Abstract
The 315-residue N-terminal T domain of colicin E3 functions in translocation of the colicin across the outer membrane through its interaction with outer membrane proteins including the OmpF porin. The first 83 residues of the T domain are known from structure studies to be disordered. This flexible translocation subdomain contains the TolB box (residues 34 to 46) that must cross the outer membrane in an early translocation event, allowing the colicin to bind to the TolB protein in the periplasm. In the present study, it was found that cytotoxicity of the colicin requires a minimum length of 19 to 23 residues between the C terminus (residue 46) of the TolB box and the end of the flexible subdomain (residue 83). Colicin E3 molecules of sufficient length display normal binding to TolB and occlusion of OmpF channels in vitro. The length of the N-terminal subdomain is critical because it allows the TolB box to cross the outer membrane and interact with TolB. It is proposed that the length constraint is a consequence of ordered structure in the downstream segment of the T domain (residues 84 to 315) that prevents its insertion through the outer membrane via a translocation pore that includes OmpF.
Transport of proteins across membranes in import and secretion is known to require an assembly of integral membrane proteins that provides passage across the hydrophobic membrane (29, 36). Such an assembly is utilized by colicins, which parasitize receptors in the outer membrane to gain entry into the cell (8, 27, 28, 46). Colicins are highly specific in that they kill only those cells that (i) contain the receptor protein to which colicin binds and (ii) do not produce the cognate immunity protein, providing a selective advantage to the colicin-producing cell in a competitive environment (37).
Colicin production is under SOS control, forming part of the stress response of the cell (20, 43). Colicins are produced in complex with a small (molecular mass of ∼10 kDa) immunity protein that protects the cell from both exogenous and endogenous colicins (7, 22). Colicins have been divided into two groups based on the protein translocation network utilized to cross the outer membrane. Group A colicins, such as the E colicins, utilize the Tol system, consisting of TolA, TolB, TolQ, TolR, and Pal proteins (15, 28, 31). Group B colicins, such as colicins B, D, Ia, and Ib, utilize the Ton system consisting of TonB, ExbB, and ExbD proteins (8, 16). Colicin E3, a 16S rRNase (5), possesses a domain structure (34, 35, 41) as found in other colicins (1, 2, 13, 14, 45). It consists of three separate domains that have distinct roles. Its 315-residue N-terminal T domain functions in cell translocation and entry (1, 2, 41). The central 135-residue receptor (R) domain binds to BtuB (25), and the C-terminal domain contains the catalytic activity (34, 35, 41). The first step of E3 entry into the cell involves binding of the R domain to BtuB (25), a 22-stranded β-barrel outer membrane receptor whose metabolic function is transport of cyanocobalamin (vitamin B12) across the outer membrane (10, 17, 24). It has been inferred that colicin is translocated across the outer membrane through OmpF (21, 26, 47), a very abundant (≥105 copies/cell) (33) outer membrane porin (12, 32), and binds to TolB protein in the periplasmic space (6). However, the details of colicin entry through OmpF are largely unknown. The cytotoxicity of colicin E3 is dependent on the interaction of the periplasmic TolB protein with the TolB box spanning residues 34 to 46 (18, 19), which is contained in the 83-residue N-terminal translocation subdomain—the T83 segment. It is likely that the high Gly content of this subdomain and its resulting flexibility (11, 41) allow it to traverse the outer membrane through a translocation complex that contains OmpF (25, 47) and to interact with the TolB protein in the periplasmic space (6).
The present studies show that the peptide segment (TolB extensor) that separates the C-terminal end of the TolB box, residue 46, from the end of the N-terminal flexible subdomain, residue 83, has a minimum length. The length of the segment is 37 residues (residue 47 to 83) in the wild-type protein. However, the minimum length of this TolB extensor that is required for cytotoxic activity is between 19 and 23 residues. It is proposed that this length constraint results from the existence of a folded conformational state of the downstream region (84 to 315) of the T domain that blocks its transfer across the outer membrane through the translocation pore (Fig. 1A and B).
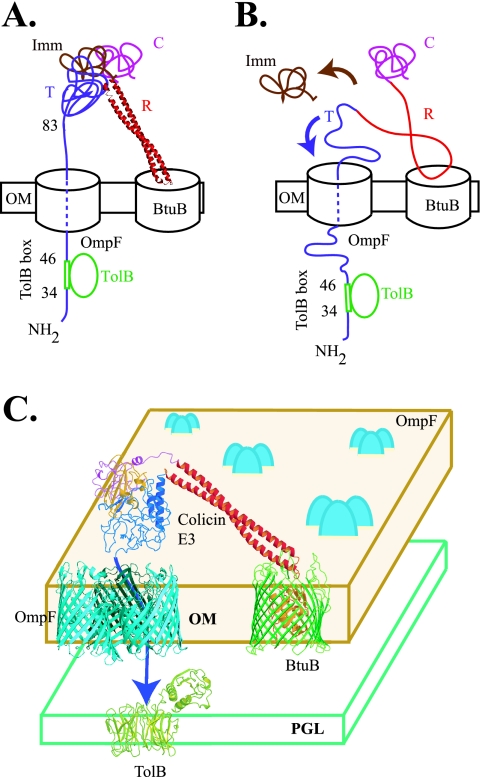
FIG. 1.
