Abstract
Myxoma virus is a rabbit-specific poxvirus pathogen that also exhibits a unique tropism for human tumor cells and is dramatically oncolytic for human cancer xenografts. Most tumor cell lines tested are permissive for myxoma infection in a fashion intimately tied to the activation state of Akt kinase. A host range factor of myxoma virus, M-T5, directly interacts with Akt and mediates myxoma virus tumor cell tropism. mTOR is a regulator of cell growth and metabolism downstream of Akt and is specifically inhibited by rapamycin. We report that treatment of nonpermissive human tumor cell lines, which normally restrict myxoma virus replication, with rapamycin dramatically increased virus tropism and spread in vitro. This increased myxoma replication is concomitant with global effects on mTOR signaling, specifically, an increase in Akt kinase. In contrast to the effects on human cancer cells, rapamycin does not increase myxoma virus replication in rabbit cell lines or permissive human tumor cell lines with constitutively active Akt. This indicates that rapamycin increases the oncolytic capacity of myxoma virus for human cancer cells by reconfiguring the internal cell signaling environment to one that is optimal for productive virus replication and suggests the possibility of a potentially therapeutic synergism between kinase signaling inhibitors and oncolytic poxviruses for cancer treatment.
The concept of oncolytic virotherapy, or using viruses to specifically infect and kill cancerous cells, is not new. Yet, recent advances in the genetic modification of many families of viruses, and the ability to more precisely deconstruct virus-host interactions, has brought this new potential therapeutic use of viruses back to the forefront of cancer research. An optimal oncolytic virus candidate would be one that has a natural and selective tropism for the cancerous tissue and would either specifically infect and destroy the tissue or effectively direct an antitumoral immune response, without causing damage to normal tissue (4, 23, 36, 37, 39, 43). To design fully selective viruses with little or no potential side effects in human patients, many current oncolytic candidates are either no longer replication competent or have been attenuated to the point of compromising their dissemination through complex tumor tissue.
Poxviruses have had a long history of therapeutic use in humans, mostly through the successful use of vaccinia virus (VACV) as a vaccine in the smallpox eradication program. Tumor-specific antigens, as well as immune stimulators such as cytokines or costimulatory molecules, have been inserted into the VACV genome and studied for their efficacy as tumor vaccines (11, 24, 33, 56). VACV also exhibits a natural proclivity for infecting rapidly multiplying tumor cells and has been explored for its oncolytic capacity (12, 29, 50, 57, 61).
Myxoma virus (MV) is a new poxvirus oncolytic candidate that has little history of direct infection in humans due to its restrictive tropism to lagomorphs (30). MV is a member of the Poxviridae family that causes a benign subclinical infection in its evolutionary host, rabbits of the Sylvilagus genus, but induces a fatal disease known as myxomatosis in the European rabbit, Oryctalagus cuniculus (19). It is nonpathogenic in all other vertebrate species tested, including humans (2, 10, 17, 28, 30). Studies examining this narrow rabbit-specific tropism have concluded that in primary murine cells, this defect in MV replication is a consequence of induced interferon responses (59). This observation then led to the discovery that MV could productively infect and kill over 70% of human tumor cell lines tested (55). The oncolytic potential of MV has also been demonstrated in an in vivo murine xenograft model of human glioblastoma (25).
The ability of MV to propagate in human tumor cells garnered interest in the viral and cellular host range factors that could be responsible for this unique cross-species tropism. To date, only the MV host range gene product M-T5 has been shown to regulate the ability of MV to optimally propagate in human tumor cells (55). M-T5 is an ankyrin repeat protein that is required for MV replication in rabbit T cells in vitro as well as for virus pathogenesis in European rabbits (35). It interacts with two distinct host proteins. One of these is Cullin-1, an E3 ubiquitin ligase that is involved in the progression through the cell cycle (18). The interaction of M-T5 and Cullin-1 protects MV-infected cells from cell cycle arrest and stress-induced cell death (18). The second cellular protein that directly interacts with M-T5 is Akt-1/protein kinase B (PKB) (60). Akt is a serine/threonine kinase that plays a critical role in controlling the balance between cell survival, proliferation, and cell death. Akt dysregulation is also commonly observed in a wide spectrum of human cancers (1, 3, 6, 7, 9, 13, 16, 20, 22, 31, 38, 44, 51, 53, 54). Many human tumor cell lines exhibit a high endogenous activation status of Akt (9, 13, 16, 54). MV appears to be uniquely suited to take advantage of this endogenous Akt activation. Human tumor cell lines that exhibit a high endogenous level of Akt activation (designated type I) are uniformly permissive to MV (60). However, cell lines with low levels of endogenous Akt kinase activity (called type II) are induced to activate Akt in an M-T5-dependent fashion. Finally, cell lines with little endogenous Akt activity that cannot be induced by viral infection (called type III cells) are nonpermissive to myxoma virus infection (60). These results prompted us to screen for drugs that affect Akt signaling pathways and thereby might alter MV tropism of human cancer cells.
The mammalian target of rapamycin, or mTOR, plays a central role in Akt-mediated cell proliferation, growth, differentiation, maturation, and survival. mTOR is a 289-kDa serine/threonine kinase that is highly conserved from yeast to humans and directly or indirectly regulates translation initiation, actin organization, membrane traffic, protein degradation, protein kinase C signaling, ribosome biogenesis, tRNA synthesis, and transcription (47). In addition, mTOR is specifically inhibited by rapamycin (sirolimus), a macrocyclic lactone isolated from the soil bacterium Streptomyces hygroscopicus (48). Rapamycin is a structural homologue of the antibiotic FK506 (tacrolimus) and, like this molecule, must bind the cellular immunophilin FK506 binding protein (FKBP12) prior to binding to its target. Rapamycin (Rapamune; Wyeth-Ayerst) is currently licensed as an immunosuppressant, based on its cytostatic effects on activated T cells, and is used as an alternative to cyclosporine treatment in transplant patients (34). In addition, studies have indicated that rapamycin has an antineoplastic effect on many types of cancer cells (51).
The effect of antineoplastic-signaling drugs on poxvirus infection has recently been explored using imatinib (Gleevec), a src/abl inhibitor that has had success in treating chronic myelogenous leukemia. This drug inhibited poxvirus (VACV) egress from infected cells in a manner similar to its ability to inhibit the invasion of tumor cells (46). Interestingly, cancer cells which feature overexpression/activation of Akt have been shown to be particularly resistant to the action of imatinib (5). In contrast, we report here that rapamycin has the ability to increase the oncolytic potential of MV and to increase Akt activity in the context of virus infection. The restored permissivity of MV M-T5 knockout virus (vMyxT5KO) in type II cells is concomitant with an increased Akt phosphorylation, as well as aberrant increases in the phosphorylation of downstream pathways. These findings help elucidate the exact mechanism by which MV, with a narrow natural tropism for rabbits, is able to circumvent the mutated intracellular signaling machinery of human tumor cells. In addition, findings indicate that rapamycin, a well-known anticancer and immunosuppressant drug, not only will increase the antitumor effectiveness of MV through the suppression of the antiviral immune response but also can increase its tumor cell tropism in vivo, expanding the potential of MV for oncolytic virotherapy.
MATERIALS AND METHODS
Cell culture, reagents, and recombinant viruses.
