Abstract
Genome sequence analysis of the bacterium Xylella fastidiosa revealed the presence of two genes, named rpoE and rseA, predicted to encode an extracytoplasmic function (ECF) sigma factor and an anti-sigma factor, respectively. In this work, an rpoE null mutant was constructed in the citrus strain J1a12 and shown to be sensitive to exposure to heat shock and ethanol. To identify the X. fastidiosa σE regulon, global gene expression profiles were obtained by DNA microarray analysis of bacterial cells under heat shock, identifying 21 σE-dependent genes. These genes encode proteins belonging to different functional categories, such as enzymes involved in protein folding and degradation, signal transduction, and DNA restriction modification and hypothetical proteins. Several putative σE-dependent promoters were mapped by primer extension, and alignment of the mapped promoters revealed a consensus sequence similar to those of ECF sigma factor promoters of other bacteria. Like other ECF sigma factors, rpoE and rseA were shown to comprise an operon in X. fastidiosa, together with a third open reading frame (XF2241). However, upon heat shock, rpoE expression was not induced, while rseA and XF2241 were highly induced at a newly identified σE-dependent promoter internal to the operon. Therefore, unlike many other ECF sigma factors, rpoE is not autoregulated but instead positively regulates the gene encoding its putative anti-sigma factor.
Regulation of gene expression is required for adaptive response of bacteria to distinct environmental stimuli. Bacterial sigma factors regulate gene expression by conferring different promoter specificities on RNA polymerase during transcription initiation. Bacterial genomes usually encode one principal sigma factor for transcription of housekeeping genes, of which sigma 70 of Escherichia coli is the prototype, and a variable number of alternative sigma factors that coordinate specific regulons (61). The extracytoplasmic function (ECF) sigma factors were initially described as a distinct group of the sigma 70 family based on sequence similarity. This group initially included the presently well-characterized ECF sigma factors σE of Streptomyces coelicolor, AlgU of Pseudomonas aeruginosa, σE and FecI of Escherichia coli, CarQ of Myxococcus xanthus, HrpL of Pseudomonas syringae, CnrH of Alcaligenes eutrophus, and SigX of Bacillus subtilis (37).
In the traditional model for regulation of ECF sigma factors, an extracytoplasmic signal releases the sigma ECF from anti-sigma factor inhibition and thereby leads to the transcription of the target genes, usually including its own operon, a mechanism of positive autoregulation that allows amplification and turn-off of the signal (26). Genome sequencing revealed that ECF sigma factors are widespread in several bacterial species, and characterization of multiple members of this group has shown that they share some common features. They recognize preferentially a promoter element with an “AAC” motif in the −35 region, are cotranscribed with an anti-sigma factor in an auto-regulated operon, and regulate functions associated with the cell envelope (26).
A combination of genetic strategies with genomewide expression experiments and bioinformatics analysis has provided a powerful way of identifying ECF regulon members. In Escherichia coli, for example, the number of genes identified as belonging to the σE regulon has increased significantly in recent years. The first three identified targets for σE were rpoH, htrA, and rpoE, defining a second heat shock regulon activated at very high temperature (18, 35, 50, 53). Many other genes have been shown to be regulated by σE, including genes that encode proteins involved in folding or degradation of polypeptides (proteases, peptidylprolyl isomerases, chaperones, and thiol-disulfide isomerases), as well as genes involved in lipopolysaccharide (LPS) biogenesis and genes of unknown function (11, 51). More recently, a study has developed a promoter prediction model for the σE regulon, enabling the identification of 89 transcription units regulated by σE in E. coli and eight related genomes. The core regulon is involved in LPS and outer membrane protein biosynthesis, while the variable set of genes is involved in functions associated with pathogenesis (52). Other examples of extensively studied ECF regulons are multiple ECF sigma factors in Bacillus subtilis, Mycobacterium tuberculosis, and Streptomyces coelicolor (26).
In Pseudomonas aeruginosa, sigma factor AlgU mutants presented increased sensitivity to extreme temperatures and reactive oxygen species (41), in addition to directly regulating an osmotically inducible gene (21). In Mycobacterium tuberculosis, 10 sigma ECF genes have been annotated, and it has been demonstrated that σE and σH are involved in the response to heat shock, oxidative, and surface stresses (38, 39).
Xylella fastidiosa is a gram-negative, xylem-limited gammaproteobacterium phylogenetically related to Xanthomonas (60). This phytopathogen, transmitted by xylem sap-feeding insects, causes disease in diverse plant hosts, including citrus variegated chlorosis (CVC) in orange trees and Pierce's disease in grapevines (49). The complete genome sequence of a citrus strain and of a grapevine strain (55, 58) and the draft sequences of the almond- and oleander-infecting strains of X. fastidiosa (7) have stimulated genetic and molecular studies of the bacterium. Transcriptome analysis has been used to compare growth in biofilm and planktonic cells (16), freshly isolated bacteria, or axenic culture (15) and in genotyping studies comparing different strains (30, 47). Analysis of the response of X. fastidiosa to heat stress was recently carried out (31), determining the genes involved in the heat shock response in the citrus strain 9a5c. A total of 261 genes were induced, and 222 genes were repressed, as determined after different times of exposure to 40°C (31).
However, a more direct assignment of gene function and evaluation of its association with pathogenesis require the analysis of mutants. Although some attempts were made to obtain mutants in citrus strains (12, 23, 29), they are more easily obtained in Pierce's disease strains (19, 24, 42, 46). In CVC strains of X. fastidiosa, mutants were obtained only by homologous recombination when vectors carrying a functional Xylella origin of replication were utilized (12, 23).
Annotation of the genome sequences of X. fastidiosa strains indicates the presence of four genes encoding putative sigma factors. The rpoN gene encodes a σ54 orthologue belonging to the sigma 54 family. The other three genes encode orthologues of the sigma 70 family: rpoD, encoding the principal sigma factor σ70; rpoH, encoding the heat shock sigma factor σ32; and rpoE, encoding the single ECF sigma factor of X. fastidiosa. Although several studies of global gene expression in response to different growth conditions were carried out, little is known regarding the regulatory networks that control gene expression in X. fastidiosa. In this work, we describe the construction of an rpoE null mutant of X. fastidiosa CVC strain J1a12 and demonstrate that the cells become sensitive to heat shock and ethanol. Using DNA microarray and real-time quantitative reverse transcription-PCR (qRT-PCR) analyses, we were able to identify members of the σE regulon and to show the involvement of this sigma factor in the regulation of the heat shock response. The increase in σE activity during heat stress is not the result of increased mRNA or protein levels and probably involves modulation of σE activity by its anti-sigma factor. This ECF sigma factor presents an unusual regulatory mechanism, since rpoE is not autoregulated but exerts positive regulation on the gene encoding the anti-sigma factor.
MATERIALS AND METHODS
Strains and growth conditions.
X. fastidiosa J1a12 (45) was grown in PW medium (13) containing 0.5% glucose (PWG). The rpoE strain was grown in PWG containing 10 μg ml−1 ampicillin (PWG/Amp) and the rpoE(pRPOE) strain was grown in 10 μg ml−1 ampicillin and 5 μg ml−1 kanamycin. Cultures were grown at 25°C with no agitation unless otherwise indicated. Transformation of X. fastidiosa was carried out as described previously (12). E. coli was grown at 37°C in LB medium supplemented with ampicillin (100 μg ml−1) or kanamycin (50 μg ml−1) as necessary. All oligonucleotides used in this study are described in Table S1 at the project web site (http://blasto.iq.usp.br/∼tkoide/Xylella/RpoE/).
Construction of an rpoE null mutant strain.
