Abstract
Sheep can be experimentally infected with bovine spongiform encephalopathy (BSE), and the ensuing disease is similar to scrapie in terms of pathogenesis and clinical signs. BSE infection in sheep is an animal and human health concern. In this study, the transmission in BoPrP-Tg110 mice of prions from BSE-infected sheep was examined and compared to the transmission of original cattle BSE in cattle and sheep scrapie prions. Our results indicate no transmission barrier for sheep BSE prions to infect BoPrP-Tg110 mice, but the course of the disease is accelerated compared to the effects of the original BSE isolate. The shortened incubation period of sheep BSE in the model was conserved in subsequent passage in BoPrP-Tg110 mice, indicating that it is not related to infectious titer differences. Biochemical signature, lesion profile, and PrPSc deposition pattern of both cattle and sheep BSE were similar. In contrast, all three sheep scrapie isolates tested showed an evident transmission barrier and further adaptation in subsequent passage. Taken together, those data indicate that BSE agent can be altered by crossing a species barrier, raising concerns about the virulence of this new prion towards other species, including humans. The BoPrP-Tg110 mouse bioassay should be considered as a valuable tool for discriminating scrapie and BSE in sheep.
Transmissible spongiform encephalopathies (TSEs) are neurodegenerative disorders occurring in sheep (scrapie), cattle (bovine spongiform encephalopathy [BSE]), or humans (Creutzfeldt-Jakob disease [CJD]). The infectious agents causing TSEs are denoted prions (30). The only known constituent of prions is PrPSc, an isoform of the cellular prion protein PrPC. The mechanism whereby PrPC is converted into PrPSc is poorly understood, although the process is characterized by conformational changes increasing the β-sheet content: polymerization and insolubility of the protein (23, 27, 31, 33, 35, 43). In most TSEs, PrPSc shows partial resistance to degradation by proteinase K, yielding an N-terminally truncated PrPres product (29).
BSE was recognized in the 1980s as a new prion disease (18). In contrast, scrapie was identified over 200 years ago (25). BSE has important implications for public health and has been linked to the emergence of a new variant form of Creutzfeldt-Jacob disease (vCJD) due to the consumption of BSE-contaminated beef products (4, 17, 39, 42). In general, prion transmission in the same mammalian species is highly efficient and cases show very similar incubation periods, whereas transmission between animals of different species is usually inefficient (this restriction is termed the species barrier). Interspecies transmission is accompanied by reduced infectivity and extended incubation periods and has the potential for new characteristics to emerge (24, 26, 32). These putative characteristics of prions after crossing the species barrier depend both on the prion strain involved and the new host, rendering new prion strains with features that differ from those of the original strains. The species barrier could be modulated by the amino acid sequence differences between the interacting PrP proteins, a host factor and the prion strain being transmitted (8, 38), such that the transmission barrier for each prion strain is specific for each donor-recipient species pair.
The possibility that BSE infection could occur in other species such as sheep and goats has important implications for public health. A goat with properties of BSE infection has been detected in France and the United Kingdom (11, 20). BSE infection in sheep has also been reported after experimental transmission (14), as has natural BSE transmission between sheep (3). The BSE susceptibility of sheep has extended to genotypes previously classed as resistant to scrapie (19). Thus, BSE could be present in sheep flocks showing similar signs to those observed in scrapie as a recurrent infection, presumably at a low incidence (13). PrPres from BSE-infected sheep shows certain differential biochemical properties distinguishing it from PrPres obtained from scrapie-infected sheep (40). However, the biological and molecular properties of the BSE strain after passage in sheep require careful evaluation. Transgenic mice expressing the corresponding host PrP protein and null for murine PrP are useful tools for analyzing prion strain properties. The BoPrP-Tg110 transgenic mouse line shows a high susceptibility to BSE prions and lacks an apparent transmission barrier (6, 7), whereas a transmission barrier is evident when these transgenic mice are inoculated with sheep scrapie prions (6, 7).
The present study was designed to compare the transmission of prions obtained from BSE-infected sheep, the original cattle BSE prions, and sheep scrapie prions in the BoPrP-Tg110 mouse model. Our results show that prions from the BSE-infected sheep isolate used are able to infect BoPrP-Tg110 mice without a transmission barrier and with enhanced virulence compared to the original BSE isolate. In contrast, all three sheep scrapie isolates tested were only transmitted to BoPrP-Tg110 mice with an evident transmission barrier. Pathological and biochemical features observed in the inoculated BoPrP-Tg110 mice were also specific for the sheep BSE isolate, revealing clear differences compared to the sheep scrapie isolates.
MATERIALS AND METHODS
Mice model.
The BoPrP-Tg110 mouse model was established as described elsewhere (6). BoPrP-Tg110 transgenic mice express bovine PrP protein under the murine PrP promoter in a murine PrP0/0 background. PrPC expression levels in this mouse line are eightfold higher than the PrPC levels found in cow brain homogenates.
TSE isolates.
BSE5 was obtained from the brainstem of one cow naturally infected with BSE (case 139) supplied by INRA (Nouzilly, France).
BSE/sheep inoculum.
Seven ARQ/ARQ sheep were intracerebrally inoculated with BSE5 in the framework of BSE in Sheep Project QLRT-2001-01309 (INRA-Nouzilly, France). The BSE/sheep inoculum was obtained by pooling brainstems from those seven sheep.
Sheep scrapie isolates.
Three different sheep scrapie isolates were also used in this study. (i) The SC-UCD-99 isolate was obtained from brain from an Irish ARQ/ARQ sheep naturally infected with scrapie (provided by the Veterinary Research Laboratory, Abbotstown, Ireland). (ii) The SC-Langlade isolate was obtained from brain from a French ARQ/ARQ sheep naturally infected with scrapie (provided by INRA, Toulouse, France). (iii) The SC-N662-97 isolate was obtained from brain from a Spanish ARQ/ARQ sheep naturally infected with scrapie. Brain homogenates from BoPrP-Tg110-inoculated and clinically affected mice were collected and pooled for further passages.
Transmission studies.
All inocula were prepared in sterile 5% glucose. To minimize the risk of bacterial infection, all inocula were preheated for 10 min at 70°C before intracerebral inoculation in mice. The transmission experiment design is illustrated in Fig. 1.
