Abstract
The differentiation and functional status of virus-specific CD8+ T cells is significantly influenced by specific and ongoing antigen recognition. Importantly, the expression profiles of the interleukin-7 receptor alpha chain (CD127) and the killer cell lectin-like receptor G1 (KLRG1) have been shown to be differentially influenced by repetitive T-cell receptor interactions. Indeed, antigen-specific CD8+ T cells targeting persistent viruses (e.g., human immunodeficiency virus and Epstein-Barr virus) have been shown to have low CD127 and high KLRG1 expressions, while CD8+ T cells targeting resolved viral antigens (e.g., FLU) typically display high CD127 and low KLRG1 expressions. Here, we analyzed the surface phenotype and function of hepatitis C virus (HCV)-specific CD8+ T cells. Surprisingly, despite viral persistence, we found that a large fraction of peripheral HCV-specific CD8+ T cells were CD127+ and KLRG1− and had good proliferative capacities, thus resembling memory cells that usually develop following acute resolving infection. Intrahepatic virus-specific CD8+ T cells displayed significantly reduced levels of CD127 expression but similar levels of KLRG1 expression compared to the peripheral blood. These results extend previous studies that demonstrated central memory (CCR7+) and early-differentiated phenotypes of HCV-specific CD8+ T cells and suggest that insufficient stimulation of virus-specific CD8+ T cells by viral antigen may be responsible for this alteration in HCV-specific CD8+ T-cell differentiation during chronic HCV infection.
Virus-specific CD8+ T-cell responses play a major role in the outcome and pathogenesis of several viral infections. In persistent viral infections, virus-specific CD8+ T cells with impaired capacities to proliferate and secrete antiviral cytokines can be identified (18, 37). Several mechanisms may contribute to the inhibition of virus-specific CD8+ T-cell function, e.g., high viral load, lack of CD4+ T-cell help, action of regulatory T cells, and defects in T-cell differentiation (8, 19, 38). Indeed, during chronic infection, antiviral CD8+ T-cell responses are characterized by complex differentiation processes due to the coexistence with the virus (18, 36). For example, early-, intermediate-, and late-differentiated virus-specific CD8+ T cells have been defined in chronic infections by the coexpression of the costimulatory molecules CD27 and CD28 (3, 4). Importantly, late differentiation stages are characterized by the loss of CD27 and CD28 and the expression of cytotoxic molecules as well as markers of replicative senescence (CD57). This effector-like phenotype suggests that late-differentiated CD8+ T cells frequently recognize and engage virus-infected cells. However, this differentiation model cannot easily distinguish among naïve, early-differentiated, and true “memory” CD8+ T-cell populations because all of them coexpress CD27 and CD28 (4). According to a model proposed by Sallusto et al. (23), the expression of the lymph node-homing receptor CCR7 distinguishes between central memory (e.g., CCR7+) and effector memory (e.g., CCR7−) T-cell populations with distinct functional capacities. Specifically, CCR7+ central memory T cells are characterized by rapid proliferation after stimulation with antigen. Of note, a large fraction of peripheral HCV-specific CD8+ T cells has also been shown to be CCR7+ (20, 21), thus displaying a central memory phenotype, whereas intrahepatic HCV-specific CD8+ T cells were reported to display an effector-like CCR7− phenotype (1). However, the mechanisms responsible for this interesting finding are still unclear.
New insights into the immunobiology of virus-host interactions came from analyses of the expression of the interleukin-7 (IL-7) receptor alpha chain (CD127) and the killer cell lectin-like receptor G1 (KLRG1) on virus-specific CD8+ T cells. CD8+ effector T cells in the acute phase of an infection that are destined to become memory T cells express CD127 and share typical functions of memory CD8+ T cells, e.g., superior proliferative capacity and long-term survival in the absence of antigen (6, 11, 17, 19). Interestingly, the establishment of CD127+ memory T cells also depends on a prolonged duration of the initial antigen exposure (5), Consequently, CD127-expressing CD8+ T cells specific for cleared pathogens (respiratory syncytial virus, influenza virus (FLU), and hepatitis B virus [HBV]) were identified in humans (6, 31), but CD8+ T cells specific for persistent viruses (cytomegalovirus [CMV], Epstein-Barr virus [EBV], and human immunodeficiency virus [HIV]) lack significant CD127 expression (6, 22, 31). This dichotomy of virus-specific CD8+ T-cell populations is further extended by the expression of KLRG1. KLRG1 identifies antigen-experienced CD8+ T cells that are impaired in their proliferative capacities but are capable of immediate effector functions (32). Of note, recently we could show that repetitive antigen stimulation leads to an increase in KLRG1 expression by virus-specific CD8+ T cells in mice and that virus-specific CD8+ T cells are mostly KLRG1+ in chronic human viral infections, such as HIV, CMV, and EBV (26). These results imply that the likelihood of a T cell to express KLRG1 increases with the number of T-cell receptor (TCR)-triggering events. CD8+ T cells specific for cleared viruses show markedly decreased KLRG1 expression compared to CD8+ T cells specific for persistent viruses (16, 26). Thus, chronic viral infections elicit virus-specific CD127− KLRG1+ CD8+ T cells, whereas cleared infections are characterized by the presence of CD127+ KLRG1− CD8+ memory T cells.
In this study, we analyzed the phenotype and function of hepatitis C virus (HCV)-specific CD8+ T cells in acute and chronic HCV infection. Surprisingly, we identified the emerging dominance of blood-derived CD127+ KLRG1− HCV-specific CD8+ T cells with good proliferative capacities in a substantial number of patients with chronic infection. As previously reported, these cells are also CCR7+, resembling CD8+ memory T cells rather than impaired CD8+ effector T cells. Importantly, the majority of HCV-specific CD8+ T cells detected in the liver did not show a complete reversion towards a CD8+ effector T-cell phenotype, although a significant reduction in CD127 expression was observed. These results suggest that a lack of sufficient stimulation of peripheral and partly intrahepatic CD8+ T cells by viral antigen may be one mechanism that contributes to viral persistence.
MATERIALS AND METHODS
Subjects.
Fifty-three patients with chronic HCV infection and three patients with acute HCV infection presenting at the Department of Medicine II, University of Freiburg, Freiburg, Germany, were included in this study after informed consent and approval of the study by the local ethics committee were obtained. All patients were serologically tested for HLA-A and -B allele expression by the Institute of Transfusion Medicine, University of Freiburg, Freiburg, Germany. The characteristics of the patients with significant detectable frequencies of peripheral CD8+ HCV-tetramer-positive cells are listed in Table 1.
TABLE 1.
