Abstract
Live vaccinia virus (VV) vaccination has been highly successful in eradicating smallpox. However, the mechanisms of immunity involved in mediating this protective effect are still poorly understood, and the roles of CD8 T-cell responses in primary and secondary VV infections are not clearly identified. By applying the concept of molecular mimicry to identify potential CD8 T-cell epitopes that stimulate cross-reactive T cells specific to lymphocytic choriomeningitis virus (LCMV) and VV, we identified after screening only 115 peptides two VV-specific immunogenic epitopes that mediated protective immunity against VV. An immunodominant epitope, VV-e7r130, did not generate cross-reactive T-cell responses to LCMV, and a subdominant epitope, VV-a11r198, did generate cross-reactive responses to LCMV. Infection with VV induced strong epitope-specific responses which were stable into long-term memory and peaked at the time virus was cleared, consistent with CD8 T cells assisting in the control of VV. Two different approaches, direct adoptive transfer of VV-e7r-specific CD8 T cells and prior immunization with a VV-e7r-expressing ubiquitinated minigene, demonstrated that memory CD8 T cells alone could play a significant role in protective immunity against VV. These studies suggest that exploiting cross-reactive responses between viruses may be a useful tool to complement existing technology in predicting immunogenic epitopes to large viruses, such as VV, leading to a better understanding of the role CD8 T cells play during these viral infections.
Prior immunity to unrelated pathogens can sometimes significantly alter the course and outcome of unrelated virus infections in mice, and this can be either beneficial or detrimental to the host (43, 47, 57, 58). For instance, immunity to lymphocytic choriomeningitis virus (LCMV) can protect mice from a lethal dose of vaccinia virus (VV), and these mice may show altered T-cell-mediated immunopathology resulting in bronchiolitis obliterans or acute fatty necrosis (8, 46). Heterologous immunity may play a role in the variability observed in human disease outcome, from subclinical to lethal, in individuals with the same infection or vaccination.
In 1980, the World Health Organization announced that smallpox (variola) had been eradicated by vaccination with VV; however, smallpox is now considered a potential bioterrorist agent (23). The use of modified VV for mixed-modality vaccines to boost immune responses that have been primed with other agents, such as DNA vaccines or adenovirus vectors expressing viral epitopes, is now being advocated (1). Concerns have arisen about the safety of VV as a vaccine. Adverse events, such as fulminant disseminating vaccinia, have occurred in immunodeficient individuals, while many significant immunity-mediated conditions, such as severe dermatological diseases (erythema multiforme and erythema nodosum), arthritis, pericarditis, myocarditis, and encephalitis, have occurred in healthy individuals following VV vaccination against smallpox (7, 19, 32, 36). Heterologous immunity may be playing a role in mediating some of this immunopathology, especially in adults, who would have large complex memory pools following a lifetime of infections. Thus, studies on heterologous immunity in the mouse model may be highly relevant to human disease, since vaccination with VV has again become prevalent.
Despite the great success of this live vaccine, the precise mechanisms of immunity associated with protection are still poorly understood. There has been evidence for both cellular immunity and humoral immunity playing a role. The level of serum-neutralizing antibody has been correlated with protective immunity. However, the observation that T-cell-deficient individuals had serious and at times fatal infections following VV immunization while agammaglobulinemic children did not suffer such complications suggests that cellular immunity plays an important role in clearing the infection (18, 36, 37, 39). It has been shown that both arms of the immune response are complementary in mediating protection against ectromelia virus (mouse pox), a poxvirus closely related to VV. CD8 T-cell responses were essential for clearing ectromelia virus early in a primary infection, while antibodies were important later in ectromelia virus infection (15, 25). The importance of CD8 T-cell responses during both primary and secondary VV infections is less clear. Previous studies using VV infection of mice have had equivocal results concerning the role of CD8 T cells, although most studies are consistent with CD8 T cells playing at least a supportive role in protective immunity (3, 13, 49, 50, 59). Ongoing research is focusing on developing new and safer vaccines (14). In order to achieve this, it is important to have a better understanding of the impact of T-cell responses on VV infection, including their roles both in protective immunity and in mediating immunopathology.
VV infection of LCMV-immune mice leads to the activation of T cells specific to many different LCMV epitopes. LCMV-NP205-specific CD8 T cells expanded most frequently, presumably due to cross-reactivity with VV (27). Interestingly, LCMV-NP205-specific CD8 T-cell responses are also cross-reactive with a similar epitope encoded by Pichinde virus (PV), PV-NP205. Six of eight amino acids are common between the LCMV and PV epitopes in sites important for interaction with the T-cell receptor (TCR) (5). CD8 T-cell responses may be cross-reactive with different antigens (5, 10, 27, 56, 58), and a common model for cross-reactivity is molecular mimicry, in which two peptides have sequence similarity at the sites of TCR recognition (16, 17). We used the concept of molecular mimicry as a premise to identify potential cross-reactive epitopes between LCMV and VV. This has led to the identification of two VV-specific CD8 T-cell epitopes in mice. Both of these epitopes induced highly effective VV-specific acute and long-term memory responses. One of these epitopes, VV-a11r198, activated cross-reactive LCMV-specific memory T cells, but its discovery unexpectedly led to the identification of a matrix of cross-reactive responses involving five different epitopes and three different viruses (M. Cornberg, S. C. Clute, F. M. Saccoccio, S. K. Kim, Y. N. Naumov, M. A. Brehm, R. M. Welsh, and L. K. Selin, submitted for publication). Interestingly, the other epitope, VV-e7r130, which is described in more detail here, did not appear to activate cross-reactive LCMV-specific memory T-cell responses, even though it was identified by using the concept of molecular mimicry. We here demonstrate that, by our use of VV-e7r130-ubiquitinated minigene immunization or adoptive transfer of VV-e7r130-specific CD8 T-cell lines, memory CD8 T cells can play a significant role in mediating protective immunity to VV during a secondary infection.
MATERIALS AND METHODS
Mice.
C57BL/6 (B6; H-2b) male mice were purchased from The Jackson Laboratory (Bar Harbor, ME), and B6.SJL-ptprca (LY5.1) congenic male mice were purchased from Taconic Farms (Germantown, NY). Mice were used at 2 to 8 months of age. All mice were maintained under specific-pathogen-free conditions in the University of Massachusetts Medical School, Department of Animal Medicine.
Cell lines.
ATCC Vero cells, used in plaque assays, were cultured in minimum essential medium (MEM; Invitrogen, Carlsbad, CA). MC57G cells, which are an H-2b-expressing methylcholanthrene-induced fibroblast cell line from B6 mice, were used as stimulators in intracellular cytokine staining (ICS) assays or as targets for 51Cr release cytotoxicity assays and were maintained in MEM. MC57G cells were infected with VV at a multiplicity of infection of 10 PFU/cell and incubated for 1 h at 37°C. The TAP-2-deficient B6-derived T-lymphoma cell line, RMA-S, kindly provided by Hans-Gustaf Ljunggren (Karolinska Institute, Stockholm, Sweden), was grown in RPMI. RMA-S cells were pulsed with 1 μM peptide for 1 h at 37°C and were then used as targets in 51Cr release cytotoxicity assays. As stimulators for CD8 T-cell lines, RMA-S cells were incubated with 1 μM peptide for 1 h at 37°C and then irradiated (3,000 rads). All cell lines were supplemented with 100 U/ml penicillin G, 100 μg/ml streptomycin sulfate, 2 mM l-glutamine, 10 mM HEPES, and 10% heat-inactivated (56°C, 30 min) fetal bovine serum (FBS; Sigma Chemical Co., St. Louis, MO).
Viruses.
The Western Reserve (WR) strain of VV, a DNA virus in the orthopoxvirus family, was propagated in L929 cells (45). LCMV (Armstrong strain), an RNA virus in the Old World arenavirus family, was propagated in BHK-21 baby hamster kidney cells (45). The mouse adapted influenza virus A/PR/8/34 (H1N1), an RNA virus in the orthomyxovirus family, was grown in the allantoic fluid of 10-day-old embryonated chicken eggs (SPAFAS, Preston, CT) (9).
Infection protocols.
For virus infections, mice were infected intraperitoneally (i.p.) with 5 × 104 PFU of LCMV or 106 PFU VV and methoxyflurane (Metofane)-anesthetized mice were challenged intranasally with 70 PFU of influenza A virus. To control for culture contaminants, VV stocks were purified through a sucrose gradient and diluted in Hanks' balanced salt solution (HBSS; Invitrogen, Carlsbad, CA) and LCMV was diluted more than 40-fold in HBSS. Mice were considered immune 6 weeks after infection or later. Control naïve mice were either left uninoculated or inoculated with tissue culture media or HBSS. The control mice were always age matched to mice of the experimental group and housed exactly the same under pathogen-free conditions.
