Abstract
Assembly of many spherical virus capsids is guided by an internal scaffolding protein or group of proteins that are often cleaved and eliminated in connection with maturation and incorporation of the genome. In cytomegalovirus there are at least two proteins that contribute to this scaffolding function; one is the maturational protease precursor (pUL80a), and the other is the assembly protein precursor (pUL80.5) encoded by a shorter genetic element within UL80a. Yeast GAL4 two-hybrid assays established that both proteins contain a carboxyl-conserved domain that is required for their interaction with the major capsid protein (pUL86) and an amino-conserved domain (ACD) that is required for their self-interaction and for their interaction with each other. In the work reported here, we demonstrate that when the ACD is deleted (δACD) or disrupted by a point mutation (L47A), the bacterially expressed mutant protein sediments as a monomer during rate-velocity centrifugation, whereas the wild-type protein sediments mainly as oligomers. We also show that the L47A mutation reduces the production of infectious virus by at least 90%, results in the formation of irregular nuclear capsids, gives rise to tube-like structures in the nucleus that resemble the capsid core in cross-section and contain UL80 proteins, slows nuclear translocation of the major capsid protein, and may slow cleavage by the maturational protease. We provide physical corroboration that mutating the ACD disrupts self-interaction of the UL80 proteins and biological support for the proposal that the ACD has a critical role in capsid assembly and production of infectious virus.
A common feature among viruses with a spherical capsid is their use of a transient internal scaffolding structure to coordinate assembly of the capsid shell (7, 11, 13, 17, 32). Among the herpes group viruses this scaffold is made up of at least two essential proteins. These are encoded by UL80a and UL80.5 in human cytomegalovirus (HCMV) (17, 38, 39) and have counterparts in the other herpesviruses (12, 22, 36, 39). UL80a, the longer of the two CMV open reading frames (ORFs), specifies the 74-kDa viral maturational protease precursor (pPR), and contains genes for three additional independently synthesized proteins. All four are in frame and carboxy coterminal, such that the amino acid sequence of the smaller proteins is identical to the sequence of the carboxyl end of the larger proteins (22, 39), as illustrated in Fig. 1. UL80.5 specifies the most abundant of the UL80 proteins, which is called the assembly protein precursor (pAP) and is the core component of the scaffold.
FIG. 1.
Genetic relationship of HCMV UL80 proteins. Four independent genetic elements encode the UL80 proteins (38). The corresponding promoters (arrows), ORF designations (above arrows), and transcripts (mRNAs) are indicated at the top. Resulting in-frame, carboxy-coterminal proteins are shown at the bottom. Protein designations and sizes are indicated. Shading illustrates the extent of shared amino acid sequence between the four proteins. The proteolytic portion of pPR (Protease; darkest shading) is joined to the scaffolding portion (Scaffold) by a “linker” sequence. The scaffolding portion is identical to the assembly protein precursor (pAP) and contains a carboxyl “tail” domain (lightest shading) that is cleaved off all four UL80 proteins by the protease. Dots at left ends of mRNAs indicate translational start sites (38); asterisks indicate the location of the ACD.
The requirement for these proteins during capsid formation and maturation has been demonstrated by using mutant viruses (10, 15, 29, 41) and a recombinant-baculovirus herpes simplex virus (HSV) capsid assembly system (33, 34). In the absence of a pAP homolog (i.e., HSV pUL26.5 or preVP22a), the formation of closed spherical capsids is disrupted, and aberrantly shaped particles accumulate in the nucleus without forming infectious virus (10, 33, 34). Without a functional protease homolog, closed capsids are formed, but there is a subsequent failure of scaffolding cleavage (e.g., pAP→AP) and of DNA packaging that likewise results in nonproductive replication, yielding no infectious virus (15, 29, 41).
The mechanisms enabling these proteins to guide the processes of capsid assembly and maturation have begun to emerge as details of their structure have been identified. Yeast two-hybrid protein-protein interaction studies showed that HCMV pAP (pUL80.5) interacts with itself through a short sequence near its amino end called the amino-conserved domain (ACD) and with the major capsid protein (MCP; pUL86) through a short sequence at its carboxyl end called the carboxyl-conserved domain (see Fig. 1) (3, 40). Similar interactions have been identified between the counterpart HSV proteins (9, 26). Deleting the ACD, or making a specific point mutation (L47A) within it, abolished HCMV pAP self-interaction and had the unexpected effect of substantially reducing the strength or stability of the pAP-MCP interaction (40). A similar, albeit lesser effect on the counterpart HSV proteins was also found (26). Coupled with results from other studies that mapped two nuclear localization signals (NLS1 and NLS2) and specific phosphorylation sites within pAP (6, 27, 28), a model of the earliest steps in the CMV capsid assembly pathway was proposed (17, 40) and elaborated (6).
Key features of the model are that (i) the capsid assembly process is initiated by pAP self-interaction, which stabilizes interaction with the MCP to form pAP-MCP protomers; (ii) the pAP-MCP protomer may continue developing in the cytoplasm, possibly reaching the complexity of a complete protocapsomer containing five or six copies of MCP; (iii) translocation of the protomers into the nucleus is enabled by NLS located in pAP; (iv) once inside the nucleus, additional interactions between protomers and other proteins (e.g., triplex and portal assemblages) take place, resulting in the formation of nascent procapsids; and (v) autoproteolytic and maturational cleavages catalyzed by pPR sever the scaffold-shell connections, enabling elimination of the scaffolding components from the capsid, perhaps facilitated by electrostatic repulsions resulting from site-specific phosphorylations. In the work reported here, we tested the hypothetical first step of this model—self-association of pAP through its ACD—and our results provide corroborating physical and biological support for its importance.
MATERIALS AND METHODS
Cells and viruses.
Viruses were propagated in human foreskin fibroblasts (HFFs) growing in Dulbecco modified Eagle medium (DMEM) supplemented with fetal calf serum, penicillin, and streptomycin as described previously (37). Parental “wild-type” virus was produced from the HCMV (strain AD169) bacmid pHB5 (lacking ORFs US2 to US6) obtained from U. Koszinowski and coworkers (5). A derivative of pHB5, containing the coding sequence for enhanced green fluorescent protein (GFP) in place of ORFs 7 to 11 (AD169 US2-11-EGFP-loxP [4]), was used as the parental bacmid for GFP-expressing virus. Mutant plasmids and bacmids were constructed as described below.
S132A-pPR, S132A/δACD-pPR, and S132A/L47A-pPR plasmids.