Gene disruption was achieved by homologous recombination using a pUC-based vector (pUCBM21; Boehringer Mannheim) harboring a 400-bp fragment (amplified by PCR with primers Ori1 and Ori2) containing the chromosomal origin of replication of X. fastidiosa 9a5c (55), generating vector pUCBM21oriC. The internal region of the rpoE gene was amplified by PCR with primers rpoE1 and rpoE2 in a 50-μl reaction mixture containing 0.5 μg DNA, 50 pmol each nucleotide, 2 mM MgCl2, 0.2 mM deoxynucleotide triphosphates, and 1 U Taq DNA polymerase (Gibco BRL) in the supplied enzyme buffer. The 450-bp BamHI/ApaI fragment was ligated to pUCBM21oriC and introduced into X. fastidiosa J1a12 by electroporation. Transformants were selected in PWG/Amp plates, and four colonies were inoculated in liquid PWG/Amp. Cultures were diluted 1:10 every 7 days in fresh medium, and this procedure was repeated several times, each time constituting one passage. At the second and fifth passages, a sample of the cells was screened for integration of the plasmid by colony PCR, using primers sigma 24-1 and M13Reverse, which amplified a fragment only if the plasmid had integrated into the rpoE gene. Once the integration was confirmed, isolated colonies were obtained and integration at the rpoE locus was confirmed by Southern blotting using a 670-bp fragment containing the rpoE coding region as a probe. The mutant rpoE strain was complemented with a copy of the rpoE gene in trans in a pUCBM21oriC vector containing a kanamycin resistance cassette isolated from pUC-4K (Amersham Biosciences) in a 1,200-bp EcoRI fragment. The 1,160-bp PstI/HindIII fragment (amplified by PCR with primers Sigma24C1 and XF2240EXT) containing the promoter and the coding regions of rpoE was sequenced and cloned into the vector (pRPOE) and introduced into the rpoE mutant strain.
Anti-σE immune serum and immunoblots.
The 670-bp BamHI/EcoRI fragment (amplified by PCR with primers Sigma24-1 and Sigma 24-2) containing the complete coding region of X. fastidiosa rpoE was cloned into vector pProEX HTc (Gibco BRL). The recombinant RpoE protein with a polyhistidine tail (His-RpoE) was purified from inclusion bodies of E. coli DH5α after solubilization in 0.3% Sarkosyl, as described previously (10a), followed by chromatography in a nickel column. The immune serum was obtained in New Zealand rabbits after four subcutaneous injections of 0.5 mg of purified protein in Freund's incomplete adjuvant. Immunoblots were performed essentially as described previously (57), using a 1:1,000 dilution of the antiserum and a secondary anti-rabbit-alkaline phosphatase conjugate (1:30,000 dilution).
Stress survival tests.
The rpoE null strain was tested for survival of heat shock by transferring a midlog-phase culture from 25°C to a water bath at 45°C and taking aliquots at several time points. Serial dilutions of the cultures were plated in PWG/Amp, and the numbers of CFU were determined by counting the colonies after 21 days at 25°C. The parental J1a12 strain was used as a control. In order to test for ethanol resistance, cultures of J1a12 and rpoE strains were grown up to midlog phase and ethanol was added to 1.5%. Growth was evaluated by measurement of optical density at 600 nm.
RNA purification.
Total RNA was extracted from cell cultures grown to midlog phase at 25°C, incubated at 40°C for 25 min, or grown in the presence of 5% ethanol for 30 min, using the TRIzol reagent (Invitrogen). A further treatment with 0.03 U RQ1 DNase I (Promega) per μg of RNA for 30 min at 37°C, followed by phenol extraction and ethanol precipitation, was carried out for RNA used in the microarrays and RT-PCR experiments. The RNA was evaluated for quantity and quality by A260 and agarose formaldehyde gel electrophoresis.
Microarray analysis.
Microarrays containing unique internal PCR-amplified fragments of 94.5% of the Xylella fastidiosa genome (2,692 genes) spotted at least in duplicate were used for the experiments. A detailed description of the array can be found in Koide et al. (30). Total RNA (20 μg) was reverse transcribed and labeled using the SuperScript Plus Indirect cDNA Labeling System (Invitrogen), according to the manufaturer's instructions. Briefly, the RNA was reverse transcribed using the enzyme SuperScriptIII in the presence of modified aminoallyl-deoxynucleotide triphosphate, an optimized nucleotide mix, buffer, dithiothreitol, and random primers. The resulting amine-modified cDNA was then chemically labeled in the aminoallyl group using Alexa Fluor 555 reactive dye, which corresponds to Cy3, and Alexa Fluor 647 reactive dye, corresponding to Cy5. Hybridization, washing, and scanning were performed as previously described (30). The fluorescence mean intensity and surrounding median background from each spot were obtained with ArrayVision v6.0 (Imaging Research, Inc). Unreliable spots having intensities too similar to the local background (mean intensity, <2 times the standard deviation of the background in the control and test samples simultaneously) or saturated (intensity, greater than 990 fluorescence units) were filtered out. Normalization was carried out by using a locally weighted linear regression (LOWESS) algorithm fitting on an M-versus-S plot, where M is the fluorescence log ratio of the test sample relative to the control condition [M = log2(rpoE−/J1a12)] and S is the log mean fluorescence intensity [S = log2(rpoE− + J1a12)/2)] (62). The complete data set is publicly available according to MIAME guidelines (3). We used intensity-dependent cutoff values for classifying a gene as differentially expressed based on self-self control experiments, as previously described (30, 59). Briefly, the self-self approach consists of simultaneously hybridizing the cDNA from the control sample labeled with either Cy3 or Cy5 to estimate the experimental noise. We used 0.99 credibility intervals, a window size of 1.0, and a window step of 0.2. A gene was classified as differentially expressed if at least 75% of its replicates were outside the intensity-dependent cutoff curves, using at least three replicates.
Real-time RT-PCR.
The primers used in this analysis were designed with PrimerExpress software (Applied Biosystems). For validation of the heat shock microarray results, a one-step qRT-PCR analysis was performed with eight open reading frames (ORFs) using the Absolute qRT-PCR SYBR green mix (ABgene). Reactions were carried out with 250 ng of RNA pretreated with DNase I, 0.1 μM each primer, and 12.5 μl of SYBR green mix. The following cycling conditions were used: 47°C for 30 min, 95°C for 15 min, 40 cycles of 95°C for 15 s, and 60°C for 1 min. Two-step qRT-PCR was used in all other experiments (rpoE mRNA level under several stresses and σE-dependent induction of specific transcripts). About 5 μg of RNA pretreated with DNase I was used as a template for total cDNA synthesis with 50 ng of random hexamers, using the SuperScript first-strand synthesis system for RT-PCR (Invitrogen). Real-time PCR was performed using 50 ng of cDNA, 0.1 μM each primer, and 12.5 μl of the Platinum SYBR green qPCR supermix UDG (Invitrogen). The cycling conditions used were 50°C for 2 min, 95°C for 2 min, 40 cycles of 95°C for 15 s, and 60°C for 1 min. Reactions were performed and analyzed in the ABI 7500 real-time system (Applied Biosystems). Nonspecific product and primer dimers were checked by dissociation curve analysis. Relative expression levels were calculated using the 2−ΔΔCT method (36). Results were normalized using the dnaQ gene (XF2157) as the endogenous control, which was shown to be constant in the samples analyzed by microarray. We were able to detect the rpoE mRNA in the rpoE mutant, since the primers used for qRT-PCR hybridized upstream of the disruption site in the rpoE gene.
Analysis of the transcription unit rpoE-rseA-XF2241 by RT-PCR.
To determine whether the genes rpoE (XF2239), rseA (XF2240), and XF2241 comprise a single transcriptional unit, RT-PCR experiments were performed. Cotranscription between XF2239 and XF2240 was analyzed with primers XF2239-1F/XF2240-705R, while cotranscription between XF2240 and XF2241 was analyzed with primers XF2240-589F/XF2241-984R. Reactions were carried out with 1 μg of RNA pretreated with DNase I isolated from cells grown at 25°C or at 40°C, using SuperScript one-step RT-PCR (Invitrogen) according to the manufacturer's instructions. The cycling conditions used were 50°C for 25 min; 95°C for 2 min; and 40 cycles of 95°C for 30 s, 55°C for 30 s, and 72°C for 1 min and 30 s, followed by incubation at 72°C for 7 min. Negative controls were made with PCR lacking reverse transcriptase to check for DNA contamination.
Mapping of the transcription start sites.
