Abstract
Mouse hepatitis virus (MHV) does not induce interferon (IFN) production in fibroblasts or bone marrow-derived dendritic cells. In this report, we show that the essential IFN-β transcription factors NF-κB and IFN regulatory factor 3 are not activated for nuclear translocation and gene induction during infection. However, MHV was unable to inhibit the activation of these factors and subsequent IFN-β production induced by poly(I:C). Further, MHV infection did not inhibit IFN-β production mediated by known host pattern recognition receptors (PRRs) (RIG-I, Mda-5, and TLR3). These results are consistent with the notion that double-stranded RNA, produced during MHV infection, is not accessible to cellular PRRs.
Coronaviruses, including mouse hepatitis virus (MHV), are large, enveloped, positive-strand RNA viruses which induce a wide variety of diseases in both humans and animals. Several strains of MHV induce acute encephalitis and acute and chronic demyelinating diseases in mice. MHV, like other viruses, must evade the innate immune response in order to replicate in a normal animal. We and others have shown previously, by using a vesicular stomatitis virus (VSV)-based bioassay, that MHV does not induce interferon (IFN) in infected fibroblasts and bone marrow (BM)-derived dendritic cells (DCs) (15, 41, 62). Similarly, the coronavirus (CoV) associated with severe acute respiratory syndrome also does not induce type I IFN (IFN-α/β) (8, 26, 63). Double-stranded RNA (dsRNA), generated during RNA virus replication, is a potent inducer of IFN-β. IFN-β, in turn, binds to the IFN-α/β receptor, resulting in activation of additional members of the type I IFN family and of other proteins involved in creating an antiviral state (reviewed in reference 40). Consistent with the important role of type I IFN in inhibiting virus replication, mice genetically deficient in the expression of the IFN-α/β receptor are highly susceptible to most viral infections, including MHV (7, 33).
The initial induction of IFN-β transcription involves activation of three transcription factors, IFN regulatory factor 3 (IRF-3), NF-κB, and activated transcription factor 2-c-Jun (ATF2-c-Jun) (28). IRF-3 is constitutively localized to the cytoplasm of noninfected cells. Upon stimulation, C-terminal serine residues of IRF-3 are phosphorylated, resulting in dimerization of IRF-3 and its translocation into the nucleus (13, 23). IRF-3 recruits p300/CREB binding protein (CBP) and, in conjunction with activated NF-κB and ATF2-c-Jun, initiates IFN-β transcription (58). Viral dsRNA is detected by multiple pattern recognition receptors (PRRs), including Toll-like receptor 3 (TLR3) and cytoplasmic RNA helicases, such as retinoic acid-inducible gene (RIG-I) and melanoma differentiation-associated gene 5 (Mda5) (19, 61; reviewed in references 21 and 40). Although initial studies suggested that TLR3 was responsible for the majority of IFN induction in response to dsRNA (1, 54), more-recent studies suggest that RIG-I and Mda5 are more important in virus-infected cells (11, 61). TLR3 is detected primarily in the endosomal compartment (29). Ligand binding to TLR3 results in the activation of IRF-3, NF-κB, and ATF2-c-Jun via the adaptor molecule TRIF (57). Alternatively, RIG-I or Mda5 binding to dsRNA recruits IPS-1 via CARD domain interactions, which results in the phosphorylation and translocation of IRF-3 (22, 31, 48). A recent report suggests that RIG-I and Mda5 recognize different types of dsRNA, i.e., RIG-I detects in vitro-transcribed dsRNA, and Mda5 recognizes poly(I:C). Further, they are critical for the response to different viral infections. For example, RIG-I is necessary in the response to paramyxoviruses, influenza virus, and Japanese encephalitis virus, whereas Mda5 is involved in picornavirus detection (20).
Due to the importance of type I IFN in the antiviral immune response, many viruses encode proteins that block interferon production through specific inhibition of factors involved in IFN-β induction, including IRF-3 (reviewed in references 14, 17, 40, and 44). Most studies have focused on the inhibition of the IFN response elicited by negative-strand RNA viruses (17). The V protein of paramyxoviruses, such as simian virus 5 and Sendai virus (SenV), targets Mda5 and subsequently inhibits IRF-3 and NF-κB activation (2). NS1 and NS2 proteins of respiratory syncytial virus, a pneumovirus, inhibit IRF-3 phosphorylation without affecting NF-κB activation (52). Ebola virus encodes a protein, VP35, which prevents IRF-3 activation mediated by either the TLR3 or the RIG-I pathway (6).
Some information about activation of these factors in MHV-infected cells is available. Banerjee et al. showed that MHV activated two mitogen-activated protein kinases (MAPKs), p38 MAPK and c-Jun N-terminal protein kinase (5). Since ATF2 is a target for phosphorylation by MAPK, it is likely that ATF2 is activated in infected cells. A recent report also suggests that levels of NF-κB mRNA are decreased and those of the inhibitor IκB are increased in infected cells (56), but it is not known whether NF-κB is activated after MHV infection. Finally, nothing is known about IRF-3 activation during MHV infection.
As a positive-strand RNA virus, MHV is known to produce dsRNA replication intermediates. Thus, the lack of IFN-β induction in MHV-infected cells indicates that the virus either must actively inhibit some step of the induction process or, alternatively, is invisible to cellular PRRs. Here, by using two different strains of MHV, we found that MHV infection does not result in activation of NF-κB or IRF-3. Furthermore, we investigated whether MHV inhibited IFN-β production induced by poly(I:C) or by overexpression of RIG-I, Mda-5, or TLR3.
MATERIALS AND METHODS
Cell cultures and viruses.
17Cl-1 cells were maintained in Dulbecco's modified Eagle's medium supplemented with 5% fetal calf serum and 5% tryptose phosphate broth. Recombinant and nonrecombinant MHV-JHM.IA (34), called MHV-JHM herein, and MHV-A59 were grown in 17Cl-1 cells, and titers were determined on HeLa cells expressing the MHV receptor CEACAM1a (16) (HeLa-MHVR cells). In all experiments in which virus titers were measured, cells and supernatants were combined prior to determination of titers. rJHM.GFP (where r denotes the recombinant virus and GFP is green fluorescent protein) was constructed as previously described (62). rA59.GFP was kindly provided by K. MacNamara and S. Weiss (University of Pennsylvania, Philadelphia). Cells were infected with MHV-JHM at a multiplicity of infection (MOI) of 10 or with MHV-A59 at an MOI of 100, unless stated otherwise in the manuscript. A recombinant virus in which gene 4 was disrupted (rJHM.gene4KO) was engineered as previously described (35). A naturally occurring gene 2a deletion mutant of MHV-JHM, Wb-1 (47), was obtained from S. Siddell (Bristol University, Bristol, United Kingdom) and J. Leibowitz (Texas A & M University, College Station). In some experiments, cells were infected with 100 hemagglutination activity units of SenV (Cantell strain; Charles River Laboratories, Wilmington, MA).
