Most bacteriocins in gram-positive bacteria are small and heat stable (peptide bacteriocins), and their antimicrobial activities are directed against a broader spectrum of bacteria than is seen for bacteriocins of gram-negative bacteria. Many excellent bacteriocin reviews have been published in recent years (10, 15, 16, 19, 27, 29, 77, 83).
The heat-stable peptide bacteriocins from lactic acid bacteria have so far been grouped into two major classes: class I, the lantibiotics, and class II, the heat-stable nonlantibiotics. In addition, a third class of bacteriocins has been suggested which includes secreted heat-labile cell wall-degrading enzymes (71, 88), but classification of such enzymes as bacteriocins has recently been disputed (19, 49). Lantibiotics contain a number of posttranslational modifications that include dehydration of serine and threonine to form 2,3-dehydroalanine (Dha) and 2,3-dehydrobutyrine (Dhb), respectively. Some of the dehydrated residues are covalently bound to the sulfur in neighboring cysteines, creating the characteristic lantionine and methyllantionine residues. It has also been shown that in a few cases the dehydroalanine can be converted to d-alanine (109, 118) and that additional modifications, such as lysinoalanine, 2-oxobutyrate, S-aminovinyl-d-cysteine, and S-aminovinyl-d-methylcysteine, are formed in some lantibiotics (59). Both class I and class II bacteriocins display great diversity with regard to their modes of action, structures, genetics, modes of secretion, choices of target organisms, etc. There is still lack of consensus on how to subdivide class I and II peptide bacteriocins further into subclasses. The lantibiotics have been divided into two subgroups, type A and type B, according to structural features (64). Type A lantibiotics (e.g., nisin, subtilin, and Pep5) are elongated molecules with a flexible structure in solution, while type B lantibiotics adapt a more rigid and globular structure (64). However, this picture is changing, since structural studies of the lantibiotic plantaricin C has been shown to hold structural elements of both type A and B lantibiotics (123). Also, nuclear magnetic resonance spectroscopy has shown that the peptides of the two-peptide lantibiotic lacticin 3247 are structurally different. While the peptide designated lacticin 3147 A1 has a specific lanthionine bridging pattern resembling the globular type B lantibiotic mersacidin, the A2 peptide is a member of the elongated type A lantibiotic subclass (80). In the present review, we refer to the A and B types of lantibiotics as one-peptide lantibiotics and mention specifically when a bacteriocin is a two-peptide lantibiotic.
Lack of consensus also exists in the differentiation between subgroups of the nonlantibiotic class II peptide bacteriocins. In this review, we retain the pediocin-like bacteriocin in class IIa, the two-peptide bacteriocins in class IIb, and the leaderless peptide bacteriocins in class IIc, and finally, we define the circular bacteriocins as class IId. This overview will discuss the dissemination of the class I and II peptide bacteriocins in enterococci and streptococci and the possibility of identifying such bacteriocins in genome sequences.
The lactic acid bacteria in fermented food have been the focus of bacteriocin research during the last 15 to 20 years. Numerous peptide bacteriocins have been characterized, and many have been used intentionally or unintentionally in food products, either through starter cultures or as food additives/supplements (15). Work on such bacteriocins has been driven by the need for new and improved natural food preservation technology and a wish to prevent food spoilage and poison-producing bacteria from growing. In recent years, the concept of probiotic bacteria has also stimulated work on bacteriocins. In light of the increased antibiotic resistance among pathogens, bacteriocins have attracted attention as an alternative means to prevent infection by pathogens. In fact, two lantibiotics, nisin and lacticin 3147, have been found useful in preventing mastitis (8, 108, 110).
The nonfood lactic acid bacteria, such as enterococci and streptococci, have also been scrutinized for bacteriocin production, and many publications have shown that they are producers of such antimicrobial peptides (Tables 1 and 2). Some enterococci are actually part of the main fermenting flora of artisan dairy and meat products, particularly in Mediterranean countries. Their presence in the fermented products is most probably doe to the relatively high temperatures found in that region (35).
TABLE 1.
Peptide bacteriocins isolated from streptococci
| Organism | Bacteriocin | Type | Mass (Da) (amino acids) | Reference(s) |
|---|---|---|---|---|
| S. salivarius | Salivaricin A | Class I | 2,315 (22) | 107, 119, 128 |
| S. salivarius | Salivaricin B | Class I | 2,733 (25) | 119 |
| S. salivarius | Salivaricin A2 | Class I | 2,364 (22) | 119 |
| S. pyogenes | Streptococcin A-FF22 | Class I | 2,795 (26) | 55, 60 |
| S. macedonicus | Macedocin | Class I | 2,795 | 40 |
| S. pyogenes | Streptin | Class I | 2,424 (23) | 68, 127 |
| S. mutans | Mutacin I, mutacin II (H-29B) | Class I, class I | 2,364 (24) 3,245 (27) | 11, 73, 92, 101 |
| S. mutans | Mutacin N | 4,806 (49) | 4 | |
| S. mutans | Mutacin B-Ny266 | Class I | 2,270 (22) | 86 |
| S. mutans | Mutacin III (Mutacin1140) | Class I | 2,263 (22) | 50, 102 |
| S. bovis | Bovicin HJ50 | Class I | 3,428 (33) | 133 |
| S. uberis | Nisin U | Class I nisin-like | 3,029 (32) | 132 |
| S. mutans | SmbA, SmbB | Class I two-peptide lantibiotic | (30, 32) | 135 |
| S. rattus | BHT-A(Smb-like); BHT-Aa, BHT-Ab | Class I Two-peptide lantibiotic | 2,802 (30), 3,375 (32) | 54 |
| S. mutans | Mutacin IV; peptide A, peptide B | Class II two-peptide bacteriocin | 4,169 (44), 4,826 (49) | 100 |
| S. thermophilus | Thermophilin 13 (A); ThmA, ThmB | Class II two-peptide bacteriocin | 5,776 (62), 3,910 (43) | 78 |
| S. bovis | Bovicin 255 | Class II | 5,968 (56) | 129 |
| S. rattus | BHT-B | Class II nonleader | 5,195 (44) | 54 |
TABLE 2.
Peptide-bacteriocins isolated from enterococci
| Organism | Bacteriocin | Type | Mass (Da) (amino acids) | Reference(s) |
|---|---|---|---|---|
| E. faecalis | Cytolysin Cyl"L, Cyl"S | Class I two-peptide lantibiotics | 3,458 (38), 2,032 (21) | 56 |
| E. faecium | Enterocin A | Class IIa pediocin-like | 4,829 (47) | 3, 93, 96 |
| E. faecium | Enterocin P | Class IIa pediocin-like | 4,493 (44) | 12 |
| E. faecium | Bac 32 | Class IIa pediocin-like | 7,998 (70) | 58 |
| E. faecium | Bacteriocin GM-1 | Class IIa pediocin-like | 4,630 (44) | 67 |
| E. faecalis | Bac 31 | Class IIa pediocin-like | (43) | 58 |
| E. mundtii | Mundticin ATO6, mundticin KS, enterocin CRL35, mundticin QU2 | Class IIa pediocin-like | 4,287 (43) | 69, 111, 138 |
| E. faecalis | Enterocin SE-K4 | Class IIa pediocin-like | 5,356.2 (43) | 28, 43 |
| E. faecium | Bacteriocin T8 | Class IIa pediocin-like | 5,090 (44) | 22 |
| E. faecium | Enterocin B | Class II (no subclass) | 5,479 (53) | 9, 36, 93 |
| E. faecalis | Enterocin 1071A, enterocin 1071B | Class IIb two-peptide bacteriocin | 4,285 (39), 3,897 (35) | 34 |
| E. faecalis | MR10A MR10B | Class IIc, leaderless | 5,202 (44), 5,208 (43) | 81 |
| E. faecium | Enterocin L50; L50A, L50B | Class IIc, leaderless | 5,190 (44), 5,178 (43) | 14 |
| E. faecium | Enterocin Q | Class IIc, leaderless | 3,980 (34) | 13 |
| E. faecalis | Enterocin EJ97 | Class IIc, leaderless | 5,328 (44) | 39, 112 |
| E. faecium | Enterocin RJ-11 | Class IIc, leaderless | 5,049 (44) | 134 |
| E. faecalis | AS-48 | Class IId circular bacteriocin | 7,166 (70) | 77 |
The enterococci are among the dominant lactic acid bacteria in the intestinal flora of mammals and other animals. The streptococci constitute part of the complex oral microflora of mammals, and some of them are pathogenic. Studies of the antimicrobial peptides from streptococci and enterococci have shown that these bacteria produce a large number and diversity of such compounds. It should be noted that identical peptide bacteriocins have been isolated by different research groups, and unfortunately, they have been given different names. In addition, many bacteriocins have been only partly characterized, and available information is often insufficient to confirm their novelty. The present review covers only the best-defined class I and class II bacteriocins from enterococci and streptococci.
DIVERSITY AND DISSEMINATION OF PEPTIDE BACTERIOCINS
Streptococcus.
Studies of the bacteriocins of streptococci go back to the 1960s (84). The more recent focus in the isolation and characterization of streptococcal bacteriocins has been on pathogenic streptococci, and most bacteriocins characterized originate from a few species (Table 1). Lantibiotics are the most prevalent peptide bacteriocins in streptococci, and the majority belong to the elongated cationic type A lantibiotics. Two-peptide lantibiotics have also been isolated from streptococci (Table 1).
