Abstract
Studies have indicated that specific heme delivery to apocytochrome c is a critical feature of the cytochrome c biogenesis pathways called system I and II. To determine directly the heme requirements of each system, including whether other metal porphyrins can be incorporated into cytochromes c, we engineered Escherichia coli so that the natural system I (ccmABCDEFGH) was deleted and exogenous porphyrins were the sole source of porphyrins (ΔhemA). The engineered E. coli strains that produced recombinant system I (from E. coli) or system II (from Helicobacter) facilitated studies of the heme concentration dependence of each system. Using this exogenous porphyrin approach, it was shown that in system I the levels of heme used are at least fivefold lower than the levels used in system II, providing an important advantage for system I. Neither system could assemble holocytochromes c with other metal porphyrins, suggesting that the attachment mechanism is specific for Fe protoporphyrin. Surprisingly, Zn and Sn protoporphyrins are potent inhibitors of the pathways, and exogenous heme competes with this inhibition. We propose that the targets are the heme binding proteins in the pathways (CcmC, CcmE, and CcmF for system I and CcsA for system II).
Cytochromes c are electron transport proteins that are essential for most aerobic and anaerobic respiratory chains, as well as many other cellular processes, such as photosynthesis and apoptosis. These cytochromes are distinguished from other cytochromes by the covalent linkage of the heme vinyl groups to specific cysteine residues present in the CXXCH motif of apocytochrome c. Three different c-type cytochrome biogenesis pathways have been described, and they are called systems I, II, and III (for reviews, see references 33, 47, and 51) (systems I and II are shown in Fig. 1). System III is the simplest of the three systems and consists of a single protein, cytochrome c heme lyase, which is found in the mitochondria of certain eukaryotes (19, 40, 46). System II consists of four proteins and is found in gram-positive bacteria (35, 44), β- and ɛ-proteobacteria (4, 30), plant chloroplasts (32, 59), and cyanobacteria (52). The most complex of the three systems, system I, consists of at least eight proteins encoded by the ccm genes and is found in α- and γ-proteobacteria (5, 15, 41, 43), archaea (1, 26), and plant mitochondria (7, 31). Systems I and II deliver heme to the site of assembly, maintain apocytochrome c in a reduced state, and facilitate energy-independent (2, 3), covalent ligation of the heme to apocytochrome c.
FIG. 1.
Diagram of the current working model for c-type cytochrome biogenesis by systems I and II. Also shown is the ChuA heme porin pathway used in this study and the first dedicated step in heme biosynthesis. ⊥ indicates targets for inhibitors used in the current study.
Progress has been made in studies on the function of many assembly proteins, but the reasons for the evolution of these three systems remain major questions. Previously, we have shown that CcmE of system I can function as a heme reservoir (24). It was also shown that the ferrochelatase inhibitor N-methyl protoporphyrin (NMPP) was able to inhibit recombinant system II at lower concentrations than it inhibited system I, leading to speculation that system I might be able to use levels of endogenous heme that are lower than the levels that system II can use. Theoretically, heme delivery (or flux) could be the limiting factor in cytochrome c biogenesis pathways under certain environmental conditions. We have recently proposed that the CcmA2B1C1 release complex of system I (23) is required for using low levels of heme in the cell. This suggests that heme is bound with high affinity to CcmC and that ATP hydrolysis by the ATP binding cassette (ABC) subunit CcmA is necessary to release this molecule (as holoCcmE) to cytochrome c synthetase (CcmF/H). System II, a genetically distinct system for synthesizing holocytochromes c, does not have an ABC transporter complex or a heme chaperone, such as holoCcmE (see Fig. 1 for the model). Rather, system II has a protein called CcsA with at least six membrane-spanning helices and a periplasmic WWD domain (a putative heme binding site), like that present in CcmC or CcmF. It is possible that system II possesses a low-affinity heme binding site (in CcsA) from which direct ligation to apocytochrome c occurs (24, 28).
To directly examine the different heme affinities for the two pathways, we engineered Escherichia coli with its eight ccm genes deleted so that endogenous heme was not synthesized (by deleting hemA). Expression of chuA, encoding an outer membrane porin selective for porphyrins, allowed the Δccm ΔhemA strain (RK105) to grow like the wild type with the addition of exogenous heme. Using this approach and recombinant systems I and II, the heme dependence of each system was quantitated. As shown here with metal porphyrins, it is feasible to analyze alternative porphyrins for attachment requirements or for the discovery of pathway inhibitors.
MATERIALS AND METHODS
Bacterial growth conditions.
All E. coli strains used in this work were grown aerobically (with shaking at 300 rpm) at 37°C in Luria-Bertani media (Difco). Antibiotics (Sigma-Aldrich) were used at the following concentrations: carbenicillin, 100 μg ml−1; tetracycline, 15 μg ml−1; chloramphenicol, 20 μg ml−1; kanamycin, 100 μg ml−1; and ampicillin, 100 μg ml−1. Aminolevulinic acid (ALA) (Sigma) was used at a concentration of 50 μg ml−1 (300 μM), unless indicated otherwise. The metalloporphyrins zinc(II) protoporphyrin IX (ZnPPIX), tin(IV) protoporphyrin IX (SnPPIX), and cobalt(III) protoporphyrin IX (CoPPIX) were obtained from Frontier Scientific (Logan, UT) and were dissolved in 0.1 N NaOH to obtain 10-mg ml−1 stock solutions. Hemin (heme; Frontier Scientific) was dissolved in 50% dimethyl sulfoxide to obtain a 10-mg ml−1 stock solution.
Construction of E. coli strain RK105.
A derivative of E. coli Δccm (RK103; eight ccm genes replaced with a kanamycin cassette [24]) with the hemA gene replaced with a kanamycin resistance cassette was constructed by the procedure of Datsenko and Wanner (14). PCR products were generated with template plasmid pKD4 containing a kanamycin resistance cassette that was flanked by FLP recombinase target sites by using a pair of oligonucleotide primers, 5′-CTATCAACGTTGGTATTATTTCCCGCAGACATGACCCTTTGTGTAGGCTGGAGCTGCTTC-3′ and 5′-TGATGTACTGCTACTCCAGCCCGAGGCTGTCGCGCAGAATCATATGAATATCCTCCTTAG-3′. These oligonucleotide primers included sequences identical to the N-terminal and C-terminal coding sequences, respectively, of the E. coli hemA gene. The 3′ ends of the oligonucleotides also included pKD4 priming sites P1 and P2, respectively. The PCR product was introduced into E. coli Δccm (RK104) cured for kanamycin resistance and expressing the λ phage Red recombinase encoded on pKD46. Transformants were selected on media with kanamycin and ALA at 37°C. Several colonies that were resistant to kanamycin but sensitive to ampicillin (indicating a loss of pKD46) were screened by PCR with oligonucleotide primers flanking the recombination site to identify strains with the hemA gene deleted.
Construction of pRGK368.
