Abstract
A feline immunodeficiency virus (FIV) provirus with a vif gene deletion (FIVΔvifATGγ) that coexpresses feline gamma interferon (IFN-γ) was tested as a proviral DNA vaccine to extend previous studies showing efficacy with an FIV-pPPRΔvif DNA vaccine. Cats were vaccinated with either FIVΔvifATGγ or FIV-pPPRΔvif proviral plasmid DNA or with both FIV-pPPRΔvif DNA and a feline IFN-γ expression plasmid (pCDNA-IFNγ). A higher frequency of FIV-specific T-cell proliferation responses was observed in cats immunized with either FIVΔvifATGγ or FIV-pPPRΔvif plus pCDNA-IFNγ, while virus-specific cytotoxic-T-lymphocyte responses were comparable between vaccine groups. Antiviral antibodies were not observed postvaccination. Virus-specific cellular and humoral responses were similar between vaccine groups after challenge with a biological FIV isolate (FIV-PPR) at 13 weeks postimmunization. All vaccinated and unvaccinated cats were infected after FIV-PPR challenge and exhibited similar plasma virus loads. Accordingly, inclusion of plasmids containing IFN-γ did not enhance the efficacy of FIV-pPPRΔvif DNA immunization. Interestingly, the lack of protection associated with FIV-pPPRΔvif DNA immunization contrasted with findings from a previous study and suggested that multiple factors, including timing of FIV-pPPRΔvif inoculations and challenge, as well as route of challenge virus delivery, may significantly impact vaccine efficacy.
Similarities between the progressive immunodeficiency syndromes described for feline immunodeficiency virus (FIV) infection in domestic cats and human immunodeficiency virus (HIV) infection in humans have validated the use of the FIV animal model for testing anti-HIV vaccine strategies (6, 9). The FIV model system has been utilized to test vaccine strategies with various degrees of success, depending on the type of immunogen and viral challenge involved. DNA immunization has emerged as a promising approach to the development of HIV type 1 vaccines based on the induction of potent virus-specific cellular immune responses observed in DNA vaccine trials in both mice and nonhuman primates (2, 18). Studies testing particular proviral DNA or multiplasmid DNA vaccines in nonhuman primates revealed protection from either virus load or disease after challenge with pathogenic virus isolates (14, 19, 29, 34, 37). Our previous studies revealed that immunization of cats with plasmid DNA containing an FIV provirus with a vif gene deletion (FIV-pPPRΔvif) that has been shown to produce a highly attenuated virus (26) resulted in protection against infection with the wild-type (WT) homologous FIV isolate (25). However, no clear immune correlates of protection could be discerned from this investigation. In other studies, defective FIV proviral DNA vaccines containing a deletion in either reverse transcriptase or integrase required coinoculation with expression plasmids encoding either gamma interferon (IFN-γ), interleukin-12 (IL-12), or IL-18 to elicit protection against WT virus challenge (13, 23).
Similarly, various studies have demonstrated enhancement of simian immunodeficiency virus (SIV) or simian-human immunodeficiency virus (SHIV) DNA vaccine-elicited immune responses (3, 10) and improved vaccine efficacy against a pathogenic SHIV isolate (4) in rhesus macaques by the incorporation of cytokine expression plasmids. Th-1 cytokines, including IL-2, IFN-γ, IL-12, and IL-15 expression plasmids, have all been shown to augment antigen-specific T-cell responses when used as adjuvants for SIV/HIV DNA vaccines in both mice and nonhuman primates, although the amplitude of augmentation was not as consistent for primates (3, 10). More novel approaches for codelivery of HIV type 1 antigen and cytokines have included bicistronic plasmids that coexpress a single antigen and cytokine and plasmids that express an antigen-cytokine fusion protein (5, 8, 28, 30). Another strategy previously reported for coexpression of viral antigens and a cytokine adjuvant involved replacement of the viral nef gene with a specific cytokine gene within an SIV or SHIV genome to allow simultaneous expression of the virus and the cytokine, while also placing expression of the viral antigen and the cytokine adjuvant under similar regulatory constraints (15, 17, 20, 22, 33, 36). These studies revealed that SIV/SHIV isolates with nef deletions that coexpressed IFN-γ provided some protection against pathogenic virus challenge, while vaccine efficacy was less consistent for isolates that coexpressed IL-2.
Based on observations from multiple studies showing a positive immunomodulatory effect of IFN-γ on DNA vaccine efficacy, we constructed a modified FIV provirus with a vif gene deletion encoding the feline IFN-γ gene (FIVΔvifATGγ), which was shown to express IFN-γ and to be severely restricted for replication in vitro (21). In this study, we compared virus-specific cellular and humoral immune responses in cats immunized using different FIV-pPPRΔvif-based DNA vaccine approaches that incorporated IFN-γ as an adjuvant, including FIVΔvifATGγ, and assessed their protection against an early challenge with a homologous WT FIV isolate. Our findings revealed that immunization with FIV-pPPRΔvif-based DNA vaccines incorporating coexpression of IFN-γ resulted in enhanced vaccine-induced cellular immune responses in comparison to vaccination with FIV-pPPRΔvif only. In contrast to a previous study that demonstrated FIV-pPPRΔvif DNA vaccine-induced protection against a later WT FIV challenge, FIV-pPPRΔvif-vaccinated cats were not protected from WT virus challenge delivered within 13 weeks after immunization. Importantly, FIVΔvifATGγ immunization was also not protective against WT FIV challenge, despite enhanced cellular immune responses.
MATERIALS AND METHODS
Plasmids for vaccination.
The FIV-pPPRΔvif vif deletion mutant carrying a 375-bp deletion within the vif gene of the WT FIV-pPPR molecular clone was described previously (26). The construction and characterization of FIVΔvifATGγ and pCDNA-IFNγ, a mammalian expression vector for feline IFN-γ, were also described previously (21), and both plasmids were confirmed to express IFN-γ. Plasmid DNA stocks were prepared using a commercial Endofree kit (DNA Maxi prep; QIAGEN, Valencia, CA) and tested for endotoxin concentrations with a commercial kit (E-Toxate; Sigma, St. Louis, MO).
DNA immunizations and virus challenge.
Twenty juvenile, specific-pathogen-free cats, aged 9 to 12 months, were obtained from a commercial vendor (Harlan, Indianapolis, IN) and placed in four experimental groups. One group (n = 5) was inoculated by the intramuscular route with 600 μg of FIV-pPPRΔvif plasmid DNA resuspended in 1 ml of sterile physiological saline, a second group (n = 5) with 600 μg of FIVΔvifATGγ plasmid DNA resuspended in 1 ml of sterile physiological saline, and a third group (n = 5) with 300 μg each of FIV-pPPRΔvif and pCDNA-IFNγ plasmid DNA. Five cats inoculated with 1 ml sterile saline served as unvaccinated controls. Animals were assessed daily by physical examination and monitored for clinical signs. Blood samples were obtained every 2 to 4 weeks for hematological, virological, and immunological assays.
