Abstract
The severe acute respiratory syndrome coronavirus (SARS-CoV) is highly pathogenic in humans, with a death rate near 10%. This high pathogenicity suggests that SARS-CoV has developed mechanisms to overcome the host innate immune response. It has now been determined that SARS-CoV open reading frame (ORF) 3b, ORF 6, and N proteins antagonize interferon, a key component of the innate immune response. All three proteins inhibit the expression of beta interferon (IFN-β), and further examination revealed that these SARS-CoV proteins inhibit a key protein necessary for the expression of IFN-β, IRF-3. N protein dramatically inhibited expression from an NF-κB-responsive promoter. All three proteins were able to inhibit expression from an interferon-stimulated response element (ISRE) promoter after infection with Sendai virus, while only ORF 3b and ORF 6 proteins were able to inhibit expression from the ISRE promoter after treatment with interferon. This indicates that N protein inhibits only the synthesis of interferon, while ORF 3b and ORF 6 proteins inhibit both interferon synthesis and signaling. ORF 6 protein, but not ORF 3b or N protein, inhibited nuclear translocation but not phosphorylation of STAT1. Thus, it appears that these three interferon antagonists of SARS-CoV inhibit the interferon response by different mechanisms.
In 2003, severe acute respiratory syndrome coronavirus (SARS-CoV) infected thousands of people throughout the world, killing hundreds. The molecular mechanisms governing virus-induced pathology have not been fully elucidated. The first immune challenge a virus must surmount in order to cause disease symptoms in people is the innate immune response. A major component of innate immunity is the interferon response. Infection of cells with virus causes the activation of several cellular transcription factors, such as IRF-3 and NF-κB, which activate the expression of the interferon genes. Once interferon is synthesized and released from cells, it binds to interferon receptors, initiating a signaling cascade of the JAK/STAT pathway that results in activated transcription factors translocating to the nucleus. These transcription factors bind to and activate genes containing an interferon-stimulated response element (ISRE) in their promoters. Activation of these genes enables the cell to combat the virus infection and can prevent viral replication (8). Many viruses have developed mechanisms to subvert the interferon response. Infection of cells with SARS-CoV does not result in the production of interferon, and pretreatment of cells with interferon prevents growthof SARS-CoV (22, 33). These results indicate that SARS-CoV has evolved to overcome the interferon response.
SARS-CoV contains a 29.7-kb single-stranded RNA genome wrapped in a helical nucleocapsid composed of multiple copies of N protein, which in turn is surrounded by an envelope containing a 180- to 190-kDa S glycoprotein, a 23-kDa M glycoprotein, an ∼30-kDa 3a glycoprotein, and a small E protein. The viral gene order is similar to that in other known coronaviruses, with the first two open reading frames (1a and 1b) encoding the viral replicase and the downstream mRNAs encoding structural proteins S, E, M, and N. These genes are interspaced with several accessory genes that are not essential for in vitro or in vitro replication (open reading frames [ORFs] 3a, 3b, 6, 7a, 7b, 8a, 8b, and 9b) (29). These accessory proteins are not homologous to any known proteins in any database. The functions of these proteins are of particular interest for understanding the pathogenesis of SARS-CoV, as the accessory proteins of other coronaviruses contribute to in vivo pathogenesis but are not essential for in vitro replication (5).
The goal of these experiments was to determine whether any of the structural and accessory proteins of SARS-CoV are interferon antagonists. Our experiments revealed that ORF 3b, ORF 6, and nucleocapsid (N) proteins are all effective at preventing the interferon response. All three of these proteins have been shown to be expressed in tissue culture cells during infection with SARS-CoV and in tissues obtained from SARS patients (4). ORF 3b protein has a length of 154 amino acids and is reported to localize to the nucleolus and the mitochondria (30, 31). ORF 6 protein is 63 amino acids long and is reported to localize to the endoplasmic reticulum (ER) (9). N is a 422-amino-acid protein that is localized to the cytoplasm (27). There are reports that N protein inhibits the progression of the cell cycle and can activate the proinflammatory factor cyclooxygenase-2 (24, 26).
The data presented here indicate that N protein inhibits interferon production, while ORF 3b and ORF 6 proteins are able to inhibit both interferon production and interferon signaling. IRF-3 activation is inhibited in cells that express ORF 3b, ORF 6, or N protein, and NF-κB is inhibited in cells expressing N protein. ORF 3b and ORF 6 proteins also effectively inhibit expression of a reporter gene under the control of a promoter containing an ISRE. STAT1 translocation is inhibited in cells expressing ORF 6 protein but not ORF 3b or N proteins, though none of these proteins reduce STAT1 phosphorylation. Taken together, our data indicate that SARS-CoV encodes at least three interferon antagonists that inhibit different aspects of the interferon response. The redundant functions of these three proteins probably augment the effective inhibition of the interferon response observed during infection with SARS-CoV. This profound inhibition of the interferon response likely contributes to the pathogenesis of SARS-CoV.
MATERIALS AND METHODS
Cells and plasmids.
293T and A549 cells were cultured in Dulbecco's modified Eagle medium (Invitrogen) containing 10% fetal bovine serum. The SARS-CoV structural and accessory genes were amplified by RT-PCR from lysates of cells infected with the Urbani strain of SARS Co-V and cloned into pCAGGS constructs with a tag encoding hemagglutinin (HA) on the C-terminal ends of the proteins. Expression of the proteins was confirmed by Western blotting (data not shown). An N gene which does not express ORF 9b protein was also cloned using the upstream primer CGCGGAATTCACCATGTCTGATAACGGACCCCAATCA, which contains a mutation in the ATG start site of the ORF 9b gene but does not change the amino acid in the N protein. pCAGGS-NS1, pCAGGS-IRF-3, p55-CIB, NF-κB-luc, Renilla luciferase, Nipah virus V, Nipah virus W, PR8 strain influenza virus NS1, STAT1-green fluorescent protein (GFP), beta interferon (IFN-β)-red fluorescent protein (RFP)/CAT, and ISRE-GFP/CAT (pHISG54-GFP/CAT) plasmids were described previously (12, 15, 20, 25).
Transfections.
293T cells were seeded in 24- or 6-well dishes for 24 h. Cells were transfected with 100 ng or 500 ng per plasmid using 0.2 or 1 μl of Lipofectamine 2000, respectively, unless otherwise indicated.
NDV-GFP assay.
A549 cells were seeded in 24-well dishes and transfected with 3 μl of Lipofectamine 2000 and 1 μg of the indicated plasmid in Fig. 1A. The larger amounts of Lipofectamine 2000 and DNA were required to stimulate interferon induction, as described previously (16). Cells were infected with Newcastle disease virus expressing green fluorescent protein (NDV-GFP) at a multiplicity of infection (MOI) of 10 at 24 h posttransfection and analyzed by microscopy at 24 h postinfection.
