Abstract
We demonstrate here that the Epstein-Barr virus (EBV) BZLF1 gene, a switch from latent infection to lytic infection, is expressed as early as 1.5 h after EBV infection in Burkitt's lymphoma-derived, EBV-negative Akata and Daudi cells and primary B lymphocytes. Since BZLF1 mRNA is expressed even when the cells are infected with EBV in the presence of anisomycin, an inhibitor of protein synthesis, its expression does not require prerequisite protein synthesis, indicating that BZLF1 is expressed as an immediate-early gene following primary EBV infection of B lymphocytes.
Epstein-Barr virus (EBV) efficiently infects human B lymphocytes and establishes latent infection, in which the entire EBV genome is maintained as an episome and restricted numbers of EBV genes are expressed (14). EBV binds to B lymphocytes through a direct interaction of the EBV glycoprotein gp350/220 with the complement receptor CD21. Additional interaction of the ternary EBV glycoprotein gp85-gp25-gp42 complex with HLA class II molecules allows endocytosis of the EBV virion into smooth vesicles. Subsequently the viral DNA is transported to the nucleus. The binding of EBV to B lymphocytes induces several signaling pathways that are required for efficient transcription of the viral genome (21). The genes encoding EBV-determined nuclear antigen 2 (EBNA2) and EBNA leader protein are the first viral genes known to be expressed following infection, and both genes have been shown to contribute to the ability of EBV to immortalize primary B lymphocytes (1, 2, 18). Protein synthesis is not required for events leading to the transcription of these genes (18), suggesting that the early stages of infection do not depend on the de novo synthesis of cellular proteins. This is consistent with the Wp promoter, which is constitutively active in B lymphocytes, being the first viral promoter used for transcription of the genes encoding EBNA2 and EBNA leader protein upon infection (26).
The BZLF1 protein is a transcriptional activator that binds to AP-1-like motifs in the promoters of early lytic genes (8, 9) and induces lytic infection in latently EBV-infected cells (5, 25). Analysis of Burkitt's lymphoma (BL)-derived Akata cells, in which lytic infection is efficiently and synchronously induced upon cross-linking of cell surface immunoglobulins (Ig) with anti-Ig antibodies, has revealed that BZLF1 is the first gene expressed in the absence of protein synthesis (22, 24). Here, we demonstrate that BZLF1 mRNA is expressed as early as 1.5 h postinfection in the absence of protein synthesis in primary EBV infection of B lymphocytes. A possible mechanism of early expression of the BZLF1 gene and its possible role in establishing latent infection are discussed.
BL-derived EBV-negative Akata and Daudi cell clones and primary B lymphocytes were used as targets for EBV infection. The Akata and Daudi cell lines were originally EBV positive. As reported previously, we isolated EBV-negative subclones from the parental Akata and Daudi cell cultures by a limiting-dilution culture (16, 20). Primary B lymphocytes were purified from peripheral blood mononuclear cells of EBV-seropositive donors by using Dynabeads CD19 and DETACHaBEAD CD19 (Dynal AS, Oslo, Norway). The purity of separated B lymphocytes was more than 95% as assessed by fluorescence-activated cell sorting analysis with CD19 and CD21 antibodies (Dako Denmark A/S, Glostrup, Denmark). Akata virus-derived recombinant EBV carrying the enhanced green fluorescence protein (EGFP) was used as a source of EBV (15). For virus production, Akata cells (2 × 106 cells) infected with EGFP-carrying EBV were suspended with 1 ml of fresh medium containing 0.5% goat