Abstract
The coexpression of human immunodeficiency virus type 1 (HIV-1) envelope glycoproteins and receptors leads to the lysis of single cells by a process that is dependent upon membrane fusion. This cell lysis was inhibited by low-molecular-weight compounds that interfere with receptor binding or with receptor-induced conformational transitions in the envelope glycoproteins. A peptide, T20, potently inhibited cell-cell fusion but had no effect on single cell lysis mediated by the HIV-1 envelope glycoproteins. Thus, critical events in the lysis of single cells by the HIV-1 envelope glycoproteins occur in intracellular compartments accessible only to small inhibitory compounds.
AIDS, which is caused by infection with human immunodeficiency virus type 1 (HIV-1), is characterized by the depletion of CD4-positive T lymphocytes (4, 10, 17, 20, 21). HIV-1 enters cells of the host immune system through the interaction of its envelope glycoproteins with cell surface receptors, CD4 and one of two chemokine receptors, CCR5 or CXCR4 (1, 9, 12, 15, 16, 18, 33, 44). In HIV-1 infection in humans and in the infection of monkeys with simian immunodeficiency virus (SIV) or simian-human immunodeficiency virus (SHIV), the CD4-positive T lymphocytes that are depleted express CD4 and the coreceptor utilized by the infecting virus (39, 46, 60). In HIV-1-infected humans, virus-producing cells exhibit much shorter half-lives than latently infected cells (29, 61), suggesting a role for a viral product in the destruction of host cells. HIV-1 infection of tissue-cultured cells leads to cytopathic effects, including syncytium formation and single cell lysis (11, 51, 58). Cytostatic or cytotoxic effects of the HIV-1 Tat, Vif, Vpr, Nef, and protease in cultured cells have been reported (5, 8, 23, 32, 38, 48, 49, 52, 53). However, lysis of cultured CD4-positive T cells infected by HIV-1 or depletion of these cells in SIV-infected monkeys has been observed in the absence of these viral proteins (2, 7, 22, 25, 26, 29, 30, 36, 47).
The expression of the HIV-1 envelope glycoproteins in cells expressing the appropriate receptors results in cytopathic effects (7, 35, 36, 40, 57). The HIV-1 gp120 envelope glycoprotein initiates virus entry by binding CD4 and either CCR5 or CXCR4; receptor binding induces conformational changes in gp120 that activate the gp41 transmembrane envelope glycoprotein to mediate the fusion of the viral and target cell membranes (28, 34, 59). Expression of the HIV-1 envelope glycoproteins on the surface of infected cells can lead to cell-cell fusion, resulting in the formation of lethal syncytia (40, 57). HIV-1 envelope glycoproteins also interact with receptors in the same cell, during the transport of these proteins through the secretory pathway (31). Through such interactions, the HIV-1 envelope glycoproteins can mediate the lysis of single, receptor-expressing cells; single-cell lysis is dependent upon the process of membrane fusion (7, 35, 36). Here we take advantage of the availability of several classes of HIV-1 entry inhibitors to investigate the possibility of interrupting the cytopathic effects of HIV-1 envelope glycoprotein expression.
MATERIALS AND METHODS
Compounds.
Compound A and BMS-806 were synthesized as previously described (19, 27, 41, 56). TAK-779 was generously provided by Takeda Pharmaceuticals. The T20 peptide was synthesized by American Peptide (Sunnyvale, CA). Compounds were dissolved in dimethyl sulfoxide at a final concentration of 10 mM and stored at −20°C. Just before use, the compounds were diluted in serum-free Dulbecco modified Eagle medium to create working stocks.
Cell lines.
293T human embryonic kidney and Cf2Th canine thymocytes (American Type Culture Collection) were grown at 37°C and 5% CO2 in Dulbecco modified Eagle medium (Invitrogen) containing 10% fetal bovine serum (Sigma) and 100 μg of penicillin-streptomycin (Mediatech, Inc.)/ml. Cf2Th cells stably expressing human CD4 and CCR5 or CXCR4 (36) were grown in medium supplemented with 0.4 mg of G418 (Invitrogen)/ml and 0.15 mg of hygromycin B (Roche Diagnostics)/ml. Cf2Th-CCR5 cells were grown in medium supplemented with 0.4 mg/ml of G418 (Invitrogen).
Recombinant luciferase viruses.
293T human embryonic kidney cells were cotransfected with plasmids expressing the pCMVΔP1Δenv HIV-1 Gag-Pol packaging construct (50), the R5 ADA or ADA ΔV1/V2 envelope glycoproteins (or the X4 HXBc2 envelope glycoproteins), and the firefly luciferase-expressing vector at a DNA ratio of 1:1:3 μg using Effectene transfection reagent (QIAGEN). Cotransfection of these plasmids produced single-round, replication-defective viruses. The virus-containing supernatants were harvested 24 to 30 h after transfection, filtered (0.45-μm pore size), divided into aliquots, and frozen at −80°C until further use. The reverse transcriptase activities of all viruses were measured as described previously (54).
Infection by single-round luciferase viruses.
Cf2Th-CD4-CCR5/CXCR4 target cells were seeded at a density of 6 × 103 cells/well in 96-well luminometer-compatible tissue culture plates (Dynex) 24 h before infection. On the day of infection, compound A (0 to 300 nM) was added to cells to a final volume of 30 μl, followed by incubation at 37°C for 1 h. Recombinant viruses (10,000 reverse transcriptase units) to a final volume of 50 μl were then added to the target cells containing compound A, followed by incubation for 48 h at 37°C; the medium was then removed from each well, and the cells were lysed by the addition of 30 μl of passive lysis buffer (Promega) and three freeze-thaw cycles. An EG&G Berthold microplate luminometer LB 96V was used to measure the luciferase activity of each well after the addition of 100 μl of luciferin buffer (15 mM MgSO4, 15 mM KPO4 [pH 7.8], 1 mM ATP, and 1 mM dithiothreitol) and 50 μl of 1 mM d-luciferin potassium salt (BD Pharmingen).
Syncytium inhibition assay.