Primer extension experiments were carried out as described previously (32), using the 32P-labeled oligonucleotides XF0644EXT, XF2594EXT, XF0167EXT, XF2239EXT, XF1694EXT, and XF2240EXT (hybridizing at the beginning of the coding region of the respective genes). The labeled primers were hybridized to 50 μg RNA isolated from J1a12, rpoE, or rpoE(pRPOE) cells grown in PWG either at 25°C or after treatment at 40°C for 25 min. A sequencing ladder was generated by M13 DNA sequencing using the 32P-labeled primer M13Forward and the Thermosequenase cycle sequencing kit (USB). The 5′ rapid amplification of cDNA ends (RACE) method was used to determine the rpoE transcription start site using the 3′/5′ RACE kit (Roche). Total RNA was extracted from strain J1a12 grown up to midlog phase at 25°C, treated with DNase I (Promega), and reverse transcribed using the rpoE sequence-specific primer XF2239SP1. The cDNA was purified with a Qiaquick PCR purification kit (QIAGEN), poly(A) tail was added, and a subsequent PCR amplification was performed using the forward dT-anchor primer and the sequence-specific reverse primer XF2239-126R (SP2). The PCR products were cloned into TOPO vector (Invitrogen) and sequenced using forward and reverse M13 universal primers.
Microarray data accession number.
The microarray data have been deposited in NCBI's Gene Expression Omnibus (GEO) database (3) (http://www.ncbi.nlm.nih.gov/geo) under the accession number GSE4960.
RESULTS AND DISCUSSION
Characterization of an rpoE null mutant strain.
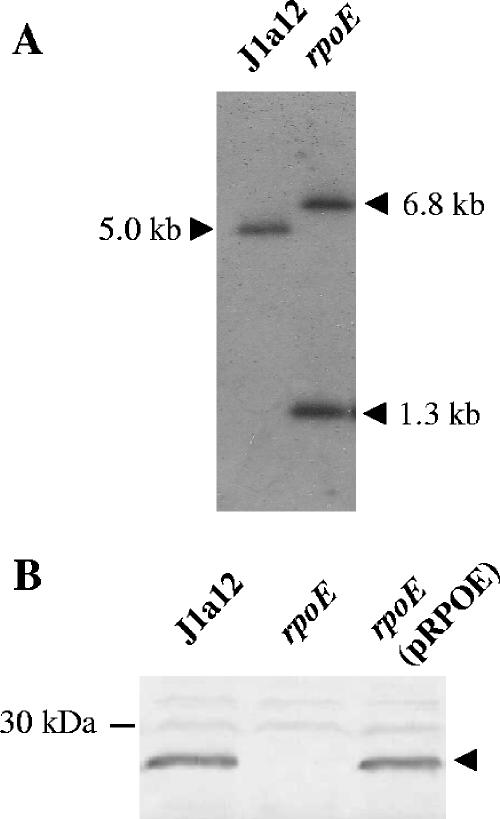
An rpoE null strain was generated by single-step homologous recombination, with integration of plasmid DNA into the rpoE gene of the X. fastidiosa CVC strain J1a12. Gene disruption by insertion duplication mutagenesis has been largely used for bacteria in which allelic exchange is not easily achieved (25, 33, 34). Detection of integration occurred only after five consecutive passages in liquid media, consistent with the low frequency of homologous recombination observed in this bacterium. This, in turn, results in a very low rate of success in obtaining mutants by targeted gene disruptions in citrus strains of X. fastidiosa, with only three reports found in the literature (12, 23, 56). Use of replicative vectors for homologous recombination, such as the system we used, has been an alternative mutagenesis method in other bacteria that are refractory to the classical suicide vector approach (5, 17). Integration into the rpoE locus, confirmed by Southern blotting (Fig. 1A), generated a null mutant strain that did not express σE, as verified by immunoblotting using a specific polyclonal antiserum (Fig. 1B). Complementation with a low-copy-number plasmid carrying the rpoE gene and its regulatory region restored the expression of the protein to levels similar to those of the parental strain (Fig. 1B). These results showed that σE is not essential for X. fastidiosa, similar to other bacteria (26) but unlike E. coli, whose single rpoE gene is essential for viability (14).
FIG. 1.
Confirmation of rpoE disruption in the mutant strain. (A) Southern blot analysis of X. fastidiosa J1a12 and rpoE strains. Total DNA was digested with EcoRI and hybridized with a probe comprising the coding region of rpoE. The original 5.0-kb fragment in J1a12 was divided into two fragments of 1.3 kb and 6.8 kb by insertion of the vector DNA. (B) A polyclonal antiserum raised against X. fastidiosa RpoE was used in an immunoblot assay of the J1a12 and rpoE strains and the mutant strain complemented with a copy of the rpoE gene in trans [rpoE(pRPOE)]. The arrowhead indicates the band corresponding to the RpoE protein.
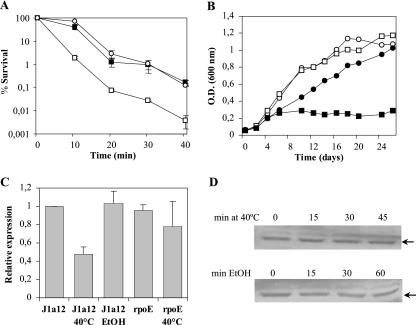
The rpoE strain was then tested for resistance to heat shock at 45°C for several periods of time. This strain showed a more severe loss of viability than the parental strain J1a12, whereas the complemented rpoE(pRPOE) strain had restored the parental levels of resistance (Fig. 2A). Growth in the presence of 1.5% ethanol was also impaired in the rpoE mutant strain (Fig. 2B), confirming the requirement for σE in the response to accumulation of misfolded proteins that are generated by these stresses. These results suggested that σE mediates the heat shock response in X. fastidiosa, probably regulating genes essential for survival under these conditions. Moreover, the rpoE mutant strain was not sensitive to either H2O2 or NaCl, as determined by growth curves and a zone diffusion assay (not shown).
FIG. 2.
Analysis of the rpoE strain in response to heat shock and ethanol. (A) Survival test of J1a12 (white circles), rpoE (white squares), and rpoE(pRPOE) (black squares) strains after incubation at 45°C. Cultures grown at 25°C to midlog phase were transferred to 45°C, and at several time points, aliquots were removed and plated for counting colonies. Survival was determined relative to the culture at 25°C (time zero). The experiments were repeated three times with independent cultures, and the results shown are the averages and standard deviations (SD) from one representative experiment in triplicate. (B) Growth curve of strain J1a12 without (white circles) or with (black circles) 1.5% ethanol and the rpoE strain without (white square) or with (black squares) 1.5% ethanol in PWG medium. The figure shows one representative experiment out of three independent replicates. (C) Determination of relative rpoE mRNA levels by qRT-PCR. Total RNA was isolated from strain J1a12 or the rpoE mutant grown without stress and incubated at 40°C for 25 min or in the presence of 5% ethanol for 30 min, and rpoE mRNA was converted to cDNA and amplified by qRT-PCR. The results show the averages and SD of two independent biological experiments done in triplicate. (D) Determination of relative σE levels under heat shock and ethanol stresses. Total protein extracts from cells grown without stress (time zero) or at several times after incubation under stress conditions (40°C or 5% ethanol) were transferred to a nitrocellulose membrane and probed with a polyclonal anti-σE serum. The arrows indicate the bands corresponding to σE.
The sensitivity of ECF sigma factor mutant strains to high temperature, oxidative stress, ethanol, and sodium dodecyl sulfate, as well as the involvement of these sigma factors in pathogenesis, has been determined for several bacteria (4). In E. coli, σE mediates the response to extreme heat shock, regulating the expression of genes important for the integrity of the cell envelope (11, 27), whereas σ32 mediates the cytoplasmic heat shock response to less severe temperatures (2). X. fastidiosa also presents the rpoH gene, encoding heat shock factor σ32 (55); therefore, σE could have a more restricted role in the extracytoplasmic response to high-temperature stress.
In order to evaluate whether rpoE expression was affected by different stresses, we measured the amount of rpoE mRNA and σE levels in strain J1a12 by qRT-PCR and immunoblot analysis, respectively. Bacteria were submitted to osmotic stress (300 mM sucrose or 250 mM NaCl), oxidative stress (0.1% H2O2), low temperature (10°C), and stationary phase. The levels of rpoE mRNA and protein were not affected under any of the conditions tested (data not shown). In addition, the roles of rpoE in oxidative and osmotic stresses have been assessed by microarray experiments with RNA from cells incubated in the presence of H2O2 and NaCl, and data indicated that these stimulons in X. fastidiosa are independent of rpoE (unpublished data).
