Abstract
Previously, we demonstrated that IL-8 induces rapid mobilization of hematopoietic progenitor cells (HPC) from the bone marrow of rhesus monkeys. Because activation of neutrophils by IL-8 induces the release of gelatinase B (MMP-9), which is involved in the degradation of extracellular matrix molecules, we hypothesized that MMP-9 release might induce stem cell mobilization by cleaving matrix molecules to which stem cells are attached. Rhesus monkeys were treated with a single i.v. injection of 0.1 mg/kg human IL-8, which resulted in a 10- to 100-fold increase in HPC within 30 min after injection. Zymographic analysis revealed a dramatic instantaneous increase in the plasma levels of MMP-9, followed by the increase in circulating HPC. Enzyme levels decreased at 2 h after injection of IL-8, simultaneously with the decrease in the numbers of circulating HPC. To test the hypothesis that MMP-9 induction was involved in HPC mobilization, rhesus monkeys were treated with a highly specific inhibitory monoclonal anti-gelatinase B antibody. Anti-gelatinase B at a dose of 1–2 mg/kg completely prevented the IL-8-induced mobilization of HPC, whereas a dose of 0.1 mg/kg had only a limited effect. Preinjection of inhibitory antibodies did not preclude the IL-8-induced production and secretion of MMP-9. Pretreatment with an irrelevant control antibody did not affect IL-8-induced mobilization, showing that the inhibition by the anti-gelatinase B antibody was specific. In summary, IL-8 induces the rapid systemic release of MMP-9 with concurrent mobilization of HPC that is prevented by pretreatment with an inhibitory anti-gelatinase B antibody, indicating that MMP-9 is involved as a mediator of the IL-8-induced mobilization of HPC.
In steady-state hematopoiesis, the retention of hematopoietic progenitor cells (HPC) is finely regulated by the bone marrow microenvironment. In addition to the cellular elements of the bone marrow stroma, adhesion to extracellular matrix (ECM) molecules has been implicated to play an important role (1–8). The mechanisms responsible for cytokine-induced mobilization of HPC are largely unknown. A prominent role has been ascribed to integrins, because these adhesion molecules are involved in the cellular interactions between HPC, stromal cells, and components of the ECM (9, 10) and because they are differentially expressed during the maturation of HPC (11, 12). Furthermore, antibodies directed to the β1-integrin very late antigen (VLA)-4 (CD29/CD49d) were able to induce the peripheralization of hematopoietic progenitors in primates and mice, indicating an important role for the VLA-4/fibronectin and/or VLA-4/vascular cell adhesion molecule (VCAM)-1 pathways in retaining HPC in the bone marrow in vivo (13, 14). We have recently demonstrated that anti-leukocyte function-associated antigen (LFA)-1 antibodies completely prevent IL-8-induced stem cell mobilization, demonstrating the major role for the β2-integrin LFA-1 (CD18/CD11a) in cytokine-induced HPC mobilization (15).
A specific class of proteolytic enzymes, the matrix metalloproteinases (MMPs), are important in degrading components of extracellular matrix molecules (16). Three types of soluble MMPs have been described: collagenases, gelatinases, and stromelysins (16). Gelatinase B (MMP-9) is produced mainly by mature neutrophils, monocytes, macrophages, and several tumor cell lines (17–19). Neutrophil MMP-9 is stored in gelatinase granules (20) and degrades denatured collagen types (gelatins) (18), which are all components of the ECM (8, 16). It is a major factor for neutrophil migration across basement membrane (21). The activity of MMPs appears to depend on a balance between the production and secretion of latent enzyme, activation of latent enzyme, and production of the naturally occurring inhibitors, the tissue inhibitors of metalloproteinases (TIMPs) (22). The mechanisms concerning the regulation of matrix enzyme activity contributing to leukocytosis and cellular influx at inflammatory sites were recently reviewed (23).
We have previously demonstrated that IL-8 induces the rapid (15- to 30-min) mobilization of HPC from the bone marrow of rhesus monkeys (24). Because activation of neutrophils by IL-8 induces the immediate release of MMP-9 (17), we hypothesized that MMP release would induce stem cell mobilization by cleaving matrix molecules to which HPC attach (1, 4, 6–8). We therefore studied the kinetics of MMP-9 induction after a bolus injection of IL-8 in a primate in relation to the rapid induction of HPC mobilization. Zymographic analysis revealed a dramatic increase in the plasma levels of MMP-9 concomitant with the increase in circulating HPC. Enzyme levels decreased after 2 h, simultaneously with the decline of circulating HPC. Moreover, a similar pattern of MMP-9 plasma levels and circulating numbers of HPC was observed after single and repeated IL-8 injections. In addition, injection of inhibitory monoclonal antibodies against gelatinase B completely inhibited the IL-8-induced mobilization, whereas injection of an irrelevant control antibody had no effect. These results indicate an important role for MMP-9 as a mediator of the IL-8-induced mobilization of HPC.
MATERIALS AND METHODS
Animals.
Rhesus monkeys (Macaca mulatta) from the Biomedical Primate Research Center (Rijswijk, The Netherlands) were used throughout this study. The age ranged from 2 to 4 years, the body weight from 2.5 to 4.5 kg. All animals were provided with food and water ad libitum throughout the study. The animals were free of intestinal parasites, herpes B, simian T cell leukemia virus (STLV), and simian immunodeficiency virus (SIV). The experimental protocol was approved by the institutional ethical committee on animal research.
IL-8.