Cultures of E. coli RK103 containing pRGK334 (system II from Helicobacter pylori) (24), pRGK332 (cytochrome c4:6×His) (24), and pHPEX2 (55) yielded inconsistent levels of cytochrome c4:6×His for unknown reasons. However, the ccsBA gene cloned from Helicobacter hepaticus yielded consistent levels of cytochrome c4:6×His even in the presence of pHPEX2. Therefore, in the studies described here we used the H. hepaticus ccsBA-encoded system II (pRGK368) (see below), unless indicated otherwise. E. coli strain TB1 was used as the initial host for cloning. pGex-4T-1 (Amersham Biosciences)-derived vectors have an N-terminal glutathione S-transferase fusion to the insert under control of the isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible tac promoter. The H. hepaticus fused ccsBA coding region was PCR amplified from genomic DNA with a pair of oligonucleotide primers, 5′-CGCGGATCCATGATGAATATAATTAAAACACTTTTTTGTT-3′ and 5′-CCGCTCGAGTTATAAATGGGGCATATCAAGCACT-3′. The amplified product was cut with BamHI and XhoI and ligated into pGEX-4T-1 to generate pRGK368 (system II plasmid). The expression of ccsBA from pRGK368 was verified by complementation of the ccm phenotype of E. coli Δccm (RK103 with ccmA-H deleted) (24) containing pRGK332 (cytochrome c4:6×His) (data not shown).
Heme addition experiments.
Cultures of E. coli RK105 (Δccm ΔhemA) harboring pRGK333 (system I; ccmA-H genes from E. coli) (24) or pRGK368 (system II), pRGK332 (cytochrome c4:6×His), and pHPEX2 (for expression of the ChuA outer membrane heme porphyrin receptor [55]) were grown in media overnight in the presence of 300 μM ALA. One-hundred-milliliter cultures were started from the overnight culture with 1% inocula in media devoid of ALA by incubation for 2.5 h. After 2.5 h, the 100-ml cultures had exhausted their cellular supply of ALA and required exogenous heme (or ALA) for further growth. The cultures in which the supply of ALA was exhausted were divided into 5-ml aliquots to which IPTG (1 mM; to induce synthesis of the system I proteins) and heme (0 μM to 100 μM; Frontier Scientific) were added. We discovered that heme at a concentration greater than 60 μM precipitated over time with no IPTG (1 mM) present and that heme at a concentration greater than 100 μM precipitated in the presence of IPTG. After 1 h, arabinose (0.2%; to induce synthesis of cytochrome c4:6×His) was added, and the cultures were grown for an additional 3 h. Cells were harvested, and protein was extracted using the B-PER protein extraction reagent (Pierce) as previously described (22). Cytochrome c4:6×His was purified from 200 μg of total protein over nickel affinity resin (Novagen) with two washes (10 column volumes each), one consisting of 0.5 M NaCl/20 mM Tris-HCl (pH 7.9) containing 5 mM imidazole and one consisting of 0.5 M NaCl/20 mM Tris-HCl (pH 7.9) containing 60 mM imidazole, to eliminate free heme, and then it was eluted with 100 mM EDTA. Twenty microliters of each sample was subjected to sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE), transferred to nitrocellulose, and heme stained as previously described (21). Curve fitting and heme recovery calculations were performed with the Origin scientific and analysis software package (OriginLab) using the Hill equation as the curve fit model.
NMPP inhibition experiments.
Inhibition experiments were performed as previously described (24), with a few modifications. One-hundred-milliliter cultures of E. coli RK103 containing pRGK333 (system I), pRGK332 (cytochrome c4:6×His), and pHPEX2 (55) were inoculated (1%, vol/vol) from an overnight culture and grown with aeration at 37°C to an optical density at 600 nm (OD600) of approximately 0.5. The mid-log-phase cultures were divided into 5-ml aliquots, to which IPTG (1 mM; to induce synthesis of the system I proteins and the chuA gene) and NMPP (0 μM to 100 μM; Frontier Scientific) were added. After 1 h, 0.2% arabinose was added (to induce synthesis of Bordetella pertussis cytochrome c4:6×His), and the cultures were incubated for an additional 3 h. After cells were harvested by centrifugation, soluble protein was extracted with the B-PER protein extraction reagent (Pierce) as previously described (22). Cytochrome c4:6×His was purified from 200 μg of total protein by using nickel affinity resin (Novagen), and 20 μl of each sample was subjected to SDS-PAGE, transferred to nitrocellulose, and heme stained as described below.
Metalloprotoporphyrin IX addition experiments.
Overnight cultures of E. coli strain RK103 harboring either pRGK333 (system I) or pRGK368 (system II), pRGK332 (cytochrome c4:6×His), and pHPEX2 (55) were diluted into fresh media (containing the appropriate antibiotics and 8 μM hemin for the E. coli RK105 strain) and incubated with aeration at 37°C until the OD600 was approximately 0.5. The mid-log-phase cultures were divided into 5-ml aliquots, to which IPTG (1 mM) and a metalloprotoporphyrin IX [Zn(II), Sn(IV), or Co(III) at the concentrations indicated below] were added. Arabinose (0.2%) was added after 1 h, and the cultures were incubated for an additional 3 h. Cells were harvested, and the soluble protein was extracted using B-PER (22). Cytochrome c4:6×His was purified from 300 μg of total protein, and 20 μl was subjected to SDS-PAGE and transferred to nitrocellulose. Prior to heme staining and cytochrome c4 immunoblotting, the nitrocellulose blots were screened for fluorescence with an LAS-1000 plus luminescent image analyzer charge-coupled device camera system (Fujifilm, Tokyo, Japan).
NMPP-ZnPPIX combined inhibition experiments.
Overnight cultures of the E. coli RK103 strain harboring pRGK333 (system I), pRGK332 (cytochrome c4:6×His), and pHPEX2 were diluted into 100 ml of fresh media containing the appropriate antibiotics and incubated at 37°C with aeration until the OD600 was approximately 0.5. The cultures were divided into 5-ml aliquots, IPTG (1 mM) and NMPP (60 μM and 100 μM) were added to separate cultures, and the cultures were incubated for 30 min. ZnPPIX (5 μM) was then added to each culture, and the cultures were incubated for an additional 30 min. Arabinose (0.2%) was added, and the cultures were incubated for an additional 3 h. Cells were harvested by centrifugation, and the soluble protein was extracted with B-PER, as previously described (22). The soluble protein was processed as described above for the metalloprotoporphyrin addition experiments.
Other methods.
Protein concentrations were determined with a bicinchoninic acid assay kit (Pierce), using bovine serum albumin as a standard. Heme staining and Western blotting were performed as previously described (21), using the SuperSignal Femto chemiluminescent substrate (Pierce). For heme stain quantitation we used LOLITA II (low light test array; raytest USA, Wilmington, NC) for standardization of detection of light intensity with an LAS-1000 plus luminescent image analyzer charge-coupled device camera system. The reduced (10 mM sodium dithionite) and oxidized (10 mM ammonium persulfate) absorption spectra were obtained with a Shimadzu UV-2101PC scanning spectrophotometer using nickel affinity-purified holocytochrome c4:6×His.
RESULTS
Exogenous porphyrin approach: characterization of E. coli Δccm ΔhemA with a porphyrin porin.