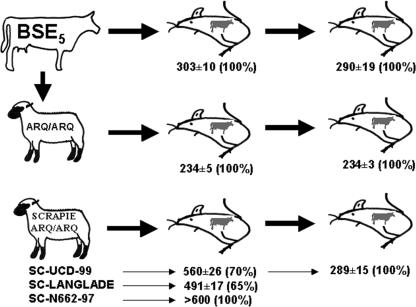
FIG. 1.
Overview of the inoculations conducted in BoPrP-Tg110 mice. Survival times (as days post inoculation) ± standard error of the mean and the percentage of animals scored PrPres positive are indicated.
Groups of 10 to 12 mice (6 to 7 weeks old, weighing approximately 20 g) were inoculated in the right parietal lobe using a disposable 25-gauge hypodermic syringe. Twenty microliters of 10% brain homogenate containing similar amounts of PrPres was delivered to each animal. To evaluate the clinical signs appearing after inoculation, mice were observed daily and their neurological status was assessed twice weekly. The presence of three signs of neurological dysfunction (using 10 different diagnostic criteria) (36, 37) was necessary to score a mouse positive for prion disease. When progression of the disease was evident, the animals were killed for ethical reasons and their brains were harvested for subsequent analysis. These specimens were used to evaluate the presence of PrPres by Western blotting (WB) and enzyme-linked immunosorbent assay (ELISA).
Western blotting.
Frozen brain tissues from mice were homogenized in 5% glucose in distilled water in grinding tubes (Bio-Rad) adjusted to 10% (wt/vol) with TeSeE Precess 48 (Bio-Rad). Complete homogenization was finally obtained by forcing the brain suspension through a 0.4-mm-diameter needle. Briefly, 175 ± 20 mg of each tissue was subjected to three 45-s cycles of homogenization (maximum speed in Ribolyser COGER) in 1.5 ml of a 5% glucose solution. One hundred microliters of brain homogenate was precleared by centrifugation at 2,000 × g for 5 min in 5% sarcosyl. Samples were treated with 20 μg/ml of proteinase K (Roche) at 37°C for 60 min, and insoluble fractions were obtained by centrifugation at 25,000 × g for 30 min. Sodium dodecyl sulfate sample loading buffer was added to all samples, and each one was boiled for 10 min before loading onto an sodium dodecyl sulfate -12% polyacrylamide gel. For the immunoblotting experiments, Sha31, 9A2, and 12B2 monoclonal antibodies (MAbs) were used at 1 μg/ml. Immunocomplexes were detected by horseradish-peroxidase conjugated antimouse immunoglobulin G (IgG) (Amersham Pharmacia Biotech). The immunoblots were developed under conditions of enhanced chemiluminescence (Amersham Pharmacia Biotech). Fujifilm LAS-3000 was used for image capture, and image analysis was performed using Image Gauge 4.0 software. SigmaPlot 2001 software was used for data analysis.
Epitope mapping antibodies.
The mouse MAb Sha31 was used in purified form, and 9A2 and 12B2 MAbs were used in the form of culture supernatants. Sha31 was raised against proteinase K-treated and nondenatured scrapie-associated fibrils of Syrian hamster infected brain (263K) and recognizes at least the 148YEDRYYRE155 epitope (numbered according to the sheep sequence) of the PrP core (12). Mouse 9A2 and 12B2 MAbs were prepared at CIDC-Lelystad from PrP-knockout mice (44) by immunizing them with a synthetic peptide covering the domain corresponding to amino acids 97 to 115 of bovine PrP. The 9A2 epitope requires residues 102WNK104 and the 12B2 epitope requires residues 93WGQGG97 (numbered according to the sheep sequence in both cases). All these sequences are conserved in sheep and bovine PrP protein.
PrPSc proteinase K resistance ELISA.
Two commercial test kits (TeSeE and TeSeE sheep/goat by Bio-Rad) were used according to the manufacturer's recommendations.
Each homogenate was incubated for 10 min at 37°C with 250 μl of proteinase K-buffer A solution. PrPSc was recovered as a pellet after addition of 250 μl of buffer B and centrifugation for 7 min at 20,000 × g at 4°C. Supernatants were discarded and pellets were dried. Finally, the pellet was solubilized in 25 μl of buffer C1 (5 min at 100°C) and diluted sixfold in R6 reagent. Aliquots of 100 μl were deposited and detected by the ELISA method. Denatured PrPSc is reacted with capture antibody and tracer antibody successively (detection kit). The signal linearity range for this ELISA was between 1.7 and 0.2 absorbance units. A recombinant ovine PrPC specifically recognized by both in the immunometric assay was used as internal standard to compare interassay results.
Each sample was diluted in negative 10% brain homogenate until a signal of 1.5 to 1.7 absorbance units was obtained. Equilibrated homogenates were aliquoted in a 12-microtube series (200 μl per microtube) and subjected to proteinase K digestion at concentrations ranging from 50 to 500 μg/mg before PrPSc precipitation. Finally the pellet was dissolved in 25 μl of buffer C1 (5 min at 100°C) and diluted 12-fold in R6 reagent. Samples were deposited as triplicates and detected using TeSeE or TeSeE sheep/goat (Bio-Rad).
Histopathology and immunohistochemistry.
All procedures involving mice brains and spleen were performed as previously described (1). Briefly, samples were fixed in neutral-buffered 10% formalin (4% formaldehyde) before paraffin embedding. After deparaffinization, 2-μm-thick tissue sections were stained with hematoxylin and eosin. Lesion profiles were established according to the standard method described by Fraser and Dickinson (15).
For PrPres immunohistochemistry, 3-μm-thick sections were deparaffinized before antigen retrieval. Briefly, sections were immersed in 98% formic acid for 7 min at room temperature and washed in running tap water before being immersed in guanidine isothiocyanate for 1 h at 4°C. Guanidine isothiocyanate treatment was followed by proteinase K digestion: TBS (50 mM Tris-HCl, 150 mM NaCl, pH 7.6) containing 20 μg/ml of proteinase K for 15 min at 37°C. Primary antibody incubation was conducted overnight at 4°C using the 2A11 MAb (2A11 MAb epitope, 171QVYYRPVDQ179 [amino acids of bovine PrP]) (5) diluted 1/400 in 10% normal goat serum. A secondary goat anti-mouse IgG biotinylated antibody (DAKO) diluted 1/200 in 10% normal goat serum was incubated for 30 min at room temperature, and an avidin-biotin-peroxidase complex (Pierce) was applied using diaminobenzidine (DAB) as a substrate. Finally, sections were counterstained with Mayer's hematoxylin for 1 min, dehydrated, and routinely mounted. Serial sections from positive controls and appropriate negative controls were included in each immunohistochemistry run.