Recombinant human IL-8 was purified from Escherichia coli expressing a synthetic gene (25) and provided by the Novartis Forschungsinstitut. IL-8 has no colony-stimulating activity, as reported previously (26). The concentration of endotoxin was less than 0.05 unit/mg as determined by the Limulus amebocyte lysate assay. For in vivo experiments, IL-8 was diluted to the desired concentration in endotoxin-free phosphate-buffered saline (PBS) with 0.1% bovine serum albumin (BSA).
Monoclonal Antibodies.
Affinity-matured murine monoclonal anti-human gelatinase B antibodies (REGA-3G12, IgG1) were purified from ascites fluid (27, 28). In immunoprecipitation experiments the antibody specifically recognized activated human and monkey MMP-9 and did not react with activated MMP-2, pro-MMP-2, or pro-MMP-9. The concentration of endotoxin was ≤3.7 units/mg of protein. In a control experiment, murine anti-human anti-CD5 was used (6.12.20.1, IgG2a), developed for in vivo treatment of humans and containing 0.07 endotoxin unit per mg of protein. Murine anti-human CD34 antibody (566, IgG1), which reacts with rhesus monkey CD34, was kindly provided by T. Egeland (University of Oslo, Norway).
Preparation of Cell Suspensions.
Blood samples were taken by venous puncture in heparin-containing tubes at various intervals, and total blood cell counts were performed on a Sysmex F1000 (Toa Medical Electronics, Kobe, Japan). Manual differential counts were performed on May–Grünwald–Giemsa-stained blood films. Plasma was separated from the blood cells by centrifugation, and the cell pellet was resuspended in an equal volume of RPMI medium 1640 containing 2% (vol/vol) fetal bovine serum (FBS; GIBCO), 500 μg/ml penicillin, and 250 μg/ml streptomycin. Erythrocytes were lysed by incubation with NH4Cl/buffer for 10 min at 0°C. Leukocytes were washed twice with the washing buffer. Cell numbers were counted and diluted to the desired concentration in Iscove’s modified Dulbecco’s medium (IMDM).
Determination of the Number of CD34+ Cells by Flow Cytometry.
After erythrocyte lysis, peripheral blood leukocytes were incubated with FITC-conjugated anti-CD34 antibodies for 30 min at 4°C in the presence of 2% (vol/vol) normal rhesus monkey serum to prevent nonspecific antibody binding. Then the cells were washed twice and resuspended in saline containing 2% fetal bovine serum. Single-color analysis was performed with a FACScan flow cytometer at 488 nm (Becton Dickinson). Data for 50,000–100,000 cells were collected in an appropriate gate with intermediate-to-high forward light scatter and low-to-intermediate right angle light scatter to exclude dead cells and mature granulocytes from the analysis. The number of CD34+ cells was measured and is shown as percentage of the total number of appropriate gated cells.
Mixed Colony-Forming Unit (CFU) Cultures.
Cells were cultured in 3.5-cm2 dishes in semisolid medium. One milliliter of IMDM contained 10 ng of recombinant human (rhu) granulocyte/macrophage colony-stimulating factor (GM-CSF), 10 ng of rhu granulocyte (G)-CSF, 10 ng of rhu stem cell factor, 2 units of rhu erythropoietin, 50 ng of rhuIL-3, 10 μM 2-mercaptoethanol, 500 ng of transferrin, 1.1% methylcellulose, and 20% (vol/vol) human plasma. Blood cell samples were plated in duplicate at concentrations of 1 × 105 and 5 × 105 cells per ml. After 7–8 days of culture in a fully humidified atmosphere at 37°C containing 5% CO2, the numbers of CFU, defined as aggregates of >20 cells, were scored by using an inverted microscope.
Detection of Gelatinase Activity.
Gelatinase B/MMP-9 activity in freshly frozen plasma samples was determined by SDS/PAGE zymography as described earlier (19). Briefly, samples were separated, without prior denaturation, on 7.5% (mass/vol) polyacrylamide gels to which 0.1% bovine gelatin (Sigma) was added and copolymerized. Stacking gels were 5% (mass/vol) polyacrylamide and did not contain gelatin substrate. Electrophoresis was performed at 4°C for approximately 16 h at 85 V. After electrophoresis, gels were washed to remove SDS, then incubated for development of enzyme activity, stained with Coomassie brilliant blue R-250, and destained in methanol/acetic acid. Gelatinase activity was detected as unstained bands on a blue background. Quantitative determination of gelatinase activity was by computerized, two-dimensional scanning densitometry (The Imager; Appligene, Pleasanton, CA). Gelatinase activity was expressed in “scanning units,” representing the scanning area under the curves, which is an integration ratio that takes into account both the brightness and width of the substrate lysis zone (17). Since gelatinase A/MMP-2 is constitutively released by most cell types and is also present in plasma, the gelatinase A/MMP-2 activity was used as a control for sample processing.
Experimental Design. Monkeys were placed in a chair-like device and blood samples were drawn from a calf vein (t = 0 sample). By the same route, IL-8 (0.1 mg/kg) was administered as a time-controlled (30 sec) bolus injection. Venous blood samples for total blood cell counts, differential cell counts, gelatinase zymography, and HPC assays were obtained at various time intervals after the injection of IL-8 at a contralateral vein (1, 5, 15, 30, 45, 60, and 120 min). The same procedure was repeated after a second injection of IL-8, which was given at several time intervals (72, 24, and 4 h) after the first injection.