Approximately 3 × 106 293T cells were seeded in an 80-mm tissue culture flask 24 h before transfection. The cells were transfected by using Polyfect transfection reagent (QIAGEN) with 2 μg of the pSVIIIenv plasmid encoding the HIV-1 envelope glycoprotein of interest and 0.2 μg of a Tat-expressing plasmid. At 24 h after transfection, the cells were lifted by using 5 mM EDTA and counted. Approximately 104 cells were incubated with 4 × 104 Cf2Th/CCR5 cells that had been seeded in 96-well plates 24 h earlier. To examine the effects of BMS-806 or T20 on the production of syncytia, transfected 293T cells were incubated with various concentrations of BMS-806 or T20 at 37°C for 15 min before their addition to Cf2Th-CCR5 cells. Syncytia were counted 24 h after the initiation of the coculture by visual inspection with a Nikon TE300 inverted microscope.
Transduction of cells and viability assay.
Recombinant HIV-1 viruses were produced in 293T cells by transfection with psrHIVenvGFP, pCMVΔP1ΔenvpA (50), pHCMV-G (64), and a Rev-expressing plasmid in a 10:10:2:1 ratio by using the calcium phosphate technique (35). At 48 h after transfection, virus-containing medium was harvested and filtered (0.45-μm pore size). Approximately 10,000 reverse transcriptase units of virus were incubated with 5 × 104 Cf2Th-CCR5 cells for 8 to 12 h as described previously (35). The cells were washed and returned to complete medium. At 72 h after transduction, plates were centrifuged at 3,000 rpm for 5 min, the medium was removed, and the cells were detached with trypsin. Approximately 85 to 90% of the cells were pelleted at 3,000 rpm (equivalent to 9,600 × g) for 5 to 10 min. Cell pellets were fixed in 3.7% formaldehyde and analyzed for green fluorescent protein (GFP) expression by flow cytometry. The remaining cells were plated in new six-well culture plates, propagated, and analyzed for GFP expression every 2 to 3 days until the completion of the experiment.
Radiolabelling of HIV-1 envelope glycoproteins.
293T cells were seeded at 3.5 × 106 cells in a T75 tissue culture flask 1 day before transfection. Cells were cotransfected with 9 μg of pSVIIIEnv(YU2) and 1 μg of pLTR-Tat by using the Polyfect transfection reagent (Qiagen). One day after transfection, the cells were labeled for 48 h with [35S]Express protein labeling mix (30 μCi/ml) (Perkin-Elmer). The supernatants were harvested 48 h later, cleared by centrifugation at 2,000 rpm for 5 min, and stored at 4°C. The amount of labeled gp120 was quantitated by immunoprecipitation with AIDS patient sera and protein A-Sepharose beads (Amersham Bio-Sciences), followed by SDS-PAGE gels and autoradiography.
Env-CCR5 binding assay.
Cf2Th cells expressing high levels of CCR5 were lifted, using 5 mM EDTA pH 7.5. The cells were washed with serum-free DMEM, added to microcentrifuge tubes (2 to 3 × 106 cells/tube), and incubated with 500 μl of labeled YU2 gp120 with soluble CD4 (sCD4) in the presence and absence of 2D7 anti-CCR5 antibody (BD Pharmingen), compound A, and BMS-806 at 37°C for 1.5 h with gentle agitation. The supernatants were removed following incubation, and the cells were washed two times with cold DMEM before lysis in 0.5 ml of IP buffer containing 0.5 M NaCl, 10 mM Tris, pH 7.5, and 0.5% [vol/vol] NP-40 and a cocktail of protease inhibitors. The cells were incubated in IP buffer for 30 min at 4°C with gentle agitation. The lysates were cleared by centrifugation at 14,000 rpm for 30 min at 4°C and immunoprecipitated with AIDS patient sera and protein A-Sepharose beads and visualized by autoradiography of a 3 to 8% SDS-polyacrylamide gel.
RESULTS
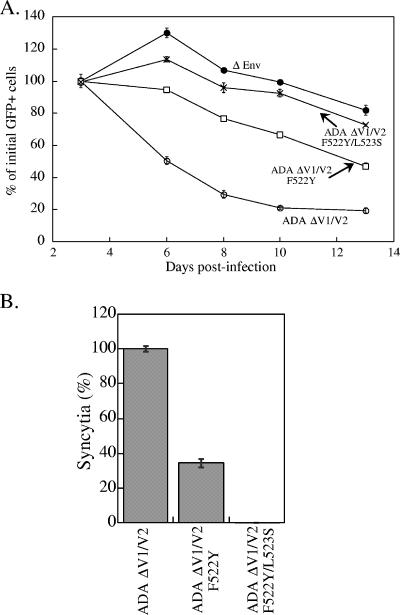
We previously established an experimental system to study single-cell lysis, using the minimal requirements for this process, i.e., expression of CD4-independent, CCR5-using (R5) HIV-1 envelope glycoproteins in cells expressing the CCR5 chemokine receptor (35, 36). Cf2Th canine thymocytes expressing the human CCR5 protein were transduced with single-round HIV-1 vectors expressing HIV-1 envelope glycoproteins and enhanced GFP. The effect of expression of the CD4-independent, CCR5-dependent ADA ΔV1/V2 envelope glycoproteins in Cf2Th cells expressing CCR5 is shown in Fig. 1A. The Cf2Th-CCR5 cells were infected with recombinant viruses coexpressing the HIV-1 envelope glycoproteins and GFP. The viability of the transduced cells is reflected in the percentage of GFP-positive cells in the culture (36). At 72 h after transduction, the percentage of GFP-positive cells ranged from 50 to 80% in all of the transduced Cf2Th cells. By day 10 after transduction, the expression of the ADA ΔV1/V2 envelope glycoproteins resulted in a significant decrease in the percentage of GFP-positive cells in the culture compared to cells transduced with the env-deleted (ΔEnv) control vector (Fig. 1A). Syncytia were rare in these cultures, suggesting that most of the decrease in GFP-positive, ADA ΔV1/V2-expressing cells results from single cell lysis, as previously observed (35, 36). The loss of GFP-positive cells was decreased or eliminated when the cells were transduced with vectors expressing the ADA ΔV1/V2 F522Y and F522Y/L523S envelope glycoproteins, respectively (Fig. 1A). These envelope glycoprotein variants have alterations in the gp41 fusion peptide that, respectively, diminish or eliminate membrane-fusing capacity. These mutant envelope glycoproteins bind CD4 and the CCR5 receptors equivalently to the ADA ΔV1/V2 envelope glycoprotein without the gp41 changes (data not shown). The syncytium-forming ability of the two gp41 mutants, which are expressed at levels comparable to that of the ADA ΔV1/V2 envelope glycoproteins, is illustrated in Fig. 1B. These results suggest that the loss of single cells coexpressing the ADA ΔV1/V2 envelope glycoproteins and GFP is dependent on membrane fusion.