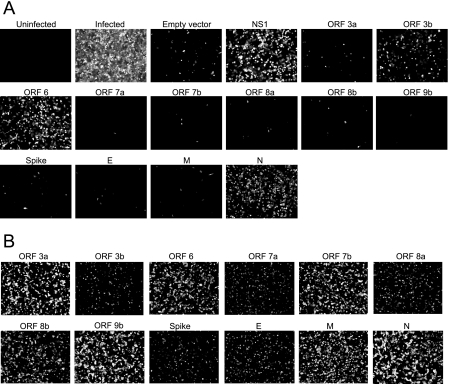
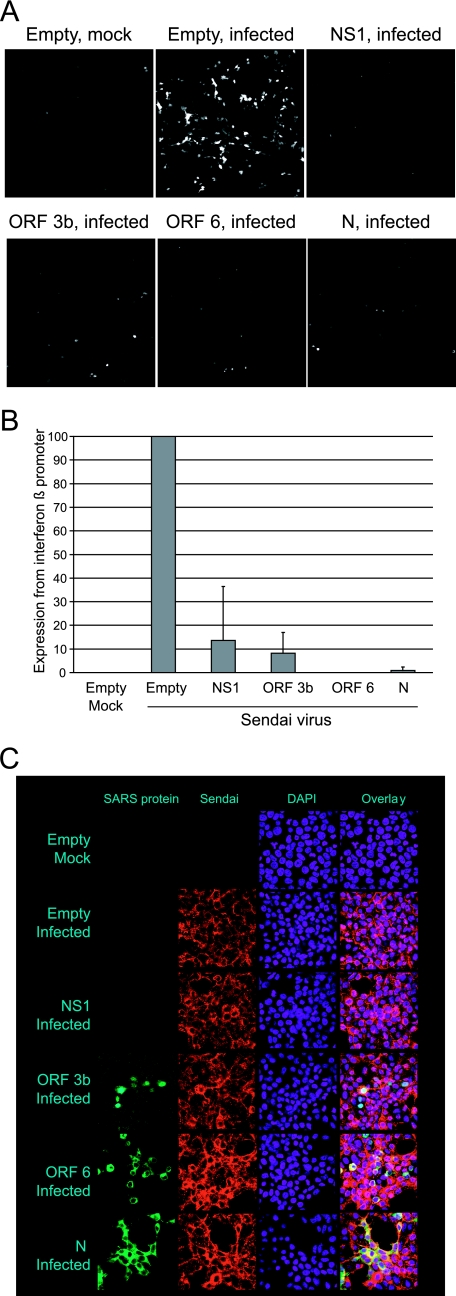
FIG. 1.
Identification of SARS-CoV ORF 3b, ORF 6, and N proteins as potential interferon antagonists. A. A549 cells were transfected with either control plasmids or plasmids expressing HA-tagged SARS-CoV proteins. At 24 h posttransfection, cells were infected with NDV-GFP, which grows in the presence of interferon antagonists. Images were obtained at 24 h postinfection using a 10× objective and are representative of three experiments. B. A549 cells were transfected with plasmids expressing HA-tagged SARS-CoV proteins for 24 h, fixed, and analyzed for expression of the SARS-CoV proteins using an antibody to the HA tag.
Apoptosis analysis.
293T cells were seeded in 24-well dishes and transfected with various GFP-tagged plasmids. At 24 h posttransfection, cells were analyzed for the morphological changes associated with apoptosis using phase-contrast and fluorescence microscopy. Cells were also harvested and analyzed for active caspase-3 as described previously (13).
Confocal microscopy.
293T cells were seeded in 24-well dishes on coverslips. At 24 h posttransfection, cells were fixed with 5% formaldehyde and permeabilized with 1% Triton X-100. Cells were incubated with blocking buffer (phosphate-buffered saline [PBS], 0.05% Tween, 0.5% bovine serum albumin, 0.8% glycine) for 5 min and then incubated with primary antibody at a dilution of 1:500 for 1 h at room temperature. Primary antibodies used were mouse anti-cytochrome c (Pharmingen), rabbit anti-protein disulfide isomerase (PDI) (kind gift from Domenico Tortorella), rabbit anti-phospho-STAT1 (Cell Signaling Technology), mouse anti-HA tag (Sigma), and mouse anti-Sendai virus antibodies 11F3 and 5F5 (1 μg/ml) (15). Cells were washed three times with blocking buffer and then incubated with either donkey anti-mouse immunoglobulin conjugated with Alexa fluor 488, donkey anti-rabbit-Alexa fluor 594, donkey anti-mouse-Alexa fluor 596, or BODIPY TR (all from Molecular Probes) at a dilution of 1:500 for 1 h. Cells were incubated with 4′,6-diamidino-2-phenylindole-dihydrochloride (DAPI) (Molecular Probes) or Hoechst 33342 (Molecular Probes) for 5 min. Cells were washed three times, and coverslips were mounted on slides using Aqua Polymount (Polysciences). Slides were analyzed by confocal microscopy with a Zeiss LSM 510 Meta microscope.
Luciferase assays.
293T cells were seeded in 24-well dishes and transfected with empty vector plasmid, plasmids encoding the SARS-CoV proteins, Renilla luciferase plasmid, and pNF-κB firefly luciferase plasmid or p55-CIB firefly luciferase plasmid. For these transfections, 0.5 μl of Fugene was used instead of Lipofectamine 2000, since this amount of Fugene does not induce detectable amounts of interferon. The NF-κB-responsive promoter plasmid contains two NF-κB binding sites, and the p55-CIB plasmid contains three IRF-3 binding sites. At 24 h posttransfection, cells were infected with Sendai virus at an MOI of 10 for 24 h. Cells were harvested, lysed, and analyzed for firefly and Renilla luciferase according to the manufacturer's protocol (Promega). Values for the samples were normalized using the Renilla luciferase values and expressed as percentages of the value for the positive control.
Quantitative microscopy analysis.
293T cells were seeded in 24-well dishes and transfected with the appropriate plasmids and either IFN-RFP or ISRE-RFP plasmids. At 24 h posttransfection, cells were infected with Sendai virus or treated with 3,000 U/ml human IFN-β (Calbiochem). Cells were analyzed by microscopy using an Olympus 1X70 microscope 24 h postinfection or posttreatment. Images from three experiments were quantitated using Image J software (NIH).
Western blots.
293T cells were seeded in 6-well dishes and transfected with empty vector plasmid or plasmids encoding the SARS-CoV proteins. For analysis of IRF-3, a plasmid encoding IRF-3 was also transfected in each sample. At 24 h posttransfection, cells were infected with Sendai virus for 6 h (see Fig. 4B) or serum starved for 4 h and treated with IFN-β for 1 h (see Fig. 8A). Cells were harvested and lysed in radioimmunoprecipitation assay buffer (PBS, 1% NP-40, 0.5% deoxycholate, 0.1% sodium dodecyl sulfate [SDS], and 1 mM EDTA) supplemented with protease inhibitor cocktail (Complete; Roche) and phosphatase inhibitors (10 mM β-glycerophosphate, 25 mM Na pyrophosphate, 50 mM NaF, 1 mM sodium orthovanadate). Lysates were spun down to remove nuclei. The protein content was determined by a Bradford assay (Bio-Rad). Aliquots of lysates representing equal amounts of protein were analyzed by SDS-polyacrylamide gel electrophoresis. The proteins were transferred to a nitrocellulose membrane and probed with anti-phospho-STAT1, recognizing phosphorylation at tyrosine 701, or anti-phospho-IRF-3, recognizing phosphorylation at serine 396 (Upstate), stripped with 0.2 N NaOH, and reprobed using anti-total IRF-3 (Santa Cruz Biotechnology) or anti-total STAT1 (Cell Signaling Technology).
