGCGCGCCCCGGGCCCAAAATTTGTTTGAT 3′ | 52.3 | 876 |
| Erm cst- B | 5′ GGCCGGCCAGTCGGCAGCGACTCATAGAAT 3′ | ||
| Real-time PCR primers | |||
| VicX-FOR | 5′ TGCTCAACCACAGTTTTACCG 3′ | 51 | 127 |
| VicX-REV | 5′ GGACTCAATCAGATAACCATCAGC 3′ | ||
| VicR-FOR | 5′ CGCAGTGGCTGAGGAAAATG 3′ | 53 | 157 |
| VicR-REV | 5′ ACCTGTGTGTGTCGCTAAGTGATG 3′ | ||
| VicK-FOR | 5′ CACTTTACGCATTCGTTTTGCC 3′ | 52 | 102 |
| VicK-REV | 5′ CGTTCTTCTTTTTCCTGTTCGGTC 3′ | ||
| GbpB-FOR | 5′ AGCAACAGAAGCACAACCATCAG 3′ | 54 | 150 |
| GbpB-REV | 5′ CCACCATTACCCCAGTAGTTTCC 3′ | ||
| GtfB-FOR | 5′ ACACTTTCGGGTGGCTTG 3′ | 49 | 127 |
| GtfB-REV | 5′ GCTTAGATGTCACTTCGGTTG 3′ | ||
| GtfC-FOR | 5′ CCAAAATGGTATTATGGCTGTCG 3′ | 51 | 136 |
| GtfC-REV | 5′ TGAGTCTCTATCAAAGTAACGCAG 3′ | ||
| 16SrRNA-FOR | 5′ CTTACCAGGTCTTGACATCCCG 3′ | 53 | 111 |
| 16SrRNA-REV | 5′ ACCCAACATCTCACGACACGAG 3′ | ||
| GyrA-FOR | 5′ ATTGTTGCTCGGGCTCTTCCAG 3′ | 58 | 105 |
| GyrA-REV | 5′ ATGCGGCTTGTCAGGAGTAACC 3′ |
Bold type indicates AscI restriction enzyme sites, and underlining indicates FseI restriction enzyme sites.
In addition to SmuvicX, another vicX-deficient mutant (designated SG440) was constructed using S. mutans strain GS5 for sucrose-dependent adhesion assays. As we later found in this study, the S. mutans GS5 wild-type strain had a prominent “sticky phenotype” that was not apparent with the UA159 strain. Therefore, chromosomal DNA isolated from SmuvicX with a DNeasy tissue kit (QIAGEN, Valencia, CA) was used to transform S. mutans GS5 in the presence of synthetic competence-stimulating peptide (CSP), whose sequence corresponds to the sequence of the natural GS5 autoinducer peptide (16). VicX-deficient erythromycin-resistant (10 μg/ml) transformants were isolated, and the allelic exchange event was confirmed by PCR using the same combination of primers that was used to confirm the vicX null mutation in the SmuvicX mutant strain.
To rule out the possibility that the vicX mutation had polar effects on vicR/K transcription, we isolated cDNAs from both vicX mutants and their UA159 and GS5 parent strains and amplified them with vicR/K/X-specific primers using quantitative real-time PCR. Since the expression of gyrA did not fluctuate in cDNA samples, we used it to normalize vicR/K/X expression results. The following experiments were conducted only after the nonpolarity of the vicX mutants was established.
Growth kinetics.
Overnight cultures of S. mutans were diluted 20-fold in fresh THYE and grown to an optical density at 600 nm (OD600) of 0.4 to 0.5. The growth was monitored for 24 h with an automated Bioscreen C microbiology reader (Bioscreen C Labsystems, Finland) as previously described (19). Briefly, 20 μl of mutant or wild-type cells was added to microtiter plate wells containing 400 μl of THYE and incubated at 37°C after the following stressors were added: sodium dodecyl sulfate (final concentration, 0.5 × 10−3% to 3 × 10−3% [wt/vol]), sodium chloride (0.1 M to 0.6 M), paraquat (methyl viologen hydrate; 0 to 100 mM), ethanol (1% to 6% [vol/vol]), and pH-buffered acidic medium (pH 7.5 to pH 5.5). Since the VicK protein has a PAS domain, which enables detection of intracellular oxidative changes, we used the SmuvicK and Smuvic+ strains in our paraquat sensitivity assays along with SmuvicX. The acronym PAS was derived from the proteins which were first used to identify the two imperfect repeat sequences in the PAS domain: the Drosophila period clock protein (PER), vertebrate aryl hydrocarbon receptor nuclear translocator (ARNT), and the Drosophila single-minded protein (SIM) (37). Wells containing S. mutans strains with THYE alone were used to monitor the growth rate without a stressor. Uninoculated wells containing THYE served as negative controls. To eliminate ambiguity, antibiotics were omitted from the culture medium in these assays.
Biofilm formation and SEM.
