Abstract
We identified the transcriptional coactivator FHL2 as a novel target of the human papillomavirus type 16 (HPV-16) E7 oncoprotein, which plays a major role in cell transformation. The interaction with FHL2 is abolished by mutations in conserved regions 1 and 2 and in the C-terminal zinc finger domain of E7, all required for its transforming potential. We found that E7 impairs the coactivator function of FHL2 on both β-catenin/LCF-dependent and AP-1-dependent promoters. Thus, the interaction with HPV-16 E7 leads to a promoter-specific impairment of FHL2 function and this may contribute to cell transformation.
Human papillomaviruses of the high-risk group are major etiological agents for human anogenital cancers (15). Their transforming potential depends largely on the expression of the E6 and E7 oncoproteins. The E7 oncoprotein of human papillomavirus type 16 (HPV-16) was shown to inactivate the pRb/E2F pathway, leading to cell cycle deregulation. However, the interaction of E7 with members of the Rb family is not sufficient for cell transformation and it is generally assumed that E7 targets additional cellular pathways (12). E7 is known to play a role in the modulation of cellular gene expression, in particular, through the activation genes driven by E2F; however, mechanisms by which E7 deregulates gene expression independent of the pRb/E2F pathway have largely been unexplored. Applying a two-hybrid screen with HPV-16 E7 as bait (16), we identified four and a half LIM domain-containing protein 2 (FHL2) as a new interaction partner for HPV-16 E7 (data not shown). FHL2 contains an autonomous transcriptional activation domain (11) but is unable to bind DNA directly; accordingly, FHL2 has been described as a coactivator that enhances transcription at a variety of different promoters, probably through its interaction with several DNA-binding transcription factors (reviewed in reference 5).
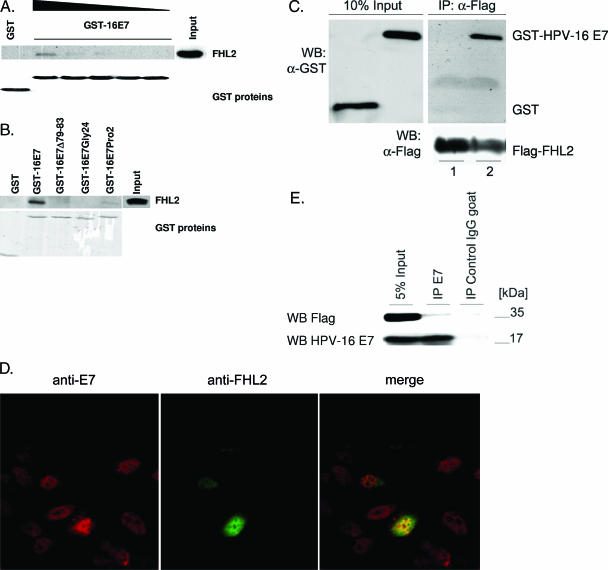
To confirm the interaction of HPV-16 E7 with FHL2, equal amounts of glutathione S-transferase (GST)-E7 fusion protein were incubated with increasing amounts of total lysates from NIH 3T3 cells that had been transfected with an FHL2 expression vector (Fig. 1A). Consistent with the two-hybrid results, FHL2 specifically bound to GST-HPV-16 E7, whereas GST alone was not able to pull down FHL2 (Fig. 1A). E7 mutants disrupting transforming domain CR1 (E7Pro2) or CR2 (E7Gly24) or the C-terminal Zn finger domain (E7Δ79-83) all displayed significantly reduced affinity for FHL2 (Fig. 1B), suggesting that the ability of E7 to bind FHL2 may be relevant for cell transformation. The failure of all three E7 mutants to interact with FHL2 might indicate that the overall structure of E7, rather than an isolated E7 subdomain, is required for the interaction.
FIG. 1.
