Abstract
Outer membrane β-barrel proteins in gram-negative bacteria, such as Escherichia coli, must be translocated from their site of synthesis in the cytoplasm to the periplasm and finally delivered to the outer membrane. At least a dozen proteins located in the cytoplasm, the periplasm, and both the inner and outer membranes are required to catalyze this complex assembly process. At normal growth temperatures and conditions the transport and assembly processes are so fast that assembly intermediates cannot be detected. Using cells grown at a low temperature to slow the assembly process and pulse-chase analysis with immunodetection methods, we followed newly synthesized LamB molecules during their transit through the cell envelope. The quality and reproducibility of the data allowed us to calculate rate constants for three different subassembly reactions. This kinetic analysis revealed that secB and secD mutants exhibit nearly identical defects in precursor translocation from the cytoplasm. However, subsequent subassembly reaction rates provided no clear evidence for an additional role for SecD in LamB assembly. Moreover, we found that surA mutants are qualitatively indistinguishable from yfgL mutants, suggesting that the products of both of these genes share a common function in the assembly process, most likely the delivery of LamB to the YaeT assembly complex in the outer membrane.
The cell envelope of gram-negative bacteria, such as Escherichia coli, is composed of an outer membrane, an inner (cytoplasmic) membrane, and an aqueous space termed the periplasm that lies between them. The outer membrane bilayer is asymmetric; the outer leaflet contains lipopolysaccharides (LPS), and the inner leaflet contains phospholipids. Generally speaking, two types of proteins are found in the outer membrane. Lipoproteins have an amino-terminal lipid modification that anchors the proteins in the inner leaflet of the outer membrane. Integral outer membrane proteins (OMPs) adopt a three-dimensional β-barrel conformation as a result of the packing of amphipathic antiparallel β-strands (16, 17, 23). We want to understand outer membrane biogenesis, a process that occurs outside the cytoplasm in an environment that lacks obvious energy sources, such as ATP. In particular, we want to understand how OMPs are targeted to and assembled in the outer membrane.
OMPs are synthesized initially as precursors (preproteins) with an amino-terminal extension known as the signal sequence (Fig. 1A). During synthesis, OMP polypeptides emerging from the ribosome are bound by the cytoplasmic chaperone SecB. SecB functions to maintain preproteins in an unfolded, translocation-competent state. In addition, SecB functions together with the signal sequence to direct the preprotein to the Sec translocation machinery in the inner membrane (18). The Sec machinery includes the membrane-associated ATPase, SecA (27), the channel-forming proteins SecYEG (26), and the associated proteins SecDF and YajC (5, 7). Although the exact role of the SecDF-YajC complex is not clear, the observation that E. coli spheroplasts prepared from SecD mutant cells have a defect in the release of processed envelope proteins into the media suggests that the SecDF-YajC complex may function at later steps in the translocation reaction (13). In the periplasm of E. coli, chaperones, such as Skp or SurA, have been implicated in the biogenesis of OMPs (2, 3, 15). By interacting with unfolded OMPs, these chaperones are thought to prevent OMP misfolding and aggregation and to deliver their protected cargo across the periplasm to the OMP assembly complex formed by the OMP YaeT and the associated lipoproteins NlpB, YfiO, and YfgL (28).
FIG. 1.
Representation of LamB biogenesis. (A) LamB is synthesized in the cytoplasm in a precursor form with a signal sequence (cross-hatched box) at the amino terminus. SecB functions to maintain precursor proteins in an export-competent conformation and to aid in their delivery to the protein translocation machinery in the cytoplasmic membrane (SecA, SecYEG, SecDF, YajC). Periplasmic chaperones, such as SurA or Skp, and DegP deliver proteins to the YaeT assembly site in the outer membrane (YaeT, YfgL, YfiO, NlpB). (B) Kinetic model for the LamB assembly pathway, showing LamB trimer and the various assembly intermediates, precursor LamB (pLamB), unfolded mature LamB (uLamB), and folded monomer (fLamB), and the reactions involved in their formation and interconversion, which are characterized by different rate constants (k1, k2, k3, k4, k11, and k12). Precursor LamB that has fallen off the assembly pathway is designated p′.
In this work we used pulse-chase analysis of wild-type and various mutant strains to determine the roles of SecB, SecD, SurA, and YfgL in the biogenesis of the E. coli LamB protein, a trimeric OMP that functions in the transport of maltose and maltose polymers and also serves as the receptor for bacteriophage λ. Our results show that SecB and SecD are required for efficient translocation of precursor LamB from the cytoplasm, but they provide no clear evidence for an additional role for SecD in the periplasm. Our results also show that SurA and YfgL are required for efficient delivery of processed LamB to the YaeT assembly complex in the outer membrane.
MATERIALS AND METHODS
Bacterial strains.
The E. coli strains used in this study are MC4100 [F− araD139 Δ(arg-lac)U169 rpsL150 relA1 flb5301 deoC1 ptsF25 rbsR] and secB::Tn5, secD57 zja Tn10, surA::Cm, and yfgL::Km mutant derivatives of this strain. Standard P1 transductions were used to construct an isogenic set of strains (24).
Media and growth conditions.
Each strain was grown in M63 minimal medium (24) supplemented with 0.4% (wt/vol) glycerol at 30°C. Overnight cultures were diluted 1/100 in 5 ml of fresh glycerol minimal medium and grown to a turbidity at 600 nm between 0.3 and 0.4. At this optical density the cells were transferred to 23°C, and 0.4% (wt/vol) maltose was added in order to induce LamB expression. The cells were incubated at this temperature for 1 h in preparation for pulse-chase experiments.