Models for different conformational (folding) states of colicin E3 when it binds to, and inserts into, a translocation pore that utilizes OmpF. (A) T domain is fully/partially folded when colicin interacts with the TolB protein. The immunity protein may or may not be bound to colicin E3 when it interacts with TolB protein. In this case, the TolB extensor region (37 residues), extending from residue 46 to 83, has no extra length, thus causing steric limitations reflected in the requirement for a minimum length of the TolB extensor. The R domain is depicted to be folded as a coiled coil. (B) Colicin/T domain is unfolded when it interacts with TolB. In this case, there is extra length in the TolB extensor. The coiled coil of the R domain is depicted as partly unfolded. (C) Outer membrane BtuB/OmpF translocon for the import of colicin E3. Abbreviations: C, catalytic domain; R, receptor binding domain; T, translocation domain; Imm, immunity protein; OM, outer membrane; PGL, peptidoglycan layer.
MATERIALS AND METHODS
Bacterial strains, media, and plasmids.
Escherichia coli XL1-Blue strain was used as the host strain for cloning of mutations and deletions. All cloning was done in the pET41b vector, with the colicin constructs containing a His8 tag at the C terminus of the immunity protein. E. coli BL21(DE3) was the host strain for the expression vector pET41b. In the pET41b vector, protein expression is under the control of a strong isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible T7 RNA polymerase promoter. All cultures were grown in LB media or on LB agar plates supplemented with antibiotic when required.
Mutagenesis and protein purification.
All cloning and mutagenesis was done using standard protocols. Colicin E3 was first amplified using upstream (Nde primer) and downstream (Xho primer) primers and then cloned between the NdeI-XhoI sites of the expression vector pET41b, so that ImmE3 contained a C-terminal polyhistidine tag to facilitate protein purification (pWC41E3). All mutagenesis experiments were carried out using this construct by an overlap two-step PCR method (38). The first and second PCR were set up, respectively, using (i) the mutation containing primers and the corresponding upstream (Nde) or downstream (Xho) primer or (ii) the Nde/Xho primers and the purified products of the first PCR as the template. This PCR product was then digested with NdeI-XhoI and cloned in pET41b. All constructs contained a C-terminal His8 tag on ImmE3. Purification of overexpressed colicin E3 constructs was carried out with a Ni-charged iminodiacetic acid-agarose column. The colicin E3 deletion constructs generated for the present study are E3 Δ65-73 and E3 Δ72-80, with 9 residues deleted; a 14-residue deletion construct, E3 Δ65-78; and E3 Δ65-83, with 19 residues missing from the TolB extensor region. Further, E3 60-64/Δ65-78, with 14 residues deleted and residues 60 to 64 mutated (S60A, G61S, H62A, G63S, N64A) was created. Also, 5 residues were added to E3 Δ65-83 to generate the construct E3 79-83/Δ65-78 which can also be considered a construct with 65 to 78 residues deleted and residues 79 to 83 mutated (G79S, N80A, L81A, S82A, A83S).
Plasmid pRJ379 containing C-terminally His-tagged TolB in the pET21d vector (9) was a generous gift from R. James and was used for the overexpression and purification of TolB. Purification of TolB was done by metal affinity chromatography using an Ni-charged iminodiacetic acid-agarose column.
Cytotoxicity.
Cytotoxicity of the different colicin E3 constructs was determined by analyzing the effect of colicin constructs on the growth rate of colicin-sensitive E. coli K17 indicator cells. One hundred milliliters of LB media was inoculated with an overnight culture of the indicator cells and grown to an OD600 (optical density at 600 nm) of 0.1. The culture was then aliquoted, and a different colicin construct was added to each sample at a concentration of 3.2 nM. Cell growth was monitored by measuring the OD600 at 1-h intervals.
Cytotoxicity of the E3 constructs was also compared by analysis of colony-forming ability. E. coli K17 cells were grown at 37°C under aerobic shaking conditions to an OD600 of 0.1, when 3.2 nM colicin was added. Cells were incubated for 1 h and then diluted and transferred to LB agar plates, from which the relative colony-forming ability was measured. The effective multiplicity (m) was calculated from the relative colony-forming ability that resulted from the cytotoxic action of each colicin construct, assuming exponential loss of the surviving cell population (S) relative to the untreated control cells (So) as a function of added colicin (i.e., S/So = e−m). It is recognized that cell survival is not truly an exponential process at low levels of cell survival. Thus, the multiplicity, m, is used as a qualitative measure of activity of the different constructs.
TolB binding.
TolB binding to the various colicin constructs was analyzed by gel filtration chromatography, using the different elution patterns of E3 and TolB proteins on a Superdex 75 column (total column volume = 23.6 ml). Each colicin construct was mixed with TolB protein in a 1:1 molar ratio (18 μM), and the binding reaction was allowed to proceed for ∼10 min. This was followed by separation on the Superdex 75 column and determination of the protein peaks in the elution profile. The total protein in each peak, represented by the area under that peak, was calculated for each colicin construct.