Since RpoE is required during heat shock and exposure to ethanol, the same analysis was carried out to determine the mRNA and protein levels of RpoE under these stresses. During heat shock, the amount of rpoE transcript decreased to 40% of that of the control, but immunoblot analysis of σE levels showed no change in the amount of the sigma factor (Fig. 2C and D), suggesting that its activation does not lead to a significant change in its own levels in the cell. Under heat shock, the rpoE mRNA levels went down but σE levels were not affected, indicating possible posttranscriptional control in response to heat stress. When cells were submitted to ethanol stress, the levels of rpoE mRNA and protein did not change (Fig. 2C and D), although RpoE was still required for survival.
Several ECF sigma factors were shown to be induced by environmental stresses at the transcriptional level: sigV of Enterococcus faecalis by glucose starvation, heat shock, and sodium dodecyl sulfate treatment (6); rpoE of Salmonella enterica serovar Typhimurium by cold shock, heat shock, osmotic stress, and stationary phase (44); and algT of Pseudomonas syringae by heat shock and osmotic and oxidative stresses (28). In these three examples, the increase in expression was dependent on an auto-regulated promoter. This was not the case for X. fastidiosa rpoE, since the levels of rpoE mRNA were not induced under any of the stresses tested and were not diminished in the rpoE strain compared to the parental strain, J1a12 (Fig. 2C), suggesting that σE is not necessary for its own transcription.
Determination of the σE heat shock regulon.
In order to determine the regulatory role of σE in the X. fastidiosa heat shock response, a global transcriptional profile analysis of the rpoE mutant compared to the parental strain was performed under heat shock. Microarray analyses were carried out using RNA isolated from cells of J1a12 and the rpoE null strain incubated at 40°C for 25 min, using three independent biological samples. Twenty-one genes were identified that showed reduced expression in the mutant in comparison to the parental strain in 17 putative distinct transcription units (Table 1). Among the genes identified as σE dependent, 11 were shown to be induced during heat shock, whereas 10 genes that were not shown to be induced by heat shock had diminished expression in the mutant at high temperature (Table 1).
TABLE 1.
Genes differentially expressed under heat shock in the rpoE strain and the parental strain J1a12
| ORFa | Product | Putative functionb | Ratio (rpoE strain/J1a12 strain)c |
|---|---|---|---|
| XF0166 | Conserved hypothetical protein | Unknown | 3.1 |
| XF0167↑ | Conserved hypothetical protein (peptidase M23) | Protein degradation | 6.5d |
| XF0513 | Phage-related endolysin | Cell wall degradation | 5.9 |
| XF0643 | Hypothetical protein | Unknown | 9.6 |
| XF0644↑ | Peptidyl-prolyl cis-trans isomerase | Protein folding | 19.3d |
| XF1212 | Peptidyl-prolyl cis-trans isomerase | Protein folding | 3.3 |
| XF1257 | Oligoribonuclease (exonuclease) | Exonuclease | 2.2d |
| XF1340 | Hypothetical protein | Unknown | 2.1 |
| XF1415 | UDP-N-acetylglucosamine 1-carboxyvinyltransferase | Peptidoglycan synthesis | 4.0d |
| XF1694 | Hypothetical protein | Unknown | 3.1d |
| XF1712 | Conserved hypothetical protein | Unknown | 4.8d |
| XF2169 | Conserved hypothetical protein | Unknown | 2.3 |
| XF2240↓ | ECF sigma factor regulator | σE negative regulator | 37.0d |
| XF2241 | Periplasmic protease | Protein degradation | 28.8d |
| XF2533↓ | Hypothetical protein | Unknown | 3.5 |
| XF2534 | Response regulator | Signal transduction | 2.6d |
| XF2594 | Conserved hypothetical protein (peptidase M48) | Protein degradation | 15.5d |
| XF2739 | Type I restriction-modification endonuclease | DNA metabolism | 10.0d |
| XFa0010 | Hypothetical protein | Unknown | 3.6 |
| XFa0059 | Plasmid replication/partition protein | Plasmid replication | 2.2 |
| XFa0061 | Single-strand binding protein | DNA binding | 3.0 |
Adjacent genes that might be cotranscribed are indicated by boldface and arrows. The direction of the arrow indicates the direction of transcription.
Predicted function based on sequence similarity.
Relative expression of the gene in the J1a12/rpoE strains incubated at 40°C for 25 min.
Genes induced by heat shock as determined in this work or in Koide et al. (31).
Several genes regulated by σE are involved in the folding and degradation of proteins, such as peptidases (XF2594 and XF0167), proteases (XF2241), and peptidylprolyl cis-trans isomerases (XF0644 and XF1212) (Table 1). Other functional categories identified were nucleic acid metabolism (XF2739 and XFa0059), cell wall synthesis (XF0513 and XF1415), and regulatory functions (XF2240 and XF2534), in addition to several proteins of unknown function. In general, these categories fit the overall pattern suggested for σE regulons of enterobacteria (52), with one relevant absence of genes involved in LPS biosynthesis.
Two genes showing the highest J1a12/rpoE expression ratio were XF2240 and XF2241, which constitute a putative single transcription unit. XF2240 encodes a putative anti-sigma factor; it is located downstream of rpoE and presents a probable σE-binding domain in its N-terminal region, showing a significant similarity (E value, 5e−45) to COG3073 (RseA; a negative regulator of sigma E activity). The N-terminal domain precedes a transmembrane region, and this protein is predicted by the PSORT-B program to be located in the inner membrane (22). Based on sequence similarity, we propose to name gene XF2240 rseA. Downstream of rseA there is a gene encoding a trypsin-like periplasmic serine protease containing a signal peptide and two PDZ domains (XF2241). This protein is a putative serine protease of the HtrA family (48), an orthologue of P. aeruginosa MucD (9).
Microarray analysis using RNA from J1a12 and the rpoE mutant cells grown at 25°C was also carried out, but only three genes showed a reduction in expression in the mutant compared to the parental strain. These genes (rseA, XF2241, and XF2594) presented a twofold reduction in expression and were also reduced in the rpoE strain under heat shock (Table 1). These results suggest that σE RNA polymerase is able to transcribe some genes at low levels in the absence of a particular stimulus and that most of its regulon is transcribed after activation by a stimulus, probably in the same way described for other ECF sigma factors, i.e., via release from an anti-sigma factor.
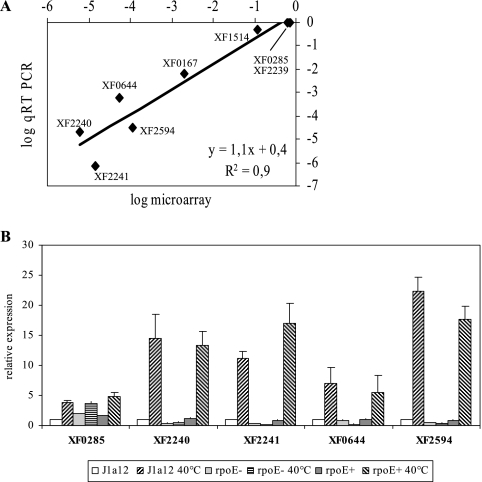
To validate microarray data, five genes downregulated in the rpoE mutant under heat shock and three genes that did not show variation in expression were analyzed by qRT-PCR, using as an internal control the dnaQ gene. The microarray and qRT-PCR data showed a high level of agreement (r = 0.90), indicating that our microarray approach was sensitive enough to identify differentially regulated genes. All five genes tested that presented lower expression in the rpoE mutant by microarray confirmed their σE dependence by RT-PCR analysis (Fig. 3A). Further analysis of some genes that presented the highest differences in expression was carried out in the J1a12, rpoE, and rpoE(pRPOE) strains at 25°C and after incubation at 40°C for 25 min (Fig. 3B). All the genes tested were induced by heat shock in a σE-dependent manner, confirming that σE mediates the heat shock induction of a subset of genes in X. fastidiosa. As a control for the experiment, a gene that was induced by heat shock in a σE-independent manner (XF0285) was also tested, confirming the pattern of expression observed in the microarray experiments.
FIG. 3.