Pulse-chase and gentle lysis.
A pulse of 20 μCi/ml of [35S]methionine was used for labeling newly synthesized proteins. In a typical experiment 3 ml of cells was labeled with 4 μl of a 15-mCi/ml [35S]Met solution. After a 1-min pulse, cold methionine (chase) was added to a final concentration of 25 mM (zero time). At this time and 2, 5, 10, and 20 min after the addition of the chase, 250-μl aliquots were transferred to Eppendorf tubes and frozen in an ethanol-dry ice bath in preparation for gentle cell lysis. For gentle lysis the samples were thawed and centrifuged, and the bacterial pellet was resuspended in 50 μl of 20 mM Tris (pH 7.5) buffer containing 1 mM EDTA and 0.5 mg/ml of lysozyme. After three cycles of freezing in ethanol-dry ice and thawing, DNase was added to a final concentration of 0.5 mg/ml. After a 5-min incubation at room temperature, 50 μl of 40 mM Tris (pH 7.5) buffer containing 20 mM EDTA and 4% (wt/vol) sodium dodecyl sulfate (SDS) was added. At this time the extracts were subjected to immunoprecipitation.
Immunoprecipitation.
Fifty-microliter portions of [35S]methionine-labeled extracts were mixed with 950 μl of 20 mM Tris (pH 7.5) buffer containing 150 mM NaCl and 0.75% (vol/vol) Tween 20 (immunoprecipitation buffer), which decreased the concentration of SDS to 0.1% (wt/vol). Polyclonal antibodies against unfolded or trimeric LamB and maltose-binding protein (MBP) (14) were added (1/5,000 and 1/10,000 dilutions for LamB and MBP antibodies, respectively), and each mixture was incubated at 4°C for 1 h in a rotatory apparatus. Protein A-Sepharose beads (GE Healthcare) were added, and the samples were incubated at 4°C for another hour. The immunocomplexes were then pelleted in an Eppendorf centrifuge and resuspended in 1 ml of immunoprecipitation buffer. After four cycles of centrifugation and resuspension in 20 mM Tris (pH 7.5) buffer containing 1 mM EDTA, the immunocomplexes were finally resuspended in 120 μl of 70 mM Tris (pH 7.5) buffer containing 10% (vol/vol) glycerol, 2% (wt/vol) SDS, 0.01% (wt/vol) bromophenol blue, and 5% (vol/vol) β-mercaptoethanol. After 2 h of incubation at room temperature, the samples were centrifuged and the supernatants were transferred to new tubes.
SDS-polyacrylamide gel electrophoresis and autoradiography.
A 10% SDS-polyacrylamide gel was prepared for electrophoresis. It was cast on a fixed-height single-gel vertical unit (16.5 by 22 cm; Sigma). Before the gel was loaded, each immunoprecipitated sample was divided into three parts, and the parts were placed in Eppendorf tubes and incubated at 40, 70, or 100°C for 10 min. Twenty-microliter samples were loaded in the lanes, and electrophoresis was performed overnight at room temperature at a constant voltage of 50 V. The next morning the gel was dried and exposed for 48 h to X-ray film.
Densitometric analysis.
After the X-ray films were scanned, the bands corresponding to LamB (precursor, mature, folded monomer, and LPS-free trimer), MBP, and OmpA were quantified using the Image J 1.33 u software (Waine Rasband, National Institutes of Health, United States).
Modeling LamB assembly.
Our mathematical model for LamB biogenesis involves the concentrations of five radioactively labeled LamB species, the precursor (p), off-pathway precursor (p′), unfolded monomer (u), folded monomer (f), and trimer (t) (Fig. 1B). We model the time dependence of the five concentrations with five linear ordinary differential equations (ODEs). The right side of each ODE consists of a positive term describing production or maturation from the previous species and a negative term describing maturation into the next species (or dilution by growth in the case of the trimer). The positive term α(t) on the right side of equation 1 for the precursor is a square pulse having amplitude α0 lasting from −1 min to zero time, representing the 1-min pulse of radioactive precursor followed by the chase. The right sides of the ODEs are linear for concentration, which corresponds to the assumption that each LamB molecule matures independently.
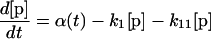 |
(1) |
 |
(1′) |
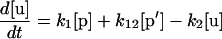 |
(2) |
 |
(3) |
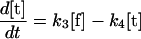 |
(4) |
The six rate constants, k1, k11, k12, k2, k3, and k4, as well as the amplitude (α0) of the pulse are fitting parameters. Note that the initial radioactive concentrations have not reached steady-state values at the time of the chase (zero time) because of the finite duration of the pulse (1 min). The best-fit parameters for each strain are obtained by numerically integrating the ODEs for different parameter choices and minimizing the sum of the squares of the deviations between theory and experiment at the various times (least-squares fit). The sum of the precursor concentration ([p]) and the off-pathway precursor concentration ([p′]) are compared to the experimental precursor concentration. For the wild-type, secB, and secD strains, our estimate for k11 indicates that there is negligible production of the off-pathway precursor, so for these strains fits are presented with k11 and k12 set to zero. Error estimates for the rate constants and α0 values are obtained as follows. For each strain, we start with the best-fit parameters. We then increase or decrease one rate constant or α0, keeping all other parameters fixed, until the least-squares error for the fit to the time course data for one of the four measured LamB species is 30% greater than its best-fit value. Table 1 shows the increase and decrease along with the best-fit value for each parameter and strain. (The use of a linear ODE may not be justified for trimer formation, which must involve at least three LamB molecules. However, the limited data do not support use of a nonlinear equation, particularly because only the radioactive concentration of LamB, not the total concentration, is measured and because the linear equation provides an adequate fit.)
