Abstract
Most adenoviruses bind to the coxsackie- and adenovirus receptor (CAR). Surprisingly, CAR is not expressed apically on polarized cells and is thus not easily available to viruses. Consequently, alternative mechanisms for entry of coxsackievirus and adenovirus into cells have been suggested. We have found that tear fluid promotes adenovirus infection, and we have identified human lactoferrin (HLf) as the tear fluid component responsible for this effect. HLf alone was found to promote binding of adenovirus to epithelial cells in a dose-dependent manner and also infection of epithelial cells by adenovirus. HLf was also found to promote gene delivery from an adenovirus-based vector. The mechanism takes place at the binding stage and functions independently of CAR. Thus, we have identified a novel binding mechanism whereby adenovirus hijacks HLf, a component of the innate immune system, and uses it as a bridge for attachment to host cells.
Human adenoviruses (Ads) cause disease of the respiratory tract, intestine, eyes, liver, and urinary tract and in lymphoid tissue (23). The most commonly isolated adenovirus serotypes belong to species C (Ad1, Ad2, Ad5, and Ad6) and cause roughly 5% of all symptomatic upper respiratory tract infections (8) and 15% of all lower respiratory tract infections (4) in children younger than 5 years. Early studies documented the isolation of species C adenoviruses following explant of human tonsils and adenoid tissue for culture (46), and it has been shown that species C adenoviruses are latent in T lymphocytes isolated from human tonsils (17).
With few exceptions, adenoviruses bind to target cells by means of an interaction between the viral fiber and its cellular receptor (34), whereas an interaction between the viral penton base protein and cellular integrins promotes internalization (52). It has been concluded that selected members of all adenovirus species except species B use the coxsackievirus and adenovirus receptor (CAR) as a cellular receptor (6, 35, 43). Most, but not all, species B adenoviruses use CD46 as a cellular receptor instead of CAR (15, 25, 36).
When CAR was initially identified as a cellular receptor for human adenoviruses, the experiments were performed using nonpolarized cell lines in vitro. In these model systems, CAR is distributed equally over the cell surface and is available to viruses. In vivo, however, epithelial cells are polarized and CAR is not readily available to virions (50). Moreover, in polarized epithelium, CAR forms lateral homodimers intercellularly, regulates cell-to-cell adhesion, and facilitates viral transport between cells in order to promote escape from the site of replication, rather then being involved in virus binding to target cells (49).
The poor ability of adenoviruses to reach their cellular receptor on polarized epithelial cells has become evident from work aimed at developing vectors for human gene therapy. Adenovirus vectors proved to be inefficient due to the low accessibility of CAR on polarized airway cells (31). Thus, it has been suggested that, alternative, apically expressed molecules are used as cellular receptors by adenovirus and coxsackievirus (11, 49, 50). In agreement with this, the roles of decay accelerating factor (DAF), which was first identified as a cellular receptor for coxsackie B viruses in 1995 (38), and CAR was recently investigated in detail during the early steps of coxsackie B virus replication. DAF was found to serve as the main attachment receptor for coxsackie B virus, whereas CAR was found to be involved in a subsequent step of the coxsackie B virus life cycle (12).
Before finding a suitable receptor, every virus has to avoid or overcome the innate immune defense present in the skin, mucosal layers, and body fluids. Human lactoferrin (HLf) is present in mucosa and most body fluids (51) and plays a role in the first line of defense against microbial infections (45). The antiviral effects of HLf are mainly related to inhibition of virus entry into host cells, either by binding to viral ligands, such as gp120 of human immunodeficiency virus (41), or by binding to cellular receptors, such as heparan sulfate glycosaminoglycans (24). In contrast, HLf has also been demonstrated to transactivate the long terminal repeat promoter of human T-lymphotropic leukemia virus 1, which is transmitted vertically through breast milk (28). Thus, in addition to having antiviral properties, HLf may also have the capacity to promote infection by certain viruses.
In this study, we set out to investigate the role of tear fluid in ocular adenovirus infections. We found an unexpected effect of tear fluid during infections by respiratory adenoviruses and decided to investigate this effect further.
MATERIALS AND METHODS
Cells and viruses.
Human epithelial lung carcinoma A549 cells and epithelial larynx carcinoma Hep2 cells were grown in Dulbecco's modified Eagle's medium (DMEM; Sigma-Aldrich) containing NaHCO3 (0.75 g/liter), 10% fetal calf serum (FCS; Sigma-Aldrich), 20 mM HEPES (Euroclone), and penicillin-streptomycin (Invitrogen) at 37°C. Human corneal epithelial (HCE) cells were grown in steroid-supplemented hormone medium (SHEM) as described previously (1). Acute lymphoblastic T-cell leukemia CEM and KE37 cells and CAR cDNA-transfected CEM and KE37 cells (CEM-CARhi and KE37-CARhi) were grown as described previously (26).
Adenovirus type 31 (Ad31; strain 13-15/63), Ad7 (Gomen), Ad11 (Slobitski), Ad1 (strain Ad71), Ad2 (strain Ad6), Ad5 (strain Ad75), Ad6 (Tonsil 99), Ad37 (1477), Ad4 (RI-67), and Ad41 (Tak) were propagated in A549 or Hep2 (Ad41) cells and purified as described elsewhere (27), with the following exception: instead of treating the cells with sonication and Arclone extraction, the cells were broken by three cycles of freeze-thawing.
Fluorescent focus assay.
Human tear fluid was induced with freshly minced onions and collected from volunteers in the laboratory. Five or sometimes 10 μl of tear fluid was mixed with virus (Ad37, 1.4 × 109 virions; Ad5, 1.8 × 108 virions), together with 500 μl SHEM (containing 1% FCS). In other experiments, tear fluid was replaced by various concentrations of HLf (Sigma), lysozyme (Sigma), or lipophilin (a kind gift of Robert Lehrer), as indicated in the figure legends. After incubation for 1 hour on ice, the mixtures were added to subconfluent cells in 24-well plates (Nunc) and incubated for another hour on ice. Unbound virions were removed by a two-step wash with SHEM (1% FCS). When experiments were performed with A549 or Hep2 cells, DMEM (including 1% FCS, penicillin-streptomycin, and 20 mM HEPES) was used instead of SHEM in all steps. The amount of virions that was added to each well was adapted to obtain approximately 10 to 20 infected cells in each viewfield (in the absence of effectors) and ranged from 4.2 × 106 virions/well (Ad5 and Ad6; A549 and Hep2 cells) to 2.6 × 109 virions/well (Ad41; A549). After 44 h of incubation at 37°C, the cells were fixed with methanol (400 μl/well) and stained first with homotypic rabbit antiadenovirus serum, produced as described elsewhere (48) and diluted 200 to 1,000 times in phosphate-buffered saline (PBS; Medicago), and then with fluorescein isothiocyanate (FITC)-conjugated swine anti-rabbit antibodies (Dako Cytomation) diluted 1:50 in PBS. Incubations with antibodies were performed in a final volume of 400 μl for 1 h at room temperature, and all washes were done in PBS twice for 15 min. When Ad5CMVeGFP vectors (Baylor College of Medicine) or enhanced green fluorescent protein (eGFP)-expressing Ad5 vectors pseudotyped with the Ad35 fiber (Ad5F35-GFP) (30) were used instead of virions, 104 vector particles were added per cell and 2% paraformaldehyde (J.T. Baker) was used for fixation. Fluorescing cells were examined and quantified using a fluorescence microscope (Axioskop2; Zeiss) at ×10 magnification and linked to a digital camera (AxioCam MRm; Zeiss) and Axiovision AC software (Zeiss).