The lantibiotic salivaricin A was the first Streptococcus salivarius lantibiotic to be characterized (107), and it strongly inhibits Streptococcus pyogenes strains. S. pyogenes (a group A Streptococcus) is a ubiquitous organism that is known to provoke a wide variety of diseases in humans. Five additional variants of the structural salivaricin A (salivaricin A1 to A5) peptide have been identified in streptococcal strains, and together with salivaricin A, these six SalA peptides seem to share an inhibition spectrum, probably because they differ from each other only by 1 or 2 amino acids (128). Among the SalA-type peptide bacteriocins, SalA1 activity was most prevalent, being produced by a broad range of species, including S. pyogenes, Streptococcus dysgalactiae, and Streptococcus agalactica. Among the 53 different M types of S. pyogenes strains, all but one carried the salA1 gene variant; however, none was found to be a bacteriocin producer. On the other hand, 77% of 36 S. salivarius strains were positive for the salA gene, as well as producing the bacteriocin (128).
Streptolysin S (SLS), which is responsible for the hallmark beta-hemolytic phenotype, is produced by group A Streptococcus. A nine-gene locus is necessary and sufficient for its biosynthesis, and the genes resemble genes required for the synthesis of some bacteriocins. Although SLS does not antagonize bacteria, the biosynthesis elements needed for its synthesis support the designation of SLS as a bacteriocin-like toxin (37, 53, 95).
Peptide bacteriocins produced by Streptococcus mutans are known as mutacins, and the frequency of such antimicrobial activities has been reported to vary from 11% to 100% among S. mutans isolates (5). A study of mutacin production in 145 oral S. mutans isolates from young children and their mothers showed that 88% of the strains produced antimicrobial activity against more than 1 of the 14 indicator strains (42).
Some S. mutans isolates produce at least three different lantibiotics named mutacin I, II, and III. Mutacin II has been shown to be structurally related to the lacticin 481 group of type A lantibiotics (73), while the structures of mutacins I and III have not been determined. A two-peptide class II peptide bacteriocin (mutacin IV) is produced by S. mutans UA140, a strain that also produces mutacin I (100). The production of mutacins I and IV by UA140 appears to be regulated by different mechanisms under different physiological conditions in the sense that mutacin I is only produced by cells growing under biofilm-forming conditions, while mutacin IV is produced in planktonic cultures. The production of two mutacins by one strain under different conditions implies that they serve different roles in the ecology of S. mutans (100).
The evolution and dissemination of such peptide bacteriocins in lactic acid bacteria were recently demonstrated by nisin, the most prominent lantibiotic, so far found only in Lactococcus lactis strains. A recent publication by Wirawan and coworkers described a nisin variant called nisin U (78% identity to nisin A) from Streptococcus uberis (132). This bacterium is primarily found on the lips and skin of cows, in raw milk, and on udder tissue and is a major cause of bovine mastitis (116, 136, 137).
Two bacteriocins, termed bovicin 255 and bovicin HJ50, have been thoroughly characterized from a rumen bacterial isolate of Streptococcus bovis (129, 133). Bovicin HJ50 is a lantibiotic, while bovicin 255 is a class II nonlantibiotic. In the same study, 7 out of 35 rumen streptococcal isolates were found to produce bacteriocin-like activity (129). The broad dissemination of bovicin 255 among rumen streptococci has recently been confirmed (18). The bovicin 255 peptide sequence showed some similarity to those of two other class II peptide bacteriocins, lactococcin A (51) and thermophilin A (thermophilin 13) (125), and it was identified in both Streptococcus gallolyticus and S. bovis isolates.
In another study, a Streptococcus rattus isolate produced two different peptide bacteriocins named BHT-A and BHT-B. The genetic loci for the BHT-A and BHT-B bacteriocins were found in six S. rattus and two S. mutans strains (54). BHT-A was found to be a variant of the two-peptide lantibiotic Smb (135), while BHT-B was a nonmodified peptide with some similarity to the tryptophan-rich and leaderless aureocin A53 from Staphylococcus aureus (54, 91).
In a screening of Streptococcus pyogenes, approximately 10% of the strains were found to inhibit the growth of nine indicators in a standardized streptococcal-bacteriocin assay (127). The bacteriocin activity was due to the type A lantibiotic streptin, and two major forms of streptin were purified to homogeneity from an S. pyogenes strain (127). The fully matured form of streptin (streptin 1) is made up of 23 amino acid residues and has a mass of 2,424 Da, while the second form of the peptide (streptin 2) has three additional amino acids (TPY) at the N terminus. The structural gene (strA) of streptin was widespread among S. pyogenes strains. Of 58 S. pyogenes isolates tested, 41 hybridized with the strA probe, but only 10 of the strains produced active streptin. The deficiency of some strains in producing streptin was ascribed to a deletion in their streptin loci, encompassing genes putatively encoding proteins involved in streptin processing (68, 127). The strA gene was found to be absent in S. salivarius (75 isolates), S. mutans (8 isolates), and S. uberis (9 isolates).
Streptococcal bacteriocins have also been characterized from food-related environments, such as food fermentation. Macedocin is a lantibiotic from the newly described species Streptococcus macedonicus, isolated from artisan cheese (40), and thermophilin 13 is a two-peptide class IIb bacteriocin produced by Streptococcus thermophilus, isolated from yogurt (78).
Peptide bacteriocin activities have been described in many more streptococcal isolates, but often the characterization is incomplete, which makes it difficult to judge if they are new bacteriocins. It is surprising to see the high frequency of peptide bacteriocin-producing streptococcal isolates, and this observation is supported by gene annotation, as well as genome mining of sequenced Streptococcus genomes (see below).
Enterococcus.
Many peptide bacteriocins from enterococci have been purified and genetically characterized over the years, and most of them have been obtained from Enterococcus faecalis and Enterococcus faecium. The best characterized ones are listed in Table 2. As observed in streptococci, identical characterized peptide bacteriocins have been given different names, such as bacteriocin AS-48, which has been named both enterocin 4 (62) and bacteriocin 21 (121).
The bacteriocins from enterococci belong almost exclusively to the heat-stable, nonlantibiotic class II bacteriocins, with the exception of cytolysin. Cytolysin is a two-peptide lantibiotic found in E. faecalis (17). It is the only peptide bacteriocin isolated from enterococci with cytolytic (hemolytic) activity (56, 61, 70). Cytolysin is a virulence factor, and consequently, it is not considered useful as an antimicrobial agent. It is interesting that the cytolysin-encoding genes are found not only in Enterococcus isolates from hospitals and patients, but also from food; animals, including houseflies; and healthy infants (65, 76, 115).
Probably the most prevalent class II enterococcal bacteriocins are pediocin-like bacteriocins (class IIa) with strong antilisterial effects (30, 31). Among these bacteriocins, enterocin A was the first to be thoroughly characterized, and this peptide bacteriocin is among those most frequently found in E. faecium strains (3, 122). The gene for enterocin A has also been identified in the partly sequenced genome of E. faecium strain DO, but the strain does not produce any bacteriocin activity (http://genome.jgi-psf.org/draft_microbes/entfa/entfa.home.html). While most class IIa bacteriocins are processed from a precursor peptide containing an N-terminal double-glycine leader sequence (between 15 and 30 amino acid residues), some peptide bacteriocins, such as enterocin P and bacteriocin 31, contain a sec-like leader sequence that is removed in the secretion process (12, 122).
The two-peptide peptide bacteriocins (class IIb) require the complementary actions of both peptides for full antimicrobial activity, and only one immunity protein is dedicated to the activity of a two-peptide bacteriocin. In some E. faecalis strains, the two-peptide bacteriocin named enterocin 1071 is encoded by plasmid-derived genes (34). The two peptides, enterocin 1071A and enterocin 1071B, constituting the bacteriocin are 39 and 34 amino acids long, respectively. The deduced amino acid sequences of the mature Ent1071A and Ent1071B peptides showed 64 and 61% homology with the α and β peptides of lactococcin G, respectively, a two-peptide bacteriocin isolated from L. lactis (94).
Several leaderless peptide bacteriocins have been identified among enterococci (Table 2). The leaderless peptide bacteriocins were first discovered in staphylococci and were shown to be hemolytic (26, 90, 91, 126); they are produced as single peptides or multiple homologous peptides, each encoded by individual genes localized in tandem repeats (91). The multiple-peptide bacteriocins (up to four individual peptides) shared 65 to 80% homology (14, 26, 91, 126). From the food isolate E. faecium strain L50, two plasmid-encoded leaderless bacteriocins were characterized. One, termed enterocin L50, was composed of two highly similar peptides sharing 72% identity. The individual peptides enterocin L50A and enterocin L50B possessed antimicrobial activity, with the L50A peptide being most active. However, in combination, the antimicrobial activity increased between 5- and 80-fold, depending on the bacterial indicator used (14). The enterocin L50-encoding genes were localized as two consecutive open reading frames (ORFs) on a 50-kb plasmid (20). The second leaderless peptide bacteriocin, enterocin Q, was isolated from the same E. faecium strain. Enterocin Q consists of only one peptide, and its gene was found on 7,383-bp plasmid (pCIZ2). Recently, two leaderless bacteriocin peptides (MR10A and MR10B) were characterized from an E. faecalis strain isolated from the holocrine glands of a bird (81). The two MR10 peptides were almost identical to the enterocin L50 peptides.
It is interesting that some leaderless peptide bacteriocins in enterococci share homology with Staphylococcus peptides that appear to be hemolytic (14). An intriguing question is, can some few amino acid substitutions render the L50 peptides cytolytic? As previously mentioned, the enterococcal lantibiotic cytolysin, as well as the SLUSH peptides (26) and the AGS peptides (126), most likely encompass both cytolytic and bactericidal activities, which suggests that such activities can coexist in the same peptide molecule.