Characteristics of the study populationa
| ID | Sex | Age | HLA types | Genotype(s) | Viral load (GE/ml) | ALT | Epitope | Liver histologyb |
|---|---|---|---|---|---|---|---|---|
| C1 | M | 37 | A2, A33, B35, B51 | 1 | 2,250,580 | 20 | 1073 NS3 | II/III |
| C2 | F | 51 | A2, A11, B39 | 1 | 1,961,194 | 58 | 1406 NS3 | I/III |
| C3 | F | 40 | A1, A2, B7, B18 | 1 | 500,000 | 33 | 2594 NS5 | 0/0 |
| C4 | M | 44 | A2, B56 | 4 | 300,000 | 62 | 1073 NS3 | I/III |
| C5 | M | 43 | A2, B18, B62 | 1 | 797,519 | 115 | 1406 NS3 | I/I |
| C6 | F | 34 | A1, A2, B7, B13 | 3 | 33,038 | 78 | 1406 NS3 | I/III |
| C11 | M | 32 | A2, A3, B18, B44 | 1 | 36,635 | 37 | 2594 NS5 | II/I |
| C15 | M | 26 | A2, A26, B7, B27 | 1 | 146,845 | 112 | tp** | II/I |
| C18 | M | 35 | A2, A26, B7, B18 | 1 | Not quantified | 31 | tp** | I/II |
| C26 | M | 26 | A2, A25, B7, B8 | 1 | 1,157,650 | 81 | tp** | I/II |
| C28 | M | 21 | A2, A29, B44 | 1 | 2032,170 | 370 | 1073 NS3 | II/I |
| C31 | M | 33 | A2, A31, B8, B35 | 1 | 442,210 | 95 | 1073 NS3 | I/II |
| C38 | M | 25 | A2, B49, B44 | 3 | 136,000 | 259 | 2594 NS5 | I/I |
| A1 | M | 25 | A2, A11, B35, B44 | 3 | 462,883 | 3,586->37* | 1073 NS3 | Not done |
| A2 | M | 26 | A26, A29, B27, B35 | 1 and 3 | 2,289,236 | 1,891->30* | 1492 NS4 | Not done |
Characteristics of 14 patients with detectable HCV-tetramer-positive CD8+ T cells are shown. A total of 56 patients were tested for significant frequencies of HCV-tetramer-positive CD8+ T cells. *, alanine aminotransferase (ALT) levels from an early and a late time point are shown for patients with acute HCV infection. **, patients were tested with a pool of all available HLA-A2-restricted tetramers because of the low frequencies of HCV-specific CD8+ T cells. GE, genome equivalents; ID, patient identification number; tp, pooled tetramers. M, male; F, female.
Liver biopsies were analyzed using the Desmet classification (expressed as degree of inflammation/degree of fibrosis).
HCV assays.
HCV antibody testing was performed using the VITROS anti-HCV assay (Ortho-Clinical Diagnostics, Neckargmünd, Germany). HCV RNA reverse transcription-PCR was performed using the Cobas Amplicor (Roche, Basel, Switzerland). HCV genotypes were identified by InnoLIPA (Innogenetics, Ghent, Belgium), and viral load was determined by using the HCV RNA 3.0 assay (bDNA) (Bayer Diagnostics, Munich, Germany). All tests were performed at the Institute of Virology, University of Freiburg.
Lymphocyte isolation.
Peripheral blood mononuclear cells (PBMCs) were isolated from EDTA-anticoagulated blood samples on a Ficoll-Histopaque density gradient (PAA, Vienna, Austria). After isolation, cells were washed twice in phosphate-buffered saline (Gibco, Auckland, New Zealand) and either were studied immediately or were cryopreserved in medium containing 80% fetal calf serum (FCS) (Gibco), 10% dimethyl sulfoxide (Sigma-Aldrich, Germany), and 10% RPMI 1640 (Gibco). The results of phenotypical and functional assays performed with fresh and thawed PBMCs from the same bleed were comparable. Intrahepatic lymphocytes (IHL) were carefully isolated as previously described (24, 27). CD8+ IHLs were enriched using a CD8+ T-cell isolation kit II (Miltenyi Biotec, Bergisch Gladbach, Germany). Analyses of intrahepatic KLRG1 and CD127 expression were limited by the yield of cells, so patients could be tested for only one marker. IHLs were always analyzed immediately after isolation.
Synthetic peptides and multimer complexes.
HCV-derived peptides previously described as HLA-restricted HCV epitopes were synthesized with free N and C termini (Biosynthan, Berlin, Germany). These peptides were dissolved in 100% dimethyl sulfoxide (Sigma-Aldrich, Germany) at 20 mg/ml and further diluted to 1 mg/ml with RPMI 1640 (Gibco) before use, as previously described (28). The amino acid sequences of the HCV and influenza epitopes used in this study as well as the corresponding tetramers are shown in Table 2. HLA-A2 tetramers corresponding to the matching HCV peptides were obtained from the National Tetramer Core Facility at Emory University, Atlanta, GA. HLA-B8 and HLA-B27 tetramers were kindly provided by Scott Ward and Paul Klenerman (Nuffield Department of Clinical Medicine, Oxford, United Kingdom). HLA-A2-matched FLU multimers corresponding to a well-described influenza matrix protein epitope were obtained commercially from ProImmune, Inc., Oxford, United Kingdom.
TABLE 2.
Peptides and tetramersa
| HLA type | AA sequence | Protein | Position |
|---|---|---|---|
| A2 | DLMGYIPLV | HCV-Core | 132 |
| A2 | CINGVCWTV | HCV-NS3 | 1073 |
| A2 | KLVALGINAV | HCV-NS3 | 1406 |
| A2 | ALYDVVTKL | HCV-NS5 | 2594 |
| B8 | HSKKKCDEL | HCV-NS3 | 1395 |
| B27 | GRWVPGAAY | HCV-NS4a | 1492 |
| A2 | GILGFVFTL | Flu-Matrix | 58 |
The amino acid (AA) sequences of the peptides and multimers used in this study are shown, as well as the corresponding protein positions.
Antibodies.
The following monoclonal antibodies were used for immunostaining and fluorescence-activated cell sorter (FACS) analysis on a BD FACSCalibur flow cytometer using either CellQuest software (BD Biosciences) or FlowJo software (Tree Star, Inc., Ashland, OR): anti-CD8-fluorescein isothiocyanate (FITC), anti-CD8-phycoerythrin (PE), anti-CD8-Cy7-PE, anti-CD8-PerCP, anti-CD8-allophycocyanin, anti-CD38-PE, anti-CD45RA-FITC, anti-CD45RO-PE, anti-granzyme-B-FITC, isotype immunoglobulin G1 (all BD Pharmingen), anti-CCR7-FITC (R&D Systems, Minneapolis, MN), anti-CD27-FITC (Hölzel Diagnostika, Cologne, Germany), anti-CD28-PE (Diaclone, Stamford, CT), anti-CD57-FITC, and anti-CD127-PE (eBioscience, San Diego, CA). Anti-KLRG1-PE and -Alexa 488 have been described previously (32).
