Abstract
Cell-to-cell viral transfer facilitates the spread of lymphotropic retroviruses such as human immunodeficiency virus (HIV) and human T-cell leukemia virus (HTLV), likely through the formation of “virological synapses” between donor and target cells. Regarding HIV replication, the importance of cell contacts has been demonstrated, but this phenomenon remains only partly characterized. In order to alter cell-to-cell HIV transmission, we have maintained cultures under continuous gentle shaking and followed viral replication in this experimental system. In lymphoid cell lines, as well as in primary lymphocytes, viral replication was dramatically reduced in shaken cultures. To document this phenomenon, we have developed an assay to assess the relative contributions of free and cell-associated virions in HIV propagation. Acutely infected donor cells were mixed with carboxyfluorescein diacetate succinimidyl ester-labeled lymphocytes as targets, and viral production was followed by measuring HIV Gag expression at different time points by flow cytometry. We report that cellular contacts drastically enhance productive viral transfer compared to what is seen with infection with free virus. Productive cell-to-cell viral transmission required fusogenic viral envelope glycoproteins on donor cells and adequate receptors on targets. Only a few syncytia were observed in this coculture system. Virus release from donor cells was unaffected when cultures were gently shaken, whereas virus transfer to recipient cells was severely impaired. Altogether, these results indicate that cell-to-cell transfer is the predominant mode of HIV spread and help to explain why this virus replicates so efficiently in lymphoid organs.
Numerous studies have demonstrated that lymphotropic retroviruses such as human T-cell leukemia virus type 1 (HTLV-1) and human immunodeficiency virus (HIV) efficiently disseminate through cell-to-cell contacts (16, 22-24, 36, 38-40). With HTLV-1, cell-to-cell transfer is probably the only means of propagation, since this virus is barely released from producer cells. The situation may be different with HIV type 1 (HIV-1), which is efficiently secreted, reaching up to a few μg of Gag p24 per ml of cell supernatant. The relative contribution of cell-free virus versus that of cell-associated virus in viral propagation is poorly known. Cell-to-cell viral transmission is, however, a rapid and potent phenomenon (11, 38, 40) (27). Cells naturally communicate by exchanging information through close contacts, which are associated with a coordinated rearrangement of receptors and other molecules at the junction region. These organized contacts, or synapses, are particularly important in immune cells, for instance promoting an adequate response of the host to pathogens (19, 33). By analogy to the immune synapse, the term “virological synapse” has been coined to designate the molecular modifications occurring during HTLV-1 transfer (22). Cell contacts induce an HTLV-1-infected cell to polarize its microtubule-organizing center towards the cell-cell junction (5, 34). HTLV-1 proteins and genome, as well as adhesion molecules, accumulate at the junction, facilitating viral spread. This is also the case for HIV. At the lymphocyte-lymphocyte junction, viral materials and adhesion molecules concentrate in donor cells, in a process involving raft microdomains, whereas on the other side, in target cells, viral receptors (CD4 and CXCR4 or CCR5) and cognate adhesion molecules accumulate in a cytoskeleton- and actin-dependent mechanism (24, 25, 36). Virological or “infectious” synapses are also formed between dendritic cells (DCs) exposed to HIV particles and target lymphocytes through the same principle (31). Productive infection of DCs is not required for viral transfer, at least during a short time frame (a few hours) after viral internalization by DCs: captured virions may be directly transmitted after trafficking through multi-vesicular-body-like compartments in DCs (3, 18, 35, 39). Virological synapses between infected cells and epithelial cells also facilitate HIV transcytosis of the latter (2).
In vivo, viral transmission through direct contact between infected cells and targets may represent an important pathway of contamination of naïve individuals (38). Subsequent DC-T-cell viral transfers likely contribute to the spread of infection after viral entry through mucosal surfaces. HIV (as well as simian immunodeficiency virus) then propagates very rapidly within secondary lymphoid tissues, particularly in the gastrointestinal tract (30) (28). The great majority of productively infected cells are CD4+ lymphocytes, and it is likely that HIV directly spreads from T cell to T cell (21, 41). During all stages of the disease, most of the virus present in the organism at a given time is localized in lymphoid tissues, although acute infection is associated with high levels of viremia (up to 106 to 107 copies of viral RNA/ml of blood). Plasma virus mainly comes from freshly infected lymphocytes, which have a short half-life (1 to 2 days) and which may have been previously infected in lymph nodes (21, 41). An obvious difference between blood and tissue lymphocytes is mobility. In the blood, the speed of free-flowing lymphocytes is dependent on the strength of the stream and may reach 15 mm/s (17). Lower speeds are observed during leukocyte recruitment from blood to tissues, a process involving cell tethering, rolling, and adhesion to vessels (32, 47). In lymph nodes, lymphocyte velocity is reduced by multiple orders of magnitude (around 1 to 10 μm/min), facilitating contacts between cells (9) (12) and thus the formation of immunological or virological synapses.
Classically, in vitro assays to assess viral replication are performed with static cultures and do not represent the situation that may be found for fluids. In this study, we have compared HIV replication kinetics in static and continuously shaken cultures. Viral growth was dramatically reduced in mobile cells. We have documented this phenomenon by developing a flow cytometry-based assay to monitor the productive infection of target cells. With this assay, we confirm previous studies showing that transfer through direct cell-cell contacts is more potent and rapid than that with cell-free virions. Interestingly, cell-to-cell viral transfer was strongly impaired by gentle shaking, demonstrating that cell-free HIV virions play a minor role in static cultures.
MATERIALS AND METHODS
Cells and viruses.
Jurkat T cells, P4 cells, and human peripheral blood mononuclear cells (PBMCs) were prepared and grown as described previously (29, 45). Primary CD4+ T lymphocytes were isolated untouched from PBMCs by use of magnetic beads (CD4+ T-cell isolation kit II; Miltenyi Biotec, Germany). For activation, primary T cells were treated with phytohemagglutinin (PHA) (1 μg/ml) for 24 h at 37°C and cultured in interleukin 2 (IL-2)-containing medium (100 IU/ml). Nonactivated T cells were kept in medium without IL-2. The production and use of wild-type (WT) HIV NL4-3 or NLAD8 strains, and of NLΔenv and NL F522Y mutants, have been described previously (29, 35, 37). NL F522Y provirus encodes a nonfusogenic gp120/g41 complex (35). To obtain Jurkat cells expressing the NLΔenv and NL F522Y mutants, these cells were exposed to viruses pseudotyped with vesicular stomatitis virus type G (VSV-G). NL4-3 was similarly produced as a control. Pseudotyped viruses were generated by cotransfection of HeLa cells with the corresponding proviruses and a VSV-G expression plasmid.
HIV infections.
Jurkat and CD4+ lymphocytes were exposed to the indicated viruses (0.1 or 1 ng p24/0.5 ml/106 cells) for 2 h at 37°C without rocking, washed, and seeded in 25-cm2 flasks or six-well plates at concentrations of 0.5 × 106/ml and 1 × 106/ml for Jurkat and primary lymphocytes, respectively. Flasks or plates were then either kept in a static position or placed on a rocker (SpeciMix; Bioblock Scientific) and gently shaken (40 movements/min). Viral release was monitored by measuring p24 production in supernatants by enzyme-linked immunosorbent assay (ELISA) (Perkin-Elmer Life Science). Gag p24 expression in infected cells was assessed by flow cytometry (see below). P4 cells were infected in suspension (5 or 0.5 ng p24/0.5 ml/0.5 × 106 cells), washed, and seeded in 24-well plates at 5 × 104 cells/well. Plates were then kept static or shaken, as for lymphocytes. Viral infection was assessed by measuring β-galactosidase activity in cell extracts 24 or 48 h postinfection (p.i.) (29). For detection of infection of P4 cells by Gag staining, cells were infected in suspension (100 ng p24/ml/106 cells), washed, and seeded in six-well plates for 2 h to allow adhesion. Plates were then kept static or shaken, and Gag expression was detected by flow cytometry at the indicated days p.i.