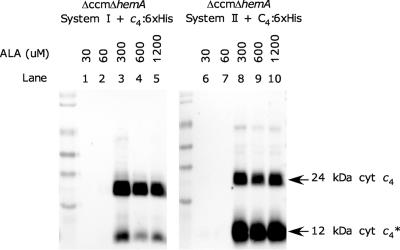
Previously, we deleted the ccmA-H genes in E. coli using a kanamycin resistance cassette (RK103) (24). To construct a heme-dependent strain, we initially screened for excision of this kanamycin resistance cassette. With the Δccm kanamycin-sensitive strain (RK104), the hemA gene was replaced with a kanamycin resistance cassette. The hemA gene codes for glutamyl-tRNA reductase, the first committed enzyme in heme biosynthesis, and E. coli cells lacking hemA require exogenous ALA for growth (58) (Fig. 1). The Δccm ΔhemA strain (RK105) requires ALA in the growth medium to form colonies (not shown). RK105 with recombinant system I (pRGK333) or system II (pRGK334) also requires exogenous ALA for growth (see Fig. S1 in the supplemental material). To determine if exogenous ALA facilitates synthesis of cytochromes c, we transformed these strains with pRGK332, which expresses a C-terminally hexahistidine-tagged B. pertussis cytochrome c4 under control of an arabinose-inducible promoter (24). E. coli RK105 with pRGK333 or pRGK334 and pRGK332 was unable to synthesize holocytochrome c4:6×His in the presence of 60 μM ALA (Fig. 2, lanes 1, 2, 6, and 7) but was able to synthesize holocytochrome c4:6×His in the presence of higher ALA concentrations (Fig. 2, lanes 3, 4, 5, 8, 9, and 10). As noted previously (24), in addition to the diheme 24-kDa cytochrome c4, a proteolyzed monoheme 12-kDa cytochrome c4:6×His* was produced. We concluded that exogenous ALA is required for growth and holocytochrome c production in RK105.
FIG. 2.
Heme staining of cytochrome c4 with increasing concentrations of ALA. E. coli RK105 cultures containing pRGK333 (lanes 1 to 5) or pRGK334 (lanes 6 to 10) were diluted into fresh media without ALA, depleting the intracellular ALA. ALA was added (concentrations are indicated above the lanes) along with 1 mM IPTG for 1 h. Cytochrome c4:6×His was induced with 0.2% arabinose for 3 h, and soluble B-PER protein extracts were prepared. System I indicates pRGK333, System II indicates pRGK334, and c4:6×His indicates pRGK332. cyt c4, cytochrome c4; cyt c4*, cytochrome c4*.
To determine if exogenous heme rather than ALA could support growth, a culture of RK105 in which ALA was exhausted was supplemented with different concentrations of heme (up to 100 μM). Growth was not detected until 4 or 5 days after the original inoculation, even at high heme concentrations. No holocytochrome c4:6×His was detected in cultures that contained either the system I or system II plasmid and the cytochrome c4:6×His reporter under these conditions.
Previous reports have indicated that the outer membranes of some strains of E. coli are poorly permeable to heme (8, 38, 57), and for this reason we expressed the heme porin (chuA) from pHPEX2 (55). The gene was obtained from E. coli O157:H7 and was expressed from the IPTG-inducible lacUV5 promoter. ChuA is a member of a class of relatively nonspecific enterobacterial heme receptors that are TonB dependent and facilitate heme acquisition (Fig. 1) (references 49 and 53 and references therein). ChuA, by analogy with the Yersinia enterocolitica HemR outer membrane heme receptor, recognizes free heme and heme bound to a variety of proteins (49). Growth of E. coli RK105 containing either pRGK333 or pRGK334 and pHPEX2 was dependent on exogenous heme (Fig. 3). Expression of chuA (pHPEX2) allowed growth at nearly wild-type levels, suggesting that there was efficient uptake of exogenous heme, whereas when the ALA in the culture was depleted and heme was not added, no growth was detectable (Fig. 3). pHPEX2 also facilitated the heme-dependent production of B. pertussis cytochrome c4:6×His when the plasmids with system I or system II were present (see below).
FIG. 3.
Growth of E. coli RK105 with exogenous heme. Overnight cultures of E. coli RK105 (Δccm ΔhemA) or cultures containing pHPEX2 and pRGK333 (A) or pRGK334 (B) were diluted into media without ALA or heme. The cultures were incubated aerobically at 37°C for 2.5 h to exhaust the ALA. Heme was added at the concentrations indicated, and growth (A600) was measured. For reference, the growth of E. coli RK103 (wild type) containing either pRGK333 or pRGK334 is shown (⧫). SysI, system I; SysII, system II.
System I acquires heme at significantly lower concentrations than system II acquires heme.
To directly examine the differences in heme acquisition between systems I and II, we added various concentrations of exogenous heme to E. coli RK105 containing pHPEX2, pRGK332, and one of the system plasmids. (As noted in Materials and Methods, in these studies we used a system II ccsBA fusion from H. hepaticus, encoded by pRGK368, which is compatible with pHPEX2, which resulted in consistent levels of the cytochrome c4:6×His reporter.) Overnight starter cultures (grown in the presence of ALA) were used to inoculate media devoid of ALA and incubated for 2.5 h to exhaust the cellular ALA (and heme); IPTG, arabinose, and heme were then added. The cytochrome c4 acted as a trap, allowing quantitation of the levels of heme that fluxed through each system. Cytochrome c4:6×His was purified over nickel chelating resin to eliminate free heme, and both the 24-kDa and 12-kDa forms were quantitated by heme staining (Fig. 4A and C). For system I, since maximum synthesis of holocytochrome c4:6×His occurs with less than 10 μM heme, in all subsequent experiments using system I we used heme levels between 0 μM and 10 μM. Averaged over four trials for system I, the holocytochrome c4:6×His levels were restored to 50% of the maximum levels achieved with exogenous heme when we used 1.9 ± 0.4 μM heme (Fig. 4B). For experiments with system II (CcsBA), increases in the holocytochrome c4:6×His signal were also observed as the heme concentration increased, and heme concentrations of 30 μM to 40 μM resulted in maximum synthesis (Fig. 4C). Averaged over five trials, the holocytochrome c4:6×His levels reached 50% of the maximum levels when we used 18.6 μM ± 8.1 μM heme for system II (Fig. 4D). We concluded that the apparent affinity for heme of system I is at least fivefold higher than the apparent affinity for heme of system II.
FIG. 4.
Exogenous heme acquisition profiles for system I and system II. An E. coli heme auxotroph (RK105) containing pRGK333 or pRGK368, pRGK332, and pHPEX2 (outer membrane heme porin) was grown and treated as described in Materials and Methods. (A and C) Heme staining of holocytochrome c4 from representative trials with system I (A) and system II (C). (B and D) Curve fits to average heme staining intensity expressed as a percentage of the maximum signal intensity with respect to the heme concentrations for system I (n = 4) (B) and system II (n = 5) (D). cytc4, cytochrome c4.
Zinc protoporphyrin IX is not incorporated into the B. pertussis cytochrome c4:6×His biogenesis reporter.
It has not been determined whether any of the three cytochrome c biogenesis systems can incorporate other metal porphyrins into the CXXCH motif of a c-type cytochrome. If they can, then iron itself is not critical for the attachment mechanism. The porphyrin porins like ChuA also facilitate permeability of other metal porphyrins (48). Using the approach described here, we tested whether heme can be replaced by other metalloporphyrins in the c-type cytochrome (cytochrome c4:6×His) reporter. We chose three metalloporphyrins to screen for incorporation into cytochrome c4:6×His: ZnPPIX, SnPPIX, and CoPPIX. These three metal protoporphyrins were selected because all three metals have been successfully inserted in vitro into horse heart cytochrome c by directly replacing the natural iron with the metal (17, 54).