Multimer and antibody staining.
A total of 1 × 106 cells per well on a 96-well plate were incubated with HLA-matched tetramers. Incubation was performed at 37°C and 5% CO2 for 15 min. Cells were washed three times with phosphate-buffered saline containing 1% FCS, blocked with pure immunoglobulin G1 for 10 min, and stained with an anti-CD8 antibody for 15 min. Cells were washed three times, followed by surface staining with monoclonal fluorophore-conjugated antibodies as detailed above, incubated for 15 min at 4°C, washed again, and fixed in 100 μl CellFIX (BD Pharmingen) per well.
Intracellular and cytokine staining.
Procedures were performed essentially as described previously. Briefly, cells (0.2 × 106 per well on a 96-well plate) were stimulated with peptides (10 μg/ml) in the presence of 50 U/ml recombinant human IL-2 (Hoffmann-La Roche, Inc., Basel, Switzerland) and 1 μg/ml brefeldin A (BD Pharmingen). After 5 h of incubation (37°C; 5% CO2), cells from each well were blocked and stained with antibodies against CD8. Prior to staining with intracellular antibodies against gamma interferon (IFN-γ) and tumor necrosis factor alpha (BD Pharmingen), cells were fixed and permeabilized by adding Cytofix/Cytoperm (BD Pharmingen). Cells were washed three times and fixed in 100 μl CellFIX (BD Pharmingen) per well. Positive and negative control assays were performed using phorbol myristate acetate-ionomycin stimulation or no stimulation at all. FACS analysis was performed on a BD FACSCalibur flow cytometer.
Antigen-specific cell proliferation and CFSE assays.
Proliferation assays were performed without or after 5,6-carboxyfluorescein diacetate succinimidyl ester (CFSE) labeling: 3 × 106 PBMCs per well were resuspended in 50 μM CFSE (Molecular Probes). After 15 min of incubation, cells were pelleted, washed extensively, and resuspended in 1 ml RPMI (Gibco) containing 10% FCS, 1% streptomycin-penicillin, and 1.5% HEPES buffer (1 M) and stimulated with 10 μg/ml of synthetic HCV or FLU peptide (in some assays, in the presence of 10 ng/ml recombinant IL-7 [Sigma Aldrich]). On day 3, 1 ml fresh medium was added. After 7 days of incubation, multimer and antibody stainings were performed. FACS analysis was performed on a BD FACSCalibur flow cytometer.
RESULTS
A large fraction of HCV-specific CD8+ T cells express CD127 despite viral persistence.
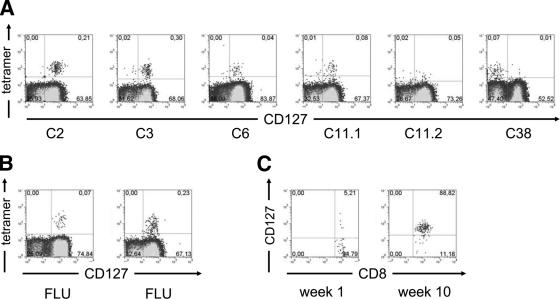
It was previously shown that CD127 expression is different on CD8+ T cells specific for persistent human viruses compared to that on CD8+ T cells specific for viruses that have been cleared (31). In addition, studies in the mouse model of chronic lymphocytic choriomeningitis virus (LCMV) infection have demonstrated a reduced CD127 expression on memory CD8+ T cells (11, 19). In this model, the emergence of CD127+ functionally competent memory T cells is compromised by high viral loads and inadequate T-cell help. To determine whether a similar phenotype is detectable on HCV-specific CD8+ T cells, we analyzed PBMCs from 10 chronically HCV-infected patients (Table 1) with detectable virus-specific CD8+ T-cell responses targeting six epitopes restricted by HLA-A2, HLA-B8, and HLA-B27 (Table 2). Surprisingly, a large fraction of HCV-specific CD8+ T cells displayed a high CD127 expression (median frequency of CD127 expression, 81%). As shown in Fig. 1 and 2, more than 70% of HCV-specific CD8+ T cells from six patients expressed CD127 and infrequent expression below 25% was observed in only two patients (C28 and C38). It is also important to note that different epitope-specific CD8+ T cells of a given patient (patient C11) showed comparable CD127 expression patterns (Fig. 1A). These results suggest that, in contrast to other persistent human viral infections, in chronic HCV infection, most virus-specific CD8+ T cells express CD127 and indeed resemble the phenotype observed on FLU-specific CD8+ T cells in resolved infection (Fig. 1A and B).
FIG. 1.
(A and B) CD127 expression on CD8+ T cells. (A) Representative plots of five patients are shown. HCV-specific CD8+ T cells were detected by positive tetramer binding. Patient C11 was analyzed using NS5 2594 (C11.1) and NS3 1406 (C11.2) tetramers. (B) In addition, two representative controls tested for CD127 expression on FLU-specific CD8+ T cells are shown. Gates were set on total CD8+ T cells. (C) Emergence of CD127 expression in acute infection. Patient A1 was analyzed for CD127 expression on HCV-specific CD8+ T cells during the acute phase of HCV infection. Upon clinical presentation, the majority of HCV-specific CD8+ tetramer-positive cells lack CD127 expression. After 10 weeks, the phenotype is reversed, with almost all HCV-specific CD8+ T cells expressing CD127. Gates were set on total tetramer-positive CD8+ T cells.
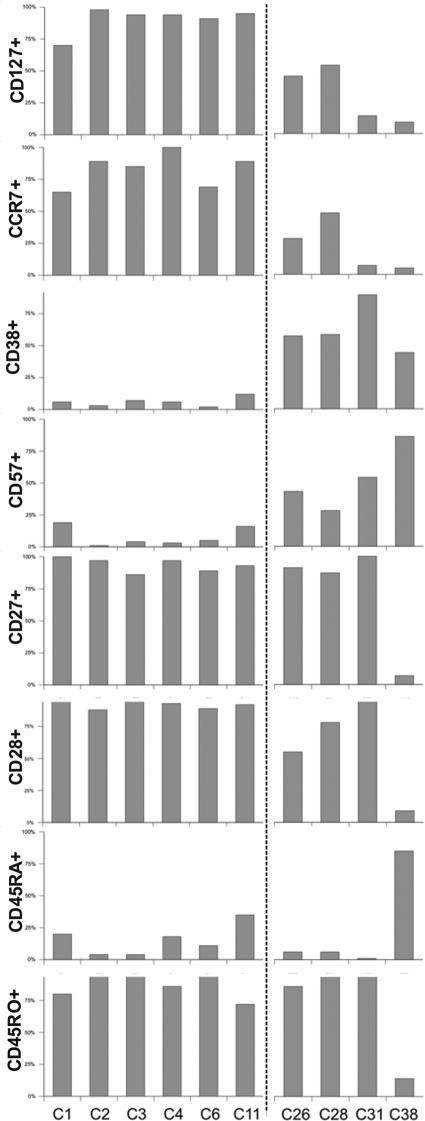
FIG. 2.
