Abstract
Previous studies demonstrated that the induction of the heat shock protein Hsp70 in response to viral infection is highly specific and differs from one cell to another and for a given virus type. However, no clear consensus exists so far to explain the likely reasons for Hsp70 induction within host cells during viral infection. We show here that upon rotavirus infection of intestinal cells, Hsp70 is indeed rapidly, specifically, and transiently induced. Using small interfering RNA-Hsp70-transfected Caco-2 cells, we observed that Hsp70 silencing was associated with an increased virus protein level and enhanced progeny virus production. Upon Hsp70 silencing, we observed that the ubiquitination of the main rotavirus structural proteins was strongly reduced. In addition, the use of proteasome inhibitors in infected Caco-2 cells was shown to induce an accumulation of structural viral proteins. Together, these results are consistent with a role of Hsp70 in the control of the bioavailability of viral proteins within cells for virus morphogenesis.
Viral infections of mammalian cells often result in alterations of the synthesis of a group of highly conserved proteins known as heat shock/stress proteins (Hsps). Hsps are ubiquitous molecular chaperones which not only facilitate correct protein folding, assembly, and intracellular transport but also appear to function in intracellular protein degradation (reviewed in references 4, 17, and 37). Relationships between the major Hsp, i.e., the heat-inducible, 72-kDa Hsp70, and viral infections have been described for a variety of experimental models with different types of viruses and host cells. Cumulative findings indicate that the induction of Hsp70 is not a general response to viral infection but, instead, a highly specific response with regard to both the infecting virus and the host cell. A clear example of this concept emerged from the comprehensive study of Phillips et al. (31) on the induction of the 70-kDa family of heat shock genes in monkey and human cells infected with different DNA viruses, such as adenovirus type 5, herpes simplex virus type 1, simian virus 40, and vaccinia virus. It appeared that only adenovirus type 5 and herpes simplex virus type 1 were able to induce Hsp70 and that, between three examined Hsps, only Hsp70 was induced, thus accounting for a highly specific response. A similar conclusion can be drawn from studies on RNA viruses, although most of these studies have shown a preferential induction of the endoplasmic reticulum (ER)-resident Hsps, such as the glucose-regulated proteins (Grp) Grp78 and Grp94 (reviewed in reference 34). Both Grps have been shown to be upregulated in response to infection with rotavirus, a double-stranded nonenveloped RNA virus that leads to age-dependent diarrhea (39). Recent studies have identified the constitutive form Hsc70 as a target protein of the multistep process required for the entry of rotavirus into epithelial intestinal cells (15, 21).
Our recent study (3) allowed us to detect the Hsp70 level in lipid rafts obtained from intestinal epithelial Caco-2 cells, whose well-polarized and enterocyte-like phenotype closely corresponds to the natural in vivo target of rotavirus (7). In these cells, both rotavirus and Hsp70 are released before any detectable cell lysis, through an atypical pathway involving lipid rafts (3, 21, 35). In the present work, we found that the Hsp70 level rapidly increases in response to rotavirus infection in Caco-2 cells and that this virus stress induction is specific to the molecular chaperone Hsp70. In order to clarify the contribution of cellular Hsp70 during rotavirus infection of intestinal epithelial Caco-2 cells, we examine here the structural rotavirus protein level under conditions where the intracellular Hsp70 level was manipulated. Hsp70-specific small interfering RNA (siRNA-Hsp70) transfection of Caco-2 cells resulted, as expected, in a strong decrease of the intracellular level of Hsp70. In that molecular context, the levels of the structural rotavirus proteins VP2, VP4, and VP6 were increased significantly 6 h after infection of Caco-2 cells, with a correlated increase in progeny virus production. Interestingly, Hsp70 silencing was also associated with a strong decrease of the ubiquitination of rotavirus structural proteins. Conversely, inhibition of proteasomal degradation was also shown to increase the level of VP4, the spike rotavirus protein. Our data are consistent with a model in which the increased level of Hsp70 in rotavirus-infected cells may represent a first cellular protective response against infection through its ability to direct virus proteins towards the ubiquitin-dependent degradation pathway.
MATERIALS AND METHODS
Materials.
