Abstract
The CbbRRS system is an atypical three-protein two-component system that modulates the expression of the cbbI CO2 fixation operon of Rhodopseudomonas palustris, possibly in response to a redox signal. It consists of a membrane-bound hybrid sensor kinase, CbbSR, with a transmitter and receiver domain, and two response regulator proteins, CbbRR1 and CbbRR2. No detectable helix-turn-helix DNA binding domain is associated with either response regulator, but an HPt domain and a second receiver domain are predicted at the C-terminal region of CbbRR1 and CbbRR2, respectively. The abundance of conserved residues predicted to participate in a His-Asp phosphorelay raised the question of their de facto involvement. In this study, the role of the multiple receiver domains was elucidated in vitro by generating site-directed mutants of the putative conserved residues. Distinct phosphorylation patterns were obtained with two truncated versions of the hybrid sensor kinase, CbbSRT189 and CbbSRR96 (CbbSR beginning at residues T189 and R96, respectively). These constructs also exhibited substantially different affinities for ATP and phosphorylation stability, which was found to be dependent on a conserved Asp residue (Asp-696) within the kinase receiver domain. Asp-696 also played an important role in defining the specificity of phosphorylation for response regulators CbbRR1 or CbbRR2, and this residue appeared to act in conjunction with residues within the region from Arg-96 to Thr-189 at the N terminus of the sensor kinase. The net effect of concerted interactions at these distinct regions of CbbSR created an internal molecular switch that appears to coordinate a unique branched phosphorelay system.
Two-component signal transduction systems regulate the expression of genes important for a myriad of physiological processes common to prokaryotes (8); such systems have also been recently identified to function in eukaryotes as well (5, 9, 13). In its most simple iteration, two-component systems consist of a membrane-bound sensor histidine kinase protein that receives an environmental cue and then transfers the signal to a single soluble response regulator protein. The sensor histidine kinase protein has a nonconserved N-terminal region, dedicated to sensing a particular signal, attached to a conserved kinase domain. The response regulator protein typically consists of a conserved receiver domain and a variable effector domain. The general mechanism of signal transduction involves a linear pattern of events whereby autophosphorylation of the kinase precedes phosphorylation of the cognate response regulator protein, ultimately leading to a change in behavior determined by the nature of the effector domain. Thus, one important manifestation is the regulation of gene expression; alternatively, the signal transduction process might lead to modification of protein-protein interactions or the modulation of enzymatic activity (13). Numerous variations of the basic modular units have been described over the years; e.g., where the kinase core might stand alone as a sensor protein (2). Alternatively, the kinase domain may be associated with one or more receiver domains on the same protein to form a hybrid sensor kinase molecule (3, 10, 14). In the latter instance, an HPt domain is often interposed to facilitate phosphotransfer reactions between separate receiver domains. By contrast, receiver domains and HPt domains, where required, may be localized on completely separate proteins (2, 7, 9).
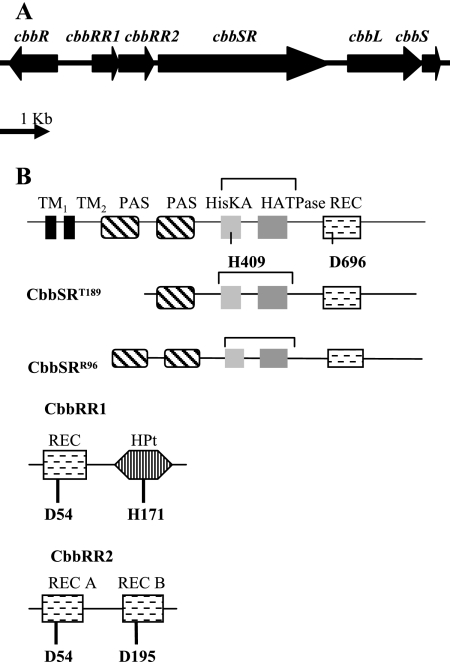
The CbbRRS (Calvin Benson Bassham response regulators sensor kinase) of Rhodopseudomonas palustris is a three-protein two-component system (11) consisting of a membrane-bound sensor kinase protein (CbbSR) and two response regulator proteins (CbbRR1 and CbbRR2) (Fig. 1). CbbSR is a large hybrid sensor kinase protein that, in addition to possessing two canonical transmembrane regions and a transmitter domain, also contains a C-terminal receiver domain. Two potential PAS motifs, predicted by PFAM analysis (http://www.ncbi.nlm.nih.gov/BLAST), are present in the N-terminal region (Fig. 1B). Response regulator 1, CbbRR1, has an HPt domain and a receiver domain. Response regulator 2, CbbRR2, has two receiver domains (Fig. 1B). Besides this modular organization, common among two-component signal transduction pathways, the components of the CbbRRS system have no homology to other known proteins (11). Collectively, the CbbRRS system was recently shown to regulate expression of the form I ribulose 1,5-bisphosphate carboxylase/oxygenase or cbbLS genes (Fig. 1A) of the cbbI operon of R. palustris (11). As the two response regulators, CbbRR1 and CbbRR2, presumably do not interact with DNA directly, due to the absence of a discernible helix-turn-helix motif, it was proposed that the response regulators posttranslationally modulate the CbbR transcription factor (encoded by the cbbR gene) (Fig. 1A), which is itself indispensable for the expression of the cbbLS genes (11). In a previous investigation, it was also shown that the full-length membrane-bound kinase, CbbSR, catalyzes phosphorylation of both response regulators (CbbRR1 and CbbRR2) independently, ruling out any absolute requirement for a multistep phosphorelay between the kinase and the two response regulator proteins. Clearly, a single sensor kinase has the potential to interact with four distinct receiver domains on three different proteins, including two different response regulator proteins. The four different receiver domains show limited amino acid identity (Fig. 2), and yet this system plays an important role with regard to the control of cbbLS transcription in R. palustris (11). To better understand what appears to be a complex signal transduction mechanism for modulating expression of genes required for CO2 assimilation under changing environmental conditions, we dissected the phosphorelay reactions in vitro and determined the role of each of the distinct functional domains of the three proteins. The likely route of the phosphoryl group following autophosphorylation of the histidine residue (His-409) on the sensor kinase was traced; also the roles of specific residues important for phosphotransfer within the receiver domain of the hybrid sensor kinase and each of the two response regulator proteins were determined. As a result of these studies, a potential internal molecular switch was identified within the sensor kinase that appeared to direct the route and specificity of phosphotransfer to the response regulator proteins.
FIG. 1.