Biofilms were formed on glass disks placed in 24-well microtiter plates containing 2 ml of 0.25× tryptone yeast extract medium (TYE) (tryptone was supplied by Bioshop, Burlington, ON, Canada) supplemented with either 12 mM glucose or 6 mM sucrose and containing no antibiotics. Each well was inoculated with 20 μl of mid-log-phase UA159 or SmuvicX cells and incubated at 37°C in the presence of 5% CO2 for 16 h. To examine biofilm architecture, the planktonic cells in the liquid phase were carefully aspirated, and scanning electron microscopy (SEM) was performed as described previously (18).
Analysis of gene expression using quantitative rtPCR.
To assess the putative effect(s) of a vicX deletion mutation on S. mutans virulence gene expression, we performed a quantitative rtPCR analysis with primers specific for gtfB/C, ftf, and gbpB using cDNAs derived from UA159 and SmuvicX total intact mRNAs. Briefly, overnight cultures grown in 10 ml of THYE (with or without erythromycin) were centrifuged, and the cell pellets were washed and resuspended in 10 ml of sterile TYE. The cell suspensions were diluted 20-fold in fresh TYE and grown to an OD600 of 0.4 after the medium was supplemented with either 1% sucrose or 1% glucose. The cells were then pelleted by centrifugation and snap-frozen in liquid nitrogen for storage at −80°C. Frozen pellets were resuspended in Trizol reagent (Invitrogen, Carlsbad, CA), and total RNA was extracted using the FastPrep system (Bio 101 Savant, Holbrook, NY) according to the supplier's instructions. Following treatment with DNase (MBI Fermentas, Hanover, MD), mRNAs were reverse transcribed, and the resulting cDNAs were used in rtPCR experiments as described previously (33). Gene expression was normalized to the expression of gyrA, which was invariable under the test conditions used (data not shown).
In addition, we investigated the expression of the vicR/K/X response to paraquat-induced intracellular oxidative stress. Briefly, overnight UA159 cultures were diluted 20-fold, grown to an OD600 of 0.4, and then supplemented with freshly prepared 50 mM paraquat for 15 min. Uninduced cultures were used as controls. Total cDNAs were extracted as described above, and an rtPCR was conducted using primers specific for vicR/K/X (Mx3000P; Stratagene, La Jolla, CA). The results were normalized using gyrA, as well as 16S rRNA gene expression. Primer efficiencies were determined using pooled cDNAs derived from equal volumes of treated and untreated samples. The relative expression of genes was calculated using results from three or more independent experiments, as previously described (25). A statistical analysis was conducted using single-factor analysis of variance (ANOVA); a P value of <0.05 was considered significant.
Sucrose-dependent adherence assay.
S. mutans GS5 and its isogenic vicX mutant (SG440) were grown overnight in 5 ml of THB and THB containing erythromycin (10 μg/ml), respectively. We used S. mutans GS5 in our adherence assays since all other strains of S. mutans that we have tested, including S. mutans UA159, did not show the level of adherence shown by this strain (data not shown). GS5 is known to have deficiencies in gbpC and pac, which encode cell wall-anchoring glucan-binding protein C and cell wall-anchored major protein antigen (22, 31). Although it is not clear whether the capacity for this form of adhesion is linked to isolation of GS5 from a carious lesion (11), we took advantage of this phenotype and its reliance on gtfB and gtfC for attachment to examine the effects of vicX. S. mutans GS5 and its isogenic vicX, gtfB, gtfC, and gtfB/C mutants were grown overnight, diluted 50-fold in fresh THB, and incubated as described above until the OD650 was 0.5. Culture aliquots (4.5 ml) were then transferred to polystyrene plates (100 by 15 mm; BD Falcon, Sparks, MD) containing either 0.5 ml of prewarmed THB or 0.5 ml of THB supplemented with sucrose (final concentration, 3%). Following 1 h of incubation, nonadherent and loosely adherent cells were removed with a pipettor, transferred to a sterile test tube, and vortexed to homogeneity. Adherent cells were scraped from the abiotic surface with a polypropylene spatula (Bel Art Product, New Jersey), resuspended in fresh THB (5 ml), and vortexed to homogeneity. The percentage of adherent cells was calculated by dividing the OD650 of the adherent fraction by the sum of the OD650 of the nonadherent and adherent cells and multiplying the quotient by 100.
Assays for genetic competence.
The transformation frequency was used to investigate the putative role of VicX in uptake of foreign DNA by S. mutans. Briefly, overnight cultures of the SmuvicX mutant and its parent strain were centrifuged, and the cell pellets were diluted 20-fold in fresh prewarmed THYE. Subsequently, the cells were grown to an OD600 of 0.15 to 0.2, and then 1 μg of closed circular plasmid DNA (pDL277, Specr) was added along with 500 ng/ml of the S. mutans synthetic CSP. The cell-DNA mixture was incubated for 2.5 h in the presence of 5% CO2 at 37°C. Samples to which CSP was not added were used to assess natural genetic transformation of the strains. Following incubation, the cell cultures were briefly sonicated, serially diluted, and plated onto THYE agar with and without antibiotics to determine the number of transformants and the total number of possible recipient cells, respectively. The transformation frequency was determined by dividing the number of spectinomycin-resistant CFU by the total number of CFU present without antibiotic selection and multiplying the quotient by 100.