Plasmids.
pLuc-IFN-β, empty vector pEF-BOS, pEF-Flag-RIG-I, and pEF-Flag-Mda5 were kindly provided by T. Fujita (Tokyo Metropolitan Institute of Medical Science, Tokyo, Japan) (57, 58). pLuc-(PRDII)2 and pLuc-(PRDIII-I)3 (13) were kindly provided by T. Maniatis (Harvard University, Cambridge, MA). pEF-Flag-TRIF and pEF-Flag-TLR3 were kindly provided by K. Fitzgerald (University of Massachusetts Medical School, Worcester) (13). pcDNA-CEACAM1a was kindly provided by T. Gallagher (Loyola University Medical Center, Maywood, IL) (16).
IFN bioassay.
L929 or 17Cl-1 cells were infected with MHV, and levels of IFN were measured using a bioassay based on inhibition of VSV growth in L929 cells. Supernatants were harvested and exposed to UV light to inactivate infectious virus. L929 cells infected with 1,000 PFU VSV were treated with dilutions of supernatants or recombinant murine IFN-β (PBL Biomedical Laboratories, Piscataway, NJ) at 30 min postinfection (p.i.). Titers of VSV were determined on Vero cells. IFN levels were calculated based on the standard curves generated with recombinant IFN-β. To verify production of IFN by L929 and 17Cl-1 cells, samples were transfected with 25 μg poly(I:C) (Amersham Biosciences, Piscataway, NJ) or infected with 100 hemagglutination activity units of SenV and supernatants assayed.
Transfection and luciferase assay.
Cells were transfected with vector DNA (0.25 μg) or poly(I:C) (25 μg) by use of Lipofectamine 2000 (Invitrogen, Carlsbad, CA). At the completion of the experiments, cells were disrupted by freeze-thawing and luciferase activity was measured using a dual-luciferase reporter assay system (Promega, Madison, WI) and a luminometer. As an internal control for the dual-luciferase assay, the Renilla luciferase construct pRL-TK (Promega) was used. In all experiments analyzing luciferase activity, data represent relative firefly luciferase activity normalized to Renilla luciferase activity. Error bars indicate standard errors of triplicate transfections in individual experiments.
Immunofluorescence analysis.
Cells were cultured on Labtek chamber slides, fixed with 4% paraformaldehyde, and permeabilized with 0.1% Triton X-100 in phosphate-buffered saline. Cells were reacted with antinucleocapsid (anti-N) monoclonal antibody (5B188.2; kindly provided by M. Buchmeier, The Scripps Research Institute, La Jolla, CA) and polyclonal anti-IRF-3 antibody or anti-NF-κB p65 antibody (Santa Cruz Biotechnology, Santa Cruz, CA), followed by fluorescein isothiocyanate-conjugated donkey anti-mouse antibody and Cy3-conjugated donkey anti-goat or donkey anti-rabbit antibody (Jackson Immunoresearch, West Grove, PA). Cells were examined using a Zeiss confocal microscope.
RESULTS
MHV does not induce IFN-β production.
Previously, we showed that IFN was not induced when either murine BM-derived DCs or L929 fibroblasts were infected with MHV-JHM (41, 62). In those experiments, both neurovirulent and attenuated strains of MHV-JHM were studied, with identical results. The closely related strain MHV-A59 grows to higher titers than MHV-JHM. Many of the experiments described herein required that most cells be infected with virus, making MHV-A59 most useful in our assays. Additionally, MHV-A59 causes the development of smaller syncytia than MHV-JHM, resulting in less cell loss at later times after infection. To show that MHV-A59 also does not induce IFN, we infected L929 cells at an MOI of 100 and measured IFN production by using a VSV-based bioassay as described in Materials and Methods. Whereas transfection with poly(I:C) resulted in IFN production (84.3 ± 10.4 U/ml, n = 3), less than 1.0 U IFN/ml was detected in the supernatants of MHV-A59-infected cells. Additionally, SenV induced significant amounts of IFN (1,236.7 ± 94.7 U/ml, n = 3) in infected L929 cells. Since 17Cl-1 cells are more susceptible to MHV than are L929 cells, we used them for most of our assays. We found that MHV-JHM and MHV-A59 did not induce detectable levels of IFN production in infected 17Cl-1 cells at 12 h p.i.; poly(I:C) transfection or SenV infection induced significant amounts of IFN (Fig. 1).
FIG. 1.

IFN is not induced in MHV-infected cells, as measured by bioassay. 17Cl-1 cells were infected with MHV-A59 or MHV-JHM for 12 h. As controls, cells were transfected with poly(I:C) or infected with SenV for the same period of time. The amount of IFN was measured by a VSV-based bioassay as described in Materials and Methods. Data are representative of two independent experiments. ND, not detectable.
To further confirm that MHV does not induce IFN-β, 17Cl-1 cells were transiently transfected with a luciferase reporter construct containing the natural IFN-β enhancer promoter (pLuc-IFN-β) (13). Cells were then infected with MHV-JHM or MHV-A59 for 12 or 24 h. Consistent with the bioassay data, equivalent levels of IFN-β were detected in mock- and virus-infected cells as measured by luciferase activity (Fig. 2A and B). As positive controls, cells were either transfected with poly(I:C) or infected with SenV, which resulted in a 10- to 20-fold or 100-fold increase, respectively, in luciferase activity compared to that for mock-treated samples. Further, cells infected with lower MOIs of MHV did not produce IFN-β (Fig. 2C). We also confirmed these results with 293T cells, which are highly susceptible to transfection. Because 293T cells are resistant to MHV infection, we cotransfected these cells with a plasmid encoding the MHV receptor molecule CEACAM1a (16) and pLuc-IFN-β and then infected them with recombinant MHV that expressed GFP (rJHM.GFP or rA59.GFP). Fifty to seventy percent of cells were infected and expressed GFP at 16 h p.i., but levels of luciferase activity were the same as those for the mock-infected cell cultures. Infection with SenV or transfection with poly(I:C) resulted in increased luciferase activity (Fig. 2D).