TABLE 1.
Parameter values (rate constants and α0) for the various LamB subassembly reactionsa
| Strain | α0 (error estimate) (min−1) | Rate constant (error estimate) (min−1)
|
|||||
|---|---|---|---|---|---|---|---|
| k1 | k11 | k12 | k2 | k3 | k4 | ||
| Wild type | 7,050 (+620, −220) | 1.53 (+0.95, −0.2) | 0.42 (+0.15, −0.05) | 0.36 (+0.21, −0.04) | 0.0 (+0.01, 0) | ||
| secB | 11,490 (+420, −10) | 0.09 (+0.01, −0.01) | 0.56 (+0.25, −0.01) | 0.69 (+6.08, −0.01) | 0.0 (+0.01, 0) | ||
| secD57 | 9,480 (+170, −1,160) | 0.18 (+0.01, −0.04) | 0.37 (+0.16, −0.11) | 0.40 (+0.1, −0.16) | 0.0 (+0.02, 0) | ||
| surA | 1,640 (+90, −40) | 2.45 (+0.21, −0.51) | 0.50 (+0.19, −0.23) | 0.01 (+0.01, −0.01) | 0.02 (+0.01, −0.01) | 0.64 (+0.1, −0.03) | 0.01 (+0.02, −0.01) |
| yfgL | 5,670 (+320, −50) | 1.87 (+0.06, −0.12) | 0.80 (+0.13, −0.07) | 0.03 (+0.02, −0.01) | 0.10 (+0.01, −0.01) | 0.28 (+0.1, −0.02) | 0.03 (+0.01, −0.01) |
Parameter values and error estimates were calculated as described in Materials and Methods.
For each strain, to quantify the error in the data, we added the radioactive label values of the four LamB species at each time. Assuming that no radioactive label is incorporated into LamB following the chase and neglecting LamB decay, the total amount of label in all four LamB species should be the same at each time. Table 2 shows the mean for the total label and the standard deviation for the five times for each strain. The standard deviation is typically ∼10% of the mean; the only exception is the standard deviation for the surA strain, which is somewhat higher. Here the lower α0 value reflects the known LamB synthesis defect in surA strains (21). Thus, despite the multistep procedure the quantitative consistency of the data over the time course of the experiment is high.
TABLE 2.
Quantification of the error in the pulse-chase experimentsa
| Strain | LamB (total ± SD) |
|---|---|
| Wild type | 8,340 ± 1,160 |
| secB | 11,950 ± 800 |
| secD | 10,140 ± 1,030 |
| surA | 1,960 ± 410 |
| yfgL | 7,650 ± 640 |
Standard deviations were calculated as described in Materials and Methods.
RESULTS
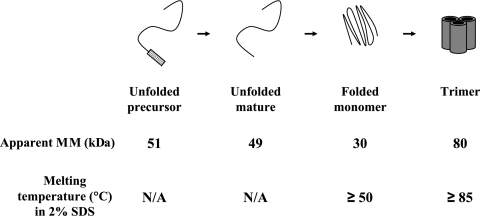
Like many OMPs, trimeric LamB and several folded assembly intermediates of LamB are stable when they are displayed on SDS-polyacrylamide gels, provided that the samples were not heated above the melting point of the folded species. During electrophoresis the folded assembly intermediates migrate with characteristic apparent molecular weights as a consequence of their compactness or multimeric state. Thus, using pulse-chase analysis and immunoprecipitation, the kinetics of LamB targeting and assembly can be monitored. Previous studies using similar methods have revealed an ordered LamB assembly pathway; unfolded precursor LamB (pLamB) is processed to an unfolded mature monomer (uLamB) that is converted to a folded monomer (fLamB) that oligomerizes into a stable trimeric species (Fig. 1 and 2) (14, 21). OmpA, a monomeric OMP that has a largely structural role, also migrates as a compact folded species (fOmpA) unless it is heat denatured (uOmpA). Although there is evidence for the existence of OmpA folding intermediates, species other than uOmpA or the precursor form (pOmpA) with its signal sequence still attached have not been detected in vivo (6, 8).
FIG. 2.
LamB assembly intermediates (21). The apparent molecular mass (MM) of each conformer and the corresponding melting temperatures in 2% SDS are indicated. N/A, not applicable.
Kinetics of LamB assembly in wild-type cells.
In wild-type cells growing at 37°C, LamB assembly is extremely fast, and consequently, assembly intermediates are present at vanishingly low levels. To slow the assembly process and allow meaningful quantification of assembly intermediates, we monitored LamB assembly in cells growing at 23°C. Exponentially growing cells were subjected to a pulse-chase and then lysed under nondenaturing conditions to retain the native OMP conformations. Samples were then immunoprecipitated using antibodies that recognize unfolded LamB (precursor and mature) or folded LamB (monomers, trimers, or trimers associated with LPS molecules). These antibodies cross-react with both folded and unfolded OmpA. Antibodies raised against the periplasmic MBP were used to provide a loading control. Before the conformers were resolved on an SDS gel, the solubilized proteins were heated to 40, 70, or 100°C for 10 min.