RESULTS
Characterization of S. mutans vicX.
The S. mutans vicX gene is part of a tricistronic operon comprised of the vicRKX genes (Fig. 1). Computational analyses were performed using the vicX gene sequence and its predicted amino acid sequence to determine the location of the gene on the chromosome, to identify putative functional motifs or domains, and to predict the subcellular localization. Analysis of the S. mutans UA159 genome (http://www.ncbi.nlm.nih.gov/genomes) localized the vicRKX genes to the minus strand of the chromosome at positions 1444056 to 1446908. We found that the vicX gene (801 bp) encodes a 267-amino-acid hypothetical protein with a predicted mass of 29,680 Da (33). BlastP search results for S. mutans VicX revealed high levels of similarity and amino acid identity to its orthologs in S. pneumoniae (accession no. NP_345691.1; 88% similarity and 77% identity), S. pyogenes (NP_268804.1; 85% and 75%), Streptococcus agalactiae (NP_687736.1; 89% and 76%), and Bacillus subtilis (NP_391917.1; 72% and 54%,). Functional motifs in VicX were predicted using the NCBI, Pfam, and ProDom databases, and the results revealed high levels of amino acid similarity to metal-dependent hydrolases belonging to the beta-lactamase superfamily; this suggested a possible role in cellular structure and maintenance, perhaps involving the bacterial cell wall.
FIG. 1.
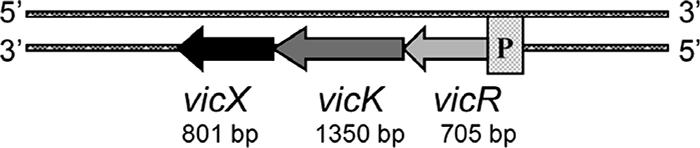
vicRKX operon of S. mutans.
In a previous study, we assigned functions to the vicRK gene products and described the organization of the genes on the UA159 chromosome (33). However, we did not find a PAS domain in the VicK protein, which we identified in this study. In prokaryotes, PAS domains are found almost exclusively in sensors belonging to signal transduction systems predominantly involved in sensing oxygen and intracellular redox potential (37). After locating this domain in the vicK gene, we included the SmuvicK, Smuvic+, and SmuvicX mutant strains and their UA159 wild-type progenitor strain in this study to investigate its putative role in oxidative stress tolerance in S. mutans.
Involvement of S. mutans VicX in oxidative stress tolerance.
To investigate whether disruption of vicX affected the growth rate and the tolerance to a variety of stresses in the environment, we performed growth kinetic studies with S. mutans UA159 and its vicX-deficient mutant using THYE supplemented with paraquat, ethanol, NaCl, or sodium dodecyl sulfate or THYE having an acidic pH. An analysis of the growth rates determined by three independent experiments showed that the mutant exhibited a reproducible growth deficiency only under paraquat-induced oxidative stress conditions. Without paraquat the doubling time of SmuvicX was greater than that of the UA159 wild-type strain (55.8 ± 1.9 versus 47.4 ± 1.34 min [means ± standard errors]). When the organisms were grown in the presence of 100 mM paraquat, the doubling times of SmuvicX and UA159 increased to 707.4 ± 45.6 and 162.1 ± 33.38 min, respectively. We also examined the growth of the SmuvicK and Smuvic+ strains in THYE with and without paraquat. Different growth patterns for these strains were apparent at a paraquat concentration as low as 25 mM (Fig. 2). The results of these studies showed that while the growth of SmuvicX and Smuvic+ was impaired when paraquat was added, the SmuvicK growth rate and yield were enhanced under these conditions compared with those of the wild type.
FIG. 2.
Effects of paraquat-induced oxidative stress on the growth of vic mutant strains and the UA159 parent strain, which were grown in THYE supplemented with 25 mM paraquat. The growth curves are representative of the curves obtained in at least three independent experimental trials. Each data point is the average OD600 obtained from triplicate experiments. ⋄, UA159; ▴, SmuvicK; ×, Smuvic+; •, SmuvicX.
S. mutans vicR/K/X expression is affected by oxidative stress induced by paraquat.
Since VicK harbors a PAS domain that potentially detects and responds to changes in intracellular oxidative stress, we measured the expression of vicK and the expression of the contiguous genes (vicR/X) using mid-log-phase cells, which were stressed by addition of paraquat for 15 min. Our first attempt to measure expression by incubation of mid-log-phase S. mutans cells with paraquat for 24 h resulted in greater RNA instability, possibly due to cell death or lysis and/or RNA degradation during treatment. After the protocol was modified, treatment of cells for 15 min before RNA was harvested eliminated this problem, as judged by the similar cycle threshold values obtained in four independent experiments in the presence and absence of paraquat (data not shown). For all three genes (vicR/K/X), an approximately 2.5-fold increase in expression (P < 0.05) was observed when paraquat was added compared with the expression in the no-treatment controls.