FIG. 1.
Dependence of cytotoxic effects of CD4-independent HIV-1 envelope glycoproteins on membrane fusion. (A) Cf2Th-CCR5 cells were infected with recombinant HIV-1 vectors expressing the ADA ΔV1/V2, ADA ΔV1/V2 F522Y, or ADA ΔV1/V2 F522Y/L523S envelope glycoproteins or with a control vector (ΔEnv) lacking the ability to express functional envelope glycoproteins. All vectors express GFP. The percentages of cells expressing GFP 72 h after viral transduction were similar for all of the vectors (data not shown), and subsequent measurements of GFP-positive cells were normalized to this initial value, which was set at 100% for each vector. (B) The syncytium-forming abilities of the ADA ΔV1/V2, ADA ΔV1/V2 F522Y, and ADA ΔV1/V2 F522Y/L523S envelope glycoproteins were compared by coculturing 293T cells transiently expressing these glycoproteins with Cf2Th-CCR5 cells. Syncytium formation by the ADA ΔV1/V2 was considered to be 100%, and the numbers of syncytia observed for the other two envelope glycoproteins were normalized to this value. The means and standard deviations of duplicate experiments are shown.
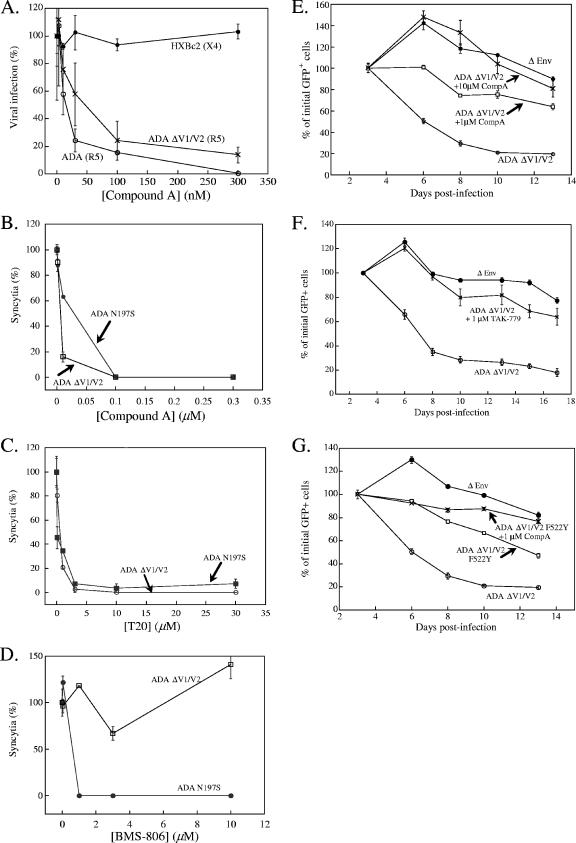
The effects of inhibitors of the HIV-1 entry process on the cytopathic consequences of HIV-1 envelope glycoprotein expression were examined. The inhibitors were chosen to block different steps in envelope glycoprotein function. Two small molecules, compound A (molecular weight 608.78 [Merck]) and TAK-779 (molecular weight 531.13 [Takeda]), bind CCR5 and block HIV-1 infection (19, 27). The specificity of compound A for the CCR5 coreceptor is illustrated in Fig. 2A. BMS-806 (molecular weight 406.4 [Bristol-Myers Squibb]) was originally proposed to block gp120-CD4 interaction (41) but has since been shown to block functionally important conformational changes in the HIV-1 envelope glycoproteins induced by receptor binding (29a, 56). T20 (molecular weight 4,492 [Trimeris/Roche]) is a peptide that mimics a gp41 region and, in a dominant-negative fashion, inhibits membrane fusion (32a, 62, 63). Compound A, TAK-779, and T20 potently inhibited syncytium formation mediated by the CD4-independent ADA ΔV1/V2 envelope glycoproteins (Fig. 2B and C and data not shown). BMS-806 did not inhibit syncytium formation mediated by the ADA ΔV1/V2 envelope glycoproteins (Fig. 2D). This was expected, because previous studies demonstrated that BMS-806 cannot inhibit the function of some HIV-1 envelope glycoproteins with deletions of the V1/V2 variable loops (43, 56). BMS-806 inhibited syncytium formation mediated by another CD4-independent envelope glycoprotein, ADA N197S (Fig. 2D). The ADA N197S gp120 envelope glycoprotein has intact V1/V2 variable loops and achieves CD4 independence by removal of the N-linked glycan at asparagine 197 (33a). BMS-806 has been previously shown to inhibit the entry of HIV-1 with the ADA N197S envelope glycoproteins (43). Thus, all of the molecules tested can inhibit the function of appropriate HIV-1 envelope glycoproteins.
FIG. 2.
CCR5 inhibitors block cytotoxicity mediated by CD4-independent HIV-1 envelope glycoproteins in CD4-CCR5+ cells. (A) Recombinant HIV-1 expressing firefly luciferase and containing the R5 ADA or ADA ΔV1/V2 envelope glycoproteins, or the X4 HXBc2 envelope glycoproteins, were used to infect cells in the presence of the indicated concentrations of compound A. Cf2Th-CD4/CCR5 cells were used as target cells for the viruses with ADA or ADA ΔV1/V2 envelope glycoproteins, whereas Cf2Th-CD4/CXCR4 cells were used for viruses with the HXBc2 envelope glycoproteins. The percentage of luciferase activity in the target cells relative to that obtained in the absence of compound is shown. The values represent the means and standard deviations of triplicate points in the assay. The results shown are typical of those obtained in two independent experiments. (B to D) 293T cells expressing the ADA ΔV1/V2 or N197S envelope glycoproteins were cocultivated with Cf2Th-CCR5 cells, and syncytia were counted as described in Materials and Methods. Some of the cocultures were incubated with the indicated concentrations of compounds. The numbers of syncytia observed 24 h later were normalized to those formed by the ADA ΔV1/V2 or N197S envelope glycoproteins in the absence of added compounds (this value was set to 100%). (E to G) Cf2Th-CCR5 cells were transduced with recombinant HIV-1 vectors expressing the ADA ΔV1/V2 envelope glycoprotein variants or a control vector (ΔEnv) in the presence or absence of compound A (E and G) or TAK-779 (F). Measurements and analyses were done as described in Materials and Methods and in the legend for Fig. 1A.