Trypsin (type IX-S, from porcine pancreas; 13 to 20 BAEE (benzoyl l-arginine ethyl ester) units/mg), Sigma-Fast o-phenylenediamine dihydrochloride (peroxidase substrate), calpain inhibitor (ALLN), and Z-Leu-Leu-Leu-Ala (MG 132) were purchased from Sigma-Aldrich (Saint Louis, MO). The protease inhibitor cocktail (Complete mini tablets) was obtained from Roche Diagnostics (Mannheim, Germany). The bicinchoninic acid reagent and bovine serum albumin for protein determination were purchased from Pierce (Rockfort, IL). Acrylamide-bisacrylamide (40% [wt/vol]) was obtained from Q-BIOgene (Montreal, Canada). Protein G Sepharose and enhanced chemiluminescence (ECL) kits were obtained from Amersham Biosciences (Buckinghamshire, United Kingdom). Phosphate-buffered saline (PBS), Dulbecco's modified Eagle medium (DMEM), fetal calf serum (FCS), nonessential amino acids (×100), and antibiotics were purchased from Invitrogen (Paisley, United Kingdom). When not mentioned otherwise, reagents were obtained from Sigma-Aldrich.
Antibodies used included monoclonal anti-Hsp70 (SPA-810); polyclonal anti-Hsp70 (SPA-812); monoclonal anti-Hsc70 (SPA-815); monoclonal anti-Hsp90 (SPA-835); monoclonal anti-Hsp110 (SPA-1103) (all of the preceding were from StressGen); monoclonal anti-VP4 from mouse ascites fluids (clone 7.7) raised against VP8*, the N-terminal cleavage product of VP4 (5, 9, 10, 29, 40); monoclonal anti-VP2 (clone E22); monoclonal anti-VP6 (clone RV133); polyclonal anti-rotavirus strain RF (3161), which recognizes several structural viral proteins, including VP4, VP2, and VP6 (kindly provided by Jean Cohen); and horseradish peroxidase-conjugated secondary antibodies (Rockland, Gilbertsville, PA, and Jackson Immunoresearch, West Grove, PA).
The pCMV-Hsp70 plasmid was kindly provided by R. I. Morimoto (Northwestern University, Evanston, IL). The pCMV-Neo plasmid control was obtained from Stratagene (La Jolla, CA). The siRNA-Hsp70 duplex was purchased from Eurogentec (Liege, Belgium), and scrambled siRNA was obtained from Dharmacon Research (Lafayette, IL).
Cell culture.
Experiments involved the human colon adenocarcinoma cell line Caco-2, obtained from the European Collection of Cell Cultures (Wiltshire, United Kingdom) and routinely cultured in DMEM supplemented with 20% FCS, 1% penicillin-streptomycin, and 1% nonessential amino acid solution in an air-10% CO2 incubator at 37°C. Cells were cultured for 21 days, changed daily (42), and used between passages 40 and 60.
Infection.
Rotavirus strain RF P6[1] and G(6), a French isolate of bovine rotavirus (isolated from calf feces) (24), were grown in embryonic African green monkey kidney cells (MA104) obtained from the European Collection of Cell Cultures. Cells were maintained in DMEM supplemented with 10% FCS and antibiotics, infected at a low multiplicity, and incubated for 72 h until cellular lysis (by freezing and thawing three times) to achieve complete virus release. Extracted viral preparations were titrated by plaque assay as previously described (30) and used to infect Caco-2 cells at a multiplicity of infection of 1 or 10 PFU per cell. For infection in the absence of transfection, Caco-2 cells cultured for 21 days were incubated in serum-free medium overnight before exposure to 10 PFU/cell of previously trypsin-activated rotavirus strain RF (0.44 μg/ml trypsin, 30 min, 37°C). Following 1 h of adsorption to the cell surface at 37°C, the virus inoculum was removed, the cells were washed twice, and the infection was continued for the indicated times in fresh medium containing trypsin (0.44 μg/ml). The time of virus removal was taken as time zero in all experiments.
Preparation of cell lysates.
Adherent cells were washed in PBS and scraped into cold lysis buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 1 mM EDTA) containing protease inhibitor cocktail (Roche Diagnostic GmbH, Mannheim, Germany).
ELISAs for Hsp70 and VP4.