To determine if ZnPPIX, SnPPIX, or CoPPIX could be inserted in place of heme into cytochrome c4:6×His, we grew cultures of E. coli RK105 containing pRGK333 (system I), pRGK332 (cytochrome c4:6×His), and pHPEX2 with heme to an OD600 of 0.5. Then IPTG and metalloporphyrin (8 μM, 12.5 μM, or 25 μM) were added, and the cultures were incubated for 1 h. Arabinose was added to a concentration of 0.2% to induce the synthesis of cytochrome c4:6×His, and the cultures were incubated for three more hours before cells were harvested. As expected, heme was detected on cytochrome c4:6×His when either 8 μM or 25 μM heme alone was added (Fig. 5, lanes 1 and 2, respectively). When ZnPPIX was present along with 8 μM heme, decreasing amounts of holocytochrome c4 were detected as the ZnPPIX concentration increased (Fig. 5, lanes 3 to 5). With 25 μM ZnPPIX (Fig. 5, lane 5) the level of heme on cytochrome c4:6×His was barely detectable. Concentrations of ZnPPIX greater than 25 μM did not further reduce the level of detectable heme (not shown). SnPPIX also reduced the level of detectable heme on cytochrome c4:6×His (see below), but CoPPIX (at concentrations up to 100 μM) had no affect. It is possible that ZnPPIX (or SnPPIX), when present with 8 μM heme, competes with heme for ChuA-dependent transport into the cell. We therefore tested an E. coli Δccm strain (RK103) in which there is endogenous heme production to determine if ZnPPIX (or SnPPIX) reduced the holocytochrome c4 levels detectable by heme staining. When cultures of E. coli RK103 with pRGK333 (system I), pRGK332, and pHPEX2 were treated with ZnPPIX, the level of holocytochrome c4:6×His decreased as the concentration of ZnPPIX was increased (Fig. 6A, lanes 1 to 5). For E. coli RK103 with pRGK368 (system II), pRGK332, and pHPEX2 there were also decreases in the level of holocytochrome c4:6×His with even lower concentrations of ZnPPIX (Fig. 6B, lanes 1 to 10) (note that the levels of ZnPPIX were nanomolar).
FIG. 5.
Heme staining of holocytochrome c4:6×His with increasing concentrations of ZnPPIX. E. coli RK105 cultures containing pRGK333, pRGK332, and pHPEX2 were grown in the presence of 8 μM heme (lanes 1 and 3 to 5) or 25 μM heme (lane 2) to an OD600 of approximately 0.5. ZnPPIX was added (lanes 3 to 5), and 1 mM IPTG was added to induce synthesis of the system I proteins. The cultures were incubated for 1 h, and 0.2% arabinose was added for 3 h to induce the synthesis of cytochrome c4. The concentrations (in μM) of heme and ZnPPIX are indicated above the lanes. SysI, pRGK333; c4:6×His, pRGK332; cyt c4, cytochrome c4.
FIG. 6.
Heme staining for detection of holocytochrome c4 synthesis in the presence of ZnPPIX. E. coli RK103 cultures containing either pRGK333, pRGK332, and pHPEX2 (A) or pRGK368, pRGK332, and pHPEX2 (B) were grown and treated as described in Materials and Methods. The concentrations of ZnPPIX are indicated above the lanes. SysI, pRGK333; SysII, pRGK368; c4:6×His, pRGK332; Δccm, E. coli RK103; cyt c4, cytochrome c4; cyt c4*, cytochrome c4*.
For SnPPIX and CoPPIX, decreases in the level of holocytochrome c4:6×His were observed as the concentration of SnPPIX was increased (see Fig. S2A, lanes 1 to 5, and Fig. 2B, lanes 1 to 10, in the supplemental material). Addition of CoPPIX (at concentrations up to 100 μM) resulted in no decrease, on average, in the level of heme on cytochrome c4:6×His, suggesting that CoPPIX is not incorporated and is not an inhibitor (see below).
Based on the results described above, we decided to focus on the effects of ZnPPIX, which at low concentrations significantly reduced the holo(heme)cytochrome c levels detected by heme staining. ZnPPIX does not react with the chemiluminescent substrate used to stain heme on holocytochrome c4 (unpublished). The absence of heme staining could indicate that ZnPPIX either (i) competes with heme and is incorporated into cytochrome c4:6×His or (ii) specifically inhibits c-type cytochrome biogenesis (i.e., attachment of heme).
Cytochrome c4 covalently bound to ZnPPIX would be highly fluorescent, a property of ZnPPIX proteins (20). We were unable to detect fluorescence of ZnPPIX in purified cytochrome c4:6×His preparations in these experiments, suggesting that ZnPPIX is not incorporated. To confirm that ZnPPIX is not incorporated into cytochrome c4, we obtained visible absorption spectra of nickel-purified cytochrome c4:6×His isolated from E. coli RK103 harboring pRGK333 (system I), pRGK332 (cytochrome c4:6×His), and pHPEX2 grown with 8 μM ZnPPIX (Fig. 7A). Note that at this concentration approximately 20% of the wild-type level of holocytochrome was produced (Fig. 6A, lane 3). The sodium hydrosulfite-reduced α peak at 552 nm is characteristic of cytochrome c4 with a c-type linkage to heme (9, 10). If ZnPPIX had been incorporated, we would have expected β and α peaks at 549 and 585 nm, respectively (54). In addition, electrospray ionization-mass spectrometry (ESI-MS) analysis of the same protein preparation showed that holocytochrome c4* (12-kDa proteolytic fragment) contained only heme, as ZnPPIX incorporation would have yielded a protein that was 10 mass units larger (Fig. 7B and inset). These results confirm that ZnPPIX is not incorporated into cytochrome c4:6×His but instead inhibits biogenesis.
FIG. 7.
Reduced and oxidized absorption spectra and ESI-MS analysis of cytochrome c4 produced in the presence of ZnPPIX. Overnight cultures of E. coli RK103 containing pRGK333 (system I), pRGK332 (cytochrome c4:6×His), and pHPEX2 were diluted into fresh media and grown to the mid-log phase. ZnPPIX (8 μM) and IPTG (1 mM) were added, and the cultures were incubated for an additional 1 h. Arabinose (0.2%) was added for 3 h to induce synthesis of cytochrome c4:6×His, and soluble B-PER extracts were prepared. (A) Reduced (sodium hydrosulfite) and oxidized (ammonium persulfate) absorption spectra. (B) ESI-MS analysis.
We also performed cytochrome c4 immunoblotting for nickel affinity-purified cytochrome c4:6×His from cultures grown as described above in the presence of SnPPIX. It has been documented that apocytochromes c in mutants in which heme is unable to attach are susceptible to natural proteases, presumably because they do not fold properly (4, 18, 29). This has been shown with the B. pertussis cytochrome c4:6×His reporter used in these studies (4, 24). Therefore, if SnPPIX is incorporated into apocytochrome c, we would expect that the holocytochrome would be stable and not subject to natural proteolysis. When antiserum to B. pertussis cytochrome c4 was used (see Fig. S2 in the supplemental material), decreases in the levels of cytochrome c4:6×His were detected for both system I (see Fig. S2A, lanes 6 to 10, in the supplemental material) and system II (see Fig. S2B, lanes 11 to 20, in the supplemental material).