Intracellular and surface molecule stainings.
Cell surface stainings were performed at 4°C for 30 min using monoclonal antibodies (mAbs) directed against the following molecules: CD4 (13B8.2 [allophycocyanin]; Beckman Coulter), CD3 (SP34-2 [peridin-chlorophyll protein complex]; BD-Pharmingen), major histocompatibility complex I (W632 [fluorescein isothiocyanate]), CXCR4 and CCR5 (12G5 and 2D7; NIH AIDS Research and Reference Reagent Program), CD11a (TS1/22; ATCC), CD18 (TS1/18; ATCC), and ICAM-1 and ICAM-3 (F10.2 and CBRR-IC3/1, respectively; Fifth Workshop on Human Leukocyte Differentiation Antigens) were a kind gift from Andres Alcover, Institut Pasteur. Gag p24 expression in infected cells was measured after permeabilization and intracellular staining with anti-Gagp24 fluorescein isothiocyanate mAb (KC57; Coulter). Isotype-matched mAbs were used as negative controls. Samples were analyzed by flow cytometry using a FACSCalibur instrument (Becton Dickinson) with CellQuest software.
Analysis of cell-to-cell HIV transfer by flow cytometry.
Donor cells were infected with the indicated strains of HIV and used a few days later, when about 10 to 75% of the cells were Gag+. The indicated target cells were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) (2.5 μM; Molecular Probes) for 10 min at 37°C. Donor and target cells were then mixed at the indicated ratio in 96-well plates at a final concentration of 1× 106/ml in a final volume of 200 μl. At the indicated time points, cells were stained for intracellular Gag expression as described above and analyzed by flow cytometry. When stated, nevirapine (NVP; 12.5 nM) was added 0.5 h before coculturing and maintained during the assay. To assess the consequence of shaking on HIV cell-to-cell transfer, cocultures of donors and targets were maintained at a final concentration of 1 × 106/ml in a volume of 1.5 ml in six-well plates and either kept static or placed on a rocker. When stated, a Transwell chamber with a virus-permeable membrane (3-μm pore size) (tissue culture inserts; Nunc) was employed, with donor cells placed on the upper part and recipient cells in the lower part.
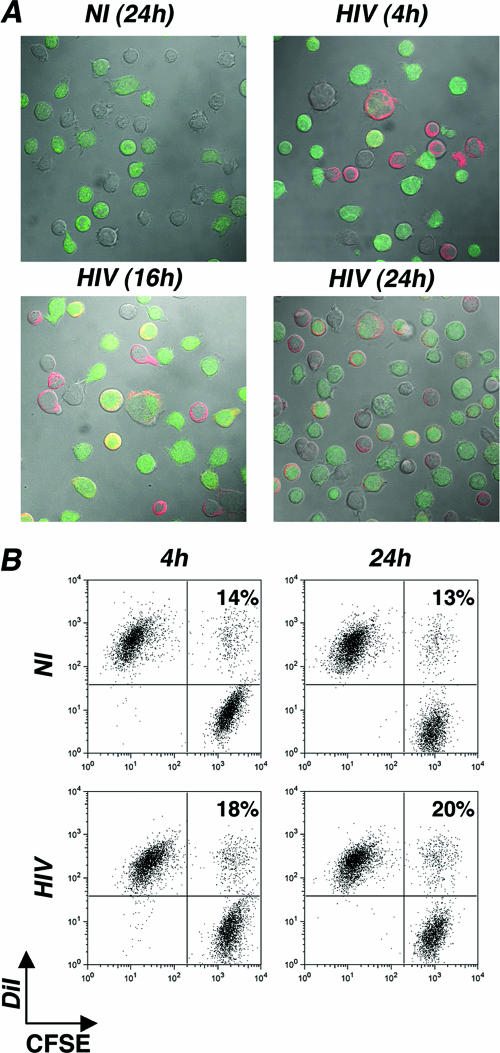
Analysis of syncytia and cell conjugates by confocal microscopy and by flow cytometry.
Donor cells were infected with the indicated strains of HIV and used a few days later, when about 10 to 75% of the cells were Gag+. For confocal microscopy, the indicated target cells were labeled with CFSE (2.5 μM; Molecular Probes) for 10 min at 37°C. Donor and target cells were then mixed at the indicated ratio in 96-well plates at a final concentration of 1 × 106/ml in a final volume of 200 μl. At the indicated time points, cells were stained for intracellular Gag expression. Confocal microscopy analysis was carried out on a Zeiss LSM510 instrument with a 63× objective. Green fluorescence and red fluorescence were acquired sequentially to the prevent the passage of fluorescence from one channel into the other. Quantitative analysis of cell-cell clustering and fusion was performed with a flow cytometry assay adapted from reference 4. Donor and target cells were labeled with CFSE (green fluorescence) and DiI (Molecular Probes, Eugene, Oregon) (red fluorescence), respectively. Cells (1 × 106 in 200 μl) were left in contact with DiI (2 μM in Dulbecco's modified Eagle's medium) for 5 min at 37°C. Labeling was ended by washing twice with phosphate-buffered saline, and cells were resuspended in culture medium. After coculturing, analysis was performed with a FACSCalibur instrument (Becton Dickinson). Only cells displaying relatively high levels of fluorescence in both green and red wavelengths were scored as double-fluorescent cells.
RESULTS
Inefficient HIV-1 replication in shaken T-cell cultures.
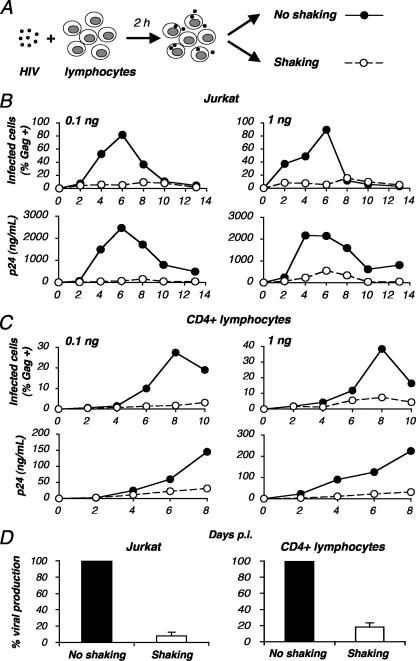
We set out to establish an experimental system to study HIV replication in mobile lymphocytes. We did this by comparing the levels of viral growth of static and shaken cell cultures by following the procedure outlined in Fig. 1A. Jurkat lymphoid cells or primary CD4+ lymphocytes were exposed to viral preparations (at two inocula, 0.1 and 1 ng of p24/106 cells, respectively) for 2 hours at 37°C, washed, and then split into two culture plates. One plate was maintained in a typical static position, whereas the other was placed on a rocker in order to obtain gentle and continuous shaking of the cells (40 movements/min). Viral replication was then assessed by measuring both p24 production in cell supernatants by ELISA and the percentage of p24+ cells by flow cytometry. In Jurkat cells under static conditions, levels of p24 production peaked at 3,000 ng/ml at days 6 and 8 p.i. with the low and high viral inocula, respectively (Fig. 1B). Strikingly, these levels were dramatically reduced in shaken cells, with peaks at 300 and 500 ng/ml, respectively. In static cultures, viral replication was associated with the progressive appearance of Gag p24+ cells, reaching, at day 8 p.i., 80% or 90% of the cell population (depending on the inoculum) (Fig. 1B). With rocking, the fraction of productively infected p24+ cells was significantly reduced, reaching only 10 to 15%.