In gelatinase B blocking experiments, all animals were first injected with a bolus of 0.1 mg/kg IL-8. Blood samples were then taken at 0, 1, 5, 15, 30, 45, and 60 min to ascertain that they were able to mobilize HPC in response to IL-8 and to assess MMP-9 induction. Five or six days later, the inhibitory anti-gelatinase B antibody (0.1, 1, or 2 mg/kg) was administered as a venous bolus injection in the calf vein. Two hours later, after a blood sample was taken, IL-8 (0.1 mg/kg) was administered i.v. Total blood cell counts, differential cell counts, gelatinase B assays, and HPC assays were performed at fixed time intervals: before administration of the blocking antibody, after 2 h, before the injection of IL-8, and 15, 30, 45, 60, and 120 min after IL-8 injection.
RESULTS
Effect of IL-8 on Gelatinase B Levels in the Circulation.
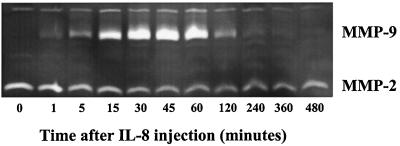
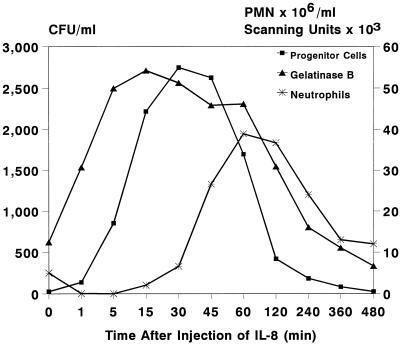
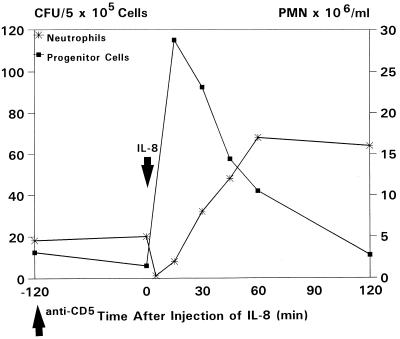
To study the IL-8-induced release of gelatinase B/MMP-9 into the circulation, levels of MMP-9 were measured in plasma samples taken at various time intervals after IL-8 injection. Zymographic analysis revealed a dramatic instantaneous increase in circulating levels of MMP-9, concomitant with the increase in circulating HPC. Enzyme levels decreased at 2 h after injection of IL-8, simultaneously with the decline in the numbers of circulating HPC (Figs. 1 and 2). Figs. 1 and 2 show the results of a single, representative, experiment; similar results were seen in all animals treated with a single injection of 100 μg/kg IL-8 (n = 10). A standard curve was constructed by using serial 3-fold dilutions of purified human neutrophil-derived MMP-9 (17). These were assayed with plasma samples on the same gel. Assuming that specific activities of monkey and human MMP-9 are equal, calculated maximum levels of circulating MMP-9 at 15–45 min after IL-8 injection are 5.5 to 6.5 μg/ml (Fig. 3).
Figure 1.
Effect of a single injection of IL-8 (0.1 mg/kg) on the induction of circulating gelatinase B (MMP-9). The zymolytic activity migrating at lower molecular mass represents circulating levels of gelatinase A/MMP-2, which is constitutively present in plasma. The zymolytic activity with a higher molecular mass represents circulating levels of gelatinase B/MMP-9 in plasma at various time intervals after IL-8 injection. Activity of gelatinase in serum samples was detected by SDS/PAGE zymography.
Figure 2.
Effect of a single injection of IL-8 (0.1 mg/kg) on numbers of circulating HPC (CFU/ml), neutrophils (PMN × 106/ml), and plasma levels of gelatinase B (scanning units). A representative experiment out of 10 is shown.
Figure 3.
MMP-9 levels were estimated quantitatively by computerized two-dimensional scanning densitometry using a standard curve. Lanes 1–5 represent a dilution of purified human MMP-9 (100, 50, 15, 5, and 1.5 ng respectively). Lanes 6–8 represent 3-μl plasma samples taken at 15, 30, and 45 min after a single bolus injection of 0.1 mg/kg IL-8.
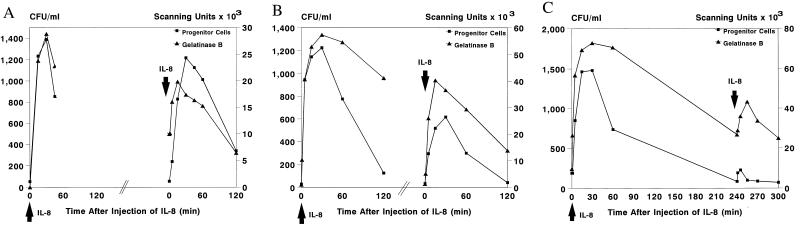
Effect of Repeated Injections of IL-8 on HPC Mobilization.
Previously (24), we observed that repeated injections of IL-8 with an interval of 72 h induced identical increments of HPC (Fig. 4A). To study the possibility of tachyphylaxis, we injected IL-8 a second time after shorter time intervals of 24 and 4 h. After 24 h, HPC mobilization was observed, although this was only 50% of the cell numbers observed after the first injection (Fig. 4B). IL-8-induced neutropenia was not affected (data not shown). With a second injection of IL-8 after 4 h, no HPC mobilization was observed (Fig. 4C), although an instant but moderate neutropenia was seen (data not shown).
Figure 4.
Effect of a second bolus injection of IL-8 (0.1 mg/kg) on mobilization of HPC (CFU/ml) and on plasma levels of gelatinase B (scanning units). The second bolus injection of IL-8 was administered with an interval of 72 h (A; n = 7), 24 h (B; n = 1), or 4 h (C; n = 1).
Effect of Repeated Injections of IL-8 on Plasma Levels of Gelatinase B.