Gene organization (A) and potential protein domains (B) found on the CbbRRS system proteins CbbSR, CbbRR1, and CbbRR2. The predicted conserved residues involved in phosphotransfer reactions are indicated. A schematic representation of the two truncated sensor kinase proteins (CbbSRT189 and CbbSRR96) is also shown.
FIG. 2.
Sequence alignment of the receiver domains present on proteins of the CbbRRS system (11). SR-REC, sensor kinase CbbSR; RR2-REC A, first receiver domain of response regulator CbbRR2; RR2-REC B, second receiver domain of response regulator CbbRR2; RR1-REC, receiver domain of response regulator CbbRR1. The asterisk indicates the conserved phosphorylation site. Conserved residues are shown in green, and identical residues are shown in yellow, whereas similar residues are shown in cyan.
MATERIALS AND METHODS
Strains, growth conditions, and plasmids.
Strains and plasmids used in this study are reported in Table 1. Escherichia coli DH5α was used for general cloning purposes as well as production of recombinant proteins. Cultures were grown at 37°C with constant shaking (200 rpm) in Luria-Bertani broth. Antibiotics were added at the following final concentrations: kanamycin, 50 μg ml−1; ampicillin, 100 μg ml−1. Standard molecular biology techniques were used for chromosomal DNA purification, restriction digests, and cloning (1). A QIAGEN Mini Prep kit was routinely employed to prepare plasmid DNA. When necessary, DNA fragments were purified from agarose gels using the Gel Purification Kit from QIAGEN. Plasmids pQE8027, pQE8024, pQE8740, pQE8797, and pQE8798 have been described elsewhere (11). The coding region corresponding to the predicted whole-cytoplasmic portion of sensor kinase CbbSR was PCR amplified by using the primers 5′-GGGGTACCCGGCGCAACGCGCACAAG-3′ and 5′-CCCAAGCTTTCAGGTCAGCTCGTG-3′ (restriction sites are underlined), in the presence of 1 U of Pfx Platinum DNA polymerase (Invitrogen), and directly cloned into pCR-Blunt II TOPO to generate plasmid pSR128. After sequencing ensured the absence of mutations, a KpnI/BamHI fragment of approximately 950 bp was ligated in frame into vector pQE8740 (11) by exploiting a unique BamHI site within the cbbSR gene to generate plasmid pQE12840 (CbbSRR96, indicating CbbSR starting from residue R96).
TABLE 1.
Strain and plasmids used in this study
| Strain or plasmid | Descriptiona | Source or reference |
|---|---|---|
| E. coli DH5α | General cloning strain and recombinant protein expression host | Invitrogen |
| Plasmids | ||
| pCR-Blunt II TOPO | General cloning plasmid for direct insertion of blunt-ended PCR products; Kmr | Invitrogen |
| pSR128 | Cytoplasmic region of the sensor kinase cbbSR starting from residue R96 cloned into pCR-Blunt II TOPO; Kmr | This study |
| pQE80 | cis-repressed, IPTG-inducible, N-terminal His6-tagged recombinant protein overexpression vector; Ampr | QIAGEN |
| pQE8027 | cbbRR1 coding region cloned into the BamHI/HindIII sites of pQE80; Ampr | 11 |
| pQE8055 | D54N cbbRR1 site-directed mutant cloned into the BamHI/HindIII sites of pQE80; Ampr | This study |
| pQE8057 | H171D cbbRR1 site-directed mutant cloned into the BamHI/HindIII sites of pQE80; Ampr | This study |
| pQE8073 | D54N H171D cbbRR1 site-directed double mutant cloned into the BamHI/HindIII sites of pQE80; Ampr | This study |
| pQE8024 | cbbRR2 coding region cloned into the BamHI/HindIII sites of pQE80; Ampr | 11 |
| pQE8060 | D54N cbbRR2 site-directed mutant cloned into the BamHI/HindIII sites of pQE80; Ampr | This study |
| pQE8061 | D195N cbbRR2 site-directed mutant cloned into the BamHI/HindIII sites of pQE80; Ampr | This study |
| pQE8075 | D54N D195N cbbRR2 site-directed double mutant cloned into the BamHI/HindIII sites of pQE80; Ampr | This study |
| pQE8740 | Truncated cbbSR coding region comprising residues 189 to 903 cloned into the KpnI/HindIII sites of pQE80; Ampr | 11 |
| pQE8797 | Truncated D696N cbbSR, starting from T189, cloned into the KpnI/HindIII sites of pQE80; Ampr | 11 |
| pQE8798 | Truncated H409D cbbSR, starting from residue T189, cloned into the KpnI/HindIII sites of pQE80; Ampr | 11 |
| pQE12840 | Truncated cbbSR coding region comprising residues 96 to 903 cloned into the KpnI/HindIII sites of pQE80; Ampr | This study |
| pQE12897 | Truncated D696N cbbSR, starting from residue R96, cloned into the KpnI/HindIII sites of pQE80; Ampr | This study |
| pQE12898 | Truncated H409D cbbSR, starting from residue R96, cloned into the KpnI/HindIII sites of pQE80; Ampr | This study |
IPTG, isopropyl-β-d-thiogalactopyranoside.
Construction of site-directed mutants.
Mutagenic PCR was performed to generate site-directed mutants of the sensor kinase and two response regulators. One specific residue was substituted at a time, using conditions recommended by the manufacturer (Quick Change site-directed mutagenic kit; Stratagene). All substitutions were verified after sequencing the mutagenic PCR products at the Plant-Microbe Genomic Facility at The Ohio State University before the products were subcloned into the pQE80 expression vector at appropriate restriction sites. Double site-directed mutants were generated by performing mutagenic PCRs on templates previously mutagenized. In particular, plasmids pQE8055 (D54NCbbRR1) and pQE8057 (H171DCbbRR1) were generated using the following mutagenic primer pairs (mutated codons are underlined): the pair 5′-CTGATCCTTAGCAACGTTTCGATGCCG-3′ and 5′-CGGCATCGAAACGTTGCTAAGG ATCAG-3′ (D54N) and the pair 5′-CGCCATGTGGCTGACAAGCTGGTCGGC-3′ and 5′-GCCGACCAGCTTGTCAGCCACATGGCG-3′ (H171D), respectively. The expression vector pQE8073 was used for the isolation of the recombinant CbbRR1 double mutant protein D54NH171DCbbRR1. Plasmids pQE8060 (D54NCbbRR2) and pQE8061 (D195NCbbRR2) were generated by using the following mutagenic primer pairs (mutated codons are underlined): the pair 5′-CTGATCATCGTCAACATCAACATGCCG-3′ and 5′-CGGCATGTTGATGTTGACGATG ATCAG-3′ (D54N) and the pair 5′-GTGGTGGTCACCAACTATCACATGCCG-3′ and 5′-CGGCATGTGATAGTTGGTGACCACCAC-3′ (D195N). The expression vector pQE8075 was used for the isolation of the CbbRR2 double mutant protein (D54ND195NCbbRR2). Plasmid pQE12897 (D696NCbbSRR96) and pQE12898 (H409NCbbSRR96) were derived from in-frame ligation of the KpnI/BamHI fragment from pSR128 into plasmids pQE8797 and pQE8798, respectively (11), as described above for the construction of pQE12840.