FIG. 1.
Inefficient HIV-1 replication in shaken cultures of lymphocytes. (A) Experimental protocol. Jurkat or primary CD4+ T cells were exposed to HIV (NL4-3 strain) for 2 h, unbound virus was removed, and cells were cultivated in static conditions (no shaking) or placed on a rocker and continuously and gently shaken (40 movements/min). Viral replication was then measured at different days p.i. (B to D) HIV-1 replication in static or shaken Jurkat (B) and primary CD4+ (C) T cells. Cells were exposed to the indicated HIV inocula (0.1 and 1 ng p24/106 cells). Viral replication is depicted as the percentage of Gag+ cells as measured by flow cytometry (upper panels) and as Gag p24 production in supernatants as measured by ELISA (lower panels). Data are representative of three independent experiments. (D) Means ± standard deviations (SD) of three independent experiments are depicted, with 100% corresponding to supernatant Gag p24 values obtained at the peak of infection (day 6 or day 8 p.i.).
A similar observation was made with primary CD4+ lymphocytes activated by PHA and maintained with IL-2 before viral exposure (Fig. 1C). Whatever the viral inoculum, there was a 5- to 10-fold decrease in the efficiency of HIV replication, as measured either by p24 release or by the percentage of Gag p24+ cells, when primary cells were continuously shaken.
In three independent experiments with shaken Jurkat or primary lymphocytes, viral replication, as assessed by the levels of p24 production in the supernatants at the peak (days 6 or 8 p.i.), corresponded to 10 to 15% of the values obtained from static cultures (Fig. 1D).
We then analyzed a panel of HIV strains in shaken cells. We used two R5-tropic strains (YU2-B and JR-CSF) and three primary strains (one X4 and two R5-tropic virus strains) directly isolated from PBMCs of HIV-infected individuals. For HIV NL4-3, as for all other viruses tested, viral replication was strongly impaired in shaken primary lymphocytes compared to what was seen for static cultures (not shown). Therefore, an inefficient viral replication in shaken lymphocytes is not a special feature of the T-cell-line-adapted strain HIV NL4-3.
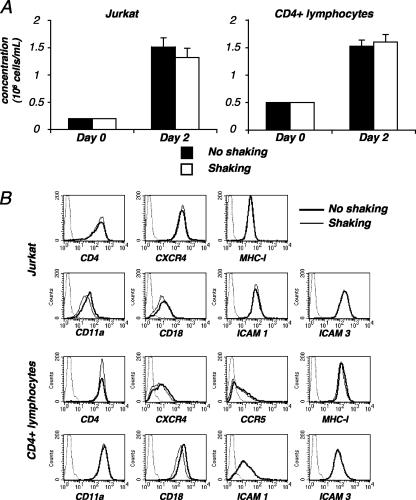
It was important to verify that the shaking did not modify the metabolism of the cells with nonspecific consequences on HIV replication. The viability of noninfected cells was apparently not affected by this continuous and gentle shaking (not shown). We also measured the growth rates of the cells (Jurkat and primary lymphocytes), and we observed similar growth values with and without rocking (Fig. 2A). The expression levels of viral receptors (CD4, CXCR4, and CCR5), of a panel of adhesion molecules including ICAM-1, ICAM-3, and LFA-1 (CD11a and CD18 chains), and of major histocompatibility complex I were similar for static and mobile cells (Fig. 2B).
FIG. 2.
Characteristics of shaken T cells. (A) Growth kinetics of shaken cells. Noninfected Jurkat cells (left panel) or primary CD4+ lymphocytes (right panel), at 2 × 105 cells/ml, were grown with or without rocking. At the indicated time points, concentrations of living cells were measured in cultures. Data are means ± SD of triplicates and are representative of four independent experiments. (B) Surface expression of various receptors in shaken lymphocytes. Noninfected Jurkat cells (upper panels) or primary CD4+ lymphocytes (lower panels) were kept static or shaken for 24 or 48 h, respectively. Cells were then stained with antibodies against the indicated surface receptors and analyzed by flow cytometry. An isotypic mAb was used as a negative control (dotted line). Data are representative of three independent experiments. MHC-I, major histocompatibility complex I.
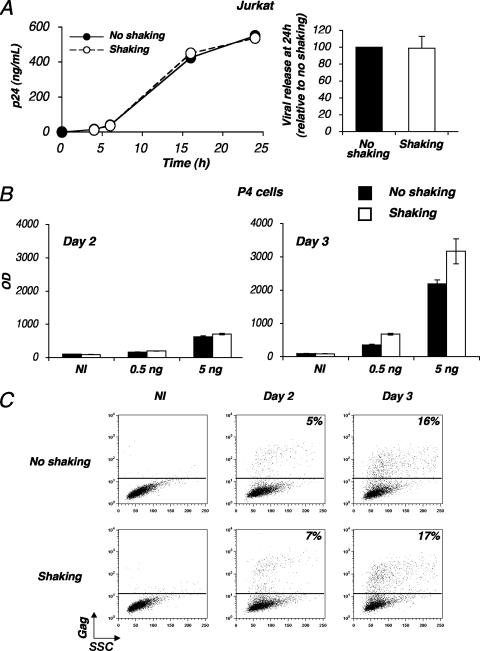
To document the consequence of cell shaking on HIV release, Jurkat cells were infected with HIV and maintained under static conditions. Two days later, when about 25% of the cells expressed HIV Gag antigens, further viral replication was stopped with the reverse transcriptase inhibitor NVP. Levels of virus production in supernatants from shaken and static cells were then compared, and no significant differences were detected in this system (Fig. 3A), indicating that cell rocking does not affect viral release.
FIG. 3.
Effects of shaking on HIV replication. (A) Shaking does not affect viral release from lymphocytes. Jurkat cells were productively infected with HIV (NL4-3 strain). After a few days of culturing, when about 25% of the cells expressed Gag antigens, cells were treated with NVP to prevent further viral spread. (Left panel) Viral release from infected cells was followed by measuring Gag p24 content in supernatants at the indicated time points. Data are representative of three independent experiments. (Right panel) Relative efficiency of each experimental condition. A mean ± SD of three independent experiments (24-h time point) is depicted, with 100% corresponding to values obtained without shaking. (B and C) HIV infection of static or shaken HeLa CD4 cells. HeLa CD4 cells (P4 clone) were infected with HIV (NL4-3 strain) at the indicated viral doses (0.5 or 5 ng p24/0.5 × 106 cells for panel B; 100 ng p24/1 × 106 cells for panel C). Cells were then kept static or were gently shaken. (B) Infection was assessed at days 2 and 3 p.i. by measuring β-galactosidase activity in cell extracts. Data are means ± SD of triplicates and are representative of three independent experiments. (C) Infection was assessed by measuring Gag p24 expression by flow cytometry. Data are representative of three independent experiments. NI, noninfected cells; OD, optical density; SSC, side scatter.
We next examined the consequences of shaking on HIV infection of adherent cells. HeLa CD4 cells carrying an integrated HIV long terminal repeat lacZ reporter cassette (P4 clone) were exposed to HIV and maintained with or without shaking. There was no inhibition of viral replication, assessed by measuring β-galactosidase activity, at days 2 and 3 p.i. (Fig. 3B). We verified that viral spread actually occurred between days 2 and 3 in P4 cells. We measured the appearance of Gag+ cells by flow cytometry over time (Fig. 3C). About 5 and 16% of the static cells were Gag+ at days 2 and 3 p.i., respectively. A similar progression was detected for cells under conditions of gentle shaking (Fig. 3C). It is noteworthy that the mean fluorescence intensities of Gag expression were similar under shaking and static conditions, strongly suggesting that viral gene expression per se is not affected.