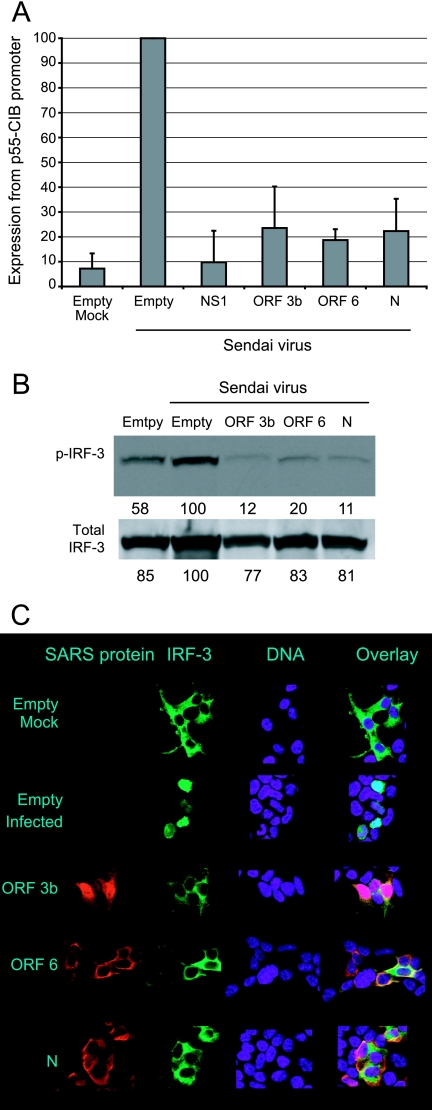
FIG. 4.
SARS-CoV ORF 3b, ORF 6, and N proteins inhibit activation of IRF-3. A. 293T cells were cotransfected with the p55-CIB promoter, which contains three IRF-3 binding sites, a plasmid that constitutively expresses Renilla luciferase, and plasmids expressing SARS-CoV proteins or the indicated control plasmids. Cells were infected with Sendai virus 24 h posttransfection. Cells were harvested at 24 h postinfection and analyzed for firefly and Renilla luciferase. Data were normalized using the Renilla luciferase values. Data are averages plus standard deviations for three experiments. A value of 100% represents approximately 10,000,000 firefly luciferase units(p55-CIB) and 6,000,000 Renilla luciferase units. B. 293T cells were transfected with the indicated plasmid and infected with Sendai virus for 6 h. Cells were harvested, and lysates were analyzed by Western blotting using a phospho-IRF-3 antibody. Quantitations are given below each band and are percentages of the value for the empty, infected control. C. 293T cells were transfected with the indicated plasmids for 24 h and then infected with Sendai virus. Cells were fixed 8 h postinfection and analyzed by microscopy using an antibody that recognizes the HA tag.
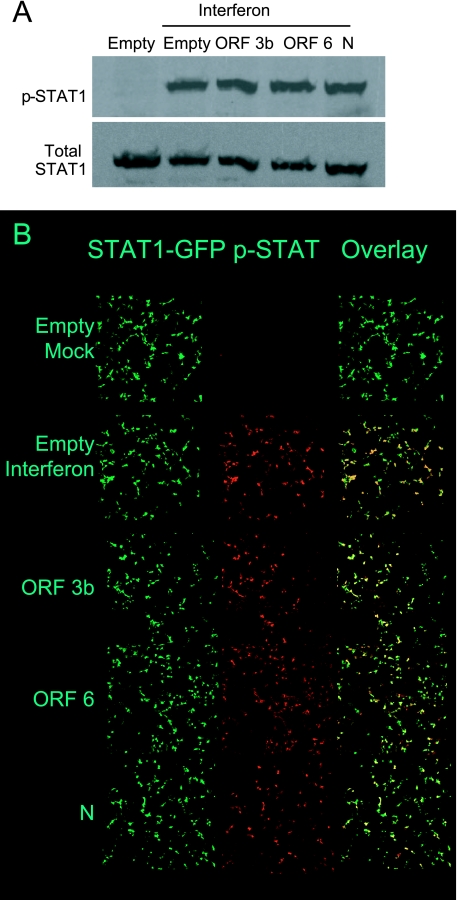
FIG. 8.
Analysis of STAT1 phosphorylation by SARS-CoV proteins. A. 293T cells were transfected with the indicated plasmid for 24 h and then treated with IFN-β for 1 h. Cells were harvested, and lysates were analyzed by Western blot analysis using antibodies recognizing the phospho- and total forms of STAT1. B. Cells were transfected with the indicated plasmid and STAT-1 GFP for 24 h and then treated with IFN-β for 1 h. Cells were fixed, permeabilized, and analyzed for phospho-STAT1 by confocal microscopy using a 10× objective. Representative images are shown.
RESULTS
Identification of SARS-CoV ORF 3b, ORF 6, and N proteins as interferon antagonists.
To determine whether the structural or accessory proteins of SARS-CoV were interferon antagonists, the proteins were screened using an interferon bioassay. In this assay, proteins are tested for their ability to complement the growth of a recombinant NDV-GFP. NDV is ideal for this assay, since it fails to replicate efficiently in the presence of a type 1 interferon response, which is induced during liposome-mediated plasmid transfection. For these experiments, A549 cells were transfected with plasmids expressing individual SARS-CoV proteins, with empty vector plasmid as a negative control, or with influenza virus NS1 protein as a positive control. At 24 h posttransfection, cells were infected with NDV-GFP, and at 24 h postinfection, the cells were analyzed by microscopy (Fig. 1). Cells infected with NDV-GFP fluoresce green (shown as white), but cells transfected with empty vector prior to infection did not display green fluorescence. Cells transfected with influenza virus NS1 protein, a known interferon antagonist, were green. Three SARS-CoV proteins—the ORF 3b, ORF 6, and N proteins—rescued the growth of NDV-GFP, indicating that they are potential interferon antagonists. The other SARS-CoV proteins tested did not rescue the growth of NDV-GFP. Expression of the SARS-CoV proteins is shown in Fig. 1B. The proteins are expressed at different levels, but there is no correlation between expression level and ability to rescue the growth of NDV-GFP, since ORF 3b is not highly expressed but does rescue the growth of NDV-GFP, while other proteins, such as ORF 7b and 9b, are highly expressed but do not rescue the growth of NDV-GFP.