Virus titration.
VV titers in each of the organs (fat pads, testes, spleens, lungs, kidneys, brains, salivary glands, hearts, and livers) were determined by plaque assays on ATCC Vero cells with the use of a 10% homogenate of tissue taken from individual mice, as described elsewhere (46).
Identification and screening for potential cross-reactive VV epitopes.
In order to identify potential VV epitopes cross-reactive with the H-2Kb-restricted LCMV-NP205 epitope (YTVKYPNL) (55), we searched the VV sequence for 8-mers which maintained the H-2Kb binding motif (i.e., Y or F in the fifth position and L or M in the eighth position) and had 30% or more sequence similarity to LCMV-NP205 by using the DNA/RNA and protein analysis software DNASIS (Hitachi Software Engineering Company, Ltd.). This identified 115 potential peptides, which were synthesized by Chiron Mimitopes (San Diego, CA) and then screened in intracellular gamma interferon (IFN-γ) assays using splenocytes or blood samples from day 8 LCMV- and day 6 VV-infected mice. Although the initial screening was done with mimotope peptides, all experiments shown here used synthetic peptides generated by Biosource International (Camarillo, CA), which were purified with reverse-phase high-pressure liquid chromatography to 90% purity. Final products were analyzed by mass spectroscopy. Two H-2Kb-restricted epitopes were identified, one in the VV protein e7r, positions 130 to 137 (STLNFNNL), and the second in the VV protein a11r, positions 198 to 205 (AIVNYANL). For controls, the influenza virus epitope NP366Db (ASNENMETM) (52) and the LCMV epitope NP396Db (FQPQNGQFI) (20) were used.
Cell surface and tetramer staining by flow cytometry.
Single cell suspensions were prepared from splenocytes, peritoneal exudates (PECs), or peripheral blood. Erythrocytes were lysed with 0.84% NH4Cl solution. Cell suspensions were incubated in fluorescence-activated cell sorter buffer (phosphate-buffered saline [PBS] containing 2% FBS and 0.2% sodium azide) with anti-mouse CD16/CD32 (Fc-block, 2.4G2) to avoid nonspecific antibody binding. Cells were washed, and surface staining was performed in 96-well plates with fluorochrome-labeled antibodies perdinin chlorophyll protein (PerCP)-anti-mouse CD8-alpha (clone 53-6.7) and fluorescein isothiocyanate-anti-mouse CD44 (clone IM7). For tetramer staining, cells were first incubated with streptavidin and Fc-block to prevent nonspecific binding, washed, and then stained with phycoerythrin (PE) and/or allophycocyanin (APC)-labeled tetramers for 60 min. After 40 min of tetramer incubation, surface antibodies were added for 20 min. Thereafter, cells were washed twice with fluorescence-activated cell sorter buffer and fixed in Cytofix (BD Pharmingen, San Diego, CA). All staining was performed on ice. Samples were analyzed with a Becton Dickinson FACSCalibur flow cytometer (San Jose, CA) and FlowJo software (Tree Star, Inc., Ashland, OR). All surface antibodies were purchased from BD Pharmingen, San Diego, CA. Major histocompatibility complex class I (MHC-I) peptide tetramers specific for VV-e7r130/H-2Kb and VV-a11R198/H-2Kb were generated as described previously (11, 27).
ICS.
Cells (106) were stimulated with medium, 5 μM synthetic peptide, 5 μg/ml anti-CD3 (145-2C11), or 105 VV-infected cells (MC57G). Stimulations were performed for 5 h at 37°C in a total volume of 200 μl RPMI medium supplemented with 10% FBS, 10 U/ml of human recombinant interleukin-2 (IL-2), and 0.2 μM of brefeldin A (GolgiPlugTM; BD Pharmingen). After incubation, the surface staining was carried out as described above. Thereafter, cells were washed twice, and then fixed and permeabilized (Cytofix/Cytoperm; BD Pharmingen, San Diego, CA). Intracellular-cytokine-producing cells were detected with PE-labeled anti-mouse IFN-γ and APC-labeled anti-mouse tumor necrosis factor alpha (TNF-α) monoclonal antibodies. Immunoglobulin G isotype antibodies labeled with the same fluorochromes were used in the same assay. Antibodies were purchased from BD Pharmingen, San Diego, CA. The samples were analyzed as described above.
Cytotoxicity assays.
Standard in vitro chromium (51Cr) release assays were performed to measure antiviral cytotoxic-T-lymphocyte (CTL) activity as described previously (45). The in vivo cytotoxicity assay was performed according to recently published techniques (2, 28), with splenocytes from B6 or congenic LY5.1 mice used as target cells. After the lysis of red blood cells, splenocytes were divided into two or four populations. One population was pulsed with 1 μM ovalbumin amino acids 257 to 264 (SIINFEKL) as a control, and other populations were pulsed with 1 μM of the indicated VV peptides for 60 min at 37°C. Each population was labeled with a different concentration (2 μM or 0.4 μM for the two-population experiment; 2 μM, 0.67 μM, 0.33 μM, or 0.11 μM for the four-population experiment) of carboxyfluorescein diacetate-succinimidyl ester (CFSE; Molecular Probes, Eugene, OR). After CFSE labeling, equal amounts of cells were mixed together, washed, and resuspended in PBS. A total of 2 × 107 cells were injected intravenously into each recipient mouse. Specific in vivo cytotoxicity was determined by harvesting splenocytes from recipient mice 16 h after intravenous cell transfer. CFSE-labeled target populations were quantified by flow cytometry. When splenocytes from LY5.1 congenic mice were used as target cells, splenocytes from recipients were costained with a PE-conjugated LY5.1-specific monoclonal antibody (CD45.1, clone A20; BD Pharmingen). By gating on LY5.1-specific cells, it was possible to include four target populations per sample. Uninfected, LCMV- and VV-infected C57BL/6 mice were used as recipients. The amount of specific in vivo killing was calculated, as described elsewhere (2), as follows:
100 − {[(% peptide pulsed in infected/% unpulsed in infected)/(% peptide pulsed in uninfected/% unpulsed in uninfected)] × 100}.
In vitro expansion of antigen-specific CTLs.
Splenocytes (107) from VV- or LCMV-immune mice were cocultured with peptide-pulsed RMA-S cells (106) in RPMI supplemented with 100 U/ml penicillin G, 100 μg/ml streptomycin sulfate, 2 mM l-glutamine, 10 mM HEPES, 1 mM sodium pyruvate, 0.1 mM MEM-nonessential amino acids, 0.05 mM β-mercaptoethanol, and 10% FBS for 4 to 5 days at 37°C at 5% CO2. The IL-2 culture supplement BD T-Stim (BD Biosciences, San Diego, CA) was added after 4 to 5 days of culture. Peptide stimulation was repeated every 4 to 5 days. After 20 to 25 days of stimulation (four or five stimulations), T-cell lines were analyzed.
Adoptive transfer of antigen-specific T cells into mice.
T-cell lines were rested without peptide stimulation for 7 days. Thereafter, live cells were separated with Lympholyte-M (Cedarlane, Hornby, Canada). T cells were suspended in HBSS at 107 cells/ml and incubated with 2 μM CFSE for 15 min at 37°C. After incubation, cells were washed twice with HBSS, and 106 cells were injected i.p. into recipient mice. Mice were infected with 106 PFU VV i.p. on the same day. Three to four days after infection, mice were sacrificed. Fat pads and/or testes were analyzed for VV titers. PECs were analyzed for immune responses and division of transferred T cells. Surface staining was performed as described above, with fluorochrome-labeled antibodies perdinin chlorophyll protein-anti-mouse CD45.2 (LY5.2, clone 104) and APC-anti-mouse CD8-alpha (clone 53-6.7). Transferred donor cells were identified as positive for CD45.2 and CD8 when congeneic mice were used.
VV-e7r130-specific minigene DNA immunization.
Ubiquitinated DNA vaccine constructs expressing VV-e7r130 epitope were prepared as described elsewhere (42). The genes for VV-e7r130 were cloned into the F3Ub expression vector kindly provided by Lindsay Whitton (Scripps Institute, San Diego, CA). Clean lipopolysaccharide-free plasmid DNA was produced by using plasmid Giga kit (QIAGEN, Valencia, CA), according to manufacturer's protocol. For immunization, the quadriceps muscles of B6 mice were injected with 100 μg (50 μg for each muscle) of DNA construct formulated in sterile 0.9% NaCl. The control mice were immunized with 100 μg of F3Ub vector or LCMV-NP396-specific minigene or were not immunized. Mice received three DNA immunizations (100 μg each) separated by two weeks. Two weeks after the last immunization, mice were infected with 106 PFU VV. Six days after VV infection, mice were sacrificed. Splenocytes and PECs were analyzed by intracellular cytokine staining and by in vitro 51Cr release assays. Fat pads were evaluated for virus titers by plaque assays.