Altogether, these results indicate that a gentle rocking of immortalized or primary lymphocytes does not alter cell growth but dramatically impairs HIV spread. Shaken lymphocytes are not affected in their ability to release HIV particles. This impairment of viral replication is not observed for adherent HeLa CD4+ cells.
Analysis of cell-to-cell HIV transfer by flow cytometry.
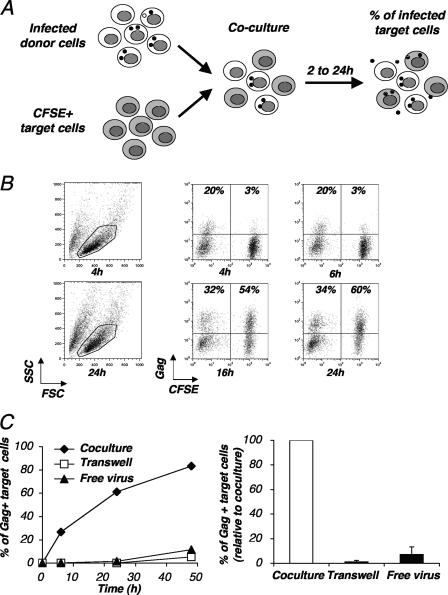
We documented the role of cellular contacts during HIV-1 spread. We designed a flow cytometry-based assay to follow the appearance of Gag p24+ cells in freshly infected cells (Fig. 4A). Donor lymphocytes were first productively infected with HIV. A few days later, a significant fraction (10 to 75%) of the cells expressed HIV antigens. These infected cells were then cocultured with target cells labeled with CFSE. The levels of Gag p24 expression were measured for CFSE− donor cells and for CFSE+ target cells at different incubation times (0 to 24 h). We first used Jurkat cells as donors and as recipients. A typical experiment, in which about 20% of the donor cells were Gag+ at the beginning of the assay, is depicted in Fig. 4B. Under this setting, target cells become very rapidly infected. At 4 to 6 h postcoculture, 3% of the targets expressed HIV Gag antigens, and this fraction increased rapidly, reaching 60% Gag p24+ cells by 24 h (Fig. 4B).
FIG. 4.
Analysis of cell-to-cell HIV transfer by flow cytometry. (A) Principle of the flow cytometry assay. Lymphocytes were productively infected with HIV. After a few days of culturing, when about 10 to 75% of the cells expressed Gag antigens, cells were cocultivated with recipient cells stained with CFSE. Gag expression was then measured for target (CFSE+) cells at various time points by flow cytometry. (B) A representative experiment. Productively infected Jurkat cells (20% of Gag+ cells at the beginning of the assay) were cocultivated with target CFSE+ Jurkat cells at a 1/1 ratio. The percentages of Gag+ cells among donors and targets are indicated at 4, 6, 16, and 24 h postcoculture. Cell viability was not significantly affected by the coculture, as visualized by side (SSC) and forward (FSC) scatter plots at 4 h and 24 h. Data are representative of at least 10 independent experiments. (C) Efficient HIV spread requires contact between infected and target lymphocytes. Productively infected Jurkat cells were cocultivated with target CFSE+ Jurkat cells at a 1/1 ratio either directly or in a Transwell chamber in which donors and recipient cells were separated by a virus-permeable membrane. Targets were also directly exposed to a high concentration of free virus (200 ng/ml). (Left panel) Results are presented as the percentages of Gag+ cells within CFSE+ Jurkat targets. (Right panel) Relative efficiency of each experimental condition. Means ± SD of three independent experiments (24-h time point) are depicted, with 100% corresponding to values obtained for target cells by direct coculturing.
We then asked whether viral transfer required a direct contact between donors and recipients. We compared the potency of viral propagation in cocultures with that in cells separated by a virus-permeable membrane in a Transwell chamber. With direct contact, infection was very efficient, with 50% and 80% of the targets becoming Gag+ by 24 h and 48 h postcontact, respectively (Fig. 4C). In the Transwell chamber, newly synthesized virus easily diffused through the membrane, reaching about 180 and 250 ng/ml of p24 (at 24 and 48 h of coculture, respectively) in the part of the chamber containing target cells. However, fewer than 10% of the recipients were productively infected at 48 h (Fig. 4C). To rule out the possibility that a slow diffusion of virions through the membrane may impair the infection of target cells, we then directly exposed lymphocytes to the same amount of cell-free virions (250 ng/ml). Stainings revealed that only 10% of the cells were Gag+ after 48 h of infection (Fig. 4C). These experiments confirmed that the kinetics of HIV-1 infection are much more rapid during cell-to-cell transmission than that with cell-free virus (16). In our flow cytometry assay, the rapid appearance of infected targets required a direct contact with donors.
Contacts between HIV-infected and receptor-expressing cells may induce cell fusion and syncytium formation. Some syncytia were visible in our cocultures, but at relatively low levels within the time frame of the experiment. Syncytia were scored by measuring, with a microscope, the fraction of multinucleated giant cells among CFSE+ Gag+ cells. By 16 to 24 h after initiation of the coculture, less than 10% of CFSE+ Gag+ Jurkat cells were engaged in syncytia (Fig. 5A). By flow cytometry, we did not observe significant numbers of large cells in forward scatter/side scatter dot plot diagrams, probably because these multinucleated cells are fragile and were in large part lost during the staining procedure (Fig. 4B).
FIG. 5.
Analysis of syncytium formation in Jurkat cells. (A) Confocal microscopy analysis. Jurkat cells were productively infected with HIV. After a few days of culturing, when about 70% expressed Gag antigens, cells were cocultivated at a 1/1.5 ratio with recipient cells stained with CFSE. Gag (red) and CFSE stainings are depicted at various time points of the coculture. One out of three representative experiments is shown. (B) Flow cytometry analysis. Productively infected Jurkat cells (20% of Gag+ cells) were stained with the fluorescent probe DiI (red) and cocultivated with target CFSE+ Jurkat cells at a 1/1 ratio. The percentages of double-fluorescent cells (DiI+ CFSE+ cells among total CFSE+ cells), which correspond to cell-cell clustering or fusion, are depicted at the indicated time points. Data are representative of three independent experiments. NI, noninfected cells.
The fusion of pairs of cells may not give rise obviously to syncytia but would nevertheless represent cell-cell fusion. The increase of Gag+ cells over time may also represent increased clustering of cells into doublets or more. To further document cell clustering and fusion, we performed a simple flow cytometry assay which detects the formation of double-fluorescent cells (4). To this end, infected donors and targets were labeled with distinct membrane-associated fluorescent probes, emitting in the red (DiI) or the green (CFSE) wavelength. Membrane exchanges during fusion or cell doublets may yield double-fluorescent objects (4). A representative experiment, in which infected Jurkat cells (20% of Gag+ cells) were stained with DiI, used as donors, and mixed with CFSE-labeled target Jurkat cells, is depicted in Fig. 5B. With control noninfected cells, the fraction of double-fluorescent objects was about 13% at 4 h and 24 h postcoculture. This fraction likely represents cell doublets, or cells having spontaneously acquired cell-derived components or debris. With infected Jurkat cells, we observed a small but reproducible increase of double-positive cells, an increase which reached 20% at 24 h (Fig. 5B). Therefore, fusion or clustering events involve only a small fraction of infected cells in this experimental system compared to what is seen for control cultures.