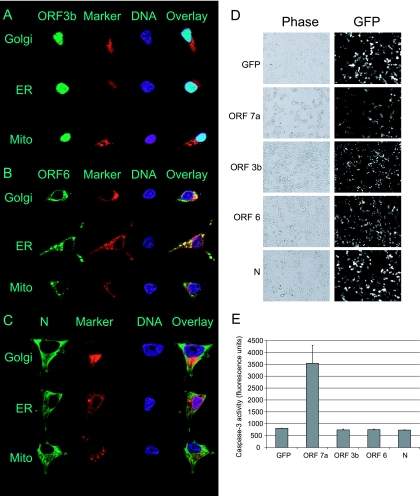
The localization of the interferon antagonists was examined (Fig. 2A to C). Cells were transfected with plasmids expressing ORF 3b (Fig. 2A), ORF 6 (Fig. 2B), or N (Fig. 2C) proteins for 24 h. Cells were fixed, permeabilized, and stained using markers for the ER, Golgi, mitochondria, and chromatin. ORF 3b protein was localized to the nucleus, ORF 6 protein was localized mainly to the ER and Golgi, and N protein was localized diffuselythroughout the cytoplasm but did not colocalize with the cellular markers. There was no evidence of mitochondrial localization of ORF 3b as had been previously reported (31). The fact that the three proteins do not localize to the same cellular compartments indicates that they may inhibit the interferon response by different mechanisms. The ability of ORF 3b, ORF 6, and N proteins to induce apoptosis was also analyzed. Cells were transfected with plasmids expressing ORF 3b, ORF 6, or N proteins for 24 h and analyzed for the morphological changes associated with apoptosis (Fig. 2D). ORF 7a protein, which has been shown to induce apoptosis and inhibit cellular gene expression, was used as a control. Cells expressing ORF 3b, ORF 6, and N proteins have morphology similar to that of cells expressing the negative control, GFP, while cells expressing ORF 7a protein displayed changes associated with apoptosis, such as rounding and shrinkage. To determine whether the SARS-CoV proteins caused biochemical changes associated with apoptosis, active caspase-3 was measured 24 h posttransfection (Fig. 2E). Cells expressing ORF 7a protein had abundant levels of active caspase-3, while cells expressing ORF 3b, ORF 6, or N protein did not have levels of active caspase-3 above that of the negative control. Previous reports stated that N protein induces apoptosis in a small percentage of cells when cells are serum starved but not when cells are grown in serum (23, 32). Thus, ORF 3b, ORF 6, and N proteins do not induce apoptosis and do not inhibit cellular gene expression (Fig. 2 and data not shown).
FIG. 2.
Localization and apoptosis analysis of SARS-CoV ORF 3b, ORF 6, and N proteins. 293T cells were transfected with plasmids expressing GFP-tagged ORF 3b (A), ORF 6 (B), or N (C) proteins for 24 h. Cells were fixed, permeabilized, and stained for either the Golgi using BODIPY TR, the ER using an antibody to PDI, the mitochondria using an antibody to cytochrome c, or chromatin using a DAPI stain. Green represents GFP-tagged protein, red represents the indicated organelle, and blue represents the chromatin. Cells were analyzed by confocal microscopy using a 63× objective, and representative images are shown. D. 293T cells were transfected with GFP or plasmids expressing GFP-tagged ORF 7a, ORF 3b, ORF 6, or N proteins for 24 h. Cells were analyzed by fluorescence and phase-contrast microscopy for morphological changes associated with apoptosis. E. 293T cells were transfected for 24 h, harvested, lysed, and analyzed for caspase-3 activity.
ORF 3b, ORF 6, and N proteins inhibit the synthesis of IFN-β.
The mechanisms of the inhibition of the interferon response by these three proteins were examined. One mechanism by which viral proteins inhibit the interferon response is by preventing expression from the interferon promoter. It has been shown that interferon is not produced in cells infected with SARS-CoV (22). To determine whether the antagonists inhibit the induction of interferon, 293T cells were transfected with a plasmid expressing RFP under the control of the IFN-β promoter. The cells were cotransfected with plasmids expressing the SARS-CoV proteins, with empty vector plasmid as a negative control, or with a plasmid expressing influenza virus NS1 protein as a positive control. At 24 h posttransfection, cells were infected with Sendai virus to induce interferon synthesis, and at 24 h postinfection, cells were analyzed by microscopy for red fluorescence (shown as white). Antagonists of interferon induction prevent the expression of RFP.
Fluorescence images are shown in Fig. 3A, and the quantitation of the RFP expression in three experiments is shown Fig. 3B. The positive control, influenza virus NS1 protein, dramatically reduces expression of RFP from the IFN-β promoter. SARS-CoV ORF 3b, ORF 6, and N proteins all reduce expression of RFP (Fig. 3A and B). This indicates that the three antagonists inhibit the induction of interferon. The N gene actually encodes two proteins, N protein and ORF 9b protein. A construct of the N gene was made which contained a mutation in the start site of the ORF 9b protein but did not alter the amino acid of the N protein. This plasmid, which expresses N protein but not ORF 9b, was able to inhibit the expression of RFP from the IFN-β promoter as effectively as the plasmid containing the unaltered N gene. This result, coupled with the result in Fig. 1 showing that ORF 9b does not rescue growth of NDV-GFP, indicate that N protein but not ORF 9b is an interferon antagonist. Expression of Sendai virus proteins in the presence of the SARS-CoV proteins is shown in Fig. 3C. None of the SARS-CoV proteins inhibit expression of Sendai virus proteins.
FIG. 3.
SARS-CoV ORF 3b, ORF 6, and N proteins inhibit production of interferon. A. 293T cells were cotransfected with a plasmid expressing red fluorescence protein under the control of the IFN-β promoter and empty vector plasmid, a plasmid expressing influenza virus NS1, or plasmids expressing SARS-CoV proteins. At 24 h posttransfection, cells were infected with Sendai virus to stimulate the production of interferon. Images were captured 24 h postinfection using a 10× objective. B. Images from three experiments described for panel A were quantitated using Image J software. Data are averages plus standard deviations for three experiments. C. Cells were transfected with the indicated plasmids for 24 h and then infected with Sendai virus for 24 h. Cells were fixed, permeabilized, and analyzed for Sendai virus proteins using monoclonal antibodies to Sendai virus.
ORF 3b, ORF 6, and N proteins inhibit the function of IRF-3, and N protein inhibits the function of NF-κB.
There are several ways in which viruses inhibit the induction of interferon. The transcription factor IRF-3 binds to the interferon promoter when activated and has been shown to be necessary for interferon induction. It was demonstrated that IRF-3 is inhibited in cells infected with SARS-CoV (22). The ability of IRF-3 to activate its DNA binding site was examined using a plasmid construct containing a firefly luciferase gene under the control of a promoter with three IRF-3 binding sites (55-CIB). Cells were cotransfected with control plasmids or with plasmids expressing the SARS-CoV proteins, with the p55-CIB-firefly luciferase plasmid, and with a plasmid that constitutively expresses Renilla luciferase. The Renilla luciferase plasmid is used to normalize expression levels of the samples. At 24 h after transfection, cells were infected with Sendai virus to stimulate interferon synthesis. Cells were harvested 24 h postinfection and analyzed for firefly and Renilla luciferase. Infection with Sendai virus activated the 55-CIB promoter in cells transfected with empty vector (Fig. 4A). The positive control, influenza virus NS1 protein, inhibits expression from the 55-CIB promoter. All three SARS-CoV proteins, ORF 3b, ORF 6, and N, also inhibit expression of the 55-CIB promoter, indicating that they prevent the activation of promoters requiring IRF-3 binding, such as interferon.
