Abstract
The human immunodeficiency virus type 1 (HIV-1) Vpu protein interacts with CD4 within the endoplasmic reticula of infected cells and targets CD4 for degradation through interaction with β-TrCP1. Mammals possess a homologue of β-TrCP1, HOS, which is also named β-TrCP2. We show by coimmunoprecipitation experiments that β-TrCP2 binds Vpu and is able to induce CD4 down-modulation as efficiently as β-TrCP1. In two different cell lines, HeLa CD4+ and Jurkat, Vpu-mediated CD4 down-modulation could not be reversed through the individual silencing of endogenous β-TrCP1 or β-TrCP2 but instead required the two genes to be silenced simultaneously.
CD4 down-modulation is essential for the production of viral infectious particles (7). In human immunodeficiency virus type 1 (HIV-1)-infected cells, CD4 undergoes rapid down-modulation through the independent action of three viral proteins: the gp160 precursor of the viral envelope glycoprotein (Env), Nef, and Vpu (6; reviewed in references 7 and 8). Vpu induces a rapid degradation of CD4 (3, 24) and promotes CD4 ubiquitination and proteasomal degradation by recruiting beta-transducing repeat-containing protein 1 (β-TrCP1) (4, 17). β-TrCP1 is the F-box protein that functions as the substrate recognition subunit of the E3 ubiquitin ligase SCFβ-TrCP (Skp1-Cullin-F-box) complex (20, 23). Mammalian SCFβ-TrCP and Drosophila sp. SCFβ-TrCP have been implicated in the regulation of NF-κB (Dorsal) and Wnt/Wingless (Armadillo) signal transduction pathways by mediating the ubiquitination and degradation of NF-κB inhibitor IκB and transcriptional coactivator β-catenin, respectively (9, 12, 13, 16, 22, 25, 26; reviewed in reference 10). β-TrCP1 is characterized by an N-terminal F-box domain (residues 139 to 186), which allows the interaction with the other components of the complex via Skp-1, and a C-terminal WD40 repeat domain (residues 253 to 545) that binds the substrate. The recognition of target proteins occurs through a phosphorylation-dependent destruction motif, DSPGΦXSP (where Φ represents a hydrophobic and X any amino acid), that is present in both IκBα and β-catenin. This motif is also present in HIV-1-encoded Vpu, an 81-amino-acid protein which is constitutively phosphorylated by casein kinase II at serine 52 and serine 56 (21). Vpu phosphorylation is necessary for the recruitment of β-TrCP1 and CD4 degradation but not for CD4 binding (3). In contrast to cellular substrates such as IκBα and β-catenin, Vpu acts as an adapter protein for targeting CD4 degradation. In infected cells, the constitutive phosphorylation of Vpu leads to a competition with the natural substrates of SCFβ-TrCP1 and a lower nuclear translocation of NF-κB upon tumor necrosis factor treatment (2). Human cells possess a homologue of BTRC (encoding β-TrCP1), FBXW11 (HUGO gene nomenclature; also named HOS or BTRCP2) (5, 9, 14), that encodes β-TrCP2. A redundant role for mammalian β-TrCP1 and β-TrCP2 in ubiquitination and degradation of IκBα and β-catenin was proposed on the basis of RNA interference analyses and data from mice with genetic ablation of BTRC (11, 15, 19). In this study, we determined whether β-TrCP2 shares with its homologue structural and functional properties that would allow it to bind Vpu and modulate CD4 expression and, thus, participate in HIV-1 pathogenesis.
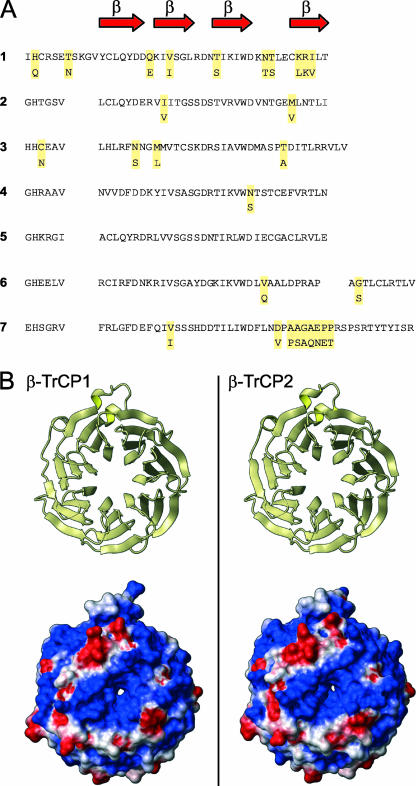
Conservation in the WD40 domains of β-TrCP1 and β-TrCP2.