Purification of recombinant proteins.
N-terminal His6-tagged recombinant proteins, hereafter simply referred to as CbbSR, CbbRR1, and CbbRR2, were synthesized in E. coli and purified by nickel column chromatography as previously described (11).
Phosphorylation reactions.
Phosphorylation reactions were performed in 50 mM Tris-Cl, pH 8.0, 5 mM MgCl2, 50 mM KCl, and 0.1 mM dithiothreitol. The final concentration of [γ-32P]ATP (specific activity of 10 mCi/mmol; 1.7 μM stock solution) (Perkin Elmer) varied depending on the type of reaction being examined and is specified in the figure legends. The phosphorylation reactions were carried out at room temperature for the desired time and were terminated by adding 3× sample buffer (37.5 mM Tris-Cl, pH 6.8, 30% glycerol, 10% sodium dodecyl sulfate [SDS; wt/vol], 0.6% β-mercaptoethanol). The reaction products were separated by electrophoresis on 8% or 12% SDS-polyacrylamide gel electrophoresis (PAGE) gels (17 by 16.5 by 0.3 cm) and were run at 4°C at 200 V constant voltage for 2 h. Gels were dried, exposed to a high-resolution phosphor screen (Kodak), and analyzed using a PhosphorImager with ImageQuant software (Molecular Dynamics 5.0; Amersham Biosciences, Pittsburgh, PA). For the label decay experiments, an excess of nonradioactive ATP (100 mM) was added to the reaction mixture following a 20-min preincubation with [γ-32P]ATP. Aliquots were taken prior to the addition of unlabeled ATP (time zero) and at various time points after the addition of cold ATP. Different dilutions of [γ-32P]ATP were used to generate, via ImageQuant, a calibration curve to quantify the label associated with the products of the phosphorylation reactions. All time-dependent quantitation results were expressed as the average of at least three independent experiments. The gel figures shown are representative of each set of experiments.
RESULTS
Phosphotransfer activities of the truncated wild-type sensor kinase.
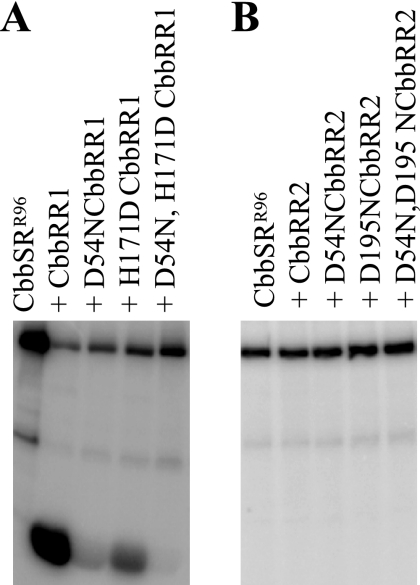
Comparisons of deduced amino acid sequences of CbbRR1, CbbRR2, and sensor kinase CbbSR to well-characterized bacterial response regulators (data not shown) resulted in the identification of residues Asp-54 and His-171 on CbbRR1 and Asp-54 and Asp-195 on CbbRR2 as potential specific sites of phosphorylation. The importance and role of these residues on each response regulator protein during phosphotransfer, along with the conserved functional residues identified on the sensor kinase (11), were assessed after purified site-directed mutant proteins were prepared. Truncated soluble His-tagged recombinant sensor kinase constructs CbbSRR96 and CbbSRT189 (CbbSR starting from residue T189) were prepared and purified using constructs that provided the cytoplasmic (but not membrane-spanning) portion of the protein (Fig. 1). As expected, CbbSRR96 catalyzed an ATP-dependent autophosphorylation reaction, followed by subsequent phosphotransfer to wild-type CbbRR1 (11) (Fig. 3A). Alteration of both Asp-54 and His-171 of CbbRR1 prevented label incorporation/phosphorylation of CbbRR1 (Fig. 3A). By contrast, considerable phosphotransfer to the H171DCbbRR1 mutant protein occurred, while the D54N construct exhibited only weak phosphorylation. These results showed that Asp-54 is the major phosphoacceptor of CbbRR1; however, the data also suggested possible phosphotransfer between residues Asp-696 from CbbSR to His-171 of CbbRR1 (further described below). No phosphorylation of either wild-type or site-directed mutant constructs of CbbRR2 by CbbSRR96 was observed (Fig. 3B).
FIG. 3.
Phosphotransfer between sensor kinase CbbSRR96 and response regulator CbbRR1 (wild-type and wild-type plus D54N, H171D, and D54NH171D mutant CbbRR1 proteins) (A) and response regulator CbbRR2 (wild-type and wild-type plus D54N, D195N, and D54ND195N mutant CbbRR2) (B). A total of 380 pmol of sensor kinase was preincubated with 20 μM [γ-32P]ATP for 20 min (far left lanes) before the addition of approximately 1 nmol of each response regulator, and the reaction mixture was incubated at room temperature for 1 h.