To examine the effects of these inhibitors on envelope glycoprotein-induced cell lysis, Cf2Th-CCR5 cells were incubated in the presence of the inhibitors with recombinant viruses coexpressing the ADA ΔV1/V2 envelope glycoprotein variants and GFP, and cultures were subsequently maintained in the presence of inhibitors until assayed for GFP expression. Both compound A and TAK-779 caused a significant reduction in cell lysis by the ADA ΔV1/V2 envelope glycoproteins (Fig. 2E and F). In addition, the moderate degree of cell lysis caused by the ADA ΔV1/V2 F522Y variant was completely eliminated by incubation with 1 μM compound A (Fig. 2G). Thus, low-molecular-weight compounds that block gp120-CCR5 interaction can prevent the lysis of single cells induced by the HIV-1 envelope glycoproteins.
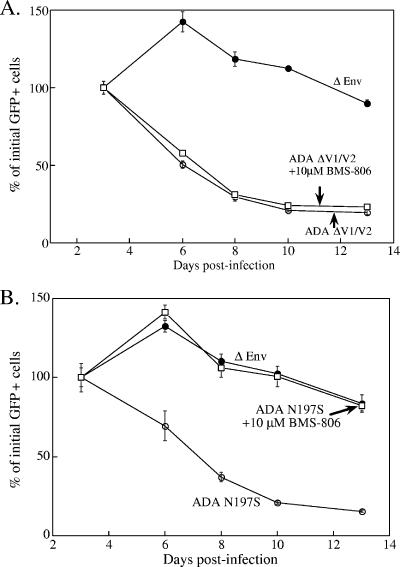
BMS-806 did not inhibit cell lysis induced by the ADA ΔV1/V2 envelope glycoproteins (Fig. 3A). This was expected because, as shown above and in previous studies (43, 56), BMS-806 cannot inhibit the function of some V1/V2 loop-deleted HIV-1 envelope glycoproteins. BMS-806 can inhibit entry (43, 56) and syncytium formation (see above) mediated by another CD4-independent ADA envelope glycoprotein variant, ADA N197S. BMS-806 completely blocked single cell lysis induced by the expression of the ADA N197S envelope glycoproteins in Cf2Th-CCR5 cells (Fig. 3B). Thus, a low-molecular-weight compound that blocks receptor-induced conformational changes in the HIV-1 envelope glycoproteins (43, 56) can interrupt the lysis of cells in this system.
FIG. 3.
Effect of BMS-806 on cell lysis by CD4-independent ADA envelope glycoprotein variants. Cf2Th-CCR5 cells were transduced with recombinant viruses expressing the ADA ΔV1/V2 (A) or ADA N197S (B) envelope glycoproteins in the presence or absence of 10 μM BMS-806. Control cells were transduced with the ΔEnv vector. Measurements and analysis were performed as described in Materials and Methods and in legend for Fig. 1A.
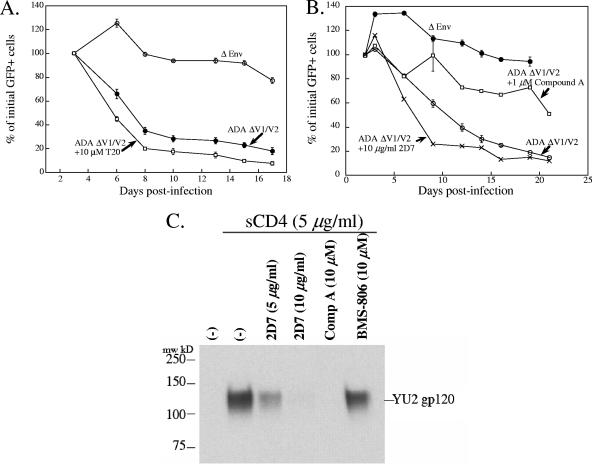
The T20 peptide did not affect the lysis of single cells associated with expression of the ADA ΔV1/V2 envelope glycoproteins in Cf2Th-CCR5 cells (Fig. 4A). The concentrations of T20 used in these experiments were identical to those that effectively inhibit viral entry and syncytium formation (3, 66) (Fig. 2C). The lysis of single cells that resulted from expression of the ADA ΔV1/V2 envelope glycoproteins in Cf2Th-CCR5 cells was not affected by incubation with the 2D7 antibody, which recognizes CCR5 (Fig. 4B). A control experiment showed that a comparable concentration of the 2D7 antibody decreased the soluble CD4-induced binding of CCR5 by the YU2 envelope glycoproteins (Fig. 4C).
FIG. 4.
Effect of T20 peptide and anti-CCR5 antibody on cell lysis by CD4-independent ADA envelope glycoproteins. (A) Cf2Th-CCR5 cells were transduced with recombinant viruses expressing the ADA ΔV1/V2 envelope glycoproteins in the presence or absence of 10 μM T20 peptide. The measurements and analyses were performed as described in Materials and Methods and in the legend for Fig. 1A. (B) Cf2Th-CCR5 cells were transduced with recombinant viruses expressing the ADA ΔV1/V2 envelope glycoproteins in the presence or absence of 2D7 anti-CCR5 antibody (10 μg/ml) and 1 μM compound A. The measurements and analyses were performed as described in Materials and Methods and in the legend to Fig. 1A. (C) Radiolabeled HIV-1 YU2 gp120 was incubated with Cf2Th-CCR5 cells either without treatment (left lane) or after treatment with 5 μg/ml of sCD4 in the presence and absence of the indicated concentrations of the 2D7 anti-CCR5 antibody, compound A, and BMS-806. The amount of gp120 bound to the Cf2Th-CCR5 cells is shown.