A unique group of peptide bacteriocins, classified as class IId in this review, are the cyclic bacteriocins. One member of this group, enterocin AS-48, has been identified in E. faecalis. Enterocin AS-48 was the first bacteriocin isolated from Enterococcus to be purified (38, 82). Screening by PCR-based technology of 15 independently isolated bacteriocin-producing Enterococcus strains for the structural genes similar to the antimicrobial peptide AS-48 gene has been carried out (63). Eight of 10 E. faecalis strains and 3 of 5 E. faecium strains gave positive results. This finding suggests that peptide bacteriocins closely related or identical to peptide AS-48 are common in enterococci (63). The almost identical cyclic enterocin AS-48RJ has been characterized from an E. faecium strain. It deviates from enterocin AS-48 only in amino acid position 20, replacing glutamine with valine (1).
In which ecological environments are bacteriocin-producing enterococci commonly found? Presently, there does not seem to be any preferential niche; wherever one finds enterococci, one also finds bacteriocin-producing enterococci. A major portion of the bacteriocin-producing enterococci have been isolated from foods (cheese, meat, fish, and vegetables), animals, and humans (33). A collection of 636 hospital-isolated vancomycin-resistant E. faecium strains were tested for bacteriocin production. It was shown that 44% of the strains were bacteriocin producers, and a significant number of these strains carried the genetic determinant for bacteriocin 32 production (58). Bacteriocin-producing enterococci have also been isolated from municipal sewage, cattle dung, ruminal content, birds, etc. (58, 74, 75, 79).
Production of multiple bacteriocins also seems to be a common feature of enterococci and streptococci. It has been reported that many isolates produce three or four bacteriocins (13, 98, 131).
PEPTIDE BACTERIOCIN GENES IN GENOME SEQUENCES
Bacterial genome sequences are revolutionizing our approach to identifying novel genes in different bacteria. More than 300 complete bacterial genome sequences are available in public-domain databases, and the number is increasing. Such sequence databases should be an extremely valuable source in which to look for genes encoding antimicrobial compounds, such as ribosomally synthesized antimicrobial peptide bacteriocins (89). The peptide bacteriocins are small molecules that are consequently encoded by small genes, and unfortunately, small ORFs are often not adequately annotated or are omitted in annotation. There is a need for improved search tools for identification of peptide bacteriocin genes, and there should be more focus on the annotation of small ORFs. In the case of Streptocococcus genomes, numerous putative peptide bacteriocin-encoding genes have been proposed. In the genome sequence of Streptococcus pneumoniae TIGR 4, seven ORFs have been annotated as bacteriocin genes, while four bacteriocin genes have been annotated in the S. pneumoniae R6 genome (http://cmr.tigr.org/tigr-scripts/CMR/CmrHomePage.cgi).
Results from an in silico screening for peptides containing the double-glycine leader sequence and their cognate transporter have been published (25). The double-glycine leader motif that is found in a majority of class II and some class I bacteriocins and the unique N-terminal peptidase C39 domain of their cognate ABC transporters were the main features used in this search for such peptides (45, 46). The study included 45 fully sequenced gram-positive genomes, and of a total of 48 GG motif candidate peptides obtained, 92% were found in lactic acid bacteria and 80% were found in the streptococcal genomes. However, one should bear in mind that peptide pheromones (including competence peptides) involved in the two-component regulatory systems are also detected in such a search (87). The peptide pheromones share most features of bacteriocins but are usually shorter and have no or poor antimicrobial activity (24).
A more general and probably more efficient peptide bacteriocin search engine has been developed (21). The genome-mining tool includes a search for lantibiotics, as well as peptide bacteriocins with different N-terminal leaders (21). This web-based genome-mining tool (with the acronym BAGEL) applies a number of ORF prediction tools that take into account the presence of genes involved in biosynthesis machinery, transport function, regulation, and immunity. These features make BAGEL unique, as well as valuable, in searches for putative bacteriocin genes and their biosynthetic operons in bacterial genomes (21).
In the annotated Streptococcus genome sequences, a number of new peptide bacteriocin genes have been proposed. In the S. pneumoniae TIGR 4 genome sequence, seven putative peptide bacteriocin genes have been annotated (SP0042, SP0109, SP0531, SP0532, SP0533, SP0539, and SP0541), but no bacteriocin activity has been characterized so far (120). Evaluation of the peptide-bacteriocin genes in S. pneumoniae TIGR 4 by use of the BAGEL algorithm (default setting) identified 11 significant peptide bacteriocin genes, including the seven original putative bacteriocin genes and four additional genes (SP974, SP0540, SP792, and SP602). The program also identified 18 other potential bacteriocin genes and a high number of ORFs (44) with some, but less, homology to bacteriocin genes. A recent published work explored the distribution of genes in eight clinical isolates of S. pneumoniae by constructing an individual genomic library for each isolate. DNA sequencing suggested that one isolate contained a gene similar to the globular lantibiotic mersacidin, probably in two copies (117).
Peptide bacteriocin genes have been identified in other sequenced streptococcal species. S. mutans UA159, a sequenced cariogenic dental pathogen, exhibits nonlantibiotic mutacin activity (2). Several putative bacteriocin ORFs in S. mutans UA159 share strong homology with ORFs found in genomes of S. bovis and S. pneumoniae strains. The annotation of S. mutans UA159 originally suggested six hypothetical peptide bacteriocin ORFs, one of which (the translated protein Q8CVC8) showed homology with bovicin 255 variants and acidocin M (66). A seventh translated ORF (the protein Q8DS95) may also encode a bacteriocin, since it shares homology with a putative bacteriocin of S. thermophilus (gi 62528196). In a recent study, it was demonstrated by bioinformatics and mutational analyses that the antimicrobial repertoires of S. mutans strain UA159 includes the two-peptide mutacin IV (SMU 150 and SMU151) and mutacin V (SMU1914c) (44).
Streptococcin A-FF22 and the streptins (streptin 1 and 2) are the only peptide bacteriocins thoroughly characterized from S. pyogenes strains (55, 60, 127). The genome sequences of seven S. pyogenes strains (MGAS1039, MGAS315, MGAS5005, MGAS6180, MGAS8232, SF370, and SSI-1) are listed in the TIGR-CMR database, and more than 30 ORFs encoding bacteriocin-like proteins have been annotated, with prevalences varying from two to seven putative bacteriocin ORFs in each strain.
Enterococci produce many different peptide bacteriocins, and purification and characterization have shown that the nonlantibiotic peptide bacteriocins dominate among them. The genome sequence of E. faecalis V583, a vancomycin-resistant clinical isolate, has been published, but it is the only complete Enterococcus genome sequence available so far (99). No putative bacteriocin ORFs were initially identified, but a hypothetical protein of 43 amino acids (EFA0015) is almost identical to the recently characterized plasmid-encoded leaderless enterocin EJ97 (112). The translated peptide EFA0015 is 97% identical to enterocin EJ97, as it lacks the threonine in position 14 of enterocin EF97. However, it remains to be seen if the protein EFA0015 is an antimicrobial compound.
It must be emphasized that identification of putative bacteriocin genes does not necessarily mean that the relevant bacterium produces antimicrobial activity, and a lack of detectable antimicrobial activity does not necessarily mean that genes involved in bacteriocin production are defective. First, it is of key importance to use a susceptible indicator, which can pose a problem, since some peptide bacteriocins act on only a narrow range of target bacteria. Secondly, the production of peptide bacteriocins is often regulated. The best-understood regulatory system for peptide bacteriocin production is the two-component system (87), but other regulatory mechanisms also exist (13, 104, 105). Deficiency in production of antimicrobial activity is often due to a dysfunctional genetic system. Occasionally, it has been observed that bacterial genomes encode only parts of the bacteriocin production system or that mutations have inactivated the functionality of the bacteriocin genes (23, 85).
REGULATION OF PEPTIDE BACTERIOCIN SYNTHESIS
Bacteriocins may play important roles in bacterial ecology, and the high incidence of bacteriocin production among streptococci and enterococci probably reflects this fact. In most cases, bacteriocin production appears to be regulated and is consequently produced only under suitable growth conditions. Therefore, the choice of the right culturing conditions (medium composition, temperature, pH, water activity, etc.) may be crucial for the outcome of bacteriocin screenings.
Some bacteriocins are produced on solid growth media but not in liquid cultures (100). Growth temperatures have also been shown to influence bacteriocin production in E. faecium. E. faecium L50 produces at least three bacteriocins, and it was demonstrated that the various bacteriocins were produced at different temperatures and had different temperatures for optimal production (13). For the production of bacteriocins from S. pyogenes, the presence of blood was essential, and the same requirement was also reported for the cytolysin of E. faecalis (17).
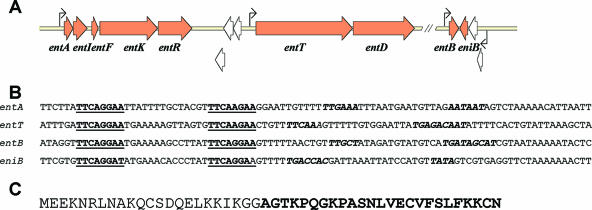
Bacteriocin production has been shown to be regulated by two-component systems in a number of lactic acid bacteria, including S. thermophilus (52) and E. faecium (93). In such cases, the inducers are highly specific autoinduced bacteriocin-like peptides that are commonly referred to as peptide pheromones (87). The regulatory circuit of enterocin A and B was among the first to be elucidated for enterococci; the individual genes involved, the sequence of the peptide pheromone, and the promoter sequences are shown in Fig. 1 (93). The induction of gene expression is achieved by an accumulation of the pheromone peptide through a low constitutive production. When a threshold concentration of the peptide pheromone has been reached, the peptides binds to its receptor (the histidine protein kinase), followed by a phosphorylation cascade leading to phosphorylation of the cognate response regulator, which binds and activates the regulated promoters (Fig. 1). A burst in the expression of genes takes place, and mass production of bacteriocin results (87).
FIG. 1.