FIG. 2.
MHV does not induce IFN-β production, as measured by luciferase assay. 17Cl-1 fibroblasts were transfected with pLuc-IFN-β for 16 to 18 h and then infected with MHV-A59 at an MOI of 100 or MHV-JHM at an MOI of 10 for 12 h (A) or 24 h (B). 17Cl-1 cells were also tested after MHV infection at an MOI of 0.1 or 1 (C). 293T cells were transfected with pcDNA-CEACAM1a and pLuc-IFN-β and then infected with MHV-A59 or MHV-JHM for 12 h (D). As positive controls, cells were transfected with poly(I:C) or infected with SenV for the same period of time. As an internal control, a Renilla luciferase construct was transfected concomitantly with pLuc-IFN-β. Cells were harvested and subjected to a dual-luciferase assay as described in Materials and Methods. Data were analyzed, and the ratio of firefly luciferase expression to Renilla luciferase activity is shown. Each sample was assayed in triplicate. Levels of luciferase in poly(I:C)-transfected or SenV-infected cells were significantly greater than levels in mock- or MHV-infected cells (P < 0.005). Data are representative of at least three independent experiments.
MHV does not activate NF-κB or IRF-3.
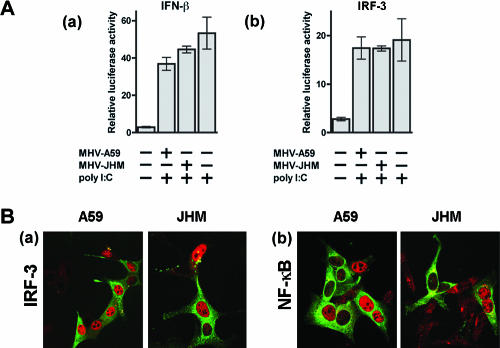
IFN-β transcription requires the activation of NF-κB, IRF-3, and ATF2-c-Jun and their subsequent binding to the IFN-β enhancer. In resting cells, NF-κB heterodimers are present in the cytoplasm, bound to IκB. Activation of NF-κB by stimuli such as poly(I:C) results in its release from this complex, translocation from the cytoplasm to the nucleus, and binding to the IFN-β promoter (30). We first measured NF-κB activation by using a luciferase reporter gene containing repeated NF-κB binding sites from the IFN-β promoter element [pLuc-(PRDII)2] (13). Luciferase expression in MHV-A59- or MHV-JHM-infected cells was similar to that detected in mock-infected cells; however, poly(I:C) transfection or SenV infection induced significantly higher levels of luciferase expression (Fig. 3A). We also investigated MHV-induced activation of NF-κB by using confocal microscopy. As expected, transfection of 17Cl-1 cells with poly(I:C) resulted in accumulation of NF-κB in the nucleus. However, after MHV infection, we observed that NF-κB remained cytoplasmic, with no evidence of activation and translocation to the nucleus (Fig. 3B).
FIG. 3.
MHV does not activate NF-κB or IRF-3. (A) 17Cl-1 fibroblasts were transfected with pLuc-(PRDII)2 or pLuc-(PRDIII-I)3 and then infected with MHV-A59 or MHV-JHM for 12 h prior to harvest for luciferase analysis. MHV did not induce luciferase activity. Levels of luciferase in poly(I:C)-transfected or SenV-infected cells were significantly greater than levels in mock- or MHV-infected cells (P < 0.005). (B) Cells were infected with MHV-A59 or MHV-JHM for 8 h. Cells were fixed with 4% paraformaldehyde and then permeabilized with 0.1% Triton X-100-phosphate-buffered saline. Samples were stained with anti-MHV (green) and anti-NF-κB p65 or anti-IRF-3 (red) antibodies. In uninfected (negative control) and in MHV-infected cells, NF-κB and IRF-3 remained in the cytoplasm. NF-κB and IRF-3 localized to the nucleus after poly(I:C) transfection (positive control). Initial magnification, ×40. Data are representative of at least three independent experiments.
Similarly, IRF-3 is also constitutively expressed in the cytoplasm. After receiving signals from upstream molecules, IRF-3 is rapidly phosphorylated, resulting in dimerization and subsequent migration into the nucleus (13, 23). In order to determine if IRF-3 activation is also inhibited after MHV infection, we investigated IRF-3 function by using a luciferase reporter gene containing repeated IRF-3 binding sites [pLuc-(PRDIII-I)3] (13). 17Cl-1 cells were transfected with this plasmid and then infected with MHV-JHM or MHV-A59. We found that luciferase activity was minimal after infection with either virus, showing that IRF-3 was not activated (Fig. 3A). By contrast, transfection with poly(I:C) or infection with SenV resulted in high levels of luciferase activity. IRF-3 binding to the IFN-β promoter is the terminal step in IFN transcription. In order to determine whether MHV inhibits IRF-3 binding to IFN-β promoter binding sites or whether the virus inhibits signaling upstream of IRF-3, the intracellular localization of IRF-3 was investigated after virus infection. IRF-3 remained localized in the cytoplasm and did not accumulate in the nucleus (Fig. 3B). In contrast, transfection of 17Cl-1 cells with poly(I:C) induced IRF-3 translocation to the nucleus.
MHV does not inhibit poly(I:C)-mediated IFN-β induction.