antihuman IgG (Dako Denmark A/S) (24) and incubated for 6 h to induce lytic replication. The culture medium was replaced with fresh medium, and 2 days later, the culture supernatant was harvested. The culture supernatant was filtered with a 0.45-μm-pore-size membrane and used as a virus solution. For EBV infection, EBV-negative Akata and Daudi cells and primary B lymphocytes (106) were suspended in 1 ml of virus solution and incubated at 37°C for 90 min with continuous gentle mixing. After the removal of the virus solution, the infected cells were suspended in fresh medium. At the designated times of incubation, portions of the cells were examined by reverse transcriptase PCR (RT-PCR) for expression of BZLF1. Cellular RNA was isolated using the Trizol reagent (Invitrogen, Carlsbad, CA) and then treated with DNase I (Invitrogen) according to the manufacturer's instructions. RNA (1 μg) was reverse transcribed by using Moloney murine leukemia virus reverse transcriptase (Invitrogen) and 100 pmol of a random primer (Takara, Otsu, Japan) at 37°C for 60 min, followed by inactivation of reverse transcriptase at 94°C for 10 min. One-tenth of the cDNA samples was then subjected to 35 cycles of PCR consisting of denaturation for 30 s at 94°C, annealing for 30 s at 55°C, and extension for 1 min at 72°C. Primers used for amplification of BZLF1 mRNA were 5′-CATGTTTCAACCGCTCCGACTGG-3′ (upstream) and 5′-GCGCAGCCTGTCATTTTCAGATG-3′. The PCR products were electrophoresed in a 2% agarose gel and visualized by ethidium bromide staining. The quality of RNA was checked by PCR amplification of glyceraldehydes-3-phosphate dehydrogenase (GAPDH) cDNA.
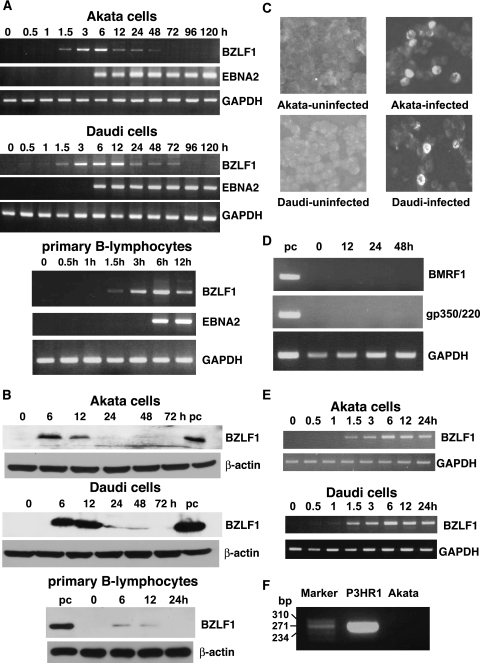
The results are shown in Fig. 1A. BZLF1 mRNA was detected as early as 1.5 h postinfection, peaked at 6 h in Akata cells and primary B lymphocytes and at 3 to 12 h in Daudi cells, and quickly decreased thereafter. EBNA2 mRNA was also investigated under the same PCR conditions with primer pairs of 5′-GCTGCTACGCATTAGAGACC-3′ (upstream) and 5′-TCCTGGTAGGGATTCGAGGG-3′. EBNA2 mRNA was first detected at 6 h postinfection and was constitutively expressed thereafter.
FIG. 1.
BZLF1 and EBNA2 expression after EBV infection of BL-derived, EBV-negative Akata and Daudi cells and peripheral B lymphocytes. (A) The cells were infected with EBV carrying the EGFP gene. At the designated time of incubation, expression of BZLF1 and EBNA2 mRNAs was examined by RT-PCR. To secure the quality of the RNA sample, GAPDH mRNA was also amplified by RT-PCR. (B) The cells were infected with EBV carrying the EGFP gene. At the designated time of incubation, expression of the BZLF1 protein was examined by immunoblot analysis using a mouse monoclonal antibody (Dako Denmark A/S). EBV-positive Akata cells treated with anti-Ig antibody were used as a positive control (pc). The β-actin protein was detected to show the quantity of each protein preparation. (C) Detection of the BZLF1 protein by immunofluorescence assay. The cells were infected with EBV carrying the EGFP gene. At 12 h postinfection, the cells were harvested and subjected to the immunofluorescence assay. (D) Analysis of early and late gene expression in primary B lymphocytes. At the designated time of incubation, expression of BMRF1 and gp350/220 mRNAs was examined by RT-PCR. (E) EBV-negative Akata and Daudi cells were infected with wild type EBV. At the designated time of incubation, expression of BZLF1 and GAPDH mRNA was examined by RT-PCR. (F) Detection of defective heterogenous EBV DNA. DNA extracted from het EBV-positive P3HR1 cells or recombinant Akata EBV was subjected to PCR. The molecular size marker (HaeIII digestion of ΦX174 DNA) is indicated in base pairs.