All cell lines were grown in Dulbecco's modified Eagle medium (DMEM; Invitrogen). Baby green monkey kidney (BGMK) cells were grown in DMEM supplemented with 10% newborn calf serum, while human tumor cell lines were grown in 10% fetal bovine serum. All cell lines were grown in medium containing 100 units/ml penicillin, 100 mg/ml streptomycin at 37°C in 5% CO2. The human tumor cells used in this study were from the NCI-60 reference collection and include cell lines 786-0 (renal cancer), HCT 116 (colon cancer), ACHN (renal cancer), HOS (osteosarcoma), PC3 (prostate cancer), MCF-7 (breast cancer), MDA-MB-435 (breast cancer), SK-MEL5 (melanoma), T47D (breast cancer), DU145 (prostate cancer), M14 (melanoma), and COLO-205 (colon cancer). In addition, the rabbit cell lines RK13 (rabbit fibroblast) and RL5 (rabbit lymphocyte cell line) were also used.
Cells were treated in vitro with rapamycin (Calbiochem) diluted in dimethyl sulfoxide (DMSO; Sigma). The parental myxoma virus used in this study, vMyxlac, is a version of myxoma virus (strain Lausanne; ATCC) containing the Escherichia coli lacZ gene inserted at an innocuous site between open reading frames M010L and M011L (41). The recombinant vMyxT5KO virus had both copies of M-T5 replaced by lacZ (35).
Viral growth curves.
The effect of rapamycin on viral growth and spread within a monolayer was analyzed. At 6 h prior to infection, cellular monolayers (at 85 to 95% confluence) were treated with 20 nM rapamycin or an appropriate dilution of DMSO. Then, vMyxlac or vMyxT5KO was added at a multiplicity of infection (MOI) of 0.1. The inoculum was allowed to adsorb for 1 h at 28°C. The virus was then removed and the well washed three times with DMEM. Supplemented DMEM containing additional rapamycin was added to the cells, which were then incubated at 37°C. Adherent cells were collected by scraping following infection at time points 12, 24, 36, 48, 72, and 96 h after infection. Cells that had detached were not collected. Following a 5-min spin at 1,500 rpm, the cells were resuspended in 500 μl of phosphate-buffered saline (PBS). To release virus from infected cells, each tube containing virus was frozen at −80°C and subsequently thawed at 37°C, and this freeze-thaw cycle was repeated twice more. The lysed cells were sonicated in a cup sonicator three times in 20-second cycles to disaggregate virus complexes.
Infectious virus at each time point was titrated on BGMK cells. Virus was diluted 1:10 in DMEM, and further serial dilutions were performed for each time point of each growth curve. The appropriately diluted virus was added to BGMK cells and allowed to adsorb for 1 h, the virus was removed, and DMEM was supplemented with serum added to each well. The inocula were allowed to proceed for 48 h, at which point the cells were fixed using neutral buffered formalin (10% formaldehyde in PBS) for 5 min and stained with X-Gal (100 mg/ml X-Gal [5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside], 500 mM Kferricyanide, 500 mM Kferrocyanide, 100 nM MgCl2 in PBS) for 4 to 8 h at room temperature. Blue foci, indicating virus replication and spread, were counted, and virus production per 105 cells was determined. Titration of each time point was done in triplicate, and the results were graphed using SlideWrite program software, with corresponding error bars.
Western blotting analysis.
Western blotting analysis was used to assess the effect of rapamycin treatment on cell signaling molecules within human tumor cells. Each cell line was treated with 20 nM rapamycin or a corresponding dilution of DMSO for 6 h prior to infection with vMyxlac or vMyxT5KO virus at an MOI of 3. Cells were collected 16 h after infection and lysed with appropriate lysis buffer containing protease inhibitors. The protein concentrations were determined by the Bradford assay, using a spectrophotometer (model DU640; Beckman). Equal amounts of protein were loaded into each lane and run on 8, 10, or 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) to resolve the desired proteins. The separated proteins were transferred onto nitrocellulose membrane (Amersham Biosciences) by either a semidry or a wet transfer apparatus (Bio-Rad) and probed with the corresponding antibodies according to indicated conditions. The proteins were visualized by horseradish-peroxidase second antibody using an enhanced-chemiluminescence detection system (Perkin-Elmer). Densitometric levels were detected by Molecular Imaging software (Kodak) and compared to the level of either Akt or β-actin. Variability between films was normalized.
Cell cycle analysis.
Cells were harvested by trypsinization, washed twice with PBS, and pelleted by centrifugation at 500 × g for 5 min. Washed cells were suspended in 1 ml cold absolute methanol added dropwise by vortexing to prevent cell clumping and fixed by incubation for a minimum of 6 h at 4°C. Fixed cells were pelleted by centrifugation, washed twice with PBS, and incubated at room temperature for 30 min in 0.5 ml of PBS containing 50 μg/ml of propidium iodide (Sigma) and 50 μg/ml RNase A (Sigma). Debris and doublets were eliminated from the analysis using pulse width/area discrimination. Following flow cytometry on a FACScalibur, data analysis was performed using ModFitLT software (Verity Software, Topsham, ME).
Superarray analysis.
Cells were harvested by trypsinization, and RNA was isolated using a GenElute Mammalian Total RNA miniprep kit (Sigma). Isolated RNA was used in an oligo GEArray human phosphoinositide 3-kinase (PI3K)-Akt signaling pathway microarray (SuperArray Bioscience Corporation, Frederick, MD) according to the manufacturer's instructions.
Statistical analysis.
A two-tailed t test was performed using InStat (GraphPad Software, San Diego, CA).
RESULTS
Rapamycin treatment rescues MV replication and spread in type II cancer cells.
We have shown that Akt kinase activity is a critical determinant of MV permissiveness in human cancer cells (60). In an effort to examine signaling pathways downstream of Akt, we tested the effect of rapamycin, an inhibitor of mTOR (mammalian target of rapamycin), an atypical serine/threonine kinase that is critical for cell growth and metabolism. To determine the effect of this drug on myxoma virus permissiveness, we performed multistep growth curves with all three designated types of cancer cells in the presence or absence of pretreatment with rapamycin. We consistently used a low dose (20 nM) of rapamycin in all experiments. Additional experiments with higher doses (100 nM and above) of rapamycin did not affect our findings, yet there are potential off-target effects of using an excess of drug in long-term cell cultures.
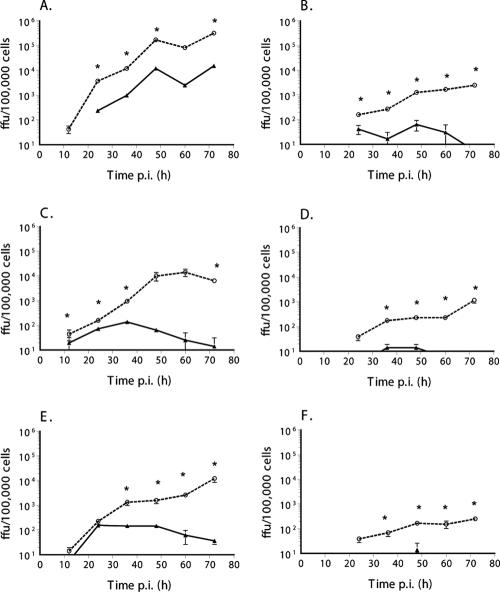
Rapamycin pretreatment resulted in a significant enhancement of MV replication and spread in type II human cancer cells (Fig. 1). In all three cell lines tested (786-0, HCT116, and ACHN), pretreatment with rapamycin significantly increased the replication and spread of vMyxlac. In addition, rapamycin pretreatment also rescued the ability of vMyxT5KO to replicate in cell lines where productive virus replication was not supported in the absence of the drug. Pretreatment with rapamycin was required to see this increase in viral replication, as simultaneous infection and rapamycin treatment did not result in significant increases in MV titer (data not shown). This indicates that, in addition to the ability of this drug to increase virus replication in cancer cells, it is also able to compensate for the essential role of M-T5 in the virus replication in these type II cells. At later time points, the effect of the drug becomes more pronounced. It is our experience that in cancer cell lines, MV replication is closely tied to viability, and the cytostatic effect of rapamycin also maintained the viability of the cells after many days in culture.
