Abstract
Neurobiological mechanisms underlying rewarding and aversive effects of drugs are often studied by examining effects of various pretreatments on acquisition of conditioned place preference (CPP) or conditioned place aversion (CPA). However, few studies have looked at effects of pretreatment with the same drug used during conditioning. Such studies might offer insight into agonist actions on conditioning while also mimicking real world contingencies experienced by drug users. Previous work from our laboratory, which showed that same drug pre-exposure interfered with acquisition of ethanol CPA but not CPP, was limited by the use of only one pre-treatment time interval (65 min). Thus, the present studies were designed to study other intervals (−5, −15, −30). Pretreatment of DBA/2J mice with ethanol (2 g/kg) reduced the activity response normally evoked by the conditioning dose (2 g/kg) at all pretreatment times, but acquisition of CPP was disrupted only by pretreatment at −5 min. The overall pattern of findings suggests that ethanol’s early pharmacological effects interfered with learning the association between the conditioned stimulus (CS) and ethanol 5 min later. Thus, one would expect ethanol agonists, when administered in close proximity to CS-ethanol pairings, to interfere with control of ethanol seeking by that CS.
Keywords: conditioned place preference, ethanol, DBA/2J mice, ethanol agonist, locomotor activity, reward, learning deficit
1. Introduction
Neurobiological mechanisms underlying the rewarding and aversive effects of drugs are often studied by examining effects of various pretreatment drugs on the acquisition of drug-seeking or drug-avoidance behavior. This strategy has gained widespread acceptance in the place conditioning procedure, which may be especially well suited for evaluating effects of pretreatment drugs due to the ability to separate effects on acquisition from effects on expression of place conditioning (e.g., Hoffman, 1989; Swerdlow et al., 1989; Tzschentke, 1998). For example, one can examine the effect of a pretreatment drug (e.g., a receptor agonist or antagonist) given shortly before each pairing of the conditioned stimulus (CS) with a rewarding drug (unconditioned stimulus or US). The effect of this treatment is later evaluated by measuring the animal’s preference for the CS in the absence of any drug treatment. If place preference is weaker in the drug pretreatment group than in a vehicle control group, it is usually assumed that the pretreatment drug interfered either with the US drug’s rewarding effect or with learning of the CS-US association (e.g., Boyce-Rustay & Cunningham, 2004).
One particularly interesting, but little studied, instance of drug pretreatment is the case in which the pretreatment drug is the same as the US drug. The question of interest in such studies is whether the recent and still present effects of the first drug exposure affect conditioning to cues paired with the second drug exposure. Effects of proximal pretreatment with the US drug are of potential interest for several reasons. First, a situation in which environmental cues are paired with multiple exposures to the same drug at short intervals may more closely approximate the natural conditioning history of habitual drug users. For example, alcohol abusers and alcoholics typically ingest their second drink (and third, etc.) while still under the influence of the previous drink. The ability of ethanol to induce conditioning to novel environmental stimuli that are first introduced at the time of later drinks in a sequence is not known. In other words, real world conditioning contingencies are not necessarily well mimicked by the usual laboratory experiment in which the CS is paired with the onset of a single contingent drug exposure and successive pairings occur a day or more apart, after effects of the previous dose have subsided. Given the possibility of acute tolerance or alterations in pharmacokinetics, one might reasonably expect a reduced conditioning effect from a second drug dose that follows closely after a previous dose. Finally, another reason for being interested in effects of same drug pre-exposure is that a drug is its own best agonist. Thus, knowing the effects of same drug pre-exposure might be helpful when interpreting the effects of other pretreatment drugs thought to be agonists at the same site of action as the US drug.
Previous studies in this laboratory were the first to show that same drug pretreatment interferes with acquisition of conditioned place aversion induced by post-CS injection of an ethanol US. However, same drug pre-exposure had absolutely no effect on acquisition of conditioned place preference produced by pre-CS injection of the same ethanol US (Cunningham et al., 2002). In both studies, the pretreatment injection occurred 65 min before the conditioning trial injection. The failure to see a pretreatment effect on acquisition of conditioned preference was surprising since measurement of activity during the conditioning trial indicated a strong residual (activity suppressing) effect of the pretreatment ethanol injection despite the 1 hr delay. The fact that the same proximal pretreatment procedure had different effects on conditioned preference and conditioned aversion produced by the same US argued against simple associative interference interpretations based on degradation of the CS-US contingency (e.g., Rescorla, 1968) or information value of the CS (Rescorla, 1972). Rather, this outcome was viewed as supporting the conclusion that ethanol pre-exposure differentially affected the motivational consequences of the unconditioned responses (URs) that are normally responsible for conditioned preference and conditioned aversion. In other words, the aversive UR was simply more sensitive to ethanol pre-exposure than the rewarding UR (Cunningham et al., 2002).
One limitation on the conclusions from this earlier study is that only one pretreatment time interval (i.e., 65 min) was examined. Thus, Experiment 1 was designed to examine the effects of several shorter pretreatment intervals (5, 15 or 30 min) on the acquisition of ethanol-induced conditioned place preference. The effects of pretreatment within this time range are of particular interest in light of the previous finding that conditioned place preference can be induced when the ethanol US injection itself is given 30 min before exposure to the CS+ (Cunningham et al., 1997). If injection of the US drug 30 min before the CS is able to condition a preference for the CS, one might expect that administration of a same drug pretreatment 30 min (or less) before the CS-US pairing would enhance acquisition of conditioned preference due to temporal summation of ethanol’s rewarding effects. Unexpectedly, however, Experiment 1 showed that ethanol pre-exposure interfered with acquisition of conditioned place preference, but only at the 5-min pretreatment interval. Experiment 2 was designed to replicate this unexpected finding and to compare the ethanol pre-exposure group with control groups that received either a saline pretreatment or no pretreatment.