Expression of BZLF1 as a protein was examined by immunoblot analysis as described previously (12). Whole-cell lysates were prepared from EBV-infected Akata and Daudi cells (106), and 1/10 of the protein sample was separated in a sodium dodecyl sulfate-polyacrylamide (10%) gel. For EBV-infected primary B lymphocytes, protein samples obtained from 1.5 × 106 cells were used for each lane. The expression of the BZLF1 protein was detected with a monoclonal antibody specific for the BZLF1 protein (Dako Denmark A/S) as the primary antibody and a peroxidase-conjugated antimouse IgG (GE Healthcare, Buckinghamshire, United Kingdom). The results indicated that the BZLF1 protein was detected in EBV-infected Akata and Daudi cells and primary B lymphocytes at 6 h and 12 h postinfection (Fig. 1B). The efficiency of EBV infection was determined by EGFP expression at 24 h postinfection. EBV-infected Akata, Daudi, and primary B lymphocytes expressed EGFP in 20%, 32%, and 45% of cells, respectively. The fraction of infected cells expressing the BZLF1 protein was assessed by immunofluorescence. Smears of cells were fixed in 3.5% formaldehyde in phosphate-buffered saline at room temperature for 10 min and treated with a polyclonal rabbit antibody for BZLF1 and Cy3-conjugated antirabbit IgG antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) sequentially. The results indicated that Akata and Daudi cells expressed the BZLF1 protein in about 8% and 10% of cells at 12 h postinfection, respectively (Fig. 1C).
Subsequently, to assess whether BZLF1-expressing cells undergo lytic replication, we analyzed early (BMRF-1) and late (gp350/220) lytic gene expression in EBV-infected primary B lymphocytes by RT-PCR. Primers used for BMRF-1 and gp350/220 mRNA expression analysis were 5′-CTAGCCGTCCTGTCCAAGTGC-3′ (upstream) and 5′-AGCCAAACAGCTCCTTGCCCA-3′ or 5′-GTCAGTACACCATCCAGAGCC-3′(upstream) and 5′-TTGGTAGACAGCCTTCGTATG-3′, respectively. cDNA samples were subjected to 25 cycles of PCR consisting of denaturation for 30 s at 94°C, annealing for 30 s at 59°C (BMRF1) or 55°C (gp350/220), and extension for 1 min at 72°C. As shown in Fig. 1D, no early and late lytic gene expression was observed in EBV-infected cells, indicating that BZLF1 expressing cells do not undergo lytic infection.
Because we performed the experiments with a recombinant EBV, in which both GFP and neomycin resistance genes were driven by a simian virus 40 promoter inserted in BXLF1, it is possible that the presence of strong exogenous enhancer could result in BZLF1 expression. To exclude this possibility, we prepared wild-type Akata EBV and repeated the experiments. As shown in Fig. 1E, we observed the same patterns of early BZLF1 expression in Akata and Daudi cells as in the cells infected with recombinant EBV. Finally, we checked whether rearranged defective EBV (het virus) is present in the Akata virus population, since the rearrangement has been reported to cause early BZLF1 expression after EBV infection (13). DNA from het EBV DNA-positive P3HR-1 cells or culture supernatant containing recombinant Akata EBV was subjected to 40 cycles of PCR consisting of denaturation for 1 min at 94°C, annealing for 1 min at 45°C and extension for 1 min at 72°C. Primers were selected that framed the junction of rearranged DNA (5′-GCACATTAGCAATGCCTGTG-3′ and 5′-GTCCAGCGCGTTTACGTAAG-3′). het DNA amplification was detected in P3HR-1 DNA but not in Akata EBV DNA (Fig. 1F). Thus, we conclude that early BZLF1 expression is not due to the rearranged defective EBV.