Western blotting.
Tear fluid proteins, and also HLf, lysozyme, and lipophilin, were separated on a 4 to 20% Criterion precast gradient gel (Bio-Rad) under denaturing conditions and transferred to a polyvinylidene difluoride membrane (Hybond-P; Amersham) using a Trans-Blot SD semidry transfer cell (Bio-Rad). The membrane was blocked with PBS in 5% nonfat dry milk (Semper) overnight at 4°C. After washing three times for 5 min in PBS-Tween (0.05% Tween; Medicago), the membrane was incubated with 9 × 109 Ad5 virions diluted in 10 ml PBS-Tween containing 1% nonfat dry milk (Bio-Rad). After 1 hour of incubation at room temperature with constant agitation, rabbit anti-Ad5 serum (diluted 1:2,000) in PBS-Tween and 1% nonfat dry milk was added to the membrane. The membrane was then incubated and washed as before and further incubated with horseradish peroxidase-conjugated swine anti-rabbit antibodies (Dako Cytomation), diluted 1:5,000 in PBS-Tween with 1% nonfat dry milk. After another round of incubation and subsequent washing, enhanced chemiluminescence Western blotting detection reagents and Hyperfilm TM enhanced chemiluminescence (both from Amersham) were used according to the instructions of the manufacturer.
Binding assay.
Cells (2 × 105) were harvested using PBS-EDTA and recovered in binding buffer (BB) consisting of DMEM, penicillin-streptomycin, 10 mM HEPES, 1% bovine serum albumin (BSA; Roche) with a 1-hour incubation at 37°C with constant agitation. Simultaneously, 35S-labeled Ad5 virions (104 particles/cell) were incubated with or without 10 μg HLf in BB (100 μl) on ice. One hour later, the virion mixtures were transferred to recovered, pelleted cells and incubated for another hour on ice (final volume, 100 μl). Unbound virions were removed by washing, and the cell-associated radioactivity was measured using a Wallac 1409 liquid scintillation counter (Perkin-Elmer). In one type of binding experiment, 35S-labeled Ad5 virions were preincubated in 100 μl of BB with or without tear fluid (1:10 dilution) before incubation with cells (see Fig. 1D, below). In another type of binding experiment (see Fig. 2B, below), the data were normalized with respect to HLf-dependent aggregation of virions: from parallel samples containing either virions only or virions and HLf, but not cells, radioactivity in the supernatant (top 95 μl) or “pellet” (remaining 5 μl) was measured with respect to radioactivity. Approximately twice as many more virions were found in the pellet fraction when virions were preincubated with HLf. In a third type of binding experiment (see Fig. 4B, below), cells were preincubated in 100 μl BB with or without 1 μg anti-CAR monoclonal antibodies E1-1 (a kind gift of Silvio Hemmi) and/or RmcB (Upstate), prior to incubation with HLf and 35S-labeled Ad5 virions (1 hour on ice).
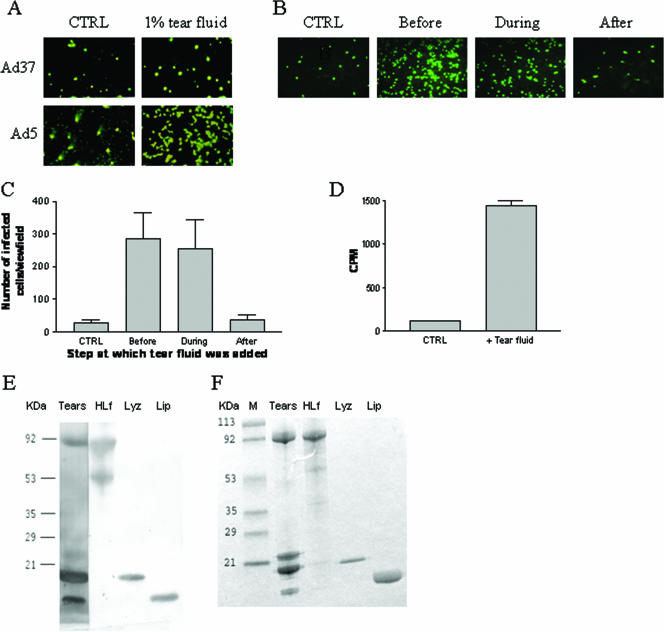
FIG. 1.
Effects of tear fluid on adenovirus infection and characterization of tear fluid components that interact with virions. (A) Tear fluid promotes infection of Ad5 virions in HCE cells. Virions were mixed with or without tear fluid (diluted 1:100) and allowed to infect cells. Forty-four hours postinfection, the cells were fixed, stained, and analyzed in a fluorescence microscope. (B and C) Tear fluid promotes infection of Ad5 in HCE cells through a preentry mechanism. Panel C is a quantification of the findings shown in panel B. Tear fluid (final dilution, 1:50) was added at different time points of the Ad5 infectious cycle. At 44 h postinfection, the cells were fixed, stained, and analyzed in a fluorescence microscope. CTRL, no tear fluid was included; “before,” tear fluid was preincubated with virions; “during,” tear fluids were incubated with cells and virions; “after,” tear fluid was added to cells after binding of virions to cells. (D) Tear fluid promotes binding of Ad5 to HCE cells. Tear fluid (diluted 1:10) was preincubated with 35S-labeled virions and then incubated with cells. After removal of unbound virions by washing, the cell-associated radioactivity was quantified with a beta counter. For panels C and D, the data shown are the results of three independent experiments, and each experiment was performed in duplicate. (E) Ad5 virions interact with different tear fluid proteins. Tear fluid (5 μl), HLf (5 μg), lysozyme (5 μg), and lipophilin (2 μg) were loaded separately onto a gel, separated, and transferred to a polyvinylidene difluoride membrane. The membrane was then treated consecutively with Ad5 virions, anti-Ad5 antibodies, and horseradish peroxidase-conjugated anti-antibodies as described in Materials and Methods. (F) Visualization of tear fluid (5 μl) and individual tear fluid proteins (HLf, 5 μg; lysozyme, 5 μg; lipophilin, 2 μg). Note that the sizes of these three proteins correspond to the sizes of the proteins detected by Ad5 virions in panel E.