HPV-16 E7 physically interacts with FHL-2. (A) Extracts (250 μg, 125 μg, 60 μg, 30 μg, and 15 μg) from FHL2-transfected NIH 3T3 cells were incubated with beads containing 5 μg of GST-HP-16 E7 and 5 μg GST, as indicated. Twenty-five micrograms of total cellular lysate was included as input. Bound proteins were analyzed by Western blotting with anti-FHL2 polyclonal antibody. The abundance of GST fusion proteins was monitored by Western blotting with anti-GST monoclonal antibody. (B) Binding of FHL2 to transformation-deficient mutants of E7 was analyzed by GST pulldown, as described for panel A. (C) Pulldown using purified GST-HPV-16 E7, GST, and Flag-FHL2 proteins. Purified Flag-tagged FHL2 was bound to anti-Flag antibody-conjugated agarose beads (Sigma, Vienna, Austria) and incubated with equimolar amounts of purified GST and GST-HPV-16 E7 (1 mg) proteins, respectively. After a wash, the probes were separated on a 12.5% sodium dodecyl sulfate-polyacrylamide gel, and the bound GST and GST-HPV-16 E7 proteins were detected by Western blotting (WB) using anti-GST antibodies (Sigma, Vienna, Austria), as indicated. Ten percent of the GST (lane 1) and GST-HPV-16 E7 (lane 2) served as input control. The Flag-FHL2 input was controlled by WB using anti-Flag antibodies (Sigma, Vienna, Austria). (D) U-2 OS cells were transiently transfected with expression vectors for HPV-16 E7 and FHL2, as indicated. At 24 h after transfection, cells were processed for indirect immunofluorescence and visualized by confocal microscopy for separate expression or coexpression of E7 and FHL2. Structures stained with anti-E7 (red) and anti-FHL2 (green) antibodies colocalized, as shown by yellow fluorescence in the merged picture. (E) Coimmunoprecipitation experiments. U-2 OS cells were cotransfected with expression vectors for Flag-tagged FHL2 and HPV-16 E7. Cell lysates were prepared and subjected to immunoprecipitation (IP) with antibodies to E7 (goat anti-HPV-16 E7; Amynon, Innsbruck, Austria) and Flag (Sigma, Vienna, Austria) and control antibodies, as indicated. Precipitated proteins were detected by WB using antibodies against HPV-16 E7 and Flag, respectively. IgG, immunoglobulin G.
To further corroborate the ability of HPV-16 E7 to bind FHL-2, additional studies were performed with purified proteins. U-2 OS cells were transiently transfected with expression vectors for Flag-tagged FHL2 (13) (a kind gift of B. Schäfer), and the Flag-tagged FHL2 was purified on anti-Flag antibody-conjugated agarose beads (Sigma, Vienna, Austria). When Flag-tagged FHL2 was incubated with affinity-purified GST-HPV-16 E7 protein, a robust interaction between both proteins was observed (Fig. 1C), suggesting that the proteins display a firm interaction under conditions where protein concentration is not a limiting factor. To analyze the E7/FHL-2 interaction within living cells, indirect immunofluorescence analyses were performed. U-2 OS osteosarcoma cells were transiently transfected with expression vectors for E7 and/or FHL2. At 24 h after transfection, cells were fixed, probed with rabbit anti-E7 and mouse anti-FHL2 antibodies, and analyzed by confocal microscopy. Separate expression of E7 and FHL2 resulted in similar staining patterns predominantly in the nucleus (data not shown), and coexpression of both proteins revealed considerable overlap, as indicated by the yellow fluorescence in the merged picture in Fig. 1D. Furthermore, immunoprecipitation of HPV-16 E7 from extracts of transiently transfected cells resulted in the coprecipitation of FHL-2 (Fig. 1E). Collectively, these results suggest that E7 and FHL2 can interact also in living cells.