2. Materials and methods
2.1. Subjects
Adult male mice (DBA/2J) were shipped from the Jackson Laboratory (Bar Harbor, ME) at 6 weeks of age and housed in groups of two to four in polycarbonate cages (n = 246). The colony was maintained at an ambient temperature of 21 ± 1°C on a 12-h light-dark cycle (lights on at 0700 hours), and experiments were carried out during the light portion of the cycle. Food and water were continuously available in the home cage. The Oregon Health & Science University IACUC approved the animal usage protocol and the experiments were conducted in compliance with the NIH Guide For Care and Use of Laboratory Animals.
2.2 Apparatus
Twelve identical acrylic and aluminum boxes (30 x 15 x 15 cm) were enclosed in individual ventilated, light- and sound-attenuating chambers (Coulbourn Instruments Model E10–20). Infrared light sources and photodetectors mounted 2.2 cm above the floor at 5 cm intervals detected general activity and location within the box. Activity and time spent on each side of the box were recorded by micrcomputer (10 ms resolution).
The tactile cues provided by two different interchangeable floor halves served as conditioned stimuli (CSs). One floor consisted of stainless steel rods (2.3-mm diameter) mounted 6.4-mm apart in acrylic rails (“grid” floor). The other floor was made from 16-ga stainless-steel perforated with 6.4-mm round holes on 9.5-mm staggered centers (“hole” floor). This combination of tactile cues was selected on the basis of many previous studies showing that groups of saline-treated control mice show approximately equal preference for each cue, i.e., the apparatus was unbiased (Cunningham et al., 2003a). The inside walls and floors of the box were wiped with a damp sponge and the litter paper beneath the flooring was changed between animals.
2.3. Experimental design
The first experiment varied the time interval between the pre-exposure injection and the conditioning trial. Mice were randomly assigned to groups (n = 22–24/group) that received an ethanol (2 g/kg) pre-exposure injection 30, 15 or 5 min before each ethanol (CS+) conditioning trial. To control for non-specific effects of these additional ethanol exposures (e.g., tolerance/ sensitization), mice assigned to a fourth group (n = 24) received the extra ethanol injection in the home cage 60 min after each CS+ trial. To control for possible effects of handling and injection, per se, each group received an additional saline injection at their assigned pre-exposure time interval on each saline (CS-) conditioning trial.
Because Experiment 1 showed a pre-exposure effect only at the 5 min time interval, the second experiment was designed to replicate that finding and to provide comparisons with two additional control groups: a group that received only saline pre-exposure injections 5 min before each type of trial (Saline Control) and a group that received no pre-exposure injections (Nothing Control). The same experimenter conducted this study in two independent replications, each of which involved 24–28 mice per group. Because initial statistical analysis showed no effects of replication, this factor was ignored in the Results section in order to simplify presentation of the findings.
2.4. Procedure
Both experiments involved three phases: habituation (one session), conditioning (eight sessions), and testing (one session). Sessions were conducted 5 days per week.
2.4.1. Habituation
This session was intended to habituate mice to handling, injection and non-specific features of the apparatus. Mice were weighed and received an intraperitoneal (IP) injection of saline immediately before being placed in the apparatus on a smooth paper floor for 5-min. No pre-exposure injections were given on the habituation day.
2.4.2. Conditioning
Mice within each of the pre-exposure groups were randomly assigned to one of two conditioning subgroups (i.e., an unbiased stimulus assignment procedure) and exposed to a differential Pavlovian conditioning procedure. For mice in the GRID+ conditioning subgroups, ethanol was injected IP just before placement on the grid floor (CS+ trials) and saline was injected just before placement on the hole floor (CS- trials). For mice in the GRID- conditioning subgroups, these floor-drug contingencies were reversed. Ethanol was diluted in 0.9% saline (20% v/v) and administered at a volume of 12.5 ml/kg and a dose of 2 g/kg. This ethanol dose has reliably induced place preference in many previous experiments (e.g., Cunningham et al., 2003a). All groups received their pre-exposure injection at the assigned time and were immediately returned to the home cage. Each animal received four 5-min conditioning trials of each type on alternating days over a period of 8 days. Order of exposure to each type of trial was counterbalanced within each group. For each trial, the floor cues were identical on both sides of the box and mice had unrestricted access to both sides.
2.4.3. Place Preference Test
A 30-min floor preference test was given 24 hrs after the last conditioning trial. All mice received a saline injection (12.5 ml/kg) just before placement in the box with both test floors (half grid/half hole). Floor position (i.e., left vs. right) was counterbalanced within each group. No pre-exposure injections were administered on the test day. The primary dependent variable was the time spent on the grid floor during the 30-min test session (Cunningham et al., 2003a).
2.5. Data Analysis
Activity and preference data from each experiment were subjected to analysis of variance (ANOVA) using Pre-Exposure Group and Conditioning Group (i.e., GRID+ versus GRID−) as between-group factors. Trial Type (CS+ versus CS−) was treated as a within-group factor in the analyses of conditioning trial activity data. To simplify presentation, activity data from the four conditioning trials of each type were averaged to create a single score for CS+ trials and CS− trials within each experiment. Post-hoc comparisons were evaluated using Fisher’s PLSD Test. The alpha level for all analyses was set at .05.
3. Results
3.1. Experiment 1
Data from three mice were removed from all analyses due to procedural errors (two mice from Group −5 and one mouse from Group −30). Final group sizes are indicated in the figure captions.
3.1.1 Conditioning Trial Activity
Pretrial injection of 2 g/kg ethanol at 5, 15 or 30 min before each CS+ trial reduced the activating effect normally produced by the CS+ trial injection of ethanol (Figure 1). Two-way ANOVA (Pre-Exposure Group x Trial Type) yielded significant main effects of Group [F(3,87) = 100.4, p < .0001] and Trial Type [F(1,87) = 367.3, p < .0001], as well as a significant interaction [F(3,87) = 143.6, p < .0001]. Separate follow-up analyses indicated that activity was greater on CS+ trials than on CS- trials in all groups [all F’s > 8.9, p < .01]. However, the Group effect was significant only on CS+ trials [F(3,87) = 157.4, p < .0001]. Post-hoc pair-wise group comparisons (Fisher’s PLSD) showed that that the +60 min group was significantly more active than any of the other three groups (all p’s < .0001), which did not differ.