Correlation of CD127 expression with other phenotypical markers. Correlation of CD127 expression on HCV-specific CD8+ T cells with other phenotypical markers of 10 patients with chronic HCV infection is shown. Two subgroups with a differential expression profile based on CD127 and CD38 expression are distinguishable.
Next, we determined whether the expression of CD127 on HCV-specific CD8+ T cells was up-regulated during the acute phase of infection. Kaech et al. have shown in the mouse model of LCMV infection that only a fraction (9 to 15%) of antigen-specific CD8+ T cells are CD127+ during acute infection, but after viral clearance, CD127+ CD8+ T cells preferentially survive and dominate the memory pool (17). To investigate whether a similar pattern can be observed during acute HCV infection, we determined the expression of CD127 on HCV-specific CD8+ T cells at the onset of infection and after the establishment of persistence in patient A1. Interestingly, at the initial presentation, CD127 expression could be identified in only 5% of HCV-specific CD8+ T cells, whereas a significant increase in CD127 expression was observed later (Fig. 1C). Thus, in HCV infection, the emergence of CD127 expression on HCV-specific CD8+ T cells occurs during the acute phase and is not compromised by viral persistence.
Expression of CD127 on HCV-specific CD8+ T cells correlates with the expression of other surface markers.
Next, we correlated the expression of CD127 with other markers of T-cell activation and differentiation by monitoring HCV-specific CD8+ T cells for the expression of the activation marker CD38 and the memory and differentiation markers CCR7, CD27, CD28, CD45RO, and CD45RA. As reported by Lucas et al., a substantial number of patients with CCR7+ HCV-specific CD8+ T cells could be identified (patients C1 to C4, C6, and C11), but patients C26, C28, and especially C31 and C38 showed reduced expression of CCR7 on HCV-specific CD8+ T cells (Fig. 2) (21). Of note, there was a clear positive correlation between the expression of CCR7 and CD127, as has previously been shown for HIV-specific CD8+ T cells (22). By contrast, the expression of the activation marker CD38 was inversely correlated with CD127. Indeed, as shown in Fig. 2, patients C1 to C4, C6, and C11 had a high expression of CD127 and almost no detectable CD38 expression, whereas patients C26, C28, C31, and C38 displayed significantly weaker expression of CD127 associated with an up-regulation of CD38 on the same virus-specific CD8+ T cells, suggesting that activation leads to a down-regulation of CD127. We also observed that CD127− CD38+ HCV-specific CD8+ T cells had a higher expression level of CD57, a marker of replicative senescence. By contrast, only a few nonactivated CD127+ CD38− HCV-specific CD8+ T cells displayed an expression of CD57. As previously described, HCV-specific CD8+ T cells in all patients except patient C38 were double positive for CD27 and CD28, high in CD45RO, and low in CD45RA expression (3, 20, 21). Thus, these cells display a phenotype previously termed early differentiated. It is also important to note that these differentiation markers were not influenced by activation status (CD38 expression) and did not correlate with the level of CD127 or CCR7 expression on HCV-specific memory CD8+ T cells. Of note, patient C38 showed a slight distinct surface profile since his HCV-specific CD8+ T cells were double negative for CD27 and CD28 and low on CD45RO but high on CD45RA expression. Overall, these results suggest the existence of different phenotypes of HCV-specific CD8+ T cells during chronic HCV infection, with a predominantly CD127+ CCR7+ nonactivated HCV-specific CD8+ memory T-cell population and a CD127 and CCR7 low-activated effector T-cell population. It is important to note that these phenotypical differences were not due to mismatches of the peptides used in the study since our analyses included patients where the autologous sequence was intact (e.g., patients C1 and C26; epitope sequence, CINGVCWTV) or cross-reactive, as we have previously shown (24) (e.g., patient C3; epitope sequence, ALYDVVSKL or patient C38; epitope sequence, ALYDVVTKL).
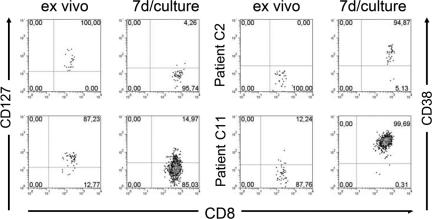
Next, we analyzed whether HCV-specific CD8+ T cells with CD127 expression despite viral persistence displayed a switch in CD127 expression in vitro following peptide stimulation. As shown in Fig. 3, significant changes in the expression of CD127 and CD38 were observed after expansion. Indeed, CD127 was almost completely down-regulated and CD38 was up-regulated, supporting the notion that activation leads to a rapid down-modulation of CD127. These results also show that the CD127+ phenotype in persistently infected patients in vivo can be easily overcome in vitro, indicating that the HCV-specific CD8+ T cells had no intrinsic differentiation block.
FIG. 3.
Effect of activation on HCV-specific CD8+ T cells. PBMCs were stimulated with epitope-specific peptide and cultured for 7 days. Analyses of CD127 and CD38 expression on HCV-specific CD8+ T cells are shown for two patients. In both, a CD127+ CD38− phenotype prior to stimulation was switched to a CD127− CD38+ phenotype after stimulation. Gates were set on the HCV-specific CD8+ tetramer-positive population.
Low KLRG1 expression on HCV-specific CD8+ T cells during chronic HCV infection.
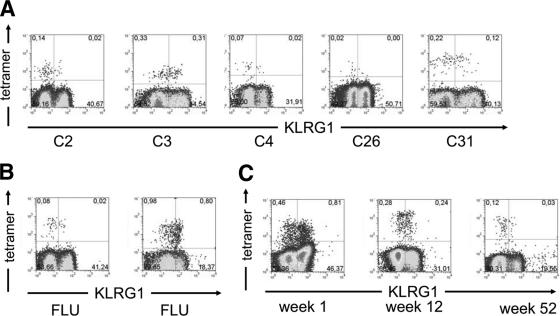
We and others have previously demonstrated that repetitive antigen stimulation leads to an increase in KLRG1 expression of virus-specific CD8+ T cells in mice and that virus-specific CD8+ T cells are in general KLRG1+ in chronic viral infection in humans, such as HIV, CMV, and EBV (16, 26). These results imply that the likelihood of a T cell to express KLRG1 increases with the number of TCR-triggering events. Since our results with CD127 also indicated the absence of TCR triggering, we determined the expression of KLRG1 on HCV-specific CD8+ T cells. Importantly, as shown in Fig. 4A and 5B, we observed a low level of KLRG1 expression (median frequency, 34%) on most HCV-specific CD8+ T cells. Of note, KLRG1 expression on HCV-specific CD8+ T cells is in the same range as on FLU-specific CD8+ T cells (median frequency, 34%) (Fig. 4A and B), as an example for a resolved infection, but significantly lower compared to HIV- (94%), CMV- (93%), and EBV- (90%) specific CD8+ T cells that represent chronic or latent viral infections (16, 26). Thus, the pattern of KLRG1 expression on HCV-specific CD8+ T cells suggests that these cells are not repetitively triggered by antigen despite viral persistence. By comparison, during acute HCV infection, a strong expression of KLRG1 is observed on HCV-specific CD8+ T cells (Fig. 4C). These results indicate that, during acute but not chronic HCV infection, stimulation with antigen is sufficient to induce the up-regulation of KLRG1 on HCV-specific CD8+ T cells, as has been observed before during acute viral infection in mice (26).