These mutations were made by site-directed mutagenesis of the HCMV UL80a ORF in vector pET17b, as described in greater detail elsewhere (E. J. Brignole and W. Gibson, unpublished data). Ser132 occurs only in the UL80a ORF and is numbered in that context; residues in the ACD are numbered according to their codon position in the UL80.5 ORF (e.g., Leu47 is Leu382 in the UL80a ORF). Mutagenic primers were used to introduce the following primary amino acid and corresponding codon changes: (i) EB6R (S132A-pPR; TGC→GCG), (ii) EB27 (S132A/L47A-pPR; CTA→GCA), and (iii) EB28 (S132A/δACD-pPR; ΔHis34-Arg52). Additional mutations introduced to simplify cloning included (i) two silent mutations in EB6R to delete an NgoMIV restriction site at Ala129 (GCC→GCG) and to introduce an XhoI site at Ser134 (GCC→GCG), (ii) a silent mutation in EB27 to introduce an EagI restriction site at Ala54 (GCA→GCC), and (iii) formation of an SpeI restriction site in EB28 after the Pro33 codon by inserting the Thr-Ser coding sequence ACT AGT. EB27 and EB28 were derived from EB6R and contain the mutations included within that construct.
L47A, L47Agr, and δACDgr bacmids.
These mutations were introduced into the viral bacmid that encodes GFP (L47Agr; δACDgr) or into the parental bacmid that does not encode GFP (L47A; δACD), via homologous recombination as described previously (8). The L47A and δACD mutations with their corresponding unique marker mutations were subcloned, from the bacterial expression plasmids EB27 and EB28 (see above), into the EB39 shuttle vector containing the UL80 gene and flanking sequences for recombination (8).
L47A/tetraCys.
The tetraCys mutation was introduced into the L47A bacmid by replacing the Pro-Gly pair in the pUL80.5 sequence (DS139PGGM) with the minimal tetraCys sequence, giving DS139CCPGCCGM. Homologous recombination between the bacmid and mutagenic oligonucleotides was done in Gam-, Beta-, Exo-expressing DY380 Escherichia coli (21) using the mutagenic oligonucleotides TATCCCGTGCCGCCGCCACCATCACCGGCCTATTACCGTCGGCGCGACTCTTGTTGCCCGGGCTGTTGC (forward) and ACGGTGACCACCGTCGTAACGCTCCCATCCGGACGGTGGTTCATCCATACCGCAACAGCCCGGGCAACA (reverse). The site-directed knockout cassette was generated by PCR using pRpsL-neo plasmid (K002; Gene Bridges, Dresden, Germany) as DNA template and the primers AGCTACGCCGGCCTCTCGCTCTCCAGCCGGCGCTGCGACGACGTGGAGGCCGGCCTGGTGATGATGGCGGGATCG (forward) and CAAAGCCACGTGTTTGAACGGCGTGGTTTCCGAGCCCGAAAGCGACGTCGCTCAGAAGAACTCGTCAAGAAGG (reverse).
Wt/tetraCys.
The mutagenic oligonucleotides used to create L47A/UL80tetraCys (above) were also used to introduce the tetraCys coding sequence into the Wt-bacmid at the same site and in the same way.
All mutations were confirmed in the bacmids by automated sequence analysis. Bacmids (3 to 5 μg of DNA) were nucleoporated into cells by using the Amaxa system and protocol with 0.5 to 1.0 × 106 HFF cells in 100 μl of nucleofector solution (VPD-1001; amaxa, Cologne, Germany). The replication of bacmids and spread of mutant viruses in cell culture was monitored by fluorescence for GFP-expressing mutants or by cytopathic effect (CPE) for those not expressing GFP. The UL80 gene of the L47A mutant virus was PCR amplified from sucrose-banded extracellular material and sequence verified in its entirety.
Virus infectivity assays.
Stocks of wild-typegr and L47Agr were prepared and determined to have titers of 9.2 × 105 and 2.3 × 105, respectively. Subconfluent HFF cultures in 10-cm dishes were infected with either wild-type or mutant virus at approximately equal multiplicities (i.e., 50 μl of wild-type stock; 250 μl of mutant stock). On days 1 to 10, 13, and 14, 1 ml of medium was removed from each dish, clarified of large particulate material by centrifugation, serially diluted in 10-fold increments to 10−6, and frozen at −80°C until assayed for infectivity. The samples were thawed, and each was added (50 μl per well) to three wells of a 96-well plate containing 150 μl of growth medium and ∼2,400 HFFs seeded the day before. Cells were monitored for fluorescence of the GFP marker for a period of 9 days, and the number of GFP-expressing plaques were counted in all wells containing between 1 and 55 plaques (i.e., single or cluster of green cells). In cases where GFP-expressing cells were detected only in the undiluted sample, and where the combined number of plaques for the three wells was fewer than three, the titer is plotted as baseline. The virus titer was calculated as the number of GFP-expressing plaques per well multiplied by the dilution factor and was then averaged for all wells counted.
Bacterial expression of pUL80a and gradient sedimentation.
Proteins were expressed in freshly transformed BL21(DE3)/pLysS cells (200132; Stratagene, La Jolla, CA), grown to an optical density at 600 nm of ∼0.6, and induced with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) at 16°C for 16 h. Pelleted cells were ruptured by using a French pressure cell, and the lysate was clarified by centrifugation at 100,000 × g (29,500 rpm in Beckman SW41 rotor) for 30 min at 4°C.
Clarified lysates were layered onto 5 to 25% gradients of glycerol (vol/vol) in lysis buffer (50 mM sodium phosphate buffer [pH 8.0], 300 mM NaCl), and the gradients were subjected to centrifugation at 200,000 × g (40,000 rpm in SW41 rotor) for 16 h at 4°C. Gradients were collected in 0.5-ml fractions from the top, by displacement from the bottom, using a gradient fractionator (model 185; ISCO, Lincoln, NE). Samples of the resulting fractions were combined (3:1) with protein sample buffer (27, 40) and stored at −80°C until analyzed.
Cell fractionation, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and Western immunoassay.