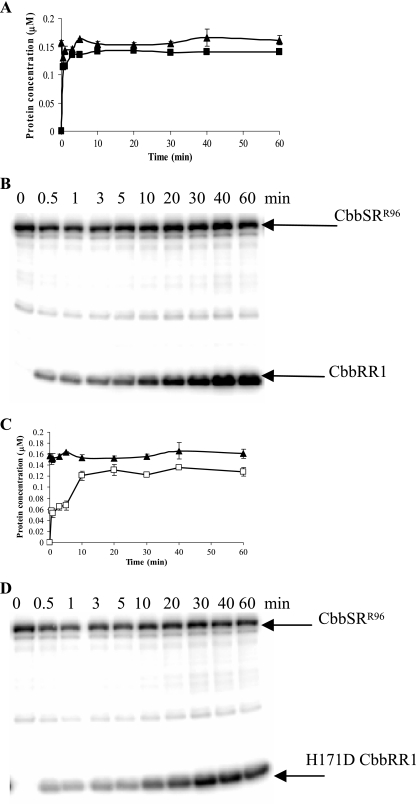
To further define phosphotransfer from the sensor kinase to CbbRR1, both the kinetics and specificity of these reactions were examined. Time course experiments indicated that CbbSRR96 catalyzed rapid transfer of γ-32P to CbbRR1 (Fig. 4A and B). Similarly, phosphotransfer from CbbSRR96 to the mutant H171DCbbRR1 protein occurred, however at a reduced initial rate (Fig. 4C and D). Both the wild-type and mutant CbbRR1 proteins reached a consistent level of phosphorylation in equilibrium with the sensor kinase. Since unincorporated [γ-32P]ATP was not removed from the reaction mixture, no significant dephosphorylation of the kinase protein was observed concomitant with the phosphorylation of CbbRR1. Analogous results were obtained with the shorter truncated kinase, CbbSRT189 (data not shown).
FIG. 4.
Time course of phosphorylation between CbbSRR96 and CbbRR1 (A and B) or H171DCbbRR1 (C and D). The concentration of phosphorylated products obtained at various times was determined (A and C) for CbbSRR96 (▴), CbbRR1 (▪), and H171DCbbRR1 (□). A total of 380 pmol of sensor kinase was preincubated with 20 μM [γ-32P]ATP for 20 min (time zero) before the addition of approximately 1 nmol of response regulator. Ten-milliliter aliquots were then removed at the time points indicated; the reactions were stopped by adding 3× SDS loading buffer and stored on ice until the end of the experiment.
Considerable differences in both the kinetics and specificity of phosphotransfer to the CbbRR1 constructs were observed with the kinase mutant D696NCbbSRR96 (Fig. 5A and B) and its more truncated homolog D696NCbbSRT189 (Fig. 5C and D). Wild-type CbbRR1 was very poorly phosphorylated in the presence of D696NCbbSRR96 (Fig. 5A) and virtually no phosphotransfer occurred to H171DCbbRR1 (Fig. 5B). Variations of the protein molar ratios over a wide range of ATP concentration and incubation times gave identical patterns of phosphorylation (data not shown). By contrast, the more truncated D696NCbbSRT189 protein exhibited activities consistent with the nonmutant CbbSRT189 construct, with rapid phosphorylation of CbbRR1 (Fig. 5C). Phosphotransfer to H171DCbbRR1 occurred both at a slower rate and to a lesser extent (Fig. 5D), with accentuated transient dephosphorylation of CbbSR and slightly decreased phosphotransfer efficiency, much like wild-type CbbSRR96 and H171DCbbRR1 (Fig. 4C and D). Incubation of the mutant kinase preparations, D696NCbbSR with D54NCbbRR1, prevented any phosphotransfer (data not shown).
FIG. 5.
Time course of phosphorylation of CbbRR1 (▪) and H171DCbbRR1 (□) catalyzed by D696NCbbSRR96 (▵) (A and B) and D696NCbbSRT189 (○) (C and D). Reaction conditions were as described in the legend of Fig. 4. Quantitation of phosphorylated products from time courses (left) was obtained from phosphotransfer reactions depicted in gels (right). The arrow indicates where CbbRR1 preparations were added to the reaction mixture.
Properties of the truncated sensor kinase proteins.
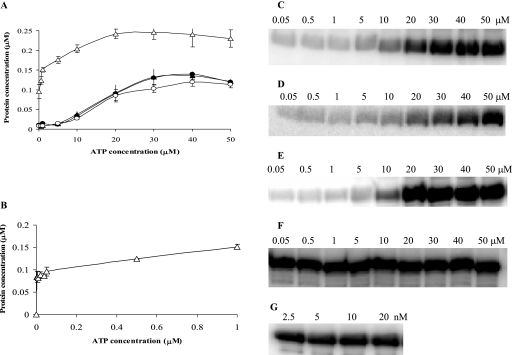
The basic autophosphorylation reactions catalyzed by all four truncated constructs—CbbSRT189, D696NCbbSRT189, CbbSRR96, and D696NCbbSRR96—were characterized. The D696NCbbSRR96 construct had a much higher affinity for ATP than the other constructs (Fig. 6A), with this protein showing optimal phosphorylation at concentrations as low as 2.5 nM ATP (Fig. 6B, F, and G) compared to CbbSRT189, D696NCbbSRT189, and CbbSRR96 (Fig. 6A, C, D, and E), all of which required at least 20 μM ATP. In addition, there was no difference in the relative rate of autophosphorylation of CbbSRR96 and D696N CbbSRR96 at 20 μM and 25 nM [γ-32P]ATP, respectively (data not shown). No significant differences in ATP affinity were noted for the CbbSRT189 wild-type and mutant constructs (11).
FIG. 6.
Kinase autophosphorylation reactions at various concentrations of [γ-32P]ATP. Each reaction was carried out with 380 pmol of protein at various concentration of [γ-32P]ATP for 20 min at room temperature in a final volume of 10 μl. The reactions were terminated by adding 5 μl of 3× sample buffer. (A) Levels of phosphorylated proteins obtained at increasing concentrations of [γ-32P]ATP for CbbSRT189 (•), D696NCbbSRT189 (○), CbbSRR96 (▴), and D696NCbbSRR96 (▵). (B) Levels of phosphorylated products obtained at increasing concentrations of [γ-32P]ATP for D696NCbbSRR96. Representative patterns of autophosphorylation at the various concentrations of [γ-32P]ATP of CbbSRT189 (C), D696NCbbSRT189 (D), CbbSRR96 (E), and D696NCbbSRR96 (F and G) at the indicated concentrations of ATP. The range of [γ-32P]ATP concentrations (μM or nM) used in the autophosphorylation assays is reported above each gel.
