Human overpopulation combined with the current lifestyle urges the rational, efficient, and sustainable use of natural resources to produce environmentally friendly plastic materials such as polyhydroxyalkanoic acids (PHAs), whose production/degradation cycle reduces undesirable wastes and emissions. In a previous article, Sabirova et al. (15) presented results of paramount importance: a PHA-hyperproducer mutant of the oil-degrading marine bacterium Alcanivorax borkumensis SK2, which deposits the PHA in the extracellular environment by a still-unknown mechanism (Fig. 1). This phenotype is observed only when the bacterium is grown on aliphatic hydrocarbons, one of the main components of petroleum.
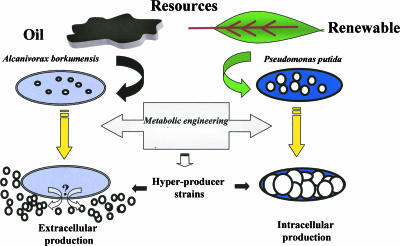
FIG. 1.
Schematic overview of some characteristics of Alcanivorax borkumensis and Pseudomonas putida mcl-PHA-hyperproducer strains. Several Pseudomonas strains are able to transform aliphatic hydrocarbons, one of the main components of petroleum, in mcl-PHA. Nevertheless, most of them can exclusively use fatty acids and carbohydrates as precursors (renewable resources). A. borkumensis SK2 is highly specialized in the assimilation of aliphatic hydrocarbons in oil-contaminated seawater, but it poorly transforms such precursors in mcl-PHA. Metabolic engineered strains are in both examples based on the accumulation of hydroxyacyl-CoA substrates for the mcl-PHA synthase. Whereas P. putida strains overaccumulated the biopolyesters intracellularly, overproduction in A. borkumensis results in deposits of the mcl-PHA in the extracellular environment by a still-unknown mechanism (indicated as a question mark). Sabirova and coworkers (15) have isolated an A. borkumensis mutant strain specialized in the biotransformation of aliphatic hydrocarbons from oil into extracellular bioplastic.
Occurrence of PHA in bacteria has been known since 1926, when Lemoigne observed that Bacillus megaterium produced an intracellular polymer of hydroxybutyrate monomers, later called polyhydroxybutyrate, which is the PHA most widely produced by bacteria (6). Six decades later, other related biopolyesters with longer side chains (medium-chain-length PHAs [mcl-PHAs]) were also described. Their production was first reported in Pseudomonas putida GPo1 (formerly known as Pseudomonas oleovorans GPo1) growing on alkanes (e.g., n-octane) (3). Since then, PHA has been detected in some archaea and a wide range of gram-positive and gram-negative bacteria in aerobic and anaerobic environments. Although there are many microorganisms that are able to produce PHA, mcl-PHA production had been repetitively restricted to Pseudomonas strains (3, 4, 7, 8, 19). As the new information provided by the sequenced bacterial genomes has started to indicate, the first conclusion that can be drawn from the contribution of Sabirova and coworkers (15) is that the genus Pseudomonas has justifiably lost its exclusiveness for this phenotype. This conclusion is also extended to the metabolic pathways acquired through evolution by the paradigmatic strain P. putida GPo1 to synthesize mcl-PHA, supported by the observation that A. borkumensis produces mcl-PHA from alkanes by a peripheral alkane oxidation pathway that links alkane catabolism to the β-oxidation central pathway (17).
The strategies designed to produce mcl-PHAs in recombinant Escherichia coli cells expressing a heterologous mcl-PHA synthase use specific mutants deficient in certain steps of the β-oxidation pathway to slow down or interrupt the ongoing cycle, which results in an accumulation of hydroxyacyl coenzyme A (hydroxyacyl-CoA) substrates for the mcl-PHA synthase (10, 11, 14). Likewise, mcl-PHA production was greatly increased in P. putida U engineered strains (fadA fadB mutants in the β-oxidation cycle), eliciting a strong intracellular accumulation of biopolyesters (9) (Fig. 1). Another novelty of what Sabirova et al. (15) discovered in their study is a new metabolic strategy to generate PHA-hyperproducer strains by inactivating a specific thioesterase that channels the hydroxyacyl-CoA intermediates towards mcl-PHA synthesis, demonstrating once more that metabolic engineering succeeds in improving microorganisms for biotechnological purposes.