DISCUSSION
During the course of HIV-1 infection, R5 isolates predominate, particularly during the early years but often throughout the course of infection (13, 55). Although originally thought to be less cytopathic than X4 or R5X4 viruses, R5 HIV-1 have been shown to be very capable of killing target cells bearing CD4 and CCR5, both in tissue culture (65) and in lymph node explants (24, 25, 45). Likewise, in vivo, CD4+ CCR5+ cells are rapidly depleted in monkeys or humans infected by R5 primate immunodeficiency viruses (4, 10, 14, 17, 21, 37). The present study and previous studies (35, 36) underscore the ability of R5 HIV-1 envelope glycoproteins to lyse target cells expressing appropriate receptors. Membrane fusion, and not just receptor binding, is essential for the induction by the HIV-1 envelope glycoproteins of the death of primary as well as immortalized cells (35). CD4 is not essential for syncytium formation or single cell lysis if the HIV-1 envelope glycoprotein expressed is capable of mediating CD4-independent membrane fusion.
Low-molecular-weight compounds, but not the relatively large peptide T20 or an anti-CCR5 antibody, effectively blocked single cell lysis mediated by the HIV-1 envelope glycoproteins; in virus entry or syncytium formation assays, these envelope glycoproteins were shown to be susceptible to inhibition by all of these agents. Low-molecular-weight compounds, by virtue of their ability to permeate the cell interior (42), may be able to access the envelope glycoproteins and/or receptors involved in single-cell lysis, whereas the nonpermeable peptide T20 and the anti-CCR5 antibody cannot. This implies that key events in envelope glycoprotein-mediated single cell lysis occur in intracellular compartments, a scenario supported by two observations: (i) intracellular complexes of the HIV-1 envelope glycoproteins and receptors are abundant in infected cells (31) and (ii) large molecules, such as antibodies, are ineffective at blocking single-cell lysis in the context of an HIV-1-infected culture (6). Thus, small molecule inhibitors of HIV-1 entry, in addition to their therapeutic potential, represent tools for the investigation of viral immunopathogenesis.
Acknowledgments
We thank Kim Lowe at the Dana-Farber Cancer Institute flow cytometry core facility for excellent technical support and Yvette McLaughlin and Elizabeth Carpelan for manuscript preparation.
This study was supported by grants from the National Institutes of Health (AI24755, AI41851, and GM56550) and by the International AIDS Vaccine Initiative, the Bristol-Myers Squibb Foundation, the William A. Haseltine Foundation for the Arts and Sciences, and the late William F. McCarty-Cooper. N.M. was supported by an NRSA postdoctoral fellowship (F32 NS43260 M) from the National Institutes of Health.
Footnotes
Published ahead of print on 30 August 2006.
REFERENCES
- 1.Alkhatib, G., C. C. Broder, and E. A. Berger. 1996. Cell type-specific fusion cofactors determine human immunodeficiency virus type 1 tropism for T-cell lines versus primary macrophages. J. Virol. 70:5487-5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baba, M., O. Nishimura, N. Kanzaki, M. Okamoto, H. Sawada, Y. Iizawa, M. Shiraishi, Y. Aramaki, K. Okonogi, Y. Ogawa, K. Meguro, and M. Fujino. 1999. A small-molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc. Natl. Acad. Sci. USA 96:5698-5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bar, S., and M. Alizon. 2004. Role of the ectodomain of the gp41 transmembrane envelope protein of human immunodeficiency virus type 1 in late steps of the membrane fusion process. J. Virol. 78:811-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barre-Sinoussi, F., J. C. Chermann, F. Rey, M. T. Nugeyre, S. Chamaret, J. Gruest, C. Dauguet, C. Axler-Blin, F. Vezinet-Brun, C. Rouzioux, W. Rozenbaum, and L. Montagnier. 1983. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science 220:868-871. [DOI] [PubMed] [Google Scholar]
- 5.Bartz, S. R., and M. Emerman. 1999. Human immunodeficiency virus type 1 Tat induces apoptosis and increases sensitivity to apoptotic signals by up-regulating FLICE/caspase-8. J. Virol. 73:1956-1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergeron, L., N. Sullivan, and J. Sodroski. 1992. Target cell-specific determinants of membrane fusion within the human immunodeficiency virus type 1 gp120 third variable region and gp41 amino terminus. J. Virol. 66:2389-2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao, J., I. W. Park, A. Cooper, and J. Sodroski. 1996. Molecular determinants of acute single-cell lysis by human immunodeficiency virus type 1. J. Virol. 70:1340-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, M., R. T. Elder, M. Yu, M. G. O'Gorman, L. Selig, R. Benarous, A. Yamamoto, and Y. Zhao. 1999. Mutational analysis of Vpr-induced G2 arrest, nuclear localization, and cell death in fission yeast. J. Virol. 73:3236-3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choe, H., M. Farzan, Y. Sun, N. Sullivan, B. Rollins, P. D. Ponath, L. Wu, C. R. Mackay, G. LaRosa, W. Newman, N. Gerard, C. Gerard, and J. Sodroski. 1996. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell 85:1135-1148. [DOI] [PubMed] [Google Scholar]
- 10.Clavel, F., D. Guetard, F. Brun-Vezinet, S. Chamaret, M. A. Rey, M. O. Santos-Ferreira, A. G. Laurent, C. Dauguet, C. Katlama, C. Rouzioux, et al. 1986. Isolation of a new human retrovirus from West African patients with AIDS. Science 233:343-346. [DOI] [PubMed] [Google Scholar]