The homologues share 75% amino acid sequence similarity (Fig. 1A). We built a model of β-TrCP2 by homology modeling based on the known structure of β-TrCP1 (Protein Data Bank code 1P22; http://www.rcsb.org/). The two homologues show striking structural similarities in their ligand-binding domains (Fig. 1B). Moreover, they show very similar electrostatic surface properties, with a conservation of the central groove covered by positively charged amino acids that could interact with the phosphorylated residues in the destruction motif of the target proteins. Both homologues are expressed in primary CD4+ T cells (data not shown).
FIG. 1.
Sequence conservation of the WD40 domain of β-TrCP1 and β-TrCP2. (A) Amino acid sequence of the WD40 domain of β-TrCP1 (residues 260 to 569; upper line of each set) and β-TrCP2 (residues 233 to 542; lower line). Red arrows represent beta sheets. For β-TrCP2, only the amino acids that differ from β-TrCP1 are shown. (B) Left, ribbon representation of the β-TrCP1 X-ray structure. Upper panel, top view of the ligand binding domain. Lower panel, solvent-accessible surface of β-TrCP1, with the color indicating the value of the electrostatic potential (red for negative regions, blue for positive, and white for neutral). Right, ribbon representation and solvent-accessible surface of the β-TrCP2 model in the same orientation.
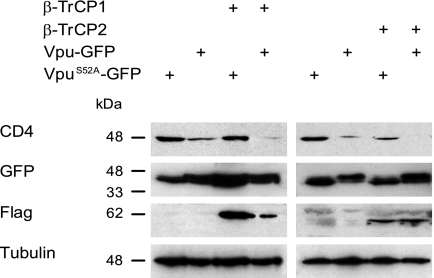
Coexpression of Vpu with β-TrCP1 or β-TrCP2 induces a decrease in total cellular CD4 content.
To construct the Vpu-green fluorescent protein (GFP) expression vector, the Vpu sequence from HIV-1 strain NL4.3 was amplified by PCR and subcloned into pEGFPN3 (Clontech). The mutation of serine 52 to alanine was performed by PCR to create the phosphorylation mutant VpuS52A-GFP. We tested whether the coexpression of the Vpu-GFP hybrid molecule with β-TrCP1 or β-TrCP2 modulated CD4 levels in HeLa CD4+ cells. We selected GFP+ cells by fluorescence-activated cell sorting (FACS) prior to cell lysis and analysis by immunoblotting. Vpu-GFP decreased total CD4 levels in the absence of any other viral proteins, and its coexpression with either β-TrCP1 or β-TrCP2 resulted in a further decrease in CD4 levels (Fig. 2). The expression of β-TrCP1 or β-TrCP2 alone had no effect on CD4 levels (data not shown).
FIG. 2.
Coexpression of Vpu and β-TrCP1 or β-TrCP2 in HeLa CD4+ cells induces a decrease in total cellular CD4 content. HeLa CD4+ cells were transfected with the indicated combinations of Vpu-GFP, VpuS52A-GFP, β-TrCP1, and β-TrCP2. GFP+ cells were isolated 24 h after transfection by FACS and lysed. The proteins were separated on sodium dodecyl sulfate-polyacrylamide gels and analyzed by Western blotting using antibodies directed against CD4, GFP, Flag, and tubulin.