Statistical analysis.
Descriptive statistics are expressed as mean values ± standard errors of the mean. Comparisons between groups were performed with the Student t test (two tailed).
RESULTS
Cross-reactive peptide motif identifies H2-Kb-restricted VV-specific CD8 T-cell epitopes.
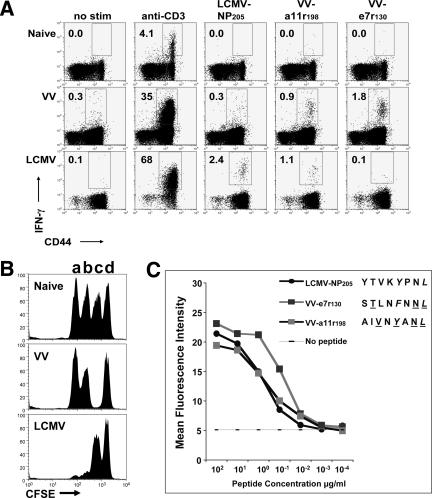
Defining VV-specific epitopes has not been a priority until recent times, as smallpox had been essentially eradicated. Identifying epitopes for VV can be a daunting task, as VV is a very large DNA virus of the orthopoxvirus family and expresses over 200 proteins, with the potential for thousands of CD8 T-cell epitopes (21). Human and mouse VV-specific CD8 T-cell epitopes have only very recently been identified (13, 33, 35, 51, 53). We took a unique approach to identifying VV-specific epitopes because our previous work had revealed selective expansion of LCMV epitope-specific T cells during VV infection, consistent with potential cross-reactive T-cell responses (8, 26, 27). In order to further examine cross-reactive responses between VV and LCMV, we had to define VV-specific epitopes. Since CD8 T cells specific for the LCMV-NP205 peptide often expanded during VV infection of LCMV-immune mice, this peptide was used as a template peptide sequence to identify potential cross-reactive epitopes with sequence similarity. By use of a general DNA/RNA and protein analysis software (DNASIS) and the criteria that the epitope should have an H-2Kb binding motif and greater than 30% sequence similarity to LCMV-NP205 (YTVKYPNL), 115 8-mer peptides were identified in the VV genome. By screening these peptides by use of an intracellular IFN-γ assay with VV- or LCMV-specific CD8 T cells, we identified two VV-specific epitopes, e7r130 (STLNFNNL) and a11r198 (AIVNYANL) (Fig. 1A). The IFN-γ responses to VV-e7r130 ranged between 1% and 9.9% (mean ± standard error of the mean, 2.2% ± 0.5%; n = 22) of all CD8 T cells six days after VV infection, whereas the IFN-γ response to VV-a11r198 ranged between 0.1 and 0.75% (mean ± standard error of the mean, 0.23% ± 0.05%; n = 17). Six days after VV infection, the VV-e7r130 and VV-a11r198 IFN-γ responses accounted for approximately 10% and 1% of the anti-CD3 response, respectively. The VV-e7r130 and VV-a11r198 peptide-specific T-cell responses accounted for approximately 20% and 2%, respectively, of the total VV-specific response as determined by stimulation with the MHC class I-matched VV-infected MC57G cells. In vivo CTL assays using peptide-pulsed splenocytes from C57BL/6 mice as targets showed about 90% specific killing (mean, 87%; range, 83 to 92%; n = 6) for VV-e7r130 and 19% (range, 15 to 23%; n = 3) for VV-a11r198 (Fig. 1B). In vitro 51Cr release assays on syngeneic target cells (RMA-S) coated with peptides, although not as sensitive, showed a similar hierarchy (data not shown). These two newly identified VV epitopes were also found to have the same relative binding affinity for H-2Kb as LCMV-NP205, the epitope used to identify them (Fig. 1C). We consider VV-e7r130 an immunodominant H-2Kb-restricted epitope for VV, while VV-a11r198 is a subdominant epitope in primary VV infection.
FIG. 1.
VV- or LCMV-specific CD8 T cells recognize the subdominant VV-a11r198 epitope, while only VV-specific CD8 T cells recognize the dominant VV-e7r130 epitope. (A) Intracellular IFN-γ assay. Splenocytes from naïve or day 6 VV- or day 8 LCMV-infected C57BL/6 mice were stimulated as indicated in an ICS assay. The percentage of CD8 T cells producing IFN-γ is recorded in the upper left quadrant (gated on CD8 cells). Data are representative of five experiments (two to six mice/group). No stim, no stimulation. (B) In vivo cytotoxicity assay. CTL activity was analyzed in naïve or day 6 VV- or day 8 LCMV-infected C57BL/6 mice using CFSE-labeled targets coated with LCMV-NP205 (a), VV-a11r198 (b), VV-e7r130 (c), or control ovalbumin SIINFEKL (d) peptide. Data are representative of two experiments (three to five mice/group). (C) RMA-S stabilization assay. The newly identified VV epitopes stabilize H-2Kb on RMA-S cells in a standard stabilization assay. The mean fluorescence intensities (MFI) of the H-2Kb expression to different concentrations of the indicated peptides are shown. Data are representative of two similar experiments.
When these peptides were tested in IFN-γ or CTL assays with splenocytes from mice infected with only LCMV 9 days earlier, VV-a11r198 induced significant IFN-γ production in up to 1% of all CD8 T cells (mean, 0.8%; range, 0.4 to 1%; n = 5) and in vivo killing was more than 80% (86%; range, 78 to 94%; n = 6), whereas VV-e7r130 failed to stimulate CD8 T cells to produce IFN-γ or demonstrate cytotoxic activity (Fig. 1A and B). Although VV-e7r130 was identified by its potential to be cross-reactive with LCMV, cells from LCMV-infected mice did not appear to recognize this epitope.
Newly identified VV CD8 T-cell epitopes e7r130 and a11r198 are maintained in the T-cell memory pool.
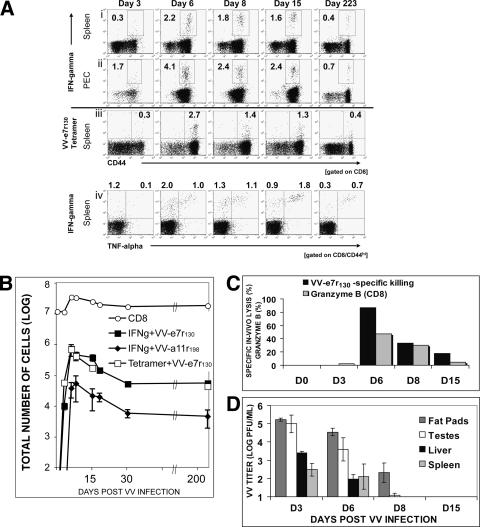
To analyze if the VV epitope-specific immune response could be maintained in the CD8 T-cell memory pool, we analyzed VV-infected C57BL/6 mice during the acute phase of infection and more than six weeks after infection. VV-e7r130 and VV-a11r198 responses in the spleen peaked at 6 days after VV infection as measured by IFN-γ production upon in vitro peptide stimulation (for e7r, a mean ± standard deviation of 2.2% ± 0.5% and a range of 1 to 9.9% [n = 22]; for a11r, a mean ± standard deviation of 0.23% ± 0.05% and a range of 0.1% to 0.75% [n = 17]) (Fig. 1 and 2A and B). CD8 T-cell responses specific to either epitope gradually declined over the first 30 days after VV infection and stabilized thereafter, as demonstrated by little change in their total number or frequencies between day 30 and 200 postinfection (Fig. 2A and B). Memory CD8 T cells specific for VV-e7r130 frequencies were stable at 0.4% ± 0.04% (range, 0.2 to 0.6% [n = 11]) and for VV-a11r198 at 0.05% ± 0.02% (range, 0.01 to 0.1% [n = 8]) for more than 200 days postinfection (Fig. 2A and B). The responses against the VV epitopes were also detectable in peripheral sites, such as the peritoneal cavity, the initial site of virus entry, at slightly higher frequencies than in the spleen (Fig. 2A, row ii). Tetramer staining with H-2Kb-containing VV-e7r130 allowed direct visualization of antigen specific CD8 T cells without in vitro stimulation and showed results similar to those determined by intracellular cytokine assays (Fig. 2A, row iii).
FIG. 2.
