Abstract
We have detected a cholesterol-dependent cytolysin, which we have named mitilysin, in a small number of Streptococcus mitis isolates. We have sequenced the mitilysin gene from seven isolates of S. mitis. Comparisons with the pneumococcal pneumolysin gene show 15 amino acid substitutions. S. mitis appear to release mitilysin extracellularly. Certain alleles of mitilysin are not recognized by a monoclonal antibody raised to the related toxin pneumolysin. Based on enzyme-linked immunosorbent assay and neutralization assay results, one isolate of S. mitis may produce a further hemolytic toxin in addition to mitilysin. As genetic exchange is known to occur between S. mitis and Streptococcus pneumoniae, this finding may have implications for the development of vaccines or therapies for pneumococcal disease that are based on pneumolysin.
Viridans group streptococci (VGS) are a group of closely related streptococci which includes the pneumococcus. The VGS belong to the normal upper respiratory tract and oral flora of humans (10). Oral streptococci contain a number of species of naturally transformable streptococci able to take up naked DNA from the extracellular environment (19). On the basis of 16S rRNA gene sequencing, the species most closely related to Streptococcus pneumoniae are Streptococcus mitis and Streptococcus oralis, which have over 99% sequence identity with S. pneumoniae (15). Pneumolysin (Ply) is a protein toxin produced by S. pneumoniae that belongs to a large protein family of cholesterol-dependent cytolysins (14). The family consists of 50- to 60-kDa single-chain proteins produced by at least seven gram-positive bacteria from the genera Streptococcus, Bacillus, Clostridium, Listeria, and Arcanobacterium (1). S. pneumoniae causes diseases such as pneumonia, bacteremia, meningitis, and otitis media (21), and pneumolysin is an important virulence factor of this human pathogen. Mutations in the ply gene can lead to reduced virulence of S. pneumoniae, and an isogenic mutant pneumococcus deficient in pneumolysin has been shown to be cleared from the blood following infection (5, 6). There is evidence of horizontal gene transfer between other VGS and S. pneumoniae, penicillin resistance has been transferred from the VGS to pneumococci by the transfer of penicillin binding proteins (9), and Ferrandiz et al. have suggested that VGS are donors in the horizontal transfer of fluoroquinolone resistance genes to S. pneumoniae (3, 11).
Whatmore et al. studied the distribution of the ply and ply-related genes in S. pneumoniae, S. mitis, and S. oralis (27). None of the S. oralis strains tested contained ply-related sequences, whereas some of the S. mitis strains were found to posses a pneumolysin homologue. The aim of the work reported here was to investigate the presence, nature, and activity of the cholesterol-dependent cytolysin in a collection of VGS.
MATERIALS AND METHODS
Bacterial strains and identification.
Two hundred nineteen isolates of VGS were screened for the presence of ply-related sequences by PCR. The isolates consisted of 16 type strains (S. sanguis NCTC 7863; S. parasanguis NCTC 15912; S. salivarius NCTC 8618; S. mutans NCTC 10449; S. gordonii NCTC 7865, 10231, 7868, and 3165; S. oralis NCTC 11427; S. morbillus NCTC 11323; S. sobrinus NCTC 10922; S. mitis NCTC 10712 and 12261; S. cristatus DSM 8249; S. peroris 12493; and S. infantium 12492), 6 isolates (COL15, COL16, COL17, COL18, COL24, and COL28) from a previous study (27), 6 isolates kindly donated by Chris Dowson (CD 1, 3, 4, 5, 6, and 7), and 191 clinical isolates. The clinical strains were isolated from blood cultures (n = 129 [from medical, surgical, hematology, and oncology wards]), respiratory tract secretions (n = 55 [from sputum, bronchoalveolar lavage fluid, and nasopharyngeal aspirates]), and acute dentoalveolar infections (n = 7).
The isolates were identified initially by colony morphology, Gram stain, optochin test, and catalase reaction. Species identifications were determined with the Rapid ID32 Strep system (API System, bioMérieux, France). Where isolates could not be fully identified by this method they, were classified as VGS for the purposes of ply gene screening. This produced 80 S. mitis, 62 S. oralis, 16 S. salivarius, 11 S. parasanguis, 8 S. sanguis, 6 S. gordonii, 5 S. vestibularis, 4 S. mutans, 1 S. sobrinus, 1 S. morbillus, and 22 VGS isolates for screening.
Isolates that were confirmed as containing ply-related sequences by PCR were further characterized by sequence analysis of the sodA gene (15). All isolates were stored at −70°C in Trypticase soy broth (BBL Microbiology Systems, Cockeysville, MD) with 15% glycerol until being tested.