FIG. 1.
Rapamycin treatment rescues myxoma virus replication and spread in type II (restrictive) tumor cell lines. The ability of myxoma virus to replicate and spread following inoculation at a low MOI was determined by multistep growth curve using restrictive cancer cell lines 786-0 (renal cancer) (A and B), HCT116 (colon cancer) (C and D) and ACHN (renal cancer) (E and F). Wild-type virus vMyxlac (A, C, and E) and the M-T5 knockout virus vMyxT5KO (B, D, and F) were used to investigate the abilities of both viruses to infect and spread throughout the monolayer, and virus titer was assessed by focus formation. Cells were pretreated for 6 h before infection with 20 nM rapamycin (dashed line) or an appropriate vehicle control (1:5,000 dilution of DMSO, solid line). *, P, <0.005 by two-tailed t test.
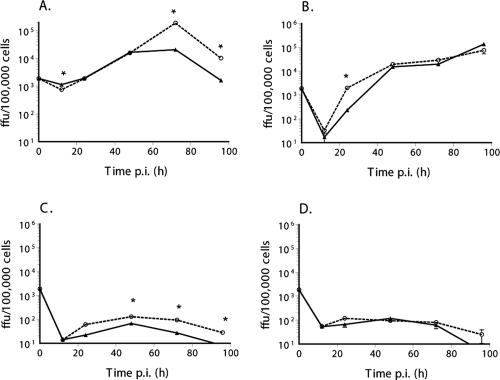
As seen in Fig. 2, rapamycin did not enhance the replication or spread of either vMyxlac or vMyxT5KO in BGMK cells (the control primate cell line) or the type I cancer cell lines (HOS and PC3) that constitutively express highly activated Akt. Similarly, type III tumor cell lines, which are nonpermissive to MV, were not rescued by rapamycin pretreatment (Fig. 3). However, the MCF-7 breast cancer cell line showed a significant increase in virus titer at later time points (Fig. 3A), and there was a modest effect on the MV titer in the COLO-205 cancer cell line (Fig. 3B), but there was little effect in either the M14 (Fig. 3C) or the MDA-MB-435 (Fig. 3D) cell line.
FIG. 2.
Rapamycin treatment does not enhance myxoma virus replication in type I (permissive) tumor cell lines. The ability of myxoma virus to replicate and spread following inoculation at a low MOI was determined by multistep growth curve, using BGMK cells (control primate cell line) (A and B) and permissive cancer cell lines HOS (osteosarcoma) (C and D) and PC3 (prostate cancer) (E and F). Wild-type virus vMyxlac (A, C, and E) and the M-T5 knockout virus vMyxT5KO (B, D, and F) were used to investigate the abilities of both viruses to infect and spread throughout the monolayer, and virus titer was assessed by focus formation. Cells were pretreated for 6 h before infection with 20 nM rapamycin (dashed line) or an appropriate vehicle control (1:5,000 dilution of DMSO, solid line). *, P, <0.005 in a two-tailed t test.
FIG. 3.
The effect of rapamycin treatment on myxoma virus replication in type III (abortive) tumor cell lines was not sufficient to rescue replication and spread. The ability of myxoma virus to replicate and spread following inoculation at a low MOI was determined by growth curves, using abortive cancer cell lines MCF-7 (breast cancer) (A), M14 (melanoma) (B), COLO-205 (colon cancer) (C), and MDA-MB-435 (breast cancer) (D). vMyxlac was used to investigate the ability of MV to infect and spread throughout the monolayer, and virus titer was assessed by focus formation. Cells were pretreated for 1 to 6 h before infection with 20 nM rapamycin (dashed line) or an appropriate vehicle control (1:5,000 dilution of DMSO, solid line). *, P, <0.005 in a two-tailed t test.
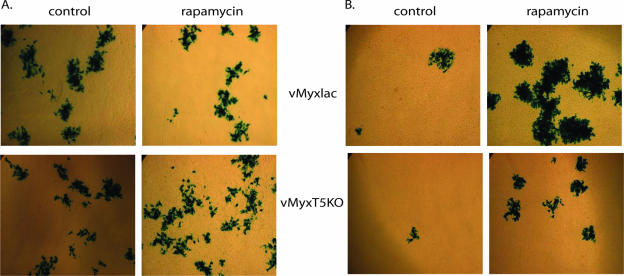
There is also a qualitative difference in the virus cell-to-cell spread in type II cells treated with rapamycin. As seen in Fig. 4, wild-type and M-T5 knockout MV focus size and number were unaffected by rapamycin treatment of BGMK cells (Fig. 4A), but the foci formed by vMyxlac or vMyxT5KO were both robustly enhanced in human 786-0 renal carcinoma cells (Fig. 4B) treated with rapamycin.
FIG. 4.
Rapamycin treatment increases focus size and number in type II cells. BGMK (A) or 786-0 (B) cells were infected with vMyxlac (top panels) or vMyxT5KO (bottom panels) at low MOIs. Cells were pretreated for 6 h before infection with 20 nM rapamycin or an appropriate vehicle control (1:5,000 dilution of DMSO). At 24 h postinfection, cellular lysates were collected, and released virus was used to infect fresh BGMK cells (shown). Foci are visualized by staining with X-Gal reagent.
As MV is a rabbit-specific virus and M-T5 is a critical determinant for the growth of MV in rabbit CD4+ T cells (line RL-5), it was important to examine the effect of rapamycin on rabbit cell lines. As seen in Fig. 5, rapamycin had little effect on the replication or spread of vMyxlac or vMyxT5KO in the rabbit fibroblast cell line RK-13, a line that is permissive to both viruses (Fig. 5A and B). In addition, rapamycin had no effect on MV replication and spread in RL-5 infected with the permissive vMyxlac virus (Fig. 5C), nor was permissiveness restored to the vMyxT5KO virus in this cell line (Fig. 5D). This indicates that the enhancing effect of rapamycin on MV replication does not include cell lines from the virus' natural host.
FIG. 5.
Rapamycin treatment did not alter myxoma virus replication in rabbit fibroblasts and lymphocytes. The ability of myxoma virus to replicate and spread following inoculation at a low MOI was determined by growth curves, using RK-13 (rabbit fibroblast cell line) (A and B) and RL-5 (rabbit lymphocyte cell line) (C and D). vMyxlac (A and C) and vMyxT5KO (B and D) were used to investigate the abilities of virus to infect and spread throughout the monolayer, and virus titer was assessed by focus formation. Cells were pretreated for 1 to 6 h before infection with 20 nM rapamycin (dashed line) or an appropriate vehicle control (1:5,000 dilution of DMSO, solid line). *, P, <0.005 as determined by two-tailed t test.
In addition to examining the effects of rapamycin in this system, we also examined the effect of tacrolimus (FK506), an immunosuppressant that binds the same cellular cofactor as rapamycin (FKBP12) yet binds calcineurin instead of mTOR to exert its immunosuppressive effects (14). We found that the pretreatment of cells with FK506 had no enhancing effect on MV growth and replication in type I or type II human tumor cells (data not shown). This indicates that the effect of rapamycin is specific to its mTOR-binding properties.