Occlusion of OmpF.
The E3 constructs were used to study occlusion of OmpF channels incorporated into planar bilayers as described previously (47). The synthetic phospholipids DOPC and DOPE were mixed in a 1:1 molar (total concentration, 10 mg/ml) ratio, dissolved in n-decane, and then used to form planar bilayers (4, 42). Bilayers were formed by painting lipids using a brush technique (30) on a 0.45-mm aperture in a partition separating two 1-ml chambers containing 10 mM HEPES (pH 7.0), 0.1 M KCl at 23°C. Occlusion was studied by measuring the current across this aperture with Ag/AgCl electrodes which are immersed into the two chambers, using a Warner BC-525C amplifier (Hamden, CT). To form channels, OmpF (1 pg/ml to 1 ng/ml) was added to the cis side of the membrane, and the solution was stirred until channels appeared. A transmembrane potential was applied to the cis side, with the trans side at electrical ground. A final concentration of 0.5 to 1 μg/ml of the colicin construct whose occlusion activity was to be tested was added to the trans or cis side of the aperture.
RESULTS
TolB binding.
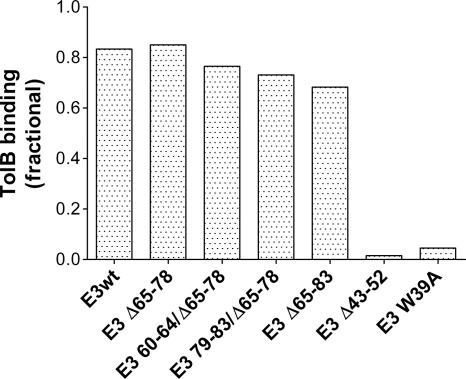
To specifically study the role of the length of the TolB extensor region (segment 47 to 83) in colicin cytotoxicity, it was necessary to make deletions that would not affect the affinity of colicin E3 for TolB. Otherwise, any decrease in cytotoxicity might be ambiguous, as it would include effects of both length and TolB binding affinity. Therefore, deletions in the TolB extensor were made as far downstream from the TolB box as possible. A colicin construct was first allowed to interact with TolB, following which the complex was separated on a Superdex 75 column. The peak fractions of colicin E3 (and constructs) and TolB protein appeared, respectively, at elution volumes of 8.2 ± 0.3 ml and 11.0 ± 0.1 ml. The colicin/TolB complex eluted at a volume of 8.0 ± 0.35 ml. Because the peak of the colicin-TolB complex overlapped that of the colicin alone, the amount of free TolB (area under of curve of TolB elution peak) was used to measure the TolB binding affinity of the colicin. Deletion of 14 (Δ65-78) or 19 (Δ65-83) residues from the TolB extensor region did not affect binding to TolB. Two additional constructs were tested that were important in the cytotoxicity studies (see below). Mutation of 5 residues, N- and C-terminal to the Δ65-78 deletion, in the two mutants, E3 60-64/Δ65-78 and E3 79-83/Δ65-78, did not affect binding to TolB. All of the deletion constructs except E3 Δ43-52 displayed wild-type affinity for TolB (Fig. 2). There was almost no binding of E3 Δ43-52 to TolB, which is expected, as this construct lacks the complete TolB box that spans residues 34 to 46 (19). The Trp39Ala (W39A) mutant was a negative control, as modifications of the TolB box prevent binding to TolB (9). Studies on the question of a critical length of the N-terminal domain were confined to the segment spanning residues 60 to 80 because deletions/mutations in this region did not affect TolB binding.
FIG. 2.
TolB binding affinity of colicin constructs measured by the area under the elution peaks formed by colicin/TolB complex separated on a Superdex 75 column, as described in Materials and Methods. The affinity is normalized to the total amount of TolB added (1.0 on the ordinate).
Cytotoxicity.
