Abstract
The purpose of this study was to clone the carocin S1 gene and express it in a non-carocin-producing strain of Erwinia carotovora. A mutant, TH22-10, which produced a high-molecular-weight bacteriocin but not a low-molecular-weight bacteriocin, was obtained by Tn5 insertional mutagenesis using H-rif-8-2 (a spontaneous rifampin-resistant mutant of Erwinia carotovora subsp. carotovora 89-H-4). Using thermal asymmetric interlaced PCR, the DNA sequence from the Tn5 insertion site and the DNA sequence of the contiguous 2,280-bp region were determined. Two complete open reading frames (ORF), designated ORF2 and ORF3, were identified within the sequence fragment. ORF2 and ORF3 were identified with the carocin S1 genes, caroS1K (ORF2) and caroS1I (ORF3), which, respectively, encode a killing protein (CaroS1K) and an immunity protein (CaroS1I). These genes were homologous to the pyocin S3 gene and the pyocin AP41 gene. Carocin S1 was expressed in E. carotovora subsp. carotovora Ea1068 and replicated in TH22-10 but could not be expressed in Escherichia coli (JM101) because a consensus sequence resembling an SOS box was absent. A putative sequence similar to the consensus sequence for the E. coli cyclic AMP receptor protein binding site (−312 bp) was found upstream of the start codon. Production of this bacteriocin was also induced by glucose and lactose. The homology search results indicated that the carocin S1 gene (between bp 1078 and bp 1704) was homologous to the pyocin S3 and pyocin AP41 genes in Pseudomonas aeruginosa. These genes encode proteins with nuclease activity (domain 4). This study found that carocin S1 also has nuclease activity.
Erwinia carotovora subsp. carotovora (Jones) Bergey et al. is a phytopathogenic enterobacterium responsible for the soft rot, blackleg, or stem rot of a number of economically important crops (20). The disease is characterized by extensive maceration of the affected tissue caused by a variety of plant cell wall-degrading enzymes secreted by the pathogen. The major pathogenicity determinants are an arsenal of extracellular pectinases, including several pectate lyase isozymes, pectin lyase, pectin methylesterase, and pectin polygalacturonase. In addition, a range of other degradative enzymes, such as cellulase and protease, are secreted, but their roles in virulence are equivocal.
Various aspects of the epidemiology of the disease caused by this phytopathogen are understood, but there is no efficient method, either chemical or otherwise, to control the global disease. Biotechnology is progressing rapidly, and science is opening avenues to solve this difficult problem. Agrochemicals are generally used for the control of the disease, but in a quest for more environmentally friendly control methods, biological control using avirulent bacteriocin-producing mutants of E. carotovora subsp. carotovora is under investigation.
Some bacteria living in a competitive environment secrete proteinaceous toxins, known as bacteriocins, that kill closely related bacteria but not the producer strain itself. According to Klaenhammer, 99% of all bacteria may make at least one bacteriocin (13). All major groups of bacteria produce these inhibitors (22). Their mode of killing can be either membrane pore formation, nonspecific degradation of cellular DNA, cleavage of 16S rRNA and tRNA, or inhibition of peptidoglycan synthesis, resulting in cell lysis (23). Other strains release bacteriocins, such as pyocin S (produced by a Pseudomonas aeruginosa strain) or colicin (produced by an Escherichia coli strain), that are soluble and sensitive to proteases. S-type pyocin, or colicin, is composed of two proteins of different sizes, one responsible for antibiotic activity (the killing protein) and the other conferring immunity (the immunity protein). A conserved consensus sequence (a P box for pyocin and an SOS box for colicin) in the 5′ upstream region of each operon may act as a regulatory element for bacteriocin production (5, 26). Among the colicins, there are two main evolutionary lineages, which also distinguish the two primary modes of killing: pore formation and nuclease activity (23).
According to Kikumoto et al. and Nakatani and Tsuyama, the antibacterial activities of two types of bacteriocin produced by avirulent bacteriocin-producing biocontrol agents may contribute to suppression of soft rot disease (11, 17). There is also strong evidence that avirulent mutant strains of Erwinia carotovora subsp. carotovora effectively control the soft rot disease of Chinese cabbage (12, 28). A biological-control agent with the trade name “Biokeeper” has also been developed for the control of this disease in Japan (Central Glass Co., Japan). In view of these reports, identification and cloning of the gene(s) controlling bacteriocin production may facilitate its use in the development of resistant cultivars of Chinese cabbage and tobacco plants, using technology to introduce the genes into plants. Among Erwinia species, high-molecular-weight bacteriocins (or large bacteriocins) have structures similar to those of bacteriophages (10). Electron microscopy showed that carotovoricin Er has an antenna-like structure, a base plate, several tail fibers (18, 19), a contractile sheath, and a flexible rod-like structure (10). Sequence comparison showed high homology between carotovoricin and phage proteins (19). To date, no genes encoding the low-molecular-weight (LMW) bacteriocin (LMWB) (or small bacteriocin) of E. carotovora have been isolated or characterized.
Here, we report the cloning and sequencing of DNA encoding one LMWB designated “carocin S1” in E. carotovora subsp. carotovora 89-H-4 and characterize its expression in a non-bacteriocin-producing strain of E. carotovora subsp. carotovora, Ea1068. A carocin S1 induction mechanism involving regulation by glucose and lactose is proposed.
MATERIALS AND METHODS
Bacterial strains, plasmids, media, and growth conditions.