Figure 1.
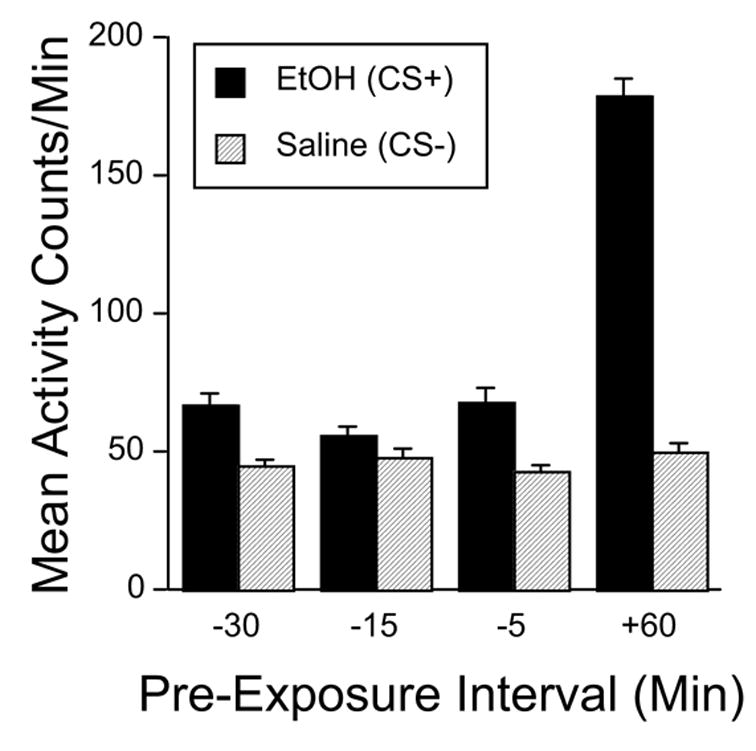
Mean (+ SEM) activity counts per minute in Experiment 1 averaged across all four CS+ (solid bars) and CS− (hatched bars) conditioning trials for each pre-exposure group. All mice received ethanol (2 g/kg) on CS+ trials and saline on CS− trials. Mice were also pretreated with ethanol (2 g/kg) at pre-exposure intervals of −30 min (n = 21), −15 min (n = 24) or −5 min (n = 22) before or +60 min (n = 24) after each CS+ pairing.
To assess whether pretreatment interval affected the pattern of activity change after the second ethanol injection, we also analyzed activity during consecutive minutes on the first and last ethanol conditioning trials. On both trials, all of the ethanol pretreatment groups showed higher activity than Group +60 during the first minute (Table 1). However, activity in ethanol pretreated groups progressively declined over time, while activity in Group +60 (which was receiving its first ethanol injection) increased over time. As a follow-up to a significant three-way interaction in an overall ANOVA [Pre-Exposure Group x Trials x Minutes: F(12,348) = 2.4, p < .005], two-way ANOVAs (Pre-Exposure Group x Minutes) were applied separately to the data from the first and last trials. Both ANOVAs showed significant main effects of Pre-Exposure Group [Fs(3,87) > 56.8, p < .0001] and Minutes [Fs(4,348) > 63.7, p < .0001] as well as the two-way interaction [F(12,348) > 44.3, p < .0001]. Simple effect follow-up analyses at each minute showed a significant Pre-Exposure Group effect at all minutes on both trials [all ps < .0001]. Post-hoc pair-wise group comparisons (Fisher’s PLSD) showed that all three ethanol pretreatment groups differed from Group +60 at all minutes on both trials [all ps < .005]. That is, all of the ethanol pretreated groups showed significantly higher activity than the control group during the first minute after the second injection, but significantly lower activity during all subsequent minutes. There were only two instances in which differences were detected among the ethanol pretreatment groups. During Minute 2 of the first trial, Group −5 was significantly less active than either Group −15 or Group −30 (both ps < .05). During Minute 1 of the last trial, Group −15 was significantly less active than either Group −5 or Group −30 (both ps < .005).
Table 1.
Mean activity counts (± SEM) during each minute of the first and last ethanol (CS+) conditioning trials in Experiment 1.
| Trial | Group | n | Minute 1 | Minute 2 | Minute 3 | Minute 4 | Minute 5 |
|---|---|---|---|---|---|---|---|
| 1 | −30 | 21 | 159.1 ± 12.3 | 73.2 ± 9.6 | 11.8 ± 5.5 | 6.3 ± 5.1 | 7.3 ± 5.5 |
| −15 | 24 | 136.5 ± 9.5 | 63.7 ± 8.7 | 19.3 ± 8.6 | 13.3 ± 8.4 | 14.7 ± 7.1 | |
| −5 | 22 | 146.2 ± 10.7 | 39.7 ± 7.8 | 45.5 ± 19.7 | 24.7 ± 9.1 | 20.9 ± 12.1 | |
| +60 | 24 | 92.1 ± 6.2 | 128.8 ± 7.2 | 192.5 ± 11.2 | 202.9 ± 8.9 | 176.9 ± 7.3 | |
| 4 | −30 | 21 | 197.4 ± 10.7 | 112.7 ± 12.4 | 34.9 ± 11.6 | 22.7 ± 9.3 | 19 ± 8.3 |
| −15 | 24 | 161.1 ± 7.4 | 104 ± 11 | 29.8 ± 9.4 | 26.7 ± 10.2 | 14.2 ± 6.5 | |
| −5 | 22 | 209.6 ± 8.6 | 85 ± 15.8 | 54 ± 16.5 | 29.2 ± 11.6 | 29.7 ± 12.5 | |
| +60 | 24 | 97.5 ± 8.1 | 181.1 ± 13.6 | 243.8 ± 14.2 | 228.5 ± 9.7 | 218.5 ± 9.4 |
3.1.2. Preference Test
As expected, the group that did not receive a pre-trial ethanol injection (Group +60) developed a strong preference for the ethanol-paired floor CS. That is, the GRID+ conditioning subgroup spent more time on the grid floor than the GRID− conditioning subgroup (Figure 2). Groups pre-treated with ethanol at 15 or 30 min also spent more time on the ethanol-paired floor. However, the group pre-treated at 5 min (Group −5) showed a trend toward place aversion. Two-way ANOVA (Pre-Exposure Group x Conditioning Subgroup) revealed a significant main effect of Conditioning Subgroup [F(1,83) = 7.0, p < .01]. The interaction fell short of the significance criterion [F(3,87) = 2.6, p = .06]. Planned comparisons (Fisher’s PLSD) between the conditioning subgroups (GRID+ versus GRID−) within each pre-exposure group indicated that there was significant conditioned place preference in Groups −15 and +60 (both p’s < .03). There was no significant conditioning effect in Groups −5 or −30, although the difference approached significance in Group − 30 (p = .08).