ZnPPIX and SnPPIX are specific inhibitors of system I and system II.
Our results suggest that ZnPPIX and SnPPIX are not incorporated into cytochrome c4:6×His by either system but instead inhibit biogenesis. The inhibition of holocytochrome c4:6×His synthesis with system I required significantly higher concentrations of ZnPPIX and SnPPIX than the inhibition of holocytochrome c4:6×His synthesis with system II required in these experiments (Fig. 6; see Fig. S2 in the supplemental material). The concentration of SnPPIX required for inhibition of holocytochrome c4:6×His synthesis was also higher than the concentration of ZnPPIX required for inhibition of holocytochrome c4:6×His synthesis for both systems. The concentration of ZnPPIX required for 50% inhibition of system I was approximately 2 μM and the concentration of ZnPPIX required for 50% inhibition of system II was approximately 25 nM, whereas the concentration of SnPPIX required for 50% inhibition of system I was approximately 65 μM and the concentration of SnPPIX required for 50% inhibition of system II was approximately 18 μM.
To determine if ZnPPIX affects some basic cellular processes, growth studies were performed with E. coli RK103 harboring pRGK333 (system I), pRGK332 (cytochrome c4:6×His), and pHPEX2 in the presence and absence of ZnPPIX. In cultures with either 12.5 μM or 25 μM ZnPPIX there was no decrease in the growth rate or yield compared to the growth rate or yield for the same strain without ZnPPIX (see Fig. S3 in the supplemental material). ZnPPIX has previously been shown to inhibit the final enzyme in heme biosynthesis, ferrochelatase, from mice at a Ki of 4.5 μM (13); however, because no growth defect was observed with 12.5 μM or 25 μM ZnPPIX, we concluded that ferrochelatase was not inhibited in our system with these concentrations of ZnPPIX. In addition, using a plasmid with cytochrome c4:Pho (B. pertussis cytochrome c4 alkaline phosphatase fusion [24]) that was induced with arabinose, alkaline phosphatase was detectable at equivalent levels in the presence of ZnPPIX (see Fig. S4A, lanes 6 and 7, in the supplemental material). These results indicate that ZnPPIX does not inhibit transcription, translation, or secretion of cytochrome c4, further suggesting that ZnPPIX specifically inhibits some step(s) in c-type cytochrome biogenesis.
ZnPPIX specifically inhibits system I c-type cytochrome biogenesis after holoCcmE synthesis.
To begin to examine where ZnPPIX inhibits system I c-type cytochrome biogenesis, we assayed for the presence of heme on cytochrome c4:6×His when both NMPP and ZnPPIX were included in the culture. NMPP is a strong inhibitor of ferrochelatase and was used to control cellular heme levels (12, 25, 39). With NMPP inhibition in E. coli (RK103) containing pRGK333 (system I) and pRGK332 (cytochrome c4:6×His), holocytochrome c synthesis was reduced to a basal level of approximately 38% (24). This result was shown to be due to the ability of the CcmE protein to act as a reservoir for heme, permitting residual (38%) synthesis of holocytochrome c4:6×His when 100 μM NMPP was added to the culture (24). Thus, in these experiments holoCcmE was present at levels that allowed 38% holocytochrome c4 production when ferrochelatase was inhibited. If ZnPPIX inhibits synthesis significantly more than NMPP inhibits synthesis, it must act after the formation of this heme reservoir since some holoCcmE was present at the time of ZnPPIX addition (Fig. 1). Cultures of E. coli (RK103) containing pRGK333 (system I), pRGK332 (cytochrome c4:6×His), and pHPEX2 were grown and treated as described in Materials and Methods (NMPP-ZnPPIX combined inhibition experiments). In addition, individual inhibition experiments were performed with cultures that contained NMPP or ZnPPIX (Fig. 8). When both NMPP and ZnPPIX (Fig. 8) were present, the holocytochrome c4:6×His levels dropped from approximately 54% with 60 μM NMPP alone (Fig. 8) to approximately 7% with 60 μM NMPP and 5 μM ZnPPIX and from approximately 30% with 100 μM NMPP (Fig. 8) to 0% with 100 μM NMPP and 5 μM ZnPPIX. These results are consistent with previous data showing that ZnPPIX inhibits holocytochrome c4:6×His production so that the levels are nearly undetectable (Fig. 6A, lane 5) and inhibits this production considerably more than NMPP inhibits it.
FIG. 8.
Holocytochrome c4 synthesis in the presence of ZnPPIX and NMPP. E. coli RK103 cultures containing pRGK333 (system I), pRGK332 (cytochrome c4:6×His), and pHPEX2 were grown and treated as described in Materials and Methods. Independent (NMPP and ZnPPIX) inhibition experiments were also performed. Holocytochrome c4 was quantified on the basis of heme staining intensity (in arbitrary units) in three independent trials.
Our results suggest that ZnPPIX inhibits downstream of holoCcmE since this “heme reservoir” is no longer available for cytochrome c4 biogenesis. Thus, it is feasible that the WWD-containing protein CcmF is the target for ZnPPIX (see Discussion) (Fig. 1). If the target for ZnPPIX is a heme binding protein (site), then exogenous heme should compete with this inhibition (i.e., ZnPPIX binding). To test this hypothesis, we used the arabinose-inducible cytochrome c4:Pho fusion, whose holocytochrome c4 component can be assayed by heme staining in E. coli RK103 harboring system I and pHPEX2. In cultures grown with ZnPPIX and increasing concentrations of exogenous heme there were corresponding increases in holocytochrome c4:Pho (see Fig. S4B, lanes 1 to 5, in the supplemental material). The same heme-stained polypeptides were confirmed to be full-length cytochrome c4:Pho fusion proteins by Western blotting (see Fig. S4A, lanes 1 to 5, in the supplemental material). We also demonstrated this heme competition with ZnPPIX using the cytochrome c4:6×His reporter. In the presence of 8 μM ZnPPIX (system I) (see Fig. S4C, left panel, in the supplemental material) or 2 μM ZnPPIX (system II) (see Fig. S4C, right panel, in the supplemental material), increasing concentrations of exogenous heme resulted in corresponding increases in holocytochrome c4:6×His levels. We concluded that heme is able to compete with ZnPPIX inhibition and that ZnPPIX is a specific inhibitor of c-type cytochrome biogenesis by either system I or system II.
DISCUSSION
Heme affinities of system I compared to those of system II.