Since after a single injection of IL-8, a dramatic increase in plasma levels of MMP-9 was seen, concomitant with HPC mobilization, we studied MMP-9 induction after repeated injections of IL-8. Administration of IL-8 at 72 or 24 h after the first injection induced a rapid second, though not equivalent, increase in plasma levels of MMP-9, concomitant with HPC mobilization (Fig. 4 A and B). Repeated administration of IL-8 after 4 h resulted in only a moderate increase in MMP-9, while no HPC mobilization was seen at the same time (Fig. 4C). These results suggested a relationship between the induction of MMP-9 and the mobilization of HPC by IL-8.
Effect of Inhibitory Monoclonal Antibody Against Gelatinase B on IL-8-Induced Mobilization of HPC.
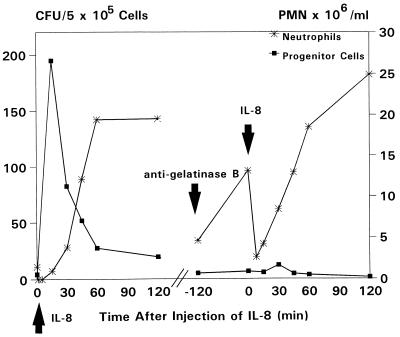
To study the role of MMP-9 in IL-8-induced HPC mobilization, we treated four rhesus monkeys with inhibitory monoclonal antibodies against gelatinase B, 2 h before the injection of 0.1 mg/kg IL-8. To demonstrate that HPC mobilization could be induced, all animals were treated first with a bolus injection of 0.1 mg/kg IL-8. Administration of the inhibitory anti-gelatinase B antibody followed 5 or 6 days later. One monkey received 0.1 mg/kg [animal A (9305)], two animals 1 mg/kg [animals B (9304) and C (KB9)], and one 2 mg/kg anti-gelatinase B antibodies [animal D (94018)]. After administration of 0.1 mg/kg anti-gelatinase B antibodies, partial inhibition of the increase in the number of circulating HPC was seen (an IL-8-induced increase from 2 CFU per 5 × 105 plated cells at baseline to 86 CFU per 5 × 105 plated cells and from 1 CFU to 24 CFU per 5 × 105 plated cells after pretreatment with anti-gelatinase B antibodies). Subsequently, the dose of antibody was increased to 1 mg/kg. In the first animal treated (animal B), a complete inhibition of the IL-8-induced HPC mobilization was observed (an IL-8-induced increase from 5 CFU at baseline to 195 CFU per 5 × 105 plated cells and from 7 CFU to 12 CFU per 5 × 105 plated cells after pretreatment with the inhibitory antibody) (Fig. 5). In a second animal (animal C) however, no inhibition of IL-8-induced HPC mobilization was seen (an IL-8-induced increase from 7 CFU to 101 CFU per 5 × 105 plated cells and from 6 CFU to 72 CFU per 5 × 105 plated cells after pretreatment with the inhibitory antibody). We have considered the possibility that the lack of response in one of the two monkeys treated with a dose of 1 mg/kg was related to the dose of the antibody. We therefore repeated the experiment with a 2-fold higher dose (2 mg/kg), which indeed showed complete inhibition of mobilization (an IL-8-induced increase from 9 CFU to 67 CFU per 5 × 105 plated cells and from 2 CFU to 7 CFU per 5 × 105 plated cells after pretreatment with the inhibitory antibody). In some experiments whole blood, after erythrocyte lysis, was incubated with FITC-labeled anti-CD34 antibody (566). To illustrate blocking of IL-8-induced mobilization of HPC, the percentage of CD34+ cells in appropriately gated populations of the animal treated with a dose of 2 mg/kg anti-gelatinase B antibodies is shown (Fig. 6). To exclude nonspecific inhibition of IL-8-induced mobilization by murine anti-human antibodies, we treated one animal [E (L177)] with 1 mg/kg non-cross-reacting murine anti-human anti-CD5 (6.12.20.1) antibody 2 h before the administration of IL-8. No inhibition of IL-8-induced mobilization of HPC was observed (an IL-8-induced increase from 6 CFU to 115 CFU per 5 × 105 plated cells) (Fig. 7). The data on the IL-8-induced mobilization of HPC from all animals treated with increasing doses of anti-gelatinase B antibodies or control anti-CD5 antibodies are summarized in Table 1.
Figure 5.
Effect of pretreatment with inhibitory anti-gelatinase B antibodies on the IL-8-induced mobilization of HPC (animal B). The animal was treated first with a bolus injection of IL-8 (0.1 mg/kg). Five days later, anti-gelatinase B antibodies (1 mg/kg) were administered 2 h before the second bolus injection of IL-8 (0.1 mg/kg). A complete inhibition of HPC mobilization was observed, whereas the IL-8-induced neutropenia was not affected.
Figure 6.
Effect of pretreatment with inhibitory anti-gelatinase B antibodies on the percentage of circulating CD34+ mononuclear cells at steady state (T = 0) and at 15 min after (T = 15) a single injection of IL-8 (0.1 mg/kg) (animal D). After erythrocyte lysis, peripheral blood leukocytes were stained with FITC-labeled anti-CD34 and analyzed by flow cytometry. The number of CD34+ cells is shown as percentage of the total number of appropriately gated cells. IL-8 induced an increase in circulating CD34+ cells from <0.1% to 0.5% at 15 min. After pretreatment with anti-gelatinase B antibodies (2 mg/kg), no increase of CD34+ cells was observed.
Figure 7.