As shown in Fig. 3A, in the sample heated to 40°C, unfolded pLamB and unfolded uLamB migrate at their true molecular weights, approximately 51,000 and 49,000, respectively. LamB folded monomers (fLamB) migrate at an apparent molecular weight of 30,000. Because of their interaction with LPS, LamB trimers migrate as a high-molecular-weight smear. The disappearance of pLamB as it is processed to uLamB and the disappearance of uLamB as it is converted to fLamB are readily apparent. In the 40°C sample shown in Fig. 3A, OmpA remained folded and migrated at an apparent molecular weight of 25,000. Even at low growth temperatures, formation of folded OmpA (fOmpA) is very fast; there is no detectable precursor or unfolded OmpA (uOmpA).
FIG. 3.
Immunoprecipitation of LamB, MBP, and OmpA from wild-type E. coli grown at low temperature. Cells were pulse-labeled and gently lysed, and extracts were immunoprecipitated as described in Materials and Methods. (A) Autoradiography showing the positions of 35S-labeled LamB, OmpA, and MBP. LamB-LPS, LPS-associated LamB; LamB trimer, LPS-free LamB trimers; pLamB, precursor LamB; uLamB, mature LamB; fLamB, folded LamB monomer; uOmpA, unfolded OmpA; fOmpA, folded OmpA. The position of MBP is also indicated. (B) Graphic representation of LamB levels obtained from band quantification (time points) or mathematical modeling (curves).
At 70°C the folded LamB monomer melts and is converted to unfolded, mature monomer, and since LamB-LPS interactions are disrupted, a major band at approximately 80 kDa corresponding to the stable LPS-free LamB trimer appears (Fig. 3A). The time-dependent appearance of stable LamB trimer is readily apparent. OmpA remains folded at this temperature.
Sample incubation at 100°C melts the LamB trimers and all assembly intermediates, converting them to unfolded mature monomers. Likewise, folded OmpA melts, forming the unfolded molecule (uOmpA), which migrates at its true molecular weight, approximately 30,000.
Previous work on the biogenesis of LamB revealed the presence of a temperature-sensitive metastable trimer, an assembly intermediate whose formation preceded the formation of the stable LamB trimer (14, 21). The metastable trimer melted when it was incubated at temperatures higher than 50°C. Under our experimental conditions, where cells are grown at a low temperature and then samples are incubated at a higher temperature, the amount of unfolded mature LamB at 70°C is equal to the sum of the amount of unfolded mature LamB and the amount of folded LamB monomer present in the 40°C sample. In other words, there is no detectable trimeric LamB species that melts at 70°C or a lower temperature because we can already account for all of the uLamB present after heating at this temperature. Thus, under our conditions, metastable LamB trimers, if they are present at all, are short-lived intermediates. For this reason metastable trimers are not considered in our calculations.
In the biogenesis of LamB and other OMPs, the level of each of the different assembly intermediates (Fig. 1B and 2) is a function not only of the rate at which it is formed but also of the rate at which it is transformed into the next intermediate. To estimate the rate constants of the partial reactions in LamB assembly, we solved five coupled differential equations (see Materials and Methods) and fit the time dependence of the levels of the intermediates to the experimental data (Table 1). As shown in Fig. 3B, a computer-generated plot for LamB assembly that was prepared using these calculated rate constants fits the experimental data well.
SecB and SecD function in translocation.
SecB is an abundant homotetrameric cytosolic protein that has multiple roles. It maintains precursor proteins in an export-competent state, and it helps target them to the secretion machinery via an interaction with SecA (18). Given the cellular location of SecB and its roles in translocation, we did not expect its absence to have any effect on the steps in LamB assembly that occur outside the cytoplasm.
In the absence of SecB, a translocation defect for LamB, OmpA, and MBP is evident (Fig. 4A). Indeed, for the strain used a pulse-chase analysis showed that the major conformer of LamB present at the start of the chase following a 1-min pulse is the precursor protein; the levels of the mature unfolded form, folded LamB monomer, and trimeric species are significantly lower. In this mutant, translocation is clearly the rate-limiting step in LamB assembly; the rate of pLamB processing (k1) is at least 10-fold lower than the wild-type rate (Table 1) (note from the error estimate that the true wild-type k1 may be higher still), and we think that this is a direct reflection of the defect in translocation, since processing cannot occur until the precursor is translocated. As expected, the rates for events that occur outside the cytoplasm, such as monomer folding (k2) and trimer assembly (k3), are not decreased (Fig. 4B and Table 1).
FIG. 4.
Translocation is compromised in secB cells, but the extracytoplasmic events leading to LamB assembly are not compromised in these cells. (A) Autoradiography showing the positions of 35S-labeled LamB, OmpA, and MBP. upOmpA, unfolded precursor OmpA; fpOmpA, folded precursor OmpA; pMBP, precursor MBP. For an explanation of other designations see the legend to Fig. 2. The position of MBP is also indicated. (B) Graphic representation of LamB levels obtained from band quantification (time points) or mathematical modeling (curves).