RESULTS
Comparison of the biological properties of BSE, sheep BSE, and scrapie in BoPrP-Tg110 mice.
BoPrP-Tg110 mice were inoculated with the BSE isolate BSE5 both before and after passage in ARQ/ARQ sheep (BSE/sheep inoculum) and compared to three sheep scrapie isolates from different sources (see Fig. 1 for a scheme describing the inoculations). BoPrP-Tg110 mice inoculated with BSE5 had a survival time of 303 ± 10 days postinoculation (dpi). A similar survival time was observed in the second passage (292 ± 15 dpi). Those values were comparable to those previously reported for another BSE isolate (BSE2; 308 ± 5 dpi) (6). All inoculated animals from both the first and second passages were PrPres positive in the central nervous system. Taken together, the 100% attack rate and the constant incubation period observed in subpassage suggest an absence of transmission barrier between cattle and BoPrP-Tg110 mice.
Inoculation of BoPrP-Tg110 mice with the three ARQ/ARQ scrapie isolates (SC-UCD-99, SC-N662-97, and SC-Langlade) indicated the existence of a clear transmission barrier in BoPrP-Tg110 mice to the TSE agent isolated in sheep. Only 70% and 65% of the animals succumbed to, respectively, SC-UCD-99 (survival time, 560 ± 26 dpi) and SC-Langlade (survival time, 491 ± 17 dpi.). None of the animals inoculated with the SC-N662-97 isolate showed clinical signs, and when euthanized (after 600 dpi), all them were PrPres positive. Second passage of SC-UCD-99 resulted in a shortened survival period (289 ± 14 days).
Strikingly, survival time of BSE/sheep-inoculated mice (generated from the same original BSE5) was 234 ± 5 dpi, which represented a 2-month reduction of the survival time when compared with the BSE5 inoculation. Second passage of BSE/sheep resulted in an equivalent survival time (234 ± 3 dpi). All animals from those two subpassages were PrPres positive in brain.
Lesion profile and PrPres distribution pattern in BoPrP-Tg110 mice.
Lesion distributions and PrPres deposit patterns were similar in BoPrP-Tg110 mice inoculated with BSE5 and BSE/sheep (Fig. 2 and 3). Those features were maintained after second passage of those isolates in BoPrP-Tg110 mice.
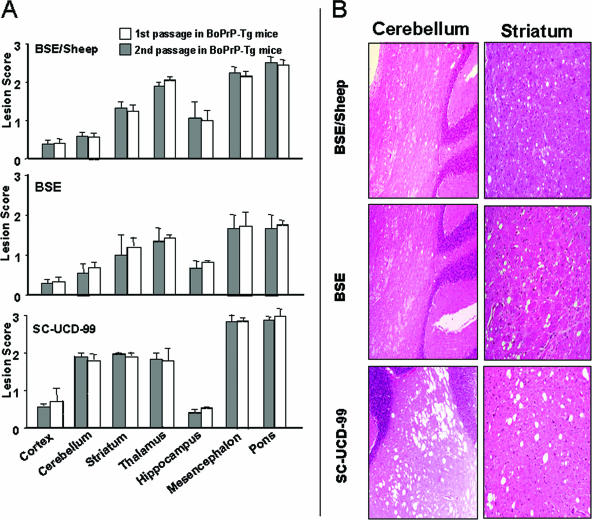
FIG. 2.
Histopathological alterations in BoPrP-Tg110 mice inoculated with BSE, BSE/sheep, or scrapie. (A) Vacuolation profiles in BSE-, BSE/sheep-, and SC-UCD-99-inoculated animals. Lesion scoring is undertaken for seven areas of the brain (cerebral cortex, cerebellum, corpus striatum, thalamus/hypothalamus, hippocampus, mesencephalon, and brainstem at the obex). Bars represent means ± standard deviation. (B) Images of vacuolation in the cerebellum and striatum of inoculated mice. The main differences are an increase in the number and size of vacuoles in these areas of animals inoculated with scrapie compared to those in either the BSE- or BSE/sheep-inoculated animals.
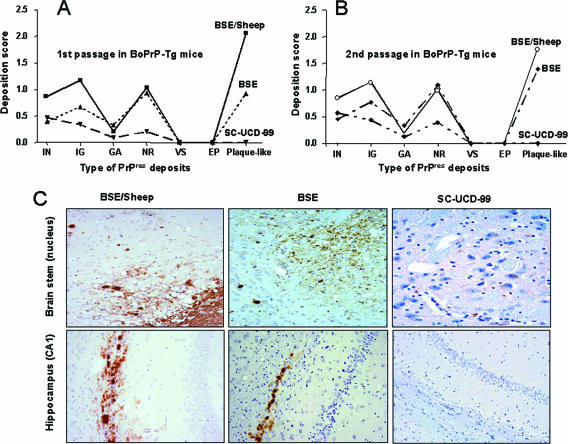
FIG. 3.
Immunohistochemical detection of PrPres in BoPrP-Tg110 mice inoculated with BSE, BSE/sheep, and scrapie. (A) Deposition scores for the different types of PrPres deposits (IN, intraneuronal; IG, intraglial; GA, glial associated deposits; NR, neuropil deposits; VS, vascular; EP, ependimary deposits; Plaque-like, amyloid plaque-like deposits) in the brain of BoPrP-Tg110 mice inoculated with BSE/sheep, BSE, or SC-UCD-99; second passages of brain material from these inoculated mice are shown in panel B. (C) Illustrations of the immunohistochemical detection of PrPres in the brain stem and hippocampus of inoculated animals. Plaque-like deposits appear in the brain stem and hippocampus of BSE- and BSE/sheep inoculated animals, but not in the hippocampus of the SC-UCD-99-inoculated animals. PrP deposition in the brain stem of SC-UCD-99-inoculated animals is observed as intraneuron staining.