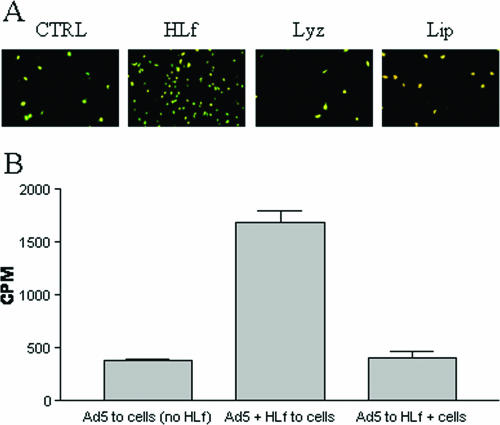
FIG. 2.
HLf promotes infection and binding of Ad5 to HCE cells. (A) HLf, but not lysozyme or lipophilin, promotes infection by Ad5 in HCE cells. Ad5 virions were preincubated with or without HLf, lysozyme, or lipophilin (6 μg/ml) and allowed to infect cells. Forty-four hours postinfection, the cells were fixed, stained, and analyzed in a fluorescence microscope. (B) Free, but not cell-associated, HLf promotes binding of Ad5 virions to HCE cells. HLf (100 μg/ml) was first preincubated with 35S-labeled virions and then incubated with cells (Ad5 + HLf to cells), or HLf (100 μg/ml) was first preincubated with cells and then with virions (Ad5 to HLf + cells). As a control, 35S-labeled virions was incubated directly with cells in the absence of HLf [Ad5 to cells (no HLf)]. After removal of unbound virions from cells by washing, the cell-associated radioactivity was quantified with a beta counter. The data are normalized with respect to the possible effect of aggregation (see Materials and Methods). For panel B, the data shown are the results of three independent experiments, and each experiment was performed in duplicate.
FIG. 4.
HLf promotes interaction of Ad5 with target cells independently of CAR. (A) Relative expression levels of cell surface CAR and HLf receptors differ between cells. Hep2, A549, or HCE cells were incubated with RmcB ascites (anti-CAR antibodies) followed by FITC-conjugated anti-antibodies, or with HLf followed by rabbit anti-HLf antibodies and FITC-conjugated anti-antibodies, and analyzed by flow cytometry. Data were normalized to 100% (A549), and HCE and Hep2 data were normalized accordingly. (B) Blocking of cell surface CAR improves the efficiency by which HLf promotes binding of Ad5 to A549 cells. 35S-labeled virions were preincubated with or without HLf (100 μg/ml) at the same time as cells were preincubated with or without RmcB and/or E1-1 anti-CAR antibodies (10 μg/ml) Thereafter, virions (with or without HLf) were incubated with cells (with or without anti-CAR antibodies). After removal of unbound virions by washing, the cell-associated radioactivity was quantified with a beta counter. (C) Lack of cell surface CAR improves the efficiency by which HLf promotes binding of Ad5 to lymphoblastic T-cell leukemia cells. 35S-labeled virions were preincubated with or without HLf (100 μg/ml) and thereafter with cells. After removal of unbound virions by washing, the cell-associated radioactivity was quantified with a beta counter. (D and E) HLf promotes GFP expression in A549 cells from adenoviruses or adenovirus vectors equipped with the Ad5 fiber, but not with the Ad35 fiber. Panel E is a quantification of the results shown in panel D. Ad5 or Ad35 virions, or Ad5 vector with Ad5 fiber (Ad5CMVeGFP) or Ad35 fiber (Ad5F35-GFP), were preincubated with or without HLf (60 μg/ml) and allowed to infect cells. At 44 h postinfection, the cells were fixed and analyzed in a fluorescence microscope. For panels A to C and E, the data shown are the results of three independent experiments, and each experiment was performed in duplicate.
Flow cytometry.
Cells were harvested using PBS-EDTA and recovered in BB, followed by a 1-hour incubation at 37°C with constant agitation. The cells (5 ×105 cells/sample) were then further incubated on ice for 1 hour in 100 μl DMEM containing 1% BSA and 0.01% NaN3, with or without HLf (4 μg) or mouse anti-CAR (RmcB ascites; a kind gift of Jeffrey M. Bergelson) diluted 1:1,000. Cells that had been preincubated with HLf were washed and resuspended in 100 μl DMEM containing 1% BSA, 0.01% NaN3, and rabbit anti-hLf (Biodesign) diluted 1:30 and incubated on ice. Thirty minutes later, these cells (and also cells preincubated with RmcB) were washed and incubated with swine anti-rabbit antibodies (in the case of cells with HLf) or rabbit anti-mouse antibodies (in the case of cells with RmcB). Both conjugates (Dako Cytomation) were FITC conjugated and diluted 1:50 in PBS with 1% BSA. After 30 min of incubation on ice, the cells were resuspended in 300 μl PBS containing propidium iodine (1 μg/ml; Sigma-Aldrich). Each incubation was followed by two washing steps. The samples were then examined using a FACScan flow cytometer and LYSIS II software (Becton Dickinson).
RESULTS
Tear fluid promotes Ad5 infection of corneal cells.
We have previously shown that Ad37 uses sialic acid as a cellular receptor (2). Unlike most other adenoviruses, the tropism of Ad37 is largely restricted to the eye (14). With this in mind, we set out to investigate the effect of tear fluid on Ad37 infection of corneal cells. As a control we used Ad5, which, like other members of species C, mainly causes tonsillitis and upper respiratory tract infections and, much less often, ocular infections (19). Tear fluid did not affect the infectivity of Ad37, but to our surprise it efficiently enhanced the infectivity of Ad5 in HCE cells (Fig. 1A). In order to investigate this unexpected finding, we first sought to identify the step in the adenovirus life cycle in which tear fluid exerted this effect. Tear fluid promoted infection when preincubated with Ad5 virions or when incubated with Ad5 virions together with cells, but not when added directly after Ad5 had bound to cells (Fig. 1B and C), suggesting that tear fluid promotes an early step in the life cycle of Ad5. In agreement with this, a 1:10 dilution of tear fluid promoted binding of 35S-labeled Ad5 to HCE cells approximately 13-fold (Fig. 1D), which led us to believe that one or more tear fluid components promote binding of Ad5 to cells through a direct interaction with virions. To test this, we performed a virus protein overlay blotting assay and found that Ad5 virions interacted with at least three different tear fluid proteins (Fig. 1E), which, according to their size and relative amounts, appeared to correspond to HLf (80 kDa), lysozyme (14 kDa), and lipophilin (5 to 8 kDa) (Fig. 1F). When blotted to larger amounts of tear fluid proteins, Ad5 virions also interacted with lipocalin, secreted immunoglobulin A, and phospholipase A2 (data not shown).
HLf promotes dose-dependent binding of Ad5 to and infection of cells that correspond to the natural tropism of Ad5.