To confirm that BZLF1 was induced by EBV infection, we examined whether UV-treated EBV could induce BZLF1 mRNA. Virus solution was exposed to 9.8 mJ of UV irradiation (7) in a UV cross-linker (Bio-Rad Laboratories, Hercules, CA) and then inoculated into EBV-negative Daudi and Akata cells and primary B lymphocytes. As shown in Fig. 2A, UV-treated EBV could not induce expression of BZLF1 as well as EBNA2 mRNAs, indicating that intact viral DNA was necessary for BZLF1 and EBNA2 expression.
FIG. 2.
(A) Effect of UV inactivation of EBV preparation on expression of BZLF1 and EBNA2 mRNAs after EBV infection. EBV-negative Daudi and Akata cells and primary B lymphocytes were infected with UV-treated EBV. At the designated time of incubation, expression of BZLF1 and EBNA2 mRNAs was examined by RT-PCR. (B) Effect of anisomycin, an inhibitor of protein synthesis, on expression of BZLF1 and EBNA2 mRNAs after EBV infection. EBV-negative Daudi cells were preincubated for 3 h in a medium containing 25 μg/ml anisomycin. The culture was then centrifuged, and the cell pellet was resuspended in virus solution containing anisomycin and incubated for 90 min. Thereafter, the cells were resuspended in fresh medium containing anisomycin, incubated for the designated time, and examined for expression of BZLF1 and EBNA2 mRNAs by RT-PCR. The effect of anisomycin on expression of the β-actin protein was examined by immunoblot analysis. (C) Effect of actinomycin D, an inhibitor of RNA synthesis, on expression of BZLF1 mRNA after EBV infection. EBV-negative Daudi cells were infected with EBV in the presence of 5 μg/ml actinomycin D, incubated for the designated time, and examined for expression of BZLF1 mRNA by RT-PCR.
The early detection of BZLF1 mRNA prompted us to examine whether it was expressed as an immediate-early gene. EBV-negative Daudi cells were preincubated for 3 h in a medium containing 25 μg/ml anisomycin (Sigma, St. Louis, MO), an inhibitor of protein synthesis. The culture was then centrifuged, and the cell pellet was resuspended in virus solution containing anisomycin and incubated for 90 min. Thereafter, the cells were resuspended in fresh medium containing anisomycin, incubated for the designated time, and examined for BZLF1 mRNA expression by RT-PCR. The results indicated that BZLF1 mRNA was detected as early as 1 h postinfection and the levels of expression were higher that those in control cells infected in the absence of anisomycin (Fig. 2B). On the other hand, anisomycin was efficient in blocking synthesis of the β-actin protein. These results demonstrated that BZLF1 was induced in the absence of protein synthesis following EBV infection and could be classified as an immediate-early gene.
To exclude the possibility that the BZLF1 RNA was packaged in the infecting virus particles, EBV-negative Daudi cells were infected with EBV in the presence of actinomycin D, an inhibitor of RNA synthesis, and expression of BZLF1 mRNA was examined by RT-PCR. The results indicated that actinomycin D completely inhibited expression of BZLF1 mRNA (Fig. 2C), indicating that BZLF1 RNA was newly synthesized after EBV infection.