In scrapie-inoculated mice, both lesion profile and PrPres deposition pattern in brain (Fig. 2 and 3) were similar for all three scrapie isolates tested but differed from BSE. Those features were also maintained after second passage of the SC-UCD-99 isolate in terms of vacuolar change and PrPres distribution (Fig. 2 and 3). Clear PrPres deposits were observed in the spleen of BoPrP-Tg110 mice inoculated with BSE or BSE/sheep (data not shown). Conversely, no PrPres accumulation was detected in the spleen of scrapie-inoculated BoPrP-Tg110 mice. Identical results were observed after a second passage in BoPrP-Tg110 mice.
PrPres biochemical signature in inoculated BoPrP-Tg110 mice is strain dependent.
In original BSE5, BSE/sheep, or BoPrP-Tg110 mice inoculated with BSE5 or BSE/sheep, no differences could be observed in terms of PrPres (i) electrophoresis mobility (Fig. 4A, lanes 1 to 6) (ii) glycoprofile (Fig. 5), or (iii) proteinase K resistance (Fig. 6A and B). Mice inoculated in second passage, using both sources of inoculum, retained similar characteristics. Taken together, those data suggest that, despite modifications of biological properties, BSE biochemical signature is highly conserved after crossing the sheep/bovine species barrier.
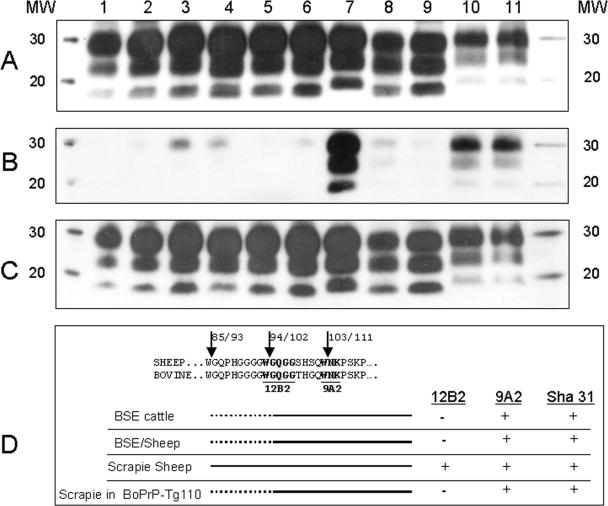
FIG. 4.
Electrophoretic profiles and antibody labeling of PrPres detected with the Sha31 (A), 12B2 (B), and 9A2 (C) MAbs. Lanes show the original BSE5 inoculum (lane 1), BSE5-infected BoPrP-Tg110 mice (lane 2), second-passage BSE5 in BoPrP-Tg110 mice (lane 3), BSE/sheep (lane 4), BSE/sheep in BoPrP-Tg110 mice (lane 5), second-passage BSE/sheep in BoPrP-Tg110 mice (lane 6), scrapie field isolate SC-UCD-99 (lane7), SC-UCD-99 in BoPrP-Tg110 mice (lane 8), second-passage SC-UCD-99 in BoPrP-Tg110 mice (lane 9), scrapie field isolate SC-Langlade (lane 10), and scrapie field isolate SC-N662-97 (lane 11). Panels A, B, and C were loaded with the same quantities of extracted PrPres from each sample. MW, molecular mass given in kilodaltons. (D) PrPres fragment yields are represented according to MAb reactivity. Arrows indicate proteinase K cleavage points, as predicted by the PeptideCuter program of the EXPASY server (http://us.expasy.org/). Numbers reflect sheep/cattle PrP sequence positions. Continuous lines represent the main PrPres fragments, and broken lines represent PrPres fragments found in minor proportions.
FIG. 5.
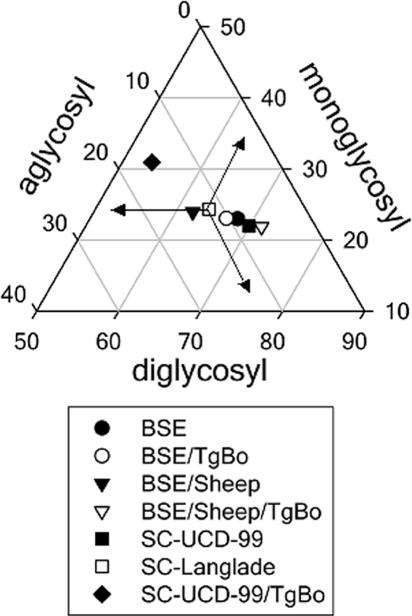
Triangular plot of the glycosyl fractions of PrPres after proteinase K digestion and Western blotting using the Sha31 antibody. The data shown are the means of six or more measurements obtained from density scans in two or more different Western blots. To interpret the plot, read the values for the diglycosyl, monoglycosyl, and aglycosyl fractions along the left, base, and right axes of the triangle, respectively. For each point, the sum of the three values is 100. As an example, the values for the individual points marked by arrows are 64, 24, and 12% for the diglycosyl, monoglycosyl, and aglycosyl fractions, respectively.
FIG. 6.
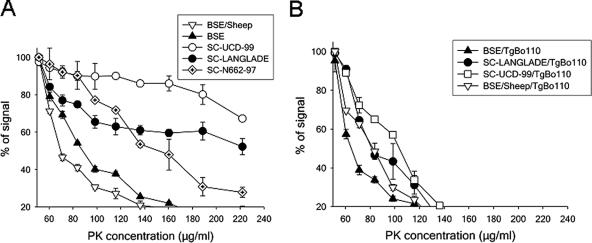
Differential proteinase K (PK) resistance ELISA for PrPSc from the inoculum (A) and from the BoPrP-Tg110-inoculated mice (B).
Using our biochemical test panel, all three original scrapie isolates were clearly distinguishable from BSE or BSE/sheep. Electrophoresis mobilities and glycoprofiles were comparable for all three isolates but different from those of BSE (Fig. 4A, lanes 7, 10, and 11; and Fig. 5). Those features are consistent with numerous reports concerning classical scrapie-BSE comparison (40). Proteinase K resistance was clearly higher in scrapie isolates than in BSE and BSE/sheep (higher proteinase K resistance) but varied between all three scrapie isolates (Fig. 6A).
The scrapie isolates inoculated in BoPrP-Tg110 mice showed different behavior in the PrPSc proteinase K resistance ELISA (Fig. 6B). Furthermore, differences were observed in the transmission of the scrapie isolates to BoPrP-Tg110 mice (Fig. 1A). These differences suggest that while these scrapie isolates share a similar classical PrPres pattern, an unrevealed biodiversity could exist among scrapie isolates.