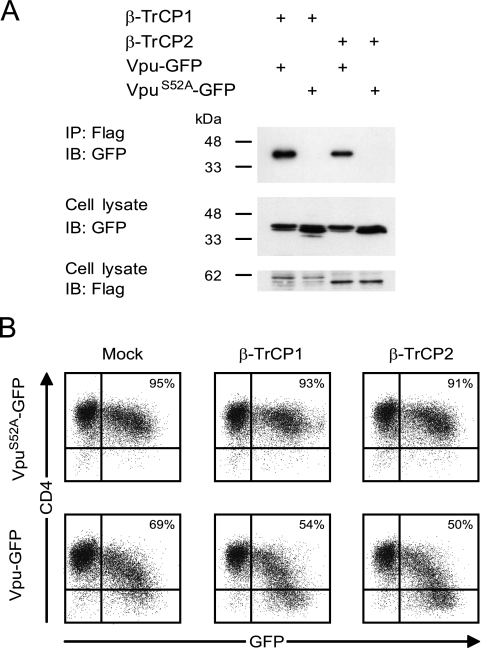
β-TrCP2 interacts with Vpu.
The coexpression of Vpu-GFP with β-TrCP2, followed by immunoprecipitation and immunoblotting analysis, revealed that β-TrCP2 associates with Vpu (Fig. 3A). In order to evaluate the extent of CD4 down-modulation induced by Vpu-GFP, alone or in combination with β-TrCP1 or β-TrCP2, we used FACS to measure surface CD4 levels on GFP+ cells. Kinetics experiments indicated that the maximal down-modulation induced by Vpu was reached 24 h posttransfection and lasted until 48 h. For this reason, and since Vpu was shown to contribute to apoptosis when expressed at longer times (1), we measured cell surface CD4 expression at 24 h posttransfection (Fig. 3B). Quantification identified a 26% decrease in the number of CD4+ cells and a 50% decrease in the mean fluorescence intensity (MFI) for cells expressing Vpu-GFP compared to cells expressing the phosphorylation mutant VpuS52A-GFP. The coexpression of Vpu-GFP with exogenous β-TrCP1 or β-TrCP2 resulted in a 39% or 41% decrease in the number of CD4+ cells and a 65% or 63% decrease in MFI, respectively. The expression of β-TrCP1 or β-TrCP2 alone had no effect on cell surface CD4 levels. These data suggest that the endogenous levels of β-TrCPs are almost sufficient to mediate CD4 down-modulation and that the addition of exogenous β-TrCPs has a minor but reproducible effect. The reduction of CD4 surface expression in this experimental approach is consistent with previous estimates of Vpu-dependent CD4 down-modulation (6).
FIG. 3.
β-TrCP2 interacts with Vpu in HeLa CD4+ cells and enhances the down-modulation of CD4 mediated by Vpu in a manner similar to that of β-TrCP1. (A) HeLa CD4+ cells were transfected with the indicated combinations of Vpu-GFP, VpuS52A-GFP, β-TrCP1, and β-TrCP2 (2 μg for each plasmid). The cells were lysed 24 h after transfection, and Flag-tagged proteins were immunoprecipitated using M2-conjugated agarose beads. The proteins were separated on 12% sodium dodecyl sulfate-polyacrylamide gels, transferred to membranes, and detected using anti-GFP and anti-Flag antibodies. IB, immunoblotting; IP, immunoprecipitation. (B) HeLa CD4+ cells were cotransfected with either VpuS52A-GFP (upper panels) or Vpu-GFP (lower panels) and an empty control vector (Mock), β-TrCP1, or β-TrCP2. Twenty-four hours posttransfection, CD4 levels on GFP+ cell surfaces were measured by flow cytometry.
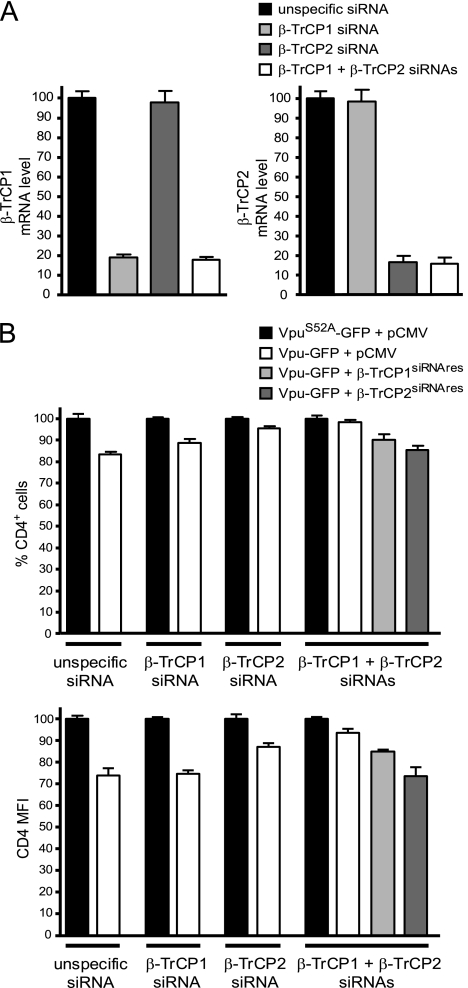
Vpu-mediated CD4 down-modulation is inhibited by double silencing of β-TrCP1 and β-TrCP2 and is restored following reexpression of silencing-resistant β-TrCP1 or β-TrCP2.