Preparation of lysates.
Several colonies from overnight growth on a blood agar plate were cultured in 15 ml of brain heart infusion broth (BHI) overnight; 500 μl of this overnight culture was added into 20 ml of BHI, and the cells were grown until mid-log phase (optical density at 600 nm [OD600], 0.5 to 0.6). Then, 15 ml of mid-log-phase culture was centrifuged at 4,000 × g for 18 min at 4°C. Culture media and cell pellets were separated. The culture medium samples (extracellular fractions) were sterilized by filtration with a Minisart syringe filter (pore size, 0.2 μm; Sartorius) and concentrated 10 times with Amicon Ultra-15, centrifugal filter devices (Millipore, Bedford, MA). The cell pellet was resuspended in 1 ml of 25 mM Tris-50 mM NaCl (pH 7.5) with protease inhibitor cocktail (Sigma). The cells were lysed by sonication (four 30-s pulses). The lysates were centrifuged to separate the lysate pellet and the lysate supernatant (intracellular fraction, cell lysate). For S. mitis and S. oralis strains, lysozyme and mutanolysin were added at 200 U and 22,000 U, respectively, and the samples were incubated for 30 min at 37°C before sonication. Total protein concentrations of intracellular and extracellular fractions were measured by Bradford's method (7) with bovine serum albumin as the standard.
Assay of hemolytic activity.
Hemolytic activity was measured by a modification of the method described by Benton et al. (4). In brief, doubling dilutions of the culture media and the lysate supernatant were performed in phosphate-buffered saline (PBS), pH 7.0 (final volume, 100 μl), on a round-bottomed 96-well plate. Next, 50 μl of 2% (vol/vol) washed defibrinated horse blood in PBS was added. The plates were incubated for 30 min at 37°C and then for 30 min at room temperature. Hemolytic units (HU) were calculated from the endpoint (defined as 50% lysis). The endpoint was estimated as the well in which the erythrocyte pellet was half the size of the control wells. PBS and purified pneumolysin were used as controls.
ELISA to measure the concentration of cholesterol-dependent cytolysin.
A modified protocol based on that described by Cima-Cabal et al. (8) to measure the concentration of Ply was used as described previously (16). Briefly, enzyme-linked immunosorbent assay (ELISA) wells were coated with 2.5 μg/ml capture monoclonal antibody (MAb) (PLY-7) and incubated overnight. Next, 100 μl of the samples was added to the wells with appropriate dilutions (1:200, 1:500, and 1:1,000) made in assay buffer (10% fetal bovine serum in PBS plus 0.05% [vol/vol] Tween 20 [PBS-T]). Samples were compared to a standard curve based on the reaction of purified pneumolysin diluted in assay buffer with doubling dilutions. The plates were incubated for 1 h at 37°C with shaking and were then washed six times with PBS-T. Next, 100 μl of detection antibody (anti-Ply rabbit polyclonal antibody [PAb]) at a 1:2,000 dilution in assay buffer was added to each well and incubated for 30 min in 37°C with shaking and was washed four times with PBS-T. Then, 100 μl of biotinylated anti-rabbit immunoglobulin G (IgG) (Amersham Biosciences) at a 1:500 dilution in assay buffer was added per well, incubated for 30 min at 37°C with shaking, and washed four times with PBS-T. Streptavidin-horseradish peroxidase (Kirkegaard & Perry Laboratories, Gaithersburg, MD) was added at a 1:2,000 dilution in assay buffer at 100 μl per well and incubated at room temperature for 30 min and then washed five times with PBS-T. Equal volumes of TMB substrates A and B were added at 100 μl per well and incubated for 3 to 5 min at room temperature. The reactions were stopped by the addition of 50 μl of 10% H2SO4 per well. The plate was read at 450 nm. The percentage of toxin in total protein and the specific activity of the toxin were calculated.
In vitro growth curves.
Bacterial growth curves were determined by incubating 20 ml of 1 × 106 CFU/ml bacterial suspensions (in BHI) at 37°C for 12 h. The OD600 was measured after every hour, and bacterial viable cell counts were made on blood agar base plates (Oxoid, Basingstoke, United Kingdom) containing 5% horse blood (E & O Laboratories, Bonnybridge, United Kingdom) after every second hour. The toxin concentration was measured in the unconcentrated culture media by ELISA after cells were separated by centrifugation. Validation work confirmed the relationship between the OD600 values and viable counts (data not shown).