The strains and plasmids used are shown in Table 1. The putative biocontrol agent produces two types of bacteriocins: low- and high-molecular-weight bacteriocins. E. carotovora subsp. carotovora strains were propagated at 28°C on 1.4% nutrient agar (NA) or with shaking in Luria-Bertani (LB) medium with 5 g rather than 10 g of NaCl per liter. E. coli strains were propagated at 37°C in LB medium with shaking. Rifampin, kanamycin, and ampicillin (all at 50 mg per liter) were added to NA and LB agars where necessary.
TABLE 1.
Bacteria and plasmids used in this study
| Bacterium or plasmid | Relevant characteristics | Source |
|---|---|---|
| E. coli | ||
| 1830 | pro met Kmr Nmr; containing transposon Tn5 on the suicide plasmid pJB4JI | Gantotti et al. (7) |
| JM101 | supE thi Δ(lac-proAB) F′ [traD36 proAB+lacIqlacZ ΔM15] | Messing et al. (15) |
| DH5 | supE44 hsdR17 recA1 endA1 gyrA1 thi-1 relA1 | Hanahan and Reusch et al. (8, 21) |
| E. carotovora subsp. carotovora | ||
| 89-H-4 | Putative biocontrol agent | Laboratory stock |
| H-rif-8-2 | 89-H-4; Rifr | This work |
| Ea1068 | Wild type | Laboratory stock |
| T-29 | Wild type | Laboratory stock |
| E108 | Wild type | Laboratory stock |
| A-100 | Wild type | Laboratory stock |
| 86-H-2 | Wild type | Laboratory stock |
| TH22-10 | H-rif-8-2; caroS1K:: Tn5 Rifr Kanr | This work |
| Plasmids | ||
| pACYC177 | Ampr Kanr; low copy number | Change et al. (2) |
| pBR322 | Ampr Kanr | Bolivar et al. (1) |
| pAYL4 | AmprcaroS1K caroS1I | This work |
Bacterial mating.
Bacterial mating was done on NA by the membrane filter mating method (7), using 0.22-μm-pore-size membrane filters (Millipore, Inc., Bedford, MA). The filters were placed on NA and incubated overnight at 28°C. Appropriate dilutions of the suspension of the progeny of the mating were spread on modified Drigalski's agar plates (27) containing 50 μg/ml rifampin and kanamycin and incubated at 28°C for 24 to 48 h before the colonies were isolated.
Bacteriocin assays.
Bacteriocin production was examined by the double-layer method of Fredericq (6), but hard and soft IFO-802 media (1% polypeptin, 0.2% yeast extract, 0.1% MgSO4 · 7H2O [pH 7.0]) containing 1.4% and 0.65% agar, respectively, were used. Growth inhibition zones around the colonies were considered an indication of bacteriocin production.
Instead of UV induction, 1 M glucose or lactose was added to induce bacteriocin synthesis.
Genetic-engineering technique.
Plasmids of E. carotovora subsp. carotovora and E. coli plasmids were isolated by the method of Sambrook et al. (24). Total DNA was isolated as previously described (16).
Oligonucleotide DNA primers were synthesized by MD Bio Inc. (Taipei, Taiwan). General PCR has been described by Sambrook et al. (24). Thermal asymmetric interlaced PCRs (TAIL-PCRs) were performed according to the method of Liu and Whittier (14), but the annealing temperature was decreased from 63°C to 60°C for specific primers. For TAIL-PCR, specific primers that are complementary to the respective sequences of Tn5 (PR-1, PR-2, PR-3, PF-1, PF-2, and PF-3) or known sequences after the first TAIL-PCR analysis (TH22-10F1 to TH22-10F4 and TH22-10R1 to TH22-10R4) were synthesized (Table 2). In addition, three arbitrary degenerate primers (N-1, N-2, and N-3) were used (Table 2).
TABLE 2.
Primers used in this studya
| Name | Sequence (5′→3′) |
|---|---|
| PR-1 | 5′-GCCGAAGAGAACACAGATTTAGCCCA |
| PR-2 | 5′-CCGCACGATGAAGAGCAGAAGTT |
| PR-3 | 5′-CAGATCTCTGGAAAACGGGAAAGG |
| PF-1 | 5′-AGAGAACACAGATTTAGCCCAGTCGG |
| PF-2 | 5′-CCGCACGATGAAGAGCAGAAGTTAT |
| PF-3 | 5′-GATCCTGGAAAACGGGAAAGGTTC |
| TH22-10F1 | 5′-GAGCATGGTGTAACAGAAGAACAG |
| TH22-10F2 | 5′-CTTCGTCCATGTAGTGGTGATTC |
| TH22-10F3 | 5′-GGGTTGTACTAATGTCTGTAAACG |
| TH22-10F4 | 5′-CTTCGTCCATGTAGTGGTGATT |
| TH22-10R1 | 5′-GAATCACCACTACATGGACGAAG |
| TH22-10R2 | 5′-CTGTTCTTCTGTTACACCATGCTC |
| TH22-10R3 | 5′-AATCACCACTACATGGACGAAG |
| TH22-10R4 | 5′-CGTTTACAGACATTAGTACAACCC |
| TH22-10R5 | 5′-GTGATCGGCCTGATAACTTCC |
| TH22-10R6 | 5′-TTGTCGTGAGCGTCCAATG |
| TH22-10R7 | 5′-CATCAAGCAAGTTAACAGGCAAC |
| TH22-10R8 | 5′-TCTCCGGAAACACCAGAATG |
| TH22-10R9 | 5′-CTCTTCCGCCATTGGTATTC |
| TH22-10R10 | 5′-TTCATCTGATGTGTCAGTGCC |
| TH22-10R11 | 5′-TGATTGGCCTGAGCTACAGC |
| TH22-10R12 | 5′-TGCCGTACCTGCCATTACC |
| N-1 | 5′-NGTCGA(G/C)(A/T)GANA(A/T)GAA |
| N-2 | 5′-GTNCGA(C/G)(A/T)CANA(A/T)GTT |
| N-3 | 5′-(A/T)GTGNAG(A/T)ANCANAGA |
| P-3 | 5′-CTCGACGTTGTCACTGAAGCGGGAAG |
| P-4 | 5′-AAAGCACGAGGAAGCGGTCAGCCCAT |
| PCAR-R2 | 5′-TCAATCTGGCATCGATAACAGGG |
| PCAR-F2 | 5′-TGCTGGATCCAGCTTACGTGG |
| PCAR-F3 | 5′-GGTTGTACTAATGTCTGTAAACG |
| PCAR-R3 | 5′-CGTTTACAGACATTAGTACAACC |
All primers were purchased from MD Bio Inc., Taipei, Taiwan.