Altogether, these results indicate that the flow cytometry assay measuring Gag transfer to CFSE+ targets represents a quantitative mean to detect the spread of productive infection to recipient cells, irrespective of syncytium or cell cluster formation.
Characteristics of HIV cell-to-cell transfer.
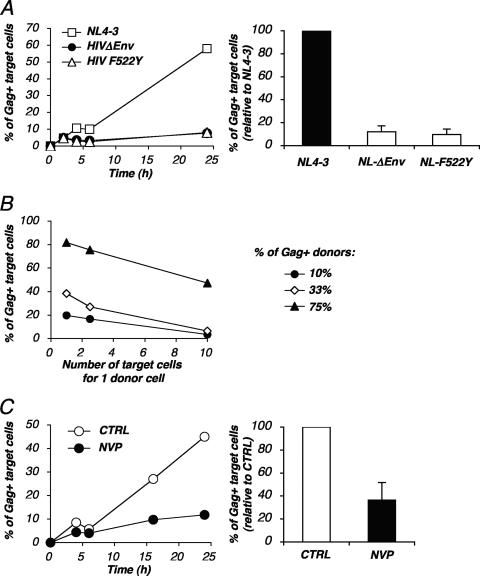
We investigated further the characteristics of HIV dissemination through cell-to-cell contacts. We next studied the role of viral envelope glycoproteins in this assay. Donor cells were infected with viruses devoid of viral envelope (HIVΔenv mutant) or carrying a fusion-defective envelope that retains its ability to bind CD4 (HIV F522Y mutant) (6, 10). The productive entry of the mutants was ensured by pseudotyping the virions with the VSV-G envelope. After a single round of infection, about 30 to 45% of the cells were Gag+ and produced noninfectious virions (not shown). These infected cells were then used as donors in the flow cytometry assay. Very few if any Gag+ cells were observed among targets with either the HIVΔenv mutant or the HIV F522Y mutant: about 5% of the recipients were positive (Fig. 6A), corresponding to a 10-fold decrease of viral transfer, compared to what was seen with the WT virus. Therefore, viral dissemination in this assay requires an envelope-dependent fusion event. The residual transfer detected with the mutant viruses may correspond to low levels of receptor-independent or fusion-independent spread of HIV, a phenomenon previously described for primary CD4 T cells (7, 8). It is also possible that in the absence of fusion, a greater fraction of incoming virions is captured by endocytosis (42).
FIG. 6.
Roles of viral envelope glycoproteins and reverse transcriptase in HIV cell-to-cell transfer. (A) HIV cell-to-cell transfer requires fusogenic envelope glycoproteins. Jurkat cells infected with wild-type (NL4-3) or envelope-deleted (HIVΔenv) viruses or with a mutant virus (HIV F522Y mutant) carrying a nonfusogenic gp120 were used as donors in the cell-to-cell transfer assay. Infection of donors was performed with VSV-G-pseudotyped viruses in order to obtain about 30 to 45% Gag+ cells. These cells were then cocultivated with target CFSE+ Jurkat cells at a 1/2.5 ratio for the indicated time points. (Left panel) Results are presented as the percentages of Gag-positive cells within CFSE+ Jurkat targets. (Right panel) Relative efficiency of each experimental condition. Means ± SD of four independent experiments (24-h time point) are depicted, with 100% corresponding to values obtained for target cells with the wild-type virus. (B) Quantitative analysis of HIV cell-to-cell transfer. Productively HIV-infected Jurkat cells (10%, 33%, and 75% Gag+ cells, respectively, at the beginning of the assay) were cocultivated with target CFSE+ Jurkat cells at the indicated number of targets for one donor cell. The percentages of Gag+ cells among CFSE+ targets at 24 h postcoculture are depicted. Data are representative of five independent experiments. (C) HIV cell-to-cell transfer is inhibited by nevirapine. Productively infected Jurkat cells were cocultivated with target CFSE+ Jurkat cells with or without NVP, a reverse transcriptase inhibitor. (Left panel) Results are presented as the percentages of Gag-positive cells within CFSE+ Jurkat targets. (Right panel) Relative efficiency of each experimental condition. A mean ± SD of four independent experiments (24-h time point) is depicted, with 100% corresponding to values obtained for target cells without NVP. CTRL, control.
We also performed a dose-response analysis of viral dissemination. We used a donor population in which the fraction of Gag+ cells varied from 10% to 75%. As expected, viral transfer was much more efficient when the donors were highly infected, even though 10% of Gag+ donors were sufficient to transmit infection to targets (Fig. 6B). We also used different ratio of donor to target cells (from 1:1 to 1:10). There was a correlation between the efficiency of viral transfer and the donor-to-target ratio (Fig. 5B). This correlation was observed at different percentages of infected cells among donors.
When target cells were incubated with the reverse transcriptase inhibitor NVP (12.5 nM), the fraction of Gag+ targets at 24 h strongly decreased (Fig. 6C). A compilation of six independent experiments, with various percentages of infected donor cells and donor cell/target cell ratios, indicated that NVP decreased the Gag signal in recipients by about 2.5-fold at 24 h postcoculture (Fig. 6C). Therefore, this Gag signal mostly originates from newly synthesized viral proteins. Interestingly, at earlier time points (4 h and 6 h) about 5% of recipient cells were already Gag+, and this signal was insensitive to NVP treatment (Fig. 4A). It likely corresponded to the transfer of incoming viral material to target cells, a process which is known to occur early after conjugate formation between infected and target cells (24).
Altogether, these results demonstrate that this sensitive assay allows a quantitative assessment of HIV transmission through direct cell-to-cell interactions.
HIV cell-to-cell transfer in primary lymphocytes.
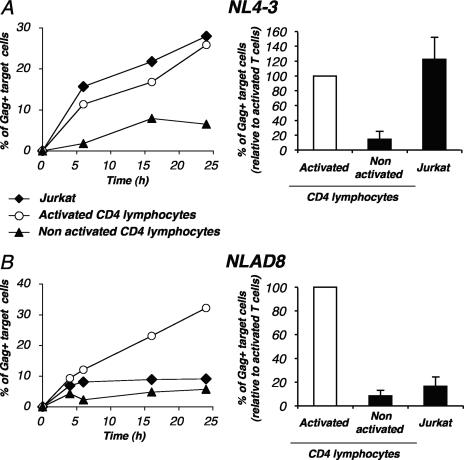
We then studied the parameters of HIV cell-to-cell transfer in primary lymphocytes. CD4+ T cells were activated with PHA and IL-2, productively infected with the X4 strain HIV NL4-3, and used as donors for various recipient cells. We first followed viral transfer from primary lymphocytes to purified autologous activated CD4 cells. Some syncytia could be detected in the 24-h coculture, albeit at lower levels than in Jurkat cells (not shown). The double-fluorescent staining (DiI and CFSE) was also used with primary CD4+ lymphocytes to detect cell fusion or clustering. The amounts of double-positive objects after 24 h of coculture between HIV-infected donor cells and recipients were similar to those observed when noninfected cells were used as donors (not shown). This confirmed that primary cells are relatively resistant to syncytium formation compared to lymphoid cell lines (40) (24).