Sequence-specific inhibition of endogenous β-TrCP1 and β-TrCP2 was performed using small interfering RNA (siRNA) (data not shown). Reverse transcription-PCR showed specific mRNA silencing of at least 80% with each target, and inhibition of the expression of both homologues was obtained with a combination of siRNAs directed against β-TrCP1 and β-TrCP2 (Fig. 4A). Silencing was effective at least up to 72 h after siRNA transfection (data not shown). mRNA silencing should result in rapid declines of β-TrCP1 and β-TrCP2 levels due to the short half-lives of the two proteins (less than 3 h in HeLa cells [data not shown]).
FIG. 4.
Silencing of β-TrCP1 and β-TrCP2 reverses Vpu-mediated CD4 down-modulation. (A) Jurkat cells were transfected with siRNAs targeting β-TrCP1 or β-TrCP2, a combination of both, or an unspecific siRNA. β-TrCP1 and β-TrCP2 mRNA levels were measured by quantitative reverse transcription-PCR 24 h later. Percentages are related to 100% β-TrCP1 or β-TrCP2 mRNA in cells transfected by the irrelevant siRNA. (B) Jurkat cells were transfected with siRNA targeting β-TrCP1 or β-TrCP2, a combination of both, or an unspecific siRNA. The cells were transfected 24 h later with the indicated combinations of vectors. The surface CD4 level on GFP+ cells was measured 24 h later by flow cytometry. The results were expressed as the percentage of CD4+ cells (upper panel) and as the MFI (lower panel) relative to those for the VpuS52A-GFP plus pCMV control sample. Shown is a representative experiment from three independent analyses.
We determined whether the CD4 down-modulation mediated by Vpu was still effective in cells in which the endogenous β-TrCPs had been previously silenced. For this experiment, we transfected a T-cell line, Jurkat (expressing endogenous levels of CD4), with either unspecific siRNA or siRNA targeting β-TrCP1 or β-TrCP2. Twenty-four hours later, the cells were transfected with Vpu-GFP or VpuS52A-GFP. The expression of Vpu-GFP induced an 18% decrease in the number of CD4+ cells and a 27% decrease in MFI (Fig. 4B). Similarly, the expression of Vpu-GFP in HeLa CD4+ cells (which express CD4 exogenously) induced a 15% decrease in the number of CD4+ cells and a 31% decrease in MFI. The small amount of CD4 down-modulation compared to the previous experiment could be explained by a different experimental procedure, i.e., the cells were transfected with synthetic siRNAs prior to the transfection with Vpu. Vpu had a minimal effect on CD4 levels in cells in which both β-TrCP1 and β-TrCP2 had been silenced. Reexpression of siRNA-resistant β-TrCP2 and β-TrCP1 restored Vpu-mediated CD4 down-modulation.
Silencing of β-TrCP2 inhibits Vpu-induced CD4 down-modulation in the context of HIV-1 infection.
We used a single-round HIV-1 replication assay by infecting GHOST cells, which express human CD4 stably (18), with HIV ΔenvΔnef pseudotyped with vesicular stomatitis virus. In the absence of nef, the CD4 down-modulation level induced by HIV-1 is comparable to that obtained with cells transfected with Vpu-GFP. CD4 down-modulation was minimal upon effective silencing of β-TrCP1 and β-TrCP2 (data not shown).