FIG. 4.
(A and B) KLRG1 expression on CD8+ T cells. (A) Representative plots of five patients are shown. HCV-specific CD8+ T cells were detected by positive tetramer binding. (B) In addition, two representative controls tested for KLRG1 expression on FLU-specific CD8+ T cells are shown. Gates were set on total CD8+ T cells. (C) KLRG1 expression in acute infection. Patient A2 was analyzed for KLRG1 expression on HCV-specific CD8+ T cells during the acute phase of HCV infection. Upon clinical presentation, the majority of HCV-specific CD8+ tetramer-positive cells expressed KLRG1. After 12 weeks, KLRG1 expression was observed on 45% of HCV-specific CD8+ T cells and 52 weeks after presentation, only 18% of tetramer-positive CD8+ cells showed KLRG1 expression. Gates were set on total CD8+ T cells.
FIG. 5.
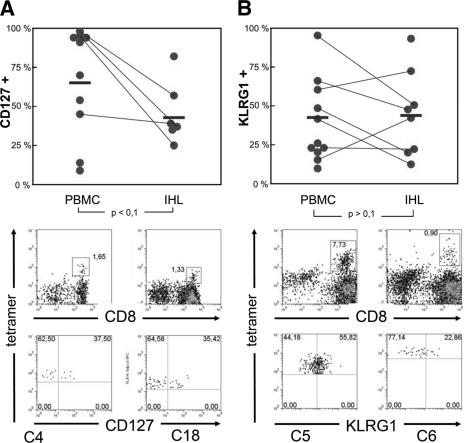
Expression of CD127 and KLRG1 on HCV-specific CD8+ T cells in blood and liver. (A) CD127 expression and (B) KLRG1 expression on HCV-specific CD8+ T cells derived from blood (PBMCs) or liver tissue (IHL) from chronically infected patients. Each circle represents one patient. Corresponding PBMC and IHL data from the same patient are connected by lines. Mean values are indicated by bars. The P value was calculated using a standard Student t test. Individual plots of patients C4 and C18 (CD127) as well as C5 and C6 (KLRG1) are shown below.
CD127+ memory HCV-specific CD8+ T cells have good proliferative capacities but only weak ex vivo IFN-γ production despite viral persistence.
To investigate the proliferative capacity of the HCV-specific CD8+ T cells at the single-cell level, we stained the cells with the fluorescent dye CFSE and determined the CFSE content of tetramer-positive cells after 7 days of in vitro stimulation with HCV peptides. As shown in Fig. 6A, CD127+ HCV-specific CD8+ T cells of patients C2, C3, C6, and C11 displayed weak CFSE signals, suggesting that they had proliferated during the days of culture. By contrast, HCV-specific CD8+ T cells that were originally CD127− still had an intense CFSE signal, indicating that these cells were viable but failed to proliferate (e.g., patient C38). These results are not entirely surprising since a low expression of CD127 is also associated with a CCR7−, CD38+, and CD57+ phenotype in our study cohort, all of which have been shown to be associated with a low proliferative capacity (2, 4). Taken together, these results suggest that a large fraction of HCV-specific CD8+ T cells have good proliferative capacities despite viral persistence that is associated with a CD127+ phenotype. By contrast, a CD127− phenotype is associated with a poor proliferative capacity. Noteworthy and as previously described (3, 34), we observed only a weak ex vivo IFN-γ production of these virus-specific memory CD8+ T cells (data not shown). However, the ability to produce IFN-γ could be restored at least partially in vitro in several patients after epitope-specific peptide stimulation (Fig. 5B).
FIG. 6.
Proliferation of HCV-specific CD8+ T cells. (A) PBMCs from several patients were preincubated with CFSE, stimulated with epitope-specific peptide, and incubated for 7 days. HCV-specific CD8+ T cells from patients with an original CD127+ phenotype proliferated vigorously (C2, C3, C6, and C11), but HCV-specific CD8+ T cells with a CD127− background failed to proliferate (C38), as measured by the decrease in CFSE fluorescence per cell. (B) IFN-γ production was tested after epitope-specific stimulation of HCV-specific CD8+ T cells. In contrast to ex vivo assays, significant IFN-γ production could be detected after in vitro culture in several patients. Gates were set on total CD8+ T cells.
Expression of CD127 and KLRG1 on intrahepatic HCV-specific CD8+ T cells.
Since viral replication during chronic HCV infection occurs primarily in the liver, we determined whether the CD127 and KLRG1 expression on HCV-specific CD8+ T cells is altered in the intrahepatic compartment. Of note, previous work has shown (12, 14) that intrahepatic HCV-specific CD8+ T cells progress to an activated (CD38+ CD69+) and relatively late differentiation phenotype (double negative for CD27 and CD28). We were able to analyze the intrahepatic HCV-specific CD8+ T-cell response in 12 patients from whom liver biopsy specimens were obtained for diagnostic purposes. Importantly, we observed a down-regulation of CD127 but no significant increase in KLRG1 expression on intrahepatic virus-specific CD8+ T cells compared to the peripheral blood (Fig. 5). These results indicate on the one hand that the HCV-specific CD8+ T-cell population was at least partially activated at the site of disease. On the other hand, they suggest that a fraction of intrahepatic virus-specific CD8+ T cells are not activated in a way to down-regulate CD127 or up-regulate KLRG1.