VicX influences S. mutans biofilm formation.
To determine if the vicX gene product influenced the formation or architecture of S. mutans biofilms, we analyzed biofilms formed by SmuvicX and its wild-type UA159 parent on glass disks. SEM analysis of biofilms grown for 16 h in 0.25× TYE supplemented with either 12 mM glucose or 6 mM sucrose revealed distinct architectural differences. Notably, exposure of SmuvicX to either sugar resulted in biofilms that were comprised of abundant cell clusters and hence appeared to be remarkably denser than the wild-type biofilms (Fig. 3). Compared with biofilms formed in glucose-supplemented medium (data not shown), the gross differences in biofilm density were most pronounced when SmuvicX was grown in the presence of sucrose. Upon closer examination of the SmuvicX biofilms at higher magnifications, no obvious alterations in exopolymer content or cell division were found compared with wild-type biofilms.
FIG. 3.
SEM micrographs of 16-h-old biofilms of S. mutans UA159 and its isogenic vicX-deficient mutant (SmuvicX) grown on glass coverslips in 0.25× TYE supplemented with sucrose.
VicX affects S. mutans gtfB/C expression.
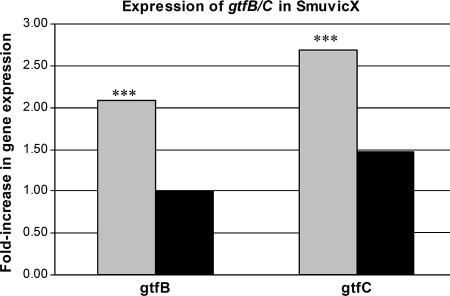
Since SmuvicX biofilms had an altered architecture compared with the architecture of wild-type biofilms, we monitored the expression of several genes known for their role in the formation of plaque biofilms. Specifically, we performed quantitative rtPCR using cDNAs derived from glucose- or sucrose-supplemented cultures and using primers specific for gtfB/C, ftf, and gbpB (Table 2). While there were no significant differences in ftf or gbpB expression between SmuvicX and wild-type cells grown in the presence of either sugar, the expression of gtfB/C was significantly up-regulated in the vicX mutant in glucose-supplemented medium (Fig. 4). More specifically, the expression of gtfB was increased 2.1-fold and the expression of gtfC was increased 2.7-fold (P < 0.05). However, when organisms were grown in the presence of sucrose, the gtfB/C expression in the mutant was approximately the same as the gtfB/C expression in the wild-type strain.
FIG. 4.
Differences between gtfB/C expression in the SmuvicX mutant and gtfB/C expression in the S. mutans UA159 wild-type parent grown in TYE supplemented with either glucose (gray bars) or sucrose (black bars). Experiments were conducted in triplicate or quadruplicate, and the results were normalized using the expression of gyrA. Differences in gtfB/C expression were calculated based on the wild-type UA159 expression, which defined as 1.0. The expression of gtfB and the expression of gtfC in SmuvicX grown in glucose-containing medium were 2.1- and 2.7-fold greater, respectively, than the expression in the wild-type UA159 strain; three asterisks indicate that the P value is <0.05.
S. mutans vicX-deficient cultures are more adherent at the mid-log phase.
Previously, other researchers have shown that the structure of a mature S. mutans biofilm (>18 h old) is both gtfB and gtfC dependent regardless of the presence of sucrose (35, 38). Moreover, we previously described a role for the vic operon in gtfB and gtfC transcription during exponential growth (33). To assess the putative contributions of VicX to this phenotype, we monitored the impact of a vicX deletion on S. mutans adherence. Measurements were obtained over the course of an hour, well before a mature biofilm could form, so that only the initial adherence was monitored. The assay was optimized for a 1-h incubation time, during which the rate of sticking was still linear over time and the signal-to-noise ratio was large enough to make valid comparisons (data not shown). Interestingly, the initial adherence of the vicX-deficient mutant strain of S. mutans to an abiotic surface was at least twofold greater than that of the wild type when the organisms were grown in a medium containing sucrose (Fig. 5). For wild-type S. mutans GS5, which showed poor adherence when it was grown in glucose-containing medium, a threefold increase in attachment was observed when cells were grown in a medium supplemented with sucrose. Interestingly, for strains deficient in gtfB, gtfC, and gtfBC we observed two- to threefold reductions in adherence and levels similar to wild-type levels in the absence of sucrose, which serves as a substrate for the glucosyltransferases. These findings indicate that VicX is involved in S. mutans adhesion that is mediated by the gtfB/C gene products.
FIG. 5.