(A) Comparison of gene expression levels determined by RT-PCR and microarray experiments. Gene expression was measured by qRT-PCR with three independent RNA samples from the rpoE and J1a12 strains exposed to 40°C for 25 min, and the levels were plotted against the corresponding microarray data values. (B) Expression of selected genes in different strains. Expression was measured by qRT-PCR using RNA samples from J1a12, rpoE, and rpoE(pRPOE) (rpoE+) strains grown at 25°C or incubated at 40°C for 25 min. The results show the averages and standard deviations of three independent biological samples.
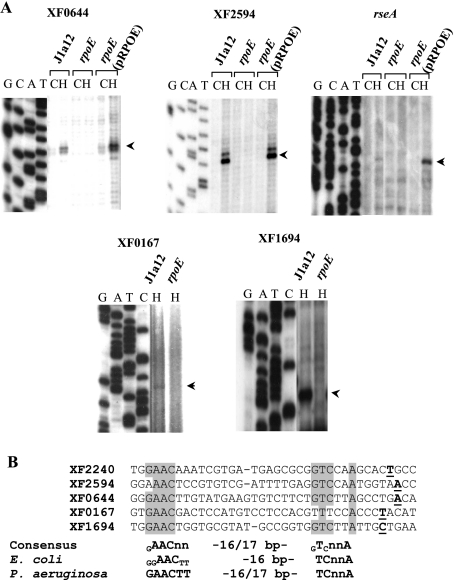
Determination of a σE-binding consensus motif.
The transcription start sites of the σE-dependent genes XF0644, XF2594, XF0167, XF1694, and rseA were determined by primer extension analysis in order to identify the σE-dependent promoter. In Fig. 4A, bands corresponding to the extension products can be seen in the lanes with RNA from strains J1a12 and rpoE(pRPOE) incubated at 40°C for 25 min, but not with the rpoE mutant RNA. The results indicated that heat shock induction was due to an increase in transcription from a σE-dependent promoter and was not the result of a second promoter in these genes.
FIG. 4.
(A) Determination of transcription start sites. Total RNAs from J1a12, rpoE, and rpoE(pRPOE) strains grown at 25°C (C) or incubated at 40°C for 25 min (H) were used as templates in primer extension experiments. Primers corresponding to genes XF0644, XF2594, rseA, XF0167, and XF1694 were 5′ end labeled with 32P and extended with reverse transcriptase. A DNA-sequencing ladder of phage M13mp18 was used as a molecular size marker. The arrowheads indicate the bands corresponding to the main start sites observed. (B) Alignment of the determined σE-dependent promoters. Identical nucleotides are shaded, and transcription start sites are boldface and underlined. A proposed consensus for an X. fastidiosa σE-binding site is indicated below, compared to E. coli σE- and P. aeruginosa AlgU binding sites (20, 51). Subscripts indicate bases of the consensus sequences conserved in less than 85% of the sequences, and n indicates any nucleotide.
A consensus for the σE-binding motif was obtained by alignment of the experimentally determined promoters (Fig. 4B). A search for this sequence was carried out manually using as parameters the sequence AAC-16/17 nucleotides-TnnA and screening about 300 nucleotides upstream of the annotated ATG of the genes identified by microarray analysis. These conserved promoter elements were identified upstream of nine other rpoE-regulated genes (Fig. 5), indicating that these genes could be directly regulated by σE. The proposed consensus X. fastidiosa σE-binding site (GAACnn-[N]16-17-GTCnnA) is very similar to E. coli σE and P. aeruginosa AlgU binding sites (20, 52). The core of the −35 sequence (AAC) and the flanking T and A of the −10 sequence are conserved through all three species and could constitute a minimum required recognition site for X. fastidiosa σE binding. It has been proposed that bacteria with few ECF sigma factors can afford more variation in the promoter sequences of their regulated genes (26), and this could also be the case for X. fastidiosa.
FIG. 5.
Alignment of putative promoter sequences of the σE-regulated genes. Transcription start sites determined by primer extension analysis are underlined. Alignment of the other sequences was carried out by manual inspection of 300 bp upstream of the ATG of the indicated ORFs. The shaded residues are those agreeing with the proposed consensus for the X. fastidiosa σE-binding site (Fig. 4B). The numbers indicate the positions of the first adenine of the conserved AAC sequence relative to the adenine of the translation initiation codon.
Regulation of the transcription unit rpoE-rseA-XF2241.
In several bacteria, ECF sigma factors are cotranscribed with their cognate anti-sigma factors, and usually this transcription unit is autoregulated by the ECF sigma factor (26). An important exception to this rule is the subgroup of ECF sigma factors that respond to iron starvation, which are not autoregulated (10). A gene encoding a putative anti-sigma factor (rseA) is located downstream of rpoE in the genome of X. fastidiosa and is probably cotranscribed with the XF2241 gene, encoding a MucD orthologue. Both rseA and XF2241 are induced by heat shock in a σE-dependent manner (Table 1 and Fig. 3B). A similar genomic organization is found in Xanthomonas sp., where genes XCC1267/XCC1268/XCC1269 encode an ECF sigma factor, a putative anti-sigma factor, and a MucD orthologue, respectively. In other bacteria, such as enterobacteria and Pseudomonas, there may be one or two genes downstream of rseA (namely, rseB/mucB and rseC/mucC), which encode accessory σE regulatory proteins (43, 54). There are no orthologues of rseB and rseC in the X. fastidiosa genome, which suggests that σE activity might be regulated only by RseA in this bacterium.
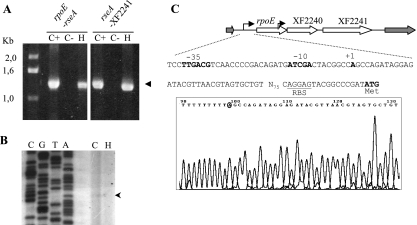
In order to investigate whether rpoE-rseA-XF2241 constitute a single transcription unit, RT-PCR experiments were carried out using primers pointing outward from each gene. As can be seen in Fig. 6A, a specific band could be obtained when primers located at the end of rpoE and the beginning of rseA were used. The same results were obtained with primers between rseA and XF2241. The amplification products were obtained from RNAs of cells growing at 25°C or exposed to 40°C for 25 min, showing that a transcript encompassing the three genes is present under both conditions. In agreement with these results, no independent transcription start site was found for XF2241 by primer extension analysis (not shown).
FIG. 6.
Determination of the rpoE/rseA/XF2241 transcription unit. (A) RT-PCR analysis of total RNA from J1a12 cells grown at 25°C (C+) or incubated at 40°C for 25 min (H) or a control without reverse transcriptase (C−). Amplification was carried out using pairs of primers between the rpoE and rseA genes (XF2239/XF2240) and between rseA and XF2241 (XF2240/XF2241). The arrowhead indicates the specific product. (B) Determination of the transcription start site of rpoE by primer extension. Total RNA from J1a12 grown at 25°C (C) or incubated at 40°C for 25 min (H) was used as a template in primer extension experiments. A primer corresponding to gene rpoE was 5′ end labeled with 32P and extended with reverse transcriptase and was also used in a DNA-sequencing reaction, shown on the left. The arrowhead indicates the band corresponding to the observed start site. (C) Determination of the transcription start site of rpoE by 5′ RACE. Total J1a12 RNA was used in a 5′-RACE amplification assay with primers anchoring at the beginning of the rpoE coding region. DNA sequencing of several amplification products identified an A as the start site (bottom). The sequence of the rpoE promoter region is shown above, and the −35/−10 region is indicated in boldface. The ribosome-binding site (RBS) is underlined, and the translational start codon of RpoE is indicated.
The transcription start site of rpoE was determined both by 5′ RACE and primer extension (Fig. 6B and C), and a single start site was found. A sequence with similarity to the consensus sequence for E. coli σ70 promoters (61) was found in the −35 region (TTGACG), suggesting that expression of this transcription unit is driven by the housekeeping sigma factor.
The results observed in microarray and qRT-PCR analyses showed that rpoE expression is not decreased in the rpoE mutant, indicating that this gene is not autoregulated, unlike most of the known orthologues. However, the downstream genes rseA and XF2241 are induced by heat shock in a σE-dependent manner and at similar levels of induction, using a σE-dependent promoter found between rpoE and rseA (Fig. 4). Despite the fact that it was long known that σE regulates the expression of the rpoE-rseABC operon in E. coli (43), it was not until recently that the existence of a second σE-dependent promoter was demonstrated upstream of the E. coli rseABC operon (52). However, unlike X. fastidiosa, in E. coli, rpoE is autoregulated by its own σE-dependent promoter (50, 53).