A faster mobility than in the original SC-UCD-99 isolate (Fig. 4A, lanes 7 and 8) was observed in the BoPrP-Tg110. This mobility change is also maintained after a second passage in BoPrP-Tg110 mice (Fig. 4A, lane 9). The glycoprofile pattern was modified in both first and second passages of scrapie in BoPrP-Tg110 mice, with a decreased proportion of diglycosylated band (Fig. 5). The proteinase K resistance assessed in our ELISA was reduced in scrapie isolates (SC-UCD-99 and SC-Langlade) passaged in BoPrP-Tg110 mice compared to that in the original isolates (Fig. 6B). All of those features clearly demonstrate that conversely to BSE or BSE/sheep prions, scrapie biochemical signature was not preserved after passage in BoPrP-Tg110 mice.
PrPres epitope mapping.
The molecular characteristics of PrPres from BoPrP-Tg110 mice inoculated with BSE, BSE/sheep, and scrapie were determined using MAbs 12B2 and 9A2 and compared to the PrPres from the original inocula. The ability of MAbs 12B2 (WGQGG epitope) and 9A2 (WNK epitope) to recognize both sheep and bovine PrPc was first confirmed using samples not treated with proteinase K (data not shown).
The 9A2 MAb allowed us to detect all the tested samples with a similar electrophoresis mobility pattern to that established using Sha31 (which is directed against the core of the protein). This indicates that the 9A2 MAb epitope is protected from proteinase K cleavage irrespective of the PrPres analyzed (Fig. 4C).
In (i) BSE, (ii) BSE/sheep, and (iii) BSE or BSE/sheep passaged in BoPrP-Tg110 mice (Fig. 4B, lanes 1 to 6), no signal or faint signal could be observed using 12B2. These results indicate that in BSE-derived prions (showing negative or faint staining for the 12B2 MAb), the 12B2 epitope is poorly protected from proteinase K digestion, while in scrapie prions, this epitope seems to be fairly well protected (see Fig. 4D for interpretation). Interestingly, after the scrapie adaptations in the BoPrP-Tg110 mice, the 12B2 epitope is not detected (Fig. 4B, lanes 8 and 9) indicating a change in the biochemical properties of the newly formed PrPres that allows proteinase K to eliminate the 12B2 epitope (see Fig. 4D for interpretation).
DISCUSSION
Species barrier/strain barrier.
The species barrier concept considers that differences in the PrP amino acid sequence confer a limitation for prion infection to occur in a different host species (32). In our study, when BoPrP-Tg110 mice were inoculated with cattle BSE, the transmission rate and stability of the incubation period between the first and second passages were consistent with the expected lack of species barrier in transmission. Meanwhile, the difficulties encountered for primary transmission of the three ARQ/ARQ sheep scrapie isolates to BoPrP-Tg110 mice and the further reduction of incubation period in second passage also sustain the species barriers concept. However, according to the species barrier hypothesis, similar difficulties should have been encountered for primary transmission into BoPrP-Tg110 of ARQ/ARQ sheep BSE versus ARQ/ARQ scrapie isolates. Conversely to this expectation, the 100% transmission rate and the stable incubation duration between the primary and secondary passages of the sheep BSE isolate into BoPrP-Tg110 are consistent with a total lack of transmission barrier.
Those observations, but also other reports (16, 21, 38) strongly support that the species barrier not only depends on the PrP amino sequence of the host and/or donor but also, with equal or greater importance, depends on the considered TSE agent. In that context, the term “strain barrier” should be used.
Alteration of virulence of the BSE agent after its propagation in sheep.
In our experiment, an obvious shorter survival period was observed in BoPrP-Tg110 mice inoculated with BSE/sheep than in those inoculated with cattle BSE. Because this feature was maintained after second passage in the mice model, it cannot be considered to be linked to a higher infectious titer in the initial inoculum nor that heterologous interaction between PrPSc from the BSE/sheep inoculum and bovine PrPC leads to more efficient conversion of PrPC than the homologous interaction, but must be considered as a modification of biological properties of the agent.
Host susceptibility to prion has been shown to be altered on passage through an intermediate species. For instance, hamsters are resistant to chronic wasting disease (CWD) from mule deer. However hamsters become highly susceptible to CWD after its passage in ferrets, and the hamster-adapted CWD is transmissible to hamsters with high efficiency (2). To our knowledge, this is the first time that propagation properties of a prion agent on its natural PrP host (harboring the PrP sequence of the species where the prion is isolated in nature) are shown to be altered by the passage in another species. Moreover, this enhanced virulence of the BSE agent after its passage into sheep showed by the isolate used raise some concern about its potential higher danger for other species, including humans. Human species have been considered resistant to sheep scrapie but susceptible to cattle BSE. In this sense, human susceptibility to the BSE agent after its passage in sheep is unknown. The potential for BSE after passage in sheep to “jump” more easily to humans than directly from cattle is presently being addressed. For that, the infectivity of sheep-passaged BSE in comparison to the original cattle BSE is being determined in transgenic mice expressing human PrP.
TSE biochemical characteristics after transmission in BoPrP-Tg110 mice.
Biochemical analysis of the PrPres from BSE and BSE/sheep and their passage in BoPrP-Tg110 mice (Fig. 4 to 6) showed the preservation of their biochemical characteristics: (i) similar apparent molecular masses, (ii) similar proteinase K resistance in the ELISA, (iii) no significant differences in glycoprofiles, and (iv) slight or null reactivity with MAb 12B2. The WB results indicated that proteinase K cleavage eliminates most of the 12B2 epitope in PrPres from all of the BSE-derived prions (amino acids 93 to 97 for sheep PrP and amino acids 101 to 105 for bovine PrP; see Fig. 4D). This cleavage is indeed a biochemical signature of BSE in sheep. Western blotting with the MAb 9A2 revealed that its corresponding epitope was present in all of the analyzed PrPres, suggesting that amino-terminal proteinase K cleavage occurs between both epitopes (Fig. 4D). The faint signal found using the 12B2 MAb in all of the BSE-derived PrPres may be due to a minor subpopulation of PrPres molecules preserving the 12B2 epitope. A recent study has shown the coexistence of PrPres bands of 19 and 21 kDa in sCJD brain samples, but these appeared in different ratios depending on the CJD type in each case (28). It is not known if in infected brains the different prion types coexist in dynamic equilibrium, or if each prion type may show different band ratios that change according to the experimental conditions, such as pH, metal ions, or proteinase K activity (22, 41, 45). Another possibility is that for each PrPSc type, nearby cleavage sites are proteolyzed in an exclusive “either/or” fashion, whereby after the primary site attack, the second site is not cleaved by the enzyme, as has been previously described for the limited proteolysis of avidin with proteinase K (10). A main proteinase K cleavage point would eliminate the 12B2 epitope, but if proteinase K previously attacks an adjacent unprotected site, this would create a portion of PrPres harboring the 12B2 epitope (in a small percentage), explaining the faint signal that may be detected with the 12B2 MAb.