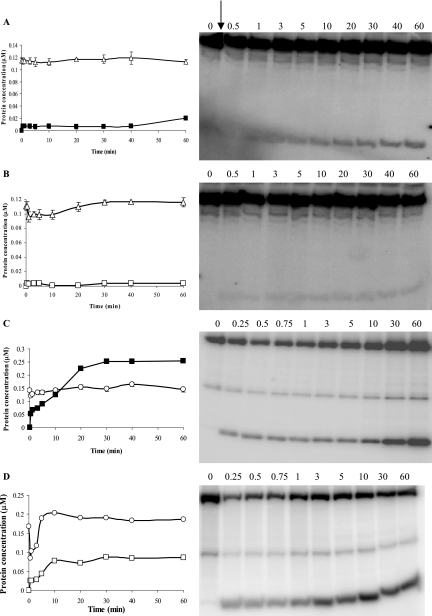
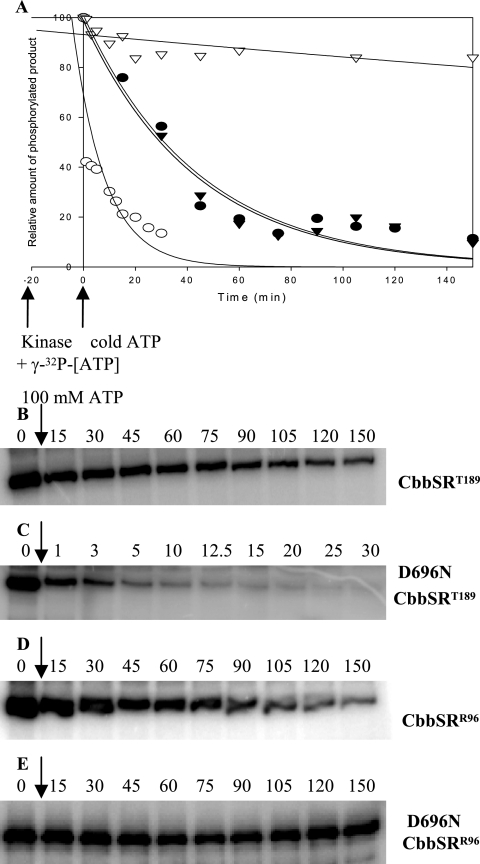
Previous studies on the chemical stability of phosphorylated CbbSRT189 constructs indicated that the wild-type sensor kinase protein was mainly acyl-phosphorylated on Asp-696, with rapid phosphotransfer from the autophosphorylated His-409 residue to Asp-696 within the receiver domain of the protein (11). This internal phosphotransfer occurred even in the presence of CbbRR1. However, after histidine autophosphorylation, the absence of a phosphoacceptor residue within the receiver domain of CbbSR and/or the absence of a response regulator protein would presumably cause dissociation of the phosphoryl group, which would then be released into solution. Thus, to further assess the properties of the four kinase constructs and their state of phosphorylation, pulse-chase experiments were performed in order to follow the time-dependent displacement of the label in the presence of a large excess of unlabeled ATP. Unlabeled ATP should compete with any labeled ATP remaining in solution as a substrate for the autophosphorylation reaction, ultimately allowing measurements of the rate of dephosphorylation. With the exception of D696NCbbSRR96, which was phosphorylated with 25 nM [γ-32P]ATP, all constructs were preincubated with 20 μM [γ-32P]ATP to allow sufficient autophosphorylation prior to the addition of 100 mM unlabeled ATP. Both wild-type truncated constructs (CbbSRT189 and CbbSRR96) showed virtually identical rates of dephosphorylation (Fig. 7A, B, and D), with gradual label decay throughout the whole experimental time frame. However, the two D696NCbbSR constructs exhibited completely different rates of γ-32P label loss; the D696NCbbSRT189 protein rapidly lost more than 50% of its label within 1 min of the addition of unlabeled ATP and maintained only about 10% of the original level of phosphorylation, that is, prior to the addition of unlabeled ATP (Fig. 7A and C). By contrast, the phosphorylation level of D696NCbbSRR96 remained substantially unchanged after 2.5 h (Fig. 7A and E). The lack of dephosphorylation shown by D696NCbbSRR96 was interesting, in particular with respect to its high affinity for ATP (Fig. 6) and weak ability to catalyze phosphotransfer to CbbRR1 (Fig. 5). Since H409DCbbSRR96, much like H409DCbbSRT189, had no autophosphorylation activity (11) and contained no label (data not shown), the high affinity of D696NCbbSRR96 for ATP was strictly dependent on its kinase activity and not an indirect effect of covalent ATP binding to the sensor region of the protein, as seen, for instance, in the sporulation master kinase, KinA (12).
FIG. 7.
Pulse-chase analysis of the CbbSRR96 and CbbSRT189 wild-type truncated histidine kinase and their D696N site-directed mutants. The phosphorylation levels of the four constructs were normalized to the maximum intensity of signal (100%) of each construct prior to the addition of an excess of unlabeled (100 mM) ATP and plotted against time. Label disappearance (γ-32P) is expressed as the percentage of the phosphorylated products obtained at any given time after the addition of unlabeled ATP. The kinase proteins were preincubated with 20 μM [γ-32P]ATP for 20 min, with the exception of D696N CbbSRR96, which was preincubated with 25 nM [γ-32P]ATP. The reaction was carried out in a 100-μl final volume. At time zero, 10 μl of the phosphorylation reaction mixture was removed, and 10 μl of unlabeled ATP (100 mM final concentration) was added. Aliquots were removed at the time points indicated. CbbSRT189, (•); D696NCbbSRT189, (○); CbbSRR96, (▾); D696NCbbSRR96, (▿). Raw data were analyzed, normalized, and plotted with SigmaPlot 8.0. Representative pulse-chase patterns of phosphorylation are shown for CbbSRT189 (B), D696NCbbSRT189 (C), CbbSRR96 (D), and D696NCbbSRR96 (E).
In summary, the results of the kinetic experiments indicated the following: (i) that the state of phosphorylation of the wild-type sensor kinase protein is most probably a combination of its autophosphorylation activity, its internal phosphotransfer activity, and the inherent stability of the phosphorylated residue; and (ii) that the N-terminal region of the sensor kinase between residues Arg-96 and Thr-189, which contains a PAS motif, must play a pivotal role in sensing the signal that affects the phosphorylation state of the protein (i.e., the in vivo signaling mode of the protein). This region apparently dictates downstream phosphotransfer reactions.
Phosphotransfer between D696NCbbSRR96 and CbbRR2.