SDS-PAGE and Western blot analysis.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was carried out as described by Laemmli (17). For Western blot analysis, samples were electrophoretically transferred from SDS-polyacrylamide gels to nitrocellulose Hybond C super membranes as described by Towbin et al. (25). Filters were probed with polyclonal anti-rabbit pneumolysin (used at a dilution of 1:1,000) or MAb PLY-7 (8) (used at dilution of 1:500) followed by donkey anti-rabbit Ig conjugated to horseradish peroxidase (Amersham Biosciences UK Limited, Buckinghamshire, United Kingdom) or sheep anti-mouse IgG conjugated to horseradish peroxidase (Amersham Biosciences UK Limited, Buckinghamshire, United Kingdom), respectively. Enzyme-labeled bands were visualized with enhanced chemiluminescence (ECL) (Amersham) Western blotting detection reagents and Hyperfilm ECL (Amersham).
Preparation of chromosomal DNA.
Chromosomal DNA was obtained by harvesting the cells from 15 ml of mid-log-phase growth in BHI (Oxoid, Basingstoke, United Kingdom) into 1 ml of 10 mM Tris base-100 mM EDTA (pH 8)-0.5% (wt/vol) SDS (1 h at 37°C). Cell lysates were obtained by sequential addition (3 h at 50°C) of proteinase K (Sigma) to a final concentration of 20 μg/ml (3 h) and RNase A (Sigma) to a final concentration of 20 μg/ml (30 min at 37°C). The resulting lysates were extracted once with phenol-chloroform-isoamyl alcohol (25:24:1) and precipitated in 0.2 volumes of 10 M ammonium acetate and 600 μl of absolute ethanol. The resulting DNA was air dried, suspended in 10 mM Tris-1 mM EDTA buffer, and stored at 4°C.
PCR and sequencing methods.
The presence of ply-related and sodA genes in the extracted DNA was determined by PCR. For initial screening of isolates for the presence of ply-related genes, primers designed against the published sequence of the ply gene (26) (designated 15C and 15D and giving a 1,500-bp product [Table 1 ]) were used. In order to determine whether the flanking genes of ply-related sequences within the S. mitis genome were conserved with S. pneumoniae, a second set of primers (designated 27R and 27S [Table 1]) was used. These PCR primers were designed against the sequences flanking the ply gene in the pneumococcal genome (24).
TABLE 1.
Synthetic oligonucleotide primers used for sequencing of the ply gene
| Primera | Sequence (5′→3′) |
|---|---|
| 27S | TACTTAGTCCAACCACGG |
| 27R | CTTGGCTACGATATTGGC |
| 4W | GATCATCAAGGTAAGGAA |
| 4V | CAATACAGAAGTGAAGGCGG |
| 4T | GTTGATCGTGCTCCGATGAC |
| 9Y | CGGGATCCGGCAAATAAAGCAGTAAATGACTTT |
| 27T | ATAAGTCATCGGAGCACG |
| 15C | GGAGGTAGAAGATGGCAAATAAAGC |
| 15D | CTAGTCATTTTCTACCTTATCCTCTACC |
Primers 27S and 27R were used for sequencing of the toxin gene in strains R75I, R76, R77, R84, COL15, and TIGR4. Primers 15C and 15D were used for sequencing of the toxin gene in strain R75II.
For ply-positive S. mitis isolates, species identification was confirmed by amplification and sequencing of the sodA gene, as previously described (15).
The sequencing of ply-related and sodA genes was performed by DBS Genomics, University of Durham, Durham, United Kingdom, or by the Sir Henry Wellcome Centre for Genomic Research, University of Glasgow, Glasgow, United Kingdom. The primers used for sequencing of the ply genes are shown in Table 1. sodA gene sequences were compared to published sequences with BLAST (http://www.ncbi.nlm.nih.gov/BLAST/). Toxin sequences were assembled and aligned with the Vector NTI package (Invitrogen). Guide trees were produced with the Clustal X algorithm of this package.
Nucleotide sequence accession numbers.
The DNA sequences of the mitilysin genes have been deposited in the GenBank database under accession no. EFO66514, EFO66515, EFO66516, EFO66517, EFO66518, EFO66519, and EFO66520.
RESULTS
Screening of VGS isolates for pneumolysin-related genes.
Initially, all isolates (n = 219) were screened for the presence of ply-related sequences by use of primers within the coding sequence of the gene. This resulted in 30 isolates with PCR products of the appropriate size (1,500 bp). Of these 30 isolates, six were isolates previously reported as containing ply by Whatmore et al. (27). The remainder were recovered from nasopharyngeal aspirates (n = 17), blood cultures from immunocompromised patients (n = 5), bronchoalveolar lavage fluid (n = 1), and a dental abscess (n = 1). Six novel positive isolates of S. mitis and the previously described strain COL15 were selected for further analysis, along with a pneumococcal type strain (TIGR4) and two reference strains of S. mitis (Table 2).