Effect of pretreatment with a monoclonal non-cross-reacting murine anti-human anti-CD5 antibody on IL-8-induced HPC mobilization (animal E). The animal was treated with anti-CD5 (1 mg/kg), 2 h before the administration of IL-8 (0.1 mg/kg).
Table 1.
Effect of anti-gelatinase B antibodies on IL-8-induced mobilization of HPC
| Time | CFU per ml
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Anti-gelatinase B antibody
|
Anti-CD5
|
||||||||
| 0.1 mg/kg (A)
|
1 mg/kg (B)
|
1 mg/kg (C)
|
2 mg/kg (D)
|
1 mg/kg (E)
|
|||||
| − | + | − | + | − | + | − | + | + | |
| Before IL-8 induction | 2 | 1 | 5 | 7 | 7 | 6 | 9 | 2 | 6 |
| After IL-8 induction | 86 | 24 | 195 | 12 | 101 | 72 | 67 | 7 | 115 |
Animals were first treated with a single bolus injection of 0.1 mg/kg IL-8 (−). Five or six days later, anti-gelatinase B antibodies were administered as a single bolus injection, followed after 2 h by a second bolus injection of 0.1 mg/kg IL-8. The maximal numbers of CFU per ml of blood at 15–30 min are reported for IL-8 alone (−) and after pretreatment with increasing doses of anti-gelatinase B antibodies (+) (animals A–D) or anti-CD5 as control (animal E).
Effect of Inhibitory Monoclonal Antibody Against Gelatinase B on MMP-9 Induction.
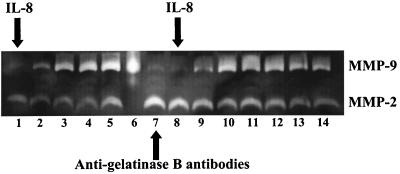
Preinjection of the inhibitory anti-gelatinase B antibody did not preclude the IL-8-induced production of MMP-9 (Fig. 8), showing that IL-8 still exerted its effect on the neutrophils.
Figure 8.
Effect of pretreatment with inhibitory anti-gelatinase B antibodies on the IL-8-induced plasma levels of gelatinase B/MMP-9 (animal B). The animal was treated first with a bolus injection of IL-8 (0.1 mg/kg). Five days later, anti-gelatinase B antibodies (1 mg/kg) were administered 2 h before the second bolus injection of IL-8 (0.1 mg/kg). The locations of gelatinase A/MMP-2 and gelatinase B/MMP-9 are indicated at the right. Lanes 1–5 represent plasma samples taken at 0, 1, 5, 15, and 30 min after IL-8 injection. Lane 6 is a positive control with 50 ng of purified neutrophil-derived MMP-9. Lanes 7–14 represent plasma samples taken before the administration of anti-gelatinase B antibodies (lane 7), before the injection of IL-8 (lane 8), and 1, 5, 15, 30, 45, and 60 min after IL-8 injection (lanes 9–14, respectively).
DISCUSSION
The increasing use of peripheral blood stem cells for transplantation emphasizes the potential practical benefits of understanding the mechanisms underlying cytokine-induced stem cell mobilization. The expression of integrins on HPC has been shown extensively, indicating a major role for these molecules in controlling cell attachment to the ECM (1–3, 4, 5, 8). However, a role for proteinases in stem cell mobilization has been less well studied. In 1972, Vos et al. (29) demonstrated that proteinases induce the circulation of hematopoietic stem cells in mice within 10 min after administration. Since MMPs are able to cleave matrix molecules, we hypothesized their involvement in the IL-8-induced mobilization of HPC. In this study we demonstrated that IL-8 induces a rapid, dramatic increase in the levels of circulating gelatinase B/MMP-9, concomitant with the mobilization of HPC. A similar pattern of circulating HPC and MMP-9 levels was observed after single and repeated injections of IL-8, suggesting a direct relationship between MMP-9 release and HPC mobilization. Moreover, injection of blocking anti-gelatinase B antibodies completely prevented IL-8-induced mobilization in several experiments. Therefore, our data indicate an important role for MMP-9 as a mediator in IL-8-induced mobilization of HPC.
Intravenous injection of IL-8 in animals induces an instant neutropenia followed by a profound neutrophilia (24, 30, 31). This systemic vascular effect of IL-8 results from its interference with neutrophil adhesion to endothelial cells (32) and is thought to be caused by the shedding of L-selectin (33), the up-regulation of Mac-1 (CD11b/CD18) (34, 35), and subsequent stiffening of the neutrophil membrane (36, 37). Furthermore, IL-8 and related CXC chemokines induce neutrophil degranulation with the subsequent release of several proteolytic enzymes (17, 38–40). MMP-9, stored in specific granules and in gelatinase granules (41), degrades components of the ECM (18) and appears to be a major factor for neutrophil migration across basement membranes (21, 23). The proteolytic activity of MMPs is usually tightly regulated through inhibition by tissue inhibitors of metalloproteinases and destructive proteolysis by neutrophil elastase (22, 23, 42). This might account for the fact that IL-8-induced HPC mobilization is short lasting and transient. The rapid and dramatic increase in circulating MMP-9 in our study is in accordance with these observations. No reinduction of MMP-9 was observed after 4 h. This lack of neutrophil response could be caused by the fact that secretory vesicles are not reformed within a period of 6 h (43) or the internalization of IL-8 receptors (44). Although the concurrent mobilization of HPC and induction of MMP-9 suggest a direct relationship, the observed absence of HPC mobilization in response to a second injection of IL-8 after 4 h could also be explained by a limited number of HPC available for mobilization.