Enzyme-linked immunosorbent assays (ELISAs) were performed to determine differences in Hsp70 and VP4 levels. For Hsp70, a monoclonal anti-Hsp70 antibody (SPA 810) (5 μg/well, overnight, 4°C) was used as the capture antibody to coat MaxiSorp 96-well plates (Nunc, Roskilde, Denmark). The wells were postcoated with PBS containing 0.1% Tween 20, washed before the addition of cell lysates or a recombinant human HSP-70 standard (StressGen, Victoria, Canada) (6.25 to 400 ng/ml, diluted in PBS containing 0.1% Tween 20), and incubated for 2 h at room temperature. Polyclonal anti-Hsp70 (SPA 812) was used as the detection antibody, diluted 1/400. The reaction was visualized and quantitated by using color development followed by horseradish peroxidase-conjugated anti-rabbit immunoglobulin G (diluted 1/20,000) and o-phenylenediamine dihydrochloride substrate. The reaction was stopped by adding 50 μl 1 M H2SO4. Absorbance was read at 492 nm in an automated ELISA reader (Labsystems Multiskan MS, Vantaa, Finland). For VP4, a similar system was developed, using monoclonal anti-VP4 (7.7; diluted 1/200) as the capture antibody and polyclonal anti-RF (3161; diluted 1/200) as the detection antibody.
pCMV and siRNA plasmids and transfection of Caco-2 cells.
The sequence of the 21-nucleotide (nt) siRNA-Hsp70 was chosen based on the rules proposed by Elbashir et al. (11) and was designed to interfere with the two mRNAs encoding Hsp70 (GenBank accession number NM_005345 for HSPA1A and BC057397 for HSPA1B; nucleotide positions 65 to 85 from the ATG) but not with the two Hsc70 mRNA variants (GenBank accession numbers NM_006597 and NM_153201). The sequences were as follows: siRNA-Hsp70 sense, 5′-CACGGCAAGGUGGAGAUCA-dTdT-3′; and siRNA-Hsp70 antisense, 5′-UGAUCUCCACCUUGCCGUG-dTdT-3′. Transfections were performed using an Amaxa Nucleofector apparatus (Amaxa, Cologne, Germany) according to the manufacturer's instructions. Caco-2 cells were harvested at 50 to 70% cell confluence and resuspended in electroporation buffer T from a Nucleofector kit (Amaxa GmbH, Koeln, Germany) at a final concentration of 5 × 106 cells/ml (12). A 0.1-ml sample of cell suspension was supplied with either 5 μg of pCMV-Neo control, 5 μg of pCMV-Hsp70, 100 nM of scrambled siRNA, or 2 μg of siRNA-Hsp70 and then transferred to a 2-mm electroporation cuvette and electroporated. After electroporation, cells were immediately diluted with 1 ml of complete Caco-2 culture medium and distributed into 24-well culture plates at a density of 5 × 105 cells per well. After 24 h, the medium was changed, and cells were cultured for 96 h until their use in experiments.
Transfected Caco-2 cells were infected with rotavirus bovine strain RF (1 PFU/cell) either 6 h or 18 h before the end of the 96-h siRNA-Hsp70 or pCMV recovery period.
Immunoprecipitation of ubiquitinated proteins and viral protein immunoblot analysis.
Cell homogenates corresponding to 40 μg proteins prepared from Caco-2 cells infected for 6 h with rotavirus (1 PFU/cell) were precleared with 70 μl of protein G Sepharose for 4 h at room temperature, with rotation. Precleared supernatants were subjected to ubiquitinated protein immunoprecipitation with 2 μl of anti-ubiquitin antibody overnight at 4°C, with rotation. Immunopurified ubiquitinated proteins were isolated by centrifugation (8,000 × g, 5 min) and three washes with lysis buffer. Bound proteins were detached from protein G Sepharose by being boiled for 3 min with sample buffer (62.5 mM Tris-HCl, pH 6.8, 10% glycerol, 2% sodium dodecyl sulfate, 0.1% bromophenol blue, and 5% β-mercaptoethanol). Detached samples were loaded onto 7.5% sodium dodecyl sulfate-polyacrylamide gels, subjected to electrophoresis, and electrotransferred to nitrocellulose membranes. After being blocked for 1 h at room temperature (1% polyvinylpyrrolidone, PBS, 0.1% Tween), membranes were sequentially incubated with mouse primary antibody (dilution, 1/5,000) anti-VP4 (clone 7.7), anti-VP2 (clone E22) (33), or anti-VP6 (clone) (22) and with a convenient secondary peroxidase-conjugated antibody.
Additional methods. (i) Protein assay.
Proteins were determined using the detergent-compatible bicinchoninic acid reagent (Pierce, Rockford, IL), with bovine serum albumin as the standard, according to the manufacturer's instructions.
(ii) Blot quantification.