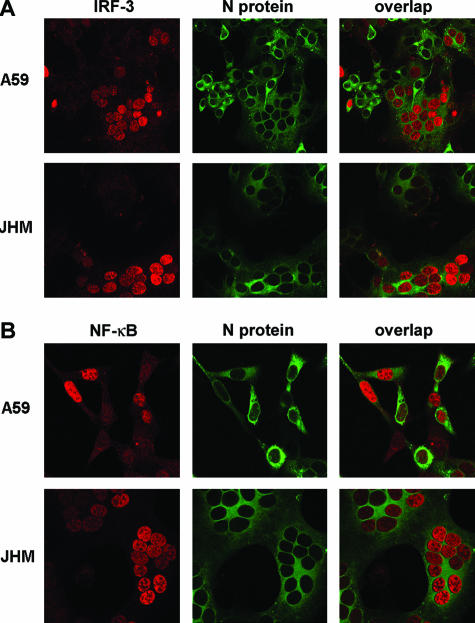
Several viruses that evade the type I IFN response are also able to inhibit poly(I:C)-induced IFN-β production. For example, in cells infected with hepatitis A virus or rhinovirus, poly(I:C) was unable to activate IRF-3 (12, 37). In order to determine whether MHV actively inhibits poly(I:C)-mediated IRF-3 activation and IFN-β synthesis, 17Cl-1 fibroblasts transfected with pLuc-IFN-β or pLuc-(PRDIII-I)3 were infected with MHV-A59 or MHV-JHM. Five to six hours after infection, cells were transfected with poly(I:C) for an additional 6 h. As shown in Fig. 4A, similar levels of luciferase were produced after poly(I:C) stimulation in MHV- or mock-infected cells, when either pLuc-IFN-β or pLuc-(PRDIII-I)3 was transfected as a reporter gene. We chose this time point because MHV inhibits host cell protein synthesis beginning at approximately 6 to 7 h p.i. and the luciferase assay is dependent upon de novo protein synthesis (39, 49). To validate these results at the single-cell level, IRF-3 and NF-κB localization in MHV-infected cells was assessed by confocal microscopy after poly(I:C) transfection for 2 h. We assayed cells at 2 h after transfection because, unlike luciferase expression, which requires treatment for 6 h to be detected, IRF-3 and NF-κB translocation can be detected after a shorter incubation period. In these experiments, cells were dually labeled with antibodies against MHV and IRF-3 or NF-κB. Evidence for IRF-3 and NF-κB translocation into the nucleus was clearly detected in single infected cells and in those that were incorporated into syncytia after either MHV-A59 or MHV-JHM infection (Fig. 4B). It is possible that the putative viral protein that inhibits poly(I:C)-induced activation of IRF-3 and NF-κB was not expressed at sufficiently high levels at 6 h p.i. To test this possibility, these experiments were repeated at 10 h p.i. (Fig. 5), when viral proteins were expressed at high levels. Transfection with poly(I:C) still resulted in IRF-3 and NF-κB translocation into the nuclei of infected cells. In these experiments, poly(I:C) transfection did not significantly inhibit MHV replication since similar numbers of syncytia were detected in the presence or absence of poly(I:C), even if cells were transfected as early as 2 h p.i. (data not shown).
FIG. 4.
MHV does not inhibit poly(I:C)-induced IRF-3 or NF-κB activation and IFN-β production. (A) Cells transfected with pLuc-IFN-β (a) or pLuc-(PRDIII-I)3 (b) were infected with MHV-A59 or MHV-JHM for 5 h and then transfected with poly(I:C) for an additional 6 h. Cells were harvested and subjected to luciferase activity analysis at 11 h p.i. MHV did not inhibit poly(I:C)-induced activation of these reporter genes (P > 0.05). (B) Six hours after MHV infection, cells were transfected with poly(I:C) for an additional 2 h prior to fixation as described above. Cells were then stained with anti-N (green) and anti-IRF-3 (a) or anti-NF-κB p65 (b) (red) antibodies. MHV infection did not inhibit IRF-3 and NF-κB nuclear accumulation occurring after stimulation with poly(I:C). Of note, NF-κB and IRF-3 appear to remain in the cytoplasm in some infected cells, but it is likely that these cells were not successfully transfected with poly(I:C). Initial magnification, ×40. Data are representative of at least three independent experiments.
FIG. 5.
MHV does not inhibit poly(I:C)-induced IRF-3 or NF-κB activation at 10 h p.i. 17Cl-1 cells were infected with MHV-A59 or MHV-JHM for 10 h and then transfected with poly(I:C) for an additional 2 h. Cells were stained with anti-N (green) and anti-IRF3 (A) or anti-NF-κB p65 (B) (red) antibodies. Poly(I:C) transfection resulted in nuclear accumulation of IRF-3 and NF-κB, even at 10 h p.i.
MHV does not inhibit RIG-I-, Mda5-, or TLR3-mediated IFN-β production.
These results show that infection with MHV did not induce IFN-β or inhibit poly(I:C)-mediated activation of this response. As described above, at least three PRRs (RIG-I, Mda5, and TLR3) are involved in dsRNA-mediated IFN-β induction, and these PRRs signal through different adaptor molecules. Different viruses inhibit different steps in these pathways (reviewed in references 14, 17, and 40). Since it is possible that MHV inhibits one pathway while poly(I:C) induces IFN-β production through another pathway, we next determined whether MHV inhibited IFN-β production mediated by RIG-I, Mda5, or TLR3. For this purpose, we overexpressed each molecule, as overexpression results in IFN-β induction in the absence of ligand (dsRNA) binding (60, 61). In most reports, constructs encoding these molecules were transfected into previously infected cells. However, our experiments were complicated by the rapid cytopathic effects induced by MHV and the comparatively long time required to induce IFN-β transcription after plasmid transfection. Thus, we were not able to test the ability of RIG-I, Mda5, or TLR3 to induce IFN-β production by transfecting infected cells with these constructs.
As an alternative approach, we transfected 17Cl-1 cells with plasmids encoding RIG-I or Mda5 together with pLuc-IFN-β 16 to 18 h prior to infection with MHV-A59. To determine whether expression of these factors inhibited MHV replication, we infected cells with a recombinant virus that expressed GFP (rA59.GFP). In preliminary experiments, we observed that transfection did not decrease the number of GFP-positive cells (data not shown). Cells were then transfected with liposomal poly(I:C) at 5 h p.i., when viral protein expression can be detected (42). As described above, cells were transfected at this time to minimize the effect of virus-induced inhibition of host cell protein synthesis. MHV-A59 was used in these experiments because, unlike MHV-JHM, MHV-A59 infected more than 90% of the cells in the culture (data not shown), making it likely that most transfected cells were also infected. Cells were harvested at 11 h p.i. and subjected to a luciferase activity assay. Consistent with previous reports (60), poly(I:C) augmented IFN-β production in cells that overexpressed RIG-I or Mda5. However, infection with MHV did not decrease this poly(I:C)-mediated enhancement of luciferase activity (Fig. 6).