- 11.Dedera, D., and L. Ratner. 1991. Demonstration of two distinct cytopathic effects with syncytium formation-defective human immunodeficiency virus type 1 mutants. J. Virol. 65:6129-6136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng, H. K., D. Unutmaz, V. N. KewalRamani, and D. R. Littman. 1997. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature 388:296-300. [DOI] [PubMed] [Google Scholar]
- 13.de Roda Husman, A. M., R. P. van Rij, H. Blaak, S. Broersen, and H. Schuitemaker. 1999. Adaptation to promiscuous usage of chemokine receptors is not a prerequisite for human immunodeficiency virus type 1 disease progression. J. Infect. Dis. 180:1106-1115. [DOI] [PubMed] [Google Scholar]
- 14.Desrosiers, R. C. 1990. The simian immunodeficiency viruses. Annu. Rev. Immunol. 8:557-578. [DOI] [PubMed] [Google Scholar]
- 15.Doranz, B. J., J. Rucker, Y. Yi, R. J. Smyth, M. Samson, S. C. Peiper, M. Parmentier, R. G. Collman, and R. W. Doms. 1996. A dualtropic primary HIV-1 isolate that uses fusin and the beta-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell 85:1149-1158. [DOI] [PubMed] [Google Scholar]
- 16.Dragic, T., V. Litwin, G. P. Allaway, S. R. Martin, Y. Huang, K. A. Nagashima, C. Cayanan, P. J. Maddon, R. A. Koup, J. P. Moore, and W. A. Paxton. 1996. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature 381:667-673. [DOI] [PubMed] [Google Scholar]
- 17.Fauci, A. S., A. M. Macher, D. L. Longo, H. C. Lane, A. H. Rook, H. Masur, and E. P. Gelmann. 1984. NIH conference: acquired immunodeficiency syndrome: epidemiologic, clinical, immunologic, and therapeutic considerations. Ann. Intern. Med. 100:92-106. [DOI] [PubMed] [Google Scholar]
- 18.Feng, Y., C. C. Broder, P. E. Kennedy, and E. A. Berger. 1996. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science 272:872-877. [DOI] [PubMed] [Google Scholar]
- 19.Finke, P. E., B. Oates, S. G. Mills, M. MacCoss, L. Malkowitz, M. S. Springer, S. L. Gould, J. A. DeMartino, A. Carella, G. Carver, K. Holmes, R. Danzeisen, D. Hazuda, J. Kessler, J. Lineberger, M. Miller, W. A. Schleif, and E. A. Emini. 2001. Antagonists of the human CCR5 receptor as anti-HIV-1 agents. Part 4. Synthesis and structure-activity relationships for 1-[N-(methyl)-N-(phenylsulfonyl)amino]-2-(phenyl)-4-(4-(N-(alkyl)-N-(benzyloxycarbonyl)amino)piperidin-1-yl)butanes. Bioorg. Med. Chem. Lett. 11:2475-2479. [DOI] [PubMed] [Google Scholar]
- 20.Furuta, R. A., C. T. Wild, Y. Weng, and C. D. Weiss. 1998. Capture of an early fusion-active conformation of HIV-1 gp41. Nat. Struct. Biol. 5:276-279. [DOI] [PubMed] [Google Scholar]
- 21.Gallo, R. C., S. Z. Salahuddin, M. Popovic, G. M. Shearer, M. Kaplan, B. F. Haynes, T. J. Palker, R. Redfield, J. Oleske, B. Safai, et al. 1984. Frequent detection and isolation of cytopathic retroviruses (HTLV-1II) from patients with AIDS and at risk for AIDS. Science 224:500-503. [DOI] [PubMed] [Google Scholar]
- 22.Gibbs, J. S., A. A. Lackner, S. M. Lang, M. A. Simon, P. K. Sehgal, M. D. Daniel, and R. C. Desrosiers. 1995. Progression to AIDS in the absence of a gene for vpr or vpx. J. Virol. 69:2378-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glushakova, S., J. Munch, S. Carl, T. C. Greenough, J. L. Sullivan, L. Margolis, and F. Kirchhoff. 2001. CD4 down-modulation by human immunodeficiency virus type 1 Nef correlates with the efficiency of viral replication and with CD4+ T-cell depletion in human lymphoid tissue ex vivo. J. Virol. 75:10113-10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grivel, J. C., and L. B. Margolis. 1999. CCR5- and CXCR4-tropic HIV-1 are equally cytopathic for their T-cell targets in human lymphoid tissue. Nat. Med. 5:344-346. [DOI] [PubMed] [Google Scholar]
- 25.Grivel, J. C., M. L. Penn, D. A. Eckstein, B. Schramm, R. F. Speck, N. W. Abbey, B. Herndier, L. Margolis, and M. A. Goldsmith. 2000. Human immunodeficiency virus type 1 coreceptor preferences determine target T-cell depletion and cellular tropism in human lymphoid tissue. J. Virol. 74:5347-5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Groux, H., G. Torpier, D. Monte, Y. Mouton, A. Capron, and J. C. Ameisen. 1992. Activation-induced death by apoptosis in CD4+ T cells from human immunodeficiency virus-infected asymptomatic individuals. J. Exp. Med. 175:331-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hale, J. J., R. J. Budhu, E. B. Holson, P. E. Finke, B. Oates, S. G. Mills, M. MacCoss, S. L. Gould, J. A. DeMartino, M. S. Springer, S. Siciliano, L. Malkowitz, W. A. Schleif, D. Hazuda, M. Miller, J. Kessler, R. Danzeisen, K. Holmes, J. Lineberger, A. Carella, G. Carver, and E. Emini. 2001. 1,3,4-Trisubstituted pyrrolidine CCR5 receptor antagonists. Part 2: lead optimization affording selective, orally bioavailable compounds with potent anti-HIV activity. Bioorg. Med. Chem. Lett. 11:2741-2745. [DOI] [PubMed] [Google Scholar]
- 28.Helseth, E., M. Kowalski, D. Gabuzda, U. Olshevsky, W. Haseltine, and J. Sodroski. 1990. Rapid complementation assays measuring replicative potential of human immunodeficiency virus type 1 envelope glycoprotein mutants. J. Virol. 64:2416-2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ho, D. D., A. U. Neumann, A. S. Perelson, W. Chen, J. M. Leonard, and M. Markowitz. 1995. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature 373:123-126. [DOI] [PubMed] [Google Scholar]