Figure 2.
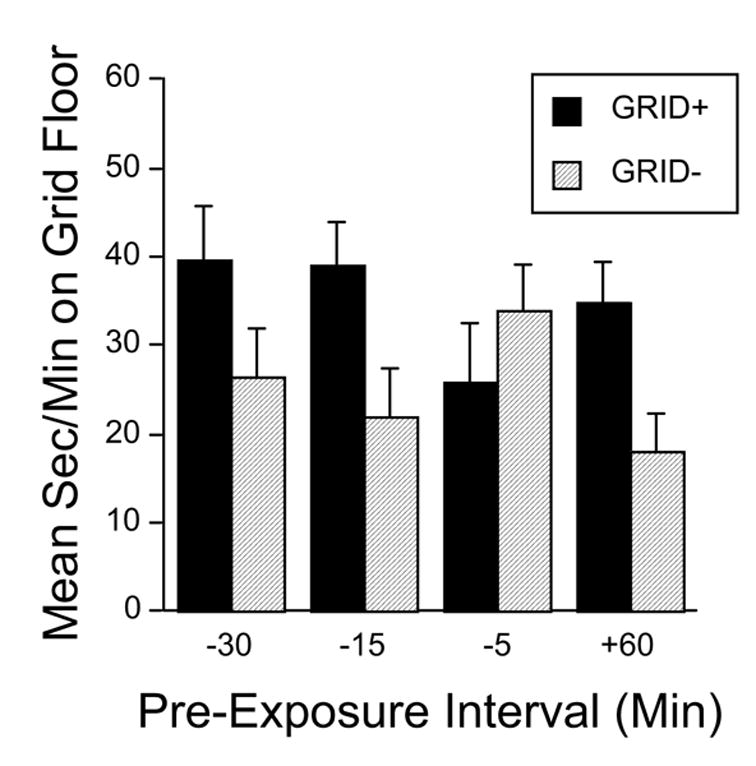
Mean (+SEM) time (sec/min) spent on the grid floor during the 30 min test session in Experiment 1. Solid bars depict the GRID+ conditioning subgroups, while hatched bars depict the GRID− conditioning subgroups (n = 10–12/subgroup). Preference is indexed by comparing the GRID+ and GRID− subgroups within each pretreatment group. Pre-exposure groups had previously received ethanol −30, −15 or −5 min before or +60 min after each CS+ trial. All mice were injected with saline only before the test session.
Test session activity was generally lower in the groups that had previously received pretreatment ethanol injections on CS+ trials compared to the group that had received ethanol 60 min after each CS+ trial. Mean ( ± SEM) activity counts per min in Groups −30, −15, −5 and +60 were 24.9 ± 1.9, 27.9 ± 1.8, 22.6 ± 1.7 and 38.3 ± 2.0, respectively. One way ANOVA yielded a significant main effect of group [F(3,87) = 14.1, p < .0001]. Post-hoc pair-wise comparisons (Fisher’s PLSD) indicated that Group +60 was significantly more active than all of the other groups (all p’s < .0001). In addition, Group −15 was significantly more active than Group −5 (p = .05).
3.2. Experiment 2
Data from three mice were removed from all analyses due to procedural errors (Two mice from the Saline Group and one mouse from the Nothing Group). Final group sizes are indicated in the figure captions.
3.2.1. Conditioning Trial Activity
As in Experiment 1, activity on ethanol (CS+) conditioning trials was quite high in the groups that did not receive ethanol pretreatment, i.e., Groups Nothing and Saline (Figure 3). In contrast, activity in the group pretreated with ethanol 5 min before the trial was substantially lower. Two-way ANOVA (Pre-Exposure Group x Trial Type) showed significant main effects of Group [F(2,146) = 181.0, p < .0001] and Trial Type [F(1,146) = 1163.4, p < .0001], and a significant interaction [F(2,146) = 183.5, p < .0001]. Within-group follow-up analyses indicated that activity was greater on CS+ trials than on CS− trials in all three groups [all F’s > 28.5, p < .0001]. One-way ANOVAs applied separately to the data from each trial type yielded a significant group effect in both cases [both F’s > 6.0, p < .01]. Post-hoc pair-wise comparisons (Fisher’s PLSD) showed that the ethanol pre-exposure group was significantly less active than either of the other groups (p’s < .01), which did not differ.
Figure 3.