NP-40 cytoplasmic and nuclear fractions were prepared by scraping the cells from a single well of a 12-well plate into the growth medium, and collecting them by centrifugation (16,000 × g, 1 min at 4°C). The cell pellet was suspended in 100 μl of NP-40 buffer (0.5% NP-40, 150 mM NaCl, and 40 mM phosphate buffer [pH 7.4], with protease inhibitor cocktail [catalog no. 1183617001] from Roche, Indianapolis, IN), the suspension was incubated on ice for 5 min, and the supernatant (cytoplasmic) and pellet (nuclear) fractions were separated after centrifugation (16,000 × g, 1 min at 4°C). The pellet was combined with 100 μl of NP-40 buffer to equal the volume of the cytoplasmic fraction, and both preparations were combined with 100 μl of protein sample buffer (3 parts NuPAGE sample buffer [NP0007; Invitrogen, Carlsbad, CA] and 2 parts 1 M dithiothreitol [DTT]).
Proteins were separated by electrophoresis in a 4 to 12% polyacrylamide gradient gels (NP0323BOX; Invitrogen), using 2-(N-morpholino)ethanesulfonic acid buffer (MES; NP0002; Invitrogen) containing SDS, as described previously (23). Protein staining after SDS-PAGE was done with SYPRO-Ruby (SYPRO-R; 170-3138; Bio-Rad, Hercules, CA), followed by imaging using a Kodak Gel Logic 200 workstation and Kodak MI software (v.4.0.0).
Western immunoassays were done as described previously (23). Proteins of interest were detected by incubating the membrane overnight at 4°C in TN buffer (10 mM Tris, 0.9% NaCl [pH 7.4]) containing 5% bovine serum albumin (BSA). The membrane was then incubated with primary antibodies, washed, incubated with 125I-labeled protein A, washed, and then dried and analyzed after phosphorimaging (BAS-2500 phosphorimager with ImageGauge v4.22; Fuji Medical Systems, Stanford, CT). Rabbit polyclonal antisera used were to synthetic peptides representing (i) the carboxy-terminal 15 residues of pUL86, the major capsid protein (anti-MCP [18]); (ii) the carboxy-terminal 15 residues of pUL85, the minor capsid protein (anti-mCP [18]); and (iii) the carboxy-terminal 13 residues of the UL80 proteins (anti-C1 [31]) or to bacterially expressed, purified (by immobilized metal affinity chromatography/SDS-PAGE) HCMV assembly protein precursor (pAP, pUL80.5) (anti-pAPhr).
Microscopy.
Fluorescence microscopy to document the spread of GFP-expressing viruses, was done using a Nikon Eclipse TE200 inverted microscope equipped with Sony DKC5000 camera as described previously (23).
Confocal microscopy was used to detect tetraCys-tagged proteins in live cells reacted with the biarsenical dye FlAsH (12589-057; Invitrogen). Prior to adding FlAsH, the cell layer was gently rinsed with phenol red-free DMEM (PR-DMEM; 21063-029; Invitrogen). After this solution was aspirated, the cells were incubated in PR-DMEM containing 1.0 μM FlAsH and 10 μM 1,2-ethanedithiol (02390; Fluka) in the dark for 60 min at 37°C. After incubation, the staining solution was removed, the cell layer was rinsed as before, and cells were incubated for 30 min in PR-DMEM containing 20 μM Disperse Blue 3 (12589-057; Invitrogen) and 750 μM 2,3-dimercaptopropanol (D1129; Sigma) at 37°C. Fixed cells were stained the same way, but the monolayer was first washed three times with 1× phosphate-buffered saline (PBS) prior to fixation in 2% paraformaldehyde plus 3 mM MgCl2. Nuclei were stained with DAPI (4′,6′-diamidino-2-phenylindole; 25 μg/ml in PBS) for 1 h at room temperature. The stained cells were inspected and photographed using a Zeiss Axiovert 200 equipped-UltraView LCI confocal system, with excitation and detection of the fluorophore at 488 and 530 nm, respectively.
Electron microscopy was done using infected cells fixed in a HEPES solution containing 2% paraformaldehyde and 2% glutaraldehyde, stained in 1% OsO4 and 2% uranyl acetate, and then embedded in Epon resin directly on the plastic culture dishes. After thin sectioning (80 to 90 nm), samples were stained with 2% uranyl acetate and 0.3% lead citrate, and visualized using a Hitachi TEM microscope equipped with a DVC1412M-FW digital camera system and AMTV542 software program. Measurements were determined while viewing the specimen by using an electronic ruler function of the system.
RESULTS
Disruption of ACD abolishes pPR self-interaction.
The importance of the ACD and Leu47 to self-interaction of the UL80 proteins was demonstrated by using yeast GAL4 two-hybrid assays (40). To corroborate this finding, we used the 74-kDa protease precursor pPR (catalytically inactive mutant, S132A-pPR) expressed in bacteria as our parental test protein. Its association state was determined by rate-velocity sedimentation and compared to those of the same protein lacking the 19-amino-acid ACD sequence (δACD) or containing the point mutation L47A. We used pPR because it is the longest member of the UL80 protein family and includes all of the sequences present in the three other proteins. In addition, it expresses well in bacteria, whereas pAP did not. Gradient sedimentation was used to evaluate oligomers because pilot studies showed that pPR and pAP bind adventitiously to most chromatography media under nondenaturing conditions (Brignole and Gibson, unpublished).
We found that some S132A-pPR, when analyzed as described above, sedimented close to the position of the BSA molecular mass marker (66 kDa), but most was present in the pellet fraction (Fig. 2, top panel). Treating the lysate with 50 mM DTT prior to centrifugation converted most of the S132A-pPR to a more soluble form that sedimented ahead of the 158-kDa aldolase marker protein, leaving comparatively little remaining in the pellet, indicating an involvement of intermolecular disulfide bonds in causing microaggregation of pPR. In contrast, even in the absence of DTT, most of the δACD and L47A mutant proteins sedimented close to the BSA marker (Fig. 2), approximating the predicted 74-kDa mass of pPR. Adding 50 mM DTT to these lysates before centrifugation had little effect on the distribution of the mutant proteins. The slightly broader distribution of the L47A mutant with DTT may be due to some imprecision in gradient fractionation rather than an actual change in sedimentation properties. Thus, the same two mutations that abolished self-interactions of pAP in the GAL4 two-hybrid assay also interfered with self-interaction of pPR in this direct physical assay. We have not further investigated the comparatively weaker effect of DTT in releasing S132A/δACD-pPR from the microaggregated fraction (Fig. 2, Pel), but we suspect this may be due to conformational changes resulting from the deletion that make the protein generally more insoluble. It is noteworthy that a triple mutation in HSV preVP22a (pUL26.5), in the region corresponding with the ACD of the CMV UL80 proteins, also results in a reduction of oligomer complexity (see Fig. 9 in reference 30).
FIG. 2.