To elucidate the role of specific tear fluid proteins during Ad5 infection, we preincubated Ad5 virions with purified HLf, lysozyme, or lipophilin before allowing Ad5 to infect HCE cells. Whereas lysozyme and lipophilin had no effect at 6 μg/ml, HLf caused an increase in the number of infected cells (Fig. 2A). Also, HLf was found to mimic the effect observed with tear fluid, in that HLf promoted infection when preincubated with Ad5 virions or when coincubated with Ad5 virions and cells, but not when added to cells directly after the virus binding step (data not shown). We also found that HLf promoted binding of Ad5 to cells only when preincubated with virions, but not when HLf was preincubated with cells (Fig. 2B), indicating that HLf must be in suspension and first interact with virions in order to be able to promote the binding of Ad5 to cells. Taken together, these findings suggest that HLf promotes Ad5 binding to and infection of target cells by serving as a bridge between the virion and the cells.
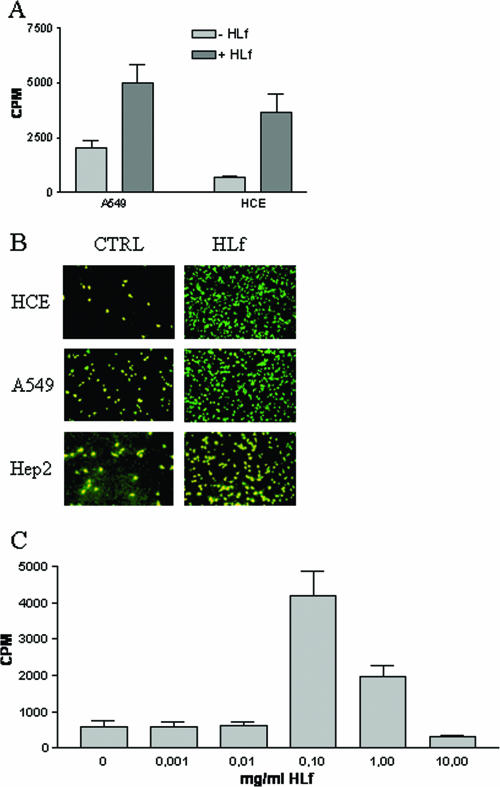
Since Ad5 causes respiratory infections more frequently than ocular infections, we hypothesized that HLf would promote the binding of Ad5 to respiratory cells also. In agreement with this, HLf alone was sufficient for promotion of Ad5 binding not only to HCE cells (5-fold), but also to the A549 cell line (2.5-fold) (Fig. 3A), which is derived from respiratory cells. Moreover, when preincubated with Ad5 virions, HLf efficiently promoted infections in ocular (HCE), respiratory (A549), and larynx (Hep2) epithelial cells (Fig. 3B). Thus, HLf promoted infection by Ad5 in cells that corresponded to the normal tropism of this virus. From previous work by others, it has been suggested that HLf inhibits Ad2 from infecting Hep2 cells (3, 13). In these studies, however, the concentrations of HLf that were inhibitory (0.5 mg/ml abolished 50% of the cytopathic effect) were higher than those we found to promote Ad5 infection in HCE, A549, and Hep2 cells. To investigate whether different concentrations exert different effects, we performed dose-dependent studies of HLf-mediated Ad5 binding to HCE cells. At concentrations lower than 0.01 mg/ml, HLf did not exert any effect on Ad5 binding to HCE cells, but at 0.1 and 1 mg/ml HLf promoted binding 7.1- and 3.3-fold, respectively (Fig. 3D). At the highest concentration tested (10 mg/ml), HLf reduced binding to a level that was lower than in its absence. One possible explanation for this is that at very high concentrations, individual HLf proteins bind to either virions or cells, but not to both, and thus HLf may be prevented from forming the bridge that is required for linking virions to cells, giving results similar to those described previously (3, 13). Assuming 2 mg/ml HLf in tear fluid (51), the dilution used here (1:10) corresponds to 0.2 mg/ml, which is within the range in which HLf was found to act as a promoter of virus binding.
FIG. 3.
HLf promotes infection and dose-dependent binding of Ad5 in respiratory and laryngeal epithelial cells. (A) HLf promotes binding of Ad5 virions to A549 and HCE cells. 35S-labeled virions were preincubated with or without HLf (100 μg/ml) and then incubated with cells. After removal of unbound virions by washing, the cell-associated radioactivity was quantified with a beta counter. Data shown are the results of three independent experiments, and each experiment was performed in duplicate. (B) HLf promotes infection of Ad5 virions in HCE, A549, and Hep2 cells. Virions were mixed with or without HLf (100 μg/ml) and allowed to infect cells. At 44 h postinfection, the cells were fixed, stained, and analyzed in a fluorescence microscope. (C) Increasing concentrations of HLf were preincubated with 35S-labeled virions and then incubated with cells. After removal of unbound virions by washing, the cell-associated radioactivity was quantified with a beta counter. For panels A to C, three independent experiments were performed, each in duplicate. Representative figures were selected.
CAR is not involved in HLf-mediated binding of Ad5 virions to cells.
Since CAR is distributed equally over the surface of nonpolarized epithelial cells in vitro and is thereby able to function efficiently as a cellular receptor for human adenoviruses, we hypothesized that the efficiency of HLf-mediated adenovirus binding to cells in vitro may depend on a combination of the presence of CAR and suitable receptors for HLf. To investigate this, we first compared the expression of CAR and HLf receptors on A549, HCE, and Hep2 cells. HLf bound to all three cell lines with similar efficiency, indicating that there are similar numbers of receptors for HLf on these epithelial cells (Fig. 4A). Anti-CAR monoclonal antibody RmcB, on the other hand, bound with highest efficiency to Hep2 cells and with least efficiency to HCE cells. To investigate the role of CAR during HLf-mediated Ad5 infection of A549 cells in greater depth, we blocked cell surface CAR with two monoclonal antibodies, RmcB and E1-1, during the adsorption step. Neither of the antibodies inhibited HLf-mediated infection (Fig. 4B). On the contrary, the HLf-mediated infectivity of Ad5 was enhanced in the presence of anti-CAR antibodies, indicating that CAR is not involved in HLf-mediated binding of Ad5 to cells. Moreover, HLf promoted Ad5 binding to CAR-negative T cells (CEM and KE37) more efficiently than to corresponding T cells transfected with the cDNA encoding human CAR (CEM-CARhi and KE37-CARhi) (Fig. 4C). Based on these experiments, we concluded that in the absence of CAR, HLf efficiently promotes binding of Ad5 to target cells.