DISCUSSION
In this study, we report the emergence and maintenance of CD127+ HCV-specific memory phenotype CD8+ T cells in patients with acute and chronic HCV infection. Importantly, previous work has shown a similar emergence of CD127+ virus-specific CD8+ T cells in acute viral infections that were followed by viral clearance. Indeed, studies of acute LCMV and Listeria infection in mice and acute HBV infection in humans have reported the emergence of CD127-expressing CD8+ T cells that arise during the effector phase and acquire the phenotypical and functional characteristics of memory T cells (5, 6, 15, 17). In contrast, in persistently LCMV-infected mice, virus-specific CD8+ T cells displayed a low CD127 expression that is associated with an activated effector phenotype and T-cell dysfunctions (11, 19). Importantly, studies with humans also showed a lack of CD127 expression on virus-specific CD8+ T cells targeting persisting viruses, such as HIV, CMV, and EBV (22, 31). The down-regulation of CD127 during these chronic and latent virus infections has been attributed to ongoing repetitive TCR stimulation, whereas the high frequency of CD127 expression on FLU-, HBV-, and RSV-specific memory CD8+ T cells has been explained by a lack of persisting antigen and ongoing TCR triggering (6, 31). Thus, the frequency of CD127 expression on peripheral HCV-specific CD8+ T cells despite viral persistence suggests the absence of ongoing TCR triggering and/or antigen recognition at least in the peripheral blood.
Importantly, KLRG1 expression on HCV-specific CD8+ T cells supports this conclusion. Indeed, we have previously demonstrated that repetitive antigen stimulation leads to an increase in KLRG1 expression on virus-specific CD8+ T cells in mice and that virus-specific CD8+ T cells are mostly KLRG1+ in chronic human viral infections, such as HIV, CMV, and EBV, but not in resolved infection (FLU) (26). In this study, we show that HCV-specific CD8+ T cells have only a weak KLRG1 expression (Fig. 4 and 5B), thus displaying a phenotype of a resolved infection, such as FLU. Taken together, the combined results of the analysis of KLRG1 and CD127 expression on HCV-specific CD8+ T cells suggest that in a substantial number of patients, a large fraction of HCV-specific CD8+ T cells display a typical phenotype of memory T cells resembling that of a resolved infection. Since both markers have been shown to be directly influenced by ongoing TCR interactions, our results suggest that these HCV-specific CD8+ T cells are not repetitively triggered by viral antigen despite viral persistence. This concept also provides a likely explanation for the findings that peripheral HCV-specific CD8+ T cells are predominantly early differentiated and CCR7+ (4, 20, 21).
The mechanisms underlying this observation are not clear from this study. However, an intrinsic arrest in HCV-specific T-cell differentiation is unlikely since we could show that the CD127+ phenotype in persistently infected patients in vivo can be easily overcome in vitro by peptide-specific stimulation, as has also been shown for other differentiation markers, such as CD27, CD28, and CCR7 (21). Further, sequence analyses of the epitopes corresponding to the peptides included in the multimers exclude a possible mismatch of the used epitopes and the autologous epitope sequences in several patients. Other possible explanations include dendritic cell dysfunctions, low antigen presentation, action of regulatory T cells, rapid deletion of CD127− effector cells in the liver, and infrequent antigen encounters (e.g., anatomic separation from antigen in the liver and the immune response). In this regard, it is important to note that we observed a significant down-regulation of CD127 but no significant change in KLRG1 expression of intrahepatic HCV-specific CD8+ T cells compared to the peripheral blood response (Fig. 5B). In this context, it has been shown previously that intrahepatic HCV-specific CD8+ T cells and CD8+ T cells, irrespective of their antigen specificity, are activated in the liver (12, 14, 33). These results are in agreement with our observation that activation is clearly associated with a down-regulation of CD127 in vivo (Fig. 2) and in vitro (Fig. 3). They also support the notion that T-cell activation and differentiation are closely related, as has been previously shown for HIV infection (22). It is not clear, however, why not all virus-specific CD8+ T cells down-regulate CD127 in the liver. Next to the tolerogenic environment of the liver, a possible small amount of intrahepatic viral antigen may also contribute to the low fraction of CD127− HCV-specific CD8+ T cells. Whatever the explanation, our results suggest that insufficient stimulation of virus-specific CD8+ T cells may represent a viral immune evasion strategy in HCV infection, next to viral escape and T-cell dysfunctions (7, 9, 10, 25, 27, 29, 34).
It is also important to note that not all HCV-specific CD8+ T cells displayed a CD127+ phenotype. Indeed, we identified four patients with a significantly lower frequency of CD127 expression, ranging from 12 to 61% (Fig. 2). The infrequent expression of CD127 on HCV-specific CD8+ T cells was associated with a low level of CCR7 expression and a CD38+ and CD57+ phenotype. Thus, these patients displayed a more differentiated and activated effector memory phenotype that was also associated with a poor proliferative capacity. Interestingly, we did not find a significant correlation between the phenotype and viral load, indicating that factors other than the simple level of viremia lead to the activation of peripheral CD8+ T cells, e.g., the expression of costimulatory molecules, dendritic cell function, or level of cross-presentation (Table 1). Patient C38 showed a slight distinct surface profile since his HCV-specific CD8+ T cells were double negative for CD27 and CD28 and low in CD45RO but high in CD45RA expression. Of note, reexpression of CD45RA has been shown to occur on virus-specific CD8+ T cells (4, 13) and to be associated with a loss of CD27, CD28, and CCR7, and a gain of CD57 expression (4). This phenotype is characteristic for late-differentiated CD8+ T cells that have only a low proliferative capacity, as we could observe in this patient (Fig. 6). Although this phenotype does not seem to occur often in chronic HCV infection (3, 20, 21), it has been described previously for one chronically HCV-infected patient (34).
Of note, Urbani et al. recently reported the emergence of CD127 expression on HCV-specific CD8+ T cells only in patients that cleared the virus, not in patients that progressed to viral persistence (30). These data seem to be in contrast to our findings; however, they can be explained as follows: first, we have reported the existence of two different HCV-specific CD8+ T-cell populations, CD127+ and CD127−, suggesting that in the study of Urbani et al., a larger fraction of the latter cohort was analyzed. Second, their cohort consisted primarily of acutely infected patients, while we primarily focused on patients with long-term chronic HCV infection. Taken together, these combined results suggest the existence of different memory T-cell populations in chronic HCV infection that differ in their phenotypical and functional characteristics.
It is, indeed, another important finding of our study that CD127+ but not CD127− HCV-specific CD8+ T cells display good proliferative capacities. These results are not entirely surprising since it has been previously shown that the expression of CD127 serves as a predictor of the functional quality of antiviral CD8+ T cells in both mice and humans (11). Our results question the general assumption, however, that virus-specific CD8+ T cells specific for persistent viruses have impaired proliferative capacities. In this respect, most HCV-specific CD8+ T cells are clearly different from those specific for HIV, CMV, or EBV (4). In addition, we observed only a weak ex vivo IFN-γ production of these virus-specific CD8+ T cells that could be overcome in vitro. Hence, it seems less likely that this may indeed reflect a true dysfunction. Rather, these findings might just reflect the characteristics of central memory cells that do have good proliferative capacities and a weak ex vivo IFN-γ production (35) with the potential to differentiate into effector cells.