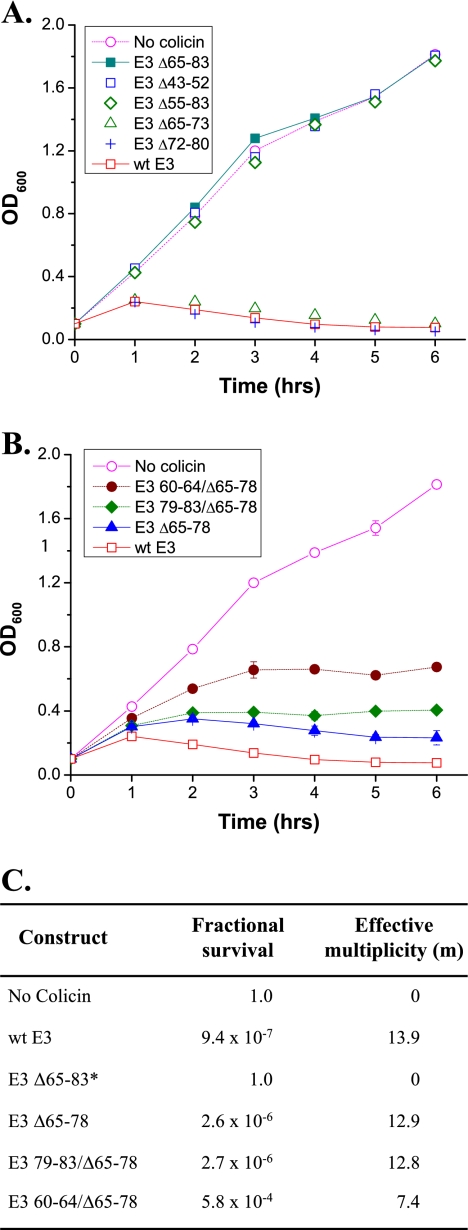
Cytotoxicity, used as an indicator of the relative activity of the constructs obtained from mutations in the colicin N-terminal subdomain, was assayed through the effect of colicin on the growth of sensitive cells (Fig. 3A and B) and the relative colony forming ability (Fig. 3C). Growth curves of wild-type cells to which no colicin or colicin E3 (3.2 nM) was added are compared (Fig. 3A). Deletion of 29 residues (E3 Δ55-83) or 19 residues (E3 Δ65-83) abolished cytotoxicity so that the growth curve was the same as the control to which no colicin was added (Fig. 3A). However, deletion of 9 residues (E3 Δ65-73 and E3 Δ72-80) at either of the two positions in the extensor region had a negligible effect on colicin cytotoxicity (Fig. 3A). Increasing the length of the deletion to 14 residues (E3 Δ65-78) resulted in a small decrease (∼7% decrease in the calculated multiplicity, m) in cytotoxicity compared to the wild-type colicin, as shown in the comparison of growth curve inhibition (Fig. 3B) and the effective multiplicities (Fig. 3C). Addition of 5 residues to the 19 residue deletion construct E3 Δ65-83, to generate a 14 residue deletion construct E3 79-83/Δ65-78, restored essentially all activity compared with E3 Δ65-78, which also has 14 residues deleted in the TolB extensor region (Fig. 3B and C). The 5 residues (Ser, Ala, Ala, Ala, Ser), added to the C-terminal end of the Δ65-83 deletion, are different from the residues present in the wild-type E3 (Gly, Asn, Leu, Ser, Ala). Thus, because deleting 19 residues from the 37-residue extensor abolishes all activity and removal of 14 residues allows almost full cytotoxicity, the minimum length of the TolB extensor is 19 to 23 residues. Deletions that further shorten this segment abolish colicin cytotoxicity.
FIG. 3.
Effect of the various colicin constructs on the growth of E. coli K17 indicator cells. (A) Colicins were added at a concentration of 3.2 nM. E3 Δ55-83, E3 Δ65-83, and E3 Δ43-52 did not inhibit cell growth, while E3 Δ65-73 and E3 Δ72-80 were as active as wild-type E3. (B) E3 Δ65-78 and E3 79-83/Δ65-78 had a similar activity, which was slightly less than that of wild-type E3. E3 60-64/Δ65-78 showed decreased cytotoxicity compared to E3 Δ65-78. Error bars are contained within symbols if they are not explicitly displayed. (C) Effective multiplicity of colicin and constructs that inhibited cell growth in panel B. *, The m value and fractional survival for E3 Δ65-83 have been assumed to be similar to the control in which no colicin was added on the basis of their identical growth curves and is shown here for purposes of comparison.
OmpF binding site.
A binding site for OmpF has been proposed in the region 60 to 80 of colicin E9 (21). No such binding site was found between residues 65 to 83 of colicin E3 because deletions or mutations in this region did not affect colicin cytotoxicity. However, when residues 60 to 64 were mutated (S60A, G61S, H62A, G63S, N64A) in the construct E3 60-64/Δ65-78, there was a decrease of ∼40% in the effective multiplicity, m, compared to colicin E3 Δ65-78 and E3 79-83/Δ65-78 (Fig. 3B and C). Thus, it cannot be excluded that residues 60 to 64 could participate in binding to OmpF.
Occlusion of OmpF channels.