Since the kinetics of HPC mobilization and MMP-9 induction after single and repeated injections of IL-8 were overlapping, we hypothesized a role of MMP-9 in IL-8-induced stem cell mobilization, and we studied the effect of blocking anti-gelatinase B antibodies on IL-8-induced mobilization of HPC. In three of four animals treated, anti-gelatinase B antibodies inhibited or completely prevented HPC mobilization. The blocking activities of the anti-gelatinase B antibody appeared to be dose-dependent. At a low dose of 0.1 mg/kg, partial inhibition of mobilization was observed; at a dose of 1 mg/kg mobilization was completely blocked in one of two animals treated. With a 2-fold higher dose of 2 mg/kg, mobilization was again completely blocked. Although we have no direct proof, these data are in accordance with the hypothesis that the lack of response in the other animal treated with 1 mg/kg was related to the dose of the antibody.
The mechanism or combinations of mechanisms, direct or indirect, through which MMP-9 exerts its effect are as yet unclear. Several possibilities can be considered. (i) Direct detachment of progenitors from the bone marrow stroma by cleavage of ECM. (ii) Degradation of the basement membrane, mainly consisting of collagen type IV, enabling HPC to egress from the bone marrow microenvironment into the bloodstream (21). (iii) Enzymatic activation of other proteases that, like MMP-9, are released as inactive proenzymes (45). (iv) Shedding of cell adhesion molecules (46, 47). Using densitometry, we calculated that the injection of IL-8 induced circulating levels of MMP-9 of approximately 6 μg/ml. These high levels require doses of 1–2 mg/kg of anti-gelatinase B antibody for neutralization. It has been reported that serine proteases such as elastase and cathepsin G, which are also released by activated neutrophils, induce the detachment of adherent epithelial cells in vitro at concentrations of 5–10 μg/ml (48). Therefore, the observed MMP-9 plasma levels are in a range that would allow in vivo biological activity—i.e., cleavage of matrix molecules. Furthermore, the binding of neutrophils to type I collagen, a major constituent of the bone marrow microenvironment, has also been reported to stimulate the release of MMP-9 (6, 49). Together, these observations indicate that IL-8-induced activation of bound neutrophils could generate high levels of MMP-9, in particular at the local level. Additional support for the involvement of MMP-9 in leukocytosis has recently been published by Masure et al. (50). After i.v. injection of recombinant mouse gelatinase B into rabbits, an almost instantaneous and transient neutropenia was observed, followed by a profound neutrophilia lasting for several hours. Concomitantly, they observed the appearance of immature myeloid cells, including circulating blast cells. The observed effects after injection of gelatinase B are strikingly similar to the effects seen after administration of IL-8 (24, 31). These observations suggest the simultaneous release of HPC, although no colony assays were reported. Thus, the observed MMP-9 levels after IL-8 injection as well as the effect of MMP-9 injection in vivo support the role of MMP-9 in stem cell mobilization. However, definitive proof requires the injection of purified human or primate MMP-9 into monkeys. Unfortunately, we were unable to obtain endotoxin-free MMP-9 in quantities sufficient to perform these experiments.
Recently, we showed that anti-LFA-1 antibodies completely prevented IL-8-induced mobilization of HPC in mice (15). This effect was not due to a direct effect of the antibodies on HPC, since LFA-1 is not expressed on HPC with colony-forming or radioprotective capacity (51–53). These data indicated that an accessory cell, expressing both LFA-1 and IL-8 receptors, has to play a central role in IL-8-induced mobilization. Our current data suggest that neutrophils, which express LFA-1 as well as IL-8 receptors, and release MMP-9 upon activation by IL-8, play such an intermediate role. In accordance with this hypothesis, Liu et al. (54) reported severely impaired IL-8-induced mobilization in mice deficient in the receptor for granulocyte colony-stimulating factor (G-CSF). Furthermore, monoclonal antibodies to LFA-1 inhibited not only the binding of neutrophils to type I collagen but also the release of MMP-9 upon stimulation (49). Taken together, our data demonstrate the involvement of the metalloproteinase gelatinase B in IL-8-induced mobilization of HPC and indicate that neutrophils play a central role in this phenomenon. Recent data point toward a role for IL-8 in G-CSF-induced stem cell mobilization (55), suggesting that neutrophils and MMPs are involved in mechanisms underlying mobilization induced by other cytokines. Further studies are needed to delineate the role of neutrophils as key regulating cells and to determine a role for MMPs in a final common pathway in cytokine-induced stem cell mobilization.
Acknowledgments
We thank Peer van Eerd, Herman Koning, and Noud Neervoort of the Biomedical Primate Research Center for their assistance and excellent handling of the animals and Dr. A. Wognum of the Department of Hematology, Erasmus University, Rotterdam, The Netherlands, for FITC labeling of the CD34 (566) antibodies. Technical assistance of Ilse Van Aelst, Maarten van der Keur, and Arie van der Marel is also greatly appreciated. This work was supported by the Dutch Cancer Society (NKB) Grant RUL 95-1091, the National Fund for Scientific Research (FWO-Vlaanderen), and the General Savings and Retirement Fund (ASLK), Belgium.