BSE and BSE/sheep showed similar behavior in the PrPSc proteinase K resistance ELISA, and this behavior was clearly different from the higher proteinase K resistance shown by the different scrapie isolates tested, allowing the discrimination between BSE and scrapie in sheep. Interestingly, when BoPrP-Tg110 mice were inoculated with scrapie, the PrPSc generated was proteinase K sensitive and behaved similarly but not identically to the BSE strain (Fig. 6), indicating that scrapie is not able to maintain its proteinase resistance when adapted to bovine PrP, as was also found by WB with the 12B2 MAb. Glycosyl fractions did not show remarkable changes in inoculated BoPrP-Tg110 mice when compared to their corresponding original inocula, except for scrapie, which showed a significant change in PrPres glycosyl fractions after passage in BoPrP-Tg110 mice (Fig. 5), in line with its adaptation after crossing the transmission barrier. Taken together, these results suggest that the biochemical and biological properties of prions generated in BoPrP-Tg110 mice are influenced by the inoculated prion strain. When the BSE prion strain (BSE or BSE/sheep) is inoculated, no transmission barrier is observed and all properties are conserved, irrespective of the inoculated PrPres amino acid sequence (ovine or bovine). On the contrary, when another strain such as sheep scrapie is used as the inoculum, the biochemical properties of the PrPres change drastically from those shown in the original inoculum and are different from the PrPres obtained when the BSE prion strain (BSE or BSE/sheep) is inoculated (Fig. 5), indicating that the scrapie strain adapts after crossing the transmission barrier.
Discrimination between scrapie and BSE in sheep using BoPrP-Tg110 mice.
Histological examination of inoculated BoPrP-Tg110 mice revealed that BSE and sheep-passaged BSE exhibit unique and similar lesion profiles and PrPres distribution patterns. Those typical features are preserved after a second passage in BoPrP-Tg110 mice but differed from those exhibited by scrapie in both first and second passages in BoPrP-Tg110 mice.
The consistent transmission barrier we observed for scrapie transmission contrasts with results reported by other authors in a different mouse bovine PrP transgenic model (no evidence of a transmission barrier) (39). These apparently contradictory results may be due to differences in (i) the scrapie isolates used (different agents or infectious titer) and (ii) the bovine-PrP transgenic mouse lines. In parallel, inoculations of scrapie isolates in cattle have been described showing transmission barrier (34) or no transmission barrier (9) but with remarkable differences in the experimental conditions used in each case.
To clarify this point, we requested the scrapie inocula used in references 38 and 39. Unfortunately our request could not be satisfied due to the limited quantity of material available. Work in progress using other scrapie isolates with different biochemical and genetic properties will improve our understanding of the behavior of scrapie prions in BoPrP-Tg110 mice and their putative potential as a tool for BSE strain discrimination.
The shorter incubation period and lack of transmission barrier observed with BSE agent compared to scrapie isolates, combined with the lesional profile found in each case, suggest that BoPrP-Tg110 mice could be a valuable and powerful tool for the identification of the BSE agent. Experiments using a large panel of natural TSE strains are ongoing in order to validate the abilities of that mouse model to identify specifically the BSE agent putatively propagated in different species.
Acknowledgments
This work was supported by a grant from the European Union (QLRT-2001-01309). The production of MAbs 9A2 and 12B2 was funded by the Dutch Ministry of Agriculture, Environmental Management and Fisheries.
The authors wish to thank J. Grassi from CEA for providing the Sha31 antibody and F. Lantier and P. Sarradin from INRA for providing the BSE and BSE/sheep inocula. Thanks are also due to Bio-Rad for supplying the ELISA TeSeE and TeSeE sheep/goat components.
Footnotes
Published ahead of print on 1 November 2006.