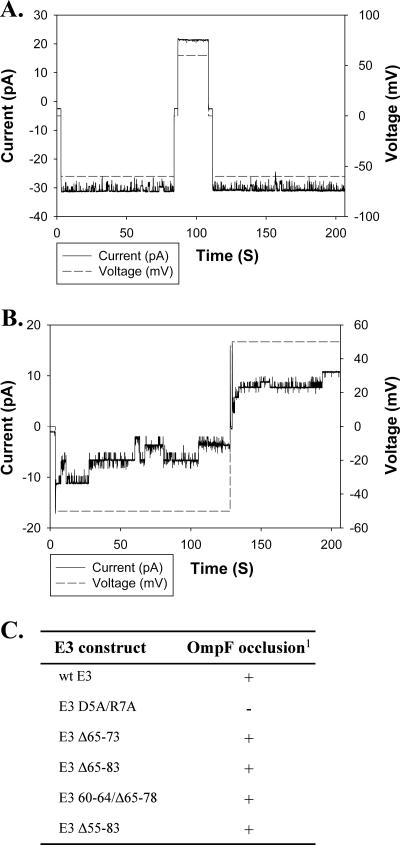
To further characterize the role of residues 60 to 80, E3 deletion constructs were also tested for their ability to occlude ion channels of OmpF porin incorporated in planar bilayer membranes. Previously, it has been shown that colicin E3 (but not E1), added from the trans side of the membrane, is able to specifically occlude OmpF channels on application of cis-negative transmembrane potential (47). The occlusion effect was negated when the transmembrane potential was changed to cis positive. Residues 5 and/or 7 were shown to be essential for occlusion of OmpF (47), and the colicin-OmpF interaction is almost abolished (reduced by 85%) when the first 7 residues are deleted (21). The ability of the different E3 constructs to occlude OmpF is summarized in Fig. 4C. E3 Δ55-83, in which most of the TolB extensor is deleted, was able to occlude OmpF when added from the trans side but not from the cis side (Fig. 4), as was observed for wild-type E3. Thus, the interaction of residues 60 to 80 with OmpF is weak or negligible. The occlusion of OmpF is dependent on residues 5 and/or 7, near the N terminus of the 83-residue translocation subdomain, rather than those in the segment of residues 60 to 80 near its C terminus.
FIG. 4.
E3 Δ55-83 occludes OmpF channels in planar bilayers. OmpF was added to the cis side of the membrane and the solution stirred until channels appeared. The transmembrane current was recorded upon application of transmembrane potentials, 50mV and −50mV (sign of the potential is that of cis side). (A) E3 Δ55-83 was added to the cis side, and no occlusion was seen upon application of potential. (B) When the colicin was added from the trans side, it occluded OmpF channels upon application of a cis-negative potential. The occlusion effect was negated upon changing the potential to cis positive. (C) OmpF occlusion by colicin E3 deletion constructs. 1, The presence (+) or absence (−) of occlusion denotes the ability of 0.5 to 1 μg/ml of colicin to block OmpF ion channels.
DISCUSSION
Formation of the E3/BtuB/OmpF/TolB translocon.
The peripheral nature of binding of the R domain of colicin E3 to BtuB seen in the X-ray structure of the complex (25), as well as the absence of channels formed by BtuB in planar bilayers in the presence or absence of colicin (47), precludes the possibility of colicin entry through any hypothetical pore of BtuB. Thus, a secondary receptor, including OmpF and perhaps other outer membrane proteins such as the OmpC porin (32), is needed through which colicin can cross the outer membrane. Genetic evidence exists for a role of OmpF in a translocon for colicin import across the outer membrane (3). OmpF is abundant in the outer membrane (≥105 copies/cell), and occlusion of OmpF channels incorporated in planar bilayers with colicin E3 has been well characterized (47). The existence of a colicin translocon involving the BtuB receptor, the bound colicin, and OmpF or an OmpF cluster has been postulated (21, 25, 47).
Minimum length requirement of the TolB extensor.
The T and R domains of nuclease colicins E2, E3, E6, E7, and E9 are highly conserved (39), suggesting that they use similar mechanisms to import C domains with different nuclease activities. The nearly identical N-terminal 83-residue subdomain of these colicins (39) is remarkable in that it is highly flexible due to the large number of glycines (∼40% of residues are Gly) and contains the residues required for interaction with the Tol system. It has been proposed that flexibility of the N-terminal region enables it to cross the outer membrane and interact with the Tol system in the periplasm (11, 44). It has been found in the present study that a critical aspect for such an interaction, along with the flexibility, is the length of the peptide segment separating the Tol recognition sequence from rest of the colicin. The requirement of a minimum length of this TolB extensor in colicin E3 was determined from the properties of mutations/deletions in the 60- to 80-residue region downstream and distal to the TolB box. Binding to TolB was assessed to ensure that deletions in this region do not affect interaction with TolB. Experiments with E3 deletion constructs show that although deletions of 9 and 14 residues did not affect cytotoxicity, deletion of 19 residues in this region causes loss of cytotoxic function. When 5 residues are added back to the 19 residue deletion construct, the colicin regains its cytotoxicity, suggesting that length, rather than details of the sequence, in this region is the critical parameter. It has been shown in a previous study that a deletion construct E9 Δ60-80 (a 21-residue deletion) did not bind to OmpF and was inactive, suggesting a second binding site for OmpF (21) in addition to an N-terminal site that includes residues 5 and/or 7 (47). However, the role of residues 60 to 80 in binding to OmpF is not clear. According to Housden et al. (21), this region is responsible for only about 15% of the colicin-OmpF interaction, with residues 1 to 7 being responsible for the remaining 85%, with deletions between residues 7 and 53 having a negligible effect on the colicin-OmpF binding. Furthermore, we have shown that deletions/mutations in the entire 60- to 80-residue region had no effect on occlusion of OmpF by colicin E3 in planar bilayer experiments. In light of the above results, it is concluded that the loss of cytotoxicity of E9 Δ60-80 arises from the length of the deletion rather than deletion of a putative OmpF binding site. Mutation of residues 60 to 64 caused a somewhat decreased cytotoxicity, reflected in a decrease of the effective multiplicity from ∼14 to 7.4 (Fig. 3B and C), suggesting that these residues could participate in a weak binding to OmpF. An ordered or partially folded structure of the C-terminal segment (residues 84 to 315) of the translocation domain could explain the minimum length requirement of the subdomain found in the present study (Fig. 1A and B). An ordered T domain can result in a steric constraint on its insertion into the OmpF translocation pore and a length restriction in the TolB extensor region. The minimum length of the TolB extensor was determined to be between 19 and 23 residues.