Remarkably exciting was the fact that mcl-PHA is deposited extracellularly (Fig. 1), since this was the first report describing this phenomenon. Other biopolymers can be accumulated intracellularly or extracellularly in bacteria, but PHA so far had been the paradigmatic example of a biopolymer that was accumulated only in the cytoplasm (3, 8). Depending on the organism, PHA production can reach levels as high as 90% of the cell dry weight (9). The cytoplasm space limits the amount of polymer that can be produced by a microbial cell, and the yield per volume is limited by the number of cells and the biopolymer fraction in the biomass. This increases the complexity of the production and downstream processes to obtain purified PHA, implying the need for cell breakage procedures as well as processes for separation of PHA from crude extracts (19). Some biotechnologically relevant biopolymers, such as poly(γ-d-glutamate) (1), alginates (12), and hyaluronic acid (18), are examples of extracellular biopolymers (16). For these polymers, the cell volume is not a bottleneck; bioreactor volume, water solubility, and biopolymer viscosity are the factors that hamper the yield. Taking into account that mcl-PHAs are water-insoluble biopolyesters, their large-scale production by recombinant strains such as A. borkumensis C9 will undoubtedly lead to the development, implementation, and optimization of new fermentation strategies using novel systems for product recovery. Therefore, the work by Sabirova et al. (15) not only shows a new biological phenomenon but, more importantly, opens new avenues for research.
PHA biodegradation is performed in bacteria by at least two different pathways. One involves an intracellular degradation process as a self-service system to reuse storage carbon sources. The other is carried out extracellularly, where exogenous PHA is utilized as a carbon/energy source (5). Previously, it was assumed that the only source of extracellular PHA was the compound released after lysis of PHA producers. The PHA that is spread into the environment can be further hydrolyzed by secreted depolymerases into water-soluble oligomers and monomers that are useful as carbon source for the microbial community (5). The mechanism used by the A. borkumensis mutant to secrete PHA is unknown at present. The electron microscopy results presented seems to rule out cell wall lysis as the mechanism, and it may well be that we are confronting a new secretion mechanism for PHA that has not been previously envisioned. Molecular insights will also provide new clues in the future to understand the machinery involved in the still-not-well-established mechanism of PHA granule formation. In this sense, the latest findings on paradigmatic PHA producers such as Pseudomonas aeruginosa support a budding model for granule formation which involves four steps: (i) the attachment of the PHA synthase to the inner surface of cytoplasmic membrane, (ii) formation of oligomers that remain bound to the polymerase and therefore associated with the inner surface of the cytoplasmic membrane, (iii) polymer elongation in the hydrophobic environment found between the phospholipid monolayers of cytoplasmic membrane, and (iv) budding granule formation probably directed by structural-granule-associated proteins (phasins) (13). Taking into account this proposal, it is not obvious how this budding mechanism can explain the PHA secretion observed in A. borkumensis C9, and therefore the mechanism for granule formation might require reconsideration.
Finally, it is now assumed that the conversion of raw material in frequently used products, such as plastics, is a major and critical goal in the development of sustainable processes. In the same way, better bioremediation strategies are required for petroleum removal in oil-polluted environments due to, for instance, crude oil spills or uncontrolled release of industrial wastes from oil refineries. Interestingly, A. borkumensis is highly specialized in the assimilation of aliphatic hydrocarbons, and it makes up the main fraction of the biomass in oil-polluted marine environments. Moreover, the availability of its complete sequenced genome makes this strain a hydrocarbonoclastic microorganism of reference for exploration of new bioremediation strategies as a practical oil-removal technology (2). The contribution of Sabirova et al. (15) allows us to envision this strain as a “superbug” able not only to devour hydrocarbons from oil-contaminated environments, but also to efficiently biotransform them into extracellular PHA for biotechnological uses. From the viewpoint of bioremediation, hydrocarbons from marine oil spill accidents might be at the same time removed and converted into a widespread utilizable carbon source for the microbial world. Could this all be just a dream?
The views expressed in this Commentary do not necessarily reflect the views of the journal or of ASM.
Footnotes
Published ahead of print on 3 November 2006.
