Abstract
FtsN, the last essential protein in the cell division localization hierarchy in Escherichia coli, has several peculiar characteristics, suggesting that it has a unique role in the division process despite the fact that it is conserved in only a subset of bacteria. In addition to suppressing temperature-sensitive mutations in ftsA, ftsK, ftsQ, and ftsI, overexpression of FtsN can compensate for a complete lack of FtsK in the cell. We examined the requirements for this phenomenon. We found that the N-terminal terminal region (cytoplasmic and transmembrane domains) is critical for suppression, while the C-terminal murein-binding domain is dispensable. Our results further suggest that FtsN and FtsK act cooperatively to stabilize the divisome.
Cell division in Escherichia coli requires the concerted action of at least 10 essential proteins at midcell. FtsZ and the FtsZ-binding proteins FtsA and ZipA assemble at midcell to form the Z-ring. The Z-ring then serves as a scaffold for assembly of the remaining late proteins, which occurs according to a linear hierarchy (FtsK → FtsQ → FtsL/B → FtsW → FtsI → FtsN). One gene, ftsN, was found to be a multicopy suppressor of a temperature-sensitive mutation in ftsA. Surprisingly, it could also act as a multicopy suppressor of a variety of other cell division defects, including temperature-sensitive alleles of ftsQ and ftsI (3). Overexpression of FtsN was even able to rescue cells in which ftsK was deleted (6).
FtsN requires multiple contacts with multiple upstream proteins in order to localize to midcell, whereas the other late division proteins associate in a simple fashion with proteins immediately upstream of them in the hierarchy (8, 9). For example, FtsI, the protein immediately upstream of FtsN, localizes normally in the absence of FtsA or FtsQ provided that FtsW is targeted to midcell using a technique known as premature targeting. In contrast, FtsN fails to localize in cells missing FtsA or FtsQ, even if all other upstream proteins are present at midcell.
We revisited the ability of FtsN overexpression to rescue the growth of cells that have lost the essential cell division protein FtsK. We reasoned that an understanding of the molecular mechanism behind this phenotype could shed light on the function of FtsN in cell division. In particular, we wanted to know (i) to what degree the functions of FtsK and FtsN are redundant, (ii) whether suppression restores relatively normal divisome assembly or allows cells to divide via a novel alternate pathway that does not require assembly of late divisomal proteins, and (iii) to what degree the nonessential domains of FtsN play a role in this process.
In the course of analyzing the localization of division proteins in cells in which FtsK is depleted, we noticed that cells expressing a green fluorescent protein (GFP)-FtsN fusion exhibited frequent midcell fluorescence and were significantly shorter than the cells of isogenic strains expressing other GFP fusions. The FtsK depletion strain JOE563 carries an ftsK::cat-Δ5 null allele at the endogenous locus and is complemented from a spectinomycin-resistant (Spcr), low-copy-number, arabinose-inducible pBAD42 plasmid (pJC85). Upon repression of the plasmid-borne copy of ftsK by growth on glucose, the FtsK protein is depleted to such an extent that the levels are undetectable by Western blotting (1). Under these conditions, GFP fusions to most downstream proteins that normally depend on FtsK (FtsK-dependent proteins), including FtsQ, FtsL, and FtsI, fail to localize and cells lose the ability to divide (2). However, we found that when a single copy of the GFP-FtsN fusion was integrated into the chromosome in an FtsK depletion strain (JOE702) and induced with 10 μM isopropyl-β-d-thiogalactopyranoside (IPTG), growth was rescued and GFP-FtsN localized efficiently to the midcell of suppressed cells (Table 1) (9).
TABLE 1.
GFP-FtsN suppression
| Constructa | Mutant background | Temp (°C) | Growth on:
|
||
|---|---|---|---|---|---|
| Ara | Glc | Glc + IPTG | |||
| GFP-FtsN | pBAD-ftsKb | 37 | +++e | ++ | +++ |
| GFP-FtsN | ΔftsKc | 37 | − | − | + |
| GFP-FtsL | pBAD-ftsK | 37 | +++ | − | − |
| GFP-FtsN | pBAD-ftsK | 42 | +++ | − | − |
| GFP-FtsN | pBAD-ftsNd | 42 | +++ | +++ | +++ |
All GFP constructs were expressed from a single-copy pDSW204 promoter.