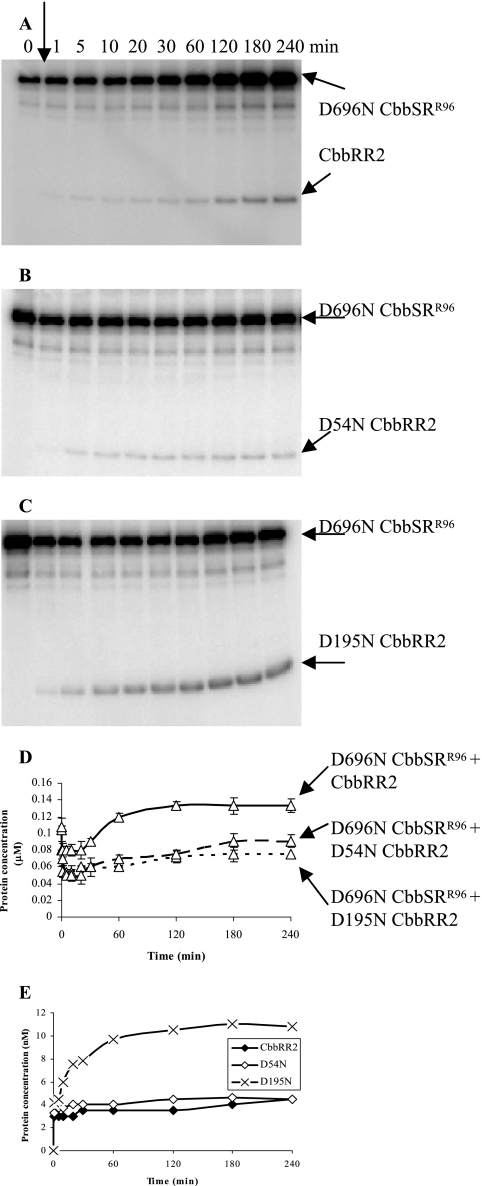
Although D696NCbbSRR96 inefficiently catalyzed phosphorylation of CbbRR1 (Fig. 5), this mutant protein, but not wild-type CbbSRR96 (Fig. 3), was able to catalyze a slow rate and moderate level of phosphotransfer to CbbRR2 (Fig. 8A). In particular, if Asp-195, one of the conserved and potential aspartate phosphoacceptor residues from the two receiver domains of CbbRR2 was mutated, i.e., by using construct D195NCbbRR2, the apparent rate of phosphotransfer from D696NCbbSRR96 to the mutated CbbRR2 proteins was markedly enhanced (Fig. 8C, D, and E). As for the reaction in the presence of wild-type CbbSRR96 (Fig. 3), concomitant mutation of both aspartate residues completely prevented phosphorylation of CbbRR2 (data not shown). Moreover, throughout the 4 h that the phosphorylation experiments were carried out in the presence of wild-type CbbRR2, further accumulation of phosphorylated D696NCbbSRR96 was observed (Fig. 8A), suggesting that possible competition for the phosphate group between residues Asp-54 and Asp-195 of CbbRR2 indirectly favored the kinase autophosphorylation reaction. Indeed, the final absolute amount of phosphorylated D696NCbbSRR96 in the presence of wild-type CbbRR2 was significantly greater than that obtained in the presence of the mutant CbbRR2 proteins (Fig. 8D). Finally, D195NCbbRR2 showed greater than double the level of phosphorylation compared to wild-type or mutant D54NCbbRR2, strongly suggesting that Asp-54 from CbbRR2 possessed the highest affinity for the phosphoryl group derived from D696NCbbSRR96 (Fig. 8E).
FIG. 8.
Time course of CbbRR2 phosphorylation. To better resolve the phosphorylation products, the phosphotransfer reaction was performed under the following conditions: 1.2 μM D696NCbbSRR96 was preincubated with 25 nM [γ-32P]ATP for 20 min (time zero), prior to the addition of 24 μM CbbRR2 (A) and its site-directed mutants, D54NCbbRR2 (B) and D195NCbbRR2 (C). Ten-microliter aliquots were removed at the time points indicated. The reaction products were resolved by 12% SDS-PAGE. The phosphorylated proteins are indicated by arrows. (D) Levels of D696NCbbSRR96 over the time course of phosphotransfer for D696NCbbSRR96+CbbRR2 (solid line), D696NCbbSRR96+D54NCbbRR2 (dashed line), and D696NCbbSRR96+D195NCbbRR2 (dotted line). (E) Levels of phosphorylation for wild-type CbbRR2 (⧫), D54NCbbRR2 (⋄), and D195NCbb RR2 (×).
Independent activity of the kinase receiver domain and phosphorylation of the CbbRR1 HPt domain.
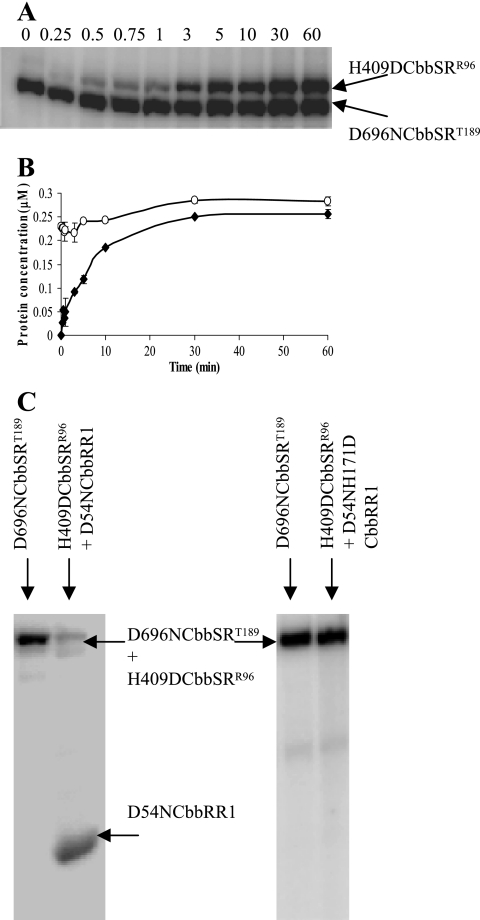
Because the receiver domain of the sensor kinase appeared to play a critical role for the reactions thus far described, its function was further addressed by assaying the phosphoacceptor activity of inactivated kinase constructs, that is, H409DCbbSR, since residue His-409 is essential for kinase activity (11). In a reaction containing 20 μM [γ-32P]ATP and equimolar concentrations of the D696NCbbSRT189 and H409DCbbSRR96 proteins (Fig. 9A and B), there was rapid and extensive phosphotransfer from His-409 of the D696NCbbSRT189 protein to the receiver domain of the inactive H409DCbbSRR96 kinase protein. A similar pattern of phosphorylation was observed with D696NCbbSRR96 and H409DCbbSRT189 (data not shown). This result clearly proves that rapid phosphotransfer occurred within the kinase protein; therefore, the kinase receiver domain functions as a discrete functional unit.
FIG. 9.