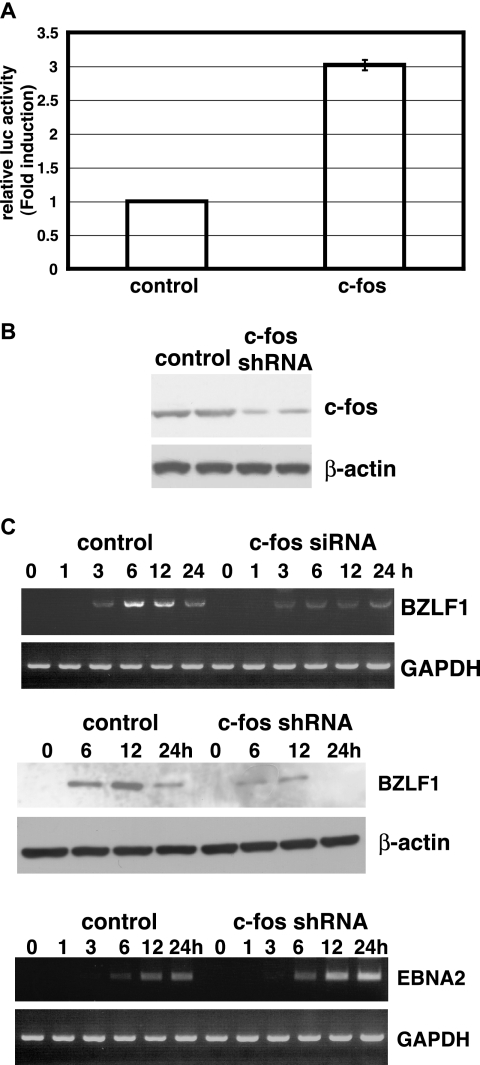
It has been established that various agents, including 12-O-tetradecanoylphorbol-13-acetate (TPA) and anti-Ig antibodies, induce lytic infection in latently EBV-infected cells (23, 28). These treatments trigger a variety of cellular signaling pathways, resulting in the activation of cellular transcription factors leading to transcription from the BZLF1 promoter. The BZLF1 protein is a lytic switch transactivator and activates the expression of EBV lytic genes necessary for lytic DNA replication. The BZLF1 promoter contains a consensus CREB/AP-1 motif, recognized by c-Jun and c-Fos, which plays a critical role in the induction of BZLF1 mRNA expression in response to TPA and anti-Ig antibodies in latently EBV-infected cells (10, 23). Since we first discovered that BZLF1 was expressed as an immediate-early gene in EBV-infected cells, we examined whether c-fos was involved in expression of BZLF1 in EBV-infected cells. At first, it was examined whether c-fos activated gene expression from the BZLF1 promoter, as described previously (12). A BZLF1-luciferase reporter plasmid (pGVB2/BZLF1) and a c-fos-expressing plasmid or control plasmid were cotransfected into EBV-negative Daudi cells using Lipofectamine 2000 (Invitrogen). After 48 h of incubation, cells were harvested and luciferase activity was determined. As shown in Fig. 3A, c -fos activated luciferase expression from the BZLF1 promoter. Next, we used small interfering RNA targeting c-fos to inhibit its expression in EBV-infected cells. EBV-negative Daudi cells (5 × 106) were transfected with 20 μg of the TransSilent Fos shRNA vector (Panomics, Redwood City, CA) or the TransSilent control vector by the electroporation method. Transfected cells were cultured for 2 days and plated in 96-well plates at 104 cells per well in medium containing 700 μg of G418/ml for selection. G418-resistant cell clones were examined for the expression of the c-fos protein by immunoblotting using a rabbit polyclonal antibody against c-Fos (Calbiochem, San Diego, CA) as the primary antibody. Although we screened more than 50 cell clones, completely c-Fos-negative cell clones could not be obtained, possibly because the c-Fos protein is necessary for cell survival and proliferation. Therefore, we chose the two cell clones with the least c-Fos expression as shown in Fig. 3B. Using these cell clones, we examined whether EBV infection induced BZLF1 expression. RT-PCR and immunoblot analysis of EBV-infected Daudi cells revealed that expression of BZLF1 mRNA and protein was repressed in c-fos-silenced Daudi cells (Fig. 3C), indicating that c-fos was necessary for expression of BZLF1 as an immediate-early gene after EBV infection. To assess whether c-fos silencing affected expression of other viral transcripts, we examined the expression of EBNA2 transcripts after EBV infection in c-fos-silenced Daudi cells. The results indicated that c-fos silencing did not affect EBNA2 expression (Fig. 3C).