Maintenance of VV-epitope-specific CD8 T-cell responses into memory and VV clearance. (A) VV-e7r-specific CD8 T-cell response measured by intracellular IFN-γ staining in the spleen (i) and in the PECs (ii) at various times after VV infection. (iii) Direct visualization of VV-e7r-specific CD8 T-cell response by tetramer staining. Cells were gated on CD8. (iv) Increase in ratio of TNF-α-to-IFN-γ production of VV-specific CD8 T cells in the spleen during VV infection. An ICS assay (gated on CD8+CD44+ T cells) indicated an increase in the ratio of TNF-α to IFN-γ ([IFN-γ+ + TNF-α+]/[IFN-γ+ + TNF-α−]) production from VV-e7r-specific CD8 T cells. Similar results were observed for a11r-specific responses (data not shown). Data are representative of three experiments. (B) Kinetics of VV epitope-specific CD8 T-cell responses. Splenic VV epitope-specific CD8 T-cell responses were analyzed by either tetramer staining or ICS (three to five mice/group). The total number of antigen-specific cells (given plus or minus the standard error of the mean) equals the frequency of peptide-reactive cells multiplied by the total number of CD8 cells. Data are representative of three experiments. (C) Effector functions of VV-specific CD8 T cells visualized by VV-e7r-specific in vivo killing (▪) and by intracellular granzyme B staining of total CD8 T cells ( ) 3 to 15 days after VV infection. (D) VV clearance. Organs were harvested at the times indicated and plaque assays were performed (five mice/time point). VV could not be detected in any organ including lung, kidneys, heart, brain, and salivary gland day 30 postinfection.
) 3 to 15 days after VV infection. (D) VV clearance. Organs were harvested at the times indicated and plaque assays were performed (five mice/time point). VV could not be detected in any organ including lung, kidneys, heart, brain, and salivary gland day 30 postinfection.
Analyzing intracellular cytokine production for both IFN-γ and TNF-α during the acute and memory phases of VV infection demonstrated that VV-specific CD8 T cells could produce both cytokines in response to VV-e7r130 and VV-a11r198. The determined ratio ([IFN-γ+ + TNF-α+]/[IFN-γ+ + TNF-α−]) was 0.5 at the peak of the VV-specific CD8 T-cell response (day 6) and greater than 2 after 15 days after VV infection. This is consistent with the previously demonstrated concept that mature memory CD8 T cells are capable of producing both cytokines (Fig. 2A, row iv) (48).
Immunodominant VV-e7r130-specific CD8 T cells elicit effector function during the acute phase of VV infection.
During the acute phase of VV infection, VV-e7r130-specific CD8 T-cell cytotoxic activity paralleled cytokine production. In vivo killing of VV-e7r130-labeled targets was 87% (range, 83 to 92%; n = 6) 6 days after VV infection and declined to 34% (range, 10 to 46%; n = 5) and 18% (range, 16 to 22%; n = 5) 8 and 15 days, respectively, after VV infection (Fig. 2C). Staining for total CD8 T-cell granzyme B was consistent with these results (Fig. 2C). Interestingly, VV-e7r130-specific in vitro killing measured by 51Cr release assay was more than fourfold lower than in vivo killing (19% versus 87%) 6 days after VV infection, indicating a much higher sensitivity for the in vivo killing assay. The kinetics of VV-specific CD8 effector functions coincided with a decrease of VV titers measured in several organs. VV titers peaked at day 3 after infection and were significantly reduced 6 days postinfection. VV was cleared in the spleen and liver by day 8 postinfection but persisted slightly longer in fat pads and testes, clearing by 15 days postinfection (Fig. 2D). VV PFU could not be detected in any organ (testis, fat pads, spleen, liver, lung, brain, or salivary gland) 30 days postinfection, suggesting that the virus had been completely cleared from the host.
VV-specific CD8 T-cell lines generated from VV-immune mice respond to VV in vitro and in vivo.
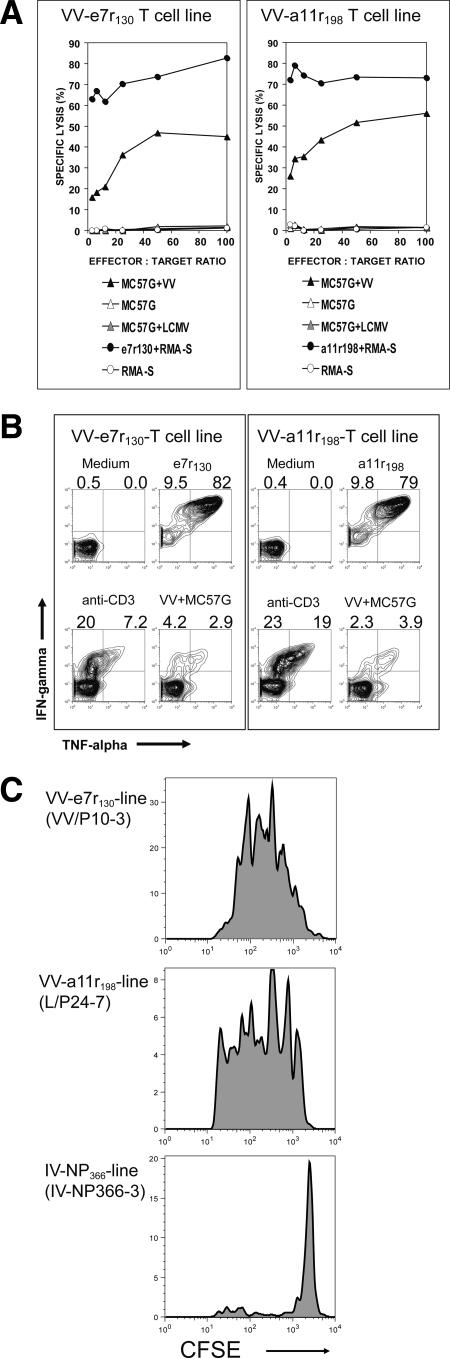
We next established VV-e7r- and -a11r-specific T-cell lines by stimulating splenocytes from VV-immune mice with the VV-specific peptides for 20 to 25 days in the presence of the IL-2. In 51Cr release cytotoxicity assays, both lines were able to lyse VV-infected targets (Fig. 3A). In vitro ICS assays also demonstrated that the VV-e7r-specific and -a11r-specific lines produce IFN-γ and TNF-α in response to VV-infected MC57G cells (Fig. 3B). However, stimulating with VV-infected MC57G cells in an ICS assay was not as sensitive a technique as peptide stimulation or the in vitro cytotoxicity 51Cr release assay using VV-infected or peptide-coated targets. Both CFSE-labeled VV epitope-specific cell lines upon adoptive transfer proliferated in vivo in the recipient mice upon VV infection, whereas control influenza A virus NP366-specific lines did not (Fig. 3C). These results demonstrate that these epitopes were processed and presented during VV infection.
FIG. 3.
VV-e7r-specific and VV-a11r-specific lines generated from VV-immune mice respond to VV in vitro and in vivo. (A) In vitro 5-h 51Cr release cytotoxicity assays demonstrate specific lysis of peptide-coated RMA-S cells and VV-infected MC57G cells. Control targets included RMA-S cells, MC57G cells, and LCMV-infected MC57G cells. (B) In vitro ICS assays show that the e7r-specific and a11r-specific lines produce IFN-γ and TNF-α in response to VV-infected MC57G cells. As a control, these lines did not produce cytokines to LCMV-NP396 (data not shown). (C) Both VV-e7r-specific and VV-a11r-specific lines proliferated in response to VV in vivo. VV-e7r-specific CD8 T-cell line (VV-p10-3, generated from a VV-immune mouse) and a11r-specific CD8 T-cell line (L/P24-7, generated from an LCMV- and VV-immune mouse) proliferated in response to VV infection i.p. as assessed by loss of CFSE by day 4 after adoptive transfer of the cell line i.p. into syngeneic C57BL/6 mice. The PECs collected from these mice were used in an ICS assay and produced IFN-γ only to the VV peptide used originally to stimulate the line and did not respond to the alternate VV peptide or the control influenza virus NP366 peptide. The control NP366-specific line generated from an influenza virus-immune mouse did not proliferate in response to VV and did not produce IFN-γ in response to stimulation with VV peptides.
VV-e7r130-specific CD8 T cells reduce VV load.