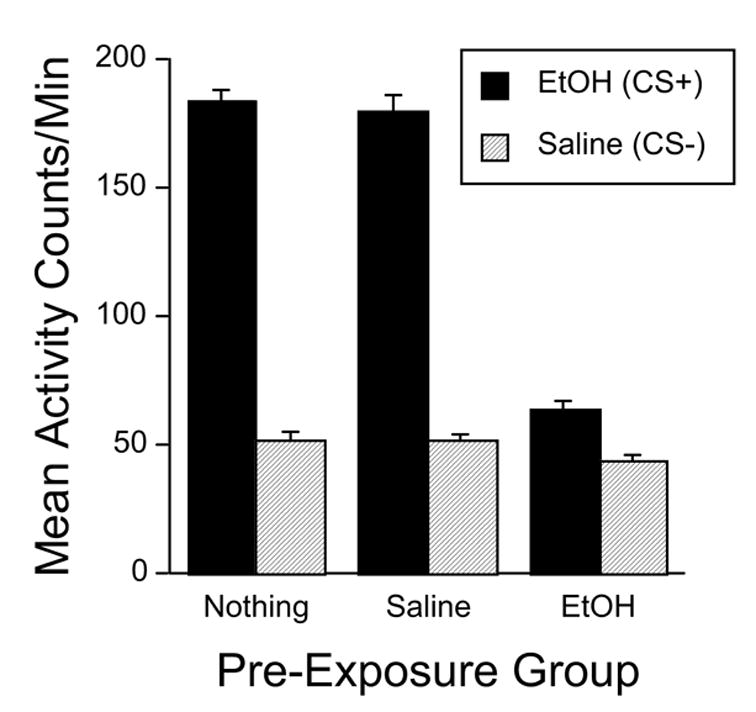
Mean (+ SEM) activity counts per minute in Experiment 2 averaged across all four CS+ (solid bars) and CS− (hatched bars) conditioning trials for each pre-exposure group. All mice received ethanol (2 g/kg) on CS+ trials and saline on CS− trials. Additionally, subjects were divided into three pre-exposure groups. The Saline (n = 50) and EtOH (n = 52) groups received an injection of saline or ethanol, respectively, 5 min before each CS+ trial. The Nothing group (n = 47) did not receive a pretreatment.
3.2.2. Preference Test
Groups that had received no pretreatment (Group Nothing) or a saline injection 5 min before each CS+ trial (Group Saline) showed a robust conditioned place preference as indicated by greater time spent on the grid floor by GRID+ subgroups than by GRID− subgroups (Figure 4). However, pretreatment with ethanol 5 min before each CS+ trial (Group EtOH) reduced the magnitude of conditioned preference. These observations were supported by a two-way ANOVA (Pre-Exposure Group x Conditioning Subgroup), which revealed a significant main effect of Conditioning Subgroup [F(1,143) = 43.1, p < .0001] and a significant interaction [F(2,143) = 3.4, p < .05]. To evaluate the source of the interaction, each pair of pretreatment groups was compared in a separate two-way ANOVA (i.e., Ethanol vs. Saline, Ethanol vs. Nothing, Saline vs. Nothing). These ANOVAs showed significant interactions for the comparisons of the ethanol group with each of the control pretreatment groups (both p’s < .05). However, comparison of the two control groups (i.e., Saline vs. Nothing) showed only a main effect of Conditioning Subgroup [F(1,93) = 62.7, p < .0001], confirming that both control groups showed a robust and equivalent conditioned preference. In contrast, post-hoc analysis showed no difference between the GRID+ and GRID− subgroups in the ethanol group (p > .15), indicating that ethanol pretreatment 5 min before each CS+ trial impaired development of conditioned place preference.
Figure 4.
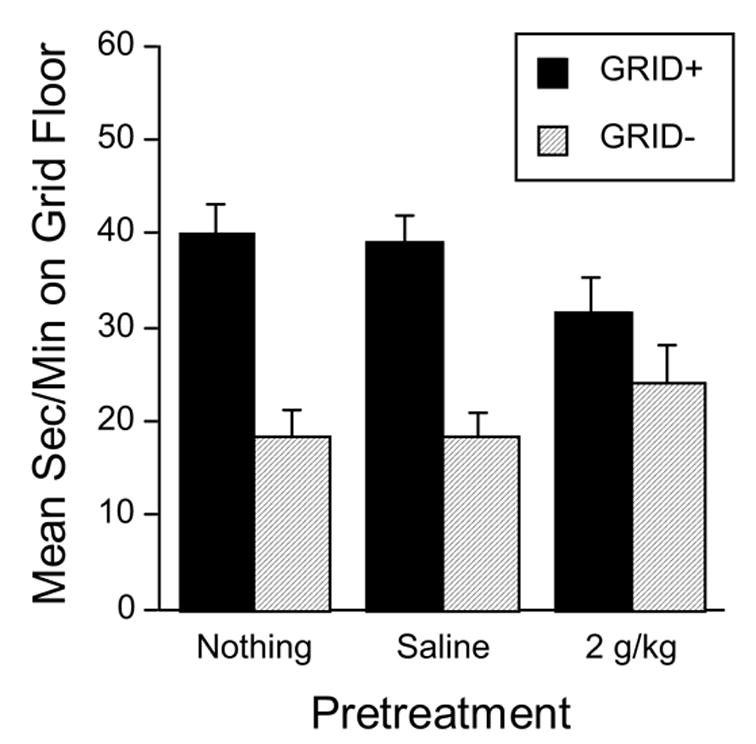
Mean (+SEM) time (sec/min) spent on the grid floor during the 30 min test session in Experiment 2. Solid bars depict the GRID+ conditioning subgroups, while hatched bars depict the GRID− conditioning subgroups (n = 23–26/subgroup). Preference is indexed by comparing the GRID+ and GRID− subgroups within each pretreatment group. The pre-exposure groups had previously received ethanol (EtOH) or saline (Saline) 5 min before each CS+ trial, or had received no pretreatment (Nothing). All mice were injected with saline only before the test session.
As in Experiment 1, test session activity was lowest in the group that had received ethanol pretreatment during the conditioning phase. Mean ( ± SEM) activity counts per min in Groups Nothing, Saline and Ethanol were 37.9.3 ± 1.1, 36.0 ± 1.0, and 26.7 ± 1.3, respectively. One-way ANOVA confirmed that the difference among groups was significant [F(2,146) = 25.8, p < .0001]. Post-hoc pair-wise comparisons (Fisher’s PLSD) indicated that the Ethanol group was significantly less active than either of the other groups (both p’s < .0001), which did not differ.