TABLE 2.
Strains used in this study
| Strain | Species | Origin | Site of isolation | Optochinb | Presence of ply-related sequencec |
|---|---|---|---|---|---|
| TIGR4 | S. pneumoniae | S | + | ||
| R75I | S. mitis | United Kingdom | NPAa | R | + |
| R75II | S. mitis | United Kingdom | NPA | R | + |
| R76 | S. mitis | United Kingdom | NPA | R | + |
| R77 | S. mitis | United Kingdom | NPA | R | + |
| 990123 | S. mitis | United Kingdom | Dental abscess | R | + |
| QH17 | S. mitis | United Kingdom | Blood culture | R | + |
| COL15 | S. mitis | United Kingdom | Sputum, chest infection | S | + |
| NCTC 10712 | S. mitis | R | − | ||
| NCTC 12261 | S. mitis | R | − |
NPA, nasopharyngeal aspirate.
S, sensitive; R, resistant.
Presence of ply gene determined by PCR.
In order to obtain a larger DNA fragment to allow the complete sequence of the gene to be determined, primers to the predicted flanking sequences of the gene were designed. These primers were based on the sequences flanking the pneumolysin gene in S. pneumoniae. Six of the seven S. mitis strains tested (Table 2) gave a fragment of the appropriate size with the flanking primers. The exception was strain R75II, which did not give a product with the flanking primers. The smaller PCR product generated from the original screen was therefore used for the sequencing of this gene. This meant that we were unable to determine the entire sequence for strain R75II.
DNA sequence analysis.
In order to examine more closely the pneumolysin-related sequences from VGS, PCR products obtained from seven S. mitis strains (Table 2) were sequenced with the primers listed in Table 1. For strain R75II, the ply gene was obtained with primers 15C and 15D, since primers 27S and 27R, used for the other strains studied, failed to produce a PCR product. The predicted amino acid sequences of the toxin genes are shown in Fig. 1. The known pneumolysin sequences from strains D39 (S. pneumoniae serotype 2) and TIGR4 (S. pneumoniae serotype 4) were used for reference. Sequence variations from the pneumolysin protein from S. pneumoniae D39 occur at 13 amino acid positions in the VGS isolates. As 11 of these are not found in pneumolysin sequences from a large number of clinical pneumococcal isolates recently sequenced in our laboratory (data not shown), we propose that the toxin from S. mitis be named mitilysin to differentiate it from pneumolysin.
FIG. 1.
Alignment of Ply from VGS with two pneumococcal Ply sequences. The epitope recognized by MAb PLY-7 is shaded.
Analysis of toxin levels in culture lysates.
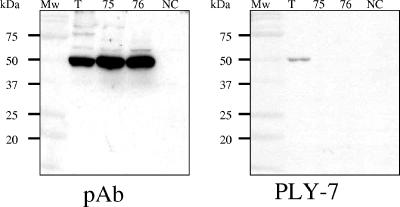
Prior to analysis, the culture lysates were adjusted to contain comparable levels of total cell protein. Levels of toxin were then measured by ELISA. For both pneumococci and S. mitis isolates, toxin was typically found to be between 0.1 to 0.3% of total cell protein. No toxin was detected by ELISA in the extracts of S. mitis strains R75II and R76. All intracellular fractions of ply PCR-positive strains were found to be hemolytic when assessed with horse erythrocytes (Table 3). The specific activity of the toxin for pneumococcal and S. mitis isolates reacting in the ELISA was calculated to be between 105 to 106 HU/mg of toxin. We were unable to calculate specific activity for the toxin from strains R75II and R76 due to the lack of recognition of the protein in the ELISA. All cell lysates from the isolates listed in Table 2 were analyzed by Western blotting with a PAb raised to pneumolysin (Ply). All pneumococcal and S. mitis isolates (except for the two S. mitis NCTC strains) gave a positive result (a band of approximately 53 kDa). As results of the Ply ELISA suggested that the toxins produced by S. mitis strains R75II and R76 were not recognized by the MAb used, we performed a Western blot analysis of the cell-associated Ply obtained from mid-log-phase cultures. Both the anti-rabbit pneumolysin PAb (Ply) and an anti-mouse pneumolysin MAb (PLY-7) were used to test strains R75II and R76 (Fig. 2). The PAb detected toxin in extracts of R75II and R76 whereas MAb PLY-7 did not.