In order for IRF-3 to bind to promoters, IRF-3 must be activated by phosphorylation. Phosphorylation of IRF-3 was analyzed in cells transfected with a plasmid expressing IRF-3 and control plasmids or plasmids expressing SARS-CoV proteins by Western blotting using an anti-phospho-IRF-3 antibody. At 24 h posttransfection, cells were infected with Sendai virus for 6 h to stimulate IRF-3 phosphorylation. ORF 3b, ORF 6, and N proteins all effectively inhibit phosphorylation of IRF-3 (Fig. 4B). Quantitation of the Western blot revealed that phosphorylation is inhibited by the SARS-CoV proteins, but IRF-3 is not degraded.
Active IRF-3 translocates from the cytoplasm to the nucleus in order to function as a transcription factor. Translocation of IRF-3 was analyzed in cells transfected with control plasmids or plasmids expressing SARS-CoV proteins and IRF-3-GFP. At 24 h posttransfection, cells were infected with Sendai virus for 8 h to stimulate IRF-3 translocation (Fig. 4C). IRF-3 is present in the cytoplasm of mock-infected cells, while it is present in the nuclei of Sendai virus-infected cells. All three SARS-CoV proteins inhibit IRF-3 translocation. Thus, the data in Fig. 4 indicate that the SARS-CoV proteins inhibit IRF-3 function.
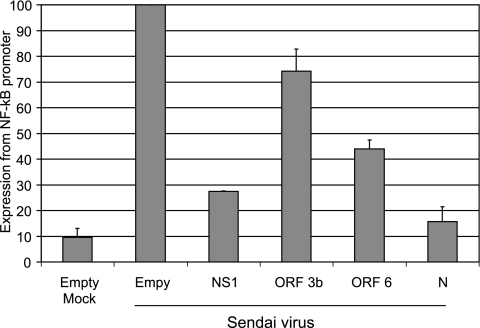
Another cellular component necessary for interferon synthesis is NF-κB. NF-κB, like IRF-3, is a transcription factor activated after viral infection and binds to and activates the interferon promoter. Activation of NF-κB was examined using a firefly luciferase construct under the control of an NF-κB-responsive promoter containing two NF-κB binding sites. Cells were cotransfected with control plasmids or with plasmids expressing the SARS-CoV proteins, with the pNF-κB-firefly luciferase plasmid, and with the Renilla plasmid. At 24 h posttransfection, cells were infected with Sendai virus, and at 24 h postinfection, cells were harvested and analyzed for firefly and Renilla luciferase. As expected, infection with Sendai activated the promoter containing NF-κB binding sites in cells transfected with empty vector, and influenza virus NS1 protein inhibited expression from this promoter (Fig. 5). SARS-CoV N protein effectively inhibited activation of the promoter, while ORF 6 protein was able to reduce expression of the NF-κB-responsive promoter to a lesser extent than N protein (P < 0.05 for both). ORF 3b protein had little effect on the NF-κB-responsive promoter.
FIG. 5.
N protein inhibits NF-κB activation. 293T cells were cotransfected with a plasmid expressing firefly luciferase under the control of an NF-κB-responsive promoter containing two NF-κB binding sites, the Renilla luciferase plasmid, and the indicated plasmids. At 24 h posttransfection, cells were infected with Sendai virus. At 24 h postinfection, cells were harvested and analyzed for firefly and Renilla luciferase activities. Cells were normalized to the Renilla luciferase control. Data are averages plus standard deviations for three experiments. A value of 100% represents approximately 8,000,000 firefly luciferase units and 6,000,000 Renilla luciferase units. The P values for N and ORF 6 proteins were less than 0.05.
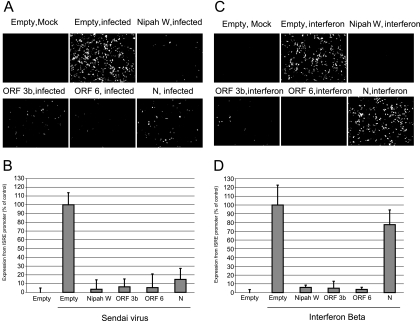
ORF 3b and ORF 6 proteins inhibit expression from an ISRE promoter.
While our data indicate that SARS-CoV ORF 3b, ORF 6, and N proteins inhibit the synthesis of interferon, we also tested whether these proteins could inhibit the cellular response to interferon (interferon signaling). Once interferon is released from the cells, it binds to an interferon receptor, and signaling occurs through a JAK/STAT pathway, resulting in the activation of genes containing an ISRE in their promoters. To determine whether ORF 3b, ORF 6, and N proteins inhibit expression of genes containing an ISRE, cells were cotransfected with the SARS-CoV proteins and with a plasmid containing GFP under the control of an ISRE promoter, the interferon-stimulated gene 54 promoter. At 24 h posttransfection, cells were either infected with Sendai virus or treated with IFN-β. Expression from the ISRE promoter after Sendai virus infection requires both interferon synthesis and interferon signaling, while expression from the ISRE promoter after treatment with interferon only requires interferon signaling. Representative images are shown in Fig. 6A and C, and quantitation of three experiments is shown in Fig. 6B and D. After infection with Sendai virus, ORF 3b, ORF 6, and N proteins all effectively inhibit expression from the ISRE promoter (Fig. 6A and B), indicating that the proteins inhibit the interferon response After treatment with IFN-β, ORF 3b and ORF 6 proteins inhibited expression from the ISRE promoter but N protein did not (Fig. 6C and D). Taken together with the data in Fig. 3, these data indicate that N protein inhibits only interferon synthesis, while ORF 3b and ORF 6 proteins inhibit both interferon synthesis and interferon signaling.
FIG. 6.
Inhibition of a promoter containing an ISRE by SARS-CoV proteins. 293T cells were cotransfected with ISRE-GFP and the indicated plasmid for 24 h. Cells were then infected with Sendai virus (A and B) or treated with IFN-β (C and D) for 24 h and then analyzed by microscopy using a 10× objective. Images were quantitated with ImageJ software (B and D). Data are averages plus standard deviations for three experiments.