Protein spot levels were determined by using Scion quantification software.
Statistics.
Data are presented as means ± standard errors of the means (SEM), and comparisons were performed using the Wilcoxon signed-rank test. P values of ≤0.05 were considered significant.
RESULTS
Rotavirus infection induces a specific increased level of Hsp70 in Caco-2 cells.
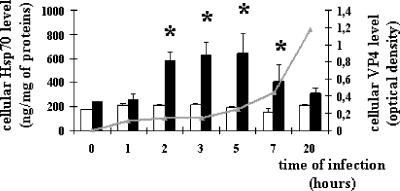
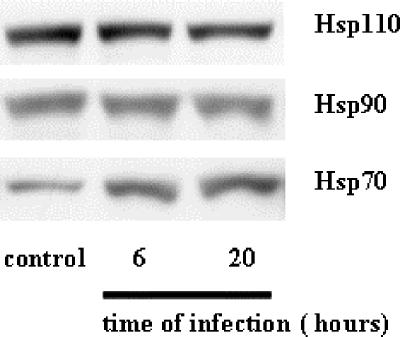
We initially investigated the impact of viral infection on the level of the stress-inducible protein Hsp70. Caco-2 cells were infected with the RF strain of rotavirus (10 PFU/cell). Cell extracts were prepared at selected times postinfection (p.i.), ranging from 1 to 20 h. As shown in Fig. 1, the Hsp70 level measured by ELISA significantly and rapidly increased in Caco-2 cells following infection, suggesting the building of a stress response. Following infection of Caco-2 cells with rotavirus, trace amounts of the spike protein VP4 were previously observed by immunoblotting as early as 3 h p.i. (35). This was confirmed here by a quantitative ELISA measurement showing detectable levels of VP4 as soon as 1 h p.i., while levels were substantial at 5 h p.i. and continued to increase until 20 h p.i. In Caco-2 cells, thermal stress has been shown previously to induce an increased level of Hsp70, with similar kinetics to those of rotavirus infection, although the maximal level reached an approximately sixfold higher value (3; data not shown). These observations were confirmed here by immunoblotting at 6 and 20 h p.i., and the results of a representative experiment are shown in Fig. 2. It should be noted that this Hsp response was specific, since the levels of other cytosolic heat-stress-inducible proteins (Hsp90 and Hsp110) were not increased upon viral infection (Fig. 2). Our results also indicated that these three Hsps escape the generalized shutoff of host transcription observed during rotavirus infection (32).
FIG. 1.
Rotavirus infection increases Hsp70 level in a time-dependent manner. Hsp70 (white and black histograms) and VP4 (gray curve) levels were analyzed by ELISA, as detailed in Materials and Methods. Caco-2 cell homogenates were harvested at selected times during the course of infection (1 h to 20 h) (10 PFU/cell) from infected cells (black histograms) or noninfected cells (white histograms). Data are expressed as means ± SEM (n = 4). *, P < 0.05. Note that the increased level of Hsp70 is only transient and that VP4 starts to be detectable as soon as 2 h p.i.
FIG. 2.
Rotavirus infection specifically increases Hsp70 level. Caco-2 cells were infected with rotavirus strain RF (10 PFU/cell) for 6 h or 20 h. Cells were harvested, and cell lysates were analyzed by immunoblotting using monoclonal anti-Hsp70 (SPA-810), monoclonal anti-Hsc70 (SPA-815), monoclonal anti-Hsp90 (SPA-835), and monoclonal anti-Hsp110 (SPA-1103) as described in Materials and Methods. A representative experiment of four total experiments is shown. Note that only the Hsp70 level increases, whereas the Hsc70, Hsp90, and Hsp110 levels remain unaffected.
In order to analyze the putative function of this specific Hsp70 induction during rotavirus infection, transfection experiments were performed to manipulate the intracellular level of Hsp70.
Specific modulation of cellular Hsp70 amount after Caco-2 cell transfection with specific siRNA.