The FtsK depletion strain was ΔftsK::cat-Δ5 complemented by pJC85 (pBAD42-ftsK).
ΔftsK::cat-Δ5 with no complementing plasmid.
FtsN depletion strain.
+++, wild-type growth; ++, slightly wrinkled colonies; +, poor growth and wrinkled colonies; −, no growth.
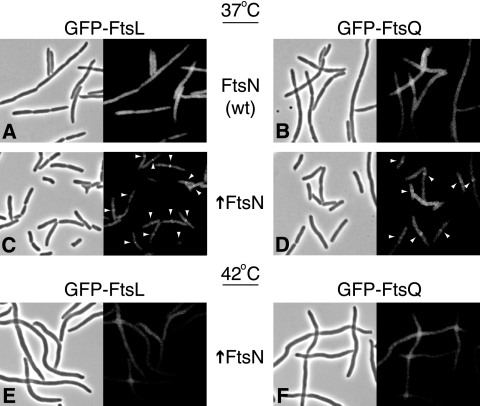
We wondered whether under suppressing conditions division occurred independent of the localization of FtsK-dependent division proteins that are normally required for FtsN′s localization. We examined the localization of two such proteins, FtsL and FtsQ, both of which fail to localize in cells in which FtsK is depleted (2). Upon overexpression of FtsN from pDSW204 in such strains (15), localization of FtsL and FtsQ to potential division sites was largely restored (Fig. 1C and D). Although suppression was not complete (the cells were somewhat longer and occasionally branched), the majority of cells (>50%) contained a fluorescent band at midcell.
FIG. 1.
FtsN overexpression restores localization of FtsL and FtsQ in cells in which FtsK is depleted at 37°C but not at 42°C. FtsK was depleted in isogenic FtsN-overexpressing cells (↑FtsN) and control cells (wt) at 37°C or 42°C for 3 to 5 h until control samples showed filamentation. Localization of GFP fusions to FtsQ and FtsL (expressed from λatt) is shown along with corresponding phase-contrast images. Expression of FtsN was from pDSW204.
This finding is consistent with prior work on FtsK suppression by pZAQ, a multicopy plasmid that increases the levels of FtsZ, FtsA, and FtsQ in the cell. Interestingly, suppression by pZAQ resulted in the localization of FtsI, indicating that division in this case was also not independent of the localization of FtsK-dependent division proteins (7). Thus, regardless of whether suppression of ΔftsK cells is provided by overexpression of proteins upstream (FtsZ, FtsA) or downstream (FtsQ, FtsN) of FtsK in the localization hierarchy, localization of FtsK-dependent proteins is restored.
Given the multiple mechanisms by which FtsK can be suppressed and the fact that none of the proteins shares a similar sequence or membrane topology with FtsK, it is unlikely that overexpression suppression is due to complementation of a specific activity that FtsK− cells lack. It has been speculated that the primary defect could be destabilization of the divisome, resulting in a failure to recruit downstream division proteins (7). FtsN may counteract this effect, stabilizing assembly via weak affinities to multiple division proteins. Such a model is consistent with recent bacterial two-hybrid studies which revealed potential interactions between FtsN and FtsA, FtsQ, FtsW, and FtsI (5, 12). As one measure of this stabilization activity, we tested the ability of FtsN to suppress at high temperatures, as higher temperatures might additionally destabilize the divisome. Strikingly, despite the fact that GFP-FtsN complemented a strain in which FtsN was depleted and localized normally at 42°C, overexpression of GFP-FtsN was unable to restore viability to a strain in which FtsK was depleted at the same temperature (Table 1). Consistent with a loss of divisome stability, both FtsL and FtsQ did not localize under these conditions, and cells were unable to divide (Fig. 1E and F).