FIG. 6.
IFN-β production mediated by intracellular helicases is not inhibited by MHV. Cells were transiently transfected with pLuc-IFN-β together with empty vector pEF-BOS (vector control), pEF-RIG, or pEF-Mda5. The ratio of plasmids was 1:1. These cells were then infected with MHV-A59 for 5 h prior to transfection with poly(I:C) for an additional 6 h. Cells were harvested at 11 h p.i. and subjected to luciferase assays. Data are representative of at least three independent experiments.
This approach could not be applied to analysis of the TLR3 pathway because we were unable to demonstrate enhancement of luciferase activity in TLR3-expressing cells after poly(I:C) transfection. Since transfection with TLR3 or its downstream adaptor molecule, TRIF, did not generally induce reporter gene expression for at least 12 h (data not shown), we transfected 17Cl-1 cells with TLR3 or TRIF and pLuc-IFN-β 6 to 10 h prior to infection with MHV-A59. Cells were harvested at 12 h p.i. and analyzed for luciferase activity. As shown in Fig. 7, transfection with TLR3 or TRIF cDNA enhanced luciferase expression compared to levels for cells transfected with empty vector. This enhancement was not decreased by MHV infection.
FIG. 7.
TLR3-mediated IFN-β production is not inhibited by MHV. Cells were transfected with pLuc-IFN-β together with empty vector pEF-BOS (vector control), pEF-TRIF, or pEF-TLR3 for 6 to 10 h prior to MHV-A59 infection. Cells were harvested at 12 h p.i. and then subjected to luciferase assays. Data are representative of at least three independent experiments.
Nonessential proteins encoded by MHV-JHM gene 2a or gene 4 are not required for inhibition of IFN synthesis.
Our results suggested that MHV did not actively inhibit IFN production by any known pathway. To further investigate MHV-mediated inhibition, we analyzed whether viruses deleted in gene 2a or gene 4 were able to induce IFN. The proteins encoded by these genes are neither essential for growth in tissue culture cells nor required for the development of MHV-induced encephalitis (35, 47, 59; unpublished data). For this purpose, we used a recombinant virus in which gene 4 was deleted (rJHM.gene4KO) (35) and a naturally occurring deletion variant lacking gene 2a protein expression (Wb-1) (47). L929 cells were infected with rJHM.gene4KO or Wb-1 at 10 PFU/cell. Supernatants were harvested at 20 h p.i., and IFN production was measured using the VSV-based bioassay as described above. Infection with the viruses deleted in either gene 2a or gene 4 induced less than 1.0 U IFN/ml. These results show that neither protein is involved in inhibition of IFN-α/β induction.
DISCUSSION
Our results confirm previous reports showing that MHV-JHM and MHV-A59 do not induce interferon production in infected cells (15, 41, 62). However, we could not demonstrate a specific step in any known pathway that was inhibited by the virus. MHV did not inhibit the induction of IFN-β by poly(I:C) or by overexpression of RIG-I, Mda5, or TLR3/TRIF (Fig. 4 to 7). MHV replication, like that of other RNA viruses, has an obligatory requirement for the generation of dsRNA, which is a potent inducer of type I IFN. Our results are consistent with the possibility that MHV does not induce IFN because viral dsRNA, generated as part of viral replication, is “invisible” to the immune system. The replication of many positive-strand RNA viruses, like MHV, occurs on intracytoplasmic double-membrane vesicles (DMV) (18, 45, 46, 50, 55). DMV in coronavirus-infected cells may originate from the endoplasmic reticulum or may occur as a result of autophagy (42, 51). These structures have been visualized previously by electron microscopy (46, 55), and it is possible that the sites of replication are isolated from the cytoplasm or endosomes, where RIG-I, Mda5, and TLR3 are located.
Other group 2 coronaviruses, such as severe acute respiratory syndrome CoV (53) and human CoV-OC43 (unpublished data), also do not induce IFN. However, group 1 coronaviruses, such as human CoV-229E and transmissible gastroenteritis virus (TGEV), do induce IFN. In the case of TGEV, IFN induction requires the transmembrane (M) protein, and mutation of a single amino acid abrogates its interferogenic ability (25). Induction is also dependent upon the glycosylation status of the M protein. M proteins from group 1 coronaviruses are N glycosylated whereas those from group 2 are O glycosylated. Comparisons of isogenic viruses expressing either N- or O-glycosylated M proteins showed that virus expressing N-glycosylated M protein induced IFN to a greater extent than virus expressing either O-glycosylated or unglycosylated M protein (9). Glycosylated viral proteins mediate IFN-α production by binding to mannose receptors present on dendritic cells (32). Of relevance to our results, IFN was not induced when virus expressed mutant versions of the M protein, showing that the presence of dsRNA in TGEV-infected cells, as in MHV-infected cells, was not sufficient for this induction. Arteriviruses, which are also members of the nidovirus family, similarly replicate on double-membrane vesicles (36). However, unlike MHV, porcine reproductive and respiratory syndrome virus, an arterivirus, does stimulate NF-κB activation in infected cells (27).
While MHV may not induce IFN because it is “hidden” from host dsRNA sensors, other positive-strand RNA viruses actively prevent IFN-β induction. In one example, Meylan et al. showed that the serine protease NS3/4A of hepatitis C virus inhibits IFN-β induction by cleavage of IPS-1, an adaptor protein required for RIG-I- and Mda5-mediated activation of IRF-3 (31). Hepatitis A virus inhibits RIG-I- and TLR3-mediated pathways of IFN-β induction at a step before IRF-3 phosphorylation (12), perhaps at the same step as hepatitis C virus. Two pestiviruses, classical swine fever virus (CSFV) and bovine viral diarrhea virus, also inhibit IFN production. However, unlike MHV, CSFV is able to inhibit IFN production induced by a heterologous virus or poly(I:C). This effect is mediated in part by the N-terminal protease of CSFV, Npro, which blocks IRF-3 transcription (24). Bovine viral diarrhea virus, on the other hand, blocks IRF-3-DNA complex formation, even when induced by a heterologous virus (3, 4). Since most positive-strand RNA viruses are postulated to replicate on DMV (46), these results collectively suggest that replication on these structures is not sufficient to shield viral RNA from detection by dsRNA sensor molecules. Alternatively, it is possible that DMV induced by coronaviruses and by other positive-strand RNA viruses have different properties.