Genomic organization of the enterocin A and B loci (3, 9, 36, 93, 96). (A) Genes involved in production of enterocin A and enterocin B in Enterococcus faecium. The enterocin A locus consists of two operons: (i) the bacteriocin operon consisting of the enterocin A gene (entA), the immunity gene of EntA (entI), the peptide pheromone gene (entF), the receptor of the peptide pheromone, the histidine protein kinease gene (entK), and the DNA binding activator, the response regulator (entR), and (ii) the second operon (transporter operon) consisting of the two genes, the ABC transporter (entT) and its accessory gene (entD), that are needed for the secretion of both the peptide pheromone and the bacteriocin. The enterocin B locus consists of two divergent operons: (i) the monocistronic operon consisting of the enterocin B gene (entB) and (ii) the second operon, containing the immunity gene (eniB). Both operons are controlled by the regulatory genes (entFKR) of enterocin A, and the processing and transport of enterocin B are probably mediated by the entT and entD genes. The four regulated promoters are indicated by arrows. ORFs of unknown function are shown as open arrows. (B) DNA sequences of the regulated promoter regions. The direct-repeat sequences that are the binding sites for the phosphorylated response regulator are in boldface and underlined. Putative −35 and −10 regions are shown in boldface italics. (C) Deduced precursor of the peptide pheromone (induction peptide) EntF. The sequence of the mature peptide pheromone is shown in boldface.
In E. faecium CTC492, enterocin A production was diminished at low pH and high concentrations of salt but was restored by the addition of inducer peptide (93). In E. faecalis, one of the two peptides of cytolysin, CylLS" peptide, induces high-level expression of the cytolysin structural genes (57). However, the second component, CylLL" peptide, can form a complex with CylLS that prevents induction. CylLL binds preferentially to target cells (erythrocytes), and in the presence of target cells, high-level cytolysin expression is induced. Thus, this autoregulatory mechanism provides the bacteria with a means to fine tune cytolysin production in response to the presence of targets (17).
TARGETS—RECEPTORS FOR BACTERIOCINS
Numerous mode-of-action studies have been performed on peptide bacteriocins. Most bacteriocins are membrane active, causing permeabilization of and eventually killing the target bacteria. Both some A- and B-type lantibiotics have been shown to kill target cells by interrupting cell wall synthesis through high-affinity binding to the lipid II molecule, a molecule that plays an essential role in the synthesis of the peptidoglycan layer (6, 7, 97). Type A lantibiotics are also able to kill bacteria by an additional mechanism: binding to the lipid II molecule and thereby forming pores in the cytoplasmic membrane of the target. The mechanisms of pore formation of type A lantibiotics are the most important killing mechanism. A similar pore formation mechanism has also been shown for the two-peptide lantibiotic lacticin 3147 (130). At present, we do not know the details of the mechanisms of action of lantibiotics from streptococci, but it seems likely that some of these lantibiotics, such as mutacin I, 1140, and B-Ny266, also use lipid II as a target molecule (10).
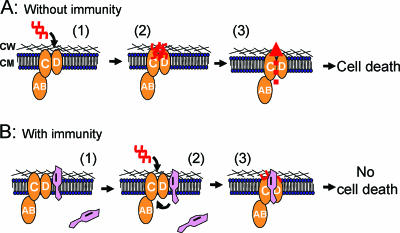
Targets for class II bacteriocins are less well known. However, genetic studies have suggested that the mannose PTS system is the target of class IIa bacteriocins (47). Based on these genetic studies and biochemical studies (D. B. Diep and H. Holo, unpublished data), a model of how class IIa bacteriocins work and how the dedicated immunity protein can provoke its activity has been developed (Fig. 2).
FIG. 2.
Model of the mechanisms behind killing (A) and immunity (B) of classIIa bacteriocins. (A) The bacteriocin (red) employs man-PTS (orange) as a target receptor upon approaching susceptible cells (1). It binds to the components IIC (C) and IID (D) of mannose-PTS (2) and somehow causes leakage of solutes across the cytoplasmic membrane (3) and eventually cell death. (B) In immune and non-bacteriocin-producing cells (1), the immunity protein (pink) is nonassociated or loosely associated with the receptor proteins. When bacteriocin is exogenously added or produced by the bacteria themselves (2), the immunity protein is tightly associated with the receptor to prevent the bound bacteriocin on the receptor from forming lethal pores in the cytoplasmic membrane (3). In all cases, the cytoplasmic component IIAB (AB) is in contact with its membrane-located partners, but without being directly involved in a receptor function or in an immunity function. CW, cell wall; CM, cytoplasmic membrane. The model is based on published work (41, 47, 103) and unpublished work (Diep and Holo, unpublished).
CONCLUDING REMARKS
Enterococci and streptococci seem to be unique in their great potential to produce peptide bacteriocins. It is interesting that while lantibiotics are by far the peptide bacteriocins most frequently found in streptococci, the class II peptide bacteriocins are dominant in enterococci. Most of the purified streptococcal bacteriocins are plasmid encoded, but bacterial chromosomally encoded bacteriocins have also been isolated, and genome mining suggests the presence of a large number of bacteriocin genes in their genomes. In spite of the great number of putative bacteriocin genes in the sequenced S. pneumoniae strains, no bacteriocin activity has been reported. This is in contrast to S. mutans, from which many bacteriocins have been purified and characterized and for which coordinated bacteriocin production and competence development have also been observed (72, 124). In the early 1970s, it was reported that bacteriocin synthesis coincided with DNA uptake competence development in Streptococcus gordonii strain Challis (113, 114), and recently, it was reported that bacteriocin/hemolysin biosynthesis was controlled by the competence regulon (48). These findings suggest that bacteriocins play an important role in providing naked DNA for uptake in competent streptococci.
The lack of direct correlation between the many putative peptide bacteriocin genes in the Streptococcus strains and antimicrobial activity expressed is puzzling. The possibility cannot be excluded that the lack of activity of such translated ORFs is simply because they are not antimicrobial peptides but serve a completely different and unknown function. In this context, one should bear in mind that peptide pheromones for competence development, as well as regulation of bacteriocin production in gram-positive bacteria (quorum sensing), share many of the physiochemical properties of bacteriocins (87). In order to determine if an ORF may encode a peptide bacteriocin, different strategies should be considered. Heterologous expression of peptide bacteriocins has been achieved, but such an approach depends on the presence of complementary genes involved in maturation and transport, in addition to immunity (106). Peptide synthesis is an alternative strategy that has been used successfully to obtain class II peptide bacteriocins (32), while chemical synthesis of lantibiotics cannot presently be obtained due to the extensive posttranslational modifications.
Correction of a mutation in a gene required for bacteriocin synthesis has been used successfully to identify a new bacteriocin in lactic acid bacteria (23). The genome of Pediococcus pentosaceus ATCC 25745 contains a gene cluster that resembles a regulated bacteriocin system. A mutated and defective peptide pheromone involved in a quorum-sensing regulatory mechanism for bacteriocin synthesis was identified. Genetic correction of the mutated peptide pheromone made it possible to express the bacteriocin.
Enterococci produce a great diversity of class II peptide bacteriocins, and most of them are plasmid encoded. Unfortunately, only one Enterococcus genome has been sequenced completely, and only one putative bacteriocin gene was identified. From this limited information, it is not possible to draw any firm conclusions about the prevalence of bacteriocin-encoding traits in enterococcal chromosomes. However, we can conclude that enterococci produce a great number of different class II bacteriocins and that most of them are apparently plasmid encoded. It is tempting to speculate that bacteriocins are found more frequently in enterococci and streptococci than in many other lactic acid bacteria, such as Lactococcus and Lactobacillus. Genome mining suggests that there is great potential to find many new bacteriocins in Streptococcus, and it will be important to follow up such findings with functional studies, which hopefully will bring new and efficient antimicrobial peptides to the market in the future.
Acknowledgments
We thank the Norwegian Research Council for funding.
Footnotes
Published ahead of print on 10 November 2006.