TABLE 3.
Analysis of the presence and activity of toxin in VGS
| Strain | Hemolytic activity
|
Toxin in cell extract (μg/ml)a | Toxin neutralized by:
|
Western blot analysis with:
|
|||
|---|---|---|---|---|---|---|---|
| Cell associated | Culture medium | MAb | PAb | MAb | PAb | ||
| S. mitis R75I | Yes | Yes | 0.065 | Not done | Yes | Not done | Positive |
| S. mitis R75II | Yes | Yes | None detected | No | Not done | Negative | Positive |
| S. mitis R76 | Yes | Yes | None detected | No | Yes | Negative | Positive |
| S. mitis R77 | Yes | Yes | 1.7 | No | No | Not done | Positive |
| S. mitis COL15 | Yes | Yes | 3.0 | Not done | Not done | Not done | Positive |
| TIGR4 | Yes | No | 1.2 | Not done | Not done | Yes | Positive |
As determined by ELISA.
FIG. 2.
Western Blot analysis of toxins from VGS strains TIGR4 (T), R75II (75), R76 (76), and the negative control NCTC 10712 (NC). The samples were cell lysates from 15 ml of mid-log-phase cultures. Pneumolysin was detected with an anti-rabbit pneumolysin PAb (Ply) and an anti-mouse pneumolysin MAb (PLY-7).
Toxin neutralization assays.
In order to investigate the functional consequences of nonrecognition of certain Ply proteins by MAb PLY-7, a neutralization assay was performed. Pneumococcal TIGR4 lysate included as a positive control showed no hemolytic activity after incubation with either PLY-7 or anti-Ply PAb. Cell lysates of S. mitis strains R75II, R76I, and R77 remained lytic after incubation with PLY-7, indicating that activity is unaffected by the presence of the MAb. This confirms the results of Western blotting and ELISA. Hemolytic activity of the S. mitis strains R75II and R76 was blocked after incubation with the polyclonal anti-Ply serum. Interestingly, the lysate of S. mitis strain R77 remained hemolytic after incubation with both PAb and PLY-7, indicating the possible presence of an additional hemolytic protein produced by this strain.
Secretion of mitilysin from S. mitis.
A hemolytic assay of the culture supernatant of five S. mitis strains following growth to mid-log phase was performed. For all five of the S. mitis strains, the culture media were found to be hemolytic (Table 3). The culture media of S. mitis strains R75I and COL15 had a 14-times-higher toxin concentration than those produced by other S. mitis strains when measured by ELISA, suggesting that S. mitis, and in particular strains R75I and COL15, actively secrete mitilysin. The pneumococcal strains D39 and TIGR4 showed no hemolytic activity in the culture supernatant. Control S. mitis strains (NCTC 10712 and NCTC12261) demonstrated no hemolytic activity.
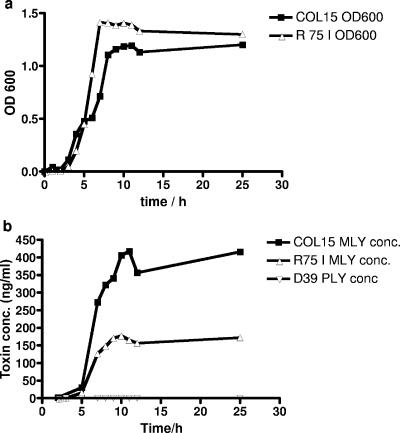
In order to confirm the possibility that S. mitis strains R75I and COL15 actively secrete mitilysin during mid-log-phase growth, an in vitro growth curve analysis was performed (Fig. 3a). This showed that an increasing amount of mitilysin is present in the culture media during growth of strains R75I and COL15 (Fig. 3b). There was no release of toxin into the culture medium by strain D39 of S. pneumoniae (Fig. 3b) or by strain TIGR4 (Table 3). The concentration of mitilysin in the culture medium of strain COL15 was found to increase up to a level of 417 ng/ml during 12 h of incubation, compared to a maximum concentration of 177 ng/ml for strain R75I. Mitilysin was first detected in the culture media of both strains after 2 h of incubation, with concentrations of 1.6 ng/ml and 0.4 ng/ml for COL15 and R75I, respectively. After 25 h of incubation, the concentration did not increase from the maximum values seen after 10 to 11 h of growth, even though the viable cell counts during this time dropped from 7.33 × 107 to 3.25 × 104 CFU/ml and 3.63 × 108 to 2.38 × 105 CFU/ml for R75I and COL15, respectively.