Taken together, our results show the emergence and maintenance of HCV-specific CD8+ T cells with a CD127+ KLRG1− proliferation-competent memory phenotype despite viral persistence in a substantial fraction of chronically HCV-infected patients. The coexistence of functional memory CD8+ T-cell populations, resembling memory T cells that develop following acute resolving infection, and virus can be best explained by a lack of sufficient stimulation of HCV-specific CD8+ T cells by persisting antigen. Our results may also be relevant for vaccine design because the induction of CD127+ proliferation-competent memory T cells is an important goal. For HCV infection, however, this requires the identification of the mechanisms underlying insufficient stimulation.
Acknowledgments
We thank the patients for donating blood after informed consent, in agreement with federal guidelines and the local ethics committee, and Natalja Nazarova for excellent technical assistance.
This study was supported by grants from the Deutsche Forschungsgemeinschaft (SFB 620, B2, and C6 and Emmy Noether-Program, TH 719/2-3) to Robert Thimme and Hanspeter Pircher and the state Baden-Wuerttemberg to Robert Thimme (Juniorprofessorenprogramm).
Footnotes
Published ahead of print on 1 November 2006.
REFERENCES
- 1.Accapezzato, D., V. Francavilla, M. Paroli, M. Casciaro, L. V. Chircu, A. Cividini, S. Abrignani, M. U. Mondelli, and V. Barnaba. 2004. Hepatic expansion of a virus-specific regulatory CD8+ T cell population in chronic hepatitis C virus infection. J. Clin. Investig. 113:963-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Accapezzato, D., V. Francavilla, P. Rawson, A. Cerino, A. Cividini, M. U. Mondelli, and V. Barnaba. 2004. Subversion of effector CD8+ T cell differentiation in acute hepatitis C virus infection: the role of the virus. Eur. J. Immunol. 34:438-446. [DOI] [PubMed] [Google Scholar]
- 3.Appay, V., P. R. Dunbar, M. Callan, P. Klenerman, G. M. Gillespie, L. Papagno, G. S. Ogg, A. King, F. Lechner, C. A. Spina, S. Little, D. V. Havlir, D. D. Richman, N. Gruener, G. Pape, A. Waters, P. Easterbrook, M. Salio, V. Cerundolo, A. J. McMichael, and S. L. Rowland-Jones. 2002. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat. Med. 8:379-385. [DOI] [PubMed] [Google Scholar]
- 4.Appay, V., and S. L. Rowland-Jones. 2004. Lessons from the study of T-cell differentiation in persistent human virus infection. Semin. Immunol. 16:205-212. [DOI] [PubMed] [Google Scholar]
- 5.Bachmann, M. F., R. R. Beerli, P. Agnellini, P. Wolint, K. Schwarz, and A. Oxenius. 2006. Long-lived memory CD8+ T cells are programmed by prolonged antigen exposure and low levels of cellular activation. Eur. J. Immunol. 36:842-854. [DOI] [PubMed] [Google Scholar]
- 6.Boettler, T., E. Panther, B. Bengsch, N. Nazarova, H. C. Spangenberg, H. E. Blum, and R. Thimme. 2006. Expression of the interleukin-7 receptor alpha chain (CD127) on virus-specific CD8+ T cells identifies functionally and phenotypically defined memory T cells during acute resolving hepatitis B virus infection. J. Virol. 80:3532-3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowen, D. G., and C. M. Walker. 2005. Adaptive immune responses in acute and chronic hepatitis C virus infection. Nature 436:946-952. [DOI] [PubMed] [Google Scholar]
- 8.Champagne, P., G. S. Ogg, A. S. King, C. Knabenhans, K. Ellefsen, M. Nobile, V. Appay, G. P. Rizzardi, S. Fleury, M. Lipp, R. Forster, S. Rowland-Jones, R. P. Sekaly, A. J. McMichael, and G. Pantaleo. 2001. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature 410:106-111. [DOI] [PubMed] [Google Scholar]
- 9.Cox, A. L., T. Mosbruger, Q. Mao, Z. Liu, X. H. Wang, H. C. Yang, J. Sidney, A. Sette, D. Pardoll, D. L. Thomas, and S. C. Ray. 2005. Cellular immune selection with hepatitis C virus persistence in humans. J. Exp. Med. 201:1741-1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erickson, A. L., Y. Kimura, S. Igarashi, J. Eichelberger, M. Houghton, J. Sidney, D. McKinney, A. Sette, A. L. Hughes, and C. M. Walker. 2001. The outcome of hepatitis C virus infection is predicted by escape mutations in epitopes targeted by cytotoxic T lymphocytes. Immunity 15:883-895. [DOI] [PubMed] [Google Scholar]
- 11.Fuller, M. J., D. A. Hildeman, S. Sabbaj, D. E. Gaddis, A. E. Tebo, L. Shang, P. A. Goepfert, and A. J. Zajac. 2005. Cutting edge: emergence of CD127high functionally competent memory T cells is compromised by high viral loads and inadequate T cell help. J. Immunol. 174:5926-5930. [DOI] [PubMed] [Google Scholar]
- 12.Grabowska, A. M., F. Lechner, P. Klenerman, P. J. Tighe, S. Ryder, J. K. Ball, B. J. Thomson, W. L. Irving, and R. A. Robins. 2001. Direct ex vivo comparison of the breadth and specificity of the T cells in the liver and peripheral blood of patients with chronic HCV infection. Eur. J. Immunol. 31:2388-2394. [DOI] [PubMed] [Google Scholar]
- 13.Hamann, D., P. A. Baars, M. H. Rep, B. Hooibrink, S. R. Kerkhof-Garde, M. R. Klein, and R. A. van Lier. 1997. Phenotypic and functional separation of memory and effector human CD8+ T cells. J. Exp. Med. 186:1407-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He, X. S., B. Rehermann, F. X. Lopez-Labrador, J. Boisvert, R. Cheung, J. Mumm, H. Wedemeyer, M. Berenguer, T. L. Wright, M. M. Davis, and H. B. Greenberg. 1999. Quantitative analysis of hepatitis C virus-specific CD8+ T cells in peripheral blood and liver using peptide-MHC tetramers. Proc. Natl. Acad. Sci. USA 96:5692-5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huster, K. M., V. Busch, M. Schiemann, K. Linkemann, K. M. Kerksiek, H. Wagner, and D. H. Busch. 2004. Selective expression of IL-7 receptor on memory T cells identifies early CD40L-dependent generation of distinct CD8+ memory T cell subsets. Proc. Natl. Acad. Sci. USA 101:5610-5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ibegbu, C. C., Y. X. Xu, W. Harris, D. Maggio, J. D. Miller, and A. P. Kourtis. 2005. Expression of killer cell lectin-like receptor G1 on antigen-specific human CD8+ T lymphocytes during active, latent, and resolved infection and its relation with CD57. J. Immunol. 174:6088-6094. [DOI] [PubMed] [Google Scholar]