Quaternary structure of pPR disrupted by ACD mutations δACD and L47A. Full-length pPR (catalytically inactivated by S132A mutation), and mutants with the ACD deleted (S132A/δACD-pPR) or mutated at the critical Leu47 within the ACD (S132A/L47A-pPR) were expressed and prepared for analysis by centrifugation as described in Results and Materials and Methods. Equal parts of each sample were treated with 50 mM DTT (+DTT) or without (−DTT). Gradients used for +DTT samples contained 50 mM DTT. Gradients were collected and samples from each fraction subjected to SDS-PAGE, followed by Western immunoassay with anti-C1. Shown here are phosphorimages of the resulting membranes. A portion of each starting lysate (Lys) and of the material pelleted onto the bottom of the centrifuge tube (Pel) were included with the respective gradient samples. The protein standards carbonic anhydrase (31 kDa), BSA (66 kDa), and aldolase (158 kDa) were added to the lysates to serve as molecular mass markers and were detected by Coomassie brilliant blue staining in a duplicate set of gels. Their positions are indicated above the image of each −DTT gradient.
δACD and L47A mutations slow the spread of mutant viruses during infection and reduce virus yield.
To evaluate whether these ACD mutations affect virus growth, we moved them into the HCMV bacmid. Within a few days of nucleoporating cells with the two mutant bacmids, and the wild-type bacmid for comparison, expression of GFP from the viral marker gene was detected in all three cultures (e.g., Fig. 3, day 7). In less than 2 weeks, cells adjacent to those initially receiving the wild-type bacmid became fluorescent and formed an increasingly large focus of viral CPE. By about 3 weeks nearly all of the cells in that same culture showed strong CPE and bright fluorescence, and by the fourth week many of the cells were ruptured and the growth medium was brightly fluorescent.
FIG. 3.
L47A mutation in HCMV UL80a gave rise to infectious virus that spread more slowly than did the wild-type virus. Cells nucleoporated with the wild-type HCMV-bacmidgr (Wt) or with the L47Agr (L47A) or the δACDgr (δACD) mutant bacmids were monitored for the expression of GFP from the marker gene in the parental bacmid. On the indicated days after nucleoporation (left of collage), fluorescence images were recorded from the same area of each culture dish. Circles enclose cells initially expressing GFP from the wild-type and mutant bacmids.
Infection initiated by the L47A bacmid progressed more slowly, requiring approximately a week longer to reach the same stage of initial focus expansion (e.g., Fig. 3, day 25 versus day 18 for wild-type) and to produce extensive CPE (e.g., day 28 versus day 21 for wild-type). The δACD mutant bacmid was apparently able to initiate replication and increase the DNA copy number, as indicated by increasing GFP fluorescence during the first 7 days, which approximated that in L47A- and wild-type bacmid-transfected cells (early data not shown). However, the δACD mutant was unable to produce infectious virus or material that could transmit the infection to other cells, and within approximately 3 weeks the fluorescent cells containing the δACD mutant blebbed, ruptured, and disappeared. Similar results not shown were obtained when the transfections were done with DNA from a different parental bacmid (pHB5) containing the L47A or δACD mutation, increasing the likelihood that the resulting phenotypes are due to the directed mutations and not to random changes that might occur during recombination or propagation in bacteria.
A time course of virus production (Fig. 4) showed that after infection with equal multiplicities of virus there was an initial plateau of infectivity around day 8, at which time the amount of wild-type virus was ∼65-fold higher than the mutant. At the time of maximal titer for each (day 10 for wild-type and day 13 for the mutant) there was still 17-fold more wild-type virus than mutant. Consistent with the slower spread of the mutant observed after transfection (Fig. 3), the sizes of its plaques in this titration assay were smaller than those of wild-type virus.
FIG. 4.
The L47A mutant produced less virus than did wild-type virus. HFF cells were infected at approximately equal multiplicities with the L47A mutant or with wild-type bacmidgr viruses, and samples were collected on the days indicated, frozen at −80°C, and assayed in triplicate as described in Materials and Methods. Shown here are graphs of the changes in virus titer, including standard deviation, over the course of the infections.
Capsid formation impaired in cells infected with virus encoding UL80 proteins with the L47A mutation.
Corresponding with the lower titer of the L47A mutant, fewer extracellular particles were produced relative to the wild type (gradient assay data not shown). Cells infected with the L47A mutant or wild-type virus were examined by electron microscopy, and three differences were noted. (i) Mutant-infected cells contained fewer cytoplasmic particles (enveloped or nonenveloped). (ii) In contrast to the symmetrical, “double-ringed,” well-defined intranuclear capsids typical of wild-type infected cells (24), intranuclear capsids in cells infected with the L47A mutant were comparatively irregular in shape; many lacked an “inner ring,” and some appeared cracked or breaking apart (Fig. 5A and B). And, (iii) nuclei of mutant-infected cells contained rod- or tube-like structures (Fig. 5B to D, arrows). When these were cut perpendicular to the surface of the culture dish (Fig. 5C, inset), the following details were more evident. First, they are uniform in appearance with two distinct rings of density. The inner ring has a diameter of ∼20 nm and the outer ring diameter is ∼60 nm. Second, their outside diameter approximates that of the inner ring of wild-type capsids. Third, the center-to-center distance between these structures is nearly constant (∼80 nm), suggesting that there may be additional material of a fixed thickness surrounding the tubules. And fourth, the number of tubules in a single such regularly spaced group varies from one to at least seven, as shown in the inset of Fig. 5C. We note for reference a report in which tubules with a 60-nm diameter, similar to the inner ring of corresponding capsids, were observed in the nuclei of cells infected with wild-type guinea pig CMV (14).
FIG. 5.
Electron microscopy of L47A mutant-infected cells. HFF cells infected with wild-type (A) or with the L47A mutant (B to D) bacmidgr viruses were fixed, stained, and embedded and thin-sectioned in situ as described in Materials and Methods. “Double-ringed” (large arrows) and empty (small arrows) nuclear capsids typical of infections with wild-type virus are seen in panel A. More irregular-looking capsids (e.g., top-left corner, Panel B) and “tubules” (arrows) are seen in panels B to D. The inset (bottom left corner of panel C) shows tubules sectioned perpendicular to the horizontal plane of cell growth seen in the other panels. (D) Immunoelectron micrograph prepared after reaction with rabbit antibodies to pAP and then 10-nm-gold (black dots)-tagged secondary antibodies.
Intranuclear L47A tubules contain UL80 proteins.