For sequencing of TAIL-PCR products, the ABI PRISM Dye Terminator Cycle Sequencing Ready Reaction Kit was used. Cycle sequencing was carried out on a GeneAmp System 9600 thermocycler. Sequencing using an automated DNA sequencer 373S (ABI) was carried out according to the manufacturer's protocol.
Southern and colony hybridizations and probe labeling and detection were performed using the digoxigenin (DIG) DNA Labeling and Detection Kit (Boehringer Mannheim GmbH, Mannheim, Germany) as described by the manufacturer. Hybridization was performed overnight, and the membrane was washed according to the manufacturer's instructions.
The DNA electrophoresis, restriction digestion, ligation, and transformation procedures for E. coli were done as described by Sambrook et al. (24). Plasmid DNA transformation for E. carotovora subsp. carotovora was performed using the methods of Hinton et al. (9) and Hanahan (8). E. carotovora subsp. carotovora cells were incubated at 35°C until the optical density of the cell suspension at 550 nm was 0.40 to 0.70 before transformation.
RNA preparation and Northern hybridization experiments.
BSM (bacteriocin screening medium; 0.5% sucrose, 0.1% NH4Cl, 0.2% KH2PO4, 0.02% MgSO4 · 7H2O, pH 7.5) was used for carocin S1 production. Total RNA was isolated from E. carotovora subsp. carotovora constructs grown in BSM without drugs at 28°C. To determine the stability of the H-rif-8-6, TH22-10, TH22-10/carocin S1, Ea1068, and Ea1068/carocin S1 strains, the bacteria were grown to a Klett value of ca. 150, at which point rifampin (0.2 mg/ml) was added to block further initiation. Culture samples (8 ml each) were then withdrawn at various time points into tubes containing 5 ml of ice-cold water, and total RNA was extracted.
Northern blot hybridization was done using 10 μg of total RNA isolated using Trizol (Invitrogen, San Diego, CA) according to the manufacturer's protocol. RNA samples were denatured at 65°C for 10 min in RNA sample buffer (250 μl of formamide, 83 μl of 37% [wt/vol] formaldehyde, 83 μl of 6× loading dye [Promega, Madison, WI], 50 μl of 10× MOPS [morpholinepropanesulfonic acid] buffer [10× MOPS buffer is 20 mM MOPS, 5 mM sodium acetate, and 1 mM EDTA, pH 7.0]) and 34 μl of distilled water. RNA samples were separated through 1% agarose gels in MOPS buffer with 2% (vol/vol) formaldehyde. DNA probes were synthesized by PCR using specific oligonucleotides, PCAR-R2 (for caroS1I) and PCAR-R3 (for caroS1K), derived from the E. carotovora subsp. carotovora sequence as a template (Table 2). Template DNAs, caroS1K and caroS1I, were obtained by PCR amplification. The probes were nonradioactively labeled by random priming using the DIG High Prime kit (Roche, Mannheim, Germany). To add the correct amount of probe for hybridization, serial dilutions of each probe (0.05 to 10 pg) were spotted on a nylon membrane, and the labeling sensitivity (the amount of labeled DNA per spot) was determined. RNA was transferred overnight to a positively charged nylon membrane (Amersham Life Science, Arlington Heights, IL) by capillary transfer using 20× SSC (20× SSC is 0.3 M NaCl plus 0.03 M sodium citrate, pH 7). Hybridization was performed for 16 h at 50°C in DIG Eazy Hyb buffer solution (Roche). The membrane was washed, and specific transcripts on the blots were detected using the DIG luminescence detection kit (Roche) according to the manufacturer's protocol.
Bacteriocin expression and purification.
Bacteria in BSM were incubated in a sterilized stainless steel box with a stainless steel cover at 28°C for 24 h without any light. After centrifugation, the medium without cells was removed. Ammonium sulfate was added to 80% saturation to precipitate the protein, and the precipitate was collected on a 0.45-μm cellulose filter. One milligram of precipitated protein was dissolved in 100 μl of bacteriocin buffer (0.1 M Tris [pH 7.5], 0.01 M dithiothreitol, and 0.5 M MgCl2).
Bacteriocin assay for nucleotidase activity.