We have shown previously that when heme biosynthesis is inhibited by the ferrochelatase inhibitor NMPP, cytochrome c assembly persists at higher inhibitor concentrations with system I than with system II (24). We concluded that lower endogenous levels of heme can be used by the system I pathway. However, with this method we were not able to directly measure heme levels, and the use of inhibitors clearly has caveats. For example, the possibility that NMPP interferes with cytochrome c biogenesis directly could not be ruled out. Here we developed an approach to directly control heme levels and quantify the product of biogenesis, cytochrome c. We found that the level of exogenous heme utilization by system I is at least fivefold lower than the level of exogenous heme utilization by system II. The heme concentration dependence curves (Fig. 4) also reveal differences in kinetics between system I and system II. System I synthesis exhibits a sigmoidal curve and is saturated at low heme levels (around 3 μM), whereas system II synthesis shows a linear response with a reduced slope, more reminiscent of a diffusional process. We argue that these heme dependence results represent a true reflection of the relative differences between systems I and II. Such comparisons must be performed with the same genetic background, as was done here. With two different organisms, each with a natural system I or system II, it would be impossible to ensure or even expect that exogenous heme levels reflect the same internal levels (for any given concentration).
With respect to the study with E. coli described here, it is likely that the recombinant, functional system II operates in an environment similar to its natural environment. Other proteobacteria (e.g., B. pertussis and Helicobacter) probably obtained their natural system II genes by lateral transfer (30), indicating that the gram-negative periplasm and inner membrane are appropriate environments. In fact, recently it has been discovered that Bordetella parapertussis has the genes for both system I and system II (47), making it even more likely that the gram-negative envelope provides an environment suitable for the use of either system. Clearly, selective pressures have maintained one system or the other, but only in this rare case have they maintained both. Here we propose that heme availability in natural habitats is an important pressure.
Because heme biosynthesis depends on central metabolism (e.g., glutamate or succinyl coenzyme A and glycine), a limiting factor for heme biosynthesis is often the free iron levels (27, 42, 56). Based on the presence of the “heme reservoir” CcmE (24) and the fivefold-higher affinity for heme, we speculate that organisms in which heme synthesis is limited, such as organisms faced with low-iron environments, would have an advantage using system I. Concerning advantages for system II, the synthesis of fewer accessory components (and not requiring ATP hydrolysis for heme flux through the system) may confer benefits to some organisms, particularly when they do not need to scavenge for heme or iron.
Metal porphyrins as inhibitors but not prosthetic groups for cytochrome c assembly.
While replacement of the central Fe in horse heart cytochrome c with Zn (54), Co (16), and Sn (54) by in vitro manipulation has been demonstrated, the use of alternative metal porphyrins by cytochrome c biogenesis systems has not been tested. Here various methods to detect ZnPPIX (and SnPPIX) insertion into apocytochrome c by either the system I or II cytochrome c biogenesis pathway were employed. No evidence for insertion was obtained, suggesting that there is specificity for iron protoporphyrin IX (FePPIX), at least for system I and system II. This inability may be related to the specific system I and system II attachment mechanism(s). Because little is known about the synthetase mechanism, a future goal is to understand the specific need for FePPIX rather than other metal protoporphyrins.
Surprisingly, ZnPPIX and SnPPIX inhibit cytochrome c biogenesis. Heme competes with these inhibitors (see Fig. S4B and C in the supplemental material), suggesting that proteins in the pathway that bind heme are the likely targets. It has also been suggested that heme binding proteins are targets of noniron metal porphyrins by Stojiljkovic and colleagues, who found that certain noniron metal porphyrins have antibacterial properties (48). It is important to note that the targets that these authors proposed (e.g., oxidases) are not the same targets that we describe here. For example, Stojiljkovic et al. showed that gallium protoporhyrin IX inhibits E. coli growth only under aerobic iron-limiting conditions. However, wild-type E. coli normally does not synthesize c-type cytochromes under any aerobic conditions. We observed no inhibition of E. coli growth with ZnPPIX under aerobic conditions in liquid media (even with a porphyrin porin). This suggests that the heme biosynthetic enzyme ferrochelatase was not the target in our studies. Ferrochelatase from mice is inhibited by ZnPPIX with a Ki of 4.5 μM (13), but apparently the bovine enzyme is not significantly inhibited (11). The E. coli enzyme has not been tested to our knowledge. Stojiljkovic and colleagues (48) showed that there was growth inhibition of Staphylococcus aureus by gallium protoporhyrin IX and ZnPPIX but not by SnPPIX. S. aureus does not have c-type cytochromes (or system I or II [34]), and thus their putative target(s) are clearly different than the targets studied here. It is feasible that the inhibition observed by Stojiljkovic et al. (48) was due to the previously demonstrated inhibition of ferrochelatase activity (13).
The system I pathway has three possible sites of interaction with ZnPPIX: the proposed heme interacting proteins CcmC and CcmF (via the WWD domain) and the “heme reservoir” on the chaperone protein CcmE. Here we show that ZnPPIX further reduces the incomplete NMPP inhibition of system I holocytochrome c biosynthesis to zero, thus inhibiting downstream of the CcmE reservoir (e.g., CcmF). (These experiments did not rule out the possibility that there is an additional interaction of ZnPPIX with the CcmC and/or the CcmE proteins.) In the case of system II, we postulate that heme diffuses to the periplasmic WWD domain of CcsA for ligation to apocytochrome c. Thus, there is only one likely target for ZnPPIX/SnPPIX inhibition in the system II pathway, which is the WWD domain of CcsA.
ZnPPIX and SnPPIX are potent inhibitors of another heme binding enzyme, heme oxygenase (HO-1) (37), which is responsible for the oxidative cleavage of heme that yields free iron, carbon monoxide, and biliverdin (50). Metalloporphyrins compete with FePPIX for binding to the heme-binding pocket of HO-1, but due to the closed shell nature of zinc and tin, these metalloporphyrins cannot undergo oxidation-reduction reactions, and thus they act as inhibitors of HO-1 rather than substrates (reference 36 and references therein). CoPPIX, recognized mainly for its ability to induce HO-1 synthesis by an unknown mechanism (45), is also a weak inhibitor of HO-1 enzymatic activity (37). Metalloporphyrins have also been found to irreversibly inhibit both caspase-3 and caspase-8, neither of which is a heme binding protein, by binding to the active site and blocking the binding of a specific substrate (for example, caspase-3 with poly-ADP ribose polymerase) (6). This inhibition was found to be mainly dependent on the porphyrin ring structure as metal-free protopophyrin IX also exhibited inhibition, but a role for the central metal ion could not be ruled out (6). Inhibition of cytochrome c assembly pathways I and II adds two more specific targets for metal porphyrins. The approach used here should facilitate further analysis of cytochrome c synthetase mechanisms and discovery of other inhibitors of these systems.
Supplementary Material
Acknowledgments
We thank Douglas Goodwin, Auburn University, for providing plasmid pHPEX2 and James Fox, Massachusetts Institute of Technology, for providing H. hepaticus.
This work was supported by NIH grant GM47909 to R.G.K.