In summary, β-TrCP1 and β-TrCP2 have a common specificity for the Vpu pseudosubstrate, and they are detected in CD4+ T cells, indicating that both homologues may play an additive role in HIV-1 pathogenesis.
Acknowledgments
This study was supported by the Swiss National Science Foundation (grant 310000-110012/1 to A.T.) and by the Leenaards Foundation.
We thank R. Benarous and Y. Ben-Neriah for providing expression vectors for β-TrCP, E. Buetti for critical readings of the manuscript, and V. Piguet and A. Trkola for commentaries.
Footnotes
Published ahead of print on 22 November 2006.
REFERENCES
- 1.Akari, H., S. Bour, S. Kao, A. Adachi, and K. Strebel. 2001. The human immunodeficiency virus type 1 accessory protein Vpu induces apoptosis by suppressing the nuclear factor kappaB-dependent expression of antiapoptotic factors. J. Exp. Med. 194:1299-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bour, S., C. Perrin, H. Akari, and K. Strebel. 2001. The human immunodeficiency virus type 1 Vpu protein inhibits NF-κB activation by interfering with βTrCP-mediated degradation of IκB. J. Biol. Chem. 276:15920-15928. [DOI] [PubMed] [Google Scholar]
- 3.Bour, S., U. Schubert, and K. Strebel. 1995. The human immunodeficiency virus type 1 Vpu protein specifically binds to the cytoplasmic domain of CD4: implications for the mechanism of degradation. J. Virol. 69:1510-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bour, S., and K. Strebel. 2003. The HIV-1 Vpu protein: a multifunctional enhancer of viral particle release. Microbes Infect. 5:1029-1039. [DOI] [PubMed] [Google Scholar]
- 5.Cenciarelli, C., D. S. Chiaur, D. Guardavaccaro, W. Parks, M. Vidal, and M. Pagano. 1999. Identification of a family of human F-box proteins. Curr. Biol. 9:1177-1179. [DOI] [PubMed] [Google Scholar]
- 6.Chen, B. K., R. T. Gandhi, and D. Baltimore. 1996. CD4 down-modulation during infection of human T cells with human immunodeficiency virus type 1 involves independent activities of vpu, env, and nef. J. Virol. 70:6044-6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doms, R. W., and D. Trono. 2000. The plasma membrane as a combat zone in the HIV battlefield. Genes Dev. 14:2677-2688. [DOI] [PubMed] [Google Scholar]
- 8.Emerman, M., and M. H. Malim. 1998. HIV-1 regulatory/accessory genes: keys to unraveling viral and host cell biology. Science 280:1880-1884. [DOI] [PubMed] [Google Scholar]
- 9.Fuchs, S. Y., A. Chen, Y. Xiong, Z. Q. Pan, and Z. Ronai. 1999. HOS, a human homolog of Slimb, forms an SCF complex with Skp1 and Cullin1 and targets the phosphorylation-dependent degradation of IkappaB and beta-catenin. Oncogene 18:2039-2046. [DOI] [PubMed] [Google Scholar]
- 10.Fuchs, S. Y., V. S. Spiegelman, and K. G. Kumar. 2004. The many faces of beta-TrCP E3 ubiquitin ligases: reflections in the magic mirror of cancer. Oncogene 23:2028-2036. [DOI] [PubMed] [Google Scholar]
- 11.Guardavaccaro, D., Y. Kudo, J. Boulaire, M. Barchi, L. Busino, M. Donzelli, F. Margottin-Goguet, P. K. Jackson, L. Yamasaki, and M. Pagano. 2003. Control of meiotic and mitotic progression by the F box protein beta-Trcp1 in vivo. Dev. Cell 4:799-812. [DOI] [PubMed] [Google Scholar]
- 12.Hart, M., J. P. Concordet, I. Lassot, I. Albert, R. del los Santos, H. Durand, C. Perret, B. Rubinfeld, F. Margottin, R. Benarous, and P. Polakis. 1999. The F-box protein beta-TrCP associates with phosphorylated beta-catenin and regulates its activity in the cell. Curr. Biol. 9:207-210. [DOI] [PubMed] [Google Scholar]