FIG. 3.
Involvement of c-fos in expression of BZLF1 mRNA after EBV infection. (A) Effect of c-fos on gene expression from BZLF1 promoter. The BZLF1-luciferase reporter plasmid (pGVB2/BZLF1) and a c-fos-expressing plasmid or control plasmid were cotransfected into EBV-negative Daudi cells by lipofection. After 48 h of incubation, cells were harvested and luciferase (luc) activity was determined. The luciferase activities obtained from c-fos-transfected cells were divided by those from control plasmid-transfected cells, and the results were expressed as the mean relative activity (fold induction) ± standard error. (B) Expression of c-fos in c-fos-silenced EBV-negative Daudi cell clones. EBV-negative Daudi cells were transfected with the TransSilent Fos shRNA vector (Panomics), and c-fos-silenced cell clones were isolated by cultivation in a selective medium. Expression of the c-Fos protein in these cell clones was examined by immunoblot analysis using a rabbit polyclonal antibody (Calbiochem). The β-actin protein was detected to show the quantity of each protein preparation. (C) Effect of c-fos silencing on expression of BZLF1 mRNA and protein after EBV infection. c-fos-silenced, EBV-negative Daudi cells were infected with EBV. At the designated time of incubation, expression of BZLF1 mRNA and protein was examined by RT-PCR and immunoblot analysis. EBV-negative Daudi cell clones stably transfected with the TransSilent control vector (Panomics) were also infected with EBV and examined for BZLF1 mRNA and protein expression as a positive control. Effect of c-fos silencing on EBNA2 mRNA expression was examined by RT-PCR.
In the present study, we demonstrated that the BZLF1 gene, a switch from latent infection to lytic infection, is expressed as early as 1.5 h after EBV infection in BL-derived, EBV-negative Akata and Daudi cells and primary B lymphocytes. Since BZLF1 mRNA was expressed even when the cells were infected with EBV in the presence of anisomycin, an inhibitor of protein synthesis, its expression did not require prerequisite protein synthesis, indicating that BZLF1 was expressed as an immediate-early gene after EBV infection. In cells lytically infected with herpes simplex virus, VP16, a tegument protein packaged in the virion, acts in trans to induce α genes, the first set of immediate-early genes to be expressed in the absence of protein synthesis (17). Analogously, virion components might act in trans to induce the BZLF1 gene.
Then what is the significance of BZLF1 expression in the early stage of EBV infection? Savard et al. (19) showed that primary EBV infection of monocytes resulted in the expression of BZLF1 transcripts as early as 2 h postinfection, followed by expression of other lytic genes. It is conceivable that BZLF1 is first expressed to induce other regulatory genes required for lytic replication. Several reports have demonstrated that the BZLF1 protein can induce expression of cell cycle regulators, including p53, and cell growth arrest (3, 4, 6). Cell cycle arrest is imposed by herpesviruses during viral lytic cycle DNA replication to prevent competition with host cell DNA synthesis for limited free nucleotides and to provide nuclear space for progeny viral DNA storage (17). From these viewpoints, BZLF1 expression during latent infection may be a remnant of lytic infection. Indeed, we observed by immunofluorescence that only the subpopulations of EBV-infected cells were BZLF1 positive, suggesting that BZLF1-expressing cells undergo lytic replication and die, whereas other cells without BZLF1 expression establish latent infection. However, we did not observe early and late lytic gene expression. Another possibility is that BZLF1-expressing cells are the only ones that survive and establish latency. Zhang and colleagues reported that the BZLF1 protein interacted directly in vitro and in vivo with p53 and inhibited p53-dependent transactivation in lymphoid cells (27). It is probable that inhibition of p53 function allows survival of the cells during the early stage of EBV infection, thus contributing to efficient establishment of latent infection. In addition, it was reported that BZLF1 expression in early-passage lymphoblastoid cell lines may contribute to tumor formation and cellular gene expression (11). This may provide insight into the role of BZLF1 in the early stage of EBV infection.