We tried several immunological approaches to determine whether the tubules contain viral capsid proteins, but the results of early experiments using indirect immunofluorescence microscopy and cryo-immunogold electron microscopy were not encouraging, with one exception. We found that when resin-embedded thin sections of L47A mutant-infected cells were reacted with rabbit antibodies to purified HCMV assembly protein precursor (anti-pAPhr), followed by gold-tagged secondary antibodies, there was a selective association of the gold particles with the tubules (Fig. 5D). Although we were unable to identify other proteins that reacted in these postembedding immuno-gold EM assays, to serve as positive controls for specificity (e.g., nuclear lamins A, B, and C), we interpreted the observed specificity of the anti-pAPhr as evidence that UL80 proteins (e.g., pAP) are present in the tubules.
As an alternative to these immunological approaches, which could be complicated by masking of the target epitopes, we tested the tetracysteine/biarsenical dye method developed by Tsien and coworkers (1, 19), in which a small-molecule fluorescent dye binds directly to a tetraCys motif within the tagged protein. We inserted a tetraCys coding sequence into the UL80 gene, downstream of the L47A mutation, as described in Materials and Methods. The doubly mutated virus (L47A/tetraCys) was viable and had no obvious phenotypic differences from its L47A parent.
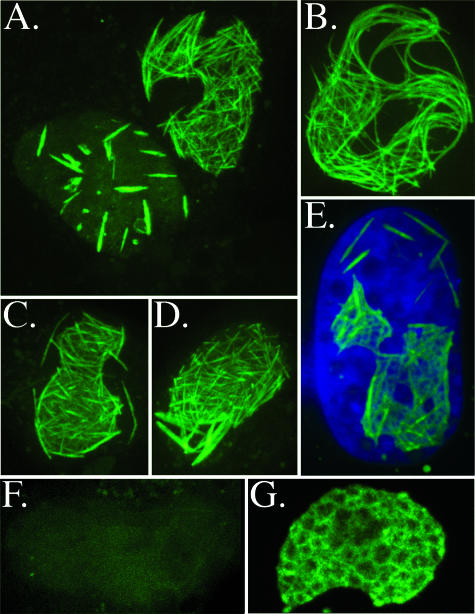
When live cells infected with the L47A/tetraCys mutant were stained with the biarsenical dye FlAsH, a pattern of strongly fluorescent intranuclear, rod- or tube-like structures was observed (Fig. 6A to E). Although the length of the tubes ranged from short and straight (e.g., Fig. 6A and C to E) to long and curvy (Fig. 6B), within individual nuclei they tended to be comparatively uniform. In cells infected with the L47A mutant lacking the tetraCys insert, the tubules could be discerned by a weak negative-staining effect after adding the dye but were not fluorescent, establishing that they have no intrinsic reactivity with the dye (Fig. 6F). Wild-type UL80 proteins with a tetraCys tag at the same position gave a strong, sponge-like pattern of fluorescence within the nuclear inclusions but no evidence of tubules (Fig. 6G). The fluorescent perimeters surrounding nonfluorescing centers in confocal images of the structures in the nuclei of wild-type virus-infected cells resemble the electron-dense surrounding electron-lucent regions routinely observed by electron microscopy as intranuclear inclusions (see, for example, references 2 and 20). These results demonstrate that UL80 proteins are concentrated in the “walls” of the intranuclear inclusions in wild-type virus-infected cells and aberrantly form tube- or rod-like structures in the L47A mutant, indicating disruption of their normal organization and localization.
FIG. 6.
Self-identification of UL80 proteins in L47A tubules by confocal microscopy, using FlAsH to specifically report the tetraCys insert. Cells infected with the L47A/tetraCys mutant (A to E), with its L47A parent virus (F), or with wild-type virus having the same tetraCys insertion (G) were stained with FlAsH and imaged by confocal microscopy. Shown here is a collage of cells with typical patterns of the intranuclear L47A tubules, ranging in appearance from comparatively slender, densely packed, and short (panels A [upper right] and C to E), to more sparse and thicker (panel A, lower left), to slender, long, and curvy (panel B). (E) Merged image showing DAPI-stained nuclei (blue) and FlAsH-stained tubules (green).
L47A mutation affects the intracellular distribution of viral capsid proteins.
Nuclear translocation of MCP requires its interaction with pAP and the strength or stability of that interaction was reduced 75% by the L47A mutation (40). To determine whether this reduction affects the relative amounts or intracellular distribution of these proteins, we compared them in cells infected with the mutant or with wild-type virus. On day 7 (extensive CPE) after infection with wild-type virus and on days 7 (modest CPE), 12, and 14 (extensive CPE) after infection with the L47A mutant the cells were collected and separated into NP-40 nuclear and cytoplasmic fractions in preparation for SDS-PAGE and Western immunoassays as described in Materials and Methods.
Duplicate sets of samples were analyzed on separate membranes. One was probed with antibodies to MCP and revealed two differences between L47A and wild-type (Fig. 7A and C). First, there was twice as much MCP in the cytoplasmic fraction of L47A-infected cells on day 7 as in the nuclear fraction (67% versus 33%), whereas the distribution of MCP in wild-type infected cells on the same day was just the opposite (37% cytoplasmic; 63% nuclear). By day 12 the cytoplasmic/nuclear ratio for L47A-infected cells was equal (50%:50%), and by day 14 when the CPE was extensive (comparable to wild-type on day 7), the distribution of MCP was essentially the same as for day 7 wild-type (i.e., 34% cytoplasmic; 66% nuclear). Second, a 118-kDa fragment of MCP, present in only trace amounts in wild-type infected cells, increased to 12 to 18% the amount of MCP in L47A-infected cells and accumulated in the nuclear fraction, along with two slightly smaller fragments (∼104 and 90 kDa). Their reactivity with anti-MCP, specific for the carboxyl end of MCP, indicates that all three fragments are truncated at their amino end. These data show that MCP translocates into the nucleus more slowly and has an increased susceptibility to proteolytic attack in the L47A mutant compared to wild-type virus.
FIG. 7.