REFERENCES
- 1.Andreoletti, O., C. Lacroux, A. Chabert, L. Monnereau, G. Tabouret, F. Lantier, P. Berthon, F. Eychenne, S. Lafond-Benestad, J. M. Elsen, and F. Schelcher. 2002. PrP(Sc) accumulation in placentas of ewes exposed to natural scrapie: influence of foetal PrP genotype and effect on ewe-to-lamb transmission. J. Gen. Virol. 83:2607-2616. [DOI] [PubMed] [Google Scholar]
- 2.Bartz, J. C., R. F. Marsh, D. I. McKenzie, and J. M. Aiken. 1998. The host range of chronic wasting disease is altered on passage in ferrets. Virology 251:297-301. [DOI] [PubMed] [Google Scholar]
- 3.Bellworthy, S. J., G. Dexter, M. Stack, M. Chaplin, S. A. Hawkins, M. M. Simmons, M. Jeffrey, S. Martin, L. Gonzalez, and P. Hill. 2005. Natural transmission of BSE between sheep within an experimental flock. Vet. Rec. 157:206. [DOI] [PubMed] [Google Scholar]
- 4.Bruce, M. E., R. G. Will, J. W. Ironside, I. McConnell, D. Drummond, A. Suttie, L. McCardle, A. Chree, J. Hope, C. Birkett, S. Cousens, H. Fraser, and C. J. Bostock. 1997. Transmissions to mice indicate that ‘new variant’ CJD is caused by the BSE agent. Nature 389:498-501. [DOI] [PubMed] [Google Scholar]
- 5.Brun, A., J. Castilla, M. A. Ramirez, K. Prager, B. Parra, F. J. Salguero, D. Shiveral, C. Sanchez, J. M. Sanchez-Vizcaino, A. Douglas, and J. M. Torres. 2004. Proteinase K enhanced immunoreactivity of the prion protein-specific monoclonal antibody 2A11. Neurosci. Res. 48:75-83. [DOI] [PubMed] [Google Scholar]
- 6.Castilla, J., A. Gutierrez Adan, A. Brun, B. Pintado, M. A. Ramirez, B. Parra, D. Doyle, M. Rogers, F. J. Salguero, C. Sanchez, J. M. Sanchez-Vizcaino, and J. M. Torres. 2003. Early detection of PrP(res) in BSE-infected bovine PrP transgenic mice. Arch. Virol. 148:677-691. [DOI] [PubMed] [Google Scholar]
- 7.Castilla, J., A. Gutierrez-Adan, A. Brun, B. Pintado, B. Parra, M. A. Ramirez, F. J. Salguero, F. Diaz San Segundo, A. Rabano, M. J. Cano, and J. M. Torres. 2004. Different behavior toward bovine spongiform encephalopathy infection of bovine prion protein transgenic mice with one extra repeat octapeptide insert mutation. J. Neurosci. 24:2156-2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, S. G., and P. Gambetti. 2002. A journey through the species barrier. Neuron 34:854-856. [DOI] [PubMed] [Google Scholar]
- 9.Cutlip, R. C., J. M. Miller, and H. D. Lehmkuhl. 1997. Second passage of a US scrapie agent in cattle. J. Comp. Pathol 117:271-275. [DOI] [PubMed] [Google Scholar]
- 10.Ellison, D., J. Hinton, S. J. Hubbard, and R. J. Beynon. 1995. Limited proteolysis of native proteins: the interaction between avidin and proteinase K. Protein Sci. 4:1337-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eloit, M., K. Adjou, M. Coulpier, J. J. Fontaine, R. Hamel, T. Lilin, S. Messiaen, O. Andreoletti, T. Baron, A. Bencsik, A. G. Biacabe, V. Beringue, H. Laude, A. Le Dur, J. L. Vilotte, E. Comoy, J. P. Deslys, J. Grassi, S. Simon, F. Lantier, and P. Sarradin. 2005. BSE agent signatures in a goat. Vet. Rec. 156:523-524. [DOI] [PubMed] [Google Scholar]
- 12.Feraudet, C., N. Morel, S. Simon, H. Volland, Y. Frobert, C. Creminon, D. Vilette, S. Lehmann, and J. Grassi. 2005. Screening of 145 anti-PrP monoclonal antibodies for their capacity to inhibit PrPSc replication in infected cells. J. Biol. Chem. 280:11247-11258. [DOI] [PubMed] [Google Scholar]
- 13.Foster, J. D., W. Goldmann, C. McKenzie, A. Smith, D. W. Parnham, and N. Hunter. 2004. Maternal transmission studies of BSE in sheep. J Gen. Virol. 85:3159-3163. [DOI] [PubMed] [Google Scholar]
- 14.Foster, J. D., J. Hope, and H. Fraser. 1993. Transmission of bovine spongiform encephalopathy to sheep and goats. Vet. Rec. 133:339-341. [DOI] [PubMed] [Google Scholar]
- 15.Fraser, H., and A. G. Dickinson. 1968. The sequential development of the brain lesion of scrapie in three strains of mice. J. Comp. Pathol. 78:301-311. [DOI] [PubMed] [Google Scholar]
- 16.Hill, A. F., and J. Collinge. 2003. Subclinical prion infection. Trends Microbiol. 11:578-584. [DOI] [PubMed] [Google Scholar]
- 17.Hill, A. F., M. Desbruslais, S. Joiner, K. C. Sidle, I. Gowland, J. Collinge, L. J. Doey, and P. Lantos. 1997. The same prion strain causes vCJD and BSE. Nature 389:448-450, 526. [DOI] [PubMed] [Google Scholar]
- 18.Hope, J., L. Ritchie, C. Farquhar, R. Somerville, and N. Hunter. 1989. Bovine spongiform encephalopathy: a scrapie-like disease of British cattle. Prog. Clin. Biol. Res. 317:659-667. [PubMed] [Google Scholar]
- 19.Houston, F., W. Goldmann, A. Chong, M. Jeffrey, L. Gonzalez, J. Foster, D. Parnham, and N. Hunter. 2003. Prion diseases: BSE in sheep bred for resistance to infection. Nature 423:498. [DOI] [PubMed] [Google Scholar]
- 20.Jeffrey, M., S. Martin, L. Gonzalez, J. Foster, J. P. Langeveld, F. G. van Zijderveld, J. Grassi, and N. Hunter. 2006. Immunohistochemical features of PrP(d) accumulation in natural and experimental goat transmissible spongiform encephalopathies. J. Comp. Pathol. 134:171-181. [DOI] [PubMed] [Google Scholar]
- 21.Nonno, R., M. A. Bari, F. Cardone, G. Vaccari, P. Fazzi, G. Dell'omo, C. Cartoni, L. Ingrosso, A. Boyle, R. Galeno, M. Sbriccoli, H. P. Lipp, M. Bruce, M. Pocchiari, and U. Agrimi. 2006. Efficient transmission and characterization of Creutzfeldt-Jakob disease strains in bank voles. PLoS Pathog. 2:e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Notari, S., S. Capellari, A. Giese, I. Westner, A. Baruzzi, B. Ghetti, P. Gambetti, H. A. Kretzschmar, and P. Parchi. 2004. Effects of different experimental conditions on the PrPSc core generated by protease digestion: implications for strain typing and molecular classification of CJD. J. Biol. Chem. 279:16797-16804. [DOI] [PubMed] [Google Scholar]