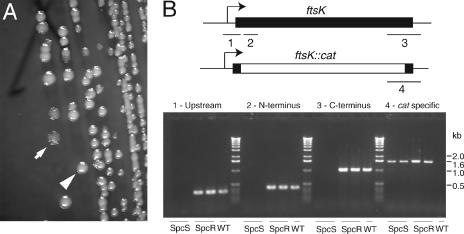
Previous studies have reported rescue of cells lacking FtsK by FtsN overexpression. However, the resulting cells grow poorly, form filaments, and show a loss of viability (6, 7). We considered the possibility that the growth defects seen in these studies compared to the relatively efficient suppression in our analysis might have been due to residual FtsK expression from the repressed pBAD42 (Spcr) plasmid pJC85. We examined whether an FtsK depletion strain expressing GFP-FtsN (JOE702) could lose the FtsK complementing plasmid when it was transformed with incompatible empty plasmid pBAD42(Kanr) and plated under GFP-FtsN overexpression conditions (with IPTG). When we plated JOE702 cells transformed with pBAD42(Kanr) on NZ arabinose plus IPTG, we found two colony types. Ninety percent of the colonies had wild-type morphology, and all of the colonies tested had not lost the complementing plasmid, pJC85 (all 24 colonies were Spcr). However, 10% of the colonies were extensively wrinkled (Fig. 2A). Cells in these colonies had lost pJC85 (none of 23 colonies were Spcr) and required IPTG (GFP-FtsN expression) for growth, regardless of the sugar present (Table 1). In contrast, when we used a control strain expressing GFP-FtsL (JOE701), none of the >200 colonies examined were wrinkled, pJC85 was maintained in all isolates tested (all 24 isolates were Spcr), and arabinose was required for growth (Table 1). PCR analysis confirmed that ftsK was not present in JOE702-derived cells that had lost the complementing plasmid and that the locus containing the ftsK::cat-Δ5 allele was intact (Fig. 2B). Inspection of cells in the colonies revealed that they were highly filamentous, and the cells grew very poorly in liquid media, consistent with previous studies of FtsN multicopy suppression (data not shown). Thus, FtsN overexpression can rescue the viability of a strain completely lacking FtsK. However, this suppression is not sufficient to provide for normal division.
FIG. 2.
Complete loss of FtsK under FtsN overexpression conditions results in a cell division defect. (A) When JOE702 (ΔftsK Δλatt::P207-ftsN/pBAD42-ftsK) was transformed with an incompatible Kanr plasmid, grown in the presence of 0.2% arabinose, 10 μM IPTG, and 40 μg/ml kanamycin, and plated on the same medium, a mixture of wrinkled and smooth colonies was obtained. The arrow indicates a wrinkled colony. The arrowhead indicates a smooth colony. (B) PCR analysis of wrinkled (Spcs) colonies, which lost the pBAD42-ftsK(Spcr) complementing plasmid, failed to show products with primer pairs specific for ftsK (products 1, 2, and 3), consistent with plasmid loss, while yielding the expected ∼1.6-kb fragment for the ftsK::cat-Δ5 allele (product 4). Smooth (Spcr) colonies which maintained the complementing plasmid contained both the ftsK::cat-Δ5 allele and the plasmid-encoded ftsK gene. PCR analysis of wild-type cells yielded products consistent with the presence of ftsK but not with the presence of the ftsK::cat-Δ5 allele. The results for two independent colonies of each colony type are shown along with the results for a single wild-type (WT) control. PCR products are mapped on the corresponding alleles (ftsK or ftsK::cat-Δ5).
Comparison of the suppression phenotype in strains in which FtsK was depleted to the phenotype of strains in which ftsK was deleted also indicated that minute quantities of FtsK in the cell (due to incomplete repression of the pBAD promoter) potentiated the ability of FtsN to rescue growth. Such synergy suggests that these proteins do not act redundantly at midcell. Rather, it seems likely that the two proteins contribute to divisome stability via distinct and potentially cooperative mechanisms.