FIG. 3.
Expression of Ply in culture media by strains COL15 and R75I. Bacteria were grown in BHI. Samples were withdrawn every hour, and the OD600 was determined (a). The cells were centrifuged down, and the pneumolysin concentration in supernatants was measured by ELISA (b).
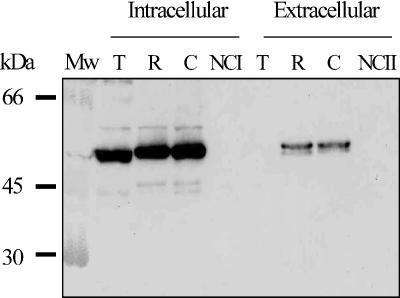
To investigate in more detail the expression of mitilysin in the culture media of strains R75I and COL15, a Western blot analysis was performed with a rabbit PAb. Mitilysin was shown to be present in high concentrations in the cell lysate and in the unconcentrated culture media of strains R75I and COL15 (Fig. 4). Pneumococcal strain TIGR4 was used as a reference strain, and no toxin was detected in the culture medium of TIGR4. Protein bands for R75I and COL15 appear to be larger than that for TIGR4. Apparent changes in molecular weight associated with sequence variations in the ply gene have been observed previously (18).
FIG. 4.
Western blot analysis of toxin expression during in vitro growth of strains TIGR4 (T), R75I (R), COL15 (C), and the negative controls lysate buffer (NCI) and BHI (NCII). The cells were collected from 15 ml of mid-log-phase cultures, resuspended in 1 ml of lysis buffer, and lysed by sonication (intracellular). The extracellular sample is unconcentrated culture medium (BHI). Toxin was detected with an anti-rabbit pneumolysin PAb.
DISCUSSION
This study has confirmed that a gene of the cholesterol cytolysin family is present in S. mitis clinical isolates and that these strains produce active toxin. Based on the fact that this gene codes for unique amino acid sequences not seen in a large collection of pneumococci, we have named it mitilysin (mly). The mly gene was found to be present in 30 out of 219 VGS isolates tested, with the majority of positive strains isolated from nasopharyngeal washings obtained from children. This is in contrast to a previous study in which S. mitis-like isolates containing ply-like genes were usually recovered in specimens from patients with respiratory disease (27). Furthermore, our collection also contained mly-positive S. mitis from blood cultures and localized oral disease, suggesting that the niche of mly-positive S. mitis may be more widespread than previously anticipated. Interestingly, a more-recent study has also detected small numbers of S. oralis and S. mitis isolates in oral mucosa swabs from preschool children that give a positive PCR result with pneumolysin-based primers (23).
We present here the first DNA sequences of mly genes from S. mitis. All of the predicted amino acid sequences of mly contain the conserved tryptophan-rich loop typical of all members of the cholesterol-dependent cytolysin family and the VPARMQYE motif important for hemolytic activity and pore formation by this family of proteins (16). However, the mly alleles expressed by S. mitis isolates in our study appear to differ in a number of ways from the pneumolysin produced by pneumococci. The S. mitis Mly alleles differ from D39 Ply at 13 amino acid positions. The mly genes also appear to be less conserved than those found in pneumococci (20; also our unpublished results). This agrees with findings from a recent study attempting to define the pneumococcus by gene content (13). The authors compared sequences of the seven housekeeping genes used for multilocus sequence typing from a reference set of pneumococci and a second set of nontypeable isolates distinct from, but closely related to, S. pneumoniae. The authors also considered the sequence of a ply gene fragment (bp 178 to 525) and found that the isolates not identified as pneumococci were a much more diverse group than the pneumococci when the mean genetic distance within the two groups was compared. It is possible that the isolates in our study identified as S. mitis belong to this diverse group; however, sodA gene sequences from our isolates were matched with S. mitis.
When strain R75II was analyzed by PCR, only the primers against the structural gene were able to amplify a product; no product was generated with primers designed against the flanking DNA of the pneumococcal toxin gene. This was in contrast to the other S. mitis strains tested and suggests that there is more diversity in the flanking regions of the toxin gene in strain R75II.
S. mitis, and in particular two of the isolates (COL15 and R75I), secrete Mly from the cells. This is in agreement with work published by Whatmore et al. (27), who reported that COL15 had “substantial” intracellular and extracellular hemolytic titers. No typical secretion signal sequences were predicted from our sequence analysis of the COL15 and R75I mly genes. Also, analysis of growth curves suggests that Mly is not released by autolysis. Balachandran et al. (2) found that in vitro log-phase Ply release from pneumococcal strain WU2 (a serotype 3 clinical isolate) was not dependent on autolysin and that Ply release does not require choline, thereby excluding other known lytic enzymes of the pneumococcus. The authors conclude that Ply release in WU2 is due to a non-type II, non-type III mechanism. A similar mechanism could be involved in the secretion of mitilysin. Another possibility is that Mly is released by the process of allolysis, recently described to release Ply from pneumococci (12).