In the absence of SecB the precursor forms of LamB, OmpA, and MBP accumulate in the cytoplasm (9). Despite this targeting defect, we noted that the precursor form of OmpA, which migrates slightly slower than the mature protein owing to the presence of the signal sequence, assumed a folded structure that appears to be similar to the structure assumed by the mature OmpA protein, which is translocated and likely targeted to the outer membrane (Fig. 4A). As in the case of the mature OmpA protein, temperatures above 70°C are required to melt the folded OmpA precursor.
Careful examination and analysis of the data for 100°C samples in Fig. 4 showed that precursor forms of OmpA and LamB are quantitatively converted to the mature forms. Despite the translocation defect caused by the lack of SecB, the precursor forms of these proteins remain translocation competent. LamB appears to remain unfolded in the cytoplasm. However, as noted above, precursor OmpA appears to assume a stably folded structure. This result is in agreement with previous reports showing that when the translocation machinery is compromised, the cytosolic precursor form of OmpA adopts a translocation-competent folded conformation that is later unfolded for translocation and processing (12). In contrast, a significant amount of precursor MBP remains in the cytoplasm. Precursor MBP folds in the cytoplasm into an export-incompetent structure (10).
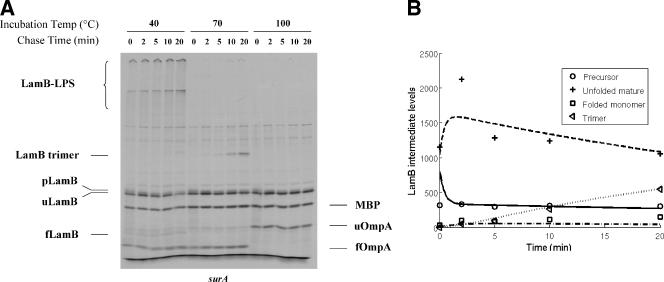
Although the exact role of the SecDF-YajC complex is not clear, the observation that spheroplasts prepared from secD mutant cells are defective for release into the medium of processed envelope proteins suggested that SecD is involved in an extracytoplasmic step in the targeting process (13). To determine if SecD plays a role not only in translocation but also in a subsequent step in LamB targeting, we employed strains carrying the cold-sensitive secD57 mutation (19). As shown in Fig. 5A, there is a clear delay in the maturation of the precursor forms of LamB, MBP, and OmpA, reflecting the known translocation defect caused by this mutation. Under conditions where SecD is largely inactive owing to the cold-sensitive secD57 mutation, the defect in LamB translocation and processing (k1) is nearly as severe as the defect seen in secB mutants (Table 1). Moreover, in the secD mutant the k2 and k3 values are indistinguishable from the wild-type values. Thus, at least for LamB, we obtained no clear evidence for an additional function for SecD beyond translocation.
FIG. 5.
Translocation is compromised in the absence of a functional SecD protein, but the extracytoplasmic events leading to LamB assembly are not compromised in the absence of this protein. (A) Autoradiography showing the positions of 35S-labeled LamB, OmpA, and MBP. For an explanation of designations see the legends to Fig. 2 and 3. (B) Graphic representation of LamB levels obtained from band quantification (time points) or mathematical modeling (curves).
SurA and YfgL function in the delivery of OMPs to the YaeT complex.
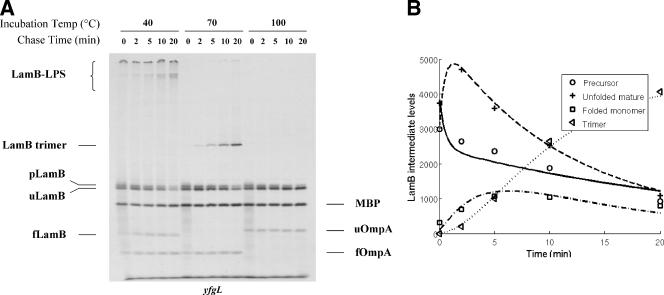
SurA, initially identified as a protein required for survival during the stationary phase (25), is a periplasmic chaperone that also has peptidyl-prolyl cis/trans-isomerase (PPIase) activity (21). However, since mutant SurA lacking its PPIase domains still retains chaperone activity and appears to be fully functional, the relevance of the PPIase activity is not yet clear (1). Mutants lacking SurA are defective in assembly of various OMPs; more specifically, they are defective in conversion of the unfolded mature LamB monomer into the folded monomer (11, 21). As expected, the pulse-chase analysis of the surA strain revealed a dramatically lower rate of conversion (k2) of the unfolded mature LamB into the folded monomer (Fig. 6A and Table 1).
FIG. 6.
Processing of pLamB is delayed in surA cells, but processing of pOmpA or pMBP is not delayed in these cells. (A) Autoradiography showing the positions of 35S-labeled LamB, OmpA, and MBP. For an explanation of designations see the legend to Fig. 2. Note that neither pOmpA nor pMBP was detected. (B) Graphic representation of LamB levels obtained from band quantification (time points) or mathematical modeling (curves).
What was not expected is that the surA mutant also showed a significant delay in the rate of processing of LamB (Fig. 6A). However, this defect in processing was limited to LamB, since the processing of OmpA and MBP was not affected by the lack of SurA. Since mutants lacking SecB or deficient in SecD exhibit defects for all three of these proteins (Fig. 4 and 5), we think that it is unlikely that SurA is directly involved in LamB translocation. Rather, we suggest that in the absence of SurA, a significant fraction of translocated LamB falls off the normal assembly pathway and assumes some conformation that hinders access to signal peptidase (p′ in Fig. 1B). This unprocessed p′LamB species was stable throughout our experiment. We observed little if any subsequent processing and return of the molecules to the normal assembly pathway (k12 in Table 1) or degradation. The calculated rate of formation of p′LamB (k11 in Fig. 1B and Table 1) suggests that about one-sixth of the total labeled LamB molecules assume this dead-end p′LamB conformation.