REFERENCES
- 1.Abriouel, H., R. Lucas, N. Ben Omar, E. Valdivia, M. Maqueda, M. Martinez-Canamero, and A. Galvez. 2005. Enterocin AS-48RJ: a variant of enterocin AS-48 chromosomally encoded by Enterococcus faecium RJ16 isolated from food. Syst. Appl. Microbiol. 28:383-397. [DOI] [PubMed] [Google Scholar]
- 2.Ajdic, D., W. M. McShan, R. E. McLaughlin, G. Savic, J. Chang, M. B. Carson, C. Primeaux, R. Tian, S. Kenton, H. Jia, S. Lin, Y. Qian, S. Li, H. Zhu, F. Najar, H. Lai, J. White, B. A. Roe, and J. J. Ferretti. 2002. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc. Natl. Acad. Sci. USA 99:14434-14439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aymerich, T., H. Holo, L. S. Havarstein, M. Hugas, M. Garriga, and I. F. Nes. 1996. Biochemical and genetic characterization of enterocin A from Enterococcus faecium, a new antilisterial bacteriocin in the pediocin family of bacteriocins. Appl. Environ. Microbiol. 62:1676-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balakrishnan, M., R. S. Simmonds, A. Carne, and J. R. Tagg. 2000. Streptococcus mutans strain N produces a novel low molecular mass non-lantibiotic bacteriocin. FEMS Microbiol. Lett. 83:165-169. [DOI] [PubMed] [Google Scholar]
- 5.Balakrishnan, M., R. S. Simmonds, M. Kilian, and J. R. Tagg. 2002. Different bacteriocin activities of Streptococcus mutans reflect distinct phylogenetic lineages. J. Med. Microbiol. 51:941-948. [DOI] [PubMed] [Google Scholar]
- 6.Bonelli, R. R., T. Schneider, H. G. Sahl, and I. Wiedemann. 2006. Insights into in vivo activities of lantibiotics from gallidermin and epidermin mode-of-action studies. Antimicrob. Agents Chemother. 50:1449-1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breukink, E., and B. de Kruijff. 2006. Lipid II as a target for antibiotics. Nat. Rev. Drug. Discov. 5:321-332. [DOI] [PubMed] [Google Scholar]
- 8.Broadbent, J. R., Y. C. Chou, K. Gillies, and J. K. Kondo. 1989. Nisin inhibits several gram-positive, mastitis-causing pathogens. J. Dairy Sci. 72:3342-3345. [DOI] [PubMed] [Google Scholar]
- 9.Casaus, P., T. Nilsen, L. M. Cintas, I. F. Nes, P. E. Hernandez, and H. Holo. 1997. Enterocin B, a new bacteriocin from Enterococcus faecium T136 which can act synergistically with enterocin A. Microbiology 143:2287-2294. [DOI] [PubMed] [Google Scholar]
- 10.Chatterjee, C., M. Paul, L. Xie, and W. A. van der Donk. 2005. Biosynthesis and mode of action of lantibiotics. Chem. Rev. 105:633-684. [DOI] [PubMed] [Google Scholar]
- 11.Chikindas, M. L., J. Novak, A. J. Driessen, W. N. Konings, K. M. Schilling, and P. W. Caufield. 1995. Mutacin II, a bactericidal antibiotic from Streptococcus mutans. Antimicrob. Agents Chemother. 39:2656-2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cintas, L. M., P. Casaus, L. S. Havarstein, P. E. Hernandez, and I. F. Nes. 1997. Biochemical and genetic characterization of enterocin P, a novel sec-dependent bacteriocin from Enterococcus faecium P13 with a broad antimicrobial spectrum. Appl. Environ. Microbiol. 63:4321-4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cintas, L. M., P. Casaus, C. Herranz, L. S. Havarstein, H. Holo, P. E. Hernandez, and I. F. Nes. 2000. Biochemical and genetic evidence that Enterococcus faecium L50 produces enterocins L50A and L50B, the sec-dependent enterocin P, and a novel bacteriocin secreted without an N-terminal extension termed enterocin Q. J. Bacteriol. 182:6806-6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cintas, L. M., P. Casaus, H. Holo, P. E. Hernandez, I. F. Nes, and L. S. Havarstein. 1998. Enterocins L50A and L50B, two novel bacteriocins from Enterococcus faecium L50, are related to staphylococcal hemolysins. J. Bacteriol. 180:1988-1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cleveland, J., T. J. Montville, I. F. Nes, and M. L. Chikindas. 2001. Bacteriocins: safe, natural antimicrobials for food preservation. Int. J. Food Microbiol. 71:1-20. [DOI] [PubMed] [Google Scholar]
- 16.Coburn, P. S., and M. S. Gilmore. 2003. The Enterococcus faecalis cytolysin: a novel toxin active against eukaryotic and prokaryotic cells. Cell Microbiol. 5:661-669. [DOI] [PubMed] [Google Scholar]
- 17.Coburn, P. S., C. M. Pillar, B. D. Jett, W. Haas, and M. S. Gilmore. 2004. Enterococcus faecalis senses target cells and in response expresses cytolysin. Science 306:2270-2272. [DOI] [PubMed] [Google Scholar]
- 18.Cookson, A. L., S. J. Noel, W. J. Kelly, and G. T. Attwood. 2004. The use of PCR for the identification and characterisation of bacteriocin genes from bacterial strains isolated from rumen or caecal contents of cattle and sheep. FEMS Microbiol. Ecol. 48:1199-1207. [DOI] [PubMed] [Google Scholar]
- 19.Cotter, P. D., C. Hill, and R. P. Ross. 2005. Bacteriocins: developing innate immunity for food. Nat. Rev. Microbiol. 3:777-788. [DOI] [PubMed] [Google Scholar]
- 20.Criado, R., D. B. Diep, A. Aakra, J. Gutierrez, I. F. Nes, P. E. Hernandez, and L. M. Cintas. 2006. Complete sequence of the enterocin Q-encoding plasmid pCIZ2 from the multiple bacteriocin producer Enterococcus faecium L50 and genetic characterization of enterocin Q. Production and immunity. Appl. Environ. Microbiol. 72:6653-6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Jong, A., S. A. F. T. van Hijum, J. J. E. Bijlsma, J. Kok, and O. P. Kuipers. 2006. BAGEL: a web-based bacteriocin genome mining tool. Nucleic Acids Res. 34:273-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Kwaadsteniet, M., T. Fraser, C. A. Van Reenen, and L. M. Dicks. 2006. Bacteriocin T8, a novel class IIa sec-dependent bacteriocin produced by Enterococcus faecium T8, isolated from vaginal secretions of children infected with human immunodeficiency virus. Appl. Environ. Microbiol. 72:4761-4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diep, D. B., L. Godager, D. Brede, and I. F. Nes. 2006. Data mining and characterization of a novel pediocin-like bacteriocin system from the genome of Pediococcus pentosaceus ATCC 25745. Microbiology 152:1649-1659. [DOI] [PubMed] [Google Scholar]
- 24.Diep, D. B., L. S. Havarstein, and I. F. Nes. 1995. A bacteriocin-like peptide induces bacteriocin synthesis in Lactobacillus plantarum C11. Mol. Microbiol. 8:631-639. [DOI] [PubMed] [Google Scholar]
- 25.Dirix, G., P. Monsieurs, B. Dombrecht, R. Daniels, K. Marchal, J. Vanderleyden, and J. Michiels. 2004. Peptide signal molecules and bacteriocins in Gram-negative bacteria: a genome-wide in silico screening for peptides containing a double-glycine leader sequence and their cognate transporters. Peptides 25:1425-1440. [DOI] [PubMed] [Google Scholar]
- 26.Donvito, B., J. Etienne, L. Denoroy, T. Greenland, Y. Benito, and F. Vandenesch. 1997. Synergistic hemolytic activity of Staphylococcus lugdunensis is mediated by three peptides encoded by a non-agr genetic locus. Infect. Immun. 65:95-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drider, D., G. Fimland, Y. Hechard, L. M. McMullen, and H. Prevost. 2006. The continuing story of class IIa bacteriocins. Microbiol. Mol. Biol. Rev. 70:564-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eguchi, T., K. Kaminaka, J. Shima, S. Kawamoto, K. Mori, S. H. Choi, K. Doi, S. Ohmomo, and S. Ogata. 2001. Isolation and characterization of enterocin SE-K4 produced by thermophilic enterococci, Enterococcus faecalis K-4. Biosci. Biotechnol. Biochem. 65:247-253. [DOI] [PubMed] [Google Scholar]
- 29.Eijsink, V. G., L. Axelsson, D. B. Diep, L. S. Havarstein, H. Holo, and I. F. Nes. 2002. Production of class II bacteriocins by lactic acid bacteria; an example of biological warfare and communication. Antonie Leeuwenhoek 81:639-654. [DOI] [PubMed] [Google Scholar]
- 30.Eijsink, V. G., M. Skeie, P. H. Middelhoven, M. B. Brurberg, and I. F. Nes. 1998. Comparative studies of class IIa bacteriocins of lactic acid bacteria. Appl. Environ. Microbiol. 64:3275-3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ennahar, S., T. Sashihara, K. Sonomoto, and A. Ishizaki. 2000. Class IIa bacteriocins: biosynthesis, structure and activity. FEMS Microbiol. Rev. 24:85-106. [DOI] [PubMed] [Google Scholar]