A model to explain these results is presented in Fig. 7. The rpoE operon is transcribed from a putative σ70-dependent promoter (Fig. 6), and some transcription is also initiated at the σE-dependent promoter upstream of rseA, since the rseA/XF2241 genes are less expressed in the rpoE strain even in the absence of stimuli (Fig. 3B). Under heat stress conditions, transcription from the σ70-dependent promoter is turned down (Fig. 2C and 6B) and transcription from the σE promoter upstream of rseA is highly induced (Fig. 3B and 4A), transcribing the genes encoding the anti-sigma factor and the protease along with other genes of the heat shock regulon. Accordingly, expression of the rseA gene was also induced in a σE-dependent manner when cells were incubated with 5% ethanol, although at a lower level (a 1.5-fold increase), as determined by qRT-PCR (not shown).
FIG. 7.
Model for the regulation of genes rpoE and rseA. The entire rpoE operon is transcribed from a σ70-dependent promoter, and some transcription also occurs from the σE internal promoter at normal temperature. During heat shock, transcription from the σ70 promoter is turned down and transcription from σE-dependent promoters is highly induced, including that from the internal promoter upstream of rseA. This results in high levels of RseA in the cell, which in turn sequestrates σE back to an inactive form, shutting down the response. R, RNA polymerase core enzyme.
It has been determined that E. coli RseA is sequentially cleaved by proteases DegS and YaeL, releasing σE in the cytoplasm to bind to the RNA polymerase core (reviewed in reference 1). XF2241 could be the protease responsible for the cleavage of the anti-sigma factor periplasmic domain; however, there is a second putative periplasmic protease containing two PDZ domains (XF0285) encoded in the X. fastidiosa 9a5c genome. Although both genes are induced by heat shock, XF0285 is not regulated by σE (Fig. 3B), but instead, its promoter presents a putative σ32 consensus sequence (31). A gene encoding an orthologue of E. coli yaeL is also present in the genome (XF1047), and therefore, we can predict that the liberation of σE from the anti-sigma factor is probably achieved by two-step proteolysis, as described for other bacteria.
A regulatory network similar to the one presented in this work was also demonstrated for the iron starvation ECF sigma factor HasI of Serratia marcescens, which is not autoregulated but exerted only partial autoregulation on the anti-sigma factor gene (8). The authors propose that ECF sigma factors that regulate multiple promoters may be required in increased amounts, while those that are limited to regulating a few promoters would not be needed. However, X. fastidiosa rpoE regulates a fair number of genes, and yet its transcription is diminished when σE is activated (Fig. 2C and 6B), whereas rseA transcription is greatly increased. A hypothesis to explain this difference in regulation could be higher stability of X. fastidiosa σE, which could substitute for the need for increased transcription by an autoregulatory feedback, such as those of other bacteria. The increase in RseA levels that results from its σE-dependent transcription could restore free σE to background levels, shutting down the response. An alternative regulatory mechanism reported for E. coli FecI/FecR factors has shown that the N-terminal fragment of the antisigma factor FecR generated by proteolytic cleavage is required for FecI sigma activity, either by changing the FecI conformation or by preventing its degradation (10). We cannot rule out the possibility that this occurs in the X. fastidiosa RpoE/RseA system as an alternative hypothesis to explain the role of RpoE-mediated induction of rseA.
Acknowledgments
This work was supported by a grant from Fundação de Amparo à Pesquisa do Estado de São Paulo. During the course of this work, J.F.S.N. and T.K. were supported by predoctoral fellowships from FAPESP. M.V.M. and S.L.G. are partly supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Footnotes
Published ahead of print on 10 November 2006.
REFERENCES
- 1.Ades, S. E. 2004. Control of the alternative sigma factor σE in Escherichia coli. Curr. Opin. Microbiol. 7:157-162. [DOI] [PubMed] [Google Scholar]
- 2.Arsene, F., T. Tomoyasu, and B. Bukau. 2000. The heat shock response of Escherichia coli. Int. J. Food Microbiol. 55:3-9. [DOI] [PubMed] [Google Scholar]
- 3.Ball, C. A., A. Brazma, H. Causton, S. Chervitz, R. Edgar, P. Hingamp, J. C. Matese, H. Parkinson, J. Quackenbush, M. Ringwald, S. A. Sansone, G. Sherlock, P. Spellman, C. Stoeckert, Y. Tateno, R. Taylor, J. White, and N. Winegarden. 2004. Submission of microarray data to public repositories. PLoS Biol. 2:e317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bashyam, M. D., and S. E. Hasnain. 2004. The extracytoplasmic function sigma factors: role in bacterial pathogenesis. Infect. Genet. Evol. 4:301-308. [DOI] [PubMed] [Google Scholar]
- 5.Baulard, A., L. Kremer, and C. Locht. 1996. Efficient homologous recombination in fast-growing and slow-growing mycobacteria. J. Bacteriol. 178:3091-3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benachour, A., C. Muller, M. Dabrowski-Coton, Y. Le Breton, J. C. Giard, A. Rince, Y. Auffray, and A. Hartke. 2005. The Enterococcus faecalis SigV protein is an extracytoplasmic function sigma factor contributing to survival following heat, acid, and ethanol treatments. J. Bacteriol. 187:1022-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhattacharyya, A., S. Stilwagen, G. Reznik, H. Feil, W. S. Feil, I. Anderson, A. Bernal, M. D'Souza, N. Ivanova, V. Kapatral, N. Larsen, T. Los, A. Lykidis, E. Selkov, Jr., T. L. Walunas, A. Purcell, R. A. Edwards, T. Hawkins, R. Haselkorn, R. Overbeek, N. C. Kyrpides, and P. F. Predki. 2002. Draft sequencing and comparative genomics of Xylella fastidiosa strains reveal novel biological insights. Genome Res. 12:1556-1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biville, F., H. Cwerman, S. Letoffe, M. S. Rossi, V. Drouet, J. M. Ghigo, and C. Wandersman. 2004. Haemophore-mediated signalling in Serratia marcescens: a new mode of regulation for an extra cytoplasmic function (ECF) sigma factor involved in haem acquisition. Mol. Microbiol. 53:1267-1277. [DOI] [PubMed] [Google Scholar]
- 9.Boucher, J. C., J. Martinez-Salazar, M. J. Schurr, M. H. Mudd, H. Yu, and V. Deretic. 1996. Two distinct loci affecting conversion to mucoidy in Pseudomonas aeruginosa in cystic fibrosis encode homologs of the serine protease HtrA. J. Bacteriol. 178:511-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braun, V., S. Mahren, and M. Ogierman. 2003. Regulation of the FecI-type ECF sigma factor by transmembrane signalling. Curr. Opin. Microbiol. 6:173-180. [DOI] [PubMed] [Google Scholar]
- 10a.Burgess, R. R., and M. W. Knuth. 1996. Purification of a recombinant protein overproduced in Escherichia coli, p. 205-274. In D. R. Marshak, J. T. Kadonaga, R. R. Burgess, M. Knuth, W. A. Brennan, and S.-H. Lin (ed.), Strategies for protein purification and characterization: a laboratory course manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 11.Dartigalongue, C., D. Missiakas, and S. Raina. 2001. Characterization of the Escherichia coli σE regulon. J. Biol. Chem. 276:20866-20875. [DOI] [PubMed] [Google Scholar]