Rapamycin treatment altered cell cycle progression in MV-infected type II cells.
M-T5 protein has been shown to interact with both Akt and Cullin-1, both of which have effects on cell cycle progression. We have previously shown that M-T5 protects MV-infected cells from cell cycle arrest in response to stress stimuli induced by virus infection (18). In that previous study, 786-0 cells were synchronized via serum starvation and then infected with vMyxlac or vMyxT5KO, with the result that wild-type vMyxlac stimulated cell cycle progression whereas vMyxT5KO did not (18). Serum starvation has been shown to inhibit mTOR activity, yet serum starvation followed by rapamycin treatment has been shown to cause apoptosis in some cancer cells (15). For this reason, in this study, we performed cell cycle analysis without serum starvation to examine the effect of rapamycin on the cell cycle in MV-infected cells. In fully permissive BGMK cells (Fig. 6A and B), there was little effect of rapamycin treatment or MV infection on the cell cycle progression of nonsynchronized cells. However, in type II 786-0 cells (Fig. 6C and D), rapamycin treatment in combination with MV infection had profound effects on cell cycle progression. In 786-0 cells without rapamycin treatment (Fig. 6C) or virus infection (Fig. 6D), the cells are rapidly cycling, as indicated by a high percentage of cells in S phase. Virus infection (vMyxlac, black bars; vMyxT5KO, gray bars) affects the cell cycle of these cells, but without cell synchronization, we do not see the clear cellular progression out of G1 and into S phase, as seen in previous studies. However, Fig. 6D shows that the treatment with rapamycin has stopped cell cycle progression, and thus, its effects are easier to discern. In the absence of MV infection, rapamycin treatment results in the majority of cells accumulating in the G1 phase, consistent with the cytostatic cell cycle blockade effects of the drug (Fig. 6D, white bar). However, in the context of wild-type MV infection, the majority of cells progress into the S phase, whereas in the absence of rapamycin (Fig. 6C, black bars), there are significantly more cells in G1. There is a similar yet less dramatic effect with vMyxT5KO, indicating that other viral proteins are involved in this antiarrest phenotype, as well as M-T5. Thus, rapamycin assists in preventing cell cycle arrest in MV-infected type II cancer cells, whereas it induces arrest in uninfected cells.
FIG. 6.
Effect of rapamycin treatment on host cell cycle in the context of myxoma virus infection. BGMK (A and B) and 786-0 (C and D) cells were pretreated with a vehicle control (1:5,000 dilution, DMSO) (A and C) or 20 nM rapamycin (B and D). Six hours later, cells were mock infected (white bars) or infected with vMyxlac (black bars) or vMyxT5KO (gray bars) at an MOI of 3. Sixteen hours later, cells were collected, fixed, and stained with propidium iodide. The percentage of cells in each phase was determined using DNA content on a FACScalibur machine. This figure is representative of at least three independent experiments.
Rapamycin induces global effects on the Akt signaling pathway in the context of MV infection.
To examine the molecular consequences of rapamycin treatment, protein and mRNA levels were measured for key Akt signaling molecules in type II (786-0) cancer cells. Cells were pretreated with either rapamycin (20 nM) or with the control vehicle DMSO for 6 h. Cells were then left uninfected (mock infected) or infected for 16 h with vMyxlac or vMyxT5KO (both at an MOI of 3). Cell lysates were analyzed for changes in levels of key Akt signaling molecules (Fig. 7; Table 1).
FIG. 7.

Global effects of rapamycin treatment on host cell signaling in the context of myxoma virus infection. 786-0 cells were pretreated with 20 nM rapamycin or a vehicle control (1:5,000 dilution, DMSO). Six hours later, cells were infected with vMyxlac or vMyxT5KO at an MOI of 3. Sixteen hours later, cells were collected and lysed and 30 mg of protein was run on 8 to 12% SDS-PAGE gel, transferred onto nitrocellulose, and probed with the indicated antibodies. (A) Akt activation, (B) signaling molecules up- and downstream of Akt, and (C) mTOR activation are shown. Densitometry levels were detected by Molecular Imaging software (Kodak) and compared to the level of either Akt (A) or β-actin (B and C). Variability between films was normalized.
TABLE 1.
Effect of rapamycin on intracellular mRNA levels in type II 786-0 human renal cancer cells in response to MV infectiona
| Gene productb | % Saturation of mock-infected cells
|
% Saturation of virus-infected cells
|
||||
|---|---|---|---|---|---|---|
| vMyxlac
|
vMyxT5KO
|
|||||
| DMSO | Rapamycin | DMSO | Rapamycin | DMSO | Rapamycin | |
| A. No change of mRNA in response to rapamycin | ||||||
| GNB1 | + | + | + | + | + | + |
| HSPCB | ++ | ++ | ++ | ++ | ++ | ++ |
| ITGB1 | ++ | ++ | ++ | ++ | ++ | ++ |
| PAB1/PABP | ++ | ++ | ++ | ++ | ++ | ++ |
| ARH12/ARHA | + | + | + | + | + | + |
| G3PD/GAPDH | +++ | +++ | +++ | +++ | +++ | +++ |
| RPS27A | +++ | +++ | + | + | +++ | +++ |
| PTEN | − | − | + | + | − | − |
| RHEB2 | ++ | ++ | + | + | ++ | ++ |
| IRAK1 | ++ | ++ | + | + | + | + |
| B. mRNA upregulation in response to rapamycin | ||||||
| ILK | − | + | + | + | − | + |
| AKT1 | − | ++ | ++ | ++ | − | − |
| IGF1R | − | + | − | − | − | − |
| GRB2 | − | + | + | + | − | + |
| BPTP3/CFC | − | + | + | + | − | + |
| C-RAF/CRAF | − | + | + | + | − | + |
| EIF3S10 | − | + | + | + | − | + |
| EIF4A1 | + | ++ | ++ | ++ | + | ++ |
| EIF4G1 | − | + | + | + | − | + |
| B2M | − | +++ | − | − | − | ++ |
| C. mRNA downregulation in response to rapamycin | ||||||
| HSPB1 | +++ | ++ | ++ | ++ | +++ | ++ |
| HSPCA | ++ | ++ | +++ | +++ | ++ | +++ |
| S6 | ++ | ++ | + | + | ++ | + |
| MLLT7 | + | − | − | − | − | − |
| PACα | + | − | − | − | − | − |
| PI3KCG/PI3K | + | − | − | − | − | − |
| GRB1/P85-β | + | − | − | − | − | − |
Key: −, less than 5% saturation; +, 5 to 30% saturation; ++, 30 to70% saturation; +++, 70 to 100% saturation.