Function of the downstream T domain: mechanism of unfolding.
The limiting size of a channel through OmpF is 7 by 11 Å (12, 23), which could, in principle, allow for the threading of an unfolded polypeptide but not a folded protein. The present studies indicate that at least 60 residues of the N-terminal subdomain are able to insert through the OmpF complex to contact TolB in the periplasmic space. It is inferred that at some distance downstream of this inserted segment, there is an ordered structure in the T domain that prevents further insertion of the colicin. This leads to the following questions. (i) What is the location of this first folded domain in the T-domain? (ii) What is the function of the remaining downstream T domain, spanning residues 84 to 315, which seems not to be required for the initial insertion into the receptor? (iii) What is the mechanism of, and energy source for, unfolding of the colicin, especially of the C domain whose entry into the cell is absolutely required for cytotoxicity?
Events in colicin import across the outer membrane.
It is not known whether the whole colicin or only the C domain of colicin E3 enters the cell. There is evidence that a proteolytic cleavage takes place between the R and C domains of colicin E7 during its entry into the cell (40). If such an event also occurs in colicin E3, as might reasonably be inferred, the C domain would be the only part of the colicin entering the cell.
In conclusion, the events believed to occur in colicin E3 import through the outer membrane BtuB/OmpF (and perhaps OmpC) translocon are summarized (Fig. 1C) as follows: colicin binds to BtuB; OmpF is recruited into the translocon through interaction of the T domain N terminus with OmpF; interaction of T83 with TolB in the periplasm; unfolding of the C-domain and its insertion into an OmpF channel (48); proteolytic cleavage of colicin between the C and R domains, allowing entry of the C domain into the cytoplasm, where it acts cytotoxically as an endoribonuclease.
Acknowledgments
These studies were supported by a grant from the NIH (GM18457) and the Henry Koffler Professorship.
We thank S. D. Zakharov and M. V. Zhalnina for helpful discussions. We also thank R. James and C. Kleanthous for providing us with the plasmid pRJ379.
Footnotes
Published ahead of print on 3 November 2006.
REFERENCES
- 1.Baty, D., M. Frenette, R. Lloubes, V. Geli, S. P. Howard, F. Pattus, and C. Lazdunski. 1988. Functional domains of colicin A. Mol. Microbiol. 2:807-811. [DOI] [PubMed] [Google Scholar]
- 2.Benedetti, H., M. Frenette, D. Baty, M. Knibiehler, F. Pattus, and C. Lazdunski. 1991. Individual domains of colicins confer specificity in colicin uptake, in pore-properties and in immunity requirement. J. Mol. Biol. 217:429-439. [DOI] [PubMed] [Google Scholar]
- 3.Benedetti, H., M. Frenette, D. Baty, R. Lloubes, V. Geli, and C. Lazdunski. 1989. Comparison of the uptake systems for the entry of various BtuB group colicins into Escherichia coli.J. Gen. Microbiol. 135:3413-3420. [DOI] [PubMed] [Google Scholar]
- 4.Benz, R., A. Schmid, and R. E. Hancock. 1985. Ion selectivity of gram-negative bacterial porins. J. Bacteriol. 162:722-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boon, T. 1972. Inactivation of ribosomes in vitro by colicin E3 and its mechanism of action. Proc. Natl. Acad. Sci. USA 69:549-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouveret, E., A. Rigal, C. Lazdunski, and H. Benedetti. 1997. The N-terminal domain of colicin E3 interacts with TolB which is involved in the colicin translocation step. Mol. Microbiol. 23:909-920. [DOI] [PubMed] [Google Scholar]
- 7.Bowman, C. M., J. Sidikaro, and M. Nomura. 1971. Specific inactivation of ribosomes by colicin E3 in vitro and mechanism of immunity in colicinogenic cells. Nat. New Biol. 234:133-137. [DOI] [PubMed] [Google Scholar]
- 8.Braun, V., S. I. Patzer, and K. Hantke. 2002. Ton-dependent colicins and microcins: modular design and evolution. Biochimie 84:365-380. [DOI] [PubMed] [Google Scholar]
- 9.Carr, S., C. N. Penfold, V. Bamford, R. James, and A. M. Hemmings. 2000. The structure of TolB, an essential component of the tol-dependent translocation system, and its protein-protein interaction with the translocation domain of colicin E9. Structure 8:57-66. [DOI] [PubMed] [Google Scholar]
- 10.Chimento, D. P., R. J. Kadner, and M. C. Wiener. 2003. The Escherichia coli outer membrane cobalamin transporter BtuB: structural analysis of calcium and substrate binding, and identification of orthologous transporters by sequence/structure conservation. J. Mol. Biol. 332:999-1014. [DOI] [PubMed] [Google Scholar]
- 11.Collins, E. S., S. B. Whittaker, K. Tozawa, C. MacDonald, R. Boetzel, C. N. Penfold, A. Reilly, N. J. Clayden, M. J. Osborne, A. M. Hemmings, C. Kleanthous, R. James, and G. R. Moore. 2002. Structural dynamics of the membrane translocation domain of colicin E9 and its interaction with TolB. J. Mol. Biol. 318:787-804. [DOI] [PubMed] [Google Scholar]