- 12.da Silva Neto, J. F., T. Koide, S. L. Gomes, and M. V. Marques. 2002. Site-directed gene disruption in Xylella fastidiosa. FEMS Microbiol. Lett. 210:105-110. [DOI] [PubMed] [Google Scholar]
- 13.Davis, M. J., W. J. French, and N. W. Schaad. 1981. Axenic culture of the bacteria associated with phony peach disease of peach and plum leaf scald. Curr. Microbiol. 6:309-314. [Google Scholar]
- 14.de Las Peñas, A. L., L. Connoly, and C. A. Gross. 1997. σE is an essential sigma factor in Escherichia coli. J. Bacteriol. 179:6862-6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Souza, A. A., M. A. Takita, H. D. Coletta-Filho, C. Caldana, G. H. Goldman, G. M. Yanai, N. H. Muto, R. C. de Oliveira, L. R. Nunes, and M. A. Machado. 2003. Analysis of gene expression in two growth states of Xylella fastidiosa and its relationship with pathogenicity. Mol. Plant-Microbe Interact. 16:867-875. [DOI] [PubMed] [Google Scholar]
- 16.de Souza, A. A., M. A. Takita, H. D. Coletta-Filho, C. Caldana, G. M. Yanai, N. H. Muto, R. C. de Oliveira, L. R. Nunes, and M. A. Machado. 2004. Gene expression profile of the plant pathogen Xylella fastidiosa during biofilm formation in vitro. FEMS Microbiol. Lett. 237:341-353. [DOI] [PubMed] [Google Scholar]
- 17.Duret, S., J. L. Danet, M. Garnier, and J. Renaudin. 1999. Gene disruption through homologous recombination in Spiroplasma citri: an scm1-disrupted motility mutant is pathogenic. J. Bacteriol. 181:7449-7456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erickson, J. W., and C. A. Gross. 1989. Identification of the σE subunit of Escherichia coli RNA polymerase: a second alternate sigma factor involved in high-temperature gene expression. Genes Dev. 3:1462-1471. [DOI] [PubMed] [Google Scholar]
- 19.Feil, H., W. S. Feil, J. C. Detter, A. H. Purcell, and S. E. Lindow. 2003. Site-directed disruption of the fimA and fimF fimbrial genes of Xylella fastidiosa. Phytopathology 93:675-682. [DOI] [PubMed] [Google Scholar]
- 20.Firoved, A. M., J. C. Boucher, and V. Deretic. 2002. Global genomic analysis of AlgU (σE)-dependent promoters (sigmulon) in Pseudomonas aeruginosa and implications for inflammatory processes in cystic fibrosis. J. Bacteriol. 184:1057-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Firoved, A. M., and V. Deretic. 2003. Microarray analysis of global gene expression in mucoid Pseudomonas aeruginosa. J. Bacteriol. 185:1071-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gardy, J. L., M. R. Laird, F. Chen, S. Rey, C. J. Walsh, M. Ester, and F. S. L. Brinkman. 2005. PSORTb v. 2.0: expanded prediction of bacterial protein subcellular localization and insights gained from comparative proteome analysis. Bioinformatics 21:617-623. [DOI] [PubMed] [Google Scholar]
- 23.Gaurivaud, P., L. C. Souza, A. C. Virgilio, A. G. Mariano, R. R. Palma, and P. B. Monteiro. 2002. Gene disruption by homologous recombination in the Xylella fastidiosa citrus variegated chlorosis strain. Appl. Environ. Microbiol. 68:4658-4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guilhabert, M. R., and B. C. Kirkpatrick. 2005. Identification of Xylella fastidiosa antivirulence genes: hemagglutinin adhesins contribute to X. fastidiosa biofilm maturation and colonization and attenuate virulence. Mol. Plant-Microbe Interact. 18:856-868. [DOI] [PubMed] [Google Scholar]
- 25.Hamilton, H. L., K. J. Schwartz, and J. P. Dillard. 2001. Insertion-duplication mutagenesis of Neisseria: use in characterization of DNA transfer genes in the gonococcal genetic island. J. Bacteriol. 183:4718-4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Helmann, J. D. 2002. The extracytoplasmic function (ECF) sigma factors. Adv. Microb. Physiol. 46:47-110. [DOI] [PubMed] [Google Scholar]
- 27.Hiratsu, K., M. Amemura, H. Nashimoto, H. Shinagawa, and K. Makino. 1995. The rpoE gene of Escherichia coli, which encodes σE, is essential for bacterial growth at high temperature. J. Bacteriol. 177:2918-2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keith, L. M., and C. L. Bender. 1999. AlgT (σ22) controls alginate production and tolerance to environmental stress in Pseudomonas syringae. J. Bacteriol. 181:7176-7184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koide, T., J. F. da Silva Neto, S. L. Gomes, and M. V. Marques. 2004. Insertional transposon mutagenesis in the Xylella fastidiosa citrus variegated chlorosis strain with transposome. Curr. Microbiol. 48:247-250. [DOI] [PubMed] [Google Scholar]
- 30.Koide, T., P. A. Zaini, L. M. Moreira, R. Z. Vencio, A. Y. Matsukuma, A. M. Durham, D. C. Teixeira, H. El-Dorry, P. B. Monteiro, A. C. da Silva, S. Verjovski-Almeida, A. M. da Silva, and S. L. Gomes. 2004. DNA microarray-based genome comparison of a pathogenic and a nonpathogenic strain of Xylella fastidiosa delineates genes important for bacterial virulence. J. Bacteriol. 186:5442-5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koide, T., R. Z. N. Vencio, and S. L. Gomes. 2006. Global gene expression analysis of the heat shock response in the phytopathogen Xylella fastidiosa. J. Bacteriol. 188:5821-5830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lang, E. A., and M. V. Marques. 2004. Identification and transcriptional control of Caulobacter crescentus genes encoding proteins containing a cold shock domain. J. Bacteriol. 186:5603-5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee, M. S., C. Seok, and D. A. Morrison. 1998. Insertion-duplication mutagenesis in Streptococcus pneumoniae: targeting fragment length is a critical parameter in use as a random insertion tool. Appl. Environ. Microbiol. 64:4796-4802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leloup, L., S. D. Ehrlich, M. Zagorec, and F. Morel-Deville. 1997. Single-crossover integration in the Lactobacillus sake chromosome and insertional inactivation of the ptsI and lacL genes. Appl. Environ. Microbiol. 63:2117-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lipinska, B., S. Sharma, and C. Georgopoulos. 1988. Sequence analysis and regulation of the htrA gene of Escherichia coli: a sigma 32-independent mechanism of heat-inducible transcription. Nucleic Acids Res. 16:10053-10067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402-408. [DOI] [PubMed] [Google Scholar]
- 37.Lonetto, M. A., K. L. Brown, K. E. Rudd, and M. J. Buttner. 1994. Analysis of the Streptomyces coelicolor sigE gene reveals the existence of a subfamily of eubacterial RNA polymerase sigma factors involved in the regulation of extracytoplasmic functions. Proc. Natl. Acad. Sci. USA 91:7573-7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manganelli, R., M. I. Voskuil, G. K. Schoolnik, and I. Smith. 2001. The Mycobacterium tuberculosis ECF sigma factor σE: role in global gene expression and survival in macrophages. Mol. Microbiol. 41:423-437. [DOI] [PubMed] [Google Scholar]
- 39.Manganelli, R., M. I. Voskuil, G. K. Schoolnik, E. Dubnau, M. Gomez, and I. Smith. 2002. Role of the extracytoplasmic-function sigma factor σH in Mycobacterium tuberculosis global gene expression. Mol. Microbiol. 45:365-374. [DOI] [PubMed] [Google Scholar]
- 40.Reference deleted.