The outer surface of the adenovirus capsid is composed of three well-exposed proteins (the hexon, the penton base, and the fiber) and two less-exposed proteins (IIIa and IX). To test whether the fiber is involved in the HLf-mediated interaction between the adenovirus capsid and cells, we used two different Ad5-based vectors that express GFP: one vector (Ad5F35-GFP) containing the Ad5 tail and the shaft and knob domain of Ad35 (a CD46 binding adenovirus, i.e., non-CAR-binding adenovirus), and another vector containing the wild-type Ad5 fiber (Ad5CMVeGFP). We observed a 5-fold increase in the number of GFP-expressing A549 cells upon preincubation of the Ad5CMVeGFP vector with HLF compared to control (no HLf) (Fig. 4D and E), whereas preincubation of the Ad5F35-GFP vector with HLf resulted only in 1.5-fold more GFP-expressing cells. Ad5 virions behaved similarly to the Ad5CMVeGFP vector in that a 5-fold increase in the number of infected cells was observed upon preincubation with HLf, whereas preincubation with HLf had only a minor effect on Ad35 infection of A549 cells (1.1-fold increase). Thus, even though it is too early to exclude other capsid proteins from being involved in the HLf-mediated interaction of Ad5 with cells, it seems likely that the fiber plays an important role in this mechanism.
HLf promotes species C-specific adenovirus infection of A549 cells.
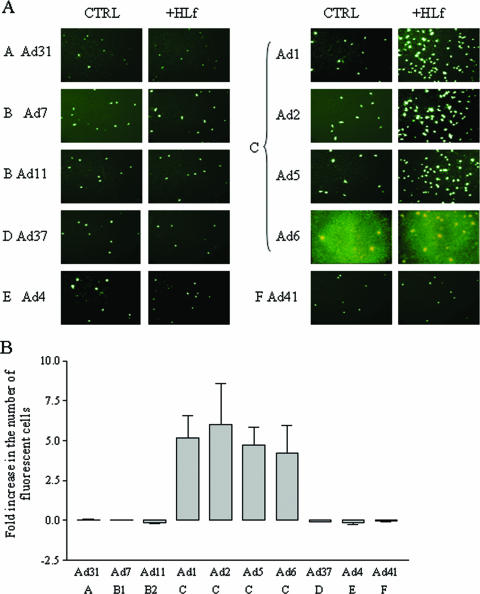
Since HLf did not promote infection of Ad35, which is one of the nine members of species B, we hypothesized that the effect of HLf could be specific for certain adenovirus species. In order to investigate this, we infected A549 cells with at least one serotype from each species and found that HLf promoted infection by all species C adenoviruses (Ad1, Ad2, Ad5, and Ad6) but not by representative adenoviruses of species A (Ad31), B (Ad7 and Ad11), D (Ad37), E (Ad4), or F (Ad41) (Fig. 5). Thus, the ability of HLf to promote adenovirus infection in A549 cells appears to be specific for adenoviruses of species C.
FIG. 5.
HLf promotes infection of A549 cells by species C adenoviruses. Panel B shows a quantification of the results in panel A. Virions of different serotypes representing all species (as indicated) were mixed with HLf (60 μg/ml) or untreated, and allowed to infect cells. At 44 h postinfection, the cells were fixed, stained, and analyzed in a fluorescence microscope. Data shown are the result of three independent experiments, and each experiment was performed in duplicate.
DISCUSSION
Species C adenoviruses are a relatively frequent cause of tonsillitis and adenoiditis in young children, and DNA of species C adenoviruses but not DNA from other species has been detected in T cells isolated from tonsils and adenoids (17). Thus, we speculated that usage of HLf as a bridge may be an important mechanism by which species C adenoviruses can cause latent/persistent infections of T cells localized in tonsils and adenoids. This idea is supported by the lack of CAR on primary T cells (10), the lack of Ad5 binding to and replication in T cells (reference 37 and this paper), and the efficient binding of Ad5 to CAR-negative T cells in the presence of HLf (this paper). Human herpesvirus type 8 (HHV8) is another virus that infects tonsils and adenoids in young children (9). An interesting parallel between Ad5 and HHV8 is that the latter has been suggested to require salivary lactoferrin for infection of target cells (20).
Species C adenoviruses are one of the most frequently used viral vectors for human gene therapy, but the in vivo results have not lived up to expectations derived from in vitro work. There are at least two possible reasons for this. First, most of the human population carries neutralizing antibodies to the Ad5-based vectors, which are used more frequently than vectors based on other serotypes. In order to circumvent this, vectors have been developed based on Ad11 (22, 40), Ad19 (42), and Ad35 (16), as their seroprevalence is considerably lower (22, 40, 47). Another recent approach to circumvent immunity, but still benefit from the characteristics of the well-studied Ad5 serotype, is to exchange the immunogenic domains on the surface of the hexon protein for corresponding domains chosen from a less common serotype (e.g., Ad48) (33). A second major reason as to why Ad5-based vectors function poorly in vivo is likely to be the absence (or low presence) of CAR on polarized epithelial cells and other cells, such as primary T cells. We predict that it might be possible to improve the efficiency of gene therapy based on Ad5 vectors simply by including HLf in gene therapy protocols.
Based on our results, and with the knowledge that CAR is absent from the apical side of polarized cells, we conclude that either CAR or HLf alone is sufficient for efficient binding of Ad5 to and infection of nonpolarized cells (i.e., in vitro) (Fig. 6). In CAR-negative cells, on the other hand (thus mimicking the in vivo situation, where epithelial cells are polarized), the interaction with HLf may be an important mechanism used by Ad5 and other species C adenoviruses for binding to and infection of cells. Because CAR is normally inaccessible from the apical surface, it has been suggested that the initial adenovirus infection may occur when a transient break in the epithelium allows luminal virus to reach its receptor or during repair of injured epithelium when CAR might be accessible (49). To the best of our knowledge, such breaks are poorly characterized, and gaining access to CAR via this pathway may not explain how adenoviruses, which are a common cause of respiratory disease, bind to and infect epithelial cells in vivo.
FIG. 6.
Model of adenovirus hijacking HLf for infection of cells in the absence and presence of apical CAR. In the presence of CAR, mimicking the in vitro situation with nonpolarized epithelial cells, species C adenoviruses can bind to and infect cells either via CAR or via the HLf-mediated pathway. In the absence of CAR, mimicking the in vivo situation with polarized epithelial cells, species C adenoviruses can bind to and infect cells via the HLf-mediated pathway alone.