Footnotes
Published ahead of print on 3 November 2006.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Allen, J. W., E. M. Harvat, J. M. Stevens, and S. J. Ferguson. 2006. A variant system I for cytochrome c biogenesis in archaea and some bacteria has a novel CcmE and no CcmH. FEBS Lett. 580:4827-4834. [DOI] [PubMed] [Google Scholar]
- 2.Barker, P. D., J. C. Ferrer, M. Mylrajan, T. M. Loehr, R. Feng, Y. Konishi, W. D. Funk, R. T. MacGillivray, and A. G. Mauk. 1993. Transmutation of a heme protein. Proc. Natl. Acad. Sci. USA 90:6542-6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barker, P. D., E. P. Nerou, S. M. Freund, and I. M. Fearnley. 1995. Conversion of cytochrome b562 to c-type cytochromes. Biochemistry 34:15191-15203. [DOI] [PubMed] [Google Scholar]
- 4.Beckett, C. S., J. A. Loughman, K. A. Karberg, G. M. Donato, W. E. Goldman, and R. G. Kranz. 2000. Four genes are required for the system II cytochrome c biogenesis pathway in Bordetella pertussis, a unique bacterial model. Mol. Microbiol. 38:465-481. [DOI] [PubMed] [Google Scholar]
- 5.Beckman, D. L., D. R. Trawick, and R. G. Kranz. 1992. Bacterial cytochromes c biogenesis. Genes Dev. 6:268-283. [DOI] [PubMed] [Google Scholar]
- 6.Blumenthal, S. B., A. K. Kiemer, G. Tiegs, S. Seyfried, M. Holtje, B. Brandt, H. D. Holtje, S. Zahler, and A. M. Vollmar. 2005. Metalloporphyrins inactivate caspase-3 and -8. FASEB J. 19:1272-1279. [DOI] [PubMed] [Google Scholar]
- 7.Bonnard, G., and J. M. Grienenberger. 1995. A gene proposed to encode a transmembrane domain of an ABC transporter is expressed in wheat mitochondria. Mol. Gen. Genet. 246:91-99. [DOI] [PubMed] [Google Scholar]
- 8.Bracken, C. S., M. T. Baer, A. Abdur-Rashid, W. Helms, and I. Stojiljkovic. 1999. Use of heme-protein complexes by the Yersinia enterocolitica HemR receptor: histidine residues are essential for receptor function. J. Bacteriol. 181:6063-6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cavazza, C., M. T. Giudici-Orticoni, W. Nitschke, C. Appia, V. Bonnefoy, and M. Bruschi. 1996. Characterisation of a soluble cytochrome c4 isolated from Thiobacillus ferrooxidans. Eur. J. Biochem. 242:308-314. [DOI] [PubMed] [Google Scholar]
- 10.Conrad, L. S., J. J. Karlsson, and J. Ulstrup. 1995. Electron transfer and spectral alpha-band properties of the di-heme protein cytochrome c4 from Pseudomonas stutzeri. Eur. J. Biochem. 231:133-141. [DOI] [PubMed] [Google Scholar]
- 11.Dailey, H. A. 1990. Conversion of coproporphyrinogen to protoheme in higher eukaryotes and bacteria: terminal three enzymes, p. 123-161. In H. A. Dailey (ed.), Biosynthesis of heme and chlorophylls. McGraw-Hill, New York, NY.
- 12.Dailey, H. A., and J. E. Fleming. 1983. Bovine ferrochelatase. Kinetic analysis of inhibition by N-methylprotoporphyrin, manganese, and heme. J. Biol. Chem. 258:11453-11459. [PubMed] [Google Scholar]
- 13.Dailey, H. A., C. S. Jones, and S. W. Karr. 1989. Interaction of free porphyrins and metalloporphyrins with mouse ferrochelatase. A model for the active site of ferrochelatase. Biochim. Biophys. Acta 999:7-11. [DOI] [PubMed] [Google Scholar]
- 14.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deshmukh, M., G. Brasseur, and F. Daldal. 2000. Novel Rhodobacter capsulatus genes required for the biogenesis of various c-type cytochromes. Mol. Microbiol. 35:123-138. [DOI] [PubMed] [Google Scholar]
- 16.Dickinson, L. C., and J. C. Chien. 1975. Cobalt-cytochrome c. I. Preparation, properties, and enzymic activity. Biochemistry 14:3526-3534. [DOI] [PubMed] [Google Scholar]
- 17.Dickinson, L. C., and J. C. Chien. 1975. Cobalt-cytochrome c. II. Magnetic resonance spectra and conformational transitions. Biochemistry 14:3534-3542. [DOI] [PubMed] [Google Scholar]
- 18.Dumont, M. D., A. J. Mathews, B. T. Nall, S. B. Baim, D. C. Eustice, and F. Sherman. 1990. Differential stability of two apo-isocytochromes c in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 265:2733-2739. [PubMed] [Google Scholar]
- 19.Dumont, M. E., J. F. Ernst, D. M. Hampsey, and F. Sherman. 1987. Identification and sequence of the gene encoding cytochrome c heme lyase in the yeast Saccharomyces cerevisiae. EMBO J. 6:235-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Falk, J. 1964. Porphyrins and metalloporphyrins, vol. 2. Elsevier Publishing Co., New York, NY.
- 21.Feissner, R., Y. Xiang, and R. G. Kranz. 2003. Chemiluminescent-based methods to detect subpicomole levels of c-type cytochromes. Anal. Biochem. 315:90-94. [DOI] [PubMed] [Google Scholar]
- 22.Feissner, R. E., C. S. Beckett, J. A. Loughman, and R. G. Kranz. 2005. Mutations in cytochrome assembly and periplasmic redox pathways in Bordetella pertussis. J. Bacteriol. 187:3941-3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feissner, R. E., C. L. Richard-Fogal, E. R. Frawley, and R. G. Kranz. 2006. ABC transporter-mediated release of a haem chaperone allows cytochrome c biogenesis. Mol. Microbiol. 61:219-231. [DOI] [PubMed] [Google Scholar]
- 24.Feissner, R. E., C. L. Richard-Fogal, E. R. Frawley, J. A. Loughman, K. W. Earley, and R. G. Kranz. 2006. Recombinant cytochromes c biogenesis systems I and II and analysis of haem delivery pathways in Escherichia coli. Mol. Microbiol. 60:563-577. [DOI] [PubMed] [Google Scholar]
- 25.Ferreira, G. C. 1994. Mammalian ferrochelatase. Overexpression in Escherichia coli as a soluble protein, purification and characterization. J. Biol. Chem. 269:4396-4400. [PubMed] [Google Scholar]
- 26.Fitz-Gibbon, S. T., H. Ladner, U. J. Kim, K. O. Stetter, M. I. Simon, and J. H. Miller. 2002. Genome sequence of the hyperthermophilic crenarchaeon Pyrobaculum aerophilum. Proc. Natl. Acad. Sci. USA 99:984-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao, T., and M. R. O'Brian. 2005. Iron-dependent cytochrome c1 expression is mediated by the status of heme in Bradyrhizobium japonicum. J. Bacteriol. 187:5084-5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldman, B. S., D. L. Beck, E. M. Monika, and R. G. Kranz. 1998. Transmembrane heme delivery systems. Proc. Natl. Acad. Sci. USA 95:5003-5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldman, B. S., D. L. Beckman, A. Bali, E. M. Monika, K. K. Gabbert, and R. G. Kranz. 1997. Molecular and immunological analysis of an ABC transporter complex required for cytochrome c biogenesis. J. Mol. Biol. 268:724-738. [DOI] [PubMed] [Google Scholar]