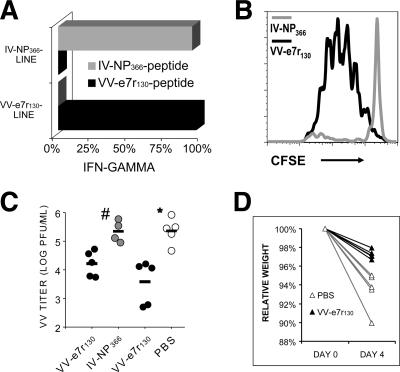
To investigate a potential protective capacity of CD8 T-cell responses against VV infection, we used two different methods, either adoptive transfer of VV-e7r-specific CD8 T cells into the peritoneal cavity or immunization with ubiquitinated minigenes expressing VV-e7r130. To directly test if VV-specific CD8 T-cell responses could protect against VV infection, rested VV-e7r130-specific CD8 T-cell lines were adoptively transferred i.p. into recipient syngeneic naïve mice, which were then infected with VV. Control mice were injected with influenza virus NP366-specific CD8 T-cell lines, which were documented not to be cross-reactive with VV. Tetramer or ICS assays demonstrated that more than 95% of each CD8 T-cell line was specific for the peptide used to generate the line (Fig. 4A). Three days after adoptive transfer, the CFSE-labeled VV-e7r-specific CD8 T cells had gone through three to six rounds of division in response to the VV infection, while the influenza virus NP366-specific cells did not proliferate (Fig. 4B). There was a significant 99% reduction in VV titers 3 days after VV infection in the fat pads of the mice injected with the VV-e7r-specific line compared to the titers in the mice injected with the control influenza virus NP366-specific line (Fig. 4C). Similar results were observed in a second experiment, in which a PBS injection was compared to injection with the VV-e7r130 cell line (Fig. 4C). Our VV dose was not lethal, but control mice appeared to be more ill, as demonstrated by significantly greater weight loss (Fig. 4D), ruffled fur, shivering, and a decrease in activity, than the mice treated by immunization with the VV-specific T-cell lines.
FIG. 4.
VV-e7r-specific CD8 T cells reduce VV titers in vivo. (A) VV-e7r-specific (VV-e7r130) or influenza virus NP366-specific (IV-NP366) CD8 T-cell lines generated from VV-immune or influenza virus-immune mice, respectively, demonstrated antigen specificity in an ICS assay (representative of two similar experiments). (B) The VV-e7r-specific CD8 T-cell line, but not the IV-NP366-specific cell line, proliferated in response to VV infection (i.p.), as assessed by loss of CFSE day 3 after adoptive transfer (i.p.) of the cell line into syngeneic C57BL/6 mice. (C) Significant reduction in VV titers in two experiments on days 3 and 4 after VV infection in mice injected with the VV-e7r-specific (black circles) or control influenza virus NP366-specific (gray circles) (experiment 1) cell line or PBS (white circles; experiment 2) (# and *, P < 0.05; four or five mice/group). (D) Significant protection from weight loss day 4 after VV infection in mice injected with VV-e7r-specific cell line compared to protection from weight loss in mice given PBS (P < 0.003; five mice/group). Weight is expressed as a percentage of the original weight prior to infection.
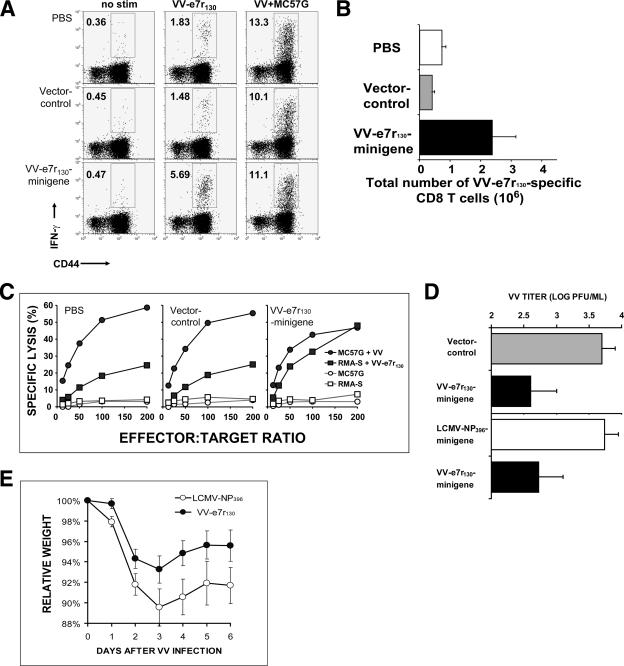
A second method utilized three intramuscular immunizations every 14 days with an ubiquitinated DNA minigene specific for the VV-e7r130 sequence to demonstrate the protective role of CD8 T-cell responses. The VV-e7r130-specific CD8 T-cell responses measured by intracellular IFN-γ (Fig. 5A and B) and in vitro cytotoxicity (Fig. 5C) assays were increased approximately three- to sixfold compared to nonimmunized mice or mice immunized with the F3Ub vector only. Six days after VV infection, the VV-e7r130-specific CD8 T-cell population was significantly higher, at 2.4 × 106 total cells (range, 1.0 × 106 to 5.1 × 106; n = 5; P = 0.04, versus the F3Ub group) in VV-e7r130-immunized mice, than the T-cell populations in F3Ub-immunized mice, at 4 × 105 cells (1 × 105 to 7 × 105; n = 5), and nonimmunized control mice, at 7 × 105 cells (4 × 105 to 13 × 105; n = 5) (Fig. 5A and B). Also, the specific lysis of VV-e7r130-labeled targets in VV-e7r130-immunized mice was higher than those in nonimmunized and F3Ub-immunized control mice (Fig. 5C). Analyzing VV titer in fat pads by plaque assays, we documented a significant 90% reduction of virus titer 6 days after VV infection in VV-e7r130-immunized mice compared to that of the group immunized with the vector only (Fig. 5D).
FIG. 5.
VV-e7r-expressing ubiquitinated DNA minigene vaccine increased e7r-specific CD8 T cells and reduced VV load. Increased frequency (A) and total number (B) of the VV-e7r130 epitope-specific CD8 T cells day 6 after VV infection in mice immunized three times intramuscularly with e7r-expressing ubiquitinated DNA minigene as assessed by ICS assay. Data are from one representative mouse (five mice/group). The total number of VV-e7r-specific cells equals the frequency of antigen specific cells multiplied by the total number of viable cells. There was a greater number of VV-e7r130 epitope-specific cells in the VV-e7r130 minigene-immunized mice than in mice given either control, minigene vector or PBS (P values of <0.05 and <0.08, respectively; n = 5). Data are representative of two experiments. No stim, no stimulation. (C) Enhanced cytotoxicity as measured by 51Cr release assays on splenocytes from the VV-e7r130 minigene-immunized mice compared to controls given minigene vector or PBS. Targets included peptide-coated RMA-S cells or VV-infected MC57G cells (five mice/group). (D and E) Significant reduction in VV titers day 6 after VV infection in mice injected with VV-e7r-specific minigenes (▪) compared to control-vector-immunized mice (▪) (log PFU/ml, 2.6 ± 0.4 versus 3.7 ± 0.2; P < 0.05; seven and five mice, respectively) or control LCMV-NP396-specific minigene-immunized mice (□) (log PFU/ml, 2.7 ± 0.4 versus 3.7 ± 0.2; P < 0.07; eight and five mice, respectively). (E) Significant protection from weight loss during VV infection of VV-e7r130-mingene immunized mice (•) compared to control LCMV-NP396 minigene-immunized mice (○) (P < 0.0004; eight and five mice, respectively; paired Student's t test). Weight is expressed as a percentage of the original weight prior to infection.
In a second experiment, we also observed a 90% reduction in VV titers (Fig. 5D), as well as significantly decreased weight loss (Fig. 5E) from VV-e7r minigene immunization compared with that from control LCMV-NP396 minigene immunization.