Neither the N-terminal domain comprising the cytoplasmic tail and transmembrane (NcytoTM) domain nor the C-terminal murein-binding domain of FtsN (NMB) is essential for growth (4, 14). We asked whether either domain is required for rescue of ftsK cells. We were particularly interested in the idea that the murein-binding element allows FtsN to bind to septal murein. Once at midcell, FtsN would be in a position to back-recruit division proteins.
In addition to full-length FtsN, we cloned two constructs: FtsN241, in which NMB was removed, and FFN, in which NcytoTM was swapped with the first 39 amino acids of MalF (which comprise the first cytoplasmic and transmembrane domains). When expressed from pDSW204 (15), all three constructs readily complemented an FtsN depletion strain at uninduced levels, consistent with prior reports of the dispensability of both the N- and C-terminal domains (Table 2).
TABLE 2.
FtsN swap suppression
| Constructa | Mutant backgroundb | Temp (°C) | Complementation with IPTG at a concn of c:
|
Notesd | |||
|---|---|---|---|---|---|---|---|
| 1 μM | 10 μM | 100 μM | 1,000 μM | ||||
| FtsN | pBAD-ftsN | 37 | +++ | +++ | +++ | +/− | |
| FFN | pBAD-ftsN | 37 | +++ | +++ | +++ | NDe | |
| FtsN241 | pBAD-ftsN | 37 | +++ | +++ | +++ | ND | |
| FtsN | ΔftsK | 37 | + | + | − | − | P1 |
| FFN | ΔftsK | 37 | − | − | − | − | P1 |
| FtsN241 | ΔftsK | 37 | + | + | − | − | P1 |
| FtsN | pBAD-ftsK | 37 | ++ | ++ | − | − | |
| FFN | pBAD-ftsK | 37 | − | − | − | − | |
| FtsN241 | pBAD-ftsK | 37 | ++ | ++ | − | − | |
| FtsN | ftsQ1(Ts) | 42 | + | +/− | − | − | |
| FFN | ftsQ1(Ts) | 42 | − | − | − | − | |
| FtsN241 | ftsQ1(Ts) | 42 | +/− | − | − | − | |
Constructs were expressed with a C-terminal 3×Myc epitope from the multicopy pDSW204 plasmid.
Same mutant backgrounds as those in Table 1.
+++, wild-type growth; ++, slightly wrinkled colonies; + and +/−, very poor growth and small wrinkled colonies (+ indicates that there was efficient recovery of transductants); −, no growth.
P1, tested by P1 transduction.
ND, not done.
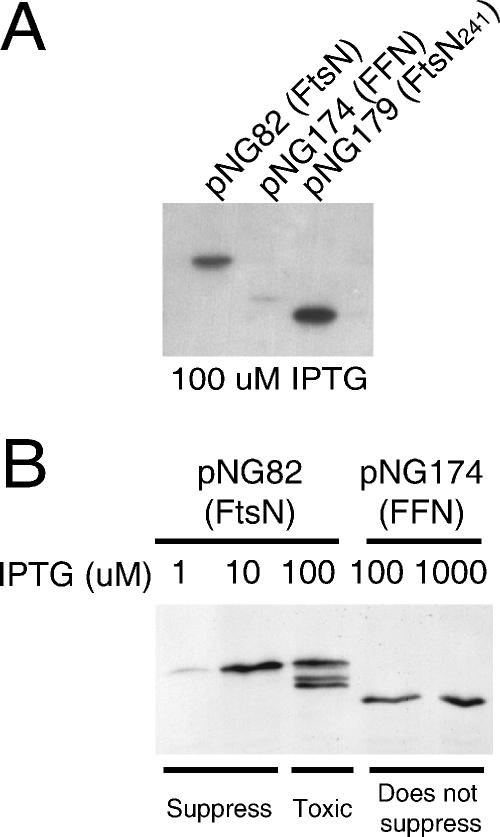
Surprisingly, while FtsN241 suppressed an FtsK depletion strain and allowed us to obtain uncomplemented ftsK::cat-Δ5 transductants, FFN was unable to suppress (Table 2). We worried that the failure to suppress could have been due to reduced levels of FFN. Indeed, Western blot analysis revealed that at equivalent IPTG levels, the FFN construct produced significantly lower levels of protein than both the wild-type FtsN construct and FtsN241 produced (Fig. 3A). However, FFN was unable to suppress even at higher IPTG levels (100 and 1,000 μM) despite the fact that it was produced at levels equivalent to or greater than the levels of the wild-type FtsN construct required for suppression (1 to 10 μM IPTG) (Table 2 and Fig. 3B). Thus, we concluded that NcytoTM, which does not show obvious conservation, is essential for suppression of an FtsK depletion strain, while the relatively conserved C-terminal murein-binding domain (NMB) is dispensable.