To determine the bacteriocin antibiotic activity, 100 μg/10 μl of the CaroS1K protein solution was added to an indicator plate containing the Ea1068 or Ea1068/pAYL4 strain growing on soft IFO-802 medium containing 0.65% agar. Growth inhibition zones at the point of addition were considered an indication of carocin S1 activity.
To confirm nucleotidase activity, 500 ng/1 μl genome DNA solution from strain Ea1068 was added to 100 μg/10 μl of the CaroS1K protein solution and incubated at 28°C for 3 h. After incubation, the samples were treated and analyzed by 1.0% agarose gel electrophoresis in Tris-acetate-EDTA buffer.
Computer analysis of sequence data.
The nucleotide sequence and the deduced amino acid sequence of carocin H1 were compared using the BLAST and FASTA programs of the National Center for Biotechnology Information server. Sequence data were compiled by DNASIS-Mac software (Hitachi, Tokyo, Japan).
RESULTS
Isolation of transposon insertion mutants.
The mating of strain H-rif-8-6 with E. coli 1830 resulted in 4,500 colonies that could grow on selective plates containing rifampin and kanamycin (50 μg/ml each). To ascertain their antibiotic resistances, colony growth on selective medium was rechecked and found to be a stable property.
Bacteriocin activities of the putative Tn5 insertional mutants.
The bacteriocin activities of the test isolates were examined. The parental strain produced an LMW bacteriocin that diffused further from the colony than did the high-molecular-weight bacteriocin. The zones of inhibition around the putative isolates (insertion mutants) were restricted compared to those of the parent strain (Fig. 1). This suggested the possibility that transposon Tn5 had been successfully inserted into the genes of the LMW bacteriocin.
FIG. 1.
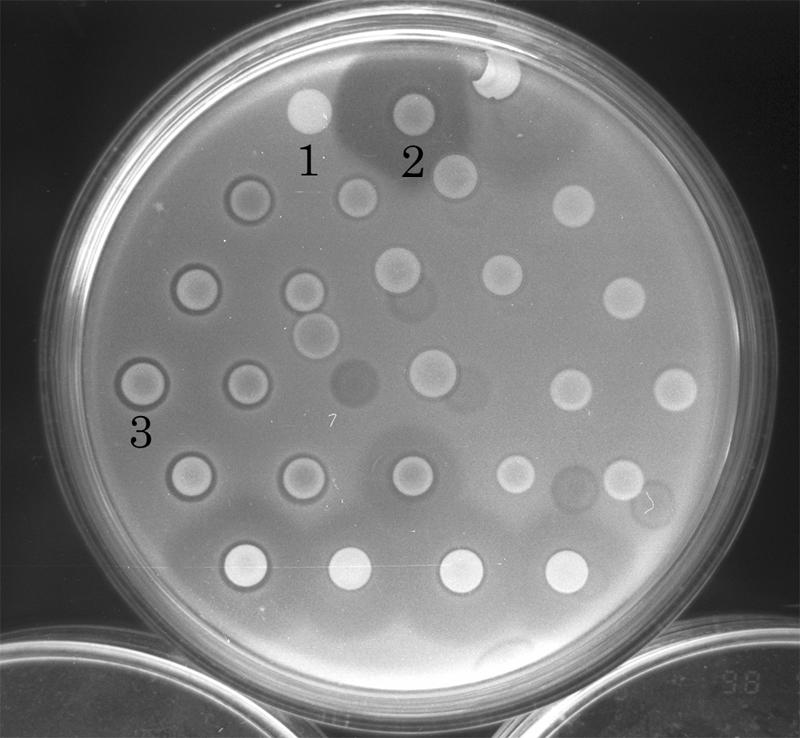
Bacteriocin activities of Tn5 insertion mutants of E. carotovora subsp. carotovora strains: 1, E. coli 1830/pBJ4JI (containing Tn5); 2, H-rif-8-6 (parent); and 3, TH22-10 (insertion mutant). The unlabeled strains are all Tn5 insertion mutants of the H-rif-8-6 parental strain. The indicator was Ea1068.
Detection of the Tn5 gene in the mutants.
To ascertain whether Tn5 was actually inserted into the putative isolates, “nested PCR” was used to amplify the nptII gene (29), using two oligonucleotide DNA primers, P-3 and P-4 (Table 2). Almost all the test isolates except H-rif-8-6 produced an ∼500-bp DNA fragment, indicating that H-rif-8-6 did not harbor the Tn5 gene. Southern blot hybridization also confirmed the above-mentioned results (data not shown).
Amplification of Tn5 insertion junction DNA and sequencing.
After the first TAIL-PCR experiment, two or more different-size bands were obtained for each sample. All of the fragment products were isolated by electrophoresis and purified, and the sequences of the recovered products were analyzed. Analysis of the respective bands showed the same sequence. On the basis of the sequence obtained from the first TAIL-PCR experiment, specific primers (left side, TH22-10F1 to TH22-10F4; right side, TH22-10R1 to TH22-10R12) were synthesized for subsequent TAIL-PCR experiments.
Sequence analysis.
A DNA fragment of 2,279 base pairs was sequenced. Analysis of the Tn5 insertions showed that two complete open reading frames (ORF2 and ORF3) were present, and Tn5 was located in ORF2 between bp 1581 and bp 1582. The 3′ end of another open reading frame, ORF1, was located upstream of ORF2. A noncoding region and a putative promoter were located between ORF1 and ORF2. Downstream from ORF3, the 5′ end of another ORF (ORF4) was found.
Homology with other genes and proteins.