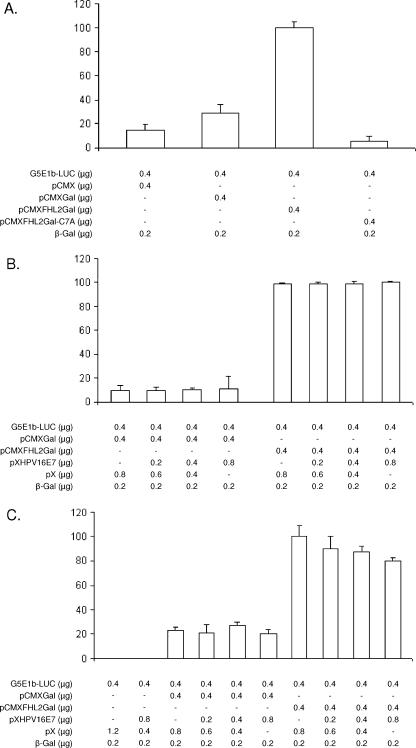
To monitor transcriptional activation by FHL2, NIH 3T3 cells were transfected with a plasmid expressing a Gal4-FHL2 fusion protein (pCMX-Gal-FHL2) and a synthetic reporter gene driven by two Gal4 binding sites (10). Transcription was activated roughly 10-fold by Gal4-FHL2, whereas a fusion protein carrying a single point mutation (C7A in FHL2) that renders the protein transcriptionally inactive had no effect (Fig. 2A), as described previously (11). When cells were cotransfected with increasing amounts of HPV-16 E7 expression plasmids, no significant effect on FHL2 transactivation activity by HPV-16 E7 was observed (Fig. 2B). When transfected cells were additionally stimulated with 1.2 μM sphingosine-1-phosphate (SPP), known to enhance nuclear translocation of the Gal4-FHL2 fusion protein (11), a weak inhibitory effect of E7 on Gal4-FHL2-dependent transcription was observed, although the effect did not reach statistical significance (Fig. 2C). The data suggest that HPV-16 E7, although highly expressed (Fig. 1C), is unable to significantly alter the function of a Gal4-FHL2 fusion protein in the context of a synthetic promoter.
FIG. 2.
E7 fails to influence transactivation by Gal4-FHL2 fusion protein. (A) Transactivation of the Gal4-dependent reporter G5E1b-Luc by Gal-FHL2 fusion protein in NIH 3T3 cells. NIH 3T3 cells were transfected by Lipofectamine with the G5E1b-Luc reporter gene construct (11) in the presence or absence of Gal-FHL2. A cytomegalovirus-driven β-galactosidase (β-Gal) expression vector was cotransfected to control for transfection efficiency. Luciferase activity was normalized to β-Gal activity. The point mutant FHL2Gal-C7A was used as the negative control. Experiments were repeated in triplicate at least three times. Shown are means ± standard deviations (SD) from one representative experiment. (B and C) E7 effects on the activity of Gal-FHL2 fusion proteins. NIH 3T3 cells were transfected with the G5E1b-Luc reporter gene construct in the presence or absence of Gal-FHL2 and increasing amounts of HPV16 E7 expression vector, as indicated. The total amount of DNA was the same (1.8 μg) for each transfection. Cells were serum starved in 0.5% fetal calf serum for 25 h and thereafter either stimulated with 1.2 μM SPP for 3 h (C) or kept in the absence of SPP (B). Luciferase activity was normalized to β-Gal activity. The results are representative of at least three independent experiments. Data shown are the averages of triplicate measurements ± SD.