Gene products are as follows: RSP27A, ribosomal protein S27a; PTEN, phosphatase and tensin homolog 1 (mutated in multiple cancers); RHEB2, Ras homolog enriched in brain; IRAK1, interleukin-1 receptor-associated kinase 1; GNB1, guanine nucleotide binding protein (G protein) beta polypeptide 1; HSPCB, heat shock 90-kDa protein 1, beta; ITGB1, integrin, beta 1 (fibronectin receptor, beta polypeptide, antigen CD29); PAB1/PABP, poly(A) binding protein, cytoplasmic 1; ARH12/ARHA (RhoA), Ras homolog gene family, member A; G3PD/GAPDH, glyceraldehyde-3-phosphate dehydrogenase; AKT1, V-akt murine thymoma viral oncogene homolog 1; IGF1R, insulin-like growth factor 1 receptor; MLLT7, myeloid/lymphoid or mixed-lineage leukemia (trithorax homolog, Drosophila sp.); PACα, P21/Cdc42/Rac1-activated kinase 1 (STE20 homolog, Saccharomyces cerevisiae); PI3KCG/PI3K, phosphoinositide-3-kinase, catalytic, gamma polypeptide; GRB1/P85-β, phosphoinositide-3-kinase, regulatory subunit, polypeptide 2 (p85 beta); GRB2, growth factor receptor-bound protein 2; BPTP3/CFC, protein tyrosine phosphatase, nonreceptor type 11 (Noonan syndrome 1); C-RAF/CRAF, V-raf-1, murine leukemia viral oncoprotein homolog 1; EIF3S10, eukaryotic translation initiation factor 3, subunit 10 theta, 150 or 170 kDa; EIF4A1, eukaryotic translation initiation factor 4A, isoform 1; EIF4G1, eukaryotic translation initiation factor 4, gamma 1; HSPB1, heat shock 27-kDa protein 1; HSPCA, heat shock 90-kDa protein, alpha; ILK, integrin-linked kinase; S6, ribosomal protein S6; B2M, beta-2-microglobulin.
An examination of mRNA levels of signaling molecules on the Akt pathway revealed that many molecules were unaffected by either virus or rapamycin treatment (Table 1, section A). These molecules include GNB1, HSPCB, ITGB1, PAB1/PABP, ARH12/ARHA, and the internal control GAPDH. Others, such as RPS27A, RHEB2, and IRAK1, exhibited decreased message signaling as a result of wild-type virus infection yet were unaffected by rapamycin treatment. Another group, including MLLT7, PACα, PI3KCG/PI3K, and GRB1/p85, expressed mRNA levels that were detected only in untreated cells (Table 1, section C), while IGFR1 mRNA was present following rapamycin treatment only in the absence of virus (Table 1, section B). These molecules give us little insight into the interplay of MV proteins and rapamycin-induced signaling leading to increased MV replication in these cells.
The activation of Akt through phosphorylation at serine 473 or threonine 308 was measured by Western blotting (Fig. 7A). We confirmed that wild-type MV, but not vMyxT5KO, could induce activation of Akt (particularly at the serine 473 site) in these type II cells (Fig. 7A, compare lanes 1, 3, and 5), and treatment with rapamycin could enhance Akt activation at either site (Fig. 7A, compare lanes 2, 4, and 6). Again, although the Akt1 mRNA levels appeared to increase with rapamycin treatment or wild-type viral infection (Table 1, section B), no difference in the native protein levels was seen with any of our treatments (Fig. 7A, Akt panel).
In general, successful MV replication results in a distinct pattern of signaling congruent with Akt hyperactivation. Our data suggest that wild-type MV infection alone or rapamycin pretreatment followed by vMyxlac or vMyxT5KO preferentially upregulates mRNA synthesis for GRB2 and ILK (Table 1, section B). Both of these signaling molecules are linked with the activation of Akt and do not become induced by vMyxT5KO infection alone (Table 1 section B, column vMyxT5KO DMSO). By Western blotting analysis, a decrease in the phosphorylation of the Akt inhibitor PTEN is observed after vMyxlac virus infection (Fig. 7B, compare lanes 1 and 3) but not after infection with the M-T5-deficient vMyxT5KO virus (Fig. 7B, lanes 1 and 5). Pretreatment with rapamycin followed by infection decreases the PTEN phosphorylation (Fig. 7B, lanes 4 and 6) in a manner similar to wild-type MV infection alone (Fig. 7B, lane 3). Although PTEN mRNA synthesis is upregulated following MV infection (Table 1, section A), there is no significant difference in the detectable protein levels among treatments (data not shown). Interestingly, neither MV nor rapamycin treatment had an effect on the phosphorylation of the Akt activator PDK-1 (Fig. 7B).
Some signaling molecules downstream of Akt exhibit patterns that are predictable based on an increased Akt activation induced by MV or rapamycin. For example, both GSK3β and Raf are downstream of Akt but are inhibited by its activation (Fig. 7B). In the context of productive wild-type MV infection, the activation of these molecules is decreased in comparison with that of mock-infected cells (Fig. 7B, compare lanes 1 and 3). However, in the context of rapamycin treatment, which increases Akt activation (Fig. 7A), the phosphorylation of both molecules is slightly increased after infection with vMyxlac (Fig. 7B, compare lanes 3 and 4) yet significantly increased in cells infected with vMyxT5KO (Fig. 7B, compare lanes 5 and 6). This finding is contrary to what may be expected as a consequence of Akt activation and indicates that other cellular or viral factors may act on these signaling molecules.
The level of activated mTOR is high in untreated cells and is modestly inhibited by treatment with rapamycin (Fig. 7C, compare lanes 1 and 2). However, its activation is dramatically inhibited by infection with wild-type MV (Fig. 7C, lanes 1 and 3) and vMyxT5KO (Fig. 7C, lanes 1 and 5). In the context of virus infection, the kinase activity of mTOR is slightly increased with vMyxlac- and vMyxT5KO-infected cells treated with rapamycin (Fig. 7C, compare lane 3 to 4 and 5 to 6). However, direct downstream effectors of mTOR, such as the p70S6 kinase (Fig. 7B), are unaffected by wild-type myxoma virus infection (Fig. 7B, compare lanes 1 and 3) yet are increased by infection with vMyxT5KO (Fig. 7B, compare lanes 1 and 5). When cells were pretreated with rapamycin followed by virus infection (Fig. 7B, compare lane 3 to 4 and 5 to 6), there was a significant decrease in activation which is contrary to that predicted by the modest kinase activity of mTOR (Fig. 7C). The mRNA synthesis of the S6 kinase was downregulated in a similar manner to that of p70S6 kinase activity observed by immunoblotting (Table 1, section C). Other mRNAs such as those for eIF-3 and eIF-4 and Hsp90 were increased by rapamycin treatment followed by virus infection, while mRNA synthesis for Hsp27 was downregulated (Table 1, sections B and C).
Taken together, these results indicate that the enhanced MV replication in the context of rapamycin treatment of type II cancer cells is linked to aberrant Akt pathway signaling induced by the synergistic activities of MV plus rapamycin.
DISCUSSION
We report the first description of the direct synergism between a known immunosuppressant and an oncolytic virus. Rapamycin, in its history of use in transplant patients, has been shown to be an efficient immunosuppressant and an alternative to cyclosporine treatment. It has also been observed that patients taking rapamycin have a lower cancer incidence as well as reduced rates of supervening cytomegalovirus infection (45). Thus, it is somewhat unexpected that this drug has a positive stimulatory effect on the replication of MV, a poxvirus oncolytic virotherapy candidate in the treatment of human cancer cells.
This apparent discrepancy can be explained by the signaling alterations that result from rapamycin interaction with mTOR, combined with the critical requirement for Akt activation for permissive MV infection of human tumor cells (60). This finding provides a compelling counterpart to a recent report showing inhibition of poxviruses by a signaling inhibitor used to treat cancer. Imatinib (Gleevec; STI-571) was shown to efficiently retard the egress of vaccinia virus from infected cells by blocking host scr/abl kinases that are co-opted by the virus to propel its virion into neighboring host cells (46). In contrast, MV also co-opts cellular kinases, but in this case, the signaling molecules frequently are aberrantly activated in human tumor cells. The Akt pathway has been shown to be either mutated or constitutively activated in a majority of human malignancies (21, 26, 32, 44, 54). This Akt activation state is a direct determinant of the ability of MV to productively infect and kill human cancer cells (60). The increased oncolytic ability of a virus, incurred by the amplification of host signaling proteins associated with tumorigenesis, is also seen in the case of reovirus (8, 27, 40, 52) and is likely a common phenomenon for other oncolytic viruses as well.