At 13 weeks following immunization, the cats were challenged by intramuscular injection with 10 50% cat infectious doses of a biological FIV-PPR virus stock prepared by sequential passage on feline peripheral blood mononuclear cells (PBMC). Blood samples were collected after challenge at time points similar to those described for vaccine DNA inoculation.
Virus detection.
Virus was isolated from primary PBMC cell cultures prepared from each cat as previously described (25). Extraction of DNA from PBMC harvested after inoculation with proviral DNA and of RNA from plasma collected after challenge was performed as described previously for assay of viral nucleic acid (31). Briefly, RNA was purified from 140 μl of plasma prepared from EDTA-treated whole blood using a QIAamp viral RNA mini kit (QIAGEN, Valencia, CA). Genomic DNA was isolated from PBMC using a QIAamp DNA blood mini kit (QIAGEN). Plasma RNA and PBMC DNA samples were assayed for FIV nucleic acid copy numbers using reverse transcription (RT) and quantitative real-time TaqMan PCR assays conducted on a 7700 ABI Prism sequence detector (Applied Biosystems, Foster City, CA) with FIV gag TaqMan probes and primer sequences by protocols previously described (16, 31).
Hematology and lymphocyte subset analysis.
Complete blood cell counts and differential leukocyte counts were performed on PBMC samples using standard methods. Enumeration of CD4 and CD8 peripheral blood lymphocyte subsets and determination of CD4:CD8 ratios were performed by flow cytometric analysis as previously described (1).
FIV-specific antibody detection.
Circulating FIV-specific antibodies in sera following inoculation and challenge were assayed by a commercial FIV antibody enzyme-linked immunosorbent assay (ELISA; IDEXX, Portland, ME). Serum samples collected from cats after vaccination and challenge were also analyzed for reactivity to native FIV envelope protein (Env) in a sensitive concanavalin A (ConA) ELISA (25). Antibody endpoint titers were calculated to be the last serial twofold dilution whose optical density at 450 nm (OD450) was twice that of normal cat serum. The avidity of plasma antibodies to native envelope proteins was determined by measuring the resistance of antibody-envelope glycoprotein complexes to 8 M urea in a ConA ELISA, adapting protocols previously described for SIV (11). The avidity index calculation was based on the following equation: (OD450 of phosphate-buffered saline-washed wells/OD450 of urea-washed wells) × 100%. The conformational dependence of FIV Env-specific antibodies was measured by comparing their reactivities to native and denatured viral glycoproteins in a ConA ELISA, adapting protocols used for assaying SIV Env proteins (11).
FIV-specific CTL assay.
FIV-specific cytotoxic-T-lymphocyte (CTL) activity was measured in PBMC isolated from cats at various time points after vaccination and challenge, as previously described (25). Briefly, the targets for CTL were autologous skin fibroblasts derived from skin biopsy specimens collected prior to vaccination. Stimulator cells prepared from autologous PBMC collected both prior to and following vaccination were infected with 5 PFU/cell of recombinant vaccinia virus expressing either FIV Gag (vFIV-gag) or FIV Env (vFIV-env) or of WT vaccinia virus (kindly provided by M. J. Burkhard, Ohio State University, Columbus, OH) and fixed with paraformaldehyde. PBMC serving as effector cells were incubated at 37°C with stimulator cells for 3 to 4 days in previously described PBMC medium (35) without IL-2 and then cultured for an additional 3 to 4 days at 37°C in PBMC medium supplemented with IL-2. Target cells were infected with WT vaccinia virus, vFIV-gag, or vFIV-env and then incubated with effector cells at various effector:target cell ratios. Cytotoxicity was measured by the release of lactate dehydrogenase using the CytoTox 96 kit (Promega Corporation, Madison, WI) according to the manufacturer's instructions.
FIV-specific T-cell proliferation assay.
FIV-specific T-cell proliferation activity in PBMC following immunization and challenge was measured with a flow cytometric method using a commercial bromodeoxyuridine (BrdU) flow kit (BD Biosciences, San Diego, CA). BrdU incorporated into proliferating cells was stained with anti-BrdU antibody labeled with fluorescein isothiocyanate and detected by flow cytometry. BrdU incorporation was coupled with staining for total DNA with 7-amino-actinomycin D. PBMC were isolated from peripheral blood of vaccinated cats at various time points after inoculation and challenge and plated at 106 cells/well in a 24-well tissue culture plate (Nunc, Inc., Naperville, IL) in PBMC medium without IL-2. Twenty-four hours later, 10 μg of T-cell mitogen ConA (Sigma), 1 μg of inactivated sucrose gradient-purified biological FIV-PPR, or 1 μg of inactivated sucrose gradient-purified tissue culture fluid (mock antigen) was added to cell cultures. Purified preparations of biological FIV-PPR were inactivated by treatment with aldrithiol-2 (Sigma) as previously described (32). Three days following incubation with viral antigen, the cells were stained overnight with 10 μM BrdU. The cells were harvested the next day, washed, fixed, and permeabilized using a BrdU flow kit according to the manufacturer's instructions. Finally, BrdU-treated cells were incubated with fluorescein isothiocyanate-labeled anti-BrdU antibody for 45 min at room temperature, stained with 7-amino-actinomycin D, and analyzed for fluorescence with a FACScan using the CellQuest program (Becton Dickinson, San Jose, CA). Data from 50,000 events for each sample were collected and analyzed.
RESULTS AND DISCUSSION
Cellular immune responses were induced by proviral DNA vaccination.