- 29a.Ho, H.-T., L. Fan, B. Nowicka-Sans, B. McAuliffe, C.-B. Li, G. Yamanaka, N. Zhou, H. Fang, I. Dicker, R. Dalterio, Y.-F. Gong, T. Wang, Z. Yin, Y. Ueda, J. Matiskella, J. Kadow, P. Clapham, J. Robinson, R. Colonno, and P.-F. Lin. 2006. Envelope conformational changes induced by human immunodeficiency virus type 1 attachment inhibitors prevent CD4 binding and downstream entry events. J. Virol. 80:4017-4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoch, J., S. M. Lang, M. Weeger, C. Stahl-Hennig, C. Coulibaly, U. Dittmer, G. Hunsmann, D. Fuchs, J. Muller, S. Sopper, et al. 1995. vpr deletion mutant of simian immunodeficiency virus induces AIDS in rhesus monkeys. J. Virol. 69:4807-4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoxie, J. A., J. D. Alpers, J. L. Rackowski, K. Huebner, B. S. Haggarty, A. J. Cedarbaum, and J. C. Reed. 1986. Alterations in T4 (CD4) protein and mRNA synthesis in cells infected with HIV. Science 234:1123-1127. [DOI] [PubMed] [Google Scholar]
- 32.Kaplan, A. H., and R. Swanstrom. 1991. Human immunodeficiency virus type 1 Gag proteins are processed in two cellular compartments. Proc. Natl. Acad. Sci. USA 88:4528-4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32a.Kilby, J. M., S. Hopkins, T. M. Venetta, B. DiMassimo, G. A. Cloud, J. Y. Lee, L. Alldredge, E. Hunter, D. Lambert, D. Bolognesi, T. Matthews, M. R. Johnson, M. A. Nowak, G. M. Shaw, and M. S. Saag. 1998. Potent suppression of HIV-1 replication in humans by T-20, a peptide inhibitor of gp41-mediated virus entry. Nat. Med. 4:1302-1307. [DOI] [PubMed] [Google Scholar]
- 33.Klatzmann, D., E. Champagne, S. Chamaret, J. Gruest, D. Guetard, T. Hercend, J. C. Gluckman, and L. Montagnier. 1984. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature 312:767-768. [DOI] [PubMed] [Google Scholar]
- 33a.Kolchinsky, P., E. Kiprilov, P. Bartley, R. Rubenstein and J. Sodroski. 2001. Loss of a single N-linked glycan allows CD4-independent human immunodeficiency virus type 1 infection by altering the position of the gp120 V1/V2 variable loops. J. Virol. 75:3435-3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kowalski, M., L. Bergeron, T. Dorfman, W. Haseltine, and J. Sodroski. 1991. Attenuation of human immunodeficiency virus type 1 cytopathic effect by a mutation affecting the transmembrane envelope glycoprotein. J. Virol. 65:281-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.LaBonte, J. A., N. Madani, and J. Sodroski. 2003. Cytolysis by CCR5-using human immunodeficiency virus type 1 envelope glycoproteins is dependent on membrane fusion and can be inhibited by high levels of CD4 expression. J. Virol. 77:6645-6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.LaBonte, J. A., T. Patel, W. Hofmann, and J. Sodroski. 2000. Importance of membrane fusion mediated by human immunodeficiency virus envelope glycoproteins for lysis of primary CD4-positive T cells. J. Virol. 74:10690-10698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Letvin, N. L., M. D. Daniel, P. K. Sehgal, R. C. Desrosiers, R. D. Hunt, L. M. Waldron, J. J. MacKey, D. K. Schmidt, L. V. Chalifoux, and N. W. King. 1985. Induction of AIDS-like disease in macaque monkeys with T-cell tropic retrovirus STLV-III. Science 230:71-73. [DOI] [PubMed] [Google Scholar]
- 38.Li, C. J., D. J. Friedman, C. Wang, V. Metelev, and A. B. Pardee. 1995. Induction of apoptosis in uninfected lymphocytes by HIV-1 Tat protein. Science 268:429-431. [DOI] [PubMed] [Google Scholar]
- 39.Li, Q., L. Duan, J. D. Estes, Z. M. Ma, T. Rourke, Y. Wang, C. Reilly, J. Carlis, C. J. Miller, and A. T. Haase. 2005. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature 434:1148-1152. [DOI] [PubMed] [Google Scholar]
- 40.Lifson, J. D., M. B. Feinberg, G. R. Reyes, L. Rabin, B. Banapour, S. Chakrabarti, B. Moss, F. Wong-Staal, K. S. Steimer, and E. G. Engleman. 1986. Induction of CD4-dependent cell fusion by the HTLV-1II/LAV envelope glycoprotein. Nature 323:725-728. [DOI] [PubMed] [Google Scholar]
- 41.Lin, P. F., W. Blair, T. Wang, T. Spicer, Q. Guo, N. Zhou, Y. F. Gong, H. G. Wang, R. Rose, G. Yamanaka, B. Robinson, C. B. Li, R. Fridell, C. Deminie, G. Demers, Z. Yang, L. Zadjura, N. Meanwell, and R. Colonno. 2003. A small molecule HIV-1 inhibitor that targets the HIV-1 envelope and inhibits CD4 receptor binding. Proc. Natl. Acad. Sci. USA 100:11013-11018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lipinski, C. A., F. Lombardo, B. W. Dominy, and P. J. Feeney. 2001. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 46:3-26. [DOI] [PubMed] [Google Scholar]
- 43.Madani, N., A. L. Perdigoto, K. Srinivasan, J. M. Cox, J. J. Chruma, J. LaLonde, M. Head, A. B. Smith III, and J. G. Sodroski. 2004. Localized changes in the gp120 envelope glycoprotein confer resistance to human immunodeficiency virus entry inhibitors BMS-806 and #155. J. Virol. 78:3742-3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maddon, P. J., A. G. Dalgleish, J. S. McDougal, P. R. Clapham, R. A. Weiss, and R. Axel. 1986. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell 47:333-348. [DOI] [PubMed] [Google Scholar]
- 45.Malkevich, N., C. Womack, P. Pandya, J. C. Grivel, A. S. Fauci, and L. Margolis. 2001. Human immunodeficiency virus type 1 (HIV-1) non-B subtypes are similar to HIV-1 subtype B in that coreceptor specificity is a determinant of cytopathicity in human lymphoid tissue infected ex vivo. J. Virol. 75:10520-10522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mattapallil, J. J., D. C. Douek, B. Hill, Y. Nishimura, M. Martin, and M. Roederer. 2005. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 434:1093-1097. [DOI] [PubMed] [Google Scholar]