4. Discussion
These experiments are the first to show that pretreatment with ethanol 5 min before each CS+ conditioning trial interferes with development of a conditioned place preference induced by ethanol given immediately before CS+ exposure. Experiment 1 suggested that this interference effect was unique to the 5-min pre-exposure interval since pre-exposure at 15 min did not interfere with place preference when compared to a group that was matched for overall ethanol exposure, but received the extra ethanol injection 60 min after each trial. Although the place preference observed after pretreatment with ethanol 30 min before the trial was statistically marginal, the general conclusion of no interference at pretreatment intervals longer than 5 min is certainly consistent with the previous report of no interference with ethanol pretreatment at 65 min (Cunningham et al., 2002). The finding that pre-exposure to a saline injection 5 min before each CS+ trial had no effect on place preference (Experiment 2) argues against an interpretation of the interference effect that simply appeals to stress induced by handling and injection. Rather, the overall pattern of findings suggests that the interference was caused by the overlap of ethanol’s early pharmacological effects (i.e., effects that occur 5–10 min after injection) with the ethanol-CS+ pairing.
Because all groups in Experiment 1 were matched for overall exposure to ethanol, the interference effect observed in Group −5 is not readily attributed to differences in chronic ethanol tolerance. However, one might argue that two closely spaced ethanol injections (i.e., 5 min apart) induce greater chronic tolerance than the same two injections given at longer intervals (e.g., 15–60 min). In other words, due to temporal summation, giving two injections 5 min apart might be functionally equivalent to giving a larger single dose, which would be expected to induce greater chronic tolerance. Although such summation is certainly possible, previous studies involving chronic pre-exposure to a larger ethanol dose (4 g/kg) have failed to show any evidence of tolerance to ethanol’s rewarding effect in the place conditioning procedure (Cunningham et al., 2002). Thus, it seems unlikely that the present findings were due to greater chronic tolerance in the 5-min ethanol pre-exposure groups.
Athough an explanation based on chronic tolerance is not compelling, one might derive an account of the time delay effect based on the phenomenon of acute functional tolerance, which refers to neuroadaptations that occur over time during a single drug experience (e.g., Radcliffe et al., 2006). For example, one might argue that groups given ethanol pretreatment at longer delays will develop greater acute tolerance to ethanol-induced sensory-motor disturbances induced by a second ethanol dose that is given before the previous dose has been eliminated. Assuming that such disturbances would interfere with development of an association between ethanol’s rewarding effect and novel cues that signal the second dose, the acute tolerance analysis predicts that interference should be greatest with a short delay. The minute-by-minute analysis of activity on the first ethanol conditioning trial provides some support for this interpretation, showing a less rapid suppression of activity in Groups −15 and −30 (during Minute 2 after the second injection) compared to Group −5. If acute tolerance to ethanol’s activity suppressing effects is correlated with acute tolerance to sensory disturbances that interfere with learning, one might expect less interference with place conditioning in Groups −15 and −30 groups than in Group −5.
The present studies do not support the predicted summation of ethanol’s rewarding effects based on the temporal proximity of the pre-exposure injection to the US injection. This prediction was based on the previous observation that injection of an ethanol US 30 min before CS exposure produces a conditioned place preference (Cunningham et al., 1997). Given that outcome, one might have expected the combination of a pretreatment injection 30 min (or less) before the US injection to produce an enhanced conditioned place preference. The failure to see temporal summation in the present situation is at odds with the previous observation of summation with two closely spaced ethanol injections when the first injection occurred just before CS exposure and the second injection was given 5 min later (Cunningham et al., 2003b). In that case, magnitude of place preference was enhanced, as would be expected when the CS is paired with a larger ethanol dose (Cunningham et al., 1992; Risinger & Oakes, 1996). Thus, the present findings suggest that temporal summation of rewarding effects does not benefit a CS that is uniquely paired with the second in a series of closely spaced ethanol exposures. Moreover, when considered together with previous findings, the present studies suggest that environmental cues associated with the onset of a lengthy drug experience may be more strongly associated with the drug’s rewarding effects than cues that signal additional drug exposure later within the same drug experience.
In contrast to the place preference data, activity data recorded during the conditioning trials provide clear evidence of temporal summation. That is, activation produced by the ethanol US injection was substantially reduced in mice that received ethanol pretreatment injections, as would be expected in DBA/2J mice that received a functionally higher dose of ethanol (Cunningham et al., 1992; Dudek & Phillips, 1990). At the same time, the general similarity of the activity effect across all three pretreatment times suggests that interference with place preference in the 5-min group was unrelated to processes involved in temporal summation of ethanol’s activating effects.
In both experiments, test session activity was lowest in the group that had received ethanol pretreatment 5 min before each CS+ trial, raising the possibility that differences in expression of conditioned place preference were secondary to effects on general test activity. However, this interpretation is not consistent with previous studies showing an inverse relationship between test session activity and magnitude of conditioned place preference (e.g., Gremel & Cunningham, submitted; Cunningham, 1995; Vezina & Stewart, 1987). That is, based on previous findings, one would expect that a reduced level of test session activity would be associated with stronger expression of conditioned preference, not weaker. Thus, it seems unlikely that the interference effect observed here was due to reduced test activity in the 5-min groups. One potentially interesting interpretation of that reduced activity is that it reflects a conditioned suppression of activity controlled by general contextual cues of the apparatus. In other words, based on the temporal contiguity of contextual cues with two closely spaced ethanol injections, those cues might have acquired the ability to elicit a conditioned suppression of activity. If true, this interpretation suggests that mice in the 5-min group were able to form an association that influenced general activity, but were unable to form the association needed to express a preference for the ethanol-associated floor.