DISCUSSION
Through the study of T-cell cross-reactivity between two heterologous viruses, we found that sequence similarity to a known epitope may be a useful tool to help predict immunogenic epitopes to large viruses, such as VV. This led to the finding that VV infection induced potent CD8 T-cell responses that were well maintained into memory and that VV-specific CD8 T cells could play a significant role in mediating protective immunity to VV. Our initial observations of VV-induced proliferation of T cells specific to an LCMV epitope, NP205, in LCMV-immune mice indicated that this LCMV epitope-specific response potentially included cross-reactive T cells that could also recognize VV (8, 26, 27). Mechanisms for CD8 T-cell cross-reactivity can be manifold. Molecular mimicry, in which a different peptide retains sites that are necessary for interaction with the TCR, is one of several paths to cross-reactive T-cell responses (43), as in the case with the NP205 epitopes of LCMV and PV (5). It had been difficult to identify VV-specific MHC-I-restricted epitopes, as VV encodes more than 200 proteins and theoretically thousands of potential epitopes (21). Basing our method on the concept of molecular mimicry, we scanned the VV genome for sequence similarities to LCMV-NP205 and identified two VV immunogenic epitopes after screening only 115 peptides. This would suggest that this technique adds another approach to identifying immunogenic epitopes and could complement more conventional methods using algorithms based on MHC binding motifs (H. G. Rammensee, J. Bachmann, and S. Stevanovic, SYFPEITHI, a database of MHC ligands and peptide motifs—epitope prediction [http://www.syfpeithi.de/Scripts/MHCServer.dll/EpitopePrediction.htm]). Paradoxically, we identified an immunodominant VV epitope, VV-e7r130, that did not generate cross-reactive T-cell responses with LCMV and a subdominant epitope, VV-a11r198, which did generate cross-reactive T-cell responses with LCMV. Further characterization of these responses in subsequent studies has confirmed these observations (Cornberg et al., submitted). Identification of these VV-specific epitopes led to a better understanding of the role CD8 T cells play during VV infection. This strategy may be useful for identifying new CD8 T-cell epitopes for large viruses such as VV. This technique is particularly useful for identifying epitopes that may be missed by the more conventional methods, such as VV-a11r198. VV-a11r198 was not identified in a recent report using algorithms to identify VV epitopes in C57BL/6 mice (35). Although the VV-a11r198 epitope is a weak epitope in naïve C57BL/6 mice infected with VV because of its ability to activate cross-reactive LCMV-specific memory CD8 T cells, its frequency can be significantly increased in some LCMV-immune mice infected with VV based on the private specificity of the memory T-cell repertoire of each mouse (27).
Both epitopes VV-e7r130 (STLNFNNL) and VV-a11r198 (AIVNYANL) were conserved among vaccinia virus strains (WR, Ankara, and Copenhagen), other poxviruses (cowpox, monkeypox, camelpox, and ectromelia virus), and also variola virus. VV-e7r is a soluble myristylated late protein of unknown function, and it is not associated with the virus membrane (30). The 36- to 40-kDa putative VV protein a11r is one of the 49 gene products that are conserved in all sequenced poxviruses (54) and was found to be associated with the putative DNA-packaging protein A32L required for virion morphogenesis (34). Recently, Resch et al. showed that the a11r protein is also a late protein that seems to be required to form normal virion membranes (40).
Although VV-specific CTLs were first described in 1975 (29), the importance of CD8 T cells in poxvirus infections still remains somewhat controversial and remains an important question, since newer vaccines are being designed to include induction of CD8 T-cell responses. Earlier studies had mixed results. Studies with ectromelia virus (mouse pox), a poxvirus closely related to VV, in mice demonstrate a consistent and absolute role for CD8 T cells in the control of primary infection, although antibody responses have been found to be important later in infection (4, 6, 15, 25). Tscharke et al. have demonstrated that immunization with a newly identified immunodominant peptide provided significant protection against a secondary lethal ectromelia infection, suggesting that CD8 memory T-cell responses could contribute to protective immunity to ectromelia virus (53). However, studies with VV in mice have been less definitive in identifying a role for CD8 T cells in both primary and secondary infections (3, 13, 49, 50, 59).
Studies examining the role of CD8 T cells during primary infection with VV have suggested that CD8 T cells may not be essential but they do play a supportive role. In one study using high-dose VV, β2-microglobulin knockout mice (β2 m−/− mice) which lack CD8 T cells were able to recover from VV infection, suggesting that CD8 T-cell responses were not essential during the primary infection, but this study did not rule out that they could play a significant role. Recovery was described as the disappearance of skin lesions and weight gain after intradermal inoculation, and VV titers were not analyzed (50). In another study, Belyakov et al. (3) showed that CD8 T cells alone in the absence of an antibody response were not sufficient to protect against VV infection during a primary infection (analyzed by weight differences). However, the depletion of CD8 T cells in the absence of an antibody response prevented late recovery, suggesting that CD8 T cells could make the difference between survival and death (3). A recent study by Xu et al. also showed that CD8 T cells can contribute to protection against VV, but CD4 T cells and antibodies may play a more important role, at least in primary infection (59).
There are less data available for the role of CD8 T cells during secondary VV infection. One study by Snyder et al. (49) documented protection against lethal secondary VV challenge in HLA-A2 transgenic mice by vaccination with an MHC-I-restricted T-cell epitope, suggesting that CD8 memory T-cell responses were important in protection against VV. These mice received the VV peptide three times subcutaneously accompanied with 50 nmol of a hepatitis B virus core helper peptide, 5 μg of granulocyte-macrophage colony-stimulating factor (GM-CSF), 800 IU of IL-2, and incomplete Freund's adjuvant. However, these mice with only a single CD8 epitope-specific memory response did not have complete protection, as some mice lost weight and some mice died. This did not occur in mice previously immunized with the whole virus (49). Another study by Daftarain et al. (12) using HLA-A2 transgenic mice to identify human immunodeficiency virus (HIV)-specific CD8 T-cell epitopes, demonstrated that immunization with a Th-CTL fusion peptide against an HIV epitope led to enhanced clearance of recombinant VV expressing the HIV epitope but was highly dependent on coadministration of peptide with cytosine-phosphate-guanine DNA (12). However, in another study by Drexler et al., immunization with HLA-A2-restricted VV-specific CD8 peptide vaccines was not able to protect HLA-A2 transgenic mice against a secondary infection with WR VV infection (13). Interestingly, research into sequential heterologous virus infections, in which there are no cross-protective neutralizing antibodies, shows that cross-reactive LCMV-specific memory CD8 T cells mediate protective immunity early in VV infection, suggesting that CD8 T cells could play an important role upon secondary VV challenge (8, 46; Cornberg et al., submitted). Our studies would strongly support the contention that CD8 memory T cells could play an important role in protection against VV during secondary infections. VV-e7r130-specific ubiquitinated DNA immunization significantly enhanced epitope-specific CD8 memory T-cell responses in vivo and resulted in a 90% reduction of VV titers on secondary challenge. Another technique allowed a more direct test of the ability of effector type memory CD8 T cells to protect against VV in a peripheral site. Epitope-specific CD8 T cells were activated in vitro and then rested into a quiescent state before transfer into the intraperitoneal cavity prior to challenge with VV. Only 106 cells were required to reduce VV titers more than 95%. These data demonstrate a number of points. First, e7r130-specific CD8 T cells recognized VV-infected targets in vivo, supporting the concept that this epitope is processed and presented. Second, CD8 T cells provided protection against VV, especially when delivered at the site of the infection. This underlines the concept that peripheral effector memory T cells can contribute to the early clearance of a virus infection by their immediate response (8, 24, 31) and justifies the use of vaccines which induce CD8 T-cell-dependent immunity. Finally, T cells can encounter their antigen presented on APCs in the periphery at the site of the infection. It also suggests that adoptive transfer of in vitro generated antigen-specific CD8 T cells could be a potential therapeutic intervention against poxviruses as has been shown for other infectious diseases (41). This model using intraperitoneal injection of CD8 T cells also provides a useful tool to study the effect of in vitro generated CD8 T cells upon VV-infected mice or in other viral infection models. To our knowledge, this is the first report of immunization with an ubiquitinated minigene expressing a VV-specific CD8 epitope or with the direct transfer of a VV-specific CD8 T-cell line leading to protective immunity without added CD4 helper peptides or adjuvants, and this clearly demonstrates that memory CD8 T cells can mediate protection against VV.
These studies are an excellent baseline for a better understanding about VV-specific memory CD8 T-cell responses in the context of other heterologous virus infections. For instance, the VV epitope-specific CD8 T cells were maintained in the memory population with the same immune hierarchy even more than 200 days after VV challenge (Fig. 2A and B). However, this analysis is not comparable with the human scenario in which one is exposed to numerous antigens during a lifetime. Encountering new infections induces immune responses to the new pathogen and results in attrition of non-cross-reactive memory T cells specific to the previous antigen (44). Studies in humans have shown that there is a 10-fold decrease in VV-specific memory over a lifetime (22). Considering the number of infections which humans can encounter, this appears to be a minimal loss. However, when memory CD8 T cells are able to generate cross-reactive responses to new antigen, their high frequency and activation state give them an advantage over naïve T cells and can lead to a preferential expansion of the cross-reactive CD8 T cells, which can alter the hierarchy of T-cell responses (5, 11). It is possible that cross-reactive VV-specific T cells could preserve VV-specific memory and can explain the maintenance of VV-specific immune responses more than 40 years after smallpox vaccination with VV (22). It is possible that cross-reactive memory responses, which may be of lower avidity to the new heterologous virus, are not always as efficient at clearing the new pathogen as higher-avidity de novo non-cross-reactive responses. These lower-avidity responses to VV may be more prone to stimulating immunopathology especially if these responses are directed at late antigens like VV-a11r. Having these systems now in place, we are better able to address the role heterologous immunity and cross-reactivity play in mediating protective immunity and/or immunopathology during VV infection a model for smallpox vaccination.