- 47.McCloskey, T. W., M. Ott, E. Tribble, S. A. Khan, S. Teichberg, M. O. Paul, S. Pahwa, E. Verdin, and N. Chirmule. 1997. Dual role of HIV Tat in regulation of apoptosis in T cells. J. Immunol. 158:1014-1019. [PubMed] [Google Scholar]
- 48.Okada, H., R. Takei, and M. Tashiro. 1997. HIV-1 Nef protein-induced apoptotic cytolysis of a broad spectrum of uninfected human blood cells independently of CD95(Fas). FEBS Lett. 414:603-606. [DOI] [PubMed] [Google Scholar]
- 49.Okada, H., R. Takei, and M. Tashiro. 1997. Nef protein of HIV-1 induces apoptotic cytolysis of murine lymphoid cells independently of CD95 (Fas) and its suppression by serine/threonine protein kinase inhibitors. FEBS Lett. 417:61-64. [DOI] [PubMed] [Google Scholar]
- 50.Parolin, C., B. Taddeo, G. Palu, and J. Sodroski. 1996. Use of cis- and trans-acting viral regulatory sequences to improve expression of human immunodeficiency virus vectors in human lymphocytes. Virology 222:415-422. [DOI] [PubMed] [Google Scholar]
- 51.Popovic, M., M. G. Sarngadharan, E. Read, and R. C. Gallo. 1984. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science 224:497-500. [DOI] [PubMed] [Google Scholar]
- 52.Purvis, S. F., J. W. Jacobberger, R. M. Sramkoski, A. H. Patki, and M. M. Lederman. 1995. HIV type 1 Tat protein induces apoptosis and death in Jurkat cells. AIDS Res. Hum. Retrovir. 11:443-450. [DOI] [PubMed] [Google Scholar]
- 53.Rasola, A., D. Gramaglia, C. Boccaccio, and P. M. Comoglio. 2001. Apoptosis enhancement by the HIV-1 Nef protein. J. Immunol. 166:81-88. [DOI] [PubMed] [Google Scholar]
- 54.Rho, H. M., B. Poiesz, F. W. Ruscetti, and R. C. Gallo. 1981. Characterization of the reverse transcriptase from a new retrovirus (HTLV) produced by a human cutaneous T-cell lymphoma cell line. Virology 112:355-360. [DOI] [PubMed] [Google Scholar]
- 55.Schuitemaker, H., M. Koot, N. A. Kootstra, M. W. Dercksen, R. E. de Goede, R. P. van Steenwijk, J. M. Lange, J. K. Schattenkerk, F. Miedema, and M. Tersmette. 1992. Biological phenotype of human immunodeficiency virus type 1 clones at different stages of infection: progression of disease is associated with a shift from monocytotropic to T-cell-tropic virus population. J. Virol. 66:1354-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Si, Z., N. Madani, J. M. Cox, J. J. Chruma, J. C. Klein, A. Schon, N. Phan, L. Wang, A. C. Biorn, S. Cocklin, I. Chaiken, E. Freire, A. B. Smith III, and J. G. Sodroski. 2004. Small-molecule inhibitors of HIV-1 entry block receptor-induced conformational changes in the viral envelope glycoproteins. Proc. Natl. Acad. Sci. USA 101:5036-5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sodroski, J., W. C. Goh, C. Rosen, K. Campbell, and W. A. Haseltine. 1986. Role of the HTLV-1II/LAV envelope in syncytium formation and cytopathicity. Nature 322:470-474. [DOI] [PubMed] [Google Scholar]
- 58.Somasundaran, M., and H. L. Robinson. 1987. A major mechanism of human immunodeficiency virus-induced cell killing does not involve cell fusion. J. Virol. 61:3114-3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stein, B. S., and E. G. Engleman. 1990. Intracellular processing of the gp160 HIV-1 envelope precursor: endoproteolytic cleavage occurs in a cis or medial compartment of the Golgi complex. J. Biol. Chem. 265:2640-2649. [PubMed] [Google Scholar]
- 60.Veazey, R. S., and A. A. Lackner. 2005. HIV swiftly guts the immune system. Nat. Med. 11:469-470. [DOI] [PubMed] [Google Scholar]
- 61.Wei, X., S. K. Ghosh, M. E. Taylor, V. A. Johnson, E. A. Emini, P. Deutsch, J. D. Lifson, S. Bonhoeffer, M. A. Nowak, B. H. Hahn, et al. 1995. Viral dynamics in human immunodeficiency virus type 1 infection. Nature 373:117-122. [DOI] [PubMed] [Google Scholar]
- 62.Wild, C., T. Oas, C. McDanal, D. Bolognesi, and T. Matthews. 1992. A synthetic peptide inhibitor of human immunodeficiency virus replication: correlation between solution structure and viral inhibition. Proc. Natl. Acad. Sci. USA 89:10537-10541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wild, C., D. C. Shugars, T. K. Greenwell, C. B. McDanal, and T. J. Matthews. 1994. Peptides corresponding to a predictive alpha-helical domain of human immunodeficiency virus type 1 gp41 are potent inhibitors of virus infection. Proc. Natl. Acad. Sci. USA 91:9770-9774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yee, J. K., A. Miyanohara, P. LaPorte, K. Bouic, J. C. Burns, and T. Friedmann. 1994. A general method for the generation of high-titer, pantropic retroviral vectors: highly efficient infection of primary hepatocytes. Proc. Natl. Acad. Sci. USA 91:9564-9568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yu, X., M. F. McLane, L. Ratner, W. O'Brien, R. Collman, M. Essex, and T. H. Lee. 1994. Killing of primary CD4+ T cells by non-syncytium-inducing macrophage-tropic human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA 91:10237-10241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yuan, W., S. Craig, Z. Si, M. Farzan, and J. Sodroski. 2004. CD4-induced T-20 binding to human immunodeficiency virus type 1 gp120 blocks interaction with the CXCR4 coreceptor. J. Virol. 78:5448-5457. [DOI] [PMC free article] [PubMed] [Google Scholar]