While MHV does not induce IFN in vitro, IFN-β mRNA (and presumably protein) is detected in the central nervous system of mice infected with either MHV-A59 or MHV-JHM (43). The etiology of this IFN-β RNA and protein is not clear. While infected fibroblasts and BM-derived DCs do not produce IFN (41, 62), it is possible that other cells, such as plasmacytoid DCs (pDCs) or glial cells, respond to infection with MHV by secreting IFN-α/β; astrocytes are a well-described target for MHV (10, 38). Alternatively, type I IFN may be induced in pDCs and other phagocytic cells after these cells ingest apoptotic bodies or viral products generated from infected cells. Of note, MHV-A59-infected pDCs were recently shown to produce type I IFN in vitro in a TLR7-dependent process (7).
In summary, our results show that MHV does not induce IFN-β production. While it is possible that this indicates that another, undefined IFN induction pathway is inhibited by MHV, we favor the conclusion that MHV RNA and proteins are shielded from detection by cellular sensor molecules. Further work will be directed at understanding the nature of this method of immune evasion.
Acknowledgments
We thank Noah Butler, Daniela Anghelina, Richard Roller, and Debra Ferraro for critical reviews of the manuscript. We thank Takashi Fujita, Tom Maniatis, Kate Fitzgerald, Shizuo Akira, Thomas Gallagher, Ann Caron, Shintaro Sato, and Sarah McWhirter for kindly providing important constructs.
This work was supported by a grant from the NIH (NS036592).
Footnotes
Published ahead of print on 1 November 2006.
REFERENCES
- 1.Alexopoulou, L., A. C. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413:732-738. [DOI] [PubMed] [Google Scholar]
- 2.Andrejeva, J., K. S. Childs, D. F. Young, T. S. Carlos, N. Stock, S. Goodbourn, and R. E. Randall. 2004. The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN-beta promoter. Proc. Natl. Acad. Sci. USA 101:17264-17269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baigent, S. J., S. Goodbourn, and J. W. McCauley. 2004. Differential activation of interferon regulatory factors-3 and -7 by non-cytopathogenic and cytopathogenic bovine viral diarrhoea virus. Vet. Immunol. Immunopathol. 100:135-144. [DOI] [PubMed] [Google Scholar]
- 4.Baigent, S. J., G. Zhang, M. D. Fray, H. Flick-Smith, S. Goodbourn, and J. W. McCauley. 2002. Inhibition of beta interferon transcription by noncytopathogenic bovine viral diarrhea virus is through an interferon regulatory factor 3-dependent mechanism. J. Virol. 76:8979-8988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banerjee, S., K. Narayanan, T. Mizutani, and S. Makino. 2002. Murine coronavirus replication-induced p38 mitogen-activated protein kinase activation promotes interleukin-6 production and virus replication in cultured cells. J. Virol. 76:5937-5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basler, C. F., A. Mikulasova, L. Martinez-Sobrido, J. Paragas, E. Muhlberger, M. Bray, H. D. Klenk, P. Palese, and A. Garcia-Sastre. 2003. The Ebola virus VP35 protein inhibits activation of interferon regulatory factor 3. J. Virol. 77:7945-7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cervantes-Barragan, L., R. Zust, F. Weber, M. Spiegel, K. S. Lang, S. Akira, V. Thiel, and B. Ludewig. 10 September 2006. Control of coronavirus infection through plasmacytoid dendritic cell-derived type I interferon. Blood doi: 10.1182/blood-2006-05-023770. [DOI] [PMC free article] [PubMed]
- 8.Cheung, C. Y., L. L. Poon, I. H. Ng, W. Luk, S. F. Sia, M. H. Wu, K. H. Chan, K. Y. Yuen, S. Gordon, Y. Guan, and J. S. Peiris. 2005. Cytokine responses in severe acute respiratory syndrome coronavirus-infected macrophages in vitro: possible relevance to pathogenesis. J. Virol. 79:7819-7826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Haan, C. A., M. de Wit, L. Kuo, C. Montalto-Morrison, B. L. Haagmans, S. R. Weiss, P. S. Masters, and P. J. Rottier. 2003. The glycosylation status of the murine hepatitis coronavirus M protein affects the interferogenic capacity of the virus in vitro and its ability to replicate in the liver but not the brain. Virology 312:395-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dubois-Dalcq, M., E. Doller, M. Haspel, and K. V. Holmes. 1982. Cell tropism and expression of mouse hepatitis viruses (MHV) in mouse spinal cord cultures. Virology 119:317-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edelmann, K. H., S. Richardson-Burns, L. Alexopoulou, K. L. Tyler, R. A. Flavell, and M. B. Oldstone. 2004. Does Toll-like receptor 3 play a biological role in virus infections? Virology 322:231-238. [DOI] [PubMed] [Google Scholar]
- 12.Fensterl, V., D. Grotheer, I. Berk, S. Schlemminger, A. Vallbracht, and A. Dotzauer. 2005. Hepatitis A virus suppresses RIG-I-mediated IRF-3 activation to block induction of beta interferon. J. Virol. 79:10968-10977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fitzgerald, K. A., S. M. McWhirter, K. L. Faia, D. C. Rowe, E. Latz, D. T. Golenbock, A. J. Coyle, S. M. Liao, and T. Maniatis. 2003. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat. Immunol. 4:491-496. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Sastre, A., and C. A. Biron. 2006. Type 1 interferons and the virus-host relationship: a lesson in detente. Science 312:879-882. [DOI] [PubMed] [Google Scholar]
- 15.Garlinghouse, L. E., Jr., A. L. Smith, and T. Holford. 1984. The biological relationship of mouse hepatitis virus (MHV) strains and interferon: in vitro induction and sensitivities. Arch. Virol. 82:19-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hemmila, E., C. Turbide, M. Olson, S. Jothy, K. V. Holmes, and N. Beauchemin. 2004. Ceacam1a−/− mice are completely resistant to infection by murine coronavirus mouse hepatitis virus A59. J. Virol. 78:10156-10165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hengel, H., U. H. Koszinowski, and K. K. Conzelmann. 2005. Viruses know it all: new insights into IFN networks. Trends Immunol. 26:396-401. [DOI] [PubMed] [Google Scholar]