REFERENCES
- 1.Birrer, G. A., A. M. Cromwick, and R. A. Gross. 1994. Gamma-poly(glutamic acid) formation by Bacillus licheniformis 9945a: physiological and biochemical studies. Int. J. Biol. Macromol. 16:265-275. [DOI] [PubMed] [Google Scholar]
- 2.de Lorenzo V. 2006. Blueprint of an oil-eating bacterium. Nat. Biotechnol. 24:952-953. [DOI] [PubMed] [Google Scholar]
- 3.de Smet, M., G. Eggink, B. Witholt, J. Kingma, and H. Wynberg. 1983. Characterization of intracellular inclusions formed by Pseudomonas oleovorans during growth on octane. J. Bacteriol. 154:870-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huisman, G. W., O. de Leeuw, G. Eggink, and B. Witholt. 1989. Synthesis of poly-3-hydroxyalkanoates is a common feature of fluorescent pseudomonads. Appl. Environ. Microbiol. 55:1949-1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jendrossek, D., and R. Handrick. 2002. Microbial degradation of polyhydroxyalkanoates. Annu. Rev. Microbiol. 56:403-432. [DOI] [PubMed] [Google Scholar]
- 6.Lemoigne, M. 1926. Produit de déshydratation et de polymérisation de l'acide b-oxybutyrique. Bull. Soc. Chim. Biol. 8:770-782. [Google Scholar]
- 7.Luengo, J. M., B. García, A. Sandoval, G. Naharro, and E. R. Olivera. 2003. Bioplastics from microorganisms. Curr. Opin. Microbiol. 6:251-260. [DOI] [PubMed] [Google Scholar]
- 8.Madison, L. L., and G. W. Huisman. 1999. Metabolic engineering of poly(3-hydroxyalkanoates): from DNA to plastic. Microbiol. Mol. Biol. Rev. 63:21-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olivera, E. R., D. Carnicero, R. Jodra, B. Minambres, B. García, G. A. Abraham, A. Gallardo, J. San Roman, J. L. García, J. L. G. Naharro, and J. M. Luengo. 2001. Genetically engineered Pseudomonas: a factory of new bioplastics with broad applications. Environ. Microbiol. 3:612-618. [DOI] [PubMed] [Google Scholar]
- 10.Prieto, M. A., M. B. Kellerhals, G. B. Bozzato, D. Radnovic, B. Witholt, and B. Kessler. 1999. Engineering of stable recombinant bacteria for production of chiral medium-chain-length poly-3-hydroxyalkanoates. Appl. Environ. Microbiol. 65:3265-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qi, Q., A. Steinbüchel, and B. H. Rehm. 1998. Metabolic routing towards polyhydroxyalkanoic acid synthesis in recombinant Escherichia coli (fadR): inhibition of fatty acid beta-oxidation by acrylic acid. FEMS Microbiol. Lett. 167:89-94. [DOI] [PubMed] [Google Scholar]
- 12.Rehm, B. H., and S. Valla. 1997. Bacterial alginates: biosynthesis and applications. Appl. Microbiol. Biotechnol. 48:281-288. [DOI] [PubMed] [Google Scholar]
- 13.Rehm, B. H. 2006. Genetics and biochemistry of polyhydroxyalkanoate granule self-assembly: the key role of polyester synthases. Biotechnol. Lett. 28:207-213. [DOI] [PubMed] [Google Scholar]
- 14.Ren, Q., N. Sierro, M. Kellerhals, B. Kessler, and B. Witholt. 2000. Properties of engineered poly-3-hydroxyalkanoates produced in recombinant Escherichia coli strains. Appl. Environ. Microbiol. 66:1311-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sabirova, J. S., M. Ferrer, H. Lunsdorf, V. Wray, R. Kalscheuer, A. Steinbuchel, K. N. Timmis, and P. N. Golyshin. 2006. Mutation in a “tesB-like” hydroxyacyl-coenzyme A-specific thioesterase gene causes hyperproduction of extracellular polyhydroxyalkanoates by Alcanivorax borkumensis SK2. J. Bacteriol. 188:8452-8459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steinbüchel, A. 2001. Perspectives for biotechnological production and utilization of biopolymers: metabolic engineering of polyhydroxyalkanoate biosynthesis pathways as a successful example. Macromol. Biosci. 1:1-24. [Google Scholar]
- 17.van Beilen, J. B., M. M. Marin, T. H. Smits, M. Rothlisberger, A. G. Franchini, B. Witholt, and F. Rojo. 2004. Characterization of two alkane hydroxylase genes from the marine hydrocarbonoclastic bacterium Alcanivorax borkumensis. Environ. Microbiol. 6:264-273. [DOI] [PubMed] [Google Scholar]
- 18.Widner, B., R. Behr, S. Von Dollen, M. Tang, T. Heu, A. Sloma, D. Sternberg, P. L. DeAngelis, P. H. Weigel, and S. Brown. 2005. Hyaluronic acid production in Bacillus subtilis. Appl. Environ. Microbiol. 71:3747-3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zinn, M., B. Witholt, and T. Egli. 2001. Occurrence, synthesis and medical application of bacterial polyhydroxyalkanoate. Adv. Drug Deliv. Rev. 53:5-21. [DOI] [PubMed] [Google Scholar]