Acknowledgments
We thank Y. Ando for technical assistance.
This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan. D.I. was supported by the Takeda Science Foundation and The Akiyama Foundation.
Footnotes
Published ahead of print on 1 November 2006.
REFERENCES
- 1.Alfieri, C., M. Birkenbach, and E. Kieff. 1991. Early events in Epstein-Barr virus infection of human B lymphocytes. Virology 181:595-608. [DOI] [PubMed] [Google Scholar]
- 2.Allday, M. J., D. H. Crawford, and B. E. Griffin. 1989. Epstein-Barr virus latent gene expression during the initiation of B-cell immortalization. J. Gen. Virol. 70:1755-1764. [DOI] [PubMed] [Google Scholar]
- 3.Cayrol, Y. N., and E. K. Flemington. 1996. The Epstein-Barr virus bZIP transcription factor Zta causes G0/G1 cell cycle arrest through induction of cyclin-dependent kinase inhibitors. EMBO J. 15:2748-2759. [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, H., J. M. Lee, Y. Wang, D. P. Huang, R. F. Ambinder, and S. D. Hayward. 1999. The Epstein-Barr virus latency BamHI-Q promoter is positively regulated by STATs and Zta interference with JAK/STAT activation leads to loss of BamHI-Q promoter activity. Proc. Natl. Acad. Sci. USA 96:9339-9344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Countryman, J., and G. Miller. 1985. Activation of expression of latent Epstein-Barr herpesvirus after gene transfer with a small cloned subfragment of heterogeneous viral DNA. Proc. Natl. Acad. Sci. USA 82:4085-4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dreyfus, D. H., M. Nagasawa, C. A. Kelleher, and E. W. Gelfand. 2000. Stable expression of Epstein-Barr virus BZLF-1-encoded ZEBRA protein activates p53-dependent transcription in human Jurkat T-lymphoblastoid cells. Blood 96:625-634. [PubMed] [Google Scholar]
- 7.Evans, T. J., M. G. Jacquemin, and P. J. Farrell. 1995. Efficient EBV superinfection of group I Burkitt's lymphoma cells distinguishes requirements for expression of the Cp viral promoter and can activate the EBV productive cycle. Virology 206:866-877. [DOI] [PubMed] [Google Scholar]
- 8.Farrell, P. J., D. T. Rowe, C. M. Rooney, and T. Kouzarides. 1989. Epstein-Barr virus BZLF1 trans-activator specifically binds to a consensus AP-1 site and is related to c-fos. EMBO J. 8:127-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flemington, E., and S. H. Speck. 1990. Epstein-Barr virus BZLF1 trans activator induces the promoter of a cellular cognate gene, c-fos. J. Virol. 64:4549-4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flemington, E., and S. H. Speck. 1990. Identification of phorbol ester response elements in the promoter of Epstein-Barr virus putative lytic switch gene BZLF1. J. Virol. 64:1217-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong, G. K., P. Kumar, L. Wang, B. Damania, M. L. Gulley, H. J. Delecluse, P. J. Polverini, and S. C. Kenney. 2005. Epstein-Barr virus lytic infection is required for efficient production of the angiogenesis factor vascular endothelial growth factor in lymphoblastoid cell lines. J. Virol. 79:13984-13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iwakiri, D., and K. Takada. 2004. Phosphatidylinositol 3-kinase is a determinant of responsiveness to B cell antigen receptor-mediated Epstein-Barr virus activation. J. Immunol. 172:1561-1566. [DOI] [PubMed] [Google Scholar]