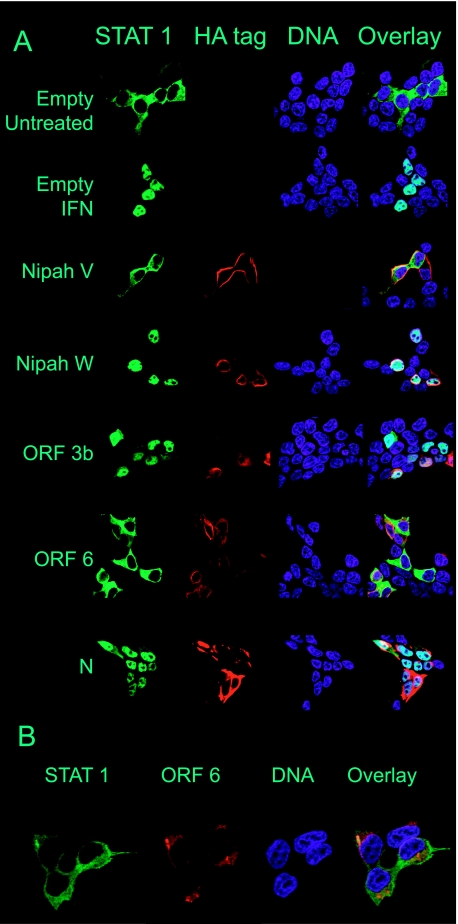
ORF 6 protein inhibits the translocation of STAT1.
Several viruses express proteins that inhibit interferon signaling by specifically targeting the transcription factor STAT1. In order for STAT1 to be activated, STAT1 is phosphorylated and then translocates to the nucleus. Translocation of STAT1 from the cytoplasm to the nucleus was analyzed in cells expressing SARS-CoV ORF 3b, ORF 6, and N proteins. Cells were cotransfected with a STAT1-GFP fusion construct and plasmids expressing ORF 3b, ORF 6, and N proteins. At 24 h posttransfection, cells were treated with IFN-β. Images were captured 1 h posttreatment, and representative images are shown in Fig. 7A. Treatment with interferon caused STAT1-GFP to translocate to the nuclei of empty-vector-transfected cells (Fig. 7A). Nipah virus proteins V and W were used as controls for viral proteins that do and do not inhibit translocation of STAT1 to the nucleus, respectively. SARS-CoV ORF 6 protein, but not ORF 3b or N protein, was able to inhibit the translocation of STAT1-GFP to the nucleus. A higher magnification of the image of ORF 6 protein in cells transfected with STAT1-GFP and treated with IFN-β shows that ORF 6 protein does not colocalize with STAT1, indicating that ORF 6 does not directly interact with STAT1 (Fig. 7B).
FIG. 7.
ORF 6 protein inhibits translocation of STAT1 to the nucleus. A and B. 293T cells were cotransfected with STAT1-GFP and the indicated plasmids. At 24 h posttransfection, cells were treated with IFN-β for 1 h and then fixed and stained. Viral proteins were visualized with an antibody to the HA tag, and DNA was visualized using Hoechst 33342 stain. Green represents STAT1-GFP, red represents the viral protein, and blue represents DNA. Cells were analyzed by confocal microscopy, and representative images are shown. All images were obtained using a 63× objective; the images in panel B were magnified using Zeiss confocal software.
To further analyze the activation of STAT1, phosphorylation of STAT1 was analyzed in cells expressing the SARS-CoV proteins. Cells were transfected for 24 h with empty vector plasmid or plasmids expressing ORF 3b, ORF 6, or N proteins. Cells were infected with Sendai virus for 6 h and then harvested and analyzed for STAT1 phosphorylation by Western blotting using an antibody specific for phospho-STAT1. A representative Western blot is shown in Fig. 8A. None of the SARS-CoV proteins reduced STAT1 phosphorylation as analyzed by Western blotting. One possibility is that phosphorylation of the untransfected cells represents a large amount of the phosphorylation observed in Fig. 8A, and thus any decrease in phosphorylation would be difficult to distinguish by Western blotting. To address this possibility, cells were cotransfected with plasmids expressing the SARS-CoV proteins and STAT1-GFP and treated with IFN-β. Cells were fixed, permeabilized, stained for phospho-STAT1, and analyzed by confocal microscopy. Representative images are shown in Fig. 8B. High rates of transfection were observed in this experiment as well as the other experiments described in this paper. Since nearly all of the phosphorylated STAT1 is observed in transfected cells, and nearly all of the transfected cells contain phosphorylated STAT1, this confirms that none of the SARS-CoV proteins inhibit STAT1 phosphorylation. There was no difference in the amounts of STAT1 phosphorylation in the ORF 3b, ORF 6, and N protein-expressing cells.
DISCUSSION
The inhibition of interferon is a common mechanism by which viruses subvert the innate host response. Viral proteins that target various aspects of the interferon pathways have been identified (7). For influenza virus, a negative-sense segmented RNA virus of the family Orthomyxoviridae, the interferon antagonist has been identified as NS1 protein (6). NS1 inhibits the interferon response by preventing cells from detecting the presence of the replicating influenza virus. NS1 binds and sequesters double-stranded RNA, a by-product of viral replication that activates several cellular antiviral proteins, such as protein kinase R and 2′,5′-oligo(A) synthetase (2, 10, 14). Nipah virus, a negative-sense nonsegmented RNA virus of the family Paramyxoviridae, encodes three proteins which antagonize interferon by inhibiting the function of STAT1 (16). Nipah virus V and P proteins act by retaining STAT1 in the cytoplasm, while the W protein sequesters STAT1 in the nucleus, creating both a cytoplasmic and a nuclear impediment for STAT1 (19, 20). Ebola virus, a negative-sense nonsegmented RNA virus of the family Filoviridae, encodes two proteins that antagonize different components of the interferon pathway. Ebola virus protein VP35 inhibits interferon by binding and sequestering double-stranded RNA, while VP24 inhibits interferon by preventing the nuclear accumulation of activated STAT1 (1, 18). Interferon is an important deterrent of SARS-CoV replication and disease in the mouse model, as SARS-CoV infection in STAT1 knockout but not wild-type animals is robust and associated with prolonged viremia, extensive lung pathology, and spread to multiple organs (11).
Our data indicate that SARS-CoV ORF 3b, ORF 6, and N proteins all function as interferon antagonists (Fig. 1). All three proteins inhibit the expression of IFN-β, and further examination revealed that all three proteins inhibited a key protein necessary for the expression of IFN-β, IRF-3 (Fig. 3 and 4). SARS-CoV ORF 3b, ORF 6, and N proteins all inhibit activation of IRF-3 by phosphorylation and binding of IRF-3 to a promoter with IRF-3 binding sites. N protein dramatically inhibited expression from an NF-κB-responsive promoter, while expression of ORF 6 protein resulted in a twofold inhibition (Fig. 5). All three proteins were able to inhibit expression from an ISRE promoter after infection of Sendai virus, while only ORF 3b and ORF 6 were able to inhibit expression from the ISRE promoter after treatment with IFN-β (Fig. 6). This indicates that N protein inhibits the synthesis of interferon but not interferon signaling. ORF 6 protein, but not ORF 3b or N protein, inhibited the translocation of STAT1 to the nucleus (Fig. 7). STAT1 is required for the activation of ISRE promoters. Interestingly, ORF 6 protein did not prevent the phosphorylation of STAT1 (Fig. 8). The interferon antagonists encoded by Nipah and Ebola viruses that inhibit STAT1 translocation do so by binding and inhibiting karyopherin-alpha (3, 21). We are investigating the mechanism by which ORF 6 protein blocks STAT1 localization to the nucleus as well as the mechanism of interferon inhibition by all three proteins. While the mechanisms of inhibition are unknown, it is possible that ORF 3b interacts with nuclear transcription factors necessary for the interferon response. ORF 6 may disrupt ER/Golgi transport necessary for the interferon response, and N protein may directly interact with double-stranded RNA or interferon pathway components in the cytoplasm.