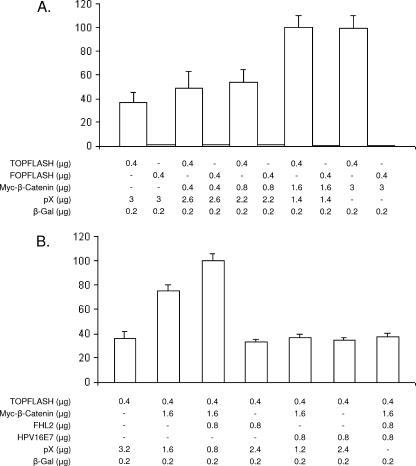
FHL2 participates in transcriptional regulation downstream of Wnt signaling, a major regulatory pathway that controls the expression of several growth-related genes and that is altered in a variety of human epithelial tumors (reviewed in reference 6a). Thus, FHL2 was found to interact with β-catenin (9) and to cooperate with β-catenin to activate T-cell factor/lymphoid enhancer factor (TCF/LEF)-driven genes in human cancer cells (8, 14). To monitor β-catenin coactivation by FHL2, human embryonic kidney 293 (HEK 293) cells were cotransfected with a plasmid encoding myc-tagged β-catenin together with a reporter gene containing three TCF/LEF-β-catenin DNA binding sites (TOPflash [8]). A high basal level of specific reporter gene activity was obtained in the absence of any cotransfected proteins (Fig. 3A), probably due to the presence of endogenous activated β-catenin in HEK 293 cells (9). Coexpression of β-catenin increased the activity of the TOPflash reporter gene in a dose-dependent manner, whereas no changes were observed with the activity of a mutant reporter gene containing mutated TCF/LEF binding sites (FOPflash), which served as a negative control (Fig. 3A). When expression plasmids for HPV-16 E7 were cotransfected in addition to β-catenin, a significant reduction of reporter gene activity was observed (Fig. 3B), and coexpression of HPV-16 E7 also impaired the FHL2-dependent increase of transcriptional activity (Fig. 3B), indicating that HPV-16 E7 can cancel both β-catenin activity and the coactivator function of FHL2 on a β-catenin-dependent promoter.
FIG.3.
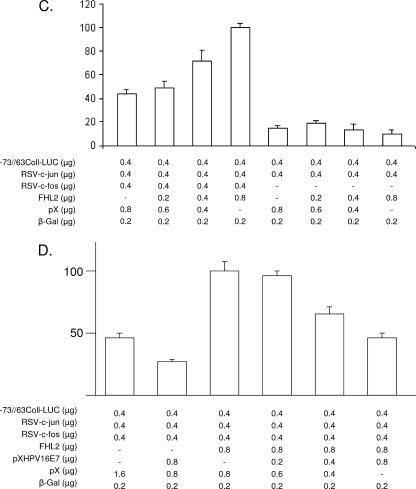
E7-dependent inhibition of FHL2 coactivator function. (A and B) E7 effects on β-catenin-dependent coactivation. (A) HEK 293 cells were transfected with TOPflash or FOPflash reporters in the presence of increasing amounts of myc-β-catenin, as indicated. The total amount of DNA was the same (3.6 μg) for each transfection. Luciferase activity was normalized to the activity of a cotransfected β-galactosidase (β-Gal) expression vector. Results shown are the averages of three independent transfections ± standard errors (SE). (B) TOPflash luciferase reporter plasmid was cotransfected with FHL2 in the presence of increasing amounts of HPV-16 E7 expression plasmid, as indicated, and evaluated as described for panel A. The total amount of DNA was the same (3.8 μg) for each transfection. Data are means of triplicates ± SE. (C and D) E7 effects on FHL2-dependent coactivation of AP-1-driven transcription. (C) NIH 3T3 cells were transiently transfected with −73/+63 Coll-Luc reporter and increasing amounts of expression vectors for FHL2, c-Jun, and c-Fos, as indicated. Luciferase activity was normalized to the activity of a cotransfected β-Gal expression vector. Results shown are the averages of three independent transfections ± SE. (D) NIH 3T3 cells were transiently transfected with −73/+63 Coll-Luc reporter and expression vectors for FHL2, c-Jun, and c-Fos, as indicated. Increasing amounts of HPV-16 E7 expression plasmid were cotransfected as indicated, and results were evaluated as described for panel C. Data are means of triplicates ± SE.