Rapamycin, which acts on the mTOR, downstream of Akt, has the ability to restore MV infection in type II cells that are infected with MV lacking M-T5 (vMyxT5KO) and to enhance the replication levels of wild-type MV. Thus, the inhibition of mTOR is able to compensate for M-T5 function in these cell lines and also to enhance MV replication in cells infected with wild-type MV. The MV-promoting effects of rapamycin can be attributed at least in part to its ability to activate Akt phosphorylation (42), likely due to an S6K-based feedback upregulation mechanism (49). Thus, a treatment that theoretically could make a tumor more aggressive by the hyperactivation of Akt instead makes it more sensitive to MV oncolysis.
This hyperactivation of Akt also interferes with the cell cycle of infected cells. We have shown previously that the interaction of M-T5 with Cullin-1 prevents the stress-induced cell arrest induced by virus infection and stimulates cell cycle progression (18). We believe that this effect may also be mediated in part by Akt phosphorylation induced by M-T5. Thus, during virus infection in the presence of rapamycin treatment, the Akt pathway is activated by both virus and treatment, leading to increased cell cycle progression, as opposed to the cell cycle arrest seen with rapamycin treatment alone that is likely mediated by the inhibition of its downstream pathways.
However, analysis of MV-infected type II 786-0 cells treated with rapamycin also revealed some unexpected trends. Signaling molecules upstream of Akt are modulated in a manner that results in an increase in Akt activation, but in the signaling molecules downstream of Akt, there appear to be selective dysregulations that favor MV replication. Pathways stimulated by GSK-3β, Raf, and mTOR should be inhibited by activated Akt, yet in MV-infected 786-0 cells treated with rapamycin, these pathways were activated in conjunction with increased Akt activation. The fact that this pattern is induced in either the presence or absence of M-T5 expression by MV indicates that either other viral proteins or a co-opted cellular factor(s) is responsible for increased MV replication as a consequence of the unusual downstream Akt signaling. This is also significant with regard to mTOR signaling, as the Ras and PI3K pathways converge to activate mTOR and stimulate cell growth (49).
We have shown previously that M-T5 binds and activates Akt in type II cancer cells (60). The atypical Akt signaling we observed here suggests that M-T5 may perturb Akt cellular localization and, thus, its ability to interact with specific downstream targets. Localization data indicate that in the context of MV infection, phosphorylated Akt shows punctuate cytoplasmic staining, similar to the localization seen for M-T5 (60). This suggests that the interaction of these proteins may orchestrate novel sustained localization of Akt. Interestingly, in the absence of M-T5 and presence of rapamycin in type II cancer cells, phosphorylated Akt colocalizes with DAPI (4′,6′-diamidino-2-phenylindole)-stained nuclei (data not shown), indicating that another viral protein independent of M-T5 may interact with Akt to mediate its nuclear localization. In addition, it has recently been shown that increased levels of phosphorylated Akt in tumor cells increase the nuclear localization of pAkt (58). This phenomenon combined with the finding that M-T5 stimulates Akt activation may indicate that one of the functions of this viral protein is to facilitate sustained Akt kinase activation at the nuclear level, where some of its targets are located. Treatment with rapamycin could also be driving this localization through increasing phosphorylated Akt levels. This could explain the complex effects on downstream Akt signaling pathways seen when vMyxT5KO was combined with rapamycin treatment.
M-T5 protein has multiple ankyrin repeats that facilitate protein-protein interactions with host proteins (18, 60). We hypothesize that M-T5 may act as a molecular scaffold that brings together diverse host and virus factors. The novel interactions of these proteins may result in functional interactions between normally distinct signaling mediators that would not naturally intersect, resulting in the activation/inhibition of pathways in cancer cells that are stimulatory for virus replication. These activating pathways clearly involve mTOR-mediated pathways, as the effect of rapamycin can increase virus replication in type II cancer cells that have a critical need for M-T5 expression for optimal MV replication. In addition, the existence of these pathways is clearly consistent with the requirement of increased Akt phosphorylation for MV tropism in human tumor cells, described previously in our laboratory.
These results indicate that a detailed understanding of how oncolytic viruses such as MV micromanipulate signaling cascades in permissive cancer cells is critical to developing the virus as a potential cancer therapy. In examining the interaction between M-T5 and Akt and its downstream pathways, including mTOR, we have uncovered a therapeutically feasible way to specifically increase virus replication in vivo by synergism with an anticancer-signaling inhibitor drug. This is also the first demonstration of a cancer drug therapy in which the efficacy of an oncolytic virus replication is increased and suggests the possibility of novel mechanisms for synergistically enhancing virotherapy for cancer.
Acknowledgments
We dedicate this manuscript to the memory of Robert (Zhen) Zhong, a pioneer in the field of xenotransplantation, who passed away on 6 September 2006 from lung cancer. Robert Zhong and members of his lab provided many helpful discussions on the properties of rapamycin.
This work was supported by grants from the National Cancer Institute of Canada and Canadian Institutes of Health Research. M.M.S. is supported by a postdoctoral fellowship provided by the Pamela Greenaway Kohlmeier Translational Breast Cancer Research Unit of the London Regional Cancer Program. G.M. holds a Canada Research Chair in Molecular Virology and is an International Scholar of The Howard Hughes Medical Institute.
Footnotes
Published ahead of print on 15 November 2006.