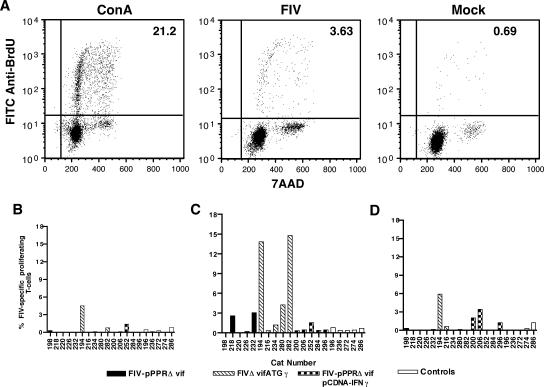
An effective antilentiviral vaccine must stimulate and sustain robust humoral and cellular immune responses to confer protection (7, 24). Accordingly, peripheral blood FIV-specific T-cell proliferation and CTL responses were compared for groups of cats vaccinated with FIV-pPPRΔvif, FIVΔvifATGγ, or FIV-pPPRΔvif and pCDNA-IFNγ. Lymphoproliferative responses to inactivated FIV were measured at 2, 6, and 10 weeks after inoculation (Fig. 1). Dot plots representative of data generated by this newly developed FIV-specific T-cell proliferation assay are shown for a vaccinated cat in Fig. 1A. Staining of BrdU-positive cells with anti-CD4 and anti-CD8 monoclonal antibodies revealed that a large proportion (60% to 70%) of the proliferating cells were CD4 T cells (data not shown), as would be expected with antigen stimulation provided by an inactivated virus preparation. Virus-specific T-cell proliferation responses were detected in only one vaccinated cat at 2 weeks after inoculation (Fig. 1B), peaked at 6 weeks after inoculation (Fig. 1C), and declined by 10 weeks after inoculation (Fig. 1D). By 6 weeks following inoculation, two cats vaccinated with FIV-pPPRΔvif and three cats vaccinated with FIVΔvifATGγ exhibited FIV-specific T-cell proliferation responses, with the responses of greater magnitude being induced by the FIVΔvifATGγ vaccine. By 10 weeks after inoculation, FIV-specific lymphoproliferative responses declined to insignificant levels for most cats, with the exception of two cats coimmunized with FIV-pPPRΔvif and pCDNA-IFNγ and one cat vaccinated with FIVΔvifATGγ. Although marked differences were not detected in lymphoproliferative responses induced by the different vaccine approaches, immunization with FIVΔvifATGγ or with FIV-pPPRΔvif and pCDNA-IFNγ produced a slightly higher frequency of FIV-specific responses than immunization with FIV-pPPRΔvif alone (Fig. 1B to D). It is important to note that all cats inoculated with FIV-pPPRΔvif or FIVΔvifATGγ remained negative for virus infection as determined by a PBMC virus isolation assay and a real-time PCR assay for PBMC-associated viral DNA (data not shown). These findings indicate that FIV-pPPRΔvif DNA-based vaccines induced FIV-specific T-cell proliferative responses in a proportion (7 out of 15) of vaccinated cats despite the lack of a detectable viremia.
FIG. 1.
Measurement of FIV-specific T-cell proliferation responses following vaccination. (A) For a depiction of a representative flow cytometric T-cell proliferation assay used to measure virus-specific T-cell proliferation responses, scatter plots that show staining for BrdU in PBMC harvested from an FIVΔvifATGγ-vaccinated cat and stimulated with ConA, aldrithiol-2-inactivated whole FIV-pPPR virus preparation (FIV), or mock antigen, are provided. The value for the percentage of PBMC that are proliferating is shown in the upper right quadrant of each scatter plot. FITC, fluorescein isothiocyanate; 7AAD, 7-amino-actinomycin D. FIV-specific T-cell proliferation responses are shown for each experimental group at 2 weeks (B), 6 weeks (C), and 10 weeks (D) following vaccination. Data shown for the percentage of FIV-specific proliferating T cells represent the value measured from PBMC stimulated with FIV minus the value measured for PBMC stimulated with mock antigen. A significant proliferation response was defined as a value equal to, or greater than, 2% FIV-specific proliferating T cells.
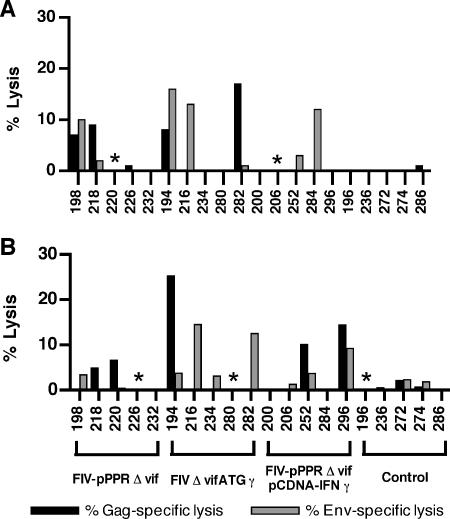
Virus-specific CTL activities of PBMC were measured for cats at 2, 6, and 10 weeks following immunization. Overall, CTL activities measured at 2 and 6 weeks after inoculation were comparable (Fig. 2). The highest frequencies of CTL activity were observed in FIVΔvifATGγ proviral DNA-vaccinated cats, with three out of five cats expressing FIV-Gag- or Env-specific CTL activity at both 2 and 6 weeks after vaccination. FIV-specific CTL responses were also observed in cats coimmunized with FIV-pPPRΔvif and pCDNA-IFNγ plasmid DNA, including one cat at 2 weeks and two cats at 6 weeks postimmunization. In contrast, CTL activity was observed in only one cat vaccinated with FIV-pPPRΔvif DNA, for one early time point postimmunization. By 10 weeks after vaccination, PBMC CTL activity was less frequent and was detectable in only two cats, both of which were in the FIV-pPPRΔvif vaccine group (data not shown). Measurable FIV-specific CTL activity was not detected in any of the unvaccinated control cats. In general, these results revealed no appreciable differences in magnitude of CTL activity, with small differences in frequency of virus-specific CTL responses elicited by the different vaccine strategies over time postimmunization. However, detection of FIV-specific CTL responses in three of the FIVΔvifATGγ-vaccinated cats at both early time points tested postvaccination suggested that the IFN-γ-expressing provirus may possibly expedite virus-specific cellular immune responses.
FIG. 2.
Detection of FIV Gag- and Env-specific CTL following inoculation. Virus-specific CTL responses were measured in restimulated lymphocytes isolated from peripheral blood at 2 weeks (A) and 6 weeks (B) following immunization. Autologous skin fibroblasts served as target cells and were infected with vFIV-env or vFIV-gag or with wild-type (data not shown) vaccinia virus stocks. Release of lactate dehydrogenase was measured to assay specific lysis as described in Materials and Methods. Results shown are the mean values for triplicate cultures at an effector-to-target cell ratio of 12.5:1 or 25:1 and represent specific lysis values measured for FIV-Env or FIV-Gag target cells after values measured for targets infected with wild-type vaccinia virus are subtracted. A significant CTL response was defined as a value greater than or equal to 10% lysis. An asterisk indicates that data for that cat are not available.
Humoral immune responses were not induced by FIV-pPPRΔvif proviral DNA immunization.
None of the cats vaccinated with an FIV-pPPRΔvif-based DNA vaccine demonstrated detectable antiviral antibody responses during the 13-week time period following inoculation (data not shown). The absence of anti-p24Gag antibody detectable by standard serological assays was previously reported for monkeys inoculated with a SIVmac239Δvif virus (12) and for cats inoculated with FIV-pPPRΔvif DNA (25). However, in both of these previous studies, detection of virus-specific antibodies was possible by using either conditions that increase assay sensitivity, as described for an SIV antibody ELISA (12), or an ultrasensitive ConA ELISA that measures FIV Env-specific antibody reactive to FIV-PPR native Env protein captured from purified virus preparations (25). In the present study, FIV Env antibody titers measured for all vaccinated and unvaccinated cats after vaccination were considered negative due to the high background reactivity of serum samples observed at a prevaccination time point. This high background reactivity prior to FIV immunization was attributed to the vaccination of all specific-pathogen-free cats within the study with a commercial feline vaccine containing antigens prepared from feline cell culture systems. Importantly, this finding contrasted with a previous observation of FIV-pPPRΔvif immunization-induced Env-specific antibody (25). A possible delay in the emergence of vaccine-induced antibody responses to a time point sometime after 12 weeks postvaccination could not be evaluated, since this study was designed to evaluate protection against an early challenge. Importantly, the absence of any detectable humoral immune responses may have resulted from a reduction of virus expression or virus replication from the proviral DNA vaccine preparations with vif deleted that were used in this study and might reflect an important reduction in vaccine immunogenicity.