The predicted amino acid sequences of ORF2 and ORF3 were compared with amino acid sequences deposited in the Swiss-Prot protein sequence database. Significant similarity was found between the sequences of ORF2 and ORF3 in E. carotovora subsp. carotovora and those of pyoS3A and pyoS3I of P. aeruginosa, respectively. Designation of ORF2 as caroS1K and ORF3 as caroS1I was therefore proposed. The two genes were called the carocin S1 genes.
Subcloning and expression of the carocin S1 gene from H-rif-8-6.
The DNA fragment of the carocin S1 gene from H-rif-8-6 was amplified by PCR. After PCR amplification of two oligonucleotide primers, CAR-F2 and CAR-R2, the carocin S1 gene was purified, digested by restriction enzymes (ClaI and BamHI), and subcloned into plasmid pACYC177 by T4 ligase. The new plasmid was designated pAYL4. One hundred transformed colonies were isolated using selective LB agar medium containing 100 μg/ml ampicillin after the transfer of pAYL4 into E. coli DH05 and JM101. The presence of the carocin S1 gene was detected by using electrophoresis after digestion with ClaI and BamHI. The caroS1 band size was 1.9 kb (data not shown). Carocin S1 activity was not detected after a bacteriocin assay using the indicator strain Ea1068 of E. carotovora subsp. carotovora. The pAYL4 plasmid DNA was isolated from DH05/pAYL4 and transferred into the insertion mutant TH20-10 and the wild-type strain of E. carotovora subsp. carotovora Ea1068 (a non-bacteriocin-producing bacterium sensitive to carocin S1). E. carotovora subsp. carotovora strains Ea1068 and E108 were used as indicators to detect bacteriocin-producing colonies. Thirty-two colonies were isolated by selection on LB medium containing kanamycin, rifampin, and ampicillin (50 μg/ml each), and the carocin S1 gene was detected as previously described.The colonies exhibited zones of growth inhibition when the host was Ea1068, and also, many colonies exhibited zones when the host was TH22-10, a mutant derived from H-rif-8-6 (Fig. 2A).
FIG. 2.
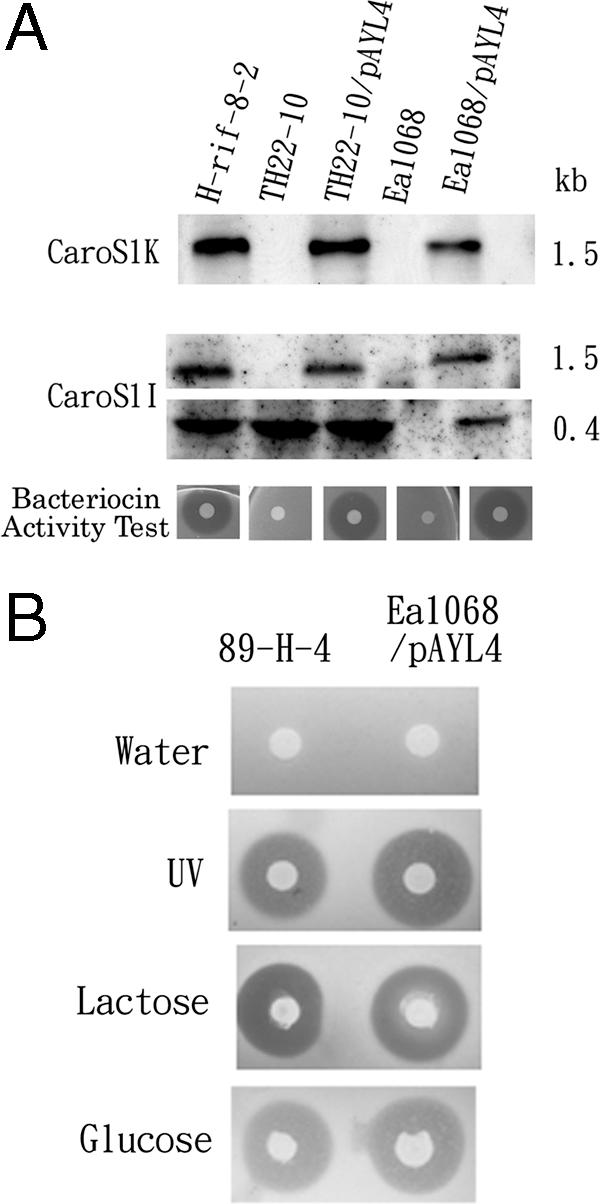
Transcription analysis of the carocin S1 gene. (A) Northern hybridization analysis of caroS1K and caroS1I. Total RNAs (20 μg) from H-rif-8-2, TH22-10, TH22-10/pAYL4, Ea1068, and Ea1068/pAYL4 cells incubated in BSM at 28°C for 24 h were subjected to Northern blot analysis. E. carotovora subsp. carotovora strain Ea1068B was used for the bacteriocin activity test. (B) Carocin S1 expression after water, UV, glucose, and lactose stimulation. The producer strains were 89-H-4 and Ea1068/pAYL4, and the indicator strain was Ea1068.
Transcription analysis of the carocin S1 genes.