Previously, we suggested two possible explanations for interference produced by same drug pre-exposure in the place conditioning procedure (Cunningham et al., 2002). One explanation was based on the assumption that drug pretreatment somehow interfered directly with the drug’s motivational effect. For example, we suggested that ethanol pre-exposure reduced conditioned place aversion produced by post-CS ethanol injection because it reduced the aversiveness of the rapid transition from the sober to the intoxicated state. However, an explanation of the present results based on direct interference with ethanol’s rewarding effect seems unlikely given the previous finding of reward summation across two injections given 5 min apart (Cunningham et al., 2003b). Rather, the present findings seem more reasonably explained by assuming that ethanol pretreatment directly interfered with the formation of an association between the CS and ethanol US 5 min later.
The exact mechanism(s) underlying this hypothesized associative interference are unknown. As noted earlier, acute tolerance to sensory-motor disturbances that interfere with learning is one possibility. However, the Pavlovian conditioning literature on proximal US pre-exposure offers several other possibilities (Domjan, 1980; Domjan & Best, 1977). One possible explanation, derived from Rescorla’s contingency analysis of Pavlovian conditioning (Rescorla, 1967, 1968), is that ethanol pretreatment 5 min before the conditioning trial degraded the excitatory CS-US contingency because ethanol pretreatment increased the probability of the US in the absence of the CS. However, this interpretation is complicated by the fact that all groups in Experiment 1 received an extra ethanol injection in the absence of the US (i.e., before or after the CS-US pairing) on each trial. Thus, the excitatory contingency was equally degraded in all groups, which leads to the (incorrect) prediction of no differences among groups. Because contingency is based only on the relative probabilities of the US in the presence and absence of the CS, this analysis cannot explain the present findings unless one modifies the definition of contingency by restricting the time window in which relative probabilities are calculated. For example, one might assume that an extra US will degrade the excitatory contingency only if it occurs within a narrow time period before CS exposure (e.g., Cannon et al., 1975).
The conditioning literature offers several other theoretical explanations that consider the time interval between the proximal US and a conditioning trial US. For example, according to opponent-process theory (Solomon, 1977), proximal ethanol exposure should initiate a time-dependent primary affective process (presumably rewarding) that evokes a delayed opponent affective process (presumably aversive) that counteracts the primary process and persists after the primary process has dissipated. Opponent-process theory can explain the time-delay effects seen in Experiment 1 only by assuming that the strength of the residual opponent process evoked by ethanol exposure at −5 min exceeded the strength of the opponent process remaining from ethanol exposure at −15 or −30 min. In that case, the net affective response to the conditioning trial US would be smallest in Group −5, consistent with the reduced conditioning seen in that group. However, this analysis is not supported by previous data suggesting that the primary affective (rewarding) process induced by a 2 g/kg ethanol injection persists for at least 30 min after injection as indicated by its ability to induce preference for a delayed CS (Cunningham et al., 1997).
Consistent with the outcome of Experiment 1, Wagner’s SOP theory (Wagner, 1975, 1981) predicts that interference produced by a proximal US will be inversely related to the time delay between US exposures. According to SOP theory, ethanol pretreatment at the short time delay would reduce the effectiveness of the ethanol US through a memory “priming” mechanism (i.e., processing of the conditioning trial US would be reduced by the recent and still ongoing processing of the proximal US). Another possible interpretation is that strong sensory experiences produced by the proximal US injection might have reduced learning about the CS due to attentional or associative competition (Best & Domjan, 1979). For example, internal stimuli produced by the pretreatment ethanol injection may have overshadowed the target CS, preventing its association with the ethanol UR. Alternatively, pretreatment ethanol may have directly prevented memory formation due to its hypothesized amnesic properties (Ryabinin, 1998). Although all of these theories offer a general explanation of the findings observed here, none of them specifies the precise form of that temporal gradient, which presumably depends on the nature, duration and intensity of the US. Regardless of the interpretation, the unique contribution of these experiments lies in showing that, in the case of ethanol conditioned place preference, the interference mechanism was optimally engaged at the 5-min pretreatment interval, but was not a factor at other pretreatment times. Additional research is needed to address these mechanisms.
The overall pattern of findings reported here is generally similar to that previously reported in studies examining effects of proximal US exposure on development of taste aversion conditioned by injection of lithium chloride in rats. For example, Domjan and Best (1977) found that interference was inversely related to the time interval between the proximal US and conditioning trial, showing a substantial reduction in conditioned taste aversion by drug pretreatment at −30 min, weaker interference at −360 min, but no interference at −24 hrs. Although the specific time course of the proximal US exposure effect on lithium-chloride induced conditioned taste aversion in rats differs from that of the proximal US exposure effect on ethanol-induced conditioned place preference in mice, the general similarity of these phenomena suggests generality of the proximal US exposure effect across species, drug and response system. Moreover, this similarity extends to the paradoxical finding that proximal US exposure produces interference during a pre-trial time interval in which a backward US-CS pairing produces conditioned taste aversion (Domjan & Best, 1977) or conditioned place preference (Cunningham et al., 1997).
Although the mechanisms underlying the interference effect are unknown, the present findings also have potentially important implications for interpreting effects of putative ethanol agonists on acquisition of ethanol-induced conditioned place preference. More specifically, these studies suggest that an ethanol agonist should interfere with acquisition, but only if administered within a relatively narrow time window just before each CS-ethanol pairing. Moreover, these studies suggest that the agonist’s effect on ethanol-induced activation during conditioning might not be predictive of its impact on conditioned place preference. Thus, in cases where a putative agonist is found to have no effect at one pretreatment interval, it would be prudent to consider other pretreatment intervals, especially relatively short ones.