FIV-pPPRΔvif-based vaccines did not elicit protection against infection after challenge with wild-type FIV-PPR.
Cats were challenged with 10 50% cat infectious doses of an infectious uncloned FIV-PPR virus stock at 13 weeks after inoculation. The animals were monitored for clinical signs of acute FIV infection, viremia, anti-FIV antibody, and PBMC FIV-specific CTL and T-cell proliferation activity until 12 weeks after challenge. Notable clinical disease or decreases in CD4:CD8 T-cell ratios were not observed after challenge in either the vaccinated or the unvaccinated control cats (data not shown). The absence of CD4:CD8 T-cell ratio alterations observed in unvaccinated control cats was consistent with previous observations in which CD4 T-cell alterations were associated with later stages of infection with the FIV-PPR biological isolate (35).
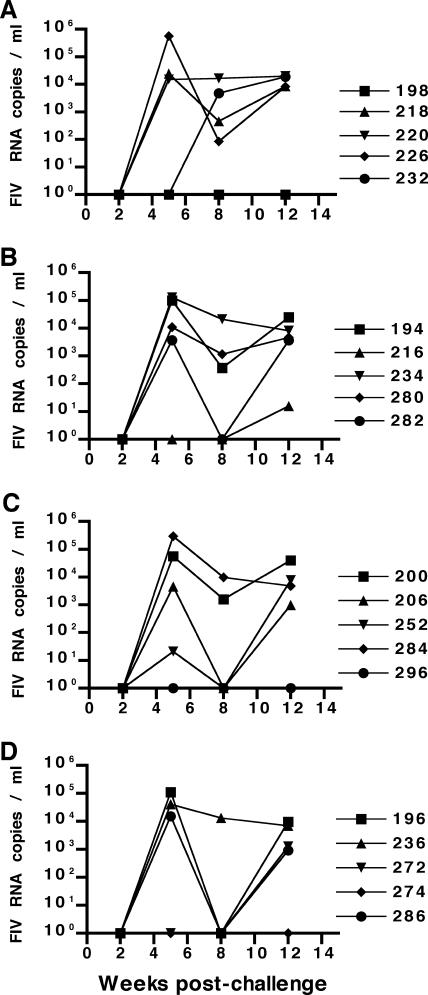
Four out of five cats in each experimental vaccine group and from the unvaccinated control group were viremic when assayed by PBMC virus isolation at 5 weeks after challenge and remained viremic through the end of the study at 12 weeks after challenge (data not shown). Virus in peripheral blood was not detected in one cat from each of the vaccine groups and also in one cat within the unvaccinated control group by either PBMC virus isolation or real-time PCR assay for plasma-associated viral RNA for the 12-week time period after challenge (Fig. 3). Given the distribution of nonviremic cats among the different vaccine and control groups after challenge, the absence of detectable virus load could not be attributed to a specific vaccine approach. However, all four nonviremic cats seroconverted following virus challenge, as measured by a commercial FIV Gag antibody ELISA (data not shown), confirming that these cats were also infected by challenge virus. Plasma viral RNA loads in viremic vaccinated cats following challenge were similar to loads measured for viremic unvaccinated control cats, with peak concentrations occurring between 5 and 12 weeks after challenge and ranging between 103 and 5 × 105 copies/ml plasma (Fig. 3). Vaccination with any of the FIV-pPPRΔvif-based vaccine approaches did not protect cats against infection or significantly reduce virus loads after an early intramuscular challenge with homologous virus delivered at 13 weeks after vaccination. Furthermore, inclusion of plasmids containing IFN-γ did not enhance the efficacy of the FIV-pPPRΔvif provirus-based DNA vaccine.
FIG. 3.
Measurement of plasma virus load (copies of viral RNA/ml plasma). Plasma viremia after challenge was assessed by a real-time RT-PCR assay for FIV gag RNA and is measured in FIV RNA copies per ml of plasma. Plasma samples harvested from cats vaccinated with FIV-pPPRΔvif (A), FIVΔvifATGγ (B), or FIV-pPPRΔvif and pCDNA-IFNγ (C) and from unvaccinated control cats (D) were tested at 2, 5, 8, and 12 weeks postchallenge. The limit of detection of this real-time RT-PCR assay for FIV RNA is 50 copies per ml. Cat identification numbers are shown on the right.
Virus load after challenge did not correlate with FIV-specific cellular immune responses.
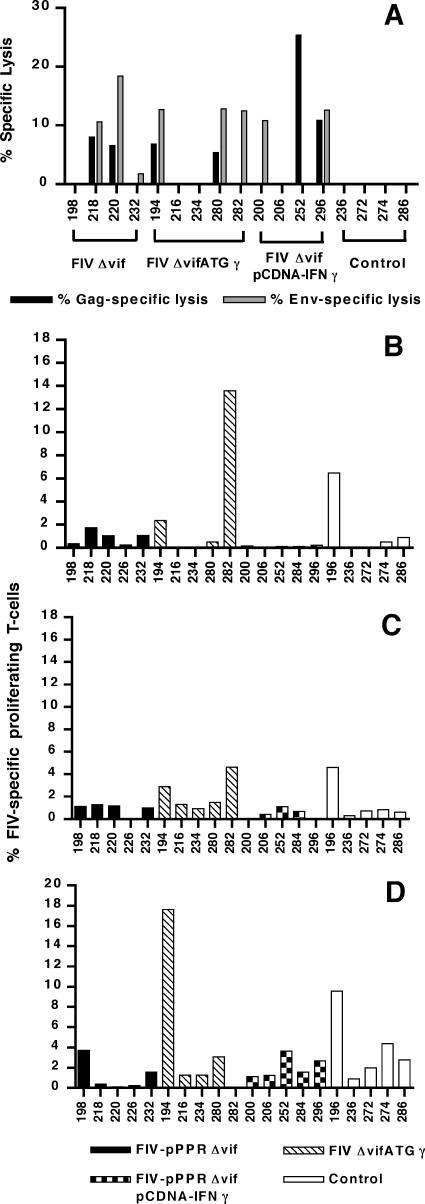
FIV-specific PBMC CTL activity responses were assayed at 1 week and at either 3 or 5 weeks after challenge, to determine the correlation of cellular responses with virus load. Appreciable differences in memory CTL activity were not observed among the vaccinated groups for early time points after challenge. By 1 week after challenge, either Env- or Gag-specific memory CTL responses were detected in only two vaccinated cats, including one cat vaccinated with FIV-pPPRΔvif (cat 18) and one cat coimmunized with FIV-pPPRΔvif and pCDNA-IFNγ (cat 284) (data not shown). Virus-specific CTL responses were detected in 5 of the 10 vaccinated cats tested at 3 weeks after challenge, including 2 cats vaccinated with FIV-pPPRΔvif and 3 cats vaccinated with FIVΔvifATGγ (Fig. 4A). Similarly, three cats coimmunized with FIV-pPPRΔvif and pCDNA-IFNγ were found to be positive for virus-specific CTL responses at 5 weeks after challenge. The absence of virus-specific CTL activity in all unvaccinated control cats by 5 weeks after challenge, despite the observation of viremia in four of these five cats, suggested that the postchallenge CTL responses detected for vaccinated cats might represent anamnestic responses. An FIV-specific CTL response after challenge was detected in only one of the three vaccinated cats that remained nonviremic after challenge, although CTL activity was detected in all three cats prior to challenge (Fig. 2). However, four vaccinated cats that were viremic after challenge also demonstrated CTL responses prior to challenge. Thus, CTL responses measured either after vaccination or after challenge did not correlate with reduced virus loads after challenge.