ABBREVIATIONS
- HPC
hematopoietic progenitor cells
- ECM
extracellular matrix
- LFA
leukocyte function-associated antigen
- MMP
matrix metalloproteinase
- CFU
colony-forming unit
- PMN
neutrophils (polymorphonuclear leukocytes)
References
- 1.Liesveld J L, Winslow J M, Kempski M C, Ryan D H, Brennan J K, Abboud C N. Exp Hematol. 1991;19:63–70. [PubMed] [Google Scholar]
- 2.Simmons P J, Zannettino A, Gronthos A, Leavesly D I. Leuk Lymphoma. 1994;12:353–363. doi: 10.3109/10428199409073776. [DOI] [PubMed] [Google Scholar]
- 3.Teixido J, Hemler M E, Greenberger J S, Anklesaria P J. J Clin Invest. 1992;90:358–367. doi: 10.1172/JCI115870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams D A, Rios M, Stephens C, Patel V P. Nature (London) 1991;352:438–441. doi: 10.1038/352438a0. [DOI] [PubMed] [Google Scholar]
- 5.Lévesque J P, Haylock D N, Simmons P J. Blood. 1996;88:1168–1176. [PubMed] [Google Scholar]
- 6.Koenigsmann M, Griffin J D, DiCarlo J, Cannistra S A. Blood. 1992;79:657–665. [PubMed] [Google Scholar]
- 7.Klein G, Müller C A, Tillet E, Chu M L, Timpi R. Blood. 1995;86:1740–1748. [PubMed] [Google Scholar]
- 8.Yoder M C, Williams D A. Exp Hematol. 1995;23:961–967. [PubMed] [Google Scholar]
- 9.Carlos M, Harlan J M. Blood. 1994;84:2068–2101. [PubMed] [Google Scholar]
- 10.Springer T A. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi M, Imamura M, Uede T, Sakurada K, Maeda S, Iwasaki H, Tsuda Y, Musashi M, Miyazaki T. Stem Cells. 1994;12:316–321. doi: 10.1002/stem.5530120307. [DOI] [PubMed] [Google Scholar]
- 12.Lund Johansen F, Terstappen L W. J Leuk Biol. 1993;54:47–55. doi: 10.1002/jlb.54.1.47. [DOI] [PubMed] [Google Scholar]
- 13.Papayannopoulou T, Nakamoto B. Proc Natl Acad Sci USA. 1993;90:9374–9378. doi: 10.1073/pnas.90.20.9374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papayannopoulou T, Craddock C, Nakamoto B, Priestley G V, Wolf N S. Proc Natl Acad Sci USA. 1995;92:9647–9651. doi: 10.1073/pnas.92.21.9647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pruijt J F M, van Kooyk Y, Figdor C G, Lindley I J D, Willemze R, Fibbe W E. Blood. 1998;91:4099–4105. [PubMed] [Google Scholar]
- 16.Matrisian L M. BioEssays. 1992;14:455–463. doi: 10.1002/bies.950140705. [DOI] [PubMed] [Google Scholar]
- 17.Masure S, Proost P, Van Damme J, Opdenakker G. Eur J Biochem. 1991;198:391–398. doi: 10.1111/j.1432-1033.1991.tb16027.x. [DOI] [PubMed] [Google Scholar]
- 18.Wilhelm S M, Collier I E, Marmer B L, Eisen A Z, Grant G A, Goldberg G I. J Biol Chem. 1989;264:17213–17221. [PubMed] [Google Scholar]
- 19.Masure S, Billiau A, Van Damme J, Opdenakker G. Biochim Biophys Acta. 1990;1054:317–325. doi: 10.1016/0167-4889(90)90103-k. [DOI] [PubMed] [Google Scholar]
- 20.Kjeldsen L, Sengolov H, Lollike K, Nielsen M H, Borregaard N. Blood. 1994;83:1640–1649. [PubMed] [Google Scholar]
- 21.Delclaux C, Delacourt C, d’Ortho M P, Boyer V, Lafuma C, Harf A. Am J Respir Cell Mol Biol. 1996;14:288–295. doi: 10.1165/ajrcmb.14.3.8845180. [DOI] [PubMed] [Google Scholar]
- 22.Denhardt D T, Feng B, Edwards D, Cocuzzi E T, Malyankar U M. Pharmacol Ther. 1993;59:329–341. doi: 10.1016/0163-7258(93)90074-n. [DOI] [PubMed] [Google Scholar]
- 23.Opdenakker G, Fibbe W E, Van Damme J. Immunol Today. 1998;19:182–189. doi: 10.1016/s0167-5699(97)01243-7. [DOI] [PubMed] [Google Scholar]
- 24.Laterveer L, Lindley I J D, Heemskerk D P M, Camps J A J, Pauwels E K J, Willemze R, Fibbe W E. Blood. 1996;87:781–788. [PubMed] [Google Scholar]
- 25.Lindley I, Aschauer H, Seifert J M, Lam C, Brunowsky W, Kownatzki E, Thelen M, Peveri P, Dewald B, von Tscharner V, Walz A, Baggiolini M. Proc Natl Acad Sci USA. 1988;85:9199–9203. doi: 10.1073/pnas.85.23.9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zwierzina H, Holzinger I, Gaggi S, Wolf H, Schollenberger S, Lam C, Bammer T, Geissler D, Lindley I J D. Scand J Immunol. 1993;37:322–328. doi: 10.1111/j.1365-3083.1993.tb02560.x. [DOI] [PubMed] [Google Scholar]
- 27.Paemen L, Martens E, Masure S, Opdenakker G. Eur J Biochem. 1995;234:759–765. doi: 10.1111/j.1432-1033.1995.759_a.x. [DOI] [PubMed] [Google Scholar]