Like other highly pathogenic viruses, such as Nipah and Ebola viruses, SARS-CoV encodes more than one protein that is able to inhibit interferon. This may enhance the ability of the viruses to inhibit the interferon response, since each viral antagonist may inhibit interferon by 90 to 95% but not completely. Thus, encoding several proteins that are antagonists increases the likelihood that the virus can completely inhibit the interferon response. Viruses, such as Ebola virus and SARS-CoV, that are able to target more than one part of the interferon response are most likely to cause a severe inhibition of interferon. This may contribute to their high pathogenicity.
Little is known about the function of ORF 3b and ORF 6 proteins. It has been shown that when mice are infected with a related coronavirus, mouse hepatitis virus (MHV), containing SARS-CoV ORF 6 protein, the mice die, in contrast to mice infected with the normally nonlethal wild type MHV (17). Although the mechanism of disease enhancement was not identified, these data suggest that SARS-CoV ORF 6 encodes important virulence determinants in SARS. Mutant SARS-CoVs that lack one or both of the ORF 3b and ORF 6 genes have been constructed. It has been reported that the deletion viruses lacking ORF 3b and/or ORF 6 genes replicate to levels similar to those of wild-type virus in several different tissue culture cell types (29). There are also no differences between cytopathogenesis of the deletion viruses and that of wild-type virus. Thus, ORF 3b and ORF 6 do not have essential roles in viral replication (29). In addition, the deletion viruses grew to levels similar to those of wild-type virus in the lungs of BALB/c mice at day 2 postinfection. This suggests that SARS-CoV is able to inhibit the host interferon response without the ORF 3b and ORF 6 genes (29). This is consistent with the results presented here, since it is likely that the N protein alone was able to inhibit the interferon response sufficiently to allow viral replication in the in vitro and in vivo systems studied thus far. While it would be interesting to test a deletion virus lacking ORF 3b, ORF 6, and N genes, it has been reported that a functional N gene is required for efficient gene expression and rescue of SARS-CoV and other coronaviruses (28). However, the interferon antagonist domain of N may be separate and distinct from the domain playing a required role in viral replication. Identification of the domain of N responsible for interferon antagonism may make production of a reverse-engineered SARS-CoV that allows replication but not IFN blocking possible.
The results presented here are especially significant since interferon antagonists have not been previously identified for any coronaviruses. Our data indicate that three viral proteins likely contribute to the inhibition of IFN-β and IRF-3 observed in cells infected with SARS-CoV (22). It also remains a possibility that one or more of the nonstructural proteins encoded by SARS-CoV also inhibit the interferon response. The SARS-CoV proteome includes 14 additional replicase ORFs that have not been evaluated for IFN antagonist activity. By encoding at least three proteins that inhibit different aspects of the interferon response, SARS-CoV has evolved to utilize different mechanisms to undermine the host interferon response. Thus, it is possible that other coronaviruses also encode proteins that inhibit multiple levels of the interferon response.
Acknowledgments
We thank Domenico Tortorella for the PDI antibody and Takashi Fujita for the p55-CIB plasmid. We thank Claire Coupillaud for help with the p55-CIB experiment.
This work was partially supported by grants from the NIH (PO1 AI059443-01A1). Confocal laser scanning microscopy was performed at the MSSM-Microscopy Shared Resource Facility, supported with funding from NIH-NCI shared resources grant 5R24 CA095823-04, NSF Major Research Instrumentation grant DBI-9724504, and NIH shared instrumentation grant 1 S10 RR0 9145-01. P.P. is a senior scholar of the Ellison Medical Foundation. S.A.K.-B. was partially supported by the NIH training grant T32 A1007645. M.B.F. was supported by NIH fellowship F32 AI66542.
Footnotes
Published ahead of print on 15 November 2006.
REFERENCES
- 1.Basler, C. F., X. Wang, E. Muhlberger, V. Volchkov, J. Paragas, H. D. Klenk, A. Garcia-Sastre, and P. Palese. 2000. The Ebola virus VP35 protein functions as a type I IFN antagonist. Proc. Natl. Acad. Sci. USA 97:12289-12294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergmann, M., A. Garcia-Sastre, E. Carnero, H. Pehamberger, K. Wolff, P. Palese, and T. Muster. 2000. Influenza virus NS1 protein counteracts PKR-mediated inhibition of replication. J. Virol. 74:6203-6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cárdenas, W. B., Y. M. Loo, M. Gale, Jr., A. L. Hartman, C. R. Kimberlin, L. Martinez-Sobrido, E. O. Saphire, and C. F. Basler. 2006. Ebola virus VP35 protein binds double-stranded RNA and inhibits alpha/beta interferon production induced by RIG-I signaling. J. Virol. 80:5168-5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan, W. S., C. Wu, S. C. Chow, T. Cheung, K. F. To, W. K. Leung, P. K. Chan, K. C. Lee, H. K. Ng, D. M. Au, and A. W. Lo. 2005. Coronaviral hypothetical and structural proteins were found in the intestinal surface enterocytes and pneumocytes of severe acute respiratory syndrome (SARS). Mod. Pathol. 18:1432-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Haan, C. A., P. S. Masters, X. Shen, S. Weiss, and P. J. Rottier. 2002. The group-specific murine coronavirus genes are not essential, but their deletion, by reverse genetics, is attenuating in the natural host. Virology 296:177-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.García-Sastre, A., A. Egorov, D. Matassov, S. Brandt, D. E. Levy, J. E. Durbin, P. Palese, and T. Muster. 1998. Influenza A virus lacking the NS1 gene replicates in interferon-deficient systems. Virology 252:324-330. [DOI] [PubMed] [Google Scholar]
- 7.García-Sastre, A. 2002. Mechanisms of inhibition of the host interferon alpha/beta-mediated antiviral responses by viruses. Microbes Infect. 4:647-655. [DOI] [PubMed] [Google Scholar]
- 8.García-Sastre, A., and C. A. Biron. 2006. Type 1 interferons and the virus-host relationship: a lesson in detente. Science 312:879-882. [DOI] [PubMed] [Google Scholar]
- 9.Geng, H., Y. M. Liu, W. S. Chan, A. W. Lo, D. M. Au, M. M. Waye, and Y. Y. Ho. 2005. The putative protein 6 of the severe acute respiratory syndrome-associated coronavirus: expression and functional characterization. FEBS Lett. 579:6763-6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hatada, E., and R. Fukuda. 1992. Binding of influenza A virus NS1 protein to dsRNA in vitro. J. Gen. Virol. 73:3325-3329. [DOI] [PubMed] [Google Scholar]