About 25% of target cells became Gag+ after 24 h of coculture, as seen in the representative experiment depicted in Fig. 7A. These levels were similar when Jurkat cells were the recipients of infection (Fig. 7A). No significant viral transfer was detectable when targets were separated from donors in a Transwell system (not shown), indicating that as for Jurkat cells, the assay detected viral transfer essentially through direct cell contacts. HIV replicates poorly in nonactivated primary CD4+ T cells (46). When nonactivated autologous CD4+ lymphocytes were used as targets, viral transfer was minimal, plateauing at 5% of Gag+ cells (Fig. 7A). This likely corresponds to the transfer of the viral inoculum in the absence of efficient productive infection. We then extended the analysis of viral transmission to an R5-tropic strain (HIV NLAD8). Primary infected lymphocytes efficiently transmitted this virus to autologous activated CD4+ cells, but not to Jurkat cells, which lack the cognate coreceptor CCR5 (Fig. 7B).
FIG. 7.
HIV cell-to-cell transfer in primary lymphocytes. Primary CD4+ T cells were activated with PHA and maintained in IL-2 and then infected with the X4 strain NL4-3 (A) or with the R5 strain NLAD8 (B) in order to obtain about 30% Gag+ cells. These cells were then used as donors in the flow cytometry-based assay of viral transfer. Infected cells were cocultivated with the indicated target CFSE+ cells, i.e., Jurkat cells, or autologous nonactivated or activated CD4 lymphocytes, at a 1/1 ratio at the indicated time points. (Left panels) Results are presented as the percentages of Gag-positive cells within CFSE+ targets. One out of four independent experiments is shown. (Right panels) Relative efficiency of each experimental condition. Means ± SD of four independent experiments (24-h time point) are depicted, with 100% corresponding to values obtained with activated CD4+ lymphocytes as targets.
Altogether, these experiments indicate that cell-to-cell transfer is the predominant mode of HIV transfer in cultured primary lymphocytes. Very low levels of coreceptor-independent transmission events were detected by flow cytometry.
HIV cell-to-cell transfer in shaken lymphocytes.
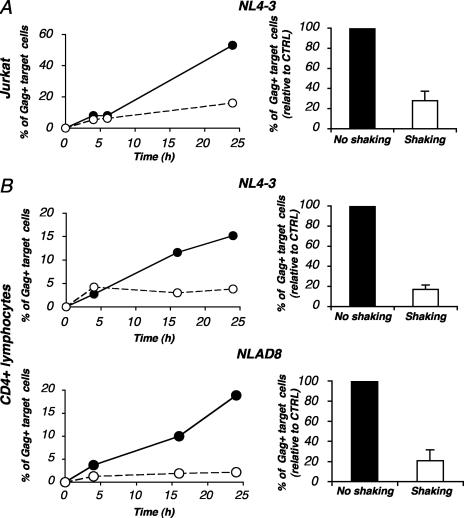
HIV poorly replicates when lymphocytes are shaken (Fig. 1). We asked whether this impaired replication was caused by an alteration of viral cell-to-cell transfer in shaken cells. We compared levels of HIV transmission from donors to CFSE+ targets under static and shaken culture conditions by using a flow cytometry-based assay. With Jurkat cells, shaking dramatically impaired the appearance of Gag+ cells in recipient cells (Fig. 8A). Similar results were obtained for primary lymphocytes with both X4 and R5 viral strains (Fig. 8B). Therefore, in shaken lymphocytes, viral replication is severely impaired as a consequence of an altered cell-to-cell virus spread. Our experiments indicate that free virus plays a minor role when HIV replication is studied by means of cell culture experiments.
FIG. 8.
Inefficient HIV cell-to-cell transfer in shaken lymphocytes. Productively infected Jurkat cells (A) or primary CD4+ cells (B) (20 to 25% Gag+ cells) were cocultivated with target CFSE+ Jurkat cells or primary CD4+ cells, respectively, with or without shaking. (Left panels) Results are presented as the percentages of Gag+ cells within CFSE+ targets. (Right panels) Relative efficiency of each experimental condition. Means ± SD of three independent experiments (24-h time point) are depicted, with 100% corresponding to values obtained for target cells without shaking. CTRL, control.
DISCUSSION
HIV is a fast-replicating virus in vivo and in cell cultures. We have established simple experimental systems to evaluate the role of cell contacts during viral spread. We first report that continuous and gentle rocking of Jurkat cells or primary CD4+ lymphocytes has profoundly deleterious effects on the ability of HIV to propagate. An altered viral replication in shaken lymphocytes was observed with a panel of laboratory-adapted and primary HIV strains with X4 or R5 tropism. Rocking did not significantly affect cell viability and metabolism, nor did it affect the surface expression of various receptors. Once productively infected, shaken cells released normal amounts of virions, indicating that the late steps of viral replication are not affected in mobile cells. We thus hypothesized that virus transfer to recipient cells was altered in shaken lymphocytes due to reduced contact times between cells. To directly demonstrate this, we have designed a flow cytometry-based assay to measure the relative contributions of free virus and cell-associated virus in the propagation of infectivity. With this assay, we confirm previous reports demonstrating that the infectivity of HIV during cell-to-cell transmission is much greater than that with free virus (11, 16, 23, 38, 40). Up to 50% of targets became productively infected within 24 h of coculturing in a process requiring a direct contact between donor cells and recipients. This assay is quantitative, the efficiency of viral transfer being dependent on the percentage of productively infected donors as well as on the donor-to-target-cell ratio. A time course analysis demonstrated a two-phase kinetics of Gag transfer and expression in targets. The first phase is rapid (2 to 4 h) and allows the detection of Gag antigens in 5 to 10% of target cells. This time frame is too short to correspond to newly synthesized viral proteins in targets and likely represents the transmission of incoming virions or viral material. This first phase was insensitive to the reverse transcriptase inhibitor NVP. The second phase is slower (12 to 24 h), in large part sensitive to NVP, and requires adequate receptors and fusogenic viral envelope glycoprotein interactions. It thus corresponds to the de novo synthesis of virus in targets.
It has been reported that a high level of coreceptor-independent HIV transfer is induced by contacts between primary CD4+ T cells (7) associated with a nonspecific endocytosis of virions in intracellular vesicles in targets. These coreceptor-independent events were barely detected in our assay but may correspond to the 5 to 10% of target cells carrying Gag antigens at early time points, after contact with cells producing viruses with either functional or defective envelope glycoproteins. The sensitivity of our assay is probably too low to detect a large number of these nonspecific events (29). The flow cytometry assay thus represents a convenient tool to follow the transfer and productive infection of targets through cell-to-cell interactions.
Small numbers of syncytia between donor and target Jurkat cells were observed by visual examination of the cocultures. Moreover, by using differential fluorescent labeling of donors and targets, we estimated that fewer than 10% of Jurkat cells in cocultures were engaged in fusion or clustering events upon analysis by flow cytometry. Syncytia were even rarer with primary lymphocytes, at least during the 24-h time frame of the survey. The kinetics of HIV Gag transfer are indeed more rapid than those of cell-cell fusion (24). With the exception of their detection in some regions of lymphoid tissue and the nervous system, syncytia are barely detectable in HIV-infected individuals. Our flow cytometry assay of Gag detection thus allows the analysis of cell-to-cell transfer without extensive syncytium formation, a situation reminiscent of the infection in vivo.
Several lines of evidence strongly suggest that Gag detection in CFSE+ targets reflects the productive transfer of infection rather than an increased clustering of cells into doublets or more. First, the number of cell-cell conjugates, as measured by double-fluorescent labeling, was not proportional to Gag expression in CFSE+ cells. Second, the nonfusogenic HIV F522Y mutant, which retains its ability to bind to its receptor, did not induce detectable Gag transfer to CFSE+ cells. Third, Gag detection was significantly decreased by NVP and was thus associated with a reverse transcription step.