The receiver domain on the sensor kinase functions as an independent catalytic unit. (A) Phosphotransfer between D696NCbbSRT189 and H409DCbbSRR96 (380 pmol each) in the presence of 20 μM [γ-32P]ATP, resolved on an 8% SDS-PAGE gel. Analogous results were obtained with D696NCbbSRR96 and H409DCbbSRT189 in the presence of 25 nM [γ-32P]ATP (data not shown). (B) Time-dependent accumulation of phosphorylated products when H409DCbbSRT189 (•) was incubated with D696NCbbSRR96 (○). (C) Residue Asp-696 is a productive phosphodonor to residue His-171 on the CbbRR1 HPt domain. D696NCbbSRT189 was preincubated with 20 μM [γ-32P]ATP for 20 min (lane 1) prior to the simultaneous addition of both H409DCbbSRR96 (380 pmol) and D54NCbbRR1 (1 nmol). The complete reaction mixture was then incubated for 1 h (lane 2). As additional controls, D696NCbbSRT189 was incubated with 20 μM [γ-32P]ATP (lane 3) prior to adding H409DCbbSRR96 and D54NH171DCbbRR1 (lane 4). The double mutant D54NH171DCbbRR1 was not phosphorylated.
The function of the CbbRR1 HPt domain was tested for its ability to receive the phosphoryl group from Asp-696 of the sensor kinase. In general, proteins containing an HPt domain have been shown to participate in multistep phosphorelay processes whereby the active histidine residue, although incapable of being autophosphorylated, participates in a phosphorylation cascade by providing a “phosphorylation” bridge between two distinct Asp residues (2, 3, 6, 14) either as a mono-domain protein or as a functional module of a tripartite hybrid kinase. To assess the interaction between CbbSR and CbbRR1, phosphotransfer was initiated by first preincubating D696NCbbSRT189 with 20 μM [γ-32P]ATP for 20 min prior to the simultaneous addition of both the H409DCbbSRR96 and D54NCbbRR1 proteins. If there was substantial phosphorylation of the CbbRR1 mutant protein, this would presumably be due to phosphotransfer from Asp-696 of the sensor kinase to His-171 of the CbbRR1 protein. Previously, it was established that Asp-54 on CbbRR1 directly received the phosphoryl group from His-409 of the sensor kinase (Fig. 4A); in addition, Asp-696 of the sensor kinase is not required for this reaction (11). The pattern of phosphorylation (Fig. 9C, lanes 1 and 2) substantiated the above reasoning. Similar results were obtained by using D696NCbbSRR96 and H409D CbbSRT189 (data not shown). A control experiment showed no CbbRR1 phosphorylation with a D54NH171DCbbRR1 double mutant (Fig. 9C, lanes 3 and 4). These studies indicated that a multistep phosphorylation reaction occurred whereby the active kinase (mutant D696NCbbSR) catalyzed phosphotransfer to the inactive kinase (mutant H409DCbbSR containing the phosphate acceptor residue Asp-696) and eventually to His-171 of the D54NCbbRR1 construct. Thus, phosphotransfer occurred from His-409 to Asp-696 on the sensor kinase to His-171 on CbbRR1. Since radiolabeled phosphate on the kinase constructs was considerably diminished by the end of the reaction (Fig. 9C, lane 2), phosphotransfer appeared to be unidirectional, and His-171 of CbbRR1 did not apparently allow reverse phosphotransfer to Asp-696 of the sensor kinase (4). Moreover, D696NCbbSRR96 reacted poorly with CbbRR1 constructs (Fig. 5). This is consistent with the idea that the kinase receiver domain and the HPt domain interact as independent functional domains. Finally, experiments with combinations of all three CbbRRS proteins (i.e., sensor kinase CbbSR and both response regulators CbbRR1 and CbbRR2, in their wild-type as well as mutant forms) indicated that phosphorylated CbbRR1, in particular, its phosphorylated His-171 residue, was incapable of passing the phosphoryl group to CbbRR2 (data not shown).
DISCUSSION
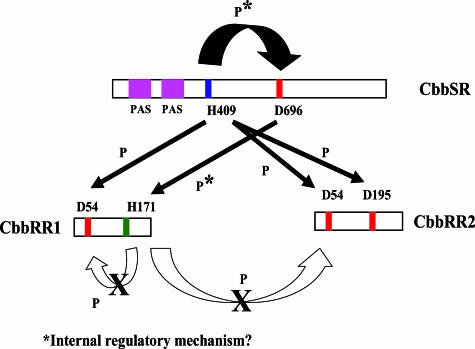
Previous results indicated that the CbbRRS system plays a significant role in the control of form I ribulose 1,5-bisphosphate carboxylase/oxygenase biosynthesis in R. palustris, with the proper balancing of sensor kinase and response regulator playing an important part in the regulatory mechanism under photoautotrophic (11) and photoheterotrophic (this laboratory, unpublished data) growth conditions. In the current study, phosphotransfer reactions catalyzed by the CbbRRS system were analyzed in vitro since a number of events were conceivable once autophosphorylation of the sensor kinase protein occurred. Since these phosphorylation events likely serve a profound function in vivo, it was particularly important to discern whether the single kinase, CbbSR, could potentially interact with four distinct receiver domains that show very limited sequence homology (Fig. 2). Thus, each of the four putative independent phosphotransfer reactions was considered here. Two truncated constructs of different lengths of CbbSR, in combination with various active-site residue mutations of CbbSR, CbbRR1, and CbbRR2, provided insight into different signaling states of the kinase protein. Based on the results of this investigation, hypothetical pathways for the CbbRRS phosphorelay were determined (Fig. 10), and several events were established upon autophosphorylation of residue His-409 of CbbSR: (i) the phosphate group may be transferred to Asp-696 within the kinase receiver domain and then to the His-171 residue of the CbbRR1 HPt domain; (ii) the phosphate group may also be transferred from His-409 of CbbSR to residue Asp-54 of the receiver domain of CbbRR1; (iii) the phosphate may be transferred from His-409 of CbbSR to either Asp-54 or Asp-195 of the two receiver domains of CbbRR2, with a preference for Asp-54. The first of these reactions occurred independently of the integrity of the N-terminal region of the sensor kinase protein; however, the second and third reactions were controlled by the presence of residues Arg-96 to Thr-189 at the N terminus of CbbSR. Furthermore, it is apparent from the in vitro data (Fig. 7) that the extent and degree of phosphorylation of the sensor kinase are influenced by the integrity of the N-terminal region, perhaps defining different conformational states of the sensor kinase. This observation raises the possibility that the phosphorylation state of the receiver domain of CbbSR, concomitant with the N-terminal region, functions as a major determinant to control the specificity of interactions of CbbSR with either response regulator CbbRR1 or CbbRR2. In the latter instance, two distinct regions of CbbSR clearly play a cooperative role in determining the signaling state of the kinase and ultimately the phosphotransfer to either CbbRR1 or CbbRR2.