Viewed in this manner, the calculations revealed that the majority (∼80%) of LamB molecules are translocated and processed with normal kinetics in the surA mutant (k1 in Table 1). Although these molecules are converted into the folded monomer slowly (k2 in Table 1), once they assume this conformation they are assembled into trimers with normal kinetics (k3 in Table 1). In other words, the only defect that we can detect for the majority of LamB molecules that remain in the assembly pathway is a defect in conversion of the unfolded monomer to the folded monomer (k2 in Table 1). These results are consistent with the idea that SurA functions to deliver LamB to the outer membrane assembly site.
YfgL is a nonessential outer membrane lipoprotein component of the essential YaeT complex (22, 28). Mutants lacking YfgL exhibit modest OMP assembly defects (22). Our kinetic analysis of LamB assembly (Fig. 7 and Table 1) showed that, as observed in cells lacking SurA, both the processing of precursor LamB and the formation of folded LamB monomer (k2) are affected by a lack of YfgL, but the processing of precursor OmpA or precursor MBP is not affected. Accordingly, we concluded that the Sec translocation machinery operates normally. Indeed, given its cellular location, it is unlikely that YfgL functions in this process. Again, calculations revealed that a fraction of the LamB molecules (about 30%) fall off the normal assembly pathway (k11 in Table 1) and that these molecules are essentially dead ended. However, the majority of the LamB molecules are translocated and processed normally in a yfgL mutant (k1 in Table 1). These molecules are converted into folded monomers slowly, but once converted, they are assembled into trimers with normal kinetics. Strikingly, in terms of LamB, OmpA, and MBP assembly, yfgL mutants are qualitatively indistinguishable from surA mutants (Fig. 6 and 7 and Table 1).
FIG. 7.
surA and yfgL cells display similar defects in pLamB processing. (A) Autoradiography showing the positions of 35S-labeled LamB, OmpA, and MBP. For an explanation of designations see the legend to Fig. 2. Note that neither pOmpA nor pMBP was detected. (B) Graphic representation of LamB levels obtained from band quantification (time points) or mathematical modeling (curves).
DISCUSSION
OMP assembly is a complex multistep process that involves cellular machinery with components in all of the cellular compartments of E. coli. The cytoplasmic chaperone SecB binds precursor molecules to prevent their misfolding and delivers them to the Sec translocation machinery in the inner membrane for translocation to the periplasm. In the aqueous periplasm, additional chaperones, such as SurA, prevent misfolding and deliver OMPs to an outer membrane assembly site which is composed of the large β-barrel protein YaeT and three lipoproteins, YfiO, YfgL, and NlpB (Fig. 1). By growing cells at a low temperature to slow the assembly process and using mutants that each lack a nonessential targeting component in one of four cellular compartments, we have been able to follow the biogenesis of LamB from the site of synthesis to the final destination.
The methods employed allow us to subdivide LamB assembly into three distinct steps, each characterized by a distinct rate constant (Fig. 1B). Step 1, characterized by the rate constant k1, is signal sequence processing, which requires translocation of the precursor molecule from the cytoplasm to form an unfolded mature LamB. Step 2 is the formation of a folded monomer, characterized by the rate constant k2. Previous work has shown that the folded monomer is present in the outer membrane (4, 14), and so k2, at least in part, describes transit through the periplasm. Step 3 is the formation of the LamB trimer, characterized by the rate constant k3. This reaction occurs in the outer membrane and requires the YaeT complex (14, 28).
Our primary goal was to probe the mechanistic role of the inner membrane Sec machinery component SecD and the YaeT complex component YfgL in LamB targeting and assembly. The chaperones SecB and SurA, which have previously been characterized in greater detail, served as controls. In particular, we wanted to determine if SecD functioned in any assembly step after translocation, a possibility raised by previous work (13). We also hoped to establish a functional role for YfgL.
The first major conclusion of our kinetic analysis of LamB assembly is that we can detect no clear differences between secB and secD mutants. In both mutants, the rate of LamB translocation and processing is decreased, as expected (k1 in Table 1). Moreover, we can detect no clear defect in k2 or k3 in either mutant. Data obtained by Matsuyama et al. (13) suggest that SecD has a role in the release of translocated proteins into the periplasm. Although our data provide no support for such a posttranslocational role for SecD, they do not exclude this possibility. For example, it is possible that SecD does have a posttranslocational role that is performed prior to precursor processing. Defects in such a proposed role would not have been detectable in our experiments unless some fraction of the translocated but unprocessed precursor fell off the assembly pathway (see below). If SecD has such a posttranslocational role, then LamB, OmpA, and MBP are likely to be affected equally since we observed comparable defects with all three proteins in both secB and secD mutants.