Confusingly, HLf has already been suggested to inhibit Ad2 from infecting Hep2 cells through a mechanism that takes place during an early step in the infection (3, 13). The contradictory results may simply be explained by the different concentrations of HLf used and the different number of HLf binding sites on virions and cells in the two different types of experiments. We speculate that in the presence of low concentrations of HLf in relation to the number of HLf binding sites on virions and cells, which was the case in most experiments of this study, HLf is allowed to bind both virions and cells at the same time, but at higher concentrations of HLf there is an excess of HLf in relation to the number of HLf binding sites on virions and cells (3, 13), resulting in binding to either virions or cells, but not both simultaneously, thus not allowing a bridge to be formed. The cutoff value at which HLf inhibits rather than promotes binding probably varies between cell types, as different cell types express different numbers of HLf receptors. When we increased the concentration of HLf above 1 mg/ml, we found that binding of Ad5 to HCE cells was reduced, thus mimicking the results obtained with Ad2 infection of Hep2 cells (3, 13). In saliva, the concentration is rather low and within the same range as we used here, indicating that use of HLf as a bridge to cells may be a functional pathway used by Ad5 during infections in vivo. We also speculate that the relative expression of HLf receptors and CAR, as well as the different concentrations of HLf found in different body fluids, may at least partially explain the tropism of adenoviruses: in tear fluid the concentration is high and HLf may therefore inhibit (or at least fail to promote) adenovirus infection. On the other hand, in saliva the concentration is much lower, and HLf may therefore promote adenovirus infection.
At low pH, the N-terminal domain of HLf, which is known as lactoferricin, is cleaved from HLf by pepsin. We did not find any difference in the activity between pepsin-cleaved and mock-treated HLf on Ad5 infection of HCE cells (data not shown). Besides being cleaved by pepsin and other proteases, HLf itself may act as a protease. The proteolytic activity of HLf that has been described previously takes place at serine residues surrounded by arginines (RSRR or RRSR) (21). However, these sites are not found in the three major Ad2 or Ad5 capsid proteins (hexon, fiber, and penton base). We therefore do not expect that HLf-mediated binding and infection of species C involves the proteolytic capacity of HLf. Moreover, HLf has very high affinity for Fe3+ ions. We compared holo-HLf (iron saturated) with apo-HLf and found that holo-HLf is a more effective promoter of Ad5 binding to HCE cells than apo-HLf (data not shown). We speculate that the ability of Fe3+ ions to affect the overall structure of HLf may affect the ability of HLf to promote binding of Ad5 to HCE cells.
Bovine lactoferrin (BLf) has been demonstrated to inhibit Ad2 infection of Hep2 cells (13). We found BLf to exert a small promoting effect of Ad5 infection of A549 cells, but not as much as HLf (data not shown). The previously described inhibitory effect of BLf has been suggested to involve the heparin binding site of BLf. We have tested whether heparan sulfate might serve as the HLf receptor responsible for HLf-mediated Ad5 binding to and infection of target cells, using heparan sulfate-deficient CHO-2241 cells (ATCC pgsB-618), but HLf promoted Ad5 infection in CHO cells with an efficiency that was similar to that in CHO-2241 cells (data not shown), thus indicating that the cell surface component that is responsible for this effect remains to be identified. However, it is obvious that there are receptors for HLf on both human epithelial cells and T cells (this paper and references 7 and 18), suggesting that HLf may indeed interact with these cells and also promote Ad5 binding to and infection of them in vivo, even in the absence of CAR.
The most commonly described mechanism used by viruses for binding to target cells usually involves a direct interaction between virions and their cell surface receptor. In addition, antibody-dependent enhancement of infection mediated by interactions between the Fc region of virus-specific immunoglobulin G and Fc receptors on certain cells has been described for a number of viruses (29). There have only been a few reports indicating that other components serve as a bridge between viruses and target cells: (i) C4BP and coagulation factor IX have been reported to promote Ad5 interactions with target cells when the CAR binding site of the Ad5 fiber has been ablated (39); (ii) dipalmitoyl phosphatidylcholine may be involved in Ad2 entry into alveolar epithelial cells (5); (iii) polymerized human serum albumin is necessary and sufficient for binding of hepatitis B virus-like particles to human liver plasma membranes (32, 44); (iv) BLf has been suggested to enhance infection of gC-negative herpes simplex viruses in mouse fibroblasts (24); and (v) HLf has been proposed to generate formation of an HHV8-lactoferrin-glycosaminoglycan-epithelial cell receptor complex, which may increase the initial infective viral dose in the vicinity of potential target cells and increase the risk of infection (20). This report further potentiates the role of lactoferrin as a soluble component with the ability to promote binding to and infection of target cells, by serving as a bridge between the virion and the surface of the target cell.
HLf has previously been shown to serve as an antimicrobial component of the innate immune system. Numerous bacteria, fungi, and viruses have been found to be inhibited or inactivated by HLf, by various mechanisms (45, 51). With few exceptions, the mechanism by which HLf inhibits viruses appears to be at the level of binding to target cells, either by blocking the cellular receptor or the viral ligand. Since binding of Ad5 to cells is promoted by free HLf, but not by cell-associated HLf, we suggest that Ad5 hijacks HLf and thereafter links the virions to the cell surface. Thus, in this case the activity of HLf is promicrobial rather than antimicrobial.
Acknowledgments
We thank Linda Gooding for the generous gift of CEM, CEM-CARhi, KE37, and KE37-CARhi cells, Jeffrey M. Bergelson for the generous gift of RmcB ascites, Silvio Hemmi for the generous gift of the monoclonal antibody E1-1, and Robert Lehrer for the generous gift of lipophilin.
This work was supported by grants from the Swedish Research Council (grants no. 2003-6008 and 2004-6174), Umeå University (biotechnology grant), the Swedish Society for Medical Research, the Jeansson Foundation, and the Stiftelsen Clas Groschinskys Foundation.
Footnotes
Published ahead of print on 1 November 2006.