Comparison of the initial adherence of S. mutans GS5 and its isogenic derivatives to an abiotic surface. Each strain was grown to the mid-logarithmic phase and added to a polystyrene petri dish containing THB or THB supplemented with sucrose. The planktonic and loosely adherent cells were compared with the cells that were firmly adherent. The percentage of adherent cells was determined by dividing the number of adherent cells by the total number of cells determined by OD650 and multiplying the quotient by 100. Each bar indicates the average of four experimental trials, and the error bars indicate standard deviations.
VicX affects natural transformation.
Previously, we determined that the VicRK signal transduction system affected the ability of S. mutans to be transformed by exogenous DNA (33). To determine if vicX contributes to the transformability of S. mutans, we examined whether SmuvicX and its wild-type UA159 parent could be transformed by a heterologous genetic marker. While no significant differences in transformation frequency were observed between these strains in the presence of CSP (data not shown), the natural transformation efficiency of the vicX mutant without added CSP was 10-fold lower (P < 0.05) than the natural transformation efficiency of the parent strain under these conditions.
DISCUSSION
Until recently, little was known about the “virulence control” (vic) locus in S. mutans, despite the fact that its orthologs in S. pneumoniae, S. pyogenes, and B. subtilis had been described. The VicRK signal transduction system is 1 of 13 TCSTSs in S. mutans and seems to be indispensable since a null mutation in the vicR component appears to be lethal. In this study, we generated a vicX null mutation and investigated its impact on S. mutans physiology. As judged by rtPCR, the mutant allele did not have any polar effect on the steady-state level of vicRK mRNA, verifying that the otherwise isogenic strains were affected solely by the vicX deficiency in the SmuvicX strain. Here we report that in the absence of vicX, the ability of S. mutans to grow under oxidative stress conditions and its ability to take up and inherit foreign DNA are drastically compromised. We also found that biofilms formed by the SmuvicX mutant were markedly denser than the wild-type biofilms, possibly due in part to the altered expression of gtfB/C genes in this strain. Also consistent with this observation is the enhanced adhesive phenotype observed for the vicX mutant when it was presented with sucrose as the carbohydrate source. In a previous study, we reported how the vicK-deficient and vicRKX-overexpressing strains contributed to biofilm formation, sucrose-dependent adhesion, and the regulation of gtfB/C/D, ftf, and gbpB expression in S. mutans (33). Since the VicK and VicX mutant phenotypes overlapped to a great extent, we used quantitative rtPCR with cDNAs derived from SmuvicK and SmuvicX to ensure that the transcript levels of the intact vic genes were not affected by mutagenesis of either the vicK or vicX gene (data not shown). Therefore, based on our studies pertaining to this operon, the vic genes are responsible for controlling several important physiological properties, including some properties (e.g., adhesion, biofilm formation, and gtfB/C expression) that are regarded as strong influences that affect the cariogenic potential of S. mutans.
We previously investigated the effects of VicRK signal transduction on the development of genetic competence and showed that there is a solid connection between the vic genes and the transformation frequency in S. mutans. Hence, we were not surprised when the natural competence of SmuvicX was severely compromised compared to that of its parent, although we were puzzled that the absence of VicX resulted in the same competence-deficient phenotype as the phenotype of the vicRKX-overexpressing Smuvic+ strain reported previously (33). Similarly, when these two strains were grown in paraquat-induced oxidative stress conditions, their growth was impaired compared to the growth of S. mutans UA159, as well as the growth of the SmuvicK strain. In other words, the SmuvicX and Smuvic+ strains had the same phenotype for transformability and oxidative stress tolerance regardless of whether vicX was overexpressed or deleted. From one point of view, it can be speculated that VicX and the VicR response regulator interact to control these and other physiological attributes in S. mutans. However, this issue may be more complex than we think, since we cannot rule out the possible involvement of other cellular proteins affecting either VicR/X or the VicR-VicX interaction. Despite this, it is important to examine if and how VicX interacts with VicR to obtain an understanding of the role of vicX in the vicRKX tricistronic operon and how it might affect the VicRK two-component signal transduction system to regulate important physiological factors in S. mutans.
The functional motifs and domains present in the VicX protein suggest that it may belong to the metallo-beta-lactamase superfamily of proteins. In addition to beta-lactamases, a number of other proteins are known to contain the metallo-beta-lactamase domain, including thiolesterases that belong to the glyoxalase II family and catalyze the hydrolysis of S-d-lactoyl-glutathione to generate glutathione and d-lactic acid (2). In Neisseria gonorrhoeae, a competence protein required for natural transformation likely is a transporter involved in DNA uptake and also has this domain (10). If vicX encodes a DNA transporter similar to that in N. gonorrhoeae, we would expect a vicX null mutant to show decreased transformability. Since the transformability of our mutant was not affected when CSP was present in the culture medium (data not shown), it is unlikely that S. mutans VicX functions as a DNA transporter unless there are other redundant uptake mechanisms.