- 32.Fimland, G., O. R. Blingsmo, K. Sletten, G. Jung, I. F. Nes, and J. Nissen-Meyer. 1996. New biologically active hybrid bacteriocins constructed by combining regions from various pediocin-like bacteriocins: the C-terminal region is important for determining specificity. Appl. Environ. Microbiol. 62:3313-3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Foulquie Moreno, M. R., P. Sarantinopoulos, E. Tsakalidou, and L. De Vuyst. 2006. The role and application of enterococci in food and health. Int. J. Food Microbiol. 106:1-24. [DOI] [PubMed] [Google Scholar]
- 34.Franz, C. M., A. Grube, A. Herrmann, H. Abriouel, J. Starke, A. Lombardi, B. Tauscher, and W. H. Holzapfel. 2002. Biochemical and genetic characterization of the two-peptide bacteriocin enterocin 1071 produced by Enterococcus faecalis FAIR-E 309. Appl. Environ. Microbiol. 68:2550-2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Franz, C. M., M. E. Stiles, K. H. Schleifer, and W. H. Holzapfel. 2003. Enterococci in foods—a conundrum for food safety. Int. J. Food Microbiol. 88:105-122. [DOI] [PubMed] [Google Scholar]
- 36.Franz, C. M., R. W. Worobo, L. E. Quadri, U. Schillinger, W. H. Holzapfel, J. C. Vederas, and M. E. Stiles. 1999. Atypical genetic locus associated with constitutive production of enterocin B by Enterococcus faecium BFE 900. Appl. Environ. Microbiol. 65:2170-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fuller, J. D., A. C. Camus, C. L. Duncan, V. Nizet, D. J. Bast, R. L. Thune, D. E. Low, and J. C. De Azavedo. 2002. Identification of a streptolysin S-associated gene cluster and its role in the pathogenesis of Streptococcus iniae disease. Infect. Immunol. 70:5730-5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galvez, A., G. Gimenez-Gallego, M. Maqueda, and E. Valdivia. 1989. Purification and amino acid composition of peptide antibiotic AS-48 produced by Streptococcus (Enterococcus) faecalis subsp. liquefaciens S-48. Antimicrob. Agents Chemother. 33:437-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galvez, A., E. Valdivia, H. Abriouel, E. Camafeita, E. Mendez, M. Martinez-Bueno, and M. Maqueda. 1998. Isolation and characterization of enterocin EJ97, a bacteriocin produced by Enterococcus faecalis EJ97. Arch. Microbiol. 171:59-65. [DOI] [PubMed] [Google Scholar]
- 40.Georgalaki, M. D., E. Van Den Berghe, D. Kritikos, B. Devreese, J. Van Beeumen, G. Kalantzopoulos, L. De Vuyst, and E. Tsakalidou. 2002. Macedocin, a food-grade lantibiotic produced by Streptococcus macedonicus ACA-DC 198. Appl. Environ. Microbiol. 68:5891-5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gravesen, A., M. Ramnath, K. B. Rechinger, N. Andersen, L. Jansch, Y. Hechard, J. W. Hastings, and S. Knochel. 2002. High-level resistance to class IIa bacteriocins is associated with one general mechanism in Listeria monocytogenes. Microbiology 148:2361-2369. [DOI] [PubMed] [Google Scholar]
- 42.Gronroos, L., M. Saarela, J. Matto, U. Tanner-Salo, A. Vuorela, and S. Alaluusua. 1998. Mutacin production by Streptococcus mutans may promote transmission of bacteria from mother to child. Infect. Immun. 66:2595-2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guchi, T., K. Kaminaka, J. Shima, S. Kawamoto, K. Mori, S.-H. Choi, K. Doi, S. Ohmomo, and S. Ogata. 2002. Isolation and characterization of enterocin SE-K4 produced by thermophilic enterococci, Enterococcus faecalis K-4. Biosci. Biotechnol. Biochem. 65:247-253. [DOI] [PubMed] [Google Scholar]
- 44.Hale, J. D., Y. T. Ting, R. W. Jack, J. R. Tagg, and N. C. Heng. 2005. Bacteriocin (mutacin) production by Streptococcus mutans genome sequence reference strain UA159: elucidation of the antimicrobial repertoire by genetic dissection. Appl. Environ. Microbiol. 71:7613-7617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Havarstein, L. S., D. B. Diep, and I. F. Nes. 1995. A family of bacteriocin ABC transporters carry out proteolytic processing of their substrates concomitant with export. Mol. Microbiol. 16:229-240. [DOI] [PubMed] [Google Scholar]
- 46.Havarstein, L. S., H. Holo, and I. F. Nes. 1994. The leader peptide of colicin V shares consensus sequences with leader peptides that are common among peptide bacteriocins produced by gram-positive bacteria. Microbiology 140:2383-2389. [DOI] [PubMed] [Google Scholar]
- 47.Hechard, Y., and H. G. Sahl. 2002. Mode of action of modified and unmodified bacteriocins from Gram-positive bacteria. Biochimie 84:545-557. [DOI] [PubMed] [Google Scholar]
- 48.Heng, N. C., J. R. Tagg, and G. R. Tompkins. 29 September 2006. Competence-dependent bacteriocin production by Streptococcus gordonii DL1 (Challis). J. Bacteriol. doi: 10.1128/JB.01174-06. [DOI] [PMC free article] [PubMed]
- 49.Heng, N. C. K., and J. R. Tagg. 2006. What's in a name? Class distinction for bacteriocins. Nat. Rev. Microbiol. doi: 10.1038/nrmicro1273-c1. [DOI]
- 50.Hillman, J. D., J. Novak, E. Sagura, J. A. Gutierrez, T. A. Brooks, P. J. Crowley, M. Hess, A. Azizi, K. Leung, D. Cvitkovitch, and A. S. Bleiweis. 1998. Genetic and biochemical analysis of mutacin 1140, a lantibiotic from Streptococcus mutans. Infect. Immun. 66:2743-2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holo, H., O. Nilssen, and I. F. Nes. 1991. Lactococcin A, a new bacteriocin from Lactococcus lactis subsp. cremoris: isolation and characterization of the protein and its gene. J. Bacteriol. 173:3879-3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hols, P., F. Hancy, L. Fontaine, B. Grossiord, D. Prozzi, N. Leblond-Bourget, B. Decaris, A. Bolotin, C. Delorme, S. Dusko Ehrlich, E. Guedon, V. Monnet, P. Renault, and M. Kleerebezem. 2005. New insights in the molecular biology and physiology of Streptococcus thermophilus revealed by comparative genomics. FEMS Microbiol. Rev. 29:435-463. [DOI] [PubMed] [Google Scholar]
- 53.Humar, D., V. Datta, D. J. Bast, B. Beall, J. C. S. De Azavedo, and V. Nizet. 2002. Streptolysin S and necrotising infections produced by group G streptococcus. Lancet 359:124-129. [DOI] [PubMed] [Google Scholar]
- 54.Hyink, O., M. Balakrishnan, and J. R. Tagg. 2005. Streptococcus rattus strain BHT produces both a class I two-component lantibiotic and a class II bacteriocin. FEMS Microbiol. Lett. 252:235-241. [DOI] [PubMed] [Google Scholar]
- 55.Hynes, W. L., J. J. Ferretti, and J. R. Tagg. 1993. Cloning of the gene encoding streptococcin A-FF22, a novel lantibiotic produced by Streptococcus pyogenes, and determination of its nucleotide sequence. Appl. Environ. Microbiol. 59:1969-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haas, W., and M. S. Gilmore. 1999. Molecular nature of a novel bacterial toxin: the cytolysin of Enterococcus faecalis. Med. Microbiol. Immunol. 187:183-190. [DOI] [PubMed] [Google Scholar]
- 57.Haas, W., B. D. Shepard, and M. S. Gilmore. 2002. Two-component regulator of Enterococcus faecalis cytolysin responds to quorum-sensing autoinduction. Nature 415:84-87. [DOI] [PubMed] [Google Scholar]
- 58.Inoue, T., H. Tomita, and Y. Ike. 2006. Bac 32, a novel bacteriocin widely disseminated among clinical isolates of Enterococcus faecium. Antimicrob. Agents Chemother. 50:1202-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jack, R. W., G. Bierbaum, and H.-G. Sahl. 1998. Lantibiotics and related peptides. Springer-Verlag, Berlin, Germany.
- 60.Jack, R. W., A. Carne, J. Metzger, S. Stefanovic, H. G. Sahl, G. Jung, and J. Tagg. 1994. Elucidation of the structure of SA-FF22, a lanthionine-containing antibacterial peptide produced by Streptococcus pyogenes strain FF22. Eur. J. Biochem. 220:455-462. [DOI] [PubMed] [Google Scholar]
- 61.Jett, B. D., M. M. Huycke, and M. S. Gilmore. 1994. Virulence of enterococci. Clin. Microbiol. Rev. 7:462-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Joosten, H. M., M. Nunez, B. Devreese, J. Van Beeumen, and J. D. Marugg. 1996. Purification and characterization of enterocin 4, a bacteriocin produced by Enterococcus faecalis INIA 4. Appl. Environ. Microbiol. 62:4220-4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Joosten, H. M., E. Rodriguez, and M. Nunez. 1997. PCR detection of sequences similar to the AS-48 structural gene in bacteriocin-producing enterococci. Lett. Appl. Microbiol. 24:40-42. [DOI] [PubMed] [Google Scholar]
- 64.Jung, G. 1991. Lantibiotics: a survey, p. 1-35. In G. Jung and H.-G. Sahl (ed.), Nisin and novel lantibiotics. ESCOM Science Publishers, Leiden, The Netherlands.