FIG. 10.
Summary of the phosphorylation reactions catalyzed by the CbbRRS system. The conserved residues and motifs on the sensor kinase, CbbSR, and the two response regulators, CbbRR1 and CbbRR2, are indicated.
The HPt domain of CbbRR1 was also intriguing. First of all, CbbRR1 represented the first investigated instance where an HPt domain was found to be part of a response regulator protein. HPt domains appear usually as either independent mono-domain proteins or as subdomains of tripartite hybrid kinases (2, 3, 7, 9, 14). The results reported here indicate that the HPt domain of CbbRR1 seems to function as a type of release device, allowing the kinase and therefore the whole CbbRRS system to reset its signaling state by removing the phosphate group from residue Asp-696 of the kinase and transferring it to His-171 of CbbRR1. This hypothesis was supported by observations with the mutant H171DCbbRR1 protein, which seemed to react with the wild-type kinase as well as CbbRR1 (Fig. 4); however, no more sequential transfer of the phosphate group occurred, either back to the kinase (Fig. 9C) or to CbbRR2 (data not shown). After transfer of the phosphate to His-171 of CbbRR1, the phosphorylated HPt domain likely decays, either by eventual spontaneous hydrolysis of the phosphate group or by means of some as yet unknown phosphatase.
In conclusion, the CbbRRS system was found to catalyze multiple phosphotransfer reactions with at least two functional checkpoints. The integrity of the N-terminal region of the sensor kinase clearly seems to influence the phosphorylation state of the kinase and specifies active complexes with either one of the two response regulators. On the other hand, the multistep phosphotransfer reaction to residue His-171 of response regulator CbbRR1 appeared to allow a turnover of the CbbRRS system, so that a new set of phosphorylation reactions might occur. Furthermore, based upon our findings, we propose that the sensor kinase protein assumes at least three separate signaling states: (i) an inactive mode, whereby phosphorylation of the kinase is ultimately terminated by phosphotransfer from His-409 to first its own receiver domain and then to the HPt domain of CbbRR1; (ii) an active mode whereby CbbSR can catalyze phosphorylation to CbbRR1; and (iii) a second active mode in which CbbSR can catalyze phosphorylation of CbbRR2. Transitions between these modes appeared to be determined by the signaling state of the N-terminal sensor region, possibly in response to some threshold level of intensity of the activating stimulus. The consequence would be the creation of an internal device to control downstream phosphotransfer reactions. Further studies will seek to identify the signal(s) that determine(s) each single step of the complex phosphorelay identified here and the physiological consequences of each of the phosphorylation steps.
Acknowledgments
This work was supported by Genomics: GTL grant DOE DE-FG02-01ER63241 from the U.S. Department of Energy (Office of Biological and Environmental Research, Office of Science) and grant GM25404 from the National Institutes of Health.
Footnotes
Published ahead of print on 27 October 2006.
REFERENCES
- 1.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 2000. Current protocols in molecular biology. Greene Publishing Associates-Wiley Intersciences, New York, NY.
- 2.Burbulys, D., K. A. Trach, and J. A. Hoch. 1991. The initiation of sporulation in Bacillus subtilis is controlled by a multicomponent phosphorelay. Cell 64:545-552. [DOI] [PubMed] [Google Scholar]
- 3.Geogellis, D., O. Kwon, P. De Wulf, and E. C. C. Lin. 1998. Signal decay through a reverse phosphorelay in the Arc two-component signal transduction system. J. Biol. Chem. 273:32864-32869. [DOI] [PubMed] [Google Scholar]
- 4.Geogellis, D., S. A. Lynch, and E. C. C. Lin. 1997. In vitro phosphorylation study of the Arc two-component signal transduction system of Escherichia coli. J. Bacteriol. 179:5429-5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grefen, C., and K. Harter. 2004. Plant two-component systems: principles, functions complexity and cross talk. Planta 219:733-742. [DOI] [PubMed] [Google Scholar]
- 6.Janiak-Spens, F., D. P. Sparling, and A. H. West. 2000. Novel role for an HPt domain in stabilizing the phosphorylated state of a response regulator domain. J. Bacteriol. 182:6673-6678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Majdalani, N., and S. Gottesman. 2005. The Rcs phosphorelay: a complex signal transduction system. Annu. Rev. Microbiol. 59:379-405. [DOI] [PubMed] [Google Scholar]
- 8.Parkinson, J. S., and E. C. Kofoid. 1992. Communication modules in bacterial signaling proteins. Annu. Rev. Genet. 26:71-112. [DOI] [PubMed] [Google Scholar]
- 9.Posas, F., S. M. Wurgler-Murphy, A. Maeda, E. A. Witten, T. C. Thai, and H. Saito. 1996. Yeast HOG1 MAP kinase cascade is regulated by a multistep phosphorelay mechanism in the SLN1-YPD1-SSK1 “two-component” osmosensor. Cell 86:865-875. [DOI] [PubMed] [Google Scholar]
- 10.Rasmussen, A. A., S. Wegener-Feldbrügge, S. L. Porter, J. P. Armitage, and L. Søgaard-Andersen. 2006. Four signalling domains in the hybrid histidine protein kinase RodK of Myxococcus xanthus are required for activity. Mol. Microbiol. 60:525-534. [DOI] [PubMed] [Google Scholar]
- 11.Romagnoli, S., and F. R. Tabita. 2006. A novel three-protein two-component system provides a regulatory twist on an established circuit to modulate expression of the cbbI region of Rhodopseudomonas palustris CGA010. J. Bacteriol. 188:2780-2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stephenson, K., and J. A. Hoch. 2001. PAS-A domain of phosphorelay sensor kinase A: a catalytic ATP-binding domain involved in the initiation of development in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 98:15251-15256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stock, A. M., V. L. Robinson, and P. N. Goudreau. 2000. Two-component signal transduction. Annu. Rev. Biochem. 69:183-215. [DOI] [PubMed] [Google Scholar]
- 14.Uhl, M. A., and J. F. Miller. 1996. Integration of multiple domains in a two-component sensor protein: the Bordetella pertussis BvgAS phosphorelay. EMBO J. 15:1028-1036. [PMC free article] [PubMed] [Google Scholar]