REFERENCES
- 1.Araki-Sasaki, K., K. Y. Ohasi, T. Sasabe, K. Hayashi, H. Watanabe, Y. Tano, and H. Handa. 1995. An SV-40-immortalized human corneal epithelial cell line and its characterization. Investig. Ophthalmol. 36:614-621. [PubMed] [Google Scholar]
- 2.Arnberg, N., K. Edlund, A. H. Kidd, and G. Wadell. 2000. Adenovirus type 37 uses sialic acid as a cellular receptor. J. Virol. 74:42-48. [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold, D., A. M. Di Biase, M. Marchetti, A. Pietrantoni, P. Valenti, L. Seganti, and F. Superti. 2002. Antiadenovirus activity of milk proteins: lactoferrin prevents viral infection. Antivir. Res. 53:153-158. [DOI] [PubMed] [Google Scholar]
- 4.Avila, M. M., G. Carballal, H. Rovaletti, B. Ebekian, M. Cusminsky, and M. Weissenbacher. 1989. Viral etiology in acute lower respiratory infections in children from a closed community. Am. Rev. Respir. Dis. 140:634-637. [DOI] [PubMed] [Google Scholar]
- 5.Balakireva, L., G. Schoehn, E. Thouvenin, and J. Chroboczek. 2003. Binding of adenovirus capsid to dipalmitoyl phosphatidylcholine provides a novel pathway for virus entry. J. Virol. 77:4858-4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergelson, J. M., J. A. Cunningham, G. Droguett, E. A. Kurt-Jones, A. Krithivas, J. S. Hong, M. S. Horwitz, R. L. Crowell, and R. W. Finberg. 1997. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science 275:1320-1323. [DOI] [PubMed] [Google Scholar]
- 7.Bi, B. Y., B. Leveugle, J. L. Liu, A. Collard, P. Coppe, A. C. Roche, N. Nillesse, M. Capron, G. Spik, and J. Mazurier. 1994. Immunolocalization of the lactotransferrin receptor on the human T lymphoblastic cell line Jurkat. Eur. J. Cell Biol. 65:164-171. [PubMed] [Google Scholar]
- 8.Brandt, C. D., H. W. Kim, A. J. Vargosko, B. C. Jeffries, J. O. Arrobio, B. Rindge, R. H. Parrott, and R. M. Chanock. 1969. Infections in 18,000 infants and children in a controlled study of respiratory tract disease. I. Adenovirus pathogenicity in relation to serologic type and illness syndrome. Am. J. Epidemiol. 90:484-500. [DOI] [PubMed] [Google Scholar]
- 9.Chagas, C. A., L. H. Endo, E. Sakano, G. A. Pinto, P. Brousset, and J. Vassallo. 2006. Detection of herpesvirus type 8 (HHV8) in children's tonsils and adenoids by immunohistochemistry and in situ hybridization. Int. J. Pediatr. Otorhinolaryngol. 70:65-72. [DOI] [PubMed] [Google Scholar]
- 10.Chen, Z., M. Ahonen, H. Hamalainen, J. M. Bergelson, V. M. Kahari, and R. Lahesmaa. 2002. High-efficiency gene transfer to primary T lymphocytes by recombinant adenovirus vectors. J. Immunol. Methods 260:79-89. [DOI] [PubMed] [Google Scholar]
- 11.Coyne, C. B., and J. M. Bergelson. 2005. CAR: a virus receptor within the tight junction. Adv. Drug Deliv. Rev. 57:869-882. [DOI] [PubMed] [Google Scholar]
- 12.Coyne, C. B., and J. M. Bergelson. 2006. Virus-induced Abl and Fyn kinase signals permit coxsackievirus entry through epithelial tight junctions. Cell 124:119-131. [DOI] [PubMed] [Google Scholar]
- 13.Di Biase, A. M., A. Pietrantoni, A. Tinari, R. Siciliano, P. Valenti, G. Antonini, L. Seganti, and F. Superti. 2003. Heparin-interacting sites of bovine lactoferrin are involved in anti-adenovirus activity. J. Med. Virol. 69:495-502. [DOI] [PubMed] [Google Scholar]
- 14.Ford, E., K. E. Nelson, and D. Warren. 1987. Epidemiology of epidemic keratoconjunctivitis. Epidemiol. Rev. 9:244-261. [DOI] [PubMed] [Google Scholar]
- 15.Gaggar, A., D. M. Shayakhmetov, and A. Lieber. 2003. CD46 is a cellular receptor for group B adenoviruses. Nat. Med. 9:1408-1412. [DOI] [PubMed] [Google Scholar]
- 16.Gao, W., P. D. Robbins, and A. Gambotto. 2003. Human adenovirus type 35: nucleotide sequence and vector development. Gene Ther. 10:1941-1949. [DOI] [PubMed] [Google Scholar]
- 17.Garnett, C. T., D. Erdman, W. Xu, and L. R. Gooding. 2002. Prevalence and quantitation of species C adenovirus DNA in human mucosal lymphocytes. J. Virol. 76:10608-10616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghio, A. J., J. D. Carter, J. M. Samet, W. Reed, J. Quay, L. A. Dailey, J. H. Richards, and R. B. Devlin. 1998. Metal-dependent expression of ferritin and lactoferrin by respiratory epithelial cells. Am. J. Physiol. 274:28-36. [DOI] [PubMed] [Google Scholar]
- 19.Gordon, Y. J., K. Aoki, and P. R. Kinchington. 1996. Adenovirus keratoconjunctivitis, p. 877-894. In J. S. Pepose, G. N. Holland, and K. R. Wilhelmus (ed.), Ocular infection and immunity. Mosby, St. Louis, Mo.
- 20.Grange, P. A., A. G. Marcelin, V. Calvez, C. Chauvel, J. P. Escande, and N. Dupin. 2005. Salivary lactoferrin is recognized by the human herpesvirus-8. J. Investig. Dermatol. 124:1249-1258. [DOI] [PubMed] [Google Scholar]
- 21.Hendrixson, D. R., J. Qiu, S. C. Shewry, D. L. Fink, S. Petty, E. N. Baker, A. G. Plaut, and J. W. R. St. Geme. 2003. Human milk lactoferrin is a serine protease that cleaves Haemophilus surface proteins at arginine-rich sites. Mol. Microbiol. 47:607-617. [DOI] [PubMed] [Google Scholar]
- 22.Holterman, L., R. Vogels, R. van der Vlugt, M. Sieuwerts, J. Grimbergen, J. Kaspers, E. Geelen, E. van der Helm, A. Lemckert, G. Gillissen, S. Verhaagh, J. Custers, D. Zuijdgeest, B. Berkhout, M. Bakker, P. Quax, J. Goudsmit, and M. Havenga. 2004. Novel replication-incompetent vector derived from adenovirus type 11 (Ad11) for vaccination and gene therapy: low seroprevalence and non-cross-reactivity with Ad5. J. Virol. 78:3207-3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horwitz, M. S. 2001. Adenoviruses, p. 2301-2326. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa. [Google Scholar]
- 24.Marchetti, M., E. Trybala, F. Superti, M. Johansson, and T. Bergstrom. 2004. Inhibition of herpes simplex virus infection by lactoferrin is dependent on interference with the virus binding to glycosaminoglycans. Virology 318:405-413. [DOI] [PubMed] [Google Scholar]
- 25.Marttila, M., D. Persson, D. Gustafsson, M. K. Liszewski, J. P. Atkinson, G. Wadell, and N. Arnberg. 2005. CD46 is a cellular receptor for all species B adenoviruses except types 3 and 7. J. Virol. 79:14429-14436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McNees, A. L., J. A. Mahr, D. Ornelles, and L. R. Gooding. 2004. Postinternalization inhibition of adenovirus gene expression and infectious virus production in human T-cell lines. J. Virol. 78:6955-6966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mei, Y. F., K. Lindman, and G. Wadell. 1998. Two closely related adenovirus genome types with kidney or respiratory tract tropism differ in their binding to epithelial cells of various origins. Virology 240:254-266. [DOI] [PubMed] [Google Scholar]