Several S. mitis strains were found to produce Mly proteins that were not recognized by the anti-Ply MAb PLY-7 described by Suárez-Álvarez et al. (22). PLY-7 recognizes an epitope, GQDLTAH, within the pneumolysin sequence stretching from amino acids 401 to 407. No mutations were found in this recognition site in any of the strains studied. However, the mitilysin sequences obtained from strains R75II, R76 990123, and QH17 showed a single amino acid change in position 398, where the aspartic acid (D) was changed to asparagine (N) (Fig. 1). This amino acid change lies three amino acids upstream from the PLY-7 recognition site and may explain why toxin from these strains is not recognized by PLY-7.
The role of mly in the pathogenicity of a subset of S. mitis isolates remains unclear. The presence of additional sources of ply-related genetic material could contribute to the genetic plasticity of pneumococcal ply when under selective pressure. Selective pressure could include the use of vaccines based on pneumolysin. Also, variations in the amino acid sequences of the proteins produced mean that MAbs raised to Ply do not recognize all versions of the protein. This finding raises concerns about the use of such MAbs as diagnostic or therapeutic tools.
Acknowledgments
We thank Chris Dowson of the University of Warwick for provision of some of the bacterial strains used in this study.
Footnotes
Published ahead of print on 27 October 2006.
REFERENCES
- 1.Alouf, J. E., C. Geoffroy, F. Pattus, and R. Verger. 1984. Surface properties of bacterial sulfhydryl-activated cytolytic toxins. Interaction with monomolecular films of phosphatidylcholine and various sterols. Eur. J. Biochem. 141:205-210. [DOI] [PubMed] [Google Scholar]
- 2.Balachandran, P., S. K. Hollingshead, J. C. Paton, and D. E. Briles. 2001. The autolytic enzyme LytA of Streptococcus pneumoniae is not responsible for releasing pneumolysin. J. Bacteriol. 183:3108-3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balsalobre, L., M. J. Ferrandiz, J. Linares, F. Tubau, and A. G. de la Campa. 2003. Viridans group streptococci are donors in horizontal transfer of topoisomerase IV genes to Streptococcus pneumoniae. Antimicrob. Agents Chemother. 47:2072-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benton, K. A., J. C. Paton, and D. E. Briles. 1997. The hemolytic and complement-activating properties of pneumolysin do not contribute individually to virulence in a pneumococcal bacteremia model. Microb. Pathog. 23:201-209. [DOI] [PubMed] [Google Scholar]
- 5.Berry, A. M., A. D. Ogunniyi, D. C. Miller, and J. C. Paton. 1999. Comparative virulence of Streptococcus pneumoniae strains with insertion-duplication, point, and deletion mutations in the pneumolysin gene. Infect. Immun. 67:981-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berry, A. M., J. Yother, D. E. Briles, D. Hansman, and J. C. Paton. 1989. Reduced virulence of a defined pneumolysin-negative mutant of Streptococcus pneumoniae. Infect. Immun. 57:2037-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 8.Cima-Cabal, M. D., F. J. Mendez, F. Vazquez, M. del Mar Garcia-Suarez, and J. R. de los Toyos. 2001. A specific and ultrasensitive chemiluminescent sandwich ELISA test for the detection and quantitation of pneumolysin. J. Immunoassay Immunochem. 22:99-112. [DOI] [PubMed] [Google Scholar]
- 9.Dowson, C. G., T. J. Coffey, and B. G. Spratt. 1994. Origin and molecular epidemiology of penicillin-binding-protein-mediated resistance to beta-lactam antibiotics. Trends Microbiol. 2:361-366. [DOI] [PubMed] [Google Scholar]