- 18.Jackson, W. T., T. H. Giddings, Jr., M. P. Taylor, S. Mulinyawe, M. Rabinovitch, R. R. Kopito, and K. Kirkegaard. 2005. Subversion of cellular autophagosomal machinery by RNA viruses. PLoS Biol. 3:e156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang, D. C., R. V. Gopalkrishnan, Q. Wu, E. Jankowsky, A. M. Pyle, and P. B. Fisher. 2002. mda-5: an interferon-inducible putative RNA helicase with double-stranded RNA-dependent ATPase activity and melanoma growth-suppressive properties. Proc. Natl. Acad. Sci. USA 99:637-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kato, H., O. Takeuchi, S. Sato, M. Yoneyama, M. Yamamoto, K. Matsui, S. Uematsu, A. Jung, T. Kawai, K. J. Ishii, O. Yamaguchi, K. Otsu, T. Tsujimura, C. S. Koh, C. Reis e Sousa, Y. Matsuura, T. Fujita, and S. Akira. 2006. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441:101-105. [DOI] [PubMed] [Google Scholar]
- 21.Kawai, T., and S. Akira. 2006. Innate immune recognition of viral infection. Nat. Immunol. 7:131-137. [DOI] [PubMed] [Google Scholar]
- 22.Kawai, T., K. Takahashi, S. Sato, C. Coban, H. Kumar, H. Kato, K. J. Ishii, O. Takeuchi, and S. Akira. 2005. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat. Immunol. 6:981-988. [DOI] [PubMed] [Google Scholar]
- 23.Kumar, K. P., K. M. McBride, B. K. Weaver, C. Dingwall, and N. C. Reich. 2000. Regulated nuclear-cytoplasmic localization of interferon regulatory factor 3, a subunit of double-stranded RNA-activated factor 1. Mol. Cell. Biol. 20:4159-4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.La Rocca, S. A., R. J. Herbert, H. Crooke, T. W. Drew, T. E. Wileman, and P. P. Powell. 2005. Loss of interferon regulatory factor 3 in cells infected with classical swine fever virus involves the N-terminal protease, Npro. J. Virol. 79:7239-7247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laude, H., J. Gelfi, L. Lavenant, and B. Charley. 1992. Single amino acid changes in the viral glycoprotein M affect induction of alpha interferon by the coronavirus transmissible gastroenteritis virus. J. Virol. 66:743-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Law, H. K., C. Y. Cheung, H. Y. Ng, S. F. Sia, Y. O. Chan, W. Luk, J. M. Nicholls, J. S. Peiris, and Y. L. Lau. 2005. Chemokine upregulation in SARS coronavirus infected human monocyte derived dendritic cells. Blood 106:2366-2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee, S. M., and S. B. Kleiboeker. 2005. Porcine arterivirus activates the NF-kappaB pathway through IkappaB degradation. Virology 342:47-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maniatis, T., J. V. Falvo, T. H. Kim, T. K. Kim, C. H. Lin, B. S. Parekh, and M. G. Wathelet. 1998. Structure and function of the interferon-beta enhanceosome. Cold Spring Harbor Symp. Quant. Biol. 63:609-620. [DOI] [PubMed] [Google Scholar]
- 29.Matsumoto, M., K. Funami, M. Tanabe, H. Oshiumi, M. Shingai, Y. Seto, A. Yamamoto, and T. Seya. 2003. Subcellular localization of Toll-like receptor 3 in human dendritic cells. J. Immunol. 171:3154-3162. [DOI] [PubMed] [Google Scholar]
- 30.May, M. J., and S. Ghosh. 1998. Signal transduction through NF-kappa B. Immunol. Today 19:80-88. [DOI] [PubMed] [Google Scholar]
- 31.Meylan, E., J. Curran, K. Hofmann, D. Moradpour, M. Binder, R. Bartenschlager, and J. Tschopp. 2005. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature 437:1167-1172. [DOI] [PubMed] [Google Scholar]
- 32.Milone, M. C., and P. Fitzgerald-Bocarsly. 1998. The mannose receptor mediates induction of IFN-alpha in peripheral blood dendritic cells by enveloped RNA and DNA viruses. J. Immunol. 161:2391-2399. [PubMed] [Google Scholar]
- 33.Muller, U., U. Steinhoff, L. F. Reis, S. Hemmi, J. Pavlovic, R. M. Zinkernagel, and M. Aguet. 1994. Functional role of type I and type II interferons in antiviral defense. Science 264:1918-1921. [DOI] [PubMed] [Google Scholar]
- 34.Ontiveros, E., T. S. Kim, T. M. Gallagher, and S. Perlman. 2003. Enhanced virulence mediated by the murine coronavirus, mouse hepatitis virus strain JHM, is associated with a glycine at residue 310 of the spike glycoprotein. J. Virol. 77:10260-10269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ontiveros, E., L. Kuo, P. S. Masters, and S. Perlman. 2001. Inactivation of expression of gene 4 of mouse hepatitis virus strain JHM does not affect virulence in the murine CNS. Virology 290:230-238. [DOI] [PubMed] [Google Scholar]
- 36.Pedersen, K. W., Y. van der Meer, N. Roos, and E. J. Snijder. 1999. Open reading frame 1a-encoded subunits of the arterivirus replicase induce endoplasmic reticulum-derived double-membrane vesicles which carry the viral replication complex. J. Virol. 73:2016-2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peng, T., S. Kotla, R. E. Bumgarner, and K. E. Gustin. 2006. Human rhinovirus attenuates the type I interferon response by disrupting activation of interferon regulatory factor 3. J. Virol. 80:5021-5031. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Perlman, S., and D. Ries. 1987. The astrocyte is a target cell in mice persistently infected with mouse hepatitis virus, strain JHM. Microb. Pathog. 3:309-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perlman, S., D. Ries, E. Bolger, L. J. Chang, and C. M. Stoltzfus. 1986. MHV nucleocapsid synthesis in the presence of cycloheximide and accumulation of negative strand MHV RNA. Virus Res. 6:261-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perry, A. K., G. Chen, D. Zheng, H. Tang, and G. Cheng. 2005. The host type I interferon response to viral and bacterial infections. Cell Res. 15:407-422. [DOI] [PubMed] [Google Scholar]