- 23.Pan, K. M., M. Baldwin, J. Nguyen, M. Gasset, A. Serban, D. Groth, I. Mehlhorn, Z. Huang, R. J. Fletterick, F. E. Cohen, et al. 1993. Conversion of alpha-helices into beta-sheets features in the formation of the scrapie prion proteins. Proc. Natl. Acad. Sci. USA 90:10962-10966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pattison, I. H. 1965. Scrapie in the Welsh mountain breed of sheep and its experimental transmission to goats. Vet. Rec. 77:1388-1390. [DOI] [PubMed] [Google Scholar]
- 25.Pattison, I. H., and K. M. Jones. 1967. The possible nature of the transmissible agent of scrapie. Vet. Rec. 80:2-9. [DOI] [PubMed] [Google Scholar]
- 26.Peretz, D., R. A. Williamson, G. Legname, Y. Matsunaga, J. Vergara, D. R. Burton, S. J. DeArmond, S. B. Prusiner, and M. R. Scott. 2002. A change in the conformation of prions accompanies the emergence of a new prion strain. Neuron 34:921-932. [DOI] [PubMed] [Google Scholar]
- 27.Peretz, D., R. A. Williamson, Y. Matsunaga, H. Serban, C. Pinilla, R. B. Bastidas, R. Rozenshteyn, T. L. James, R. A. Houghten, F. E. Cohen, S. B. Prusiner, and D. R. Burton. 1997. A conformational transition at the N terminus of the prion protein features in formation of the scrapie isoform. J. Mol. Biol. 273:614-622. [DOI] [PubMed] [Google Scholar]
- 28.Polymenidou, M., K. Stoeck, M. Glatzel, M. Vey, A. Bellon, and A. Aguzzi. 2005. Coexistence of multiple PrP(Sc) types in individuals with Creutzfeldt-Jakob disease. Lancet Neurol. 4:805-814. [DOI] [PubMed] [Google Scholar]
- 29.Prusiner, S. B. 1991. Molecular biology of prion diseases. Science 252:1515-1522. [DOI] [PubMed] [Google Scholar]
- 30.Prusiner, S. B. 1998. Prions. Proc. Natl. Acad. Sci. USA 95:13363-13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prusiner, S. B., M. P. McKinley, K. A. Bowman, D. C. Bolton, P. E. Bendheim, D. F. Groth, and G. G. Glenner. 1983. Scrapie prions aggregate to form amyloid-like birefringent rods. Cell 35:349-358. [DOI] [PubMed] [Google Scholar]
- 32.Prusiner, S. B., M. Scott, D. Foster, K. M. Pan, D. Groth, C. Mirenda, M. Torchia, S. L. Yang, D. Serban, G. A. Carlson, et al. 1990. Transgenetic studies implicate interactions between homologous PrP isoforms in scrapie prion replication. Cell 63:673-686. [DOI] [PubMed] [Google Scholar]
- 33.Riek, R., S. Hornemann, G. Wider, M. Billeter, R. Glockshuber, and K. Wuthrich. 1996. NMR structure of the mouse prion protein domain PrP(121-321). Nature 382:180-182. [DOI] [PubMed] [Google Scholar]
- 34.Robinson, M. M., W. J. Hadlow, D. P. Knowles, T. P. Huff, P. A. Lacy, R. F. Marsh, and J. R. Gorham. 1995. Experimental infection of cattle with the agents of transmissible mink encephalopathy and scrapie. J. Comp. Pathol 113:241-251. [DOI] [PubMed] [Google Scholar]
- 35.Safar, J., P. P. Roller, D. C. Gajdusek, and C. J. Gibbs, Jr. 1993. Conformational transitions, dissociation, and unfolding of scrapie amyloid (prion) protein. J. Biol. Chem. 268:20276-20284. [PubMed] [Google Scholar]
- 36.Scott, M., D. Foster, C. Mirenda, D. Serban, F. Coufal, M. Walchli, M. Torchia, D. Groth, G. Carlson, S. J. DeArmond, et al. 1989. Transgenic mice expressing hamster prion protein produce species-specific scrapie infectivity and amyloid plaques. Cell 59:847-857. [DOI] [PubMed] [Google Scholar]
- 37.Scott, M., D. Groth, D. Foster, M. Torchia, S. L. Yang, S. J. DeArmond, and S. B. Prusiner. 1993. Propagation of prions with artificial properties in transgenic mice expressing chimeric PrP genes. Cell 73:979-988. [DOI] [PubMed] [Google Scholar]
- 38.Scott, M. R., D. Peretz, H.-O. B. Nguyen, S. J. DeArmond, and S. B. Prusiner. 2005. Transmission barriers for bovine, ovine, and human prions in transgenic mice. J. Virol. 79:5259-5271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scott, M. R., R. Will, J. Ironside, H. O. Nguyen, P. Tremblay, S. J. DeArmond, and S. B. Prusiner. 1999. Compelling transgenetic evidence for transmission of bovine spongiform encephalopathy prions to humans. Proc. Natl. Acad. Sci. USA 96:15137-15142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thuring, C. M. A., J. H. F. Erkens, J. G. Jacobs, A. Bossers, L. J. M. Van Keulen, G. J. Garssen, F. G. Van Zijderveld, S. J. Ryder, M. H. Groschup, T. Sweeney, and J. P. Langeveld. 2004. Discrimination between scrapie and bovine spongiform encephalopathy in sheep by molecular size, immunoreactivity, and glycoprofile of prion protein. J. Clin. Microbiol. 42:972-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wadsworth, J. D., A. F. Hill, S. Joiner, G. S. Jackson, A. R. Clarke, and J. Collinge. 1999. Strain-specific prion-protein conformation determined by metal ions. Nat. Cell Biol. 1:55-59. [DOI] [PubMed] [Google Scholar]
- 42.Will, R. G., J. W. Ironside, M. Zeidler, S. N. Cousens, K. Estibeiro, A. Alperovitch, S. Poser, M. Pocchiari, A. Hofman, and P. G. Smith. 1996. A new variant of Creutzfeldt-Jakob disease in the UK. Lancet 347:921-925. [DOI] [PubMed] [Google Scholar]
- 43.Wille, H., M. D. Michelitsch, V. Guenebaut, S. Supattapone, A. Serban, F. E. Cohen, D. A. Agard, and S. B. Prusiner. 2002. Structural studies of the scrapie prion protein by electron crystallography. Proc. Natl. Acad. Sci. USA 99:3563-3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yull, H. M., D. L. Ritchie, J. P. Langeveld, F. G. van Zijderveld, M. E. Bruce, J. W. Ironside, and M. W. Head. 2006. Detection of type 1 prion protein in variant Creutzfeldt-Jakob disease. Am. J. Pathol. 168:151-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zanusso, G., A. Farinazzo, M. Fiorini, M. Gelati, A. Castagna, P. G. Righetti, N. Rizzuto, and S. Monaco. 2001. pH-dependent prion protein conformation in classical Creutzfeldt-Jakob disease. J. Biol. Chem. 276:40377-40380. [DOI] [PubMed] [Google Scholar]