- 13.Kitagawa, M., S. Hatakeyama, M. Shirane, M. Matsumoto, N. Ishida, K. Hattori, I. Nakamichi, A. Kikuchi, and K. Nakayama. 1999. An F-box protein, FWD1, mediates ubiquitin-dependent proteolysis of beta-catenin. EMBO J. 18:2401-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koike, J., N. Sagara, H. Kirikoshi, A. Takagi, T. Miwa, M. Hirai, and M. Katoh. 2000. Molecular cloning and genomic structure of the betaTRCP2 gene on chromosome 5q35.1. Biochem. Biophys. Res. Commun. 269:103-109. [DOI] [PubMed] [Google Scholar]
- 15.Lang, V., J. Janzen, G. Z. Fischer, Y. Soneji, S. Beinke, A. Salmeron, H. Allen, R. T. Hay, Y. Ben-Neriah, and S. C. Ley. 2003. βTrCP-mediated proteolysis of NF-κB1 p105 requires phosphorylation of p105 serines 927 and 932. Mol. Cell. Biol. 23:402-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Latres, E., D. S. Chiaur, and M. Pagano. 1999. The human F box protein beta-Trcp associates with the Cul1/Skp1 complex and regulates the stability of beta-catenin. Oncogene 18:849-854. [DOI] [PubMed] [Google Scholar]
- 17.Margottin, F., S. P. Bour, H. Durand, L. Selig, S. Benichou, V. Richard, D. Thomas, K. Strebel, and R. Benarous. 1998. A novel human WD protein, h-beta TrCp, that interacts with HIV-1 Vpu connects CD4 to the ER degradation pathway through an F-box motif. Mol. Cell 1:565-574. [DOI] [PubMed] [Google Scholar]
- 18.Mörner, A., A. Björndal, J. Albert, V. N. KewalRamani, D. R. Littman, R. Inoue, R. Thorstensson, E. M. Fenyö, and E. Björling. 1999. Primary human immunodeficiency virus type 2 (HIV-2) isolates, like HIV-1 isolates, frequently use CCR5 but show promiscuity in coreceptor usage. J. Virol. 73:2343-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakayama, K., S. Hatakeyama, S. Maruyama, A. Kikuchi, K. Onoe, R. A. Good, and K. I. Nakayama. 2003. Impaired degradation of inhibitory subunit of NF-kappa B (I kappa B) and beta-catenin as a result of targeted disruption of the beta-TrCP1 gene. Proc. Natl. Acad. Sci. USA 100:8752-8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petroski, M. D., and R. J. Deshaies. 2005. Function and regulation of cullin-RING ubiquitin ligases. Nat. Rev. Mol. Cell Biol. 6:9-20. [DOI] [PubMed] [Google Scholar]
- 21.Schubert, U., and K. Strebel. 1994. Differential activities of the human immunodeficiency virus type 1-encoded Vpu protein are regulated by phosphorylation and occur in different cellular compartments. J. Virol. 68:2260-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spencer, E., J. Jiang, and Z. J. Chen. 1999. Signal-induced ubiquitination of IkappaBalpha by the F-box protein Slimb/beta-TrCP. Genes Dev. 13:284-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Willems, A. R., M. Schwab, and M. Tyers. 2004. A hitchhiker's guide to the cullin ubiquitin ligases: SCF and its kin. Biochim. Biophys. Acta 1695:133-170. [DOI] [PubMed] [Google Scholar]
- 24.Willey, R. L., F. Maldarelli, M. A. Martin, and K. Strebel. 1992. Human immunodeficiency virus type 1 Vpu protein induces rapid degradation of CD4. J. Virol. 66:7193-7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Winston, J. T., P. Strack, P. Beer-Romero, C. Y. Chu, S. J. Elledge, and J. W. Harper. 1999. The SCFbeta-TRCP-ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IkappaBalpha and beta-catenin and stimulates IkappaBalpha ubiquitination in vitro. Genes Dev. 13:270-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yaron, A., A. Hatzubai, M. Davis, I. Lavon, S. Amit, A. M. Manning, J. S. Andersen, M. Mann, F. Mercurio, and Y. Ben-Neriah. 1998. Identification of the receptor component of the IkappaBalpha-ubiquitin ligase. Nature 396:590-594. [DOI] [PubMed] [Google Scholar]