- 17.Kaech, S. M., J. T. Tan, E. J. Wherry, B. T. Konieczny, C. D. Surh, and R. Ahmed. 2003. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 4:1191-1198. [DOI] [PubMed] [Google Scholar]
- 18.Klenerman, P., and A. Hill. 2005. T cells and viral persistence: lessons from diverse infections. Nat. Immunol. 6:873-879. [DOI] [PubMed] [Google Scholar]
- 19.Lang, K. S., M. Recher, A. A. Navarini, N. L. Harris, M. Lohning, T. Junt, H. C. Probst, H. Hengartner, and R. M. Zinkernagel. 2005. Inverse correlation between IL-7 receptor expression and CD8 T cell exhaustion during persistent antigen stimulation. Eur. J. Immunol. 35:738-745. [DOI] [PubMed] [Google Scholar]
- 20.Lauer, G. M., E. Barnes, M. Lucas, J. Timm, K. Ouchi, A. Y. Kim, C. L. Day, G. K. Robbins, D. R. Casson, M. Reiser, G. Dusheiko, T. M. Allen, R. T. Chung, B. D. Walker, and P. Klenerman. 2004. High resolution analysis of cellular immune responses in resolved and persistent hepatitis C virus infection. Gastroenterology 127:924-936. [DOI] [PubMed] [Google Scholar]
- 21.Lucas, M., A. L. Vargas-Cuero, G. M. Lauer, E. Barnes, C. B. Willberg, N. Semmo, B. D. Walker, R. Phillips, and P. Klenerman. 2004. Pervasive influence of hepatitis C virus on the phenotype of antiviral CD8+ T cells. J. Immunol. 172:1744-1753. [DOI] [PubMed] [Google Scholar]
- 22.Paiardini, M., B. Cervasi, H. Albrecht, A. Muthukumar, R. Dunham, S. Gordon, H. Radziewicz, G. Piedimonte, M. Magnani, M. Montroni, S. M. Kaech, A. Weintrob, J. D. Altman, D. L. Sodora, M. B. Feinberg, and G. Silvestri. 2005. Loss of CD127 expression defines an expansion of effector CD8+ T cells in HIV-infected individuals. J. Immunol. 174:2900-2909. [DOI] [PubMed] [Google Scholar]
- 23.Sallusto, F., D. Lenig, R. Forster, M. Lipp, and A. Lanzavecchia. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401:708-712. [DOI] [PubMed] [Google Scholar]
- 24.Spangenberg, H. C., S. Viazov, N. Kersting, C. Neumann-Haefelin, D. McKinney, M. Roggendorf, F. von Weizsacker, H. E. Blum, and R. Thimme. 2005. Intrahepatic CD8+ T-cell failure during chronic hepatitis C virus infection. Hepatology 42:828-837. [DOI] [PubMed] [Google Scholar]
- 25.Tester, I., S. Smyk-Pearson, P. Wang, A. Wertheimer, E. Yao, D. M. Lewinsohn, J. E. Tavis, and H. R. Rosen. 2005. Immune evasion versus recovery after acute hepatitis C virus infection from a shared source. J. Exp. Med. 201:1725-1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thimme, R., V. Appay, M. Koschella, E. Panther, E. Roth, A. D. Hislop, A. B. Rickinson, S. L. Rowland-Jones, H. E. Blum, and H. Pircher. 2005. Increased expression of the NK cell receptor KLRG1 by virus-specific CD8 T cells during persistent antigen stimulation. J. Virol. 79:12112-12116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thimme, R., J. Bukh, H. C. Spangenberg, S. Wieland, J. Pemberton, C. Steiger, S. Govindarajan, R. H. Purcell, and F. V. Chisari. 2002. Viral and immunological determinants of hepatitis C virus clearance, persistence, and disease. Proc. Natl. Acad. Sci. USA 99:15661-15668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thimme, R., D. Oldach, K. M. Chang, C. Steiger, S. C. Ray, and F. V. Chisari. 2001. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J. Exp. Med. 194:1395-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Timm, J., G. M. Lauer, D. G. Kavanagh, I. Sheridan, A. Y. Kim, M. Lucas, T. Pillay, K. Ouchi, L. L. Reyor, J. S. Zur Wiesch, R. T. Gandhi, R. T. Chung, N. Bhardwaj, P. Klenerman, B. D. Walker, and T. M. Allen. 2004. CD8 epitope escape and reversion in acute HCV infection. J. Exp. Med. 200:1593-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Urbani, S., B. Amadei, P. Fisicaro, D. Tola, A. Orlandini, L. Sacchelli, C. Mori, G. Missale, and C. Ferrari. 2006. Outcome of acute hepatitis C is related to virus-specific CD4 function and maturation of antiviral memory CD8 responses. Hepatology 44:126-139. [DOI] [PubMed] [Google Scholar]
- 31.van Leeuwen, E. M., G. J. de Bree, E. B. Remmerswaal, S. L. Yong, K. Tesselaar, I. J. ten Berge, and R. A. van Lier. 2005. IL-7 receptor alpha chain expression distinguishes functional subsets of virus-specific human CD8+ T cells. Blood 106:2091-2098. [DOI] [PubMed] [Google Scholar]
- 32.Voehringer, D., M. Koschella, and H. Pircher. 2002. Lack of proliferative capacity of human effector and memory T cells expressing killer cell lectinlike receptor G1 (KLRG1). Blood 100:3698-3702. [DOI] [PubMed] [Google Scholar]
- 33.Ward, S. M., J. R. Jonsson, S. Sierro, A. D. Clouston, M. Lucas, A. L. Vargas, E. E. Powell, and P. Klenerman. 2004. Virus-specific CD8+ T lymphocytes within the normal human liver. Eur. J. Immunol. 34:1526-1531. [DOI] [PubMed] [Google Scholar]
- 34.Wedemeyer, H., X. S. He, M. Nascimbeni, A. R. Davis, H. B. Greenberg, J. H. Hoofnagle, T. J. Liang, H. Alter, and B. Rehermann. 2002. Impaired effector function of hepatitis C virus-specific CD8+ T cells in chronic hepatitis C virus infection. J. Immunol. 169:3447-3458. [DOI] [PubMed] [Google Scholar]
- 35.Wherry, E. J., and R. Ahmed. 2004. Memory CD8 T-cell differentiation during viral infection. J. Virol. 78:5535-5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wherry, E. J., D. L. Barber, S. M. Kaech, J. N. Blattman, and R. Ahmed. 2004. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc. Natl. Acad. Sci. USA 101:16004-16009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wherry, E. J., J. N. Blattman, K. Murali-Krishna, R. van der Most, and R. Ahmed. 2003. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 77:4911-4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zajac, A. J., J. N. Blattman, K. Murali-Krishna, D. J. Sourdive, M. Suresh, J. D. Altman, and R. Ahmed. 1998. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188:2205-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]