The results of transcriptional assays confirmed that vicX mutation has an effect on gtfB/C expression. In contrast to the wild-type strain, in which expression of gtfB and gtfC was increased when the carbohydrate source was switched from glucose to sucrose, gtfB/C expression in SmuvicX remained at a level equivalent to the level in sucrose-induced wild-type cells. This was likely due to derepression of gtfB/C transcription in the presence of glucose in the vicX null mutant. Recently, it was shown that vicR and gtfB/C were regulated differentially depending on the bacterial growth phase (i.e., early exponential versus late exponential), as well as the type of sugar added to the culture medium (e.g., glucose, sucrose, fructose, mannitol, sorbitol, etc.) (34). The researchers showed that the type of carbohydrate added during growth had a significant influence on the expression of the gtf genes, whereas the vicR expression was more dependent on the growth phase than on the carbohydrate source. While we tested the effects of only two carbohydrates on the expression of gtfBC in this study, we clearly observed significantly increased expression of these genes in the presence of glucose in SmuvicX compared to the expression in the wild type, suggesting that a vicX-mediated negative influence on gtfBC expression was dependent on the carbohydrate source in the growth medium.
We also investigated the role of vicX in cell adhesion using a functional adherence assay that relied almost exclusively on gtfB and gtfC expression, which showed that the initial adherence of the VicX mutant was remarkably increased compared to the initial adherence of the parent strain in sucrose-supplemented medium. In this study, we found that the adherence of a gtfC-deficient strain of S. mutans was more compromised than the adherence of an isogenic gtfB-deficient strain was. This result was similar to the result obtained by Tsumori and Kuramitsu, who reported that inactivation of gtfC drastically reduced S. mutans adherence to smooth surfaces in an in vitro sucrose-dependent biofilm assay (38). Hence, it appears that GtfC plays a more dominant role in both initial adherence and biofilm maturation. This may be because GtfC can catalyze the synthesis of substantial amounts of α-1,3- and α-1,6-linked glucans (13), which can become cross-linked.
Another important finding of this study is the link between the vicRKX genes and oxidative stress tolerance in S. mutans, which exhibited altered growth as well as enhanced expression of these genes under paraquat-induced oxidative stress conditions. The bacterial response to oxidative stress is especially important in the case of S. mutans, since it is a catalase-negative facultative anaerobe that has to combat reactive oxidation species in mouths for survival and persistence. Hence, it is useful and important from a scientific point of view, as well as from a therapeutic point of view, to obtain insight into how S. mutans can detect and respond to oxidative stress. In the 13 TCSTSs in S. mutans, only the VicK protein harbors a PAS domain, and PAS domains are almost exclusively sensors of oxygen and intracellular redox potential (37). In E. coli the aerotaxis transducer Aer has a PAS domain in its N-terminal region that promotes cell migration to a microenvironment with a preferred redox potential (26, 27, 36, 41). As S. mutans is a member of the human oral microbial consortium that is usually exposed to various oxidative stresses, the ability of this organism to monitor intracellular oxygen levels is important because reactive oxygen radicals can be highly toxic to the cell. However, further studies to investigate the mode of action and the downstream target genes controlled by the vicRKX are warranted so that we can better understand the oxidative stress tolerance response of S. mutans.
Given the multiple physiological properties that appear to be controlled by the vicRKX signal transduction system (e.g., a strong transformation phenotype), it is likely that these genes cross-communicate with other signal transduction pathways in S. mutans. The availability of the S. mutans UA159 genome sequence, in combination with improvements in molecular and genetic tools for examining gene functions and biochemical pathways, has facilitated the characterization of various genetic networks in S. mutans. We have only just begun to appreciate the complexity of these regulatory networks. An improved understanding of their interrelatedness and the mechanisms that control their expression may improve current therapeutic strategies aimed at controlling caries initiation. Here we present evidence that supports the hypothesis that VicX has a role in initial attachment, biofilm formation, competence development, and oxidative stress tolerance. However, additional experiments to determine if and/or how the vicX gene product controls these phenotypes are warranted.
Acknowledgments
We thank Richard Mair for assistance with bioinformatics, Bob Chernecky and Jian Wang for assistance with SEM analyses, and Anousheh Persadmehr for help with rtPCR.
This study was supported by NIH grant RO1DE013230 and CIHR grant MT-15431 to D.G.C., who is a recipient of a Canada Research Chair. S.D.G. was supported by NIH grant R01DE13965. M.D.S. is a CIHR Strategic Training Fellow supported by training grant STP-53877, an OGSST scholarship, and a Harron fellowship.
Footnotes
Published ahead of print on 17 November 2006.