This efficient viral spread through direct cell contacts is likely mediated by the induction of virological synapses (22, 24, 26, 39). Virological synapses are defined by a macromolecular and structural organization of the junction zone between infected cells and recipients. These synapses involve cellular and retroviral proteins, as well as components of rafts and cytoskeleton (5, 22, 25, 34). However, it is not formally demonstrated that HIV Gag movement across the synaptic junction corresponds to productive infection (24). The combined use of our flow cytometry assay, which provides a functional assessment of productive viral transfer, and of the analysis of synapse formation by fluorescent microscopy will help to decipher the functional role of virological synapses. For instance, it will be worthwhile to examine the effects of chemicals (i.e., raft and cytoskeleton disorganizers, etc.), negative transdominant proteins, and small interfering RNA against cellular proteins of interest, from both functional and structural points of view.
The influence of viral proteins can also be easily studied with our functional assay of productive cell-to-cell viral transfer. We have shown here that a fusogenic envelope is required in this test. It will be of interest to analyze other Env mutants, for instance those previously described to alter the polarized budding of HIV in lymphocytes (14, 15). The Vpu protein is known to facilitate virion release from the cell. Interestingly, a vpu-defective virus has been previously identified in an in vitro assay selecting rapidly spreading viral strains (20). Accordingly, we observed that a Δvpu HIV strain is transmitted in our assay at an efficiency similar or even better than that of its WT counterpart (not shown). Another protein of interest is Nef, which is known to increase the infectivity of free virions, whereas WT and Δnef viruses replicate similarly in most cell culture systems (1, 13, 43, 44). This apparent discrepancy may be due to the cell-to-cell propagation of infection in cultures, which may not require Nef. Current experiments in our laboratory are aimed at examining this point.
In conclusion, we report here that HIV replication is dramatically impaired in shaken cells. We show that shaking prevents efficient interactions between cells and hence viral transfer. We also demonstrate that cell-to-cell transmission is the predominant mode of viral propagation in cultures. These findings help to explain why, in infected individuals, HIV replicates mostly in lymphoid tissues, where high lymphocyte concentrations and slow movements will facilitate cellular cross talk and viral transmission. In contrast, circulating, highly motile lymphocytes will probably transmit HIV infection much less efficiently. Finally, from a practical point of view, the flow cytometry assay of productive HIV transfer may prove useful in studying the effects of antiviral drugs or neutralizing antibodies in a physiologically relevant situation of viral propagation.
Acknowledgments
We thank Simon Wain-Hobson and Arnaud Moris for critical reading of the manuscript, Céline Trouillet for excellent technical help, Andres Alcover and the NIH AIDS Research and Reference Reagent Program for the kind gift of reagents, and Gianfranco Pancino for the kind gift of primary HIV isolates.
This work was supported by grants from the Agence Nationale de Recherche sur le SIDA (ANRS), SIDACTION, Fondation de France, the CNRS, the European Community, and Institut Pasteur.
Footnotes
Published ahead of print on 1 November 2006.
REFERENCES
- 1.Aiken, C., and D. Trono. 1995. Nef stimulates human immunodeficiency virus type 1 proviral DNA synthesis. J. Virol. 69:5048-5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alfsen, A., H. Yu, A. Magerus-Chatinet, A. Schmitt, and M. Bomsel. 2005. HIV-1-infected blood mononuclear cells form an integrin- and agrin-dependent viral synapse to induce efficient HIV-1 transcytosis across epithelial cell monolayer. Mol. Biol. Cell 16:4267-4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arrighi, J. F., M. Pion, E. Garcia, J. M. Escola, Y. van Kooyk, T. B. Geijtenbeek, and V. Piguet. 2004. DC-SIGN-mediated infectious synapse formation enhances X4 HIV-1 transmission from dendritic cells to T cells. J. Exp. Med. 200:1279-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bar, S., and M. Alizon. 2004. Role of the ectodomain of the gp41 transmembrane envelope protein of human immunodeficiency virus type 1 in late steps of the membrane fusion process. J. Virol. 78:811-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnard, A. L., T. Igakura, Y. Tanaka, G. P. Taylor, and C. R. Bangham. 2005. Engagement of specific T-cell surface molecules regulates cytoskeletal polarization in HTLV-1-infected lymphocytes. Blood 106:988-995. [DOI] [PubMed] [Google Scholar]
- 6.Bergeron, L., N. Sullivan, and J. Sodroski. 1992. Target cell-specific determinants of membrane fusion within the human immunodeficiency virus type 1 gp120 third variable region and gp41 amino terminus. J. Virol. 66:2389-2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blanco, J., B. Bosch, M. T. Fernandez-Figueras, J. Barretina, B. Clotet, and J. A. Este. 2004. High level of coreceptor-independent HIV transfer induced by contacts between primary CD4 T cells. J. Biol. Chem. 279:51305-51314. [DOI] [PubMed] [Google Scholar]
- 8.Bosch, B., J. Blanco, E. Pauls, I. Clotet-Codina, M. Armand-Ugon, B. Grigorov, D. Muriaux, B. Clotet, J. L. Darlix, and J. A. Este. 2005. Inhibition of coreceptor-independent cell-to-cell human immunodeficiency virus type 1 transmission by a CD4-immunoglobulin G2 fusion protein. Antimicrob. Agents Chemother. 49:4296-4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bousso, P., and E. A. Robey. 2004. Dynamic behavior of T cells and thymocytes in lymphoid organs as revealed by two-photon microscopy. Immunity 21:349-355. [DOI] [PubMed] [Google Scholar]
- 10.Buseyne, F., S. Le Gall, C. Boccaccio, J. P. Abastado, J. D. Lifson, L. O. Arthur, Y. Rivière, J. M. Heard, and O. Schwartz. 2001. MHC-I-restricted presentation of HIV-1 virion antigens without viral replication. Nat. Med. 7:344-349. [DOI] [PubMed] [Google Scholar]
- 11.Carr, J. M., H. Hocking, P. Li, and C. J. Burrell. 1999. Rapid and efficient cell-to-cell transmission of human immunodeficiency virus infection from monocyte-derived macrophages to peripheral blood lymphocytes. Virology 265:319-329. [DOI] [PubMed] [Google Scholar]
- 12.Celli, S., Z. Garcia, and P. Bousso. 2005. CD4 T cells integrate signals delivered during successive DC encounters in vivo. J. Exp. Med. 202:1271-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chowers, M. Y., M. W. Pandori, C. A. Spina, D. D. Richman, and J. C. Guatelli. 1995. The growth advantage conferred by HIV-1 nef is determined at the level of viral DNA formation and is independent of CD4 downregulation. Virology 212:451-457. [DOI] [PubMed] [Google Scholar]