FIG. 4.
Detection of FIV-specific cellular immune responses after challenge. (A) FIV Gag- and Env-specific CTL activity was measured at 3 or 5 weeks postchallenge. Values for percentage of lysis shown for cats vaccinated with FIV-pPPRΔvif and FIVΔvifATGγ reflect CTL activity measured at 3 weeks after challenge. Values shown for cats vaccinated with FIV-pPPRΔvif and pCDNA-IFNγ and for control cats represent CTL activity measured at 5 weeks after challenge. A significant CTL response was defined as a value greater than or equal to 10% lysis. Data for cats 196, 226, and 284 are not available. FIV-specific T-cell proliferation was assessed at 1 week (B), 3 weeks (C), and 6 weeks (D) following challenge. A significant proliferation response was defined as a value greater than or equal to 2% FIV-specific proliferating T cells.
FIV-specific peripheral blood T-cell proliferative responses were also assayed in cats at 1, 3, and 6 weeks following challenge. At 1 and 3 weeks after challenge, proliferative responses were detected in two of the FIVΔvifATGγ-vaccinated cats and one unvaccinated control cat (Fig. 4B and C). By 6 weeks after challenge, T-cell proliferative responses were detected in one cat vaccinated with FIV-pPPRΔvif, two cats vaccinated with FIVΔvifATGγ, two cats coimmunized with FIV-pPPRΔvif and pCDNA-IFNγ, and four unvaccinated control cats (Fig. 4D). Although the frequency of proliferative responses at early time points after challenge (1 and 3 weeks) was higher for the FIVΔvifATGγ-vaccinated group, these responses did not correlate with protection from viremia after challenge. Furthermore, detection of a strong T-cell proliferation response in an unvaccinated cat at 1 week postchallenge suggested that proliferation activity did not represent anamnestic T-cell responses but instead resulted from ongoing challenge virus replication in the host. Interestingly, three of the four antibody-positive, nonviremic cats exhibited T-cell proliferative responses at 6 weeks after challenge. Detection of FIV-specific lymphoproliferative activity, along with antiviral antibody (see below), in these nonviremic cats most likely reflected low-level challenge virus replication. Also of importance, vaccinated cats remaining nonviremic after challenge had not exhibited FIV-specific lymphoproliferative responses before challenge. Therefore, detection of FIV-specific T-cell proliferation responses after either vaccination or challenge did not correlate with resistance to challenge virus infection. Collectively, these data do not show a relationship between cellular immune responses measured postvaccination and postchallenge and peripheral blood virus load after challenge. However, assays of virus-specific CTL and T-cell proliferative responses in lymphoid tissues were not performed and may be necessary to fully elucidate and compare cellular immune responses associated with the different vaccine strategies and with resistance to challenge.
Major differences in virus-specific humoral immune responses between vaccine groups were not observed following challenge.
As stated above, all cats (vaccinated and unvaccinated) seroconverted following virus challenge, based on the results of a commercial ELISA for detection of FIV Gag antibody (data not shown). This finding confirmed that the four cats remaining nonviremic after challenge were infected by the challenge virus. FIV Env-specific antibodies were also detected in vaccinated and control cats after challenge when tested with a ConA ELISA based on native FIV-PPR Env protein captured from purified virus. However, significant differences in antibody titers measured for vaccinated compared to unvaccinated control cats were not observed (Fig. 5). The time of appearance of Env-specific antibodies varied from 2 to 12 weeks after challenge, and no apparent differences were observed in time of emergence of these antibodies between vaccinated and unvaccinated cats. Two of the four cats that remained nonviremic after challenge exhibited FIV Env-specific antibody titers comparable to those measured for virus-positive cats. Interestingly, the two remaining nonviremic cats (cats 216 and 296) exhibited very low FIV Env antibody titers at 12 weeks after challenge. In the face of such low Env antibody titers, serum samples from these two cats were not assayed for Env antibody avidity and conformation dependence.
FIG. 5.
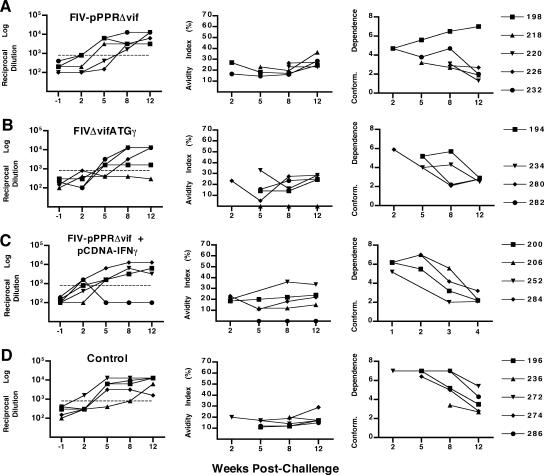
Measurement of envelope-specific antibody responses. Envelope-specific serum antibody responses measured by an FIV ConA ELISA are shown for time points 12 weeks postvaccination (or 1 week prior to challenge) and 2, 5, 8, and 12 weeks postchallenge for cats vaccinated with FIV-pPPRΔvif (A), FIVΔvifATGγ (B), or FIV-pPPRΔvif and pCDNA-IFNγ (C) and for unvaccinated control cats (D). The first column of graphs shows reciprocal endpoint FIV Env antibody titers as measured by reactivity to native viral glycoprotein captured from purified FIV-PPR virus in a ConA ELISA. Due to the high background reactivity measured at preimmunization time points for a proportion of the cats, a reciprocal log dilution value greater than 800 (horizontal dashed line) is considered significant. The second column of graphs shows FIV Env antibody avidity indices measured by a modified ConA ELISA for each experimental group, as described in Materials and Methods. Conformational dependence of serum antibodies to FIV Env is shown in the third column of graphs and reflects the ratio of serum reactivity to native viral glycoproteins to that to denatured viral glycoproteins, as measured by the ConA ELISA. Avidity and conformational dependence of FIV Env antibodies were determined only at postchallenge time points and for those serum samples demonstrating an OD at 450 nm of 1.0 or greater at a 1:50 dilution of serum (generally at Env antibody titers of 1:1,000 or greater). As a result, avidity indices and conformation ratio values for serum samples from cats 216 (B) and 296 (C) were not determined. Results shown represent the mean values for two or more assays. Cat identification numbers are to the right.