- 30.Goldman, B. S., and R. G. Kranz. 1998. Evolution and horizontal transfer of an entire biosynthetic pathway for cytochrome c biogenesis: Helicobacter, Deinococcus, Archae and more. Mol. Microbiol. 27:871-873. [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez, D. H., G. Bonnard, and J. M. Grienenberger. 1993. A gene involved in the biogenesis of c-type cytochromes is co-transcribed with a ribosomal protein gene in wheat mitochondria [corrected]. Curr. Genet. 24:248-255. [DOI] [PubMed] [Google Scholar]
- 32.Inoue, K., B. W. Dreyfuss, K. L. Kindle, D. B. Stern, S. Merchant, and O. A. Sodeinde. 1997. Ccs1, a nuclear gene required for the post-translational assembly of chloroplast c-type cytochromes. J. Biol. Chem. 272:31747-31754. [DOI] [PubMed] [Google Scholar]
- 33.Kranz, R., R. Lill, B. Goldman, G. Bonnard, and S. Merchant. 1998. Molecular mechanisms of cytochrome c biogenesis: three distinct systems. Mol. Microbiol. 29:383-396. [DOI] [PubMed] [Google Scholar]
- 34.Kranz, R. G., C. S. Beckett, and B. S. Goldman. 2002. Genomic analyses of bacterial respiratory and cytochrome c assembly systems: Bordetella as a model for the system II cytochrome c biogenesis pathway. Res. Microbiol. 153:1-6. [DOI] [PubMed] [Google Scholar]
- 35.Le Brun, N. E., J. Bengtsson, and L. Hederstedt. 2000. Genes required for cytochrome c synthesis in Bacillus subtilis. Mol. Microbiol. 36:638-650. [DOI] [PubMed] [Google Scholar]
- 36.Maines, M. D. 2004. The heme oxygenase system: past, present, and future. Antioxid. Redox Signal. 6:797-801. [DOI] [PubMed] [Google Scholar]
- 37.Maines, M. D. 1981. Zinc protoporphyrin is a selective inhibitor of heme oxygenase activity in the neonatal rat. Biochim. Biophys. Acta 673:339-350. [DOI] [PubMed] [Google Scholar]
- 38.Mills, M., and S. M. Payne. 1995. Genetics and regulation of heme iron transport in Shigella dysenteriae and detection of an analogous system in Escherichia coli O157:H7. J. Bacteriol. 177:3004-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moody, M. D., and H. A. Dailey. 1985. Ferric iron reductase of Rhodopseudomonas sphaeroides. J. Bacteriol. 163:1120-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nargang, F. E., M. E. Drygas, P. L. Kwong, D. W. Nicholson, and W. Neupert. 1988. A mutant of Neurospora crassa deficient in cytochrome c heme lyase activity cannot import cytochrome c into mitochondria. J. Biol. Chem. 263:9388-9394. [PubMed] [Google Scholar]
- 41.Page, M. D., and S. J. Ferguson. 1997. Paracoccus denitrificans CcmG is a periplasmic protein-disulphide oxidoreductase required for c- and aa3-type cytochrome biogenesis; evidence for a reductase role in vivo. Mol. Microbiol. 24:977-990. [DOI] [PubMed] [Google Scholar]
- 42.Pope, C. D., W. O'Connell, and N. P. Cianciotto. 1996. Legionella pneumophila mutants that are defective for iron acquisition and assimilation and intracellular infection. Infect. Immun. 64:629-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramseier, T. M., H. V. Winteler, and H. Hennecke. 1991. Discovery and sequence analysis of bacterial genes involved in the biogenesis of c-type cytochromes. J. Biol. Chem. 266:7793-7803. [PubMed] [Google Scholar]
- 44.Schiott, T., C. von Wachenfeldt, and L. Hederstedt. 1997. Identification and characterization of the ccdA gene, required for cytochrome c synthesis in Bacillus subtilis. J. Bacteriol. 179:1962-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shan, Y., R. W. Lambrecht, and H. L. Bonkovsky. 2004. Identification of key elements that are responsible for heme-mediated induction of the avian heme oxygenase-1 gene. Biochim. Biophys. Acta 1679:87-94. [DOI] [PubMed] [Google Scholar]
- 46.Steiner, H., G. Kispal, A. Zollner, A. Haid, W. Neupert, and R. Lill. 1996. Heme binding to a conserved Cys-Pro-Val motif is crucial for the catalytic function of mitochondrial heme lyases. J. Biol. Chem. 271:32605-32611. [DOI] [PubMed] [Google Scholar]
- 47.Stevens, J. M., O. Daltrop, J. W. Allen, and S. J. Ferguson. 2004. C-type cytochrome formation: chemical and biological enigmas. Acc. Chem. Res. 37:999-1007. [DOI] [PubMed] [Google Scholar]
- 48.Stojiljkovic, I., V. Kumar, and N. Srinivasan. 1999. Non-iron metalloporphyrins: potent antibacterial compounds that exploit haem/Hb uptake systems of pathogenic bacteria. Mol. Microbiol. 31:429-442. [DOI] [PubMed] [Google Scholar]
- 49.Stojiljkovic, I., and D. Perkins-Balding. 2002. Processing of heme and heme-containing proteins by bacteria. DNA Cell Biol. 21:281-295. [DOI] [PubMed] [Google Scholar]
- 50.Tenhunen, R., H. S. Marver, and R. Schmid. 1968. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc. Natl. Acad. Sci. USA 61:748-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thony-Meyer, L. 2002. Cytochrome c maturation: a complex pathway for a simple task? Biochem. Soc. Trans. 30:633-638. [DOI] [PubMed] [Google Scholar]
- 52.Tichy, M., and W. Vermaas. 1999. Accumulation of pre-apocytochrome f in a Synechocystis sp. PCC 6803 mutant impaired in cytochrome c maturation. J. Biol. Chem. 274:32396-32401. [DOI] [PubMed] [Google Scholar]
- 53.Torres, A. G., and S. M. Payne. 1997. Haem iron-transport system in enterohaemorrhagic Escherichia coli O157:H7. Mol. Microbiol. 23:825-833. [DOI] [PubMed] [Google Scholar]
- 54.Vanderkooi, J. M., F. Adar, and M. Erecinska. 1976. Metallocytochromes c: characterization of electronic absorption and emission spectra of Sn4+ and Zn2+ cytochromes c. Eur. J. Biochem. 64:381-387. [DOI] [PubMed] [Google Scholar]
- 55.Varnado, C. L., and D. C. Goodwin. 2004. System for the expression of recombinant hemoproteins in Escherichia coli. Protein Expr. Purif. 35:76-83. [DOI] [PubMed] [Google Scholar]
- 56.Viswanathan, V. K., S. Kurtz, L. L. Pedersen, Y. Abu-Kwaik, K. Krcmarik, S. Mody, and N. P. Cianciotto. 2002. The cytochrome c maturation locus of Legionella pneumophila promotes iron assimilation and intracellular infection and contains a strain-specific insertion sequence element. Infect. Immun. 70:1842-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wandersman, C., and I. Stojiljkovic. 2000. Bacterial heme sources: the role of heme, hemoprotein receptors and hemophores. Curr. Opin. Microbiol. 3:215-220. [DOI] [PubMed] [Google Scholar]
- 58.Wulff, D. L. 1967. Delta-aminolevulinic acid-requiring mutant from Escherichia coli. J. Bacteriol. 93:1473-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xie, Z., and S. Merchant. 1998. A novel pathway for cytochromes c biogenesis in chloroplasts. Biochim. Biophys. Acta 1365:309-318. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.