REFERENCES
- 1.Aoki, H., T. Shiroza, M. Hayakawa, S. Sato, and H. K. Kuramitsu. 1986. Cloning of a Streptococcus mutans glucosyltransferase gene coding for insoluble glucan synthesis. Infect. Immun. 53:587-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aravind, L. 1999. An evolutionary classification of the metallo-beta-lactamase fold proteins. In Silico Biol. 1:69-91. [PubMed] [Google Scholar]
- 3.Banas, J. A. 2004. Virulence properties of Streptococcus mutans. Front. Biosci. 9:1267-1277. [DOI] [PubMed] [Google Scholar]
- 4.Burne, R. A., Y. Y. Chen, D. L. Wexler, H. Kuramitsu, and W. H. Bowen. 1996. Cariogenicity of Streptococcus mutans strains with defects in fructan metabolism assessed in a program-fed specific-pathogen-free rat model. J. Dent. Res. 75:1572-1577. [DOI] [PubMed] [Google Scholar]
- 5.Cvitkovitch, D. G. 2001. Genetic competence and transformation in oral streptococci. Crit. Rev. Oral Biol. Med. 12:217-243. [DOI] [PubMed] [Google Scholar]
- 6.Cvitkovitch, D. G., Y. H. Li, and R. P. Ellen. 2003. Quorum sensing and biofilm formation in streptococcal infections. J. Clin. Investig. 112:1626-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dowson, C. G., V. Barcus, S. King, P. Pickerill, A. Whatmore, and M. Yeo. 1997. Horizontal gene transfer and the evolution of resistance and virulence determinants in Streptococcus. Soc. Appl. Bacteriol. Symp. Ser. 26:42S-51S. [PubMed] [Google Scholar]
- 8.Dowson, C. G., T. J. Coffey, C. Kell, and R. A. Whiley. 1993. Evolution of penicillin resistance in Streptococcus pneumoniae; the role of Streptococcus mitis in the formation of a low affinity PBP2B in S. pneumoniae. Mol. Microbiol. 9:635-643. [DOI] [PubMed] [Google Scholar]
- 9.Dubnau, D. 1999. DNA uptake in bacteria. Annu. Rev. Microbiol. 53:217-244. [DOI] [PubMed] [Google Scholar]
- 10.Facius, D., and T. F. Meyer. 1993. A novel determinant (comA) essential for natural transformation competence in Neisseria gonorrhoeae and the effect of a comA defect on pilin variation. Mol. Microbiol. 10:699-712. [DOI] [PubMed] [Google Scholar]
- 11.Gibbons, R. J., K. S. Berman, P. Knoettner, and B. Kapimalis. 1966. Dental caries and alveolar bone loss in gnotobiotic rats infected with capsule forming streptococci of human origin. Arch. Oral Biol. 11:549-560. [DOI] [PubMed] [Google Scholar]
- 12.Hamada, S., and H. D. Slade. 1980. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol. Rev. 44:331-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanada, N., and H. K. Kuramitsu. 1988. Isolation and characterization of the Streptococcus mutans gtfC gene, coding for synthesis of both soluble and insoluble glucans. Infect. Immun. 56:1999-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanada, N., and H. K. Kuramitsu. 1989. Isolation and characterization of the Streptococcus mutans gtfD gene, coding for primer-dependent soluble glucan synthesis. Infect. Immun. 57:2079-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kazor, C. E., P. M. Mitchell, A. M. Lee, L. N. Stokes, W. J. Loesche, F. E. Dewhirst, and B. J. Paster. 2003. Diversity of bacterial populations on the tongue dorsa of patients with halitosis and healthy patients. J. Clin. Microbiol. 41:558-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kreth, J., J. Merritt, W. Shi, and F. Qi. 2005. Co-ordinated bacteriocin production and competence development: a possible mechanism for taking up DNA from neighbouring species. Mol. Microbiol. 57:392-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lau, P. C., C. K. Sung, J. H. Lee, D. A. Morrison, and D. G. Cvitkovitch. 2002. PCR ligation mutagenesis in transformable streptococci: application and efficiency. J. Microbiol. Methods 49:193-205. [DOI] [PubMed] [Google Scholar]
- 18.Li, Y. H., P. C. Lau, J. H. Lee, R. P. Ellen, and D. G. Cvitkovitch. 2001. Natural genetic transformation of Streptococcus mutans growing in biofilms. J. Bacteriol. 183:897-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li, Y. H., P. C. Lau, N. Tang, G. Svensater, R. P. Ellen, and D. G. Cvitkovitch. 2002. Novel two-component regulatory system involved in biofilm formation and acid resistance in Streptococcus mutans. J. Bacteriol. 184:6333-6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loesche, W. J. 1986. Role of Streptococcus mutans in human dental decay. Microbiol. Rev. 50:353-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munro, C., S. M. Michalek, and F. L. Macrina. 1991. Cariogenicity of Streptococcus mutans V403 glucosyltransferase and fructosyltransferase mutants constructed by allelic exchange. Infect. Immun. 