REFERENCES
- 1.Amornphimoltham, P., V. Sriuranpong, V. Patel, F. Benavides, C. J. Conti, J. Sauk, E. A. Sausville, A. A. Molinolo, and J. S. Gutkind. 2004. Persistent activation of the Akt pathway in head and neck squamous cell carcinoma: a potential target for UCN-01. Clin. Cancer Res. 10:4029-4037. [DOI] [PubMed] [Google Scholar]
- 2.Andrewes, C. H., and S. Harisijades. 1955. Propagation of myxoma virus in one-day old mice. Br. J. Exp. Pathol. 36:18-21. [PMC free article] [PubMed] [Google Scholar]
- 3.Aoki, M., E. Blazek, and P. K. Vogt. 2001. A role of the kinase mTOR in cellular transformation induced by the oncoproteins P3k and Akt. Proc. Natl. Acad. Sci. USA 98:136-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bell, J. C., B. Lichty, and D. Stojdl. 2003. Getting oncolytic virus therapies off the ground. Cancer Cell 4:7-11. [DOI] [PubMed] [Google Scholar]
- 5.Burchert, A., Y. Wang, D. Cai, N. von Bubnoff, P. Paschka, S. Muller-Brusselbach, O. G. Ottmann, J. Duyster, A. Hochhaus, and A. Neubauer. 2005. Compensatory PI3-kinase/Akt/mTor activation regulates imatinib resistance development. Leukemia 19:1774-1782. [DOI] [PubMed] [Google Scholar]
- 6.Chen, Y. L., P. Y. Law, and H. H. Loh. 2005. Inhibition of PI3K/Akt signaling: an emerging paradigm for targeted cancer therapy. Curr. Med. Chem. Anticancer Agents 5:575-589. [DOI] [PubMed] [Google Scholar]
- 7.Chow, L. M., and S. J. Baker. 2006. PTEN function in normal and neoplastic growth. Cancer Lett. 241:184-196. [DOI] [PubMed] [Google Scholar]
- 8.Coffey, M. C., J. E. Strong, P. A. Forsyth, and P. W. Lee. 1998. Reovirus therapy of tumors with activated Ras pathway. Science 282:1332-1334. [DOI] [PubMed] [Google Scholar]
- 9.Dahia, P. L., R. C. Aguiar, J. Alberta, J. B. Kum, S. Caron, H. Sill, D. J. Marsh, J. Ritz, A. Freedman, C. Stiles, and C. Eng. 1999. PTEN is inversely correlated with the cell survival factor Akt/PKB and is inactivated via multiple mechanisms in haematological malignancies. Hum. Mol. Genet. 8:185-193. [DOI] [PubMed] [Google Scholar]
- 10.Fenner, F. 2000. Adventures with poxviruses of vertebrates. FEMS Microbiol. Rev. 24:123-133. [DOI] [PubMed] [Google Scholar]
- 11.Guo, Z. S., and D. L. Bartlett. 2004. Vaccinia as a vector for gene delivery. Expert Opin. Biol. Ther. 4:901-917. [DOI] [PubMed] [Google Scholar]
- 12.Guo, Z. S., A. Naik, M. E. O'Malley, P. Popovic, R. Demarco, Y. Hu, X. Yin, S. Yang, H. J. Zeh, B. Moss, M. T. Lotze, and D. L. Bartlett. 2005. The enhanced tumor selectivity of an oncolytic vaccinia lacking the host range and antiapoptosis genes SPI-1 and SPI-2. Cancer Res. 65:9991-9998. [DOI] [PubMed] [Google Scholar]
- 13.Haas-Kogan, D., N. Shalev, M. Wong, G. Mills, G. Yount, and D. Stokoe. 1998. Protein kinase B (PKB/Akt) activity is elevated in glioblastoma cells due to mutation of the tumor suppressor PTEN/MMAC. Curr. Biol. 8:1195-1198. [DOI] [PubMed] [Google Scholar]
- 14.Hafizi, S., V. N. Mordi, K. M. Andersson, A. H. Chester, and M. H. Yacoub. 2004. Differential effects of rapamycin, cyclosporine A, and FK506 on human coronary artery smooth muscle cell proliferation and signalling. Vascul. Pharmacol. 41:167-176. [DOI] [PubMed] [Google Scholar]
- 15.Huang, S., L. N. Liu, H. Hosoi, M. B. Dilling, T. Shikata, and P. J. Houghton. 2001. p53/p21(CIP1) cooperate in enforcing rapamycin-induced G(1) arrest and determine the cellular response to rapamycin. Cancer Res. 61:3373-3381. [PubMed] [Google Scholar]
- 16.Hyun, T., A. Yam, S. Pece, X. Xie, J. Zhang, T. Miki, J. S. Gutkind, and W. Li. 2000. Loss of PTEN expression leading to high Akt activation in human multiple myelomas. Blood 96:3560-3568. [PubMed] [Google Scholar]
- 17.Jackson, E. W., C. R. Dorn, J. K. Saito, and D. G. McKercher. 1966. Absence of serological evidence of myxoma virus infection in humans exposed during an outbreak of myxomatosis. Nature 211:313-314. [DOI] [PubMed] [Google Scholar]
- 18.Johnston, J. B., G. Wang, J. W. Barrett, S. H. Nazarian, K. Colwill, M. Moran, and G. McFadden. 2005. Myxoma virus M-T5 protects infected cells from the stress of cell cycle arrest through its interaction with host cell cullin-1. J. Virol. 79:10750-10763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kerr, P., and G. McFadden. 2002. Immune responses to myxoma virus. Viral Immunol. 15:229-246. [DOI] [PubMed] [Google Scholar]
- 20.Kim, C. S., V. V. Vasko, Y. Kato, M. Kruhlak, M. Saji, S. Y. Cheng, and M. D. Ringel. 2005. AKT activation promotes metastasis in a mouse model of follicular thyroid carcinoma. Endocrinology 146:4456-4463. [DOI] [PubMed] [Google Scholar]
- 21.Kurose, K., X. P. Zhou, T. Araki, S. A. Cannistra, E. R. Maher, and C. Eng. 2001. Frequent loss of PTEN expression is linked to elevated phosphorylated Akt levels, but not associated with p27 and cyclin D1 expression, in primary epithelial ovarian carcinomas. Am. J. Pathol. 158:2097-2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li, L., M. M. Ittmann, G. Ayala, M. J. Tsai, R. J. Amato, T. M. Wheeler, B. J. Miles, D. Kadmon, and T. C. Thompson. 2005. The emerging role of the PI3-K-Akt pathway in prostate cancer progression. Prostate Cancer Prostatic Dis. 8:108-118. [DOI] [PubMed] [Google Scholar]
- 23.Lin, E., and J. Nemunaitis. 2004. Oncolytic viral therapies. Cancer Gene Ther. 11:643-664. [DOI] [PubMed] [Google Scholar]
- 24.Liu, M., B. Acres, J. M. Balloul, N. Bizouarne, S. Paul, P. Slos, and P. Squiban. 2004. Gene-based vaccines and immunotherapeutics. Proc. Natl. Acad. Sci. USA 101:S14567-S14571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lun, X., W. Yang, T. Alain, Z. Q. Shi, H. Muzik, J. W. Barrett, G. McFadden, J. Bell, M. G. Hamilton, D. L. Senger, and P. A. Forsyth. 2005. Myxoma virus is a novel oncolytic virus with significant antitumor activity against experimental human gliomas. Cancer Res. 65:9982-9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lynch, H. T., M. J. Casey, J. Lynch, T. E. White, and A. K. Godwin. 1998. Genetics and ovarian carcinoma. Semin. Oncol. 25:265-280. [PubMed] [Google Scholar]
- 27.Marcato, P., M. Shmulevitz, and P. W. Lee. 2005. Connecting reovirus oncolysis and Ras signaling. Cell Cycle 4:556-559. [DOI] [PubMed] [Google Scholar]
- 28.McCabe, V. J., I. Tarpey, and N. Spibey. 2002. Vaccination of cats with an attenuated recombinant myxoma virus expressing feline calicivirus capsid protein. Vaccine 20:2454-2462. [DOI] [PubMed] [Google Scholar]
- 29.McCart, J. A., J. M. Ward, J. Lee, Y. Hu, H. R. Alexander, S. K. Libutti, B. Moss, and D. L. Bartlett. 2001. Systemic cancer therapy with a tumor-selective vaccinia virus mutant lacking thymidine kinase and vaccinia growth factor genes. Cancer Res. 61:8751-8757. [PubMed] [Google Scholar]