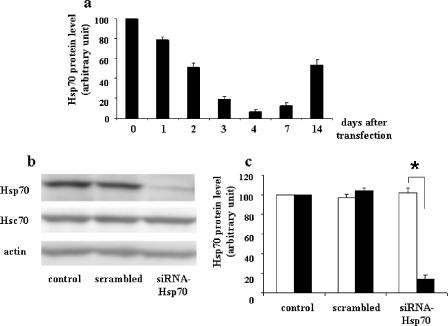
Figure 3a shows the Hsp70 level in Caco-2 cells after siRNA electroporation as a function of time posttransfection. The effect of siRNA was time dependent, and the smallest amount of Hsp70 was observed after 96 h. The Hsp70 cellular level then steadily escaped silencing by siRNA-Hsp70. Immunoblot analysis (Fig. 3b) and blot quantification (Fig. 3c) confirmed the low Hsp70 level at 96 h compared to that with the siRNA control (scrambled) and the specificity of Hsp70 silencing, since no change was observed in the amount of either Hsc70, the constitutive form of the heat stress protein, or actin.
FIG. 3.
Transient and specific inhibition of Hsp70 in Caco-2 cells. (a) Kinetics of inhibition of the Hsp70 protein level after specific siRNA targeting (0 to 14 days). A Caco-2 cell suspension of 5 × 105 cells was supplied with 2 μg of siRNA-Hsp70 and then electroporated. Cells were plated and cultured at the indicated times after electroporation. Cells were harvested, and lysates were analyzed by immunoblotting as described in Materials and Methods. Hsp70 levels were quantified by immunoblot scanning. Results of this quantification are means ± SEM (n = 4). Note that the maximal inhibition of the Hsp70 level was observed 4 days after siRNA electroporation. (b) Specificity of siRNA-Hsp70. Caco-2 cells were plated and cultured for 96 h in the presence of 100 pmol scrambled siRNA in 100 μl of T solution from Amaxa, and 5 × 105 cells were subjected to electroporation and 2 μg of siRNA-Hsp70. Cell lysates were harvested and analyzed by immunoblotting as described in Materials and Methods, using monoclonal anti-Hsp70 or anti-Hsc70 antibodies. A representative experiment of four total experiments is shown. Only the Hsp70 level was affected by siRNA treatment, whereas the Hsc70 level remained unaffected. (c) Quantitative analysis of Hsp70 levels in siRNA-Hsp70-transfected cells. Caco-2 cells were treated as described for panel b, and the resulting immunoblots (four independent experiments) were quantified using immunoblot scanning. Hsc70 (white histograms) and Hsp70 (black histograms) were measured. *, P < 0.05. Note that at this time (4 days after electroporation) only the Hsp70 level decreased, whereas the Hsc70 level remained unaffected.
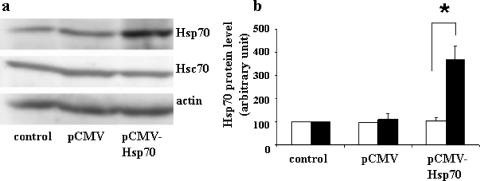
Conversely, transient transfection of pCMV-Hsp70 resulted in a fourfold increase of Hsp70 after 96 h, without an effect on the constitutive Hsc70 protein (Fig. 4a and b). Kinetics and dose-effect experiments have been carried out with pCMV-Hsp70 and indicated that it was not possible to obtain more overexpression of Hsp70 in Caco-2 cells because of its toxic effects on cells (not shown).
FIG. 4.
Transient, specific overexpression of Hsp70 in Caco-2 cells. (a) A suspension of 5 × 105 Caco-2 cells was supplied with 5 μg of pCMV-Neo (control) or 5 μg of pCMV-Hsp70 and then electroporated. Cells were plated and cultured for 96 h. Cells were harvested, and lysates were subjected to immunoblotting as described in the legend to Fig. 3. A representative experiment of four total experiments is shown. (b) Quantitative analysis of Hsp70 levels in pCMV-Hsp70-transfected cells. Caco-2 cells were treated as described for panel a, and the resulting immunoblots (four independent experiments) were quantified using immunoblot scanning. Hsc70 (white histograms) and Hsp70 (black histograms) were measured. *, P < 0.05. Note that the Hsp70 level increased 96 h after pCMV-Hsp70 electroporation, whereas the Hsc70 level was not affected.
These transfection models were subsequently used to study the link between rotavirus infection and Hsp70 in intestinal epithelial cells. It should be mentioned that Hsc70, which is implicated during rotavirus entry into host cells (15), remained mostly unchanged upon Hsp70 down modulation.
Decreased level of Hsp70 results in increased levels of structural viral proteins in infected cells.