The avidity and conformational dependence of anti-FIV Env antibodies detected after challenge were also assayed, and the findings for the vaccine groups were compared. The Env-specific antibody avidity index was measured by the relative stabilities of the antigen-antibody complexes exposed to 8 M urea to assess the maturation of humoral immune responses. Increasing avidity values after challenge were observed over time for FIV Env antibodies in the majority of vaccinated cats, with increases observed by 8 to 12 weeks after challenge (Fig. 5A to C), compared to 12 weeks postchallenge for unvaccinated control cats (Fig. 5D). The conformation ratio is a direct measure of the conformational dependence of a particular antibody sample, where conformation ratios of >1 reflect a predominant reactivity with native envelope glycoproteins and conformation ratios of <1 reflect a predominant antibody reactivity with denatured envelope glycoproteins. The predominant trend for all vaccinated groups after immunization was a decrease in conformation ratios by 8 to 12 weeks after challenge, indicating a broadening of FIV-Env antibody response. Furthermore, decreases in conformation ratios by 8 weeks postchallenge were observed predominantly in cats vaccinated with FIVΔvifATGγ or covaccinated with FIV-pPPRΔvif and pCDNA-IFNγ. Therefore, although no measurable antibody responses were detected in vaccinated cats after immunization, vaccination with FIV-pPPRΔvif-based DNA vaccines appeared to prime the immune system so that the secondary antibody responses were slightly more rapid in vaccinated cats. However, differences in Env antibody responses measured for vaccinated cats compared to those for unvaccinated cats were small and did not correlate with protection against virus load after FIV challenge.
The vaccine efficacy observed for the FIV-pPPRΔvif-based DNA vaccines tested in this investigation was abrogated in comparison to that observed in a previous FIV-pPPRΔvif DNA vaccine study (25). However, a comparison of the two vaccine studies reveals differences in experimental variables, including differences in experimental design and methods of preparation of vaccine DNA. In the present study, all vaccinated cats were administered an early wild-type FIV challenge at 13 weeks following a single priming immunization. In contrast, FIV-pPPRΔvif-vaccinated cats were challenged approximately 10 months after inoculation in the previous study and received a booster DNA immunization prior to challenge. Immunization by a single inoculation of FIV-pPPRΔvif DNA 13 weeks prior to challenge may not be sufficient for developing efficacious immune responses against challenge virus. Maturation of vaccine-induced immune responses may require a time period longer than 13 weeks as well as vaccine boosting (12). Another variable includes differences in route of challenge virus delivery. Challenge virus was administered by intramuscular inoculation for the present study and by intraperitoneal injection for the previous study. Differences in vaccine efficacy imposed by intraperitoneal versus intramuscular routes of challenge virus delivery have not been well examined in FIV vaccine studies, but a few reports (12a, 27) have described differences in vaccine efficacy based on route of challenge. Lastly, vaccine plasmid DNA stocks were prepared by centrifugation in cesium chloride-ethidium bromide gradients for the previous study and were associated with a transient endotoxin response upon the initial priming immunization (25). Plasmid DNA used for immunization in the present study was prepared with a commercial Endofree kit (QIAGEN). Proviral plasmid DNA prepared by a cesium chloride gradient may differ in either quantity or type of bacterial impurities in comparison to DNA prepared by an endotoxin-free commercial kit. Contaminating bacterial impurities, including bacterial DNA with CpG motifs, lipopolysaccharides, or other bacterial proteins, may have altered the immunogenicity of the vaccine plasmid DNA and may have affected early virus expression or replication after proviral DNA inoculation. This possibility is further supported by the absence of vaccine-induced FIV Env antibody in the present study, compared to a positive detection of vaccine-induced Env antibody by the same ConA ELISA in the previous FIV-pPPRΔvif DNA vaccine study (25). Altogether, these variables, including maturation and boosting of proviral DNA vaccine-induced immune responses, routes for delivery of challenge virus, methods of vaccine DNA preparation, and absence of vaccine-induced Env antibodies, warrant future examination as factors for the efficacy of a vif deletion proviral DNA vaccine.
Despite limited evidence that IFN-γ augmented virus-specific cellular immune responses, FIV-pPPRΔvif-based proviral DNA vaccines incorporating IFN-γ did not induce protection or resistance to wild-type FIV challenge or achieve reduced virus loads after challenge. Although obvious differences in replication for FIV-pPPRΔvif and FIVΔvifATGγ were not observed in vitro (21), coexpression of IFN-γ may have induced a subtle restriction of virus expression of an already severely attenuated FIV-pPPRΔvif provirus in vivo, due to its potent antiviral properties. Further reduction of virus replication or expression imposed by coexpression of IFN-γ could potentially reduce or counteract the positive adjuvant effects of this cytokine and thereby produce a negative effect on the efficacy of this particular proviral DNA vaccine. However, it is important to note that the efficacy of IFN-γ as an adjuvant could differ with alternative vaccine protocols or challenge conditions. Regardless, these findings indicate the need to assess other cytokines that may enhance both cellular and humoral immune responses for use as an adjuvant for this DNA vaccine approach. Highly attenuated mutant FIV proviruses that encode a cytokine provide an opportunity to evaluate vaccines incorporating lentivirus-regulated expression of cytokines as vaccine adjuvants and warrant further testing in this animal model.
Acknowledgments
We gratefully acknowledge the expert technical assistance of Anthony Dang, Hung Kieu, Joanne Higgins, and Jenni Byerly. We also acknowledge Mary Jo Burkard for the provision of vaccinia virus stocks, Carol Oxford for technical assistance with flow cytometric assays, Jill Mikovich for animal care, Gary Rhodes and Paul Luciw for insightful suggestions for the project, and Niels Pedersen for constructive comments on the manuscript.
These studies were supported by the George and Phyllis Miller Feline Health Fund; Center for Companion Animal Health, School of Veterinary Medicine, University of California, Davis; and by National Institutes of Health grants R01AI40896 and R21AI46306 (E. E. Sparger) and R01AI29243 (K. S. Cole).
Footnotes
Published ahead of print on 1 November 2006.