Acknowledgments
This research was supported by NIH-NIAAA grant AA07702. Thanks are extended to Rachel Smith for assistance in data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Best MR, Domjan M. Characteristics of the lithium-mediated proximal US-preexposure effect in flavor-aversion conditioning. Animal Learning and Behavior. 1979;7:433–440. doi: 10.3758/bf03209697. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Cunningham CL. The role of NMDA receptor binding sites in ethanol place conditioning. Behav Neurosci. 2004;118:822–834. doi: 10.1037/0735-7044.118.4.822. [DOI] [PubMed] [Google Scholar]
- Cannon DS, Berman RF, Baker TB, Atkinson CA. Effect of preconditioning unconditioned stimulus experience on learned taste aversions. Journal of Experimental Psychology: Animal Behavior Processes. 1975;104:270–284. doi: 10.1037//0097-7403.1.3.270. [DOI] [PubMed] [Google Scholar]
- Cunningham CL. Localization of genes influencing ethanol-induced conditioned place preference and locomotor activity in BXD recombinant inbred mice. Psychopharmacology. 1995;120:28–41. doi: 10.1007/BF02246142. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Ferree NK, Howard MA. Apparatus bias and place conditioning with ethanol in mice. Psychopharmacology. 2003a;170:409–422. doi: 10.1007/s00213-003-1559-y. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Niehus DR, Malott DH, Prather LK. Genetic differences in the rewarding and activating effects of morphine and ethanol. Psychopharmacology. 1992;107:385–393. doi: 10.1007/BF02245166. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Okorn DM, Howard CE. Interstimulus interval determines whether ethanol produces conditioned place preference or aversion in mice. Animal Learning & Behavior. 1997;25:31–42. [Google Scholar]
- Cunningham CL, Smith R, McMullin C. Competition between ethanol-induced reward and aversion in place conditioning. Learn Behav. 2003b;31:273–280. doi: 10.3758/bf03195988. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Tull LE, Rindal KE, Meyer PJ. Distal and proximal pre-exposure to ethanol in the place conditioning task: tolerance to aversive effect, sensitization to activating effect, but no change in rewarding effect. Psychopharmacology. 2002;160:414–424. doi: 10.1007/s00213-001-0990-1. [DOI] [PubMed] [Google Scholar]
- Domjan M. Ingestional aversion learning: Unique and general processes. In: Rosenblatt JS, Hinde RA, Beer C, Busnel MC, editors. Advances in the Study of Behavior. Vol. 11. New York: Academic Press; 1980. pp. 275–336. [Google Scholar]
- Domjan M, Best MR. Paradoxical effects of proximal unconditioned stimulus preexposure: Interference with and conditioning of a taste aversion. Journal of Experimental Psychology: Animal Behavior Processes. 1977;3:310–321. doi: 10.1037//0097-7403.3.4.310. [DOI] [PubMed] [Google Scholar]
- Dudek BC, Phillips TJ. Distinctions among sedative, disinhibitory, and ataxic propeties of ethanol in inbred and selectively bred mice. Psychopharmacology. 1990;101:93–99. doi: 10.1007/BF02253724. [DOI] [PubMed] [Google Scholar]
- Gremel CM, Cunningham CL. Role of test activity in ethanol-induced disruption of place preference expression in mice. Submitted. [DOI] [PubMed] [Google Scholar]
- Hoffman DC. The use of place conditioning in studying the neuropharmacology of drug reinforcement. Brain Research Bulletin. 1989;23:373–387. doi: 10.1016/0361-9230(89)90224-4. [DOI] [PubMed] [Google Scholar]
- Radcliffe RA, Floyd KL, Lee MJ. Rapid ethanol tolerance mediated by adaptations in acute tolerance in inbred mouse strains. Pharmacol Biochem Behav. 2006;84:524–534. doi: 10.1016/j.pbb.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Pavlovian conditioning and its proper control procedures. Psychological Review. 1967;74:71–80. doi: 10.1037/h0024109. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Probability of shock in the presence and absence of CS in fear conditioning. J Comp Physiol Psychol. 1968;66:1–5. doi: 10.1037/h0025984. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Informational variables in Pavlovian conditioning. In: Bower GH, editor. The Psychology of Learning and Motivation. Vol. 6. Academic Press; 1972. pp. 1–46. [Google Scholar]
- Risinger FO, Oakes RA. Dose- and conditioning trial-dependent ethanol-induced conditioned place preference in Swiss-Webster mice. Pharmacol Biochem Behav. 1996;55:117–123. doi: 10.1016/0091-3057(96)00069-x. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE. Role of hippocampus in alcohol-induced memory impairment: implications from behavioral and immediate early gene studies. Psychopharmacology. 1998;139:34–43. doi: 10.1007/s002130050687. [DOI] [PubMed] [Google Scholar]
- Solomon RL. An opponent-process theory of acquired motivation: The affective dynamics of addiction. In: Seligman MEP, Maser JD, editors. Psychopathology: Experimental Models. San Francisco: W. H. Freeman and Company; 1977. pp. 66–103. [Google Scholar]
- Swerdlow NR, Gilbert D, Koob GF. Conditioned drug effects on spatial preference: Critical evaluation. In: Boulton AA, Baker GB, Greenshaw AJ, editors. Psychopharmacology (Neuromethods Vol 13) Clifton, NJ: Humana Press; 1989. pp. 399–446. [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- Vezina P, Stewart J. Morphine conditioned place preference and locomotion: the effect of confinement during training. Psychopharmacology. 1987;93:257–260. doi: 10.1007/BF00179944. [DOI] [PubMed] [Google Scholar]
- Wagner AR. Priming in STM: An information processing mechanism for self-generated or retrieval-generated depression in performance. In: Tighe TJ, Leaton RN, editors. Habituation: Perspectives from Child Development, Animal Behavior, and Neurophysiology. Hillsdale, NJ: Lawrence Erlbaum Associates; 1975. pp. 1–62. [Google Scholar]
- Wagner AR. SOP: A model of automatic memory processing in animal behavior. In: Spear NE, Miller RR, editors. Information processing in animals: Memory mechanisms. Hillsdale: NJ: Lawrence Erlbaum Associates; 1981. pp. 5–47. [Google Scholar]