The plasmid pAYL4, which contains the carocin S1 gene, is expressed in E. carotovora subsp. carotovora Ea1068 and TH22-10 strains from their native promoters. Northern blots of total RNAs from H-rif-8-2, TH22-10, TH22-10/pAYL4, Ea1068, and Ea1068/pAYL4 cells incubated in BSM at 28°C for 24 h are shown in Fig. 2A. PCR products specific for caroS1K and caroS1I were used as probes in hybridizations. In both cases, the caroS1K gene was expressed and detected in H-rif-8-2, TH22-10/pAYL4, and Ea1068/pAYL4 cells. However, the caroS1I gene was also detected in all host cells except Ea1068 cells. Only a 0.4-kb RNA band was detected in the Tn5 insertional mutant strain, TH22-10. Sequence analysis found similarity to the consensus sequence of the E. coli cyclic AMP receptor protein binding site (−312 bp) upstream of the start codon. To prove this finding, the 89-H-4 and Ea1068/pAYL4 cells were treated with water, UV, or lactose. Carocin S1 gene expression was stimulated by either lactose or UV (Fig. 2B).
Carocin S1 purification and nucleotidase assay.
Carocin S1 was collected and purified, and its activity was tested (Fig. 3B). This product, produced from 89-H-4, was highly toxic. Homology analysis of the protein showed similarities to pyocin S3, which has nucleotidase activity (Fig. 3A). Here, carocin S1 was shown to inhibit growth of the indicator strain Ea1068 and to have nucleotidase activity.
FIG. 3.
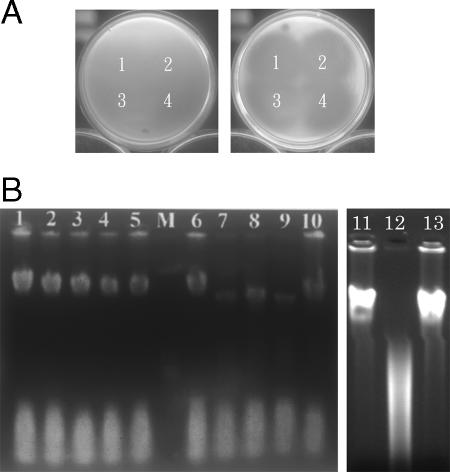
DNase activity of carocin S1 against genomic DNA from E. carotovora subsp. carotovora Ea1068. (A) Analysis of the killing activity of purified carocin S1. Carocin S1 was purified from 89-H-4 (1), H-rif-8-6 (2), TH22-10/pAYL4 (3), and Ea1068/pAYL4 (4) strains and then added to the indicator plates to test its killing activity. The indicator strains were Ea1068/pAYL4 in the left plate and Ea1068 in the right plate. (B) The reaction mixture (50 μl) containing 1 μg of DNA and 20 μl of sample solution in 10 mM Tris, pH 7.5, 4 mM MnCl2 was incubated at 28°C for 2 h. Samples 1 to 5 were collected from Ea1068, and samples 6 to 10 were collected from Ea1068/pAYL4. Lanes 1 and 6 were from the 11th fraction tubes, lanes 2 and 7 from the 12th fraction tubes, lanes 3 and 8 from the 13th fraction tubes, lanes 4 and 9 from the 14th fraction tubes, and lanes 5 and 10 from the 15th fraction tubes. Lane 11 was genomic DNA isolated from Ea1068. Lane 12 was the positive control (DNA mixed with EcoRI and buffer), and lane 13 was the negative control (DNA and buffer). Samples were treated and analyzed by agarose gel electrophoresis.
Nucleotide sequence accession number.
The GenBank accession number of the sequence of the carocin S1 gene is AF205141.
DISCUSSION
The results described here show that E. carotovora subsp. carotovora 89-H-4 has a functional antibacterial gene. Expression of the gene in a non-bacteriocin-producing strain of E. carotovora subsp. carotovora, strain Ea1068, resulted in the production of a bacteriocin (named carocin S1) that was released into the growth medium. This study is the first to find, clone, and express an LMWB gene of an Erwinia species. The carocin S1 genetic determinant consists of two structural genes, caroS1K and caroS1I, which have homology to the pyocin S3 and pyocin AP41 genes that encode the killer protein and immunity protein of P. aeruginosa strains.
Analysis of the caroS1K gene showed a potential Shine-Dalgarno sequence (ATGGAA), which may be a ribosome binding site, 5′ of the putative ATG start codon at position −44 bp. Several E. coli sigma 70-like promoter sequences were found 5′ of this putative ribosome binding site. A possible CTGATA(17 bp)CAGTAT was found at positions −186 to −214 bp relative to the translational start codon of the E. coli sigma 70 binding consensus sequence. This may be the promoter for caroS1K and could be expressed only in E. carotovora subsp. carotovora, but not in E. coli.
Upstream of the translational start codon, no sequence resembling the consensus sequence for an SOS box (the site for binding of LexA, the repressor of the DNA damage-inducible genes of E. coli) (30) was found. This may explain why caroS1 could not be expressed in E. coli HB101 but was successfully expressed in E. carotovora subsp. carotovora strain Ea1068 (a non-bacteriocin-producing strain).
Analysis of the genomic sequence around the carocin S1 gene revealed a sequence similar to the consensus sequence of the E. coli cyclic AMP receptor protein binding site (−312 bp) upstream of the start codon, which is activated by lactose. From our studies, glucose, as well as SOS agents, can also induce the carocin S1 gene.
Similar to caroS1K, caroS1I had a putative Shine-Dalgarno sequence (AAGGAA) located at the 3′ end of the caroS1K gene. This sequence was 13 nucleotides from the stop codon, TAA, of caroS1K and 15 nucleotides from the initiation codon of the caroS1I gene. Also, several E. coli sigma 70-like promoter sequences were found at the 5′ end of this putative ribosome binding site. A putative TAGAAC(19 bp)TAAACT was found at positions −25 to −55 bp relative to the translational start codon of the E. coli sigma 70 binding consensus sequence. This may also be the promoter for the caroS1I gene. However, its sequence is very similar to that of the pyocin S3 gene of P. aeruginosa (25, 26). With this structure, ribosomes can remain in simultaneous contact with the termination codon of the first gene (caroS1K) and the initiation codon of the second (caroS1I). Thus, caroS1K and caroS1I can be transcribed as a two-gene operon, because the two genes are translationally coupled.