FHL2 is also known to coactivate activator protein-1 (AP-1)-driven transcription (10). AP-1 is composed by homodimers of Jun family members or by heterodimers of the Jun and Fos families of transcription factors. Jun and Fos family members are important regulators of cellular gene expression and also play a key role in a variety of human tumors (1). To address the influence of HPV-16 E7 on the ability of FHL2 to coactivate AP-1-driven transcription, the AP-1 responsive promoter −73/+63 Coll-Luc (9) was used. When increasing amounts of FHL2 were cotransfected with expression vectors for c-Jun and c-Fos, FHL2 consistently stimulated the transcriptional activity of c-Jun/c-Fos heterodimers in a dose-dependent manner but had no significant effect on reporter gene activity in the absence of c-Fos (Fig. 3C). This result suggests that FHL2 specifically provides coactivation function to Fos/Jun heterodimers, as described before (10). When increasing amounts of HPV-16 E7 expression vector were cotransfected, activation of transcription by FHL2 was abrogated, indicating that FHL2 is unable to coactivate AP-1-dependent transcription in the presence of HPV-16 E7 (Fig. 3D). When E7 was cotransfected with Jun/Fos in the absence of FHL-2, transcription of the collagenase promoter was repressed (Fig. 3D), suggesting that the coactivator function of endogenous FHL-2 is abrogated by E7. It is of potential interest that transcription of the HPV-16 E7 gene is controlled in part by AP-1 sites present in the viral promoter (3). The findings reported here suggest that transcriptional regulation by E7 may also influence the viral life cycle, in agreement with results from others (4).
Others have shown that E7 directly interacts with c-Jun and enhances the transcriptional activity of c-Jun homodimers on AP-1-driven promoters and concluded that synergistic activation of AP-1-driven genes by c-Jun and E7 may play a role in viral oncogenesis (2). However, the FHL-2 coactivator function is detectable only in the presence of both Jun and Fos (Fig. 3C), as was also reported by others before (10), and E7-mediated stimulation of AP-1-responsive promoters seems to be restricted to Jun/Jun homodimers. Our current data strongly suggest that E7 represses transcription from AP-1-driven promoters, at least in the case of promoters driven by Jun/Fos heterodimeric complexes that contain the FHL-2 coactivator. It was shown previously that levels of AP-1 activity determine E7 expression in HPV-infected stratified epithelia (7), and our finding that E7 can block the coactivator function of FHL2 on c-Jun/c-Fos heterodimers could be related to the well-known ability of E7 to inhibit keratinocyte differentiation (6). Together, the data suggest that the functional interplay between E7 and Jun/Fos family members is more complex than anticipated previously.
Data in the literature link FHL2 function with two major signaling cascades involved in epithelial cell transformation, namely, the Wnt pathway and signal transduction upstream of AP-1, and data reported here suggest that HPV-16 E7 can antagonize this important function of FHL2. FHL2 is considered an adaptor protein without any known enzymatic activity, and its major role might be to participate in and potentially control the architecture of multiprotein complexes that regulate gene expression (5). We favor a model that HPV-16 E7, by directly binding to FHL2, interferes with that function and compromises the ability of FHL2 to function as coactivator. A further analysis of how E7 disrupts the integrator role of FHL2 in several signaling pathways should provide additional insight into the association between HPV infection and the development of malignant disease.
Acknowledgments
We are grateful to Roland Schüle and Beat Schäfer for FHL2 expression vectors and several reporter gene constructs, Jürgen Behrens for TOPflash/FOPflash, and Peter Angel for AP-1 constructs.
This work was supported by the European Union (Oncodeath Research Training Network) and the Austrian Science Funds through SFB 021 to P.J.-D. and by the Austrian Science Funds (FWF project P15383), the Austrian Cancer Society—Tyrol, and the European Union (INCA project LSHC-CT-2005-018704) to W.Z.
Footnotes
Published ahead of print on 8 November 2006.