- 41.Pewe, L., H. Zhou, J. Netland, C. Tangadu, H. Olivares, L. Shi, D. Look, T. M. Gallagher, and S. Perlman. 2005. A severe acute respiratory syndrome-associated coronavirus-specific protein enhances virulence of an attenuated murine coronavirus. J. Virol. 79:11335-11342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prentice, E., W. G. Jerome, T. Yoshimori, N. Mizushima, and M. R. Denison. 2004. Coronavirus replication complex formation utilizes components of cellular autophagy. J. Biol. Chem. 279:10136-10141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rempel, J. D., S. J. Murray, J. Meisner, and M. J. Buchmeier. 2004. Differential regulation of innate and adaptive immune responses in viral encephalitis. Virology 318:381-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sarkar, S. N., and G. C. Sen. 2004. Novel functions of proteins encoded by viral stress-inducible genes. Pharmacol. Ther. 103:245-259. [DOI] [PubMed] [Google Scholar]
- 45.Schwartz, M., J. Chen, M. Janda, M. Sullivan, J. den Boon, and P. Ahlquist. 2002. A positive-strand RNA virus replication complex parallels form and function of retrovirus capsids. Mol. Cell 9:505-514. [DOI] [PubMed] [Google Scholar]
- 46.Schwartz, M., J. Chen, W. M. Lee, M. Janda, and P. Ahlquist. 2004. Alternate, virus-induced membrane rearrangements support positive-strand RNA virus genome replication. Proc. Natl. Acad. Sci. USA 101:11263-11268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwarz, B., E. Routledge, and S. G. Siddell. 1990. Murine coronavirus nonstructural protein ns2 is not essential for virus replication in transformed cells. J. Virol. 64:4784-4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seth, R. B., L. Sun, C. K. Ea, and Z. J. Chen. 2005. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell 122:669-682. [DOI] [PubMed] [Google Scholar]
- 49.Siddell, S., H. Wege, A. Barthel, and V. ter Meulen. 1981. Coronavirus JHM: intracellular protein synthesis. J. Gen. Virol. 53:145-155. [DOI] [PubMed] [Google Scholar]
- 50.Sims, A. C., J. Ostermann, and M. R. Denison. 2000. Mouse hepatitis virus replicase proteins associate with two distinct populations of intracellular membranes. J. Virol. 74:5647-5654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Snijder, E. J., Y. van der Meer, J. Zevenhoven-Dobbe, J. J. Onderwater, J. van der Meulen, H. K. Koerten, and A. M. Mommaas. 2006. Ultrastructure and origin of membrane vesicles associated with the severe acute respiratory syndrome coronavirus replication complex. J. Virol. 80:5927-5940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spann, K. M., K. C. Tran, and P. L. Collins. 2005. Effects of nonstructural proteins NS1 and NS2 of human respiratory syncytial virus on interferon regulatory factor 3, NF-κB, and proinflammatory cytokines. J. Virol. 79:5353-5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spiegel, M., A. Pichlmair, L. Martinez-Sobrido, J. Cros, A. Garcia-Sastre, O. Haller, and F. Weber. 2005. Inhibition of beta interferon induction by severe acute respiratory syndrome coronavirus suggests a two-step model for activation of interferon regulatory factor 3. J. Virol. 79:2079-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tabeta, K., P. Georgel, E. Janssen, X. Du, K. Hoebe, K. Crozat, S. Mudd, L. Shamel, S. Sovath, J. Goode, L. Alexopoulou, R. A. Flavell, and B. Beutler. 2004. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc. Natl. Acad. Sci. USA 101:3516-3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van der Meer, Y., E. J. Snijder, J. C. Dobbe, S. Schleich, M. R. Denison, W. J. Spaan, and J. K. Locker. 1999. Localization of mouse hepatitis virus nonstructural proteins and RNA synthesis indicates a role for late endosomes in viral replication. J. Virol. 73:7641-7657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Versteeg, G. A., O. Slobodskaya, and W. J. Spaan. 2006. Transcriptional profiling of acute cytopathic murine hepatitis virus infection in fibroblast-like cells. J. Gen. Virol. 87:1961-1975. [DOI] [PubMed] [Google Scholar]
- 57.Yamamoto, M., S. Sato, H. Hemmi, K. Hoshino, T. Kaisho, H. Sanjo, O. Takeuchi, M. Sugiyama, M. Okabe, K. Takeda, and S. Akira. 2003. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 301:640-643. [DOI] [PubMed] [Google Scholar]
- 58.Yang, H., G. Ma, C. H. Lin, M. Orr, and M. G. Wathelet. 2004. Mechanism for transcriptional synergy between interferon regulatory factor (IRF)-3 and IRF-7 in activation of the interferon-beta gene promoter. Eur. J. Biochem. 271:3693-3703. [DOI] [PubMed] [Google Scholar]
- 59.Yokomori, K., and M. M. C. Lai. 1991. Mouse hepatitis virus S RNA sequence reveals that nonstructural protein ns4 and ns5a are not essential for murine coronavirus replication. J. Virol. 65:5605-5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yoneyama, M., M. Kikuchi, K. Matsumoto, T. Imaizumi, M. Miyagishi, K. Taira, E. Foy, Y. M. Loo, M. Gale, Jr., S. Akira, S. Yonehara, A. Kato, and T. Fujita. 2005. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J. Immunol. 175:2851-2858. [DOI] [PubMed] [Google Scholar]
- 61.Yoneyama, M., M. Kikuchi, T. Natsukawa, N. Shinobu, T. Imaizumi, M. Miyagishi, K. Taira, S. Akira, and T. Fujita. 2004. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5:730-737. [DOI] [PubMed] [Google Scholar]
- 62.Zhou, H., and S. Perlman. 2006. Preferential infection of mature dendritic cells by mouse hepatitis virus strain JHM. J. Virol. 80:2506-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ziegler, T., S. Matikainen, E. Ronkko, P. Osterlund, M. Sillanpaa, J. Siren, R. Fagerlund, M. Immonen, K. Melen, and I. Julkunen. 2005. Severe acute respiratory syndrome coronavirus fails to activate cytokine-mediated innate immune responses in cultured human monocyte-derived dendritic cells. J. Virol. 79:13800-13805. [DOI] [PMC free article] [PubMed] [Google Scholar]