Intracellular distribution of viral proteins is disrupted in L47A mutant-infected cells. Cells were infected with either wild-type (Wt) or L47A mutant (L47A) virus, separated into NP-40 cytoplasmic (Cytoplasm), and nuclear (Nucleus) fractions on days 7, 12, or 14 after infection and subjected to Western immunoassay using either anti-MCP (A) or anti-pAPhr (B), all as described in Results and Materials and Methods. Phosphorimages of the two resulting membranes are shown in panels A and B. (C) Calculations based on measurements from these membranes show the relative percentages of proteins of interest in the NP-40 cytoplasmic and nuclear fractions. Measurements for L47A from panel A included the MCP and “Frag” bands for all six samples and also the two smaller fragments (see asterisks) in “Nucleus” samples from days 12 and 14. Abbreviations indicate the 118-kDa fragment of MCP (Frag.), smaller fragments of MCP (*), protease precursor (pPR), protease precursor cleaved at M site and lacking carboxyl “tail” (PR, §), carboxyl fragment of pPR resulting from R-site cleavage (pPRc, linker+scaffold in Fig. 1), pPRc after removal of the carboxyl “tail” by M-site cleavage (−), assembly protein precursor (pAP), precursor of pUL80.4 (⋄), product of pAP cleavage at M site (AP), and precursor of pUL80.3 (p80.3).
The duplicate membrane was probed with antibodies to the UL80 proteins (anti-pAPhr). Calculations based on measurements from a phosphorimage of this membrane (Fig. 7B and C) show that for each of the UL80 proteins the ratio of precursor forms (i.e., pPR and pAP) to their respective products (i.e., PR and AP) was higher for the mutant than for the wild type. This difference indicates that M-site cleavage is less efficient in mutant-infected cells than in the wild type. In contrast to the slower nuclear translocation of MCP in mutant-infected cells, movement of L47A-pAP into the nucleus was essentially the same over the course of the experiment and indistinguishable from that of wild-type pAP (Fig. 7C). The distribution of L47A-AP (cleaved form of pAP) was also unchanged over this period in mutant-infected cells. However, unlike wild-type infected cells, which contained more nuclear than cytoplasmic AP, cells infected with the L47A mutant contained more cytoplasmic than nuclear AP (Fig. 7B and C).
DISCUSSION
Self-interaction of the UL80 scaffolding proteins is mediated by the ACD and has been proposed, based on results of GAL4 two-hybrid assays, to be an initiating event in HCMV capsid assembly (16, 17, 40). We now provide supporting evidence that the ACD is essential for the self-association of these internal procapsid constituents and demonstrate, by using a virus mutated at a critical amino acid within the ACD (L47A), that this mutation interferes with nuclear translocation of the MCP, intranuclear capsid formation, and the production of infectious virus.
Self-interaction of UL80 proteins.
To verify that the ACD is required for self-interaction of the UL80 proteins, we used mutant forms of pPR expressed in bacteria and assayed by rate-velocity sedimentation in glycerol gradients. We found that, whereas nearly all of the S132A-pPR expressed and analyzed in this way under nonreducing conditions is microaggregated and passes through the gradient and onto the bottom of the centrifuge tube, both ACD mutants (δACD and L47A) sedimented predominantly as monomers. Their smaller-than-predicted mass (∼66 kDa versus 74 kDa predicted) indicates an extended or rod-like shape, as also implied by radial density distributions in the CMV B-capsids (35) and HSV procapsids (25). Although there was little change in the sedimentation pattern of the ACD mutant proteins under strong reducing conditions, the pattern for S132A-pPR showed a dramatic shift of protein from aggregates at the bottom of the tube to slower-moving complexes, suggesting that ACD self-association precedes microaggregation. Correcting for the observed versus predicted mass of the L47A-pPR monomer, the highest concentration of protein in these DTT-treated S132A-pPR preparations sedimented as expected for homo-trimers or tetramers. The same patterns of monomers (δACD-pPR, and L47A-pPR) and oligomers (S132A-pPR) were found when these proteins were affinity purified under denaturing conditions (i.e., 8 M urea) and refolded (Brignole and Gibson, unpublished), supporting the conclusion that pPR forms self-associating complexes whose quaternary structure requires a functional ACD.
ACD impact on capsid formation.
The same two mutations when moved into viruses to determine the biological requirement for the ACD gave rise to multiple phenotypic defects, all relating to capsid formation and maturation. The bacmid carrying the more severe of the two mutations (δACD) failed to yield infectious virus able to spread between cells after transfection (Fig. 3), possibly due to abolishing the critical pAP-MCP interaction or possibly due to gross conformational changes resulting from the deletion. Virus with the L47A point mutation was viable but spread through the culture more slowly and produced less (≥17-fold) virus than did the wild type (Fig. 3 and 4).
Consistent with the lower yield of infectious virus, electron microscopy showed an overall reduction in the number of particles in mutant-infected cells. Fewer capsids were present in the nucleus, and most of those seen differed from wild-type capsids in being less symmetrical and lacking an inner ring (Fig. 5B), demonstrating that the mutation interferes with capsid formation. There were also fewer intranuclear capsids containing DNA (e.g., darkly stained centers) and even fewer enveloped nucleocapsids in the cytoplasm, indicating that the L47A mutation reduces the efficiency of DNA packaging, and capsid egress or envelopment, or both. These last two deficiencies are probable secondary complications of the primary defect in capsid formation. Thus, in addition to abolishing the self-interaction (Fig. 2) (40) of the UL80 proteins, the L47A mutation disrupts capsid formation, DNA packaging, and nucleocapsid envelopment, resulting in reduced production of infectious virus.
An interesting feature of cells infected with the L47A mutant is the appearance of tube-like structures in the nucleus. The similarity of their diameter to that of the inner ring of nuclear capsids lacking DNA, as well as the presence of UL80 proteins in them (Fig. 5 and 6), suggests that they are aberrant forms of the capsid scaffolding structure. More information about their composition and the interactions that underlie their formation and elongation may provide insight into whether and how the UL80 proteins impart curvature and closure to the capsid shell.
Slowed MCP nuclear translocation.
A cell fractionation experiment, done to test effects of the L47A mutation on nuclear translocation of the MCP, provided evidence that it is less efficient in cells infected with the mutant virus than with wild-type virus. In contrast, no difference in the rate of pAP nuclear localization was apparent between the two viruses, even though it is pAP that carries the L47A mutation. These findings are consistent with the hypothesis, based on two-hybrid and transfection assays (27, 40), that nuclear translocation of MCP is mediated by critical interactions of pAP with itself and with MCP, both of which are impaired by the L47A mutation (Fig. 2) (40). The findings are also fully compatible with the fact that pAP is small, contains two nuclear localization signals, and should enter the nucleus whether or not blocked for self-interaction by the L47A mutation. The differential interference with transport of MCP (slowed) versus pAP (normal) supports it being a selective effect rather than a generalized slowdown of protein trafficking into the nucleus of L47A-infected cells.