- 65.Jurkovic, D., L. Krizkova, R. Dusinsky, A. Belicova, M. Sojka, J. Krajcovic, and L. Ebringer. 2006. Identification and characterization of enterococci from bryndza cheese. Lett. Appl. Microbiol. 42:553-559. [DOI] [PubMed] [Google Scholar]
- 66.Kanatani, K., T. Tahara, M. Oshimura, K. Sano, and C. Umezawa. 1995. Identification of the replication region of Lactobacillus acidophilus plasmid pLA103. FEMS Microbiol. Lett. 133:127-130. [DOI] [PubMed] [Google Scholar]
- 67.Kang, J. H., and M. S. Lee. 2005. Characterization of a bacteriocin produced by Enterococcus faecium GM-1 isolated from an infant. J. Appl. Microbiol. 98:1169-1176. [DOI] [PubMed] [Google Scholar]
- 68.Karaya, K., T. Shimizu, and A. Taketo. 2001. New gene cluster for lantibiotic streptin possibly involved in streptolysin S formation. J. Biochem. 129:769-775. [DOI] [PubMed] [Google Scholar]
- 69.Kawamoto, S., J. Shima, R. Sato, T. Eguchi, S. Ohmomo, J. Shibato, N. Horikoshi, K. Takeshita, and T. Sameshima. 2002. Biochemical and genetic characterization of mundticin KS, an antilisterial peptide produced by Enterococcus mundtii NFRI 7393. Appl. Environ. Microbiol. 68:3830-3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kayaoglu, G., and D. Orstavik. 2004. Virulence factors of Enterococcus faecalis: relationship to endodontic disease. Crit. Rev. Oral Biol. Med. 15:308-320. [DOI] [PubMed] [Google Scholar]
- 71.Klaenhammer, T. R. 1993. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol. Rev. 12:39-85. [DOI] [PubMed] [Google Scholar]
- 72.Kreth, J., J. Merritt, W. Shi, and F. Qi. 2005. Co-ordinated bacteriocin production and competence development: a possible mechanism for taking up DNA from neighbouring species. Mol. Microbiol. 57:392-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Krull, R. E., P. Chen, J. Novak, M. Kirk, S. Barnes, J. Baker, N. R. Krishna, and P. W. Caufield. 2000. Biochemical structural analysis of the lantibiotic mutacin II. J. Biol. Chem. 275:15845-15850. [DOI] [PubMed] [Google Scholar]
- 74.Laukova, A., S. Czikkova, Z. Vasilkova, P. Juris, and M. Marekova. 1998. Occurrence of bacteriocin production among environmental enterococci. Lett. Appl. Microbiol. 27:178-182. [DOI] [PubMed] [Google Scholar]
- 75.Laukova, A., and M. Marekova. 2001. Production of bacteriocins by different enterococcal isolates. Folia Microbiol. 46:49-52. [DOI] [PubMed] [Google Scholar]
- 76.Macovei, L., and L. Zurek. 2006. Ecology of antibiotic resistance genes: characterization of enterococci from houseflies collected in food settings. Appl. Environ. Microbiol. 72:4028-4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maqueda, M., A. Galvez, M. M. Bueno, M. J. Sanchez-Barrena, C. Gonzalez, A. Albert, M. Rico, and E. Valdivia. 2004. Peptide AS-48: prototype of a new class of cyclic bacteriocins. Curr. Protein Pept. Sci. 5:399-416. [DOI] [PubMed] [Google Scholar]
- 78.Marciset, O., M. C. Jeronimus-Stratingh, B. Mollet, and B. Poolman. 1997. Thermophilin 13, a nontypical antilisterial poration complex bacteriocin, that functions without a receptor. J. Biol. Chem. 272:14277-14284. [DOI] [PubMed] [Google Scholar]
- 79.Marekova, M., A. Laukova, L. DeVuyst, M. Skaugen, and I. F. Nes. 2003. Partial characterization of bacteriocins produced by environmental strain Enterococcus faecium EK13. J. Appl. Microbiol. 94:523-530. [DOI] [PubMed] [Google Scholar]
- 80.Martin, N. I., T. Sprules, M. R. Carpenter, P. D. Cotter, C. Hill, R. P. Ross, and J. C. Vederas. 2004. Structural characterization of lacticin 3147, a two-peptide lantibiotic with synergistic activity. Biochemistry 43:3049-3056. [DOI] [PubMed] [Google Scholar]
- 81.Martin-Platero, A. M., E. Valdivia, M. Ruiz-Rodriguez, J. J. Soler, M. Martin-Vivaldi, M. Maqueda, and M. Martinez-Bueno. 2006. Characterization of antimicrobial substances produced by Enterococcus faecalis MRR 10-3, isolated from the uropygial gland of the hoopoe (Upupa epops). Appl. Environ. Microbiol. 72:4245-4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Martinez-Bueno, M., M. Maqueda, A. Galvez, B. Samyn, J. Van Beeumen, J. Coyette, and E. Valdivia. 1994. Determination of the gene sequence and the molecular structure of the enterococcal peptide antibiotic AS-48. J. Bacteriol. 176:6334-6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McAuliffe, O., R. P. Ross, and C. Hill. 2001. Lantibiotics: structure, biosynthesis and mode of action. FEMS Microbiol. Rev. 25:285-308. [DOI] [PubMed] [Google Scholar]
- 84.Mindich, L. 1966. Bacteriocins of Diplococcus pneumoniae. I. Antagonistic relationships and genetic transformations. J. Bacteriol. 92:1090-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moretro, T., K. Naterstad, E. Wang, I. M. Aasen, S. Chaillou, M. Zagorec, and L. Axelsson. 2005. Sakacin P non-producing Lactobacillus sakei strains contain homologues of the sakacin P gene cluster. Res. Microbiol. 156:949-960. [DOI] [PubMed] [Google Scholar]
- 86.Mota-Meira, M., C. Lacroix, G. LaPointe, and M. C. Lavoie. 1997. Purification and structure of mutacin B-Ny266: a new lantibiotic produced by Streptococcus mutans. FEBS Lett. 410:275-279. [DOI] [PubMed] [Google Scholar]
- 87.Nes, I. F., and V. G. H. Eijsink. 1999. Regulation of group II peptide bacteriocin synthesis by quorum-Sensing mechansims, p. 175-192. In G. M. Dunny and S. C. Winans (ed.), Cell-cell signaling in bacteria. ASM Press, Washington, DC.
- 88.Nes, I. F., and H. Holo. 2000. Class II antimicrobial peptides from lactic acid bacteria. Peptide Sci. 55:50-61. [DOI] [PubMed] [Google Scholar]
- 89.Nes, I. F., and O. Johnsborg. 2004. Exploration of antimicrobial potential in LAB by genomics. Curr. Opin. Biotechnol. 15:100-104. [DOI] [PubMed] [Google Scholar]
- 90.Netz, D. J., R. Pohl, A. G. Beck-Sickinger, T. Selmer, A. J. Pierik, C. Bastos Mdo, and H. G. Sahl. 2002. Biochemical characterisation and genetic analysis of aureocin A53, a new, atypical bacteriocin from Staphylococcus aureus. J. Mol. Biol. 319:745-756. [DOI] [PubMed] [Google Scholar]
- 91.Netz, D. J., H. G. Sahl, R. Marcelino, J. dos Santos Nascimento, S. S. de Oliveira, M. B. Soares, and M. do Carmo de Freire Bastos. 2001. Molecular characterisation of aureocin A70, a multi-peptide bacteriocin isolated from Staphylococcus aureus. J. Mol. Biol. 311:939-949. [DOI] [PubMed] [Google Scholar]
- 92.Nicolas, G., H. Morency, G. LaPointe, and M. C. Lavoie. 2006. Mutacin H-29B is identical to mutacin II (J-T8). BMC Microbiol. 6:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nilsen, T., I. F. Nes, and H. Holo. 1998. An exported inducer peptide regulates bacteriocin production in Enterococcus faecium CTC492. J. Bacteriol. 180:1848-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nissen-Meyer, J., H. Holo, L. S. Havarstein, K. Sletten, and I. F. Nes. 1992. A novel lactococcal bacteriocin whose activity depends on the complementary action of two peptides. J. Bacteriol. 174:5686-5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nizet, V., B. Beall, D. J. Bast, V. Datta, L. Kilburn, D. E. Low, and J. C. De Azavedo. 2000. Genetic locus for streptolysin S production by group A streptococcus. Infect. Immun. 68:4245-4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.O'Keeffe, T., C. Hill, and R. P. Ross. 1999. Characterization and heterologous expression of the genes encoding enterocin A production, immunity, and regulation in Enterococcus faecium DPC1146. Appl. Environ. Microbiol. 65:1506-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pag, U., and H. G. Sahl. 2002. Multiple activities in lantibiotics—models for the design of novel antibiotics. Curr. Pharm. Des. 8:815-833. [DOI] [PubMed] [Google Scholar]
- 98.Park, S. H., K. Itoh, and T. Fujisawa. 2003. Characteristics and identification of enterocins produced by Enterococcus faecium JCM 5804T. J. Appl. Microbiol. 95:294-300. [DOI] [PubMed] [Google Scholar]
- 99.Paulsen, I. T., L. Banerjei, G. S. Myers, K. E. Nelson, R. Seshadri, T. D. Read, D. E. Fouts, J. A. Eisen, S. R. Gill, J. F. Heidelberg, H. Tettelin, R. J. Dodson, L. Umayam, L. Brinkac, M. Beanan, S. Daugherty, R. T. DeBoy, S. Durkin, J. Kolonay, R. Madupu, W. Nelson, J. Vamathevan, B. Tran, J. Upton, T. Hansen, J. Shetty, H. Khouri, T. Utterback, D. Radune, K. A. Ketchum, B. A. Dougherty, and C. M. Fraser. 2003. Role of mobile DNA in the evolution of vancomycin-resistant Enterococcus faecalis. Science 299:2071-2074. [DOI] [PubMed] [Google Scholar]
- 100.Qi, F., P. Chen, and P. W. Caufield. 2001. The group I strain of Streptococcus mutans, UA140, produces both the lantibiotic mutacin I and a nonlantibiotic bacteriocin, mutacin IV. Appl. Environ. Microbiol. 67:15-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Qi, F., P. Chen, and P. W. Caufield. 2000. Purification and biochemical characterization of mutacin I from the group I strain of Streptococcus mutans, CH43, and genetic analysis of mutacin I biosynthesis genes. Appl. Environ. Microbiol. 66:3221-3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Qi, F., P. Chen, and P. W. Caufield. 1999. Purification of mutacin III from group III Streptococcus mutans UA787 and genetic analyses of mutacin III biosynthesis genes. Appl. Environ. Microbiol. 65:3880-3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ramnath, M., S. Arous, A. Gravesen, J. W. Hastings, and Y. Hechard. 2004. Expression of mptC of Listeria monocytogenes induces sensitivity to class IIa bacteriocins in Lactococcus lactis. Microbiology 150:2663-2668. [DOI] [PubMed] [Google Scholar]