- 28.Moriuchi, M., and H. Moriuchi. 2001. A milk protein lactoferrin enhances human T cell leukemia virus type I and suppresses HIV-1 infection. J. Immunol. 166:4231-4236. [DOI] [PubMed] [Google Scholar]
- 29.Munakata, Y., I. Kato, T. Saito, T. Kodera, K. K. Ishii, and T. Sasaki. 2006. Human parvovirus B19 infection of monocytic cell line U937 and antibody-dependent enhancement. Virology 345:251-257. [DOI] [PubMed] [Google Scholar]
- 30.Nilsson, M., J. Ljungberg, J. Richter, T. Kiefer, M. Magnusson, A. Lieber, B. Widegren, S. Karlsson, and X. Fan. 2004. Development of an adenoviral vector system with adenovirus serotype 35 tropism; efficient transient gene transfer into primary malignant hematopoietic cells. J. Gene Med. 6:631-641. [DOI] [PubMed] [Google Scholar]
- 31.Pickles, R. J., D. McCarty, H. Matsui, P. J. Hart, S. H. Randell, and R. C. Boucher. 1998. Limited entry of adenovirus vectors into well-differentiated airway epithelium is responsible for inefficient gene transfer. J. Virol. 72:6014-6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pontisso, P., M. A. Petit, M. J. Bankowski, and M. E. Peeples. 1989. Human liver plasma membranes contain receptors for the hepatitis B virus pre-S1 region and, via polymerized human serum albumin, for the pre-S2 region. J. Virol. 63:1981-1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roberts, D. M., A. Nanda, M. J. Havenga, P. Abbink, D. M. Lynch, B. A. Ewald, J. Liu, A. R. Thorner, P. E. Swanson, D. A. Gorgone, M. A. Lifton, A. A. Lemckert, L. Holterman, B. Chen, A. Dilraj, A. Carville, K. G. Mansfield, J. Goudsmit, and D. H. Barouch. 2006. Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature 441:239-243. [DOI] [PubMed] [Google Scholar]
- 34.Roelvink, P. W., I. Kovesdi, and T. J. Wickham. 1996. Comparative analysis of adenovirus fiber-cell interaction: adenovirus type 2 (Ad2) and Ad9 utilize the same cellular fiber receptor but use different binding strategies for attachment. J. Virol. 70:7614-7621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roelvink, P. W., A. Lizonova, J. G. Lee, Y. Li, J. M. Bergelson, R. W. Finberg, D. E. Brough, I. Kovesdi, and T. J. Wickham. 1998. The coxsackievirus-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J. Virol. 72:7909-7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Segerman, A., J. P. Atkinson, M. Marttila, V. Dennerquist, G. Wadell, and N. Arnberg. 2003. Adenovirus type 11 uses CD46 as a cellular receptor. J. Virol. 77:9183-9191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Segerman, A., K. Lindman, Y. F. Mei, A. Allard, and G. Wadell. 2006. Adenovirus types 11p and 35 attach to and infect primary lymphocytes and monocytes, but hexon expression in T-cells requires prior activation. Virology 349:96-111. [DOI] [PubMed] [Google Scholar]
- 38.Shafren, D. R., R. C. Bates, M. V. Agrez, R. L. Herd, G. F. Burns, and R. D. Barry. 1995. Coxsackieviruses B1, B3, and B5 use decay accelerating factor as a receptor for cell attachment. J. Virol. 69:3873-3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shayakhmetov, D. M., A. Gaggar, S. Ni, Z. Y. Li, and A. Lieber. 2005. Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity. J. Virol. 79:7478-7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stone, D., S. Ni, Z. Y. Li, A. Gaggar, N. DiPaolo, Q. Feng, V. Sandig, and A. Lieber. 2005. Development and assessment of human adenovirus type 11 as a gene transfer vector. J. Virol. 79:5090-5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swart, P. J., M. E. Kuipers, C. Smit, R. Pauwels, M. P. deBethune, E. de Clercq, D. K. Meijer, and J. G. Huisman. 1996. Antiviral effects of milk proteins: acylation results in polyanionic compounds with potent activity against human immunodeficiency virus types 1 and 2 in vitro. AIDS Res. Hum. Retrovir. 12:769-775. [DOI] [PubMed] [Google Scholar]
- 42.Thirion, C., H. Lochmuller, Z. Ruzsics, M. Boelhauve, C. Konig, C. Thedieck, S. Kutik, C. Geiger, S. Kochanek, C. Volpers, and H. G. Burgert. 2006. Adenovirus vectors based on human adenovirus type 19a have high potential for human muscle-directed gene therapy. Hum. Gene Ther. 17:193-205. [DOI] [PubMed] [Google Scholar]
- 43.Tomko, R. P., R. Xu, and L. Philipson. 1997. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc. Natl. Acad. Sci. USA 94:3352-3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trevisan, A., F. Gudat, R. Guggenheim, G. Krey, U. Durmuller, G. Luond, M. Duggelin, J. Landmann, P. Tondelli, and L. Bianchi. 1982. Demonstration of albumin receptors on isolated human hepatocytes by light and scanning electron microscopy. Hepatology 2:832-835. [DOI] [PubMed] [Google Scholar]
- 45.van der Strate, B. W., L. Beljaars, G. Molema, M. C. Harmsen, and D. K. Meijer. 2001. Antiviral activities of lactoferrin. Antivir. Res. 52:225-239. [DOI] [PubMed] [Google Scholar]
- 46.van der Veen, J., and M. Lambriex. 1973. Relationship of adenovirus to lymphocytes in naturally infected human tonsils and adenoids. Infect. Immun. 7:604-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vogels, R., D. Zuijdgeest, R. van Rijnsoever, E. Hartkoorn, I. Damen, M. P. de Bethune, S. Kostense, G. Penders, N. Helmus, W. Koudstaal, M. Cecchini, A. Wetterwald, M. Sprangers, A. Lemckert, O. Ophorst, B. Koel, M. van Meerendonk, P. Quax, L. Panitti, J. Grimbergen, A. Bout, J. Goudsmit, and M. Havenga. 2003. Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: efficient human cell infection and bypass of preexisting adenovirus immunity. J. Virol. 77:8263-8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wadell, G., A. Allard, and J. C. Hierholzer. 1999. Adenoviruses, p. 970-982. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. ASM Press, Washington, D.C.
- 49.Walters, R. W., P. Freimuth, T. O. Moninger, I. Ganske, J. Zabner, and M. J. Welsh. 2002. Adenovirus fiber disrupts CAR-mediated intercellular adhesion allowing virus escape. Cell 110:789-799. [DOI] [PubMed] [Google Scholar]
- 50.Walters, R. W., T. Grunst, J. M. Bergelson, R. W. Finberg, M. J. Welsh, and J. Zabner. 1999. Basolateral localization of fiber receptors limits adenovirus infection from the apical surface of airway epithelia. J. Biol. Chem. 274:10219-10226. [DOI] [PubMed] [Google Scholar]
- 51.Weinberg, E. D. 2001. Human lactoferrin: a novel therapeutic with broad spectrum potential. J. Pharm. Pharmacol. 53:1303-1310. [DOI] [PubMed] [Google Scholar]
- 52.Wickham, T. J., P. Mathias, D. A. Cheresh, and G. R. Nemerow. 1993. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell 73:309-319. [DOI] [PubMed] [Google Scholar]