Acknowledgments
We thank K. A. Daniels and F. Jeffe for their technical assistance and F. A. Ennis, H. Wedemeyer, E. Szomolanyi-Tsuda, and R. M. Welsh for helpful discussions.
This study was supported by NIH grant AI-46578 (L.K.S.) and DFG fellowship CO 310/1-1 (M.C.) (Germany).
The contents of this publication are solely the responsibility of the authors and do not represent the official view of the NIH.
Footnotes
Published ahead of print on 1 November 2006.
REFERENCES
- 1.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. [DOI] [PubMed] [Google Scholar]
- 2.Barber, D. L., E. J. Wherry, and R. Ahmed. 2003. Cutting edge: rapid in vivo killing by memory CD8 T cells. J. Immunol. 171:27-31. [DOI] [PubMed] [Google Scholar]
- 3.Belyakov, I. M., P. Earl, A. Dzutsev, V. A. Kuznetsov, M. Lemon, L. S. Wyatt, J. T. Snyder, J. D. Ahlers, G. Franchini, B. Moss, and J. A. Berzofsky. 2003. Shared modes of protection against poxvirus infection by attenuated and conventional smallpox vaccine viruses. Proc. Natl. Acad. Sci. USA 100:9458-9463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blanden, R. V. 1970. Mechanisms of recovery from a generalized viral infection: mousepox. I. The effects of anti-thymocyte serum. J. Exp. Med. 132:1035-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brehm, M. A., A. K. Pinto, K. A. Daniels, J. P. Schneck, R. M. Welsh, and L. K. Selin. 2002. T cell immunodominance and maintenance of memory regulated by unexpectedly cross-reactive pathogens. Nat. Immunol. 3:627-634. [DOI] [PubMed] [Google Scholar]
- 6.Buller, R. M., K. L. Holmes, A. Hugin, T. N. Frederickson, and H. C. Morse III. 1987. Induction of cytotoxic T-cell responses in vivo in the absence of CD4 helper cells. Nature 328:77-79. [DOI] [PubMed] [Google Scholar]
- 7.Check, E. 2004. Side effects leave smallpox vaccine in limbo. Nature 428:789. [DOI] [PubMed] [Google Scholar]
- 8.Chen, H. D., A. E. Fraire, I. Joris, M. A. Brehm, R. M. Welsh, and L. K. Selin. 2001. Memory CD8+ T cells in heterologous antiviral immunity and immunopathology in the lung. Nat. Immunol. 2:1067-1076. [DOI] [PubMed] [Google Scholar]
- 9.Chen, H. D., A. E. Fraire, I. Joris, R. M. Welsh, and L. K. Selin. 2003. Specific history of heterologous virus infections determines anti-viral immunity and immunopathology in the lung. Am. J. Pathol. 163:1341-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clute, S. C., L. B. Watkin, M. Cornberg, Y. N. Naumov, J. L. Sullivan, K. Luzuriaga, R. M. Welsh, and L. K. Selin. 2005. Cross-reactive influenza virus-specific CD8+ T cells contribute to lymphoproliferation in Epstein-Barr virus-associated infectious mononucleosis. J. Clin. Investig. 115:3602-3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cornberg, M., A. T. Chen, L. A. Wilkinson, M. A. Brehm, S. K. Kim, C. Calcagno, D. Ghersi, R. Puzone, F. Celada, R. M. Welsh, and L. K. Selin. 2006. Narrowed TCR repertoire and viral escape as a consequence of heterologous immunity. J. Clin. Investig. 116:1443-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daftarian, P., S. Ali, R. Sharan, S. F. Lacey, R. C. La, J. Longmate, C. Buck, R. F. Siliciano, and D. J. Diamond. 2003. Immunization with Th-CTL fusion peptide and cytosine-phosphate-guanine DNA in transgenic HLA-A2 mice induces recognition of HIV-infected T cells and clears vaccinia virus challenge. J. Immunol. 171:4028-4039. [DOI] [PubMed] [Google Scholar]
- 13.Drexler, I., C. Staib, W. Kastenmuller, S. Stevanovic, B. Schmidt, F. A. Lemonnier, H. G. Rammensee, D. H. Busch, H. Bernhard, V. Erfle, and G. Sutter. 2003. Identification of vaccinia virus epitope-specific HLA-A*0201-restricted T cells and comparative analysis of smallpox vaccines. Proc. Natl. Acad. Sci. USA 100:217-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Earl, P. L., J. L. Americo, L. S. Wyatt, L. A. Eller, J. C. Whitbeck, G. H. Cohen, R. J. Eisenberg, C. J. Hartmann, D. L. Jackson, D. A. Kulesh, M. J. Martinez, D. M. Miller, E. M. Mucker, J. D. Shamblin, S. H. Zwiers, J. W. Huggins, P. B. Jahrling, and B. Moss. 2004. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature 428:182-185. [DOI] [PubMed] [Google Scholar]
- 15.Fang, M., and L. J. Sigal. 2005. Antibodies and CD8+ T cells are complementary and essential for natural resistance to a highly lethal cytopathic virus. J. Immunol. 175:6829-6836. [DOI] [PubMed] [Google Scholar]
- 16.Fujinami, R. S., and M. B. Oldstone. 1985. Amino acid homology between the encephalitogenic site of myelin basic protein and virus: mechanism for autoimmunity. Science 230:1043-1045. [DOI] [PubMed] [Google Scholar]
- 17.Fujinami, R. S., and M. B. Oldstone. 1989. Molecular mimicry as a mechanism for virus-induced autoimmunity. Immunol. Res. 8:3-15. [DOI] [PubMed] [Google Scholar]
- 18.Fulginiti, V. A., C. H. Kempe, W. E. Hathaway, D. Pearlman, O. Sieber, Jr., J. Eller, J. Joyner, Sr., and A. Robinson. 1968. Progressive vaccinia in immunologically deficient individuals. Birth Defects 4:129-145. [Google Scholar]
- 19.Fulginiti, V. A., A. Papier, J. M. Lane, J. M. Neff, and D. A. Henderson. 2003. Smallpox vaccination: a review, part II. Adverse events. Clin. Infect. Dis. 37:251-271. [DOI] [PubMed] [Google Scholar]
- 20.Gairin, J. E., H. Mazarguil, D. Hudrisier, and M. B. Oldstone. 1995. Optimal lymphocytic choriomeningitis virus sequences restricted by H-2Db major histocompatibility complex class I molecules and presented to cytotoxic T lymphocytes. J. Virol. 69:2297-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goebel, S. J., G. P. Johnson, M. E. Perkus, S. W. Davis, J. P. Winslow, and E. Paoletti. 1990. The complete DNA sequence of vaccinia virus. Virology 179:247-263. [DOI] [PubMed] [Google Scholar]
- 22.Hammarlund, E., M. W. Lewis, S. G. Hansen, L. I. Strelow, J. A. Nelson, G. J. Sexton, J. M. Hanifin, and M. K. Slifka. 2003. Duration of antiviral immunity after smallpox vaccination. Nat. Med. 9:1131-1137. [DOI] [PubMed] [Google Scholar]
- 23.Henderson, D. A., T. V. Inglesby, J. G. Bartlett, M. S. Ascher, E. Eitzen, P. B. Jahrling, J. Hauer, M. Layton, J. McDade, M. T. Osterholm, T. O'Toole, G. Parker, T. Perl, P. K. Russell, K. Tonat, et al. 1999. Smallpox as a biological weapon: medical and public health management. JAMA 281:2127-2137. [DOI] [PubMed] [Google Scholar]
- 24.Hogan, R. J., E. J. Usherwood, W. Zhong, A. A. Roberts, R. W. Dutton, A. G. Harmsen, and D. L. Woodland. 2001. Activated antigen-specific CD8+ T cells persist in the lungs following recovery from respiratory virus infections. J. Immunol. 166:1813-1822. [DOI] [PubMed] [Google Scholar]
- 25.Karupiah, G., R. M. Buller, N. Van Rooijen, C. J. Duarte, and J. Chen. 1996. Different roles for CD4+ and CD8+ T lymphocytes and macrophage subsets in the control of a generalized virus infection. J. Virol. 70:8301-8309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim, S. K., M. A. Brehm, R. M. Welsh, and L. K. Selin. 2002. Dynamics of memory T cell proliferation under conditions of heterologous immunity and bystander stimulation. J. Immunol. 169:90-98. [DOI] [PubMed] [Google Scholar]
- 27.Kim, S. K., M. Cornberg, X. Z. Wang, H. D. Chen, L. K. Selin, and R. M. Welsh. 2005. Private specificities of CD8 T cell responses control patterns of heterologous immunity. J. Exp. Med. 201:523-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim, S. K., and R. M. Welsh. 2004. Comprehensive early and lasting loss of memory CD8 T cells and functional memory during acute and persistent viral infections. J. Immunol. 172:3139-3150. [DOI] [PubMed] [Google Scholar]