The second major conclusion of our kinetic analysis of LamB assembly is that surA and yfgL mutants are qualitatively indistinguishable. Both surA and yfgL mutations have strong slowing effects on k2 but no effect on k3. Because the defects in yfgL and surA mutants are largely indistinguishable, we suggest that the SurA and YfgL proteins share a common function in the assembly process. In particular, since k3 (i.e., LamB trimerization) is not clearly affected in either mutant but k2 is reduced in both mutants, we think that SurA and YfgL are involved in the delivery of LamB to the YaeT assembly complex in the outer membrane. As described below, we believe that the effect of surA and yfgL mutations on LamB precursor processing is due to a fraction of the LamB molecules that fall off the assembly pathway.
A role for SurA in the delivery of OMPs to the YaeT assembly complex is not surprising. SurA is a well-characterized periplasmic chaperone that is known to play an important role in LamB assembly (11, 21). Since LamB must transit the periplasm on its way to the outer membrane and since periplasmic chaperone activity appears to be required (11, 20, 21), SurA is a likely candidate to fulfill this delivery role. SurA is not essential, because in its absence other periplasmic chaperones, such as Skp or DegP, can perform this role (20).
YfgL is an outer membrane lipoprotein, the bulk of its mass faces the periplasm, and it interacts directly with YaeT (28). Thus, this protein is in the proper position to function in OMP delivery to the outer membrane. YfgL cannot be the sole component of the delivery site complex because LamB delivery still occurs, albeit at a reduced rate, in its absence. Furthermore, YfgL is not essential. However, we suspect that a role in the delivery of LamB and other OMPs to YaeT is an important function of YfgL. LamB trimerization, a step that occurs after delivery to the outer membrane, proceeds normally in the absence of this lipoprotein. Again, although the assay is less sensitive, it appears that OmpA delivery to the outer membrane is not affected by the absence of YfgL. Thus, it is possible that YfgL is required for the efficient delivery of only a subset of OMPs.
Surprisingly, both surA and yfgL mutants show a significant delay in the processing of precursor LamB (Fig. 6 and 7). Since YfgL is an outer membrane protein, we think that it is unlikely that this protein plays a role in LamB translocation and processing. Although we cannot exclude the possibility that SurA has a specific role in LamB translocation, we think that such a role is unlikely as well. If precursor LamB remained associated with the Sec machinery, we would expect that the function of this machinery would be generally affected, and this is clearly not the case. OmpA and MBP are translocated normally in surA and yfgL mutants. We think that it is more likely that in the absence of SurA or YfgL, some fraction of translocated LamB falls off the normal assembly pathway and, as a consequence, productive interaction with signal peptidase is hindered. Indeed, calculations accounting for the fraction of LamB molecules that fall off the assembly pathway (p′LamB) revealed a normal translocation and processing rate for the remaining fraction of LamB molecules in both surA and yfgL mutants (k1 in Table 1). We suggest that SurA interacts with LamB before its translocation is complete. This interaction prevents precursor LamB from misfolding (the formation of p′LamB), allowing efficient interaction with signal peptidase. After processing, SurA then delivers LamB to the YaeT assembly complex via YfgL.
We propose that in the yfgL mutant the delivery defect causes a backup of unassembled LamB, and this assembly pathway overload likely titrates SurA. We think that it is this decrease in functional SurA that is responsible for the LamB precursor-processing defect for reasons described above. Indeed, we propose that the formation of p′LamB is diagnostic for a lack of functional SurA.
It is possible that the k3 value in the surA mutant is slightly greater than the wild-type value and that the k3 value in the yfgL mutant is slightly less than the wild-type value. The difference is more pronounced if the k3 values in the surA and yfgL mutants are compared, and this may be the more relevant comparison. In surA mutants, defects in outer membrane protein synthesis (21) and defects in delivery would decrease the protein traffic that reaches the YaeT complex in the outer membrane. Such a decrease in traffic might increase the availability of components of the YaeT complex, thus increasing the rate of outer membrane protein assembly (k3). If the sole function of YfgL was to accept delivery of proteins from SurA, then we would expect a similar defect in traffic in the yfgL mutant since the SurA function would be quickly titrated by the assembly intermediates that accumulate in the absence of an important delivery destination. Yet we did not observe a similar increase in k3 in the yfgL mutant. This could indicate that YfgL has an additional function in assembly steps downstream from delivery.
SurA and YfgL are both required for the efficient formation of LamB folded monomer, and evidence that the folded monomer is present in the outer membrane has been presented previously (4, 14). Therefore, we suspect that SurA delivers unfolded monomer to YfgL and the YaeT assembly complex. In other words, we suspect that the YaeT complex is required for all aspects of LamB folding, including folding of the monomer, insertion into the outer membrane, and probably even trimerization. Now that the components of this assembly machine have been identified, we hope that insights into these folding reactions will be forthcoming.
Acknowledgments
We thank members of the Silhavy lab, particularly Natividad Ruiz, for their helpful suggestions and Susan DiRenzo for her assistance in preparing the manuscript.
This work was supported by grant GM34821 to T.J.S. from the National Institute of General Medical Sciences.
Footnotes
Published ahead of print on 27 October 2006.