- 30.McFadden, G. 2005. Poxvirus tropism. Nat. Rev. Microbiol. 3:201-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Medema, R. H., G. J. Kops, J. L. Bos, and B. M. Burgering. 2000. AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature 404:782-787. [DOI] [PubMed] [Google Scholar]
- 32.Mizuki, M., R. Fenski, H. Halfter, I. Matsumura, R. Schmidt, C. Muller, W. Gruning, K. Kratz-Albers, S. Serve, C. Steur, T. Buchner, J. Kienast, Y. Kanakura, W. E. Berdel, and H. Serve. 2000. Flt3 mutations from patients with acute myeloid leukemia induce transformation of 32D cells mediated by the Ras and STAT5 pathways. Blood 96:3907-3914. [PubMed] [Google Scholar]
- 33.Moroziewicz, D., and H. L. Kaufman. 2005. Gene therapy with poxvirus vectors. Curr. Opin. Mol. Ther. 7:317-325. [PubMed] [Google Scholar]
- 34.Morris, P. J. 1991. Cyclosporine, FK-506 and other drugs in organ transplantation. Curr. Opin. Immunol. 3:748-751. [DOI] [PubMed] [Google Scholar]
- 35.Mossman, K., S. F. Lee, M. Barry, L. Boshkov, and G. McFadden. 1996. Disruption of M-T5, a novel myxoma virus gene member of poxvirus host range superfamily, results in dramatic attenuation of myxomatosis in infected European rabbits. J. Virol. 70:4394-4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mullen, J. T., and K. K. Tanabe. 2002. Viral oncolysis. Oncologist 7:106-119. [DOI] [PubMed] [Google Scholar]
- 37.Nemunaitis, J. 1999. Oncolytic viruses. Investig. New Drugs 17:375-386. [DOI] [PubMed] [Google Scholar]
- 38.Nicholson, K. M., and N. G. Anderson. 2002. The protein kinase B/Akt signalling pathway in human malignancy. Cell Signal. 14:381-395. [DOI] [PubMed] [Google Scholar]
- 39.Norman, K. L., F. Farassati, and P. W. Lee. 2001. Oncolytic viruses and cancer therapy. Cytokine Growth Factor Rev. 12:271-282. [DOI] [PubMed] [Google Scholar]
- 40.Norman, K. L., K. Hirasawa, A. D. Yang, M. A. Shields, and P. W. Lee. 2004. Reovirus oncolysis: the Ras/RalGEF/p38 pathway dictates host cell permissiveness to reovirus infection. Proc. Natl. Acad. Sci. USA 101:11099-11104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Opgenorth, A., K. Graham, N. Nation, D. Strayer, and G. McFadden. 1992. Deletion analysis of two tandemly arranged virulence genes in myxoma virus, M11L and myxoma growth factor. J. Virol. 66:4720-4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Reilly, K. E., F. Rojo, Q. B. She, D. Solit, G. B. Mills, D. Smith, H. Lane, F. Hofmann, D. J. Hicklin, D. L. Ludwig, J. Baselga, and N. Rosen. 2006. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 66:1500-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parato, K. A., D. Senger, P. A. Forsyth, and J. C. Bell. 2005. Recent progress in the battle between oncolytic viruses and tumours. Nat. Rev. Cancer 5:965-976. [DOI] [PubMed] [Google Scholar]
- 44.Pedrero, J. M., D. G. Carracedo, C. M. Pinto, A. H. Zapatero, J. P. Rodrigo, C. S. Nieto, and M. V. Gonzalez. 2005. Frequent genetic and biochemical alterations of the PI 3-K/AKT/PTEN pathway in head and neck squamous cell carcinoma. Int. J. Cancer 114:242-248. [DOI] [PubMed] [Google Scholar]
- 45.Ponticelli, C. 2004. The pleiotropic effects of mTor inhibitors. J. Nephrol. 17:762-768. [PubMed] [Google Scholar]
- 46.Reeves, P. M., B. Bommarius, S. Lebeis, S. McNulty, J. Christensen, A. Swimm, A. Chahroudi, R. Chavan, M. B. Feinberg, D. Veach, W. Bornmann, M. Sherman, and D. Kalman. 2005. Disabling poxvirus pathogenesis by inhibition of Abl-family tyrosine kinases. Nat. Med. 11:731-739. [DOI] [PubMed] [Google Scholar]
- 47.Schmelzle, T., and M. N. Hall. 2000. TOR, a central controller of cell growth. Cell 103:253-262. [DOI] [PubMed] [Google Scholar]
- 48.Sehgal, S. N., H. Baker, and C. Vezina. 1975. Rapamycin (AY-22,989), a new antifungal antibiotic. II. Fermentation, isolation and characterization. J. Antibiot. (Tokyo) 28:727-732. [DOI] [PubMed] [Google Scholar]
- 49.Shaw, R. J., and L. C. Cantley. 2006. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature 441:424-430. [DOI] [PubMed] [Google Scholar]
- 50.Shen, Y., and J. Nemunaitis. 2005. Fighting cancer with vaccinia virus: teaching new tricks to an old dog. Mol. Ther. 11:180-195. [DOI] [PubMed] [Google Scholar]
- 51.Shi, Y., J. Gera, L. Hu, J. H. Hsu, R. Bookstein, W. Li, and A. Lichtenstein. 2002. Enhanced sensitivity of multiple myeloma cells containing PTEN mutations to CCI-779. Cancer Res. 62:5027-5034. [PubMed] [Google Scholar]
- 52.Shmulevitz, M., P. Marcato, and P. W. Lee. 2005. Unshackling the links between reovirus oncolysis, Ras signaling, translational control and cancer. Oncogene 24:7720-7728. [DOI] [PubMed] [Google Scholar]
- 53.Staal, F. J., R. B. van der Luijt, M. R. Baert, J. van Drunen, H. van Bakel, E. Peters, I. de Valk, H. K. van Amstel, M. J. Taphoorn, G. H. Jansen, C. W. van Veelen, B. Burgering, and G. E. Staal. 2002. A novel germline mutation of PTEN associated with brain tumours of multiple lineages. Br. J. Cancer 86:1586-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stiles, B., V. Gilman, N. Khanzenzon, R. Lesche, A. Li, R. Qiao, X. Liu, and H. Wu. 2002. Essential role of AKT-1/protein kinase Bα in PTEN-controlled tumorigenesis. Mol. Cell. Biol. 22:3842-3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sypula, J., F. Wang, Y. Ma, J. C. Bell, and G. McFadden. 2004. Myxoma virus tropism in human tumor cells. Gene Ther. Mol. Biol. 8:108-114. [Google Scholar]
- 56.Thorne, S. H., D. L. Bartlett, and D. H. Kirn. 2005. The use of oncolytic vaccinia viruses in the treatment of cancer: a new role for an old ally? Curr. Gene Ther. 5:429-443. [DOI] [PubMed] [Google Scholar]
- 57.Timiryasova, T. M., J. Li, B. Chen, D. Chong, W. H. Langridge, D. S. Gridley, and I. Fodor. 1999. Antitumor effect of vaccinia virus in glioma model. Oncol. Res. 11:133-144. [PubMed] [Google Scholar]
- 58.Trotman, L. C., A. Alimonti, P. P. Scaglioni, J. A. Koutcher, C. Cordon-Cardo, and P. P. Pandolfi. 2006. Identification of a tumour suppressor network opposing nuclear Akt function. Nature 441:523-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang, F., Y. Ma, J. W. Barrett, X. Gao, J. Loh, E. Barton, H. W. Virgin, and G. McFadden. 2004. Disruption of Erk-dependent type I interferon induction breaks the myxoma virus species barrier. Nat. Immunol. 5:1266-1274. [DOI] [PubMed] [Google Scholar]
- 60.Wang, G., J. W. Barrett, M. Stanford, S. J. Werden, J. B. Johnston, X. Gao, M. Sun, J. Q. Cheng, and G. McFadden. 2006. Infection of human cancer cells with myxoma virus requires Akt activation via interaction with a viral ankyrin-repeat host range factor. Proc. Natl. Acad. Sci. USA 103:4640-4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zeh, H. J., and D. L. Bartlett. 2002. Development of a replication-selective, oncolytic poxvirus for the treatment of human cancers. Cancer Gene Ther. 9:1001-1012. [DOI] [PubMed] [Google Scholar]