- 29.Koszinowski, U., and R. Thomssen. 1975. Target cell-dependent T cell-mediated lysis of vaccinia virus-infected cells. Eur. J. Immunol. 5:245-251. [DOI] [PubMed] [Google Scholar]
- 30.Martin, K. H., D. W. Grosenbach, C. A. Franke, and D. E. Hruby. 1997. Identification and analysis of three myristylated vaccinia virus late proteins. J. Virol. 71:5218-5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masopust, D., V. Vezys, A. L. Marzo, and L. Lefrancois. 2001. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291:2413-2417. [DOI] [PubMed] [Google Scholar]
- 32.Matheis, H. 1971. Skin complications of smallpox vaccination. Dermatologica 142:340-343. (In German.) [PubMed] [Google Scholar]
- 33.Mathew, A., M. Terajima, K. West, S. Green, A. L. Rothman, F. A. Ennis, and J. S. Kennedy. 2005. Identification of murine poxvirus-specific CD8+ CTL epitopes with distinct functional profiles. J. Immunol. 174:2212-2219. [DOI] [PubMed] [Google Scholar]
- 34.McCraith, S., T. Holtzman, B. Moss, and S. Fields. 2000. Genome-wide analysis of vaccinia virus protein-protein interactions. Proc. Natl. Acad. Sci. USA 97:4879-4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moutaftsi, M., B. Peters, V. Pasquetto, D. C. Tscharke, J. Sidney, H. H. Bui, H. Grey, and A. Sette. 2006. A consensus epitope prediction approach identifies the breadth of murine T(CD8+)-cell responses to vaccinia virus. Nat. Biotechnol. 24:817-819. [DOI] [PubMed] [Google Scholar]
- 36.Murphy, J. G., R. S. Wright, G. K. Bruce, L. M. Baddour, M. A. Farrell, W. D. Edwards, H. Kita, and L. T. Cooper. 2003. Eosinophilic-lymphocytic myocarditis after smallpox vaccination. Lancet 362:1378-1380. [DOI] [PubMed] [Google Scholar]
- 37.O'Connell, C., D. Karzon, A. Barron, M. Plaut, and V. Ali. 1964. Progressive vaccinia with normal antibodies. A case possible due to deficient cellular immunity. Ann. Intern. Med. 60:282-289. [DOI] [PubMed] [Google Scholar]
- 38.Reference deleted.
- 39.Redfield, R. R., D. C. Wright, W. D. James, T. S. Jones, C. Brown, and D. S. Burke. 1987. Disseminated vaccinia in a military recruit with human immunodeficiency virus (HIV) disease. N. Engl. J. Med. 316:673-676. [DOI] [PubMed] [Google Scholar]
- 40.Resch, W., A. S. Weisberg, and B. Moss. 2005. Vaccinia virus nonstructural protein encoded by the A11R gene is required for formation of the virion membrane. J. Virol. 79:6598-6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Riddell, S. R., and P. D. Greenberg. 1995. Principles for adoptive T cell therapy of human viral diseases. Annu. Rev. Immunol. 13:545-586. [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez, F., J. Zhang, and J. L. Whitton. 1997. DNA immunization: ubiquitination of a viral protein enhances cytotoxic T-lymphocyte induction and antiviral protection but abrogates antibody induction. J. Virol. 71:8497-8503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Selin, L. K., M. Cornberg, M. A. Brehm, S. K. Kim, C. Calcagno, D. Ghersi, R. Puzone, F. Celada, and R. M. Welsh. 2004. CD8 memory T cells: cross-reactivity and heterologous immunity. Semin. Immunol. 16:335-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Selin, L. K., M. Y. Lin, K. A. Kraemer, D. M. Pardoll, J. P. Schneck, S. M. Varga, P. A. Santolucito, A. K. Pinto, and R. M. Welsh. 1999. Attrition of T cell memory: selective loss of LCMV epitope-specific memory CD8 T cells following infections with heterologous viruses. Immunity 11:733-742. [DOI] [PubMed] [Google Scholar]
- 45.Selin, L. K., S. R. Nahill, and R. M. Welsh. 1994. Cross-reactivities in memory cytotoxic T lymphocyte recognition of heterologous viruses. J. Exp. Med. 179:1933-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Selin, L. K., S. M. Varga, I. C. Wong, and R. M. Welsh. 1998. Protective heterologous antiviral immunity and enhanced immunopathogenesis mediated by memory T cell populations. J. Exp. Med. 188:1705-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Selin, L. K., and R. M. Welsh. 2004. Plasticity of T cell memory responses to viruses. Immunity 20:5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Slifka, M. K., and J. L. Whitton. 2000. Activated and memory CD8+ T cells can be distinguished by their cytokine profiles and phenotypic markers. J. Immunol. 164:208-216. [DOI] [PubMed] [Google Scholar]
- 49.Snyder, J. T., I. M. Belyakov, A. Dzutsev, F. Lemonnier, and J. A. Berzofsky. 2004. Protection against lethal vaccinia virus challenge in HLA-A2 transgenic mice by immunization with a single CD8+ T-cell peptide epitope of vaccinia and variola viruses. J. Virol. 78:7052-7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spriggs, M. K., B. H. Koller, T. Sato, P. J. Morrissey, W. C. Fanslow, O. Smithies, R. F. Voice, M. B. Widmer, and C. R. Maliszewski. 1992. Beta 2-microglobulin-, CD8+ T-cell-deficient mice survive inoculation with high doses of vaccinia virus and exhibit altered IgG responses. Proc. Natl. Acad. Sci. USA 89:6070-6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Terajima, M., J. Cruz, G. Raines, E. D. Kilpatrick, J. S. Kennedy, A. L. Rothman, and F. A. Ennis. 2003. Quantitation of CD8+ T cell responses to newly identified HLA-A*0201-restricted T cell epitopes conserved among vaccinia and variola (smallpox) viruses. J. Exp. Med. 197:927-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Townsend, A. R., J. Rothbard, F. M. Gotch, G. Bahadur, D. Wraith, and A. J. McMichael. 1986. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell 44:959-968. [DOI] [PubMed] [Google Scholar]
- 53.Tscharke, D. C., G. Karupiah, J. Zhou, T. Palmore, K. R. Irvine, S. M. Haeryfar, S. Williams, J. Sidney, A. Sette, J. R. Bennink, and J. W. Yewdell. 2005. Identification of poxvirus CD8+ T cell determinants to enable rational design and characterization of smallpox vaccines. J. Exp. Med. 201:95-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Upton, C., S. Slack, A. L. Hunter, A. Ehlers, and R. L. Roper. 2003. Poxvirus orthologous clusters: toward defining the minimum essential poxvirus genome. J. Virol. 77:7590-7600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van der Most, R. G., K. Murali-Krishna, J. L. Whitton, C. Oseroff, J. Alexander, S. Southwood, J. Sidney, R. W. Chesnut, A. Sette, and R. Ahmed. 1998. Identification of Db- and Kb-restricted subdominant cytotoxic T-cell responses in lymphocytic choriomeningitis virus-infected mice. Virology 240:158-167. [DOI] [PubMed] [Google Scholar]
- 56.Wedemeyer, H., E. Mizukoshi, A. R. Davis, J. R. Bennink, and B. Rehermann. 2001. Cross-reactivity between hepatitis C virus and influenza A virus determinant-specific cytotoxic T cells. J. Virol. 75:11392-11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Welsh, R. M., and L. K. Selin. 2002. No one is naive: the significance of heterologous T-cell immunity. Nat. Rev. Immunol. 2:417-426. [DOI] [PubMed] [Google Scholar]
- 58.Welsh, R. M., L. K. Selin, and E. Szomolanyi-Tsuda. 2004. Immunological memory to viral infections. Annu. Rev. Immunol. 22:711-743. [DOI] [PubMed] [Google Scholar]
- 59.Xu, R., A. J. Johnson, D. Liggitt, and M. J. Bevan. 2004. Cellular and humoral immunity against vaccinia virus infection of mice. J. Immunol. 172:6265-6271. [DOI] [PubMed] [Google Scholar]