FIG. 3.
Western blot of FtsN swap constructs. In panel A, FtsN, FFN, and FtsN241 were induced with 100 μM IPTG. In panel B, FtsN and FFN were induced with various levels of IPTG, as indicated above the lanes. In all cases, equal cell volumes were loaded and proteins were detected by their C-terminal 3×Myc tags using polyclonal anti C-myc antibody (Sigma). “Suppress” and “Does not suppress” refer to the abilities of the constructs to suppress a FtsK depletion strain at the levels of IPTG indicated above the lanes.
We also asked whether NcytoTM is required for FtsN overexpression rescue of other division defects as well. In fact, we found that NcytoTM is also necessary to suppress the ftsQ1(Ts) allele as FFN failed to rescue at all levels of induction. Similar to the case with the FtsK depletion strain and in contrast to FFN, FtsN241 was also able to suppress ftsQ1(Ts). However, in this case, the suppression was somewhat less than that seen with full-length FtsN (Table 2). Thus, NcytoTM, previously postulated to be important only for promoting translocation and anchoring of the periplasmic domain in the appropriate cellular compartment, turns out to play a general role in facilitating divisome assembly. In contrast, although we obtained some evidence that NMB allows FtsN to suppress ftsQ1(Ts) more efficiently, if NMB does in fact play a role in cell division, its contribution is likely to be minor or redundant.
Previous work on mutant cell division proteins has focused primarily on testing for the abilities of these proteins to complement null alleles. Such an approach has yielded significant insights into the essential domains of the division proteins, providing a framework for understanding protein function. This work, however, highlights the limitations of relying on complementation for growth and division as the only measure of fts protein function, particularly because our understanding of the ultimate functions of many of the division proteins is incomplete. Here, by assaying the role of FtsN with a nonstandard assay (suppression of the ΔftsK::cat-Δ5 allele) we were able to discover a novel role for a nonessential domain of FtsN.
This finding adds to the increasing number of observations of a specific role for the cytoplasmic and/or transmembrane segments of cell division proteins in divisome assembly. This role appears to be established even though, at least in the case of the bitopic membrane proteins (FtsQ, FtsL, FtsB, FtsI, and FtsN), there is no obvious conservation in these regions and in several cases (FtsQ and FtsN) these N-terminal regions have been shown to be “nonessential” for function in standard complementation assays (7, 10, 11, 13, 16). Together, these observations point to a model in which the N-terminal cytoplasmic and/or transmembrane domains of (most likely all) division proteins contribute to the interactions among divisomal components.
Acknowledgments
We thank members of the Beckwith laboratory for critical comments and general assistance.
N.W.G. was a Howard Hughes Predoctoral Fellow. C.R. holds a Marie Curie Outgoing International Fellowship. This work was supported by grant GM38922 from the National Institute of General Medical Sciences. J.B. is an American Cancer Society Professor.
Footnotes
Published ahead of print on 27 October 2006.