- 12.Cowan, S. W., T. Schirmer, G. Rummel, M. Steiert, R. Ghosh, R. A. Pauptit, J. N. Jansonius, and J. P. Rosenbusch. 1992. Crystal structures explain functional properties of two E. coli porins. Nature 358:727-733. [DOI] [PubMed] [Google Scholar]
- 13.Cramer, W. A., J. B. Heymann, S. L. Schendel, B. N. Deriy, F. S. Cohen, P. A. Elkins, and C. V. Stauffacher. 1995. Structure-function of the channel-forming colicins. Annu. Rev. Biophys. Biomol. Struct. 24:611-641. [DOI] [PubMed] [Google Scholar]
- 14.Dankert, J. R., Y. Uratani, C. Grabau, W. A. Cramer, and M. Hermodson. 1982. On a domain structure of colicin E1. A COOH-terminal peptide fragment active in membrane depolarization. J. Biol. Chem. 257:3857-3863. [PubMed] [Google Scholar]
- 15.Davies, J. K., and P. Reeves. 1975. Genetics of resistance to colicins in Escherichia coli K-12: cross-resistance among colicins of group A. J. Bacteriol. 123:102-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davies, J. K., and P. Reeves. 1975. Genetics of resistance to colicins in Escherichia coli K-12: cross-resistance among colicins of group B. J. Bacteriol. 123:96-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Masi, D. R., J. C. White, C. A. Schnaitman, and C. Bradbeer. 1973. Transport of vitamin B12 in Escherichia coli: common receptor sites for vitamin B12 and the E colicins on the outer membrane of the cell envelope. J. Bacteriol. 115:506-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garinot-Schneider, C., C. N. Penfold, G. R. Moore, C. Kleanthous, and R. James. 1997. Identification of residues in the putative TolA box which are essential for the toxicity of the endonuclease toxin colicin E9. Microbiology 143:(Pt 9)2931-2938. [DOI] [PubMed] [Google Scholar]
- 19.Hands, S. L., L. E. Holland, M. Vankemmelbeke, L. Fraser, C. J. Macdonald, G. R. Moore, R. James, and C. N. Penfold. 2005. Interactions of TolB with the translocation domain of colicin E9 require an extended TolB box. J. Bacteriol. 187:6733-6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hardy, K. G., and G. G. Meynell. 1972. “Induction” of colicin factor E2-P9 by mitomycin C. J. Bacteriol. 112:1007-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Housden, N. G., S. R. Loftus, G. R. Moore, R. James, and C. Kleanthous. 2005. Cell entry mechanism of enzymatic bacterial colicins: porin recruitment and the thermodynamics of receptor binding. Proc. Natl. Acad. Sci. USA 102:13849-13854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jakes, K. S., and N. D. Zinder. 1974. Highly purified colicin E3 contains immunity protein. Proc. Natl. Acad. Sci. USA 71:3380-3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeanteur, D., T. Schirmer, D. Fourel, V. Simonet, G. Rummel, C. Widmer, J. P. Rosenbusch, F. Pattus, and J. M. Pages. 1994. Structural and functional alterations of a colicin-resistant mutant of OmpF porin from Escherichia coli.Proc. Natl. Acad. Sci. USA 91:10675-10679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kadner, R. J. 1978. Repression of synthesis of the vitamin B12 receptor in Escherichia coli. J. Bacteriol. 136:1050-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurisu, G., S. D. Zakharov, M. V. Zhalnina, S. Bano, V. Y. Eroukova, T. I. Rokitskaya, Y. N. Antonenko, M. C. Wiener, and W. A. Cramer. 2003. The structure of BtuB with bound colicin E3 R-domain implies a translocon. Nat. Struct. Biol. 10:948-954. [DOI] [PubMed] [Google Scholar]
- 26.Law, C. J., C. N. Penfold, D. C. Walker, G. R. Moore, R. James, and C. Kleanthous. 2003. OmpF enhances the ability of BtuB to protect susceptible Escherichia coli cells from colicin E9 cytotoxicity. FEBS Lett. 545:127-132. [DOI] [PubMed] [Google Scholar]
- 27.Lazdunski, C. J. 1995. Colicin import and pore formation: a system for studying protein transport across membranes? Mol. Microbiol. 16:1059-1066. [DOI] [PubMed] [Google Scholar]
- 28.Lazzaroni, J. C., J. F. Dubuisson, and A. Vianney. 2002. The Tol proteins of Escherichia coli and their involvement in the translocation of group A colicins. Biochimie 84:391-397. [DOI] [PubMed] [Google Scholar]
- 29.Mokranjac, D., and W. Neupert. 2005. Protein import into mitochondria. Biochem. Soc. Trans. 33:1019-1023. [DOI] [PubMed] [Google Scholar]
- 30.Mueller, P., D. O. Rudin, H. T. Tien, and W. C. Wescott. 1962. Reconstitution of cell membrane structure in vitro and its transformation into an excitable system. Nature 194:979-980. [DOI] [PubMed] [Google Scholar]
- 31.Nagel de Zwaig, R., and S. E. Luria. 1967. Genetics and physiology of colicin-tolerant mutants of Escherichia coli.J. Bacteriol. 94:1112-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nikaido, H. 2003. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 67:593-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nikaido, H., and M. Vaara. 1987. Outer membrane, p. 7-22. In F. C. Neidhardt (ed.), Escherichia coli and Salmonella: cellular and molecular biology. American Society for Microbiology, Washington, DC.