- 13.Jenson, H. B., M. S. Rabson, and G. Miller. 1986. Palindromic structure and polypeptide expression of 36 kilobase pairs of heterogeneous Epstein-Barr virus (P3HR-1) DNA. J. Virol. 58:475-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kieff, E., and A. B. Rickinson. 2001. Epstein-Barr virus and its replication, p. 2511-2573. In M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 15.Maruo, S., L. Yang, and K. Takada. 2001. Roles of Epstein-Barr virus glycoproteins gp350 and gp25 in the infection of human epithelial cells. J. Gen. Virol. 82:2373-2383. [DOI] [PubMed] [Google Scholar]
- 16.Nanbo, A., K. Inoue, K. Adachi-Takasawa, and K. Takada. 2002. Epstein-Barr virus RNA confers resistance to interferon-alpha-induced apoptosis in Burkitt's lymphoma. EMBO. J. 21:954-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roizman, B., and D. M. Knipe. 2001. Herpes simplex viruses and their replication, p. 2399-2459. In M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 18.Rooney, C., J. G. Howe, S. H. Speck, and G. Miller. 1989. Influences of Burkitt's lymphoma and primary B cells in latent gene expression by the nonimmortalizing P3J-HR-1 strain of Epstein-Barr virus. J. Virol. 63:1531-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savard, M., C. Belanger, M. Tardif, P. Gourde, L. Flamand, and J. Gosselin. 2000. Infection of primary human monocytes by Epstein-Barr virus. J. Virol. 74:2612-2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimizu, N., A. Tanabe-Tochikura, Y. Kuroiwa, and K. Takada. 1994. Isolation of Epstein-Barr virus (EBV)-negative cell clones from the EBV-positive Burkitt's lymphoma (BL) line Akata: malignant phenotypes of BL cells are dependent on EBV. J. Virol. 68:6069-6073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sinclair, A. J., and P. J. Farrell. 1995. Host cell requirements for efficient infection of quiescent primary B lymphocytes by Epstein-Barr virus. J. Virol. 69:5461-5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sinclair, A. J., M. Brimmell, F. Shanahan, and P. J. Farrell. 1991. Pathways of activation of the Epstein-Barr virus productive cycle. J. Virol. 65:2237-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takada, K. 1984. Cross-linking of cell surface immunoglobulins induces Epstein-Barr virus in Burkitt lymphoma lines. Int. J. Cancer 33:27-32. [DOI] [PubMed] [Google Scholar]
- 24.Takada, K., and Y. Ono. 1989. Synchronous and sequential activation of latently infected Epstein-Barr virus genomes. J. Virol. 63:445-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takada, K., N. Shimizu, S. Sakuma, and Y. Ono. 1986. trans activation of the latent Epstein-Barr virus (EBV) genome after transfection of the EBV DNA fragment. J. Virol. 57:1016-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woisetschlaeger, M., X. W. Jin, C. N. Yandava, L. A. Furmanski, J. L. Strominger, and S. H. Speck. 1991. Role for the Epstein-Barr virus nuclear antigen 2 in viral promoter switching during initial stages of infection. Proc. Natl. Acad. Sci. USA 88:3942-3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang, Q., D. Gutsch, and S. Kenney. 1994. Functional and physical interaction between p53 and BZLF1: implications for Epstein-Barr virus latency. Mol. Cell. Biol. 14:1929-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.zur Hausen, H., F. J. O'Neill, U. K. Freese, and E. Hecker. 1978. Persisting oncogenic herpesvirus induced by the tumour promotor TPA. Nature 272:373-375. [DOI] [PubMed] [Google Scholar]