- 41.Martin, D. W., M. J. Schurr, H. Yu, and V. Deretic. 1994. Analysis of promoters controlled by the putative sigma factor AlgU regulating conversion to mucoidy in Pseudomonas aeruginosa: relationship to σE and stress response. J. Bacteriol. 176:6688-6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meng, Y., Y. Li, C. D. Galvani, G. Hao, J. N. Turner, T. J. Burr, and H. C. Hoch. 2005. Upstream migration of Xylella fastidiosa via pilus-driven twitching motility. J. Bacteriol. 187:5560-5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Missiakas, D., M. P. Mayer, M. Lemaire, C. Georgopoulos, and S. Raina. 1997. Modulation of the Escherichia coli σE (RpoE) heat-shock transcription-factor activity by the RseA, RseB and RseC proteins. Mol. Microbiol. 24:355-371. [DOI] [PubMed] [Google Scholar]
- 44.Miticka, H., G. Rowley, B. Rezuchova, D. Homerova, S. Humphreys, J. Farn, M. Roberts, and J. Kormanec. 2003. Transcriptional analysis of the rpoE gene encoding extracytoplasmic stress response sigma factor σE in Salmonella enterica serovar Typhimurium. FEMS Microbiol. Lett. 226:307-314. [DOI] [PubMed] [Google Scholar]
- 45.Monteiro, P. B., D. C. Teixeira, R. R. Palma, M. Garnier, J.-M. Bové, and J. Renaudin. 2001. Stable transformation of the Xylella fastidiosa citrus variegated chlorosis strain with oriC plasmids. Appl. Environ. Microbiol. 67:2263-2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Newman, K. L., R. P. Almeida, A. H. Purcell, and S. E. Lindow. 2004. Cell-cell signaling controls Xylella fastidiosa interactions with both insects and plants. Proc. Natl. Acad. Sci. USA 101:1737-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nunes, L. R., Y. B. Rosato, N. H. Muto, G. M. Yanai, V. S. da Silva, D. B. Leite, E. R. Goncalves, A. A. de Souza, H. D. Coletta-Filho, M. A. Machado, S. A. Lopes, and R. C. de Oliveira. 2003. Microarray analyses of Xylella fastidiosa provide evidence of coordinated transcription control of laterally transferred elements. Genome Res. 13:570-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pallen, M. J., and B. W. Wren. 1997. The HtrA family of serine proteases. Mol. Microbiol. 26:209-221. [DOI] [PubMed] [Google Scholar]
- 49.Purcell, A. H., and D. L. Hopkins. 1996. Fastidious xylem-limited bacterial plant pathogens. Annu. Rev. Phytopathol. 34:131-151. [DOI] [PubMed] [Google Scholar]
- 50.Raina, S., D. Missiakas, and C. Georgopoulous. 1995. The rpoE gene encoding the sigma E (sigma 24) heat shock sigma factor of Escherichia coli. EMBO J. 14:1043-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rezuchova, B., H. Miticka, D. Homerova, M. Roberts, and J. Kormanec. 2003. New members of the Escherichia coli σE regulon identified by a two-plasmid system. FEMS Microbiol. Lett. 225:1-7. [DOI] [PubMed] [Google Scholar]
- 52.Rhodius, V. A., W. C. Suh, G. Nonaka, J. West, and C. A. Gross. 2006. Conserved and variable functions of the σE stress response in related genomes. PLoS Biol. 4:e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rouviere, P. E., A. De Las Penas, J. Mecsas, C. Z. Lu, K. E. Rudd, and C. A. Gross. 1995. rpoE, the gene encoding the second heat-shock sigma factor, sigma E, in Escherichia coli. EMBO J. 14:1032-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schurr, M. J., H. Yu, J. M. Martinez-Salazar, J. C. Boucher, and V. Deretic. 1996. Control of AlgU, a member of the σE-like family of stress sigma factors, by the negative regulators MucA and MucB and Pseudomonas aeruginosa conversion to mucoidy in cystic fibrosis. J. Bacteriol. 178:4997-5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Simpson, A. J., F. C. Reinach, P. Arruda, F. A. Abreu, M. Acencio, R. Alvarenga, L. M. Alves, J. E. Araya, G. S. Baia, C. S. Baptista, M. H. Barros, E. D. Bonaccorsi, S. Bordin, J. M. Bove, M. R. Briones, M. R. Bueno, A. A. Camargo, L. E. Camargo, D. M. Carraro, H. Carrer, N. B. Colauto, C. Colombo, F. F. Costa, M. C. Costa, C. M. Costa-Neto, L. L. Coutinho, M. Cristofani, E. Dias-Neto, C. Docena, H. El-Dorry, A. P. Facincani, A. J. Ferreira, V. C. Ferreira, J. A. Ferro, J. S. Fraga, S. C. Franca, M. C. Franco, M. Frohme, L. R. Furlan, M. Garnier, G. H. Goldman, M. H. Goldman, S. L. Gomes, A. Gruber, P. L. Ho, J. D. Hoheisel, M. L. Junqueira, E. L. Kemper, J. P. Kitajima, J. E. Krieger, E. E. Kuramae, F. Laigret, M. R. Lambais, L. C. Leite, E. G. Lemos, M. V. Lemos, S. A. Lopes, C. R. Lopes, J. A. Machado, M. A. Machado, A. M. Madeira, H. M. Madeira, C. L. Marino, M. V. Marques, E. A. Martins, E. M. Martins, A. Y. Matsukuma, C. F. Menck, E. C. Miracca, C. Y. Miyaki, C. B. Monteriro-Vitorello, D. H. Moon, M. A. Nagai, A. L. Nascimento, L. E. Netto, A. Nhani, Jr., F. G. Nobrega, L. R. Nunes, M. A. Oliveira, M. C. de Oliveira, R. C. de Oliveira, D. A. Palmieri, A. Paris, B. R. Peixoto, G. A. Pereira, H. A. Pereira, Jr., J. B. Pesquero, R. B. Quaggio, P. G. Roberto, V. Rodrigues, A. J. de M. Rosa, V. E. de Rosa, Jr., R. G. de Sa, R. V. Santelli, H. E. Sawasaki, A. C. da Silva, A. M. da Silva, F. R. da Silva, W. A. da Silva, Jr., J. F. da Silveira, et al. 2000. The genome sequence of the plant pathogen Xylella fastidiosa. Nature 406:151-157. [DOI] [PubMed] [Google Scholar]
- 56.Souza, L. C., N. A. Wulff, P. Gaurivaud, A. G. Mariano, A. C. Virgilio, J. L. Azevedo, and P. B. Monteiro. 2006. Disruption of Xylella fastidiosa CVC gumB and gumF genes affects biofilm formation without a detectable influence on exopolysaccharide production. FEMS Microbiol. Lett. 257:236-242. [DOI] [PubMed] [Google Scholar]
- 57.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van Sluys, M. A., M. C. de Oliveira, C. B. Monteiro-Vitorello, C. Y. Miyaki, L. R. Furlan, L. E. Camargo, A. C. da Silva, D. H. Moon, M. A. Takita, E. G. Lemos, M. A. Machado, M. I. Ferro, F. R. da Silva, M. H. Goldman, G. H. Goldman, M. V. Lemos, H. El-Dorry, S. M. Tsai, H. Carrer, D. M. Carraro, R. C. de Oliveira, L. R. Nunes, W. J. Siqueira, L. L. Coutinho, E. T. Kimura, E. S. Ferro, R. Harakava, E. E. Kuramae, C. L. Marino, E. Giglioti, I. L. Abreu, L. M. Alves, A. M. do Amaral, G. S. Baia, S. R. Blanco, M. S. Brito, F. S. Cannavan, A. V. Celestino, A. F. da Cunha, R. C. Fenille, J. A. Ferro, E. F. Formighieri, L. T. Kishi, S. G. Leoni, A. R. Oliveira, V. E. Rosa, Jr., F. T. Sassaki, J. A. Sena, A. A. de Souza, D. Truffi, F. Tsukumo, G. M. Yanai, L. G. Zaros, E. L. Civerolo, A. J. Simpson, N. F. Almeida, Jr., J. C. Setubal, and J. P. Kitajima. 2003. Comparative analyses of the complete genome sequences of Pierce's disease and citrus variegated chlorosis strains of Xylella fastidiosa. J. Bacteriol. 185:1018-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vencio, R. Z., and T. Koide. 2005. HTself: self-self based statistical test for low replication microarray studies. DNA Res. 12:211-214. [DOI] [PubMed] [Google Scholar]
- 60.Wells, J. M., B. C. Raju, H. Y. Hung, W. G. Weisburg, L. Mandelco-Paul, and D. J. Brenner. 1987. Xylella fastidiosa gen. nov., sp. nov.: gram-negative, xylem-limited, fastidious plant bacteria related to Xanthomonas spp. Int. J. Syst. Bacteriol. 37:136-143. [Google Scholar]
- 61.Wosten, M. M. 1998. Eubacterial sigma-factors. FEMS Microbiol. Rev. 22:127-150. [DOI] [PubMed] [Google Scholar]
- 62.Yang, Y. H., S. Dudoit, P. Luu, D. M. Lin, V. Peng, J. Ngai, and T. P. Speed. 2002. Normalization for cDNA microarray data: a robust composite method addressing single and multiple slide systematic variation. Nucleic Acids Res. 30:e15. [DOI] [PMC free article] [PubMed] [Google Scholar]