REFERENCES
- 1.Buddelmeijer, N., and J. Beckwith. 2004. A complex of the Escherichia coli cell division proteins FtsL, FtsB and FtsQ forms independently of its localization to the septal region. Mol. Microbiol. 52:1315-1327. [DOI] [PubMed] [Google Scholar]
- 2.Chen, J. C., and J. Beckwith. 2001. FtsQ, FtsL and FtsI require FtsK, but not FtsN, for co-localization with FtsZ during Escherichia coli cell division. Mol. Microbiol. 42:395-413. [DOI] [PubMed] [Google Scholar]
- 3.Dai, K., Y. Xu, and J. Lutkenhaus. 1993. Cloning and characterization of ftsN, an essential cell division gene in Escherichia coli isolated as a multicopy suppressor of ftsA12(Ts). J. Bacteriol. 175:3790-3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dai, K., Y. Xu, and J. Lutkenhaus. 1996. Topological characterization of the essential Escherichia coli cell division protein FtsN. J. Bacteriol. 178:1328-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Lallo, G., M. Fagioli, D. Barionovi, P. Ghelardini, and L. Paolozzi. 2003. Use of a two-hybrid assay to study the assembly of a complex multicomponent protein machinery: bacterial septosome differentiation. Microbiology 149:3353-3359. [DOI] [PubMed] [Google Scholar]
- 6.Draper, G. C., N. McLennan, K. Begg, M. Masters, and W. D. Donachie. 1998. Only the N-terminal domain of FtsK functions in cell division. J. Bacteriol. 180:4621-4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geissler, B., and W. Margolin. 2005. Evidence for functional overlap among multiple bacterial cell division proteins: compensating for the loss of FtsK. Mol. Microbiol. 58:596-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goehring, N. W., M. D. Gonzalez, and J. Beckwith. 2006. Premature targeting of cell division proteins to midcell reveals hierarchies of protein interactions involved in divisome assembly. Mol. Microbiol. 61:33-45. [DOI] [PubMed] [Google Scholar]
- 9.Goehring, N. W., F. Gueiros-Filho, and J. Beckwith. 2005. Premature targeting of a cell division protein to midcell allows dissection of divisome assembly in Escherichia coli. Genes Dev. 19:127-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goehring, N. W., I. Petrovska, D. Boyd, and J. Beckwith. 2007. Mutants, suppressors, and wrinkled colonies: mutant alleles of the cell division gene ftsQ point to functional domains in FtsQ and a role for domain 1C of FtsA in divisome assembly. J. Bacteriol. 189:633-645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guzman, L. M., D. S. Weiss, and J. Beckwith. 1997. Domain-swapping analysis of FtsI, FtsL, and FtsQ, bitopic membrane proteins essential for cell division in Escherichia coli. J. Bacteriol. 179:5094-5103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karimova, G., N. Dautin, and D. Ladant. 2005. Interaction network among Escherichia coli membrane proteins involved in cell division as revealed by bacterial two-hybrid analysis. J. Bacteriol. 187:2233-2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piette, A., C. Fraipont, T. Den Blaauwen, M. E. Aarsman, S. Pastoret, and M. Nguyen-Disteche. 2004. Structural determinants required to target penicillin-binding protein 3 to the septum of Escherichia coli. J. Bacteriol. 186:6110-6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ursinus, A., F. van den Ent, S. Brechtel, M. de Pedro, J. V. Holtje, J. Lowe, and W. Vollmer. 2004. Murein (peptidoglycan) binding property of the essential cell division protein FtsN from Escherichia coli. J. Bacteriol. 186:6728-6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiss, D. S., J. C. Chen, J. M. Ghigo, D. Boyd, and J. Beckwith. 1999. Localization of FtsI (PBP3) to the septal ring requires its membrane anchor, the Z ring, FtsA, FtsQ, and FtsL. J. Bacteriol. 181:508-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wissel, M. C., J. L. Wendt, C. J. Mitchell, and D. S. Weiss. 2005. The transmembrane helix of the Escherichia coli division protein FtsI localizes to the septal ring. J. Bacteriol. 187:320-328. [DOI] [PMC free article] [PubMed] [Google Scholar]