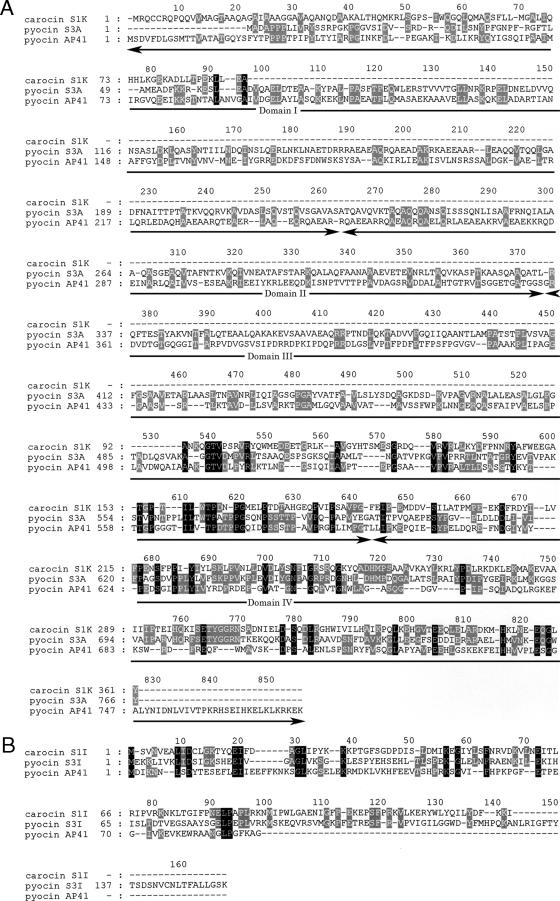
The homology search found that the carocin S1 gene was homologous to the pyocin S3 and AP41 genes in P. aeruginosa from positions 1078 to 1704; these genes encode nuclease (domain 4) (27) (Fig. 4). This suggests that the carocin S1 gene may also encode a similar nuclease. Although the remaining sequence (622 to 1078 bp) had low homology with domain 1 or 3, it may encode functions that are the same or similar to those of domain 1 (receptor binding domain) or domain 3 (translocation domain) or both (27).
FIG. 4.
Alignment of the amino acid sequences of carocin S1, pyocin S3A, and pyocin AP41. (A) Killer protein. (B) Immunity protein. The numbers refer to the positions of the amino acid residues in the sequence of each protein. The putative structural domains (I to IV) of carocin S1K are indicated.
The LMWB of Erwinia species was not induced by mitomycin C (data not shown), which is a DNA-damaging agent commonly used for colicin production in E. coli, but was induced by UV exposure and glucose. Recently, similarity between an LMWB receptor in Erwinia carotovora subsp. carotovora and receptors for lipopolysaccharide, sucrose, and glucose was reported (23), but more study is needed to determine how receptor interaction affects bacteriocin induction.
Here, we proved that carocin S1 has nucleotidase activity and can be induced by glucose. The activity of each domain of carocin S1 and the effect of the promoter region will need further study to determine the regulatory mechanism of glucose induction.
To our knowledge, this is the first time that an LMWB gene (the carocin S1 gene) from E. carotovora subsp. carotovora has been cloned and expressed. This gene can now be introduced into tobacco, Chinese cabbage, or other plant species by transgenic techniques to protect them against soft rot disease.
Footnotes
Published ahead of print on 27 October 2006.
REFERENCES
- 1.Bolivar, F., R. L. Rodriguez, P. J. Greene, M. C. Betlach, H. L. Heyneker, and H. W. Boyer. 1977. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene 2:95-113. [PubMed] [Google Scholar]
- 2.Chang, A. C. Y., and S. N. Cohen. 1978. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J. Bacteriol. 134:1141-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chuang, D.-Y., A. G. Kyeremeh, Y. Gunji, Y. Takahara, Y. Ehara, and T. Kikumoto. 1999. Identification and cloning of an Erwinia carotovora subsp. carotovora bacteriocin regulator gene by insertional mutagenesis. J. Bacteriol. 181:1953-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chuang, D.-Y., Y. Gunji, A. G. Kyeremeh, Y. Takahara, and T. Kikumoto. 1998. Cloning of bacteriocin regulator gene (brg) from Erwinia carotovora subsp. carotovora, abstr. 251, p. 389-390. Abstr. Annu. Meet. Soc. 1998. Phytopathological Society of Japan, Sapporo, Japan. (In Japanese.)