REFERENCES
- 1.Angel, P., and M. Karin. 1991. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim. Biophys. Acta 1072:129-157. [DOI] [PubMed] [Google Scholar]
- 2.Antinore, M. J., M. J. Birrer, D. Patel, L. Nader, and D. J. McCance. 1996. The human papillomavirus type 16 E7 gene product interacts with and trans-activates the AP1 family of transcription factors. EMBO J. 15:1950-1960. [PMC free article] [PubMed] [Google Scholar]
- 3.Chan, W. K., T. Chong, H. U. Bernard, and G. Klock. 1990. Transcription of the transforming genes of the oncogenic human papillomavirus-16 is stimulated by tumor promotors through AP1 binding sites. Nucleic Acids Res. 18:763-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collins, A. S., T. Nakahara, A. Do, and P. F. Lambert. 2005. Interactions with pocket proteins contribute to the role of human papillomavirus type 16 E7 in the papillomavirus life cycle. J. Virol. 79:14769-14780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johannessen, M., S. Moller, T. Hansen, U. Moens, and M. Van Ghelue. 2006. The multifunctional roles of the four-and-a-half-LIM only protein FHL2. Cell. Mol. Life Sci. 63:268-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones, D. L., R. M. Alani, and K. Munger. 1997. The human papillomavirus E7 oncoprotein can uncouple cellular differentiation and proliferation in human keratinocytes by abrogating p21Cip1-mediated inhibition of cdk2. Genes Dev. 11:2101-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6a.Karim, R., G. Tse, T. Putti, R. Scolyer, and S. Lee. 2004. The significance of the Wnt pathway in the pathology of human cancers. Pathology. 36:120-128. [DOI] [PubMed] [Google Scholar]
- 7.Kyo, S., D. J. Klumpp, M. Inoue, T. Kanaya, and L. A. Laimins. 1997. Expression of AP1 during cellular differentiation determines human papillomavirus E6/E7 expression in stratified epithelial cells. J. Gen. Virol. 78:401-411. [DOI] [PubMed] [Google Scholar]
- 8.Labalette, C., C. A. Renard, C. Neuveut, M. A. Buendia, and Y. Wei. 2004. Interaction and functional cooperation between the LIM protein FHL2, CBP/p300, and beta-catenin. Mol. Cell. Biol. 24:10689-10702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin, B., R. Schneider, S. Janetzky, Z. Waibler, P. Pandur, M. Kuhl, J. Behrens, K. von der Mark, A. Starzinski-Powitz, and V. Wixler. 2002. The LIM-only protein FHL2 interacts with beta-catenin and promotes differentiation of mouse myoblasts. J. Cell Biol. 159:113-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morlon, A., and P. Sassone-Corsi. 2003. The LIM-only protein FHL2 is a serum-inducible transcriptional coactivator of AP-1. Proc. Natl. Acad. Sci. USA 100:3977-3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muller, J. M., E. Metzger, H. Greschik, A. K. Bosserhoff, L. Mercep, R. Buettner, and R. Schule. 2002. The transcriptional coactivator FHL2 transmits Rho signals from the cell membrane into the nucleus. EMBO J. 21:736-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munger, K., and P. M. Howley. 2002. Human papillomavirus immortalization and transformation functions. Virus Res. 89:213-228. [DOI] [PubMed] [Google Scholar]
- 13.Scholl, F. A., P. McLoughlin, E. Ehler, C. de Giovanni, and B. W. Schafer. 2000. DRAL is a p53-responsive gene whose four and a half LIM domain protein product induces apoptosis. J. Cell Biol. 151:495-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei, Y., C. A. Renard, C. Labalette, Y. Wu, L. Levy, C. Neuveut, X. Prieur, M. Flajolet, S. Prigent, and M. A. Buendia. 2003. Identification of the LIM protein FHL2 as a coactivator of beta-catenin. J. Biol. Chem. 278:5188-5194. [DOI] [PubMed] [Google Scholar]
- 15.zur Hausen, H. 2002. Papillomaviruses and cancer: from basic studies to clinical application. Nat. Rev. Cancer 2:342-350. [DOI] [PubMed] [Google Scholar]
- 16.Zwerschke, W., S. Mazurek, P. Massimi, L. Banks, E. Eigenbrodt, and P. Jansen-Dürr. 1999. Modulation of type M2 pyruvate kinase activity by the human papillomavirus type 16 E7 oncoprotein. Proc. Natl. Acad. Sci. USA 96:1291-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]