- 104.Rawlinson, E. L., I. F. Nes, and M. Skaugen. 2005. Identification of the DNA-binding site of the Rgg-like regulator LasX within the lactocin S promoter region. Microbiology 151:813-823. [DOI] [PubMed] [Google Scholar]
- 105.Rawlinson, E. L., I. F. Nes, and M. Skaugen. 2002. LasX, a transcriptional regulator of the lactocin S biosynthetic genes in Lactobacillus sakei L45, acts both as an activator and a repressor. Biochimie 84:559-567. [DOI] [PubMed] [Google Scholar]
- 106.Rodriguez, J. M., M. I. Martinez, N. Horn, and H. M. Dodd. 2003. Heterologous production of bacteriocins by lactic acid bacteria. Int. J. Food Microbiol. 80:101-116. [DOI] [PubMed] [Google Scholar]
- 107.Ross, K. F., C. W. Ronson, and J. R. Tagg. 1993. Isolation and characterization of the lantibiotic salivaricin A and its structural gene salA from Streptococcus salivarius 20P3. Appl. Environ. Microbiol. 59:2014-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ross, R. P., M. Galvin, O. McAuliffe, S. M. Morgan, M. P. Ryan, D. P. Twomey, W. J. Meaney, and C. Hill. 1999. Developing applications for lactococcal bacteriocins. Antonie Leeuwenhoek 76:337-346. [PubMed] [Google Scholar]
- 109.Ryan, M. P., R. W. Jack, M. Josten, H. G. Sahl, G. Jung, R. P. Ross, and C. Hill. 1999. Extensive post-translational modification, including serine to d-alanine conversion, in the two-component lantibiotic, lacticin 3147. J. Biol. Chem. 274:37544-37550. [DOI] [PubMed] [Google Scholar]
- 110.Ryan, M. P., W. J. Meaney, R. P. Ross, and C. Hill. 1998. Evaluation of lacticin 3147 and a teat seal containing this bacteriocin for inhibition of mastitis pathogens. Appl. Environ. Microbiol. 64:2287-2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Saavedra, L., C. Minahk, A. P. de Ruiz Holgado, and F. Sesma. 2004. Enhancement of the enterocin CRL35 activity by a synthetic peptide derived from the NH2-terminal sequence. Antimicrob. Agents Chemother. 48:2778-2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sanchez-Hidalgo, M., M. Maqueda, A. Galvez, H. Abriouel, E. Valdivia, and M. Martinez-Bueno. 2003. The genes coding for enterocin EJ97 production by Enterococcus faecalis EJ97 are located on a conjugative plasmid. Appl. Environ. Microbiol. 69:1633-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schlegel, R., and H. D. Slade. 1972. Bacteriocin production by transformable group H streptococci. J. Bacteriol. 112:824-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schlegel, R., and H. D. Slade. 1973. Properties of a Streptococcus sanguis (group H) bacteriocin and its separation from the competence factor of transformation. J. Bacteriol. 115:655-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Semedo, T., M. Almeida Santos, P. Martins, M. F. Silva Lopes, J. J. Figueiredo Marques, R. Tenreiro, and M. T. Barreto Crespo. 2003. Comparative study using type strains and clinical and food isolates to examine hemolytic activity and occurrence of the cyl operon in enterococci. J. Clin. Microbiol. 41:2569-2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sharma, R. M., and R. A. Packer. 1970. Occurrence and ecologic features of Streptococcus uberis in the dairy cow. Am. J. Vet. Res. 31:1197-1202. [PubMed] [Google Scholar]
- 117.Shen, K., J. Gladitz, P. Antalis, B. Dice, B. Janto, R. Keefe, J. Hayes, A. Ahmed, R. Dopico, N. Ehrlich, J. Jocz, L. Kropp, S. Yu, L. Nistico, D. P. Greenberg, K. Barbadora, R. A. Preston, J. C. Post, G. D. Ehrlich, and F. Z. Hu. 2006. Characterization, distribution, and expression of novel genes among eight clinical isolates of Streptococcus pneumoniae. Infect. Immun. 74:321-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Skaugen, M., J. Nissen-Meyer, G. Jung, S. Stevanovic, K. Sletten, C. Inger, M. Abildgaard, and I. F. Nes. 1994. In vivo conversion of l-serine to d-alanine in a ribosomally synthesized polypeptide. J. Biol. Chem. 269:27183-27185. [PubMed] [Google Scholar]
- 119.Tagg, J. R. 2004. Prevention of streptococcal pharyngitis by anti-Streptococcus pyogenes bacteriocin-like inhibitory substances (BLIS) produced by Streptococcus salivarius. Indian J. Med. Res. 119:13-16. [PubMed] [Google Scholar]
- 120.Tettelin, H., K. E. Nelson, I. T. Paulsen, J. A. Eisen, T. D. Read, S. Peterson, J. Heidelberg, R. T. DeBoy, D. H. Haft, R. J. Dodson, A. S. Durkin, M. Gwinn, J. F. Kolonay, W. C. Nelson, J. D. Peterson, L. A. Umayam, O. White, S. L. Salzberg, M. R. Lewis, D. Radune, E. Holtzapple, H. Khouri, A. M. Wolf, T. R. Utterback, C. L. Hansen, L. A. McDonald, T. V. Feldblyum, S. Angiuoli, T. Dickinson, E. K. Hickey, I. E. Holt, B. J. Loftus, F. Yang, H. O. Smith, J. C. Venter, B. A. Dougherty, D. A. Morrison, S. K. Hollingshead, and C. M. Fraser. 2001. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science 293:498-506. [DOI] [PubMed] [Google Scholar]
- 121.Tomita, H., S. Fujimoto, K. Tanimoto, and Y. Ike. 1997. Cloning and genetic and sequence analyses of the bacteriocin 21 determinant encoded on the Enterococcus faecalis pheromone-responsive conjugative plasmid pPD1. J. Bacteriol. 179:7843-7855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tomita, H., S. Fujimoto, K. Tanimoto, and Y. Ike. 1996. Cloning and genetic organization of the bacteriocin 31 determinant encoded on the Enterococcus faecalis pheromone-responsive conjugative plasmid pYI17. J. Bacteriol. 178:3585-3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Turner, D. L., L. Brennan, H. E. Meyer, C. Lohaus, C. Siethoff, H. S. Costa, B. Gonzalez, H. Santos, and J. E. Suarez. 1999. Solution structure of plantaricin C, a novel lantibiotic. Eur. J. Biochem. 264:833-839. [DOI] [PubMed] [Google Scholar]
- 124.van der Ploeg, J. R. 2005. Regulation of bacteriocin production in Streptococcus mutans by the quorum-sensing system required for development of genetic competence. J. Bacteriol. 187:3980-3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ward, D. J., and G. A. Somkuti. 1995. Characterization of a bacteriocin produced by Streptococcus thermophilus ST134. Appl. Microbiol. Biotechnol. 43:330-335. [DOI] [PubMed] [Google Scholar]
- 126.Watson, D. C., M. Yaguchi, J. G. Bisaillon, R. Beaudet, and R. Morosoli. 1988. The amino acid sequence of a gonococcal growth inhibitor from Staphylococcus haemolyticus. Biochem. J. 252:87-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wescombe, P. A., and J. R. Tagg. 2003. Purification and characterization of streptin, a type A1 lantibiotic produced by Streptococcus pyogenes. Appl. Environ. Microbiol. 69:2737-2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wescombe, P. A., M. Upton, K. P. Dierksen, N. L. Ragland, S. Sivabalan, R. E. Wirawan, M. A. Inglis, C. J. Moore, G. V. Walker, C. N. Chilcott, H. F. Jenkinson, and J. R. Tagg. 2006. Production of the lantibiotic salivaricin A and its variants by oral streptococci and use of a specific induction assay to detect their presence in human saliva. Appl. Environ. Microbiol. 72:1459-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Whitford, M. F., M. A. McPherson, R. J. Forster, and R. M. Teather. 2001. Identification of bacteriocin-like inhibitors from rumen Streptococcus spp. and isolation and characterization of bovicin 255. Appl. Environ. Microbiol. 67:569-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wiedemann, I., T. Bottiger, R. R. Bonelli, A. Wiese, S. O. Hagge, T. Gutsmann, U. Seydel, L. Deegan, C. Hill, P. Ross, and H. G. Sahl. 2006. The mode of action of the lantibiotic lacticin 3147—a complex mechanism involving specific interaction of two peptides and the cell wall precursor lipid II. Mol. Microbiol. 61:285-296. [DOI] [PubMed] [Google Scholar]
- 131.Wirawan, R. E., R. W. Jack, and J. R. Tagg. 2006. The antimicrobial (bacteriocin) repertoire of Streptococcus uberis, abstr. B14. Streptococcal Genet. Conf., Saint-Malo, France, 18 to 21 June 2006. American Society for Microbiology, Washington, DC.
- 132.Wirawan, R. E., N. A. Klesse, R. W. Jack, and J. R. Tagg. 2006. Molecular and genetic characterization of a novel nisin variant produced by Streptococcus uberis. Appl. Environ. Microbiol. 72:1148-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Xiao, H., X. Chen, M. Chen, S. Tang, X. Zhao, and L. Huan. 2004. Bovicin HJ50, a novel lantibiotic produced by Streptococcus bovis HJ50. Microbiology 150:103-108. [DOI] [PubMed] [Google Scholar]
- 134.Yamamoto, Y., Y. Togawa, M. Shimosaka, and M. Okazaki. 2003. Purification and characterization of a novel bacteriocin produced by Enterococcus faecalis strain RJ-11. Appl. Environ. Microbiol. 69:5746-5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yonezawa, H., and H. K. Kuramitsu. 2005. Genetic analysis of a unique bacteriocin, Smb, produced by Streptococcus mutans GS5. Antimicrob. Agents Chemother. 49:541-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zadoks, R. N., H. G. Allore, H. W. Barkema, O. C. Sampimon, Y. T. Grohn, and Y. H. Schukken. 2001. Analysis of an outbreak of Streptococcus uberis mastitis. J. Dairy Sci. 84:590-599. [DOI] [PubMed] [Google Scholar]
- 137.Zadoks, R. N., L. L. Tikofsky, and K. J. Boor. 2005. Ribotyping of Streptococcus uberis from a dairy's environment, bovine feces and milk. Vet. Microbiol. 109:257-265. [DOI] [PubMed] [Google Scholar]
- 138.Zendo, T., N. Eungruttanagorn, S. Fujioka, Y. Tashiro, K. Nomura, Y. Sera, G. Kobayashi, J. Nakayama, A. Ishizaki, and K. Sonomoto. 2005. Identification and production of a bacteriocin from Enterococcus mundtii QU 2 isolated from soybean. J. Appl. Microbiol. 99:1181-1190. [DOI] [PubMed] [Google Scholar]