- 34.Ohno, S., Y. Ohno-Iwashita, K. Suzuki, and K. Imahori. 1977. Purification and characterization of active component and active fragment of colicin E3. J. Biochem. (Tokyo) 82:1045-1053. [DOI] [PubMed] [Google Scholar]
- 35.Ohno-Iwashita, Y., and K. Imahori. 1980. Assignment of the functional loci in colicin E2 and E3 molecules by the characterization of their proteolytic fragments. Biochemistry 19:652-659. [DOI] [PubMed] [Google Scholar]
- 36.Pfanner, N., N. Wiedemann, C. Meisinger, and T. Lithgow. 2004. Assembling the mitochondrial outer membrane. Nat. Struct. Mol. Biol. 11:1044-1048. [DOI] [PubMed] [Google Scholar]
- 37.Riley, M. A., and D. M. Gordon. 1999. The ecological role of bacteriocins in bacterial competition. Trends Microbiol. 7:129-133. [DOI] [PubMed] [Google Scholar]
- 38.Sarkar, G., and S. S. Sommer. 1990. The “megaprimer” method of site-directed mutagenesis. BioTechniques 8:404-407. [PubMed] [Google Scholar]
- 39.Sharma, O., S. D. Zakharov, and W. A. Cramer. 2006. Colicins, p. 115-123. In A. J. Kastin (ed.), Handbook of biologically active peptides, 1st ed. Academic press, San Diego, CA.
- 40.Shi, Z., K. F. Chak, and H. S. Yuan. 2005. Identification of an essential cleavage site in ColE7 required for import and killing of cells. J. Biol. Chem. 280:24663-24668. [DOI] [PubMed] [Google Scholar]
- 41.Soelaiman, S., K. Jakes, N. Wu, C. Li, and M. Shoham. 2001. Crystal structure of colicin E3: implications for cell entry and ribosome inactivation. Mol. Cell 8:1053-1062. [DOI] [PubMed] [Google Scholar]
- 42.Song, J., C. A. Minetti, M. S. Blake, and M. Colombini. 1998. Successful recovery of the normal electrophysiological properties of PorB (class 3) porin from Neisseria meningitidis after expression in Escherichia coli and renaturation. Biochim. Biophys. Acta 1370:289-298. [DOI] [PubMed] [Google Scholar]
- 43.Tyler, J., and D. J. Sherratt. 1975. Synthesis of E colicins in Escherichia coli.Mol. Gen. Genet. 140:349-353. [DOI] [PubMed] [Google Scholar]
- 44.Vetter, I. R., M. W. Parker, A. D. Tucker, J. H. Lakey, F. Pattus, and D. Tsernoglou. 1998. Crystal structure of a colicin N fragment suggests a model for toxicity. Structure 6:863-874. [DOI] [PubMed] [Google Scholar]
- 45.Wiener, M., D. Freymann, P. Ghosh, and R. M. Stroud. 1997. Crystal structure of colicin Ia. Nature 385:461-464. [DOI] [PubMed] [Google Scholar]
- 46.Zakharov, S. D., and W. A. Cramer. 2004. On the mechanism and pathway of colicin import across the E. coli outer membrane. Front. Biosci. 9:1311-1317. [DOI] [PubMed] [Google Scholar]
- 47.Zakharov, S. D., V. Y. Eroukova, T. I. Rokitskaya, M. V. Zhalnina, O. Sharma, P. J. Loll, H. I. Zgurskaya, Y. N. Antonenko, and W. A. Cramer. 2004. Colicin occlusion of OmpF and TolC channels: outer membrane translocons for colicin import. Biophys. J. 87:3901-3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zakharov, S. D., M. Zhalnina, O. Sharma, and W. A. Cramer. 2006. The colicin E3 outer membrane tranclocon: immunity protein release allows interaction of the cytotoxic domain with OmpF porin. Biochemistry 45:10199-10207. [DOI] [PubMed] [Google Scholar]