- 5.Duport, C., C. Baysse, and Y. Michel-Briand. 1995. Molecular characterization of pyocin S3, a novel S-type pyocin from Pseudomonas aeruginosa. J. Biol. Chem. 270:8920-8927. [DOI] [PubMed] [Google Scholar]
- 6.Fredericq, P. 1957. Colicins. Annu. Rev. Microbiol. 11:7-22. [DOI] [PubMed] [Google Scholar]
- 7.Gantotti, B. V., K. L. Kindle, and S. V. Beer. 1981. Transfer of the drug-resistance transposon Tn5 to Erwinia herbicola and the induction of insertion mutations. Curr. Microbiol. 6:417-425. [Google Scholar]
- 8.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 9.Hinton, J. C. D., M. C. M. Perombelon, and G. P. C. Salmond. 1985. Efficient transformation of Erwinia carotovora subsp. carotovora and E. carotovora subsp. atroseptica. J. Bacteriol. 161:786-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Itoh, Y., K. Izaki, H. Takahashi, and S. Kamimiya. 1979. Bacteriocins of genus Erwinia—especially on the bacteriocins from E. carotovora. Tanpakushitsu Kakusan Koso 24:888-894. (In Japanese.) [PubMed] [Google Scholar]
- 11.Kikumoto, T., S. Ma, and Y. Takahara. 1993. Biological control of the soft rot disease of Chinese cabbage. 3. Interactions of avirulent and virulent strains of Erwinia carotovora subsp. carotovora on the petiole of Chinese cabbage, abstr. 195, p. 315-316. In Abstr. Annu. Meet. Soc. 1993. Phytopathological Society of Japan, Tokyo, Japan. (In Japanese.)
- 12.Kikumoto, T., A. G. Kyeremeh, D.-Y. Chuang, and Y. Gunji. 1997. Biological control of the soft rot disease of Chinese cabbage with avirulent mutant strains of Erwinia carotovora subsp. carotovora, p. 118-119. In A. Ogoshi, K. Kobayashi, Y. Homma, F. Kodama, N. Kondo, and S. Akino (ed.), Proceedings of the Fourth International Workshop on Plant Growth-Promoting Rhizobacteria. Japan-OECD Joint Workshop, Sapporo, Japan.
- 13.Klaenhammer, T. R. 1988. Bacteriocins of lactic acid bacteria. Biochimie 70:337-349. [DOI] [PubMed] [Google Scholar]
- 14.Liu, Y.-G., and R. F. Whittier. 1995. Thermal asymmetric interlaced PCR: automatable amplification and sequencing of insert end fragments from P1 and YAC clones for chromosome walking. Genomics 25:674-681. [DOI] [PubMed] [Google Scholar]
- 15.Messing, J. 1979. A multipurpose cloning system based on single-stranded DNA bacteriophage M13. Recomb. DNA Tech. Bull. 2:43. [Google Scholar]
- 16.Miyashita, K. 1992. DNA probes, p. 163-172. In Experimental methods in soil microbiology. Youkendou Publishing Co., Tokyo, Japan. (In Japanese.)
- 17.Nakatani, F., and H. Tsuyama. 1973. Production of two kinds of antibacterial agents by isolates of Erwinia carotovora. J. Fac. Agric. Iwate Univ. 11:245-253. [Google Scholar]
- 18.Nguyen, A. H., T. Tomita, M. Hirota, T. Sato, and Y. Kamio. 1999. A simple purification method and morphology and component analyses for carotovoricin Er, a phage-tail-like bacteriocin from the plant pathogen Erwinia carotovora Er. Biosci. Biotechnol. Biochem. 63:1360-1369. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen, A. H., T. Tomita, M. Hirota, J. Kaneko, T. Hayashi, and Y. Kamio. 2001. DNA inversion in the tail fiber gene alters the host range specificity of carotovoricin Er, a phage-tail-like bacteriocin of phytopathogenic Erwinia carotovora subsp. carotovora Er. J. Bacteriol. 183:6274-6281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perombelon, M. C. M., and A. Kelman. 1980. Ecology of the soft rot erwinias. Annu. Rev. Phytopathol. 18:361-397. [Google Scholar]
- 21.Reusch, R. N., T. W. Hiske, and H. L. Sadoff. 1986. Poly-beta-hydroybutyrate membrane structure and its relationship to genetic transformability in Escherichia coli. J. Bacteriol. 168:553-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riley, M. A., and J. E. Wertz. 2002. Bacteriocins: evolution, ecology, and application. Annu. Rev. Microbiol. 56:117-137. [DOI] [PubMed] [Google Scholar]
- 23.Riley, M. A. 1998. Molecular mechanism bacteriocin evolution. Annu. Rev. Genet. 32:255-278. [DOI] [PubMed] [Google Scholar]
- 24.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 25.Sano, Y. 1993. The inherent DNase of pyocin AP41 causes breakdown of chromosomal DNA. J. Bacteriol. 175:912-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sano, Y., H. Matsui, M. Kobayashi, and M. Kageyama. 1993. Molecular structures and functions of pyocins S1 and S2 in Pseudomonas aeruginosa. J. Bacteriol. 175:2907-2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sano, Y., M. Kobayashi, and M. Kageyama. 1993. Functional domains of S-type pyocins deduced from chimeric molecules. J. Bacteriol. 175:6179-6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takahara, Y. 1994. Development of the microbial pesticide for soft-rot disease. PSJ Biocont. Rep. 4:1-7. (In Japanese.) [Google Scholar]
- 29.Tsushima, S., A. Hasebe, Y. Komoto, J. P. Charter, K. Miyashita, K. Yokoyama, and R. W. Pickup. 1995. Detection of genetically engineered microorganisms in paddy soil using a simple and rapid “nested” polymerase chain reaction method. Soil Biol. Biochem. 27:219-227. [Google Scholar]
- 30.Van Gijsegem, F. 1989. Relationship between the pel genes of the pelADE cluster in Erwinia chrysanthemi strain B374. Mol. Microbiol. 3:1415-1424. [DOI] [PubMed] [Google Scholar]