REFERENCES
- 1.Barlough, J. E., C. D. Ackley, J. W. George, N. Levy, R. Acevedo, P. F. Moore, B. A. Rideout, M. D. Cooper, and N. C. Pedersen. 1991. Acquired immune dysfunction in cats with experimentally induced feline immunodeficiency virus infection: comparison of short-term and long-term infections. J. Acquir. Immune Defic. Syndr. 4:219-227. [PubMed] [Google Scholar]
- 2.Barouch, D. H., and M. L. Letvin. 2000. DNA vaccination for HIV-1 and SIV. Intervirology 43:282-287. [DOI] [PubMed] [Google Scholar]
- 3.Barouch, D. H., N. L. Letvin, and R. A. Seder. 2004. The role of cytokine DNAs as vaccine adjuvants for optimizing cellular immune responses. Immunol. Rev. 202:266-274. [DOI] [PubMed] [Google Scholar]
- 4.Barouch, D. H., S. Santra, J. E. Schmitz, M. J. Kuroda, T.-M. Fu, W. Wagner, M. Bilska, A. Craiu, X. X. Zheng, G. R. Krivulka, K. Beaudry, M. A. Lifton, C. E. Nickerson, W. L. Trigona, K. Punt, D. C. Freed, L. Guan, S. Dubey, D. Casimiro, A. Simon, M.-E. Davies, M. Chastain, T. B. Strom, R. S. Gelman, D. C. Montefiori, M. G. Lewis, E. A. Emini, J. W. Shiver, and N. L. Letvin. 2000. Control of viremia and prevention of clinical AIDS in rhesus macaques by cytokine-augmented DNA vaccination. Science 290:486-491. [DOI] [PubMed] [Google Scholar]
- 5.Barouch, D. H., S. Santra, K. Tenner-Racz, P. Racz, M. J. Kuroda, J. E. Schmitz, S. S. Jackson, M. A. Lifton, D. C. Freed, H. C. Perry, M. E. Davies, J. W. Shiver, and N. L. Letvin. 2002. Potent CD4+ T cell responses elicited by a bicistronic HIV-1 DNA vaccine expressing gp120 and GM-CSF. J. Immunol. 168:562-568. [DOI] [PubMed] [Google Scholar]
- 6.Bendinelli, M., M. Pistello, S. Lombari, A. Poli, C. Garzelli, D. Matteucci, L. Ceccherini-Nelli, G. Malvaldi, and F. Tozzini. 1995. Feline immunodeficiency virus: an interesting model for AIDS studies and an important cat pathogen. Clin. Microbiol. Rev. 8:87-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berzofsky, J. A., J. D. Ahlers, J. Janik, J. Morris, S. Oh, M. Terabe, and I. M. Belyakov. 2004. Progress on new vaccine strategies against chronic viral infections. J. Clin. Investig. 114:450-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biragyn, A., I. M. Belyakov, Y. H. Chow, D. S. Dimitrov, J. A. Berzofsky, and L. W. Kwak. 2002. DNA vaccines encoding human immunodeficiency virus-1 glycoprotein 120 fusions with proinflammatory chemoattractants induce systemic and mucosal immune responses. Blood 100:1153-1159. [DOI] [PubMed] [Google Scholar]
- 9.Burkhard, M. J., and G. A. Dean. 2003. Transmission and immunopathogenesis of FIV in cats as a model for HIV. Curr. HIV Res. 1:15-29. [DOI] [PubMed] [Google Scholar]
- 10.Calarota, S. A., and D. B. Weiner. 2004. Enhancement of human immunodeficiency virus type 1-DNA vaccine potency through incorporation of T-helper 1 molecular adjuvants. Immunol. Rev. 199:84-99. [DOI] [PubMed] [Google Scholar]
- 11.Cole, K. S., J. L. Rowles, B. A. Jagerski, M. Murphey-Corb, T. Unangst, J. E. Clements, J. Robinson, M. S. Wyand, R. C. Desrosiers, and R. C. Montelaro. 1997. Evolution of envelope-specific antibody responses in monkeys experimentally infected or immunized with simian immunodeficiency virus and its association with the development of protective immunity. J. Virol. 71:5069-5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desrosiers, R. C., J. D. Lifson, J. S. Gibbs, S. C. Czajak, A. Y. M. Howe, L. O. Arthur, and R. P. Johnson. 1998. Identification of highly attenuated mutants of simian immunodeficiency virus. J. Virol. 72:1431-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12a.Dunham, S., J. Bruce, S. MacKay, M. Golder, O. Jarrett, and J. Neil. 2004. Abstr. 7th Int. Feline Retrovir. Res. Symp., abstr. Va 1.2. Polo Didattico G. Carmignani, Pisa, Italy.
- 13.Dunham, S. T., J. N. Flynn, M. A. Rigby, J. MacDonald, J. Bruce, C. Cannon, M. C. Golder, L. Hanlon, D. A. Harbour, N. A. Mackay, N. Spibey, O. Jarrett, and J. C. Neil. 2002. Protection against feline immunodeficiency virus using replication defective proviral DNA vaccines with feline interleukin-12 and -18. Vaccine 20:1483-1496. [DOI] [PubMed] [Google Scholar]
- 14.Egan, M. A., W. A. Charini, M. J. Kuroda, J. E. Schmitz, P. Racz, K. Tenner-Racz, K. Manson, M. Wyand, M. A. Lifton, C. E. Nickerson, T. Fu, J. W. Shiver, and N. L. Letvin. 2000. Simian immunodeficiency virus (SIV) gag DNA-vaccinated rhesus monkeys develop secondary cytotoxic T-lymphocyte responses and control viral replication after pathogenic SIV infection. J. Virol. 74:7485-7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Enose, Y., M. Kita, T. Yamamoto, H. Suzuki, A. Miyake, R. Horiuchi, K. Ibuki, K. Kaneyasu, T. Kuwata, E. Takahashi, K. Sakai, K. Shinohara, T. Miura, and M. Hayami. 2004. Protective effects of nef-deleted SHIV or that having IFN-gamma against disease induced with a pathogenic virus early after vaccination. Arch. Virol. 149:1705-1720. [DOI] [PubMed] [Google Scholar]
- 16.Gemeniano, M. C., E. T. Sawai, C. M. Leutenegger, and E. E. Sparger. 2003. Feline immunodeficiency virus Orf-A is required for virus particle formation and virus infectivity. J. Virol. 77:8819-8830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giavedoni, L. D., and T. Yilma. 1996. Construction and characterization of replication-competent simian immunodeficiency virus vectors that express gamma interferon. J. Virol. 70:2247-2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giri, M., K. E. Ugen, and D. B. Weiner. 2004. DNA vaccines against human immunodeficiency virus type 1 in the past decade. Clin. Microbiol. Rev. 17:370-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gorelick, R. J., R. E. Benveniste, J. D. Lifson, J. L. Yovandich, W. R. Morton, L. Kuller, B. M. Flynn, B. A. Fisher, J. L. Rossio, M. Piatak, Jr., J. W. Bess, Jr., L. E. Henderson, and L. O. Arthur. 