- 10.Facklam, R. 2002. What happened to the streptococci: overview of taxonomic and nomenclature changes. Clin. Microbiol. Rev. 15:613-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrandiz, M. J., A. Fenoll, J. Linares, and A. G. De La Campa. 2000. Horizontal transfer of parC and gyrA in fluoroquinolone-resistant clinical isolates of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 44:840-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guiral, S., T. J. Mitchell, B. Martin, and J. P. Claverys. 2005. Competence-programmed predation of noncompetent cells in the human pathogen Streptococcus pneumoniae: genetic requirements. Proc. Natl. Acad. Sci. USA 102:8710-8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanage, W. P., T. Kaijalainen, E. Herva, A. Saukkoriipi, R. Syrjanen, and B. G. Spratt. 2005. Using multilocus sequence data to define the pneumococcus. J. Bacteriol. 187:6223-6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jedrzejas, M. J. 2001. Pneumococcal virulence factors: structure and function. Microbiol. Mol. Biol. Rev. 65:187-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawamura, Y., R. A. Whiley, S.-E. Shu, T. Ezaki, and J. M. Hardie. 1999. Genetic approaches to the identification of the mitis group within the genus Streptococcus. Microbiology 145:2605-2613. [DOI] [PubMed] [Google Scholar]
- 16.Kirkham, L. A., A. R. Kerr, G. R. Douce, G. K. Paterson, D. A. Dilts, D. F. Liu, and T. J. Mitchell. 2006. Construction and immunological characterization of a novel nontoxic protective pneumolysin mutant for use in future pneumococcal vaccines. Infect. Immun. 74:586-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 18.Lock, R. A., Q. Y. Zhang, A. M. Berry, and J. C. Paton. 1996. Sequence variation in the Streptococcus pneumoniae pneumolysin gene affecting haemolytic activity and electrophoretic mobility of the toxin. Microb. Pathog. 21:71-83. [DOI] [PubMed] [Google Scholar]
- 19.Lorenz, M. G., and W. Wackernagel. 1994. Bacterial gene transfer by natural genetic transformation in the environment. Microbiol. Rev. 58:563-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitchell, T. J., F. Mendez, J. C. Paton, P. W. Andrew, and G. J. Boulnois. 1990. Comparison of pneumolysin genes and proteins from Streptococcus pneumoniae types 1 and 2. Nucleic Acids Res. 18:4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rossjohn, J., R. J. Gilbert, D. Crane, P. J. Morgan, T. J. Mitchell, A. J. Rowe, P. W. Andrew, J. C. Paton, R. K. Tweten, and M. W. Parker. 1998. The molecular mechanism of pneumolysin, a virulence factor from Streptococcus pneumoniae. J. Mol. Biol. 284:449-461. [DOI] [PubMed] [Google Scholar]
- 22.Suarez-Alvarez, B., M. Garcia-Suarez Mdel, F. J. Mendez, and J. R. de los Toyos. 2003. Characterisation of mouse monoclonal antibodies for pneumolysin: fine epitope mapping and V gene usage. Immunol. Lett. 88:227-239. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki, N., M. Seki, Y. Nakano, Y. Kiyoura, M. Maeno, and Y. Yamashita. 2005. Discrimination of Streptococcus pneumoniae from viridans group streptococci by genomic subtractive hybridization. J. Clin. Microbiol. 43:4528-4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tettelin, H., K. E. Nelson, I. T. Paulsen, J. A. Eisen, T. D. Read, S. Peterson, J. Heidelberg, R. T. DeBoy, D. H. Haft, R. J. Dodson, A. S. Durkin, M. Gwinn, J. F. Kolonay, W. C. Nelson, J. D. Peterson, L. A. Umayam, O. White, S. L. Salzberg, M. R. Lewis, D. Radune, E. Holtzapple, H. Khouri, A. M. Wolf, T. R. Utterback, C. L. Hansen, L. A. McDonald, T. V. Feldblyum, S. Angiuoli, T. Dickinson, E. K. Hickey, I. E. Holt, B. J. Loftus, F. Yang, H. O. Smith, J. C. Venter, B. A. Dougherty, D. A. Morrison, S. K. Hollingshead, and C. M. Fraser. 2001. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science 293:498-506. [DOI] [PubMed] [Google Scholar]
- 25.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walker, J. A., R. L. Allen, P. Falmagne, M. K. Johnson, and G. J. Boulnois. 1987. Molecular cloning, characterization, and complete nucleotide sequence of the gene for pneumolysin, the sulfhydryl-activated toxin of Streptococcus pneumoniae. Infect. Immun. 55:1184-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whatmore, A. M., A. Efstratiou, A. P. Pickerill, K. Broughton, G. Woodard, D. Sturgeon, R. George, and C. G. Dowson. 2000. Genetic relationships between clinical isolates of Streptococcus pneumoniae, Streptococcus oralis, and Streptococcus mitis: characterization of “atypical” pneumococci and organisms allied to S. mitis harboring S. pneumoniae virulence factor-encoding genes. Infect. Immun. 68:1374-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]