- 14.Day, J. R., C. Munk, and J. C. Guatelli. 2004. The membrane-proximal tyrosine-based sorting signal of human immunodeficiency virus type 1 gp41 is required for optimal viral infectivity. J. Virol. 78:1069-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deschambeault, J., J. P. Lalonde, G. Cervantes-Acosta, R. Lodge, E. A. Cohen, and G. Lemay. 1999. Polarized human immunodeficiency virus budding in lymphocytes involves a tyrosine-based signal and favors cell-to-cell viral transmission. J. Virol. 73:5010-5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dimitrov, D. S., R. L. Willey, H. Sato, L. J. Chang, R. Blumenthal, and M. A. Martin. 1993. Quantitation of human immunodeficiency virus type 1 infection kinetics. J. Virol. 67:2182-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dixon, J. B., D. C. Zawieja, A. A. Gashev, and G. L. Cote. 2005. Measuring microlymphatic flow using fast video microscopy. J. Biomed. Opt. 10:064016. [DOI] [PubMed] [Google Scholar]
- 18.Garcia, E., M. Pion, A. Pelchen-Matthews, L. Collinson, J. F. Arrighi, G. Blot, F. Leuba, J. M. Escola, N. Demaurex, M. Marsh, and V. Piguet. 2005. HIV-1 trafficking to the dendritic cell-T-cell infectious synapse uses a pathway of tetraspanin sorting to the immunological synapse. Traffic 6:488-501. [DOI] [PubMed] [Google Scholar]
- 19.Grakoui, A., S. K. Bromley, C. Sumen, M. M. Davis, A. S. Shaw, P. M. Allen, and M. L. Dustin. 1999. The immunological synapse: a molecular machine controlling T cell activation. Science 285:221-227. [DOI] [PubMed] [Google Scholar]
- 20.Gummuluru, S., C. M. Kinsey, and M. Emerman. 2000. An in vitro rapid-turnover assay for human immunodeficiency virus type 1 replication selects for cell-to-cell spread of virus. J. Virol. 74:10882-10891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haase, A. T. 1999. Population biology of HIV-1 infection: viral and CD4+ T cell demographics and dynamics in lymphatic tissues. Annu. Rev. Immunol. 17:625-656. [DOI] [PubMed] [Google Scholar]
- 22.Igakura, T., J. C. Stinchcombe, P. K. Goon, G. P. Taylor, J. N. Weber, G. M. Griffiths, Y. Tanaka, M. Osame, and C. R. Bangham. 2003. Spread of HTLV-I between lymphocytes by virus-induced polarization of the cytoskeleton. Science 299:1713-1716. [DOI] [PubMed] [Google Scholar]
- 23.Johnson, D. C., and M. T. Huber. 2002. Directed egress of animal viruses promotes cell-to-cell spread. J. Virol. 76:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jolly, C., K. Kashefi, M. Hollinshead, and Q. J. Sattentau. 2004. HIV-1 cell to cell transfer across an Env-induced, actin-dependent synapse. J. Exp. Med. 199:283-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jolly, C., and Q. J. Sattentau. 2005. Human immunodeficiency virus type 1 virological synapse formation in T cells requires lipid raft integrity. J. Virol. 79:12088-12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jolly, C., and Q. J. Sattentau. 2004. Retroviral spread by induction of virological synapses. Traffic 5:643-650. [DOI] [PubMed] [Google Scholar]
- 27.Karageorgos, L., P. Li, and C. J. Burrell. 1995. Stepwise analysis of reverse transcription in a cell-to-cell human immunodeficiency virus infection model: kinetics and implications. J. Gen. Virol. 76:1675-1686. [DOI] [PubMed] [Google Scholar]
- 28.Li, Q., L. Duan, J. D. Estes, Z. M. Ma, T. Rourke, Y. Wang, C. Reilly, J. Carlis, C. J. Miller, and A. T. Haase. 2005. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature 434:1148-1152. [DOI] [PubMed] [Google Scholar]
- 29.Maréchal, V., F. Clavel, J. M. Heard, and O. Schwartz. 1998. Cytosolic Gag p24 as an index of productive entry of human immunodeficiency virus type 1. J. Virol. 72:2208-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mattapallil, J. J., D. C. Douek, B. Hill, Y. Nishimura, M. Martin, and M. Roederer. 2005. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 434:1093-1097. [DOI] [PubMed] [Google Scholar]
- 31.McDonald, D., L. Wu, S. M. Bohks, V. N. KewalRamani, D. Unutmaz, and T. J. Hope. 2003. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science 300:1295-1297. [DOI] [PubMed] [Google Scholar]
- 32.Mempel, T. R., M. L. Scimone, J. R. Mora, and U. H. von Andrian. 2004. In vivo imaging of leukocyte trafficking in blood vessels and tissues. Curr. Opin. Immunol. 16:406-417. [DOI] [PubMed] [Google Scholar]
- 33.Monks, C. R., B. A. Freiberg, H. Kupfer, N. Sciaky, and A. Kupfer. 1998. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature 395:82-86. [DOI] [PubMed] [Google Scholar]
- 34.Nejmeddine, M., A. L. Barnard, Y. Tanaka, G. P. Taylor, and C. R. Bangham. 2005. Human T-lymphotropic virus, type 1, tax protein triggers microtubule reorientation in the virological synapse. J. Biol. Chem. 280:29653-29660. [DOI] [PubMed] [Google Scholar]
- 35.Nobile, C., C. Petit, A. Moris, K. Skrabal, J. P. Abastado, F. Mammano, and O. Schwartz. 2005. Covert human immunodeficiency virus replication in dendritic cells and in DC-SIGN-expressing cells promotes long-term transmission to lymphocytes. J. Virol. 79:5386-5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pearce-Pratt, R., D. Malamud, and D. M. Phillips. 1994. Role of the cytoskeleton in cell-to-cell transmission of human immunodeficiency virus. J. Virol. 68:2898-2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petit, C., F. Buseyne, C. Boccaccio, J. P. Abastado, J. M. Heard, and O. Schwartz. 2001. Nef is required for efficient HIV-1 replication in cocultures of dendritic cells and lymphocytes. Virology 286:225-236. [DOI] [PubMed] [Google Scholar]
- 38.Phillips, D. M. 1994. The role of cell-to-cell transmission in HIV infection. AIDS 8:719-731. [DOI] [PubMed] [Google Scholar]
- 39.Piguet, V., and Q. Sattentau. 2004. Dangerous liaisons at the virological synapse. J. Clin. Investig. 114:605-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sato, H., J. Orenstein, D. Dimitrov, and M. Martin. 1992. Cell-to-cell spread of HIV-1 occurs within minutes and may not involve the participation of virus particles. Virology 186:712-724. [DOI] [PubMed] [Google Scholar]
- 41.Schacker, T., S. Little, E. Connick, K. Gebhard, Z. Q. Zhang, J. Krieger, J. Pryor, D. Havlir, J. K. Wong, R. T. Schooley, D. Richman, L. Corey, and A. T. Haase. 2001. Productive infection of T cells in lymphoid tissues during primary and early human immunodeficiency virus infection. J. Infect. Dis. 183:555-562. [DOI] [PubMed] [Google Scholar]
- 42.Schaeffer, E., V. B. Soros, and W. C. Greene. 2004. Compensatory link between fusion and endocytosis of human immunodeficiency virus type 1 in human CD4 T lymphocytes. J. Virol. 78:1375-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwartz, O., V. Maréchal, O. Danos, and J. M. Heard. 1995. Human immunodeficiency virus type 1 Nef increases the efficiency of reverse transcription in the infected cell. J. Virol. 69:4053-4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwartz, O., Y. Rivière, J. M. Heard, and O. Danos. 1993. Reduced cell surface expression of processed HIV-1 envelope glycoprotein in the presence of Nef. J. Virol. 67:3274-3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sol-Foulon, N., C. Esnault, Y. Percherancier, F. Porrot, P. Metais-Cunha, F. Bachelerie, and O. Schwartz. 2004. The effects of HIV-1 Nef on CD4 surface expression and viral infectivity in lymphoid cells are independent of rafts. J. Biol. Chem. 279:31398-31408. [DOI] [PubMed] [Google Scholar]
- 46.Stevenson, M., T. L. Stanwick, M. P. Dempsey, and C. A. Lamonica. 1990. HIV-1 replication is controlled at the level of T cell activation and proviral integration. EMBO J. 9:1551-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.von Andrian, U. H., and T. R. Mempel. 2003. Homing and cellular traffic in lymph nodes. Nat. Rev. Immunol. 3:867-878. [DOI] [PubMed] [Google Scholar]