59:2316-2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murakami, Y., Y. Nakano, Y. Yamashita, and T. Koga. 1997. Identification of a frameshift mutation resulting in premature termination and loss of cell wall anchoring of the Pac antigen of Streptococcus mutans GS-5. Infect. Immun. 65:794-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paster, B. J., S. K. Boches, J. L. Galvin, R. E. Ericson, C. N. Lau, V. A. Levanos, A. Sahasrabudhe, and F. E. Dewhirst. 2001. Bacterial diversity in human subgingival plaque. J. Bacteriol. 183:3770-3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paster, B. J., W. A. Falkler, Jr., C. O. Enwonwu, E. O. Idigbe, K. O. Savage, V. A. Levanos, M. A. Tamer, R. L. Ericson, C. N. Lau, and F. E. Dewhirst. 2002. Prevalent bacterial species and novel phylotypes in advanced noma lesions. J. Clin. Microbiol. 40:2187-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pfaffl, M. W. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rebbapragada, A., M. S. Johnson, G. P. Harding, A. J. Zuccarelli, H. M. Fletcher, I. B. Zhulin, and B. L. Taylor. 1997. The Aer protein and the serine chemoreceptor Tsr independently sense intracellular energy levels and transduce oxygen, redox, and energy signals for Escherichia coli behavior. Proc. Natl. Acad. Sci. USA 94:10541-10546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Repik, A., A. Rebbapragada, M. S. Johnson, J. O. Haznedar, I. B. Zhulin, and B. L. Taylor. 2000. PAS domain residues involved in signal transduction by the Aer redox sensor of Escherichia coli. Mol. Microbiol. 36:806-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rolla, G. 1989. Why is sucrose so cariogenic? The role of glucosyltransferase and polysaccharides. Scand. J. Dent. Res. 97:115-119. [DOI] [PubMed] [Google Scholar]
- 29.Rolla, G., A. A. Scheie, and J. E. Ciardi. 1985. Role of sucrose in plaque formation. Scand. J. Dent. Res. 93:105-111. [DOI] [PubMed] [Google Scholar]
- 30.Sakamoto, M., M. Umeda, and Y. Benno. 2005. Molecular analysis of human oral microbiota. J. Periodontal Res. 40:277-285. [DOI] [PubMed] [Google Scholar]
- 31.Sato, Y., K. Okamoto, and H. Kizaki. 2002. gbpC and pac gene mutations detected in Streptococcus mutans strain GS-5. Oral Microbiol. Immunol. 17:263-266. [DOI] [PubMed] [Google Scholar]
- 32.Schroeder, V. A., S. M. Michalek, and F. L. Macrina. 1989. Biochemical characterization and evaluation of virulence of a fructosyltransferase-deficient mutant of Streptococcus mutans V403. Infect. Immun. 57:3560-3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Senadheera, M. D., B. Guggenheim, G. A. Spatafora, Y. C. Huang, J. Choi, D. C. Hung, J. S. Treglown, S. D. Goodman, R. P. Ellen, and D. G. Cvitkovitch. 2005. A VicRK signal transduction system in Streptococcus mutans affects gtfBCD, gbpB, and ftf expression, biofilm formation, and genetic competence development. J. Bacteriol. 187:4064-4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shemesh, M., A. Tam, M. Feldman, and D. Steinberg. 2006. Differential expression profiles of Streptococcus mutans ftf, gtf and vicR genes in the presence of dietary carbohydrates at early and late exponential growth phases. Carbohydr Res. 341:2090-2097. [DOI] [PubMed] [Google Scholar]
- 35.Tamesada, M., S. Kawabata, T. Fujiwara, and S. Hamada. 2004. Synergistic effects of streptococcal glucosyltransferases on adhesive biofilm formation. J. Dent. Res. 83:874-879. [DOI] [PubMed] [Google Scholar]
- 36.Taylor, B. L., A. Rebbapragada, and M. S. Johnson. 2001. The FAD-PAS domain as a sensor for behavioral responses in Escherichia coli. Antioxid. Redox. Signal. 3:867-879. [DOI] [PubMed] [Google Scholar]
- 37.Taylor, B. L., and I. B. Zhulin. 1999. PAS domains: internal sensors of oxygen, redox potential, and light. Microbiol. Mol. Biol. Rev. 63:479-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsumori, H., and H. Kuramitsu. 1997. The role of the Streptococcus mutans glucosyltransferases in the sucrose-dependent attachment to smooth surfaces: essential role of the GtfC enzyme. Oral Microbiol. Immunol. 12:274-280. [DOI] [PubMed] [Google Scholar]
- 39.Wagner, C., A. Saizieu Ad, H. J. Schonfeld, M. Kamber, R. Lange, C. J. Thompson, and M. G. Page. 2002. Genetic analysis and functional characterization of the Streptococcus pneumoniae vic operon. Infect. Immun. 70:6121-6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamashita, Y., W. H. Bowen, R. A. Burne, and H. K. Kuramitsu. 1993. Role of the Streptococcus mutans gtf genes in caries induction in the specific-pathogen-free rat model. Infect. Immun. 61:3811-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhulin, I. B., B. L. Taylor, and R. Dixon. 1997. PAS domain S-boxes in Archaea, Bacteria and sensors for oxygen and redox. Trends Biochem. Sci. 22:331-333. [DOI] [PubMed] [Google Scholar]