The apparent cleavage of a 30-kDa fragment from the amino end of MCP in L47A-infected cells is currently unexplained but is likely to be a secondary effect, since the same cleavage is found to an even greater extent in cells infected with viruses carrying mutations in the pAP nuclear localization sequences (unpublished data). We speculate, on the basis of these and other preliminary findings, that the interaction of pAP with MCP results in a conformational change that exposes an otherwise sequestered MCP sequence to proteolytic attack. Nuclear translocation of the pAP-MCP complex is suggested to be fast enough in wild-type infected cells that cleavage of the “sensitized” MCP is minimal. In cells infected with pAP mutants that slow the movement of MCP into the nucleus, however, there is a longer opportunity for proteolytic attack and a greater portion of MCP is cleaved. The protease involved is of interest but has not been identified.
In summary, these studies were done to test predicted functional involvements of the CMV ACD in the earliest steps of the capsid assembly pathway. The results from this work make the following contributions to that objective. First, we corroborated the two-hybrid evidence for self-interaction of the UL80 proteins, determined that full-length pPR (pUL80a) associates into homo-oligomeric trimers or tetramers, and demonstrated the requirement for an intact ACD to form these oligomers. Second, we established the biological importance of this domain by demonstrating that virus carrying the L47A mutation shows replication deficiencies, including cleavage and impaired nuclear translocation of the MCP and delayed activation of the maturational protease, that result in disrupted capsid formation and reduced production of infectious virus. The negative impact of this ACD point mutation on viral replication, considered together with our observation that small molecules (e.g., FlAsH dye) can access and interact with structurally involved UL80 proteins in infected cells, raises the possibility that this essential sequence has potential as an antiviral target.
Acknowledgments
We thank Neal Copeland at the NCI for the DY380 cells, Mike Delannoy of The Johns Hopkins University School of Medicine Microscope Facility for help with the electron microscopy that resulted in the images shown in Fig. 4A to C, Jian Zhang for assistance with other microscopy work and, in particular, for his contributions to Fig. 3 and 4D, and Ron Schnaar for use of his inverted fluorescence microscope. We also thank Larry Gerace for kindly providing anti-nuclear lamin antibodies to test in the immuno-EM experiments.
E.J.B. was in the Biochemistry, Cellular, and Molecular Biology graduate program; N.L.N. is a fellow in the Anti-Cancer Drug Development program, supported by PHS grant T32 CA09243. This research was aided by PHS grants AI13718 and AI32957 to W.G.
Footnotes
Published ahead of print on 1 November 2006.
REFERENCES
- 1.Adams, S. R., R. E. Campbell, L. A. Gross, B. R. Martin, G. K. Walkup, Y. Yao, J. Llopis, and R. Y. Tsien. 2002. New biarsenical ligands and tetracysteine motifs for protein labeling in vitro and in vivo: synthesis and biological applications. J. Am. Chem. Soc. 124:6063-6076. [DOI] [PubMed] [Google Scholar]
- 2.Albrecht, T., T. Cavallo, N. L. Cole, and K. Graves. 1980. Cytomegalovirus: development and progression of cytopathic effects in human cell culture. Lab. Investig. 42:1-7. [PubMed] [Google Scholar]
- 3.Beaudet-Miller, M., R. Zhang, J. Durkin, W. Gibson, A. D. Kwong, and Z. Hong. 1996. Viral specific interaction between the human cytomegalovirus major capsid protein and the C terminus of the precursor assembly protein. J. Virol. 70:8081-8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borst, E., and M. Messerle. 2000. Development of a cytomegalovirus vector for somatic gene therapy. Bone Marrow Transplant 25(Suppl. 2):S80-S82. [DOI] [PubMed] [Google Scholar]
- 5.Borst, E. M., G. Hahn, U. H. Koszinowski, and M. Messerle. 1999. Cloning of the human cytomegalovirus (HCMV) genome as an infectious bacterial artificial chromosome in Escherichia coli: a new approach for construction of HCMV mutants. J. Virol. 73:8320-8329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casaday, R. J., J. R. Bailey, S. R. Kalb, E. J. Brignole, A. N. Loveland, R. J. Cotter, and W. Gibson. 2004. Assembly protein precursor (pUL80.5 homolog) of simian cytomegalovirus is phosphorylated at a glycogen synthase kinase 3 site and its downstream “priming” site: phosphorylation affects interactions of protein with itself and with major capsid protein. J. Virol. 78:13501-13511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casjens, S., and J. King. 1975. Virus assembly. Annu. Rev. Biochem. 44:555-611. [DOI] [PubMed] [Google Scholar]
- 8.Chan, C. K., E. J. Brignole, and W. Gibson. 2002. Cytomegalovirus assemblin (pUL80a): cleavage at internal site not essential for virus growth; proteinase absent from virions. J. Virol. 76:8667-8674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desai, P., and S. Person. 1996. Molecular interactions between the HSV-1 capsid proteins as measured by the yeast two-hybrid system. Virology 220:516-521. [DOI] [PubMed] [Google Scholar]
- 10.Desai, P., S. C. Watkins, and S. Person. 1994. The size and symmetry of B capsids of herpes simplex virus type 1 are determined by the gene products of the UL26 open reading frame. J. Virol. 68:5365-5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dokland, T. 1999. Scaffolding proteins and their role in viral assembly. Cell Mol. Life Sci. 56:580-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donaghy, G., and R. Jupp. 1995. Characterization of the Epstein-Barr virus proteinase and comparison with the human cytomegalovirus proteinase. J. Virol. 69:1265-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fane, B. A., and P. E. Prevelige, Jr. 2003. Mechanism of scaffolding-assisted viral assembly. Adv. Protein Chem. 64:259-299. [DOI] [PubMed] [Google Scholar]
- 14.Fong, C. K., F. Bia, G. D. Hsiung, P. Madore, and P. W. Chang. 1979. Ultrastructural development of guinea pig cytomegalovirus in cultured guinea pig embryo cells. J. Gen. Virol. 42:127-140. [DOI] [PubMed] [Google Scholar]
- 15.Gao, M., L. Matusick-Kuman, W. Hurlburt, S. F. DiTusa, W. W. Newcomb, J. C. Brown, P. J. McCann, I. Deckman, and R. J. Colonno. 1994. The protease of herpes simplex virus type 1 is essential for functional capsid formation and viral growth. J. Virol. 68:3702-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gibson, W. 2006. Assembly and maturation of the capsid, p. 231-244. In M. J. Reddehase (ed.), Cytomegaloviruses: molecular biology and immunology. Caister Academic Press, Norfolk, United Kingdom.