REFERENCES
- 1.Behrens, S., R. Maier, H. de Cock, F. X. Schmid, and C. A. Gross. 2001. The SurA periplasmic PPIase lacking its parvulin domains functions in vivo and has chaperone activity. EMBO J. 20:285-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bos, M. P., and J. Tommassen. 2004. Biogenesis of the Gram-negative bacterial outer membrane. Curr. Opin. Microbiol. 7:610-616. [DOI] [PubMed] [Google Scholar]
- 3.Duguay, A. R., and T. J. Silhavy. 2004. Quality control in the bacterial periplasm. Biochim. Biophys. Acta 1694:121-134. [DOI] [PubMed] [Google Scholar]
- 4.Duguay, A. R., and T. J. Silhavy. 2002. Signal sequence mutations as tools for the characterization of LamB folding intermediates. J. Bacteriol. 184:6918-6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duong, F., and W. Wickner. 1997. Distinct catalytic roles of the SecYE, SecG and SecDFyajC subunits of preprotein translocase holoenzyme. EMBO J. 16:2756-2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freudl, R., H. Schwarz, Y. D. Stierhof, K. Gamon, I. Hindennach, and U. Henning. 1986. An outer membrane protein (OmpA) of Escherichia coli K-12 undergoes a conformational change during export. J. Biol. Chem. 261:11355-11361. [PubMed] [Google Scholar]
- 7.Gardel, C., K. Johnson, A. Jacq, and J. Beckwith. 1990. The secD locus of E. coli codes for two membrane proteins required for protein export. EMBO J. 9:3209-3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kleinschmidt, J. H., and L. K. Tamm. 1996. Folding intermediates of a β-barrel membrane protein. Kinetic evidence for a multi-step membrane insertion mechanism. Biochemistry 35:12993-13000. [DOI] [PubMed] [Google Scholar]
- 9.Kumamoto, C. A. 1989. Escherichia coli SecB protein associates with exported protein precursors in vivo. Proc. Natl. Acad. Sci. USA 86:5320-5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumamoto, C. A., and P. M. Gannon. 1988. Effects of Escherichia coli secB mutations on pre-maltose binding protein conformation and export kinetics. J. Biol. Chem. 263:11554-11558. [PubMed] [Google Scholar]
- 11.Lazar, S. W., and R. Kolter. 1996. SurA assists the folding of Escherichia coli outer membrane proteins. J. Bacteriol. 178:1770-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lecker, S. H., A. J. Driessen, and W. Wickner. 1990. ProOmpA contains secondary and tertiary structure prior to translocation and is shielded from aggregation by association with SecB protein. EMBO J. 9:2309-2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsuyama, S., Y. Fujita, and S. Mizushima. 1993. SecD is involved in the release of translocated secretory proteins from the cytoplasmic membrane of Escherichia coli. EMBO J. 12:265-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Misra, R., A. Peterson, T. Ferenci, and T. J. Silhavy. 1991. A genetic approach for analyzing the pathway of LamB assembly into the outer membrane of Escherichia coli. J. Biol. Chem. 266:13592-13597. [PubMed] [Google Scholar]
- 15.Mogensen, J. E., and D. E. Otzen. 2005. Interactions between folding factors and bacterial outer membrane proteins. Mol. Microbiol. 57:326-346. [DOI] [PubMed] [Google Scholar]
- 16.Nikaido, H. 2003. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 67:593-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nikaido, H. 1996. Outer membrane, p. 29-47. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella typhimurium: cellular and molecular biology, 2nd ed. ASM Press, Washington, DC.
- 18.Randall, L. L., and S. J. Hardy. 2002. SecB, one small chaperone in the complex milieu of the cell. Cell. Mol. Life Sci. 59:1617-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riggs, P. D., A. I. Derman, and J. Beckwith. 1988. A mutation affecting the regulation of a secA-lacZ fusion defines a new sec gene. Genetics 118:571-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rizzitello, A. E., J. R. Harper, and T. J. Silhavy. 2001. Genetic evidence for parallel pathways of chaperone activity in the periplasm of Escherichia coli. J. Bacteriol. 183:6794-6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rouviere, P. E., and C. A. Gross. 1996. SurA, a periplasmic protein with peptidyl-prolyl isomerase activity, participates in the assembly of outer membrane porins. Genes Dev. 10:3170-3182. [DOI] [PubMed] [Google Scholar]
- 22.Ruiz, N., B. Falcone, D. Kahne, and T. J. Silhavy. 2005. Chemical conditionality: a genetic strategy to probe organelle assembly. Cell 121:307-317. [DOI] [PubMed] [Google Scholar]
- 23.Ruiz, N., D. Kahne, and T. J. Silhavy. 2006. Advances in understanding bacterial outer-membrane biogenesis. Nat. Rev. Microbiol. 4:57-66. [DOI] [PubMed] [Google Scholar]
- 24.Silhavy, T., M. L. Berman, and L. W. Enquist. 1984. Experiments with gene fusions. Cold Spring Harbor Laboratory Press, Plainview, NY.
- 25.Tormo, A., M. Almiron, and R. Kolter. 1990. surA, an Escherichia coli gene essential for survival in stationary phase. J. Bacteriol. 172:4339-4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Veenendaal, A. K., C. van der Does, and A. J. Driessen. 2004. The protein-conducting channel SecYEG. Biochim. Biophys. Acta 1694:81-95. [DOI] [PubMed] [Google Scholar]
- 27.Vrontou, E., and A. Economou. 2004. Structure and function of SecA, the preprotein translocase nanomotor. Biochim. Biophys. Acta 1694:67-80. [DOI] [PubMed] [Google Scholar]
- 28.Wu, T., J. Malinverni, N. Ruiz, S. Kim, T. J. Silhavy, and D. Kahne. 2005. Identification of a multicomponent complex required for outer membrane biogenesis in Escherichia coli. Cell 121:235-245. [DOI] [PubMed] [Google Scholar]