- 11.Hogan, R. J., G. Gao, T. Rowe, P. Bell, D. Flieder, J. Paragas, G. P. Kobinger, N. A. Wivel, R. G. Crystal, J. Boyer, H. Feldmann, T. G. Voss, and J. M. Wilson. 2004. Resolution of primary severe acute respiratory syndrome-associated coronavirus infection requires Stat1. J. Virol. 78:11416-11421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jennings, S., L. Martinez-Sobrido, A. Garcia-Sastre, F. Weber, and G. Kochs. 2005. Thogoto virus ML protein suppresses IRF3 function. Virology 331:63-72. [DOI] [PubMed] [Google Scholar]
- 13.Kopecky-Bromberg, S. A., L. Martinez-Sobrido, and P. Palese. 2006. 7a protein of severe acute respiratory syndrome coronavirus inhibits cellular protein synthesis and activates p38 mitogen-activated protein kinase. J. Virol. 80:785-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu, Y., M. Wambach, M. G. Katze, and R. M. Krug. 1995. Binding of the influenza virus NS1 protein to double-stranded RNA inhibits the activation of the protein kinase that phosphorylates the elF-2 translation initiation factor. Virology 214:222-228. [DOI] [PubMed] [Google Scholar]
- 15.Martínez-Sobrido, L., E. I. Zuniga, D. Rosario, A. Garcia-Sastre, and J. C. de la Torre. 2006. Inhibition of the type I interferon response by the nucleoprotein of the prototypic arenavirus lymphocytic choriomeningitis virus. J. Virol. 80:9192-9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park, M. S., M. L. Shaw, J. Munoz-Jordan, J. F. Cros, T. Nakaya, N. Bouvier, P. Palese, A. Garcia-Sastre, and C. F. Basler. 2003. Newcastle disease virus (NDV)-based assay demonstrates interferon-antagonist activity for the NDV V protein and the Nipah virus V, W, and C proteins. J. Virol. 77:1501-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pewe, L., H. Zhou, J. Netland, C. Tangudu, H. Olivares, L. Shi, D. Look, T. Gallagher, and S. Perlman. 2005. A severe acute respiratory syndrome-associated coronavirus-specific protein enhances virulence of an attenuated murine coronavirus. J. Virol. 79:11335-11342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reid, S. P., L. W. Leung, A. L. Hartman, O. Martinez, M. L. Shaw, C. Carbonnelle, V. E. Volchkov, S. T. Nichol, and C. F. Basler. 2006. Ebola virus VP24 binds karyopherin α1 and blocks STAT1 nuclear accumulation. J. Virol. 80:5156-5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriguez, J. J., C. D. Cruz, and C. M. Horvath. 2004. Identification of the nuclear export signal and STAT-binding domains of the Nipah virus V protein reveals mechanisms underlying interferon evasion. J. Virol. 78:5358-5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shaw, M. L., A. Garcia-Sastre, P. Palese, and C. F. Basler. 2004. Nipah virus V and W proteins have a common STAT1-binding domain yet inhibit STAT1 activation from the cytoplasmic and nuclear compartments, respectively. J. Virol. 78:5633-5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaw, M. L., W. B. Cardenas, D. Zamarin, P. Palese, and C. F. Basler. 2005. Nuclear localization of the Nipah virus W protein allows for inhibition of both virus- and toll-like receptor 3-triggered signaling pathways. J. Virol. 79:6078-6088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spiegel, M., A. Pichlmair, L. Martinez-Sobrido, J. Cros, A. Garcia-Sastre, O. Haller, and F. Weber. 2005. Inhibition of beta interferon induction by severe acute respiratory syndrome coronavirus suggests a two-step model for activation of interferon regulatory factor 3. J. Virol. 79:2079-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Surjit, M., B. Liu, S. Jameel, V. T. Chow, and S. K. Lal. 2004. The SARS coronavirus nucleocapsid protein induces actin reorganization and apoptosis in COS-1 cells in the absence of growth factors. Biochem. J. 383:13-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Surjit, M., B. Liu, V. T. Chow, and S. K. Lal. 2006. The nucleocapsid protein of severe acute respiratory syndrome-coronavirus inhibits the activity of cyclin-cyclin-dependent kinase complex and blocks S phase progression in mammalian cells. J. Biol. Chem. 281:10669-10681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Talon, J., C. M. Horvath, R. Polley, C. F. Basler, T. Muster, P. Palese, and A. Garcia-Sastre. 2000. Activation of interferon regulatory factor 3 is inhibited by the influenza A virus NS1 protein. J. Virol. 74:7989-7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan, X., Q. Hao, Y. Mu, K. A. Timani, L. Ye, Y. Zhu, and J. Wu. 2006. Nucleocapsid protein of SARS-CoV activates the expression of cyclooxygenase-2 by binding directly to regulatory elements for nuclear factor-kappa B and CCAAT/enhancer binding protein. Int. J. Biochem. Cell. Biol. 38:1417-1428. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.You, J., B. K. Dove, L. Enjuanes, M. L. DeDiego, E. Alvarez, G. Howell, P. Heinen, M. Zambon, and J. A. Hiscox. 2005. Subcellular localization of the severe acute respiratory syndrome coronavirus nucleocapsid protein. J. Gen. Virol. 86:3303-3310. [DOI] [PubMed] [Google Scholar]
- 28.Yount, B., K. M. Curtis, E. A. Fritz, L. E. Hensley, P. B. Jahrling, E. Prentice, M. R. Denison, T. W. Geisbert, and R. S. Baric. 2003. Reverse genetics with a full-length infectious cDNA of severe acute respiratory syndrome coronavirus. Proc. Natl. Acad. Sci. USA 100:12995-13000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yount, B., R. S. Roberts, A. C. Sims, D. Deming, M. B. Frieman, J. Sparks, M. R. Denison, N. Davis, and R. S. Baric. 2005. Severe acute respiratory syndrome coronavirus group-specific open reading frames encode nonessential functions for replication in cell cultures and mice. J. Virol. 79:14909-14922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuan, X., Z. Yao, Y. Shan, B. Chen, Z. Yang, J. Wu, Z. Zhao, J. Chen, and Y. Cong. 2005. Nucleolar localization of non-structural protein 3b, a protein specifically encoded by the severe acute respiratory syndrome coronavirus. Virus Res. 114:70-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuan, X., Y. Shan, Z. Yao, J. Li, Z. Zhao, J. Chen, and Y. Cong. 2006. Mitochondrial location of severe acute respiratory syndrome coronavirus 3b protein. Mol. Cell 21:186-191. [PubMed] [Google Scholar]
- 32.Zhao, G., S. Q. Shi, Y. Yang, and J. P. Peng. 2006. M and N proteins of SARS coronavirus induce apoptosis in HPF cells. Cell Biol. Toxicol. 22:313-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng, B., M. L. He, K. L. Wong, C. T. Lum, L. L. Poon, Y. Peng, Y. Guan, M. C. Lin, and H. F. Kung. 2004. Potent inhibition of SARS-associated coronavirus (SCOV) infection and replication by type I interferons (IFN-alpha/beta) but not by type II interferon (IFN-gamma). J. Interferon Cytokine Res. 24:388-390. [DOI] [PubMed] [Google Scholar]