- 28.Zhou N, Paemen L, Opdenakker G, Froyen G. FEBS Lett. 1997;414:562–566. doi: 10.1016/s0014-5793(97)01072-7. [DOI] [PubMed] [Google Scholar]
- 29.Vos O, Buurman W A, Ploemacher R E. Cell Tissue Kinet. 1972;5:467–479. doi: 10.1111/j.1365-2184.1972.tb00385.x. [DOI] [PubMed] [Google Scholar]
- 30.Van Damme J, Van Beeumen J, Opdenakker G, Billiau A. J Exp Med. 1988;167:1364–1376. doi: 10.1084/jem.167.4.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Zee K J, Fischer E, Hawes A S, Hebert C A, Terrell G E, Baker J B, Lowry S F, Moldawer L L. J Immunol. 1992;148:1746–1752. [PubMed] [Google Scholar]
- 32.Hechtman D H, Cybulski M I, Fuchs H J, Baker J B, Gimbrone M E. J Immunol. 1991;147:883–892. [PubMed] [Google Scholar]
- 33.Smith C W, Kishimoto T K, Abbass O, Hughes B, Rothlein R, McIntyre L V, Butcher E, Anderson D C. J Clin Invest. 1991;87:609–618. doi: 10.1172/JCI115037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carveth H J, Bohnsack J F, McIntyre T M, Baggiolini M, Prescott S M, Zimmerman G A. Biochem Biophys Res Commun. 1989;162:387–393. doi: 10.1016/0006-291x(89)92009-3. [DOI] [PubMed] [Google Scholar]
- 35.Detmers P A, Lo S K, Olsen-Egbert E, Walz A, Baggiolini M, Cohn Z A. J Exp Med. 1990;171:1155–1162. doi: 10.1084/jem.171.4.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Worthen G S, Schwab B, III, Elson E L, Downey G P. Science. 1989;245:183–186. doi: 10.1126/science.2749255. [DOI] [PubMed] [Google Scholar]
- 37.Rot A. Immunol Today. 1992;13:291–294. doi: 10.1016/0167-5699(92)90039-A. [DOI] [PubMed] [Google Scholar]
- 38.Schröder J M, Mrowietz U, Morita E, Christophers E. J Immunol. 1987;139:3474–3483. [PubMed] [Google Scholar]
- 39.Peveri P, Walz A, Dewald B, Baggiolini M. J Exp Med. 1988;167:1547–1559. doi: 10.1084/jem.167.5.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wuyts A, Haelens A, Proost P, Lenaerts J P, Conings R, Opdenakker G, Van Damme J. J Immunol. 1996;157:1736–1743. [PubMed] [Google Scholar]
- 41.Borregaard N, Cowland J B. Blood. 1997;89:3503–3521. [PubMed] [Google Scholar]
- 42.Rice A, Banda M J. Biochemistry. 1995;34:9249–9256. doi: 10.1021/bi00028a038. [DOI] [PubMed] [Google Scholar]
- 43.Sengolov H, Follin P, Kjeldsen L, Lolike K, Dahlgren C, Borregaard N. J Immunol. 1995;154:4157–4165. [PubMed] [Google Scholar]
- 44.Khandaker M H, Mitchell G, Xu L, Andrews J D, Singh R, Leung H, Madrenas J, Ferguson S S G, Feldman R D, Kelvin D J. Blood. 1999;93:2173–2185. [PubMed] [Google Scholar]
- 45.Werb Z. Cell. 1997;91:439–442. doi: 10.1016/s0092-8674(00)80429-8. [DOI] [PubMed] [Google Scholar]
- 46.Arribas J, Coodly L, Vollmer P, Kishimoto T K, Rose-John S, Massagué J. J Biol Chem. 1996;271:11376–11382. doi: 10.1074/jbc.271.19.11376. [DOI] [PubMed] [Google Scholar]
- 47.Preece G, Murphy G, Ager A. J Biol Chem. 1996;271:11634–11640. doi: 10.1074/jbc.271.20.11634. [DOI] [PubMed] [Google Scholar]
- 48.Van Wetering S, Mannesse-Lazeroms S P G, Dijkman J H, Hiemstra P S. J Leuk Biol. 1997;62:217–226. doi: 10.1002/jlb.62.2.217. [DOI] [PubMed] [Google Scholar]
- 49.Garnotel R, Monboisse J C, Randoux A, Haye B, Borel J P. J Biol Chem. 1995;270:27495–27503. doi: 10.1074/jbc.270.46.27495. [DOI] [PubMed] [Google Scholar]
- 50.Masure S, Paemen L, Van Aelst I, Fiten P, Proost P, Billiau A, Van Damme J, Opdenakker G. Eur J Biochem. 1997;244:21–30. doi: 10.1111/j.1432-1033.1997.00021.x. [DOI] [PubMed] [Google Scholar]
- 51.Gunji Y, Nakamura M, Hagiwara T, Hayakawa K, Matshushita H, Osawa H, Nagayoshi K, Nakauchi H, Yanagisawa M, Miura Y, Suda T. Blood. 1992;80:429–436. [PubMed] [Google Scholar]
- 52.Torensma R, Raymakers R A P, van Kooyk Y, Figdor C G. Blood. 1996;87:4120–4128. [PubMed] [Google Scholar]
- 53.Pruijt J F M, van Kooyk Y, Figdor C G, Willemze R, Fibbe W E. Blood. 1999;93:107–112. [PubMed] [Google Scholar]
- 54.Liu F L, Poursine-Laurent J, Link D C. Blood. 1997;90:2526–2528. [PubMed] [Google Scholar]
- 55.Wanatabe T, Kawano Y, Kanamaru S, Onishi T, Kaneko S, Wakata Y, Nakagawa R, Makimoto A, Kuroda Y, Takaue Y, Talmadge J E. Blood. 1999;93:1157–1163. [PubMed] [Google Scholar]