2000. Protection of Macaca nemestrina from disease following pathogenic simian immunodeficiency virus (SIV) challenge: utilization of SIV nucleocapsid mutant DNA vaccines with and without an SIV protein boost. J. Virol. 74:11935-11949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gundlach, B. R., H. Linhart, U. Dittmer, S. Sopper, S. Reiprich, D. Fuchs, B. Fleckenstein, G. Hunsmann, C. Stahl-Hennig, and K. Überla. 1997. Construction, replication, and immunogenic properties of a simian immunodeficiency virus expressing interleukin-2. J. Virol. 71:2225-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta, S., C. Leutenegger, G. Dean, and E. Sparger. 2006. Construction and characterization of feline immunodeficiency proviral mutants that co-express interferon gamma and green fluorescent protein. AIDS Res. Hum. Retrovir. 22:342-349. [DOI] [PubMed] [Google Scholar]
- 22.Horiuchi, R., W. Akahata, T. Kuwata, Y. Enose, E. Ido, H. Suzuki, A. Miyake, N. Saito, K. Ibuki, T. Goto, T. Miura, and M. Hayami. 2006. DNA vaccination of macaques by a full-genome SHIV plasmid that has an IL-2 gene and produces non-infectious virus particles. Vaccine 24:3677-3685. [DOI] [PubMed] [Google Scholar]
- 23.Hosie, M. J., J. N. Flynn, M. A. Rigby, C. Cannon, T. Dunsford, N. A. Mackay, D. Argyle, B. J. Willett, T. Miyazawa, D. E. Onions, O. Jarrett, and J. C. Neil. 1998. DNA vaccination affords significant protection against feline immunodeficiency virus infection without inducing detectable antiviral antibodies. J. Virol. 72:7310-7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Letvin, N. L. 2005. Progress toward an HIV vaccine. Annu. Rev. Med. 56:213-223. [DOI] [PubMed] [Google Scholar]
- 25.Lockridge, K., M. Chien, P. A. Luciw, and E. E. Sparger. 2000. Protective immunity against feline immunodeficiency virus induced by inoculation with vif-deleted proviral DNA. Virology 273:67-79. [DOI] [PubMed] [Google Scholar]
- 26.Lockridge, K., S. Himathongkham, E. T. Sawai, M. Chien, and E. E. Sparger. 1999. The feline immunodeficiency virus vif gene is required for productive infection of feline peripheral blood mononuclear cells and monocyte-derived macrophages. Virology 261:25-30. [DOI] [PubMed] [Google Scholar]
- 27.Matteucci, D., M. Pistello, P. Mazzetti, S. Giannecchini, P. Isola, A. Merico, L. Zaccaro, A. Rizzuti, and M. Bendinelli. 1999. AIDS vaccination studies using feline immunodeficiency virus as a model: immunisation with inactivated whole virus suppresses viraemia levels following intravaginal challenge with infected cells but not following intravenous challenge with cell-free virus. Vaccine 18:119-130. [DOI] [PubMed] [Google Scholar]
- 28.McCormick, A. L., M. S. Thomas, and A. W. Heath. 2001. Immunization with an interferon-gamma-gp120 fusion protein induces enhanced immune responses to human immunodeficiency virus gp120. J. Infect. Dis. 184:1423-1430. [DOI] [PubMed] [Google Scholar]
- 29.Muthumani, K., M. Bagarazzi, D. Conway, D. S. Hwang, K. Manson, R. Ciccarelli, Z. Israel, D. C. Montefiori, K. Ugen, N. Miller, J. Kim, J. Boyer, and D. B. Weiner. 2003. A Gag-Pol/Env-Rev SIV239 DNA vaccine improves CD4 counts, and reduces viral loads after pathogenic intrarectal SIV(mac)251 challenge in rhesus Macaques. Vaccine 21:629-637. [DOI] [PubMed] [Google Scholar]
- 30.Nimal, S., A. L. McCormick, M. S. Thomas, and A. W. Heath. 2005. An interferon gamma-gp120 fusion delivered as a DNA vaccine induces enhanced priming. Vaccine 23:3984-3990. [DOI] [PubMed] [Google Scholar]
- 31.Pedersen, N. C., C. M. Leutenegger, J. C. Woo, and J. Higgins. 2001. Virulence differences between two field isolates in feline immunodeficiency virus (FIV-APetaluma and FIV-CpGammar) in young adult specific pathogen free cats. Vet. Immunol. Immunopathol. 79:53-67. [DOI] [PubMed] [Google Scholar]
- 32.Rossio, J. L., M. T. Esser, K. Suryanarayana, D. K. Schneider, J. W. Bess, G. M. Vasquez, T. A. Wiltrout, E. Chertova, M. K. Grimes, Q. Sattentau, L. O. Arthur, L. E. Henderson, and A. R. Lifson. 1998. Inactivation of human immunodeficiency virus type 1 infectivity with preservation of conformational and functional integrity of virion surface proteins. J. Virol. 72:7992-8001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sawai, E. T., M. S. Hamza, M. Ye, K. E. S. Shaw, and P. A. Luciw. 2000. Pathogenic conversion of live attenuated simian immunodeficiency virus vaccines is associated with expression of truncated Nef. J. Virol. 74:2038-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh, D. K., Z. Liu, D. Sheffer, G. A. Mackay, M. Smith, S. Dhillon, R. Hegde, F. Jia, I. Adany, and O. Narayan. 2005. A noninfectious simian/human immunodeficiency virus DNA vaccine that protects macaques against AIDS. J. Virol. 79:3419-3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sparger, E. E., A. M. Beebe, N. Dua, J. Elder, S. Himathongkam, M. Torten, and J. Higgins. 1994. Infection of cats with molecularly cloned and biological isolates of the feline immunodeficiency virus. Virology 205:546-553. [DOI] [PubMed] [Google Scholar]
- 36.Stahl-Hennig, C., B. R. Gundlach, U. Dittmer, P. Haaft, J. Heeney, W. Zou, D. Emilie, S. Sopper, and K. Uberla. 2003. Replication, immunogenicity, and protective properties of live-attenuated simian immunodeficiency viruses expressing interleukin-4 and interferon-γ. Virology 305:473-485. [DOI] [PubMed] [Google Scholar]
- 37.Wang, S.-W., P. A. Kozlowski, G. Schmelz, K. Manson, M. S. Wyand, R. Glickman, D. Montefiori, J. D. Lifson, R. P. Johnson, M. R. Neutra, and A. Aldovini. 2000. Effective induction of simian immunodeficncy virus-specific systemic and mucosal immune responses in primates by vaccination with proviral DNA producing intact but noninfectious virions. J. Virol. 74:10514-10522. [DOI] [PMC free article] [PubMed] [Google Scholar]