- 17.Gibson, W. 1996. Structure and assembly of the virion. Intervirology 39:389-400. [DOI] [PubMed] [Google Scholar]
- 18.Gibson, W., M. K. Baxter, and K. S. Clopper. 1996. Cytomegalovirus “missing” capsid protein identified as heat-aggregable product of human cytomegalovirus UL46. J. Virol. 70:7454-7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffin, B. A., S. R. Adams, and R. Y. Tsien. 1998. Specific covalent labeling of recombinant protein molecules inside live cells. Science 281:269-272. [DOI] [PubMed] [Google Scholar]
- 20.Haguenau, F., and S. Michelson-Fiske. 1975. Cytomegalovirus: nucleocapsid assembly and core structure. Intervirology 5:293-299. [DOI] [PubMed] [Google Scholar]
- 21.Lee, E. C., D. Yu, J. Martinez de Velasco, L. Tessarollo, D. A. Swing, D. L. Court, N. A. Jenkins, and N. G. Copeland. 2001. A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics 73:56-65. [DOI] [PubMed] [Google Scholar]
- 22.Liu, F., and B. Roizman. 1991. The herpes simplex virus 1 gene encoding a protease also contains within its coding domain the gene encoding the more abundant substrate. J. Virol. 65:5149-5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loveland, A. N., C. K. Chan, E. J. Brignole, and W. Gibson. 2005. Cleavage of human cytomegalovirus protease pUL80a at internal and cryptic sites is not essential but enhances infectivity. J. Virol. 79:12961-12968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGavran, M. H., and M. G. Smith. 1965. Ultrastructural, cytochemical, and microchemical observations on cytomegalovirus (salivary gland virus) infection of human cells in tissue culture. Exp. Mol. Pathol. 76:1-10. [DOI] [PubMed] [Google Scholar]
- 25.Newcomb, W. W., B. L. Trus, N. Cheng, A. C. Steven, A. K. Sheaffer, D. J. Tenney, S. K. Weller, and J. C. Brown. 2000. Isolation of herpes simplex virus procapsids from cells infected with a protease-deficient mutant virus. J. Virol. 74:1663-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pelletier, A., F. Do, J. J. Brisebois, L. Lagace, and M. G. Cordingley. 1997. Self-association of herpes simplex virus type 1 ICP35 is via coiled-coil interactions and promotes stable interaction with the major capsid protein. J. Virol. 71:5197-5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plafker, S. M., and W. Gibson. 1998. Cytomegalovirus assembly protein precursor and proteinase precursor contain two nuclear localization signals that mediate their own nuclear translocation and that of the major capsid protein. J. Virol. 72:7722-7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plafker, S. M., A. S. Woods, and W. Gibson. 1999. Phosphorylation of simian cytomegalovirus assembly protein precursor (pAPNG.5) and proteinase precursor (pAPNG1): multiple attachment sites identified, including two adjacent serines in a casein kinase II consensus sequence. J. Virol. 73:9053-9062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Preston, V. G., J. A. Coates, and F. J. Rixon. 1983. Identification and characterization of a herpes simplex virus gene product required for encapsidation of virus DNA. J. Virol. 45:1056-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Preston, V. G., and I. M. McDougall. 2002. Regions of the herpes simplex virus scaffolding protein that are important for intermolecular self-interaction. J. Virol. 76:673-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schenk, P., A. S. Woods, and W. Gibson. 1991. The 45-kDa protein of cytomegalovirus (Colburn) B-capsids is an amino-terminal extension form of the assembly protein. J. Virol. 65:1525-1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steven, A. C., and P. G. Spear. 1997. Herpesvirus capsid assembly and envelopment, p. 312-351. In W. Chiu, R. M. Burnett, and R. L. Garcea (ed.), Structural biology of viruses. Oxford University Press, New York, NY.
- 33.Tatman, J. D., V. G. Preston, P. Nicholson, R. M. Elliott, and F. J. Rixon. 1994. Assembly of herpes simplex virus type 1 capsids using a panel of recombinant baculoviruses. J. Gen. Virol. 75:1101-1113. [DOI] [PubMed] [Google Scholar]
- 34.Thomsen, D. R., L. L. Roof, and F. L. Homa. 1994. Assembly of herpes simplex virus (HSV) intermediate capsids in insect cells infected with recombinant baculoviruses expressing HSV capsid proteins. J. Virol. 68:2442-2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trus, B. L., W. Gibson, N. Cheng, and A. C. Steven. 1999. Capsid structure of simian cytomegalovirus from cryoelectron microscopy: evidence for tegument attachment sites. J. Virol. 73:2181-2192. (Erratum, 73:4530.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Unal, A., T. R. Pray, M. Lagunoff, M. W. Pennington, D. Ganem, and C. S. Craik. 1997. The protease and the assembly protein of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8). J. Virol. 71:7030-7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang, J., A. N. Loveland, L. M. Kattenhorn, H. L. Ploegh, and W. Gibson. 2006. High-molecular-weight protein (pUL48) of human cytomegalovirus is a competent deubiquitinating protease: mutant viruses altered in its active-site cysteine or histidine are viable. J. Virol. 80:6003-6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Welch, A. R., L. M. McNally, and W. Gibson. 1991. Cytomegalovirus assembly protein nested gene family: four 3′-coterminal transcripts encode four in-frame, overlapping proteins. J. Virol. 65:4091-4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Welch, A. R., A. S. Woods, L. M. McNally, R. J. Cotter, and W. Gibson. 1991. A herpesvirus maturational protease, assemblin: identification of its gene, putative active site domain, and cleavage site. Proc. Natl. Acad. Sci. USA 88:10792-10796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wood, L. J., M. K. Baxter, S. M. Plafker, and W. Gibson. 1997. Human cytomegalovirus capsid assembly protein precursor (pUL80.5) interacts with itself and with the major capsid protein (pUL86) through two different domains. J. Virol. 71:179-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu, X., P. Trang, S. Shah, I. Atanasov, Y. H. Kim, Y. Bai, Z. H. Zhou, and F. Liu. 2005. Dissecting human cytomegalovirus gene function and capsid maturation by ribozyme targeting and electron cryomicroscopy. Proc. Natl. Acad. Sci. USA 102:7103-7108. [DOI] [PMC free article] [PubMed] [Google Scholar]