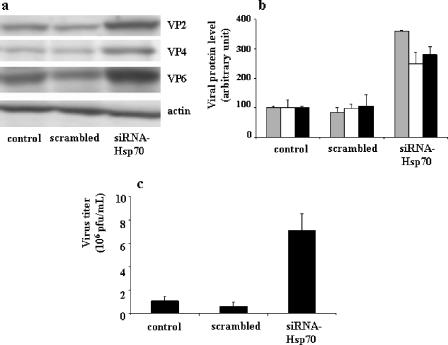
The most striking results observed upon Hsp70 silencing in infected Caco-2 cells (1 PFU/cell, 6 h) were the significantly increased levels of rotavirus structural proteins (Fig. 5a and b): the VP4 level increased 250%, the VP2 level increased 360%, and the VP6 level increased 280%.
FIG. 5.
Effects of siRNA-Hsp70 transfection on rotavirus protein level (a and b) and on progeny virus production (c) in infected Caco-2 cells. (a) A cell suspension of 5 × 105 Caco-2 cells was supplied with 2 μg of siRNA-Hsp70 and then electroporated. Transfected cells were infected with rotavirus strain RF (1 PFU/cell) for 6 h as described in Materials and Methods. Cells were harvested, and lysates were subjected to immunoblotting using monoclonal anti-VP4 (clone 7.7) and anti-VP6 (clone RV133) as described in Materials and Methods. A representative experiment of four total experiments is shown. (b) Quantitative analysis of VP2, VP4, and VP6 levels in siRNA-transfected cells. Caco-2 cells were treated as described for panel a and subjected to immunoblot analysis (a representative experiment of four total experiments is displayed). VP2 (gray histograms), VP4 (white histograms), and VP6 (black histograms) were measured. (c) Quantification of virus production by siRNA-transfected and infected Caco-2 cells. Caco-2 cells were treated as described for panel a (1 PFU/cell, 18-h infection). Supernatants were titrated by a standard plaque assay with MA104 cells, as described in Materials and Methods (22). Results are expressed as PFU/ml and represent means ± SEM for six experiments. Note that in siRNA-transfected Caco-2 cells, the levels of virus structural proteins (VP2, VP4, and VP6) were significantly increased and associated with an increase of virus production.
Accordingly, Hsp70 silencing resulted in increased virus morphogenesis. This was tested by measuring progeny virus production at 18 h postinfection in Caco-2 cells transfected with Hsp70 siRNA for 96 h. Virus production measured by plaque assay increased eightfold compared with that of control cells (Fig. 5c). This significant increase of virus production correlated with the increased levels of structural virus proteins, with the increases being on the same order of magnitude.
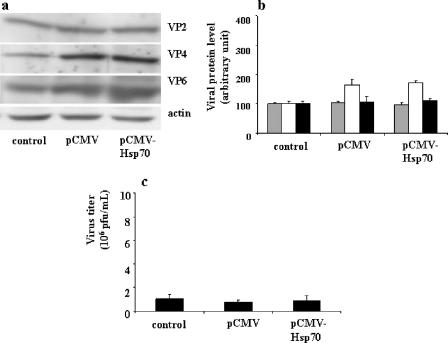
A parallel experiment was performed in which Caco-2 cells were transiently transfected with pCMV-Hsp70 for 90 h (resulting in an increased Hsp70 level) (Fig. 4) and subsequently infected with rotavirus (1 PFU/cell, 6 h). Interestingly, no significant changes were observed in the amounts of VP2, VP4, and VP6, as shown in Fig. 6a and b. Overexpression of Hsp70 did not induce a significant change in new virus production (Fig. 6c).
FIG. 6.
Effects of pCMV-Hsp70 transfection on rotavirus protein level (a and b) and on progeny virus production (c) in infected Caco-2 cells. (a) A suspension of 5 × 105 Caco-2 cells was supplied with 5 μg of pCMV-Neo (control) or 5 μg of pCMV-Hsp70 and then electroporated. Cells were plated and cultured for 90 h and then infected with rotavirus strain RF (1 PFU/cell for 6 h), as described in Materials and Methods. Cells were harvested, and lysates were subjected to immunoblotting as described in the legend to Fig. 5b. A representative experiment of four total experiments is shown. (b) Quantitative analysis of VP2, VP4, and VP6 levels in pCMV-Hsp70-transfected cells. Caco-2 cells were treated as described for panel a and subjected to immunoblot analysis (a representative experiment of four total experiments is displayed). VP2 (gray histograms), VP4 (white histograms), and VP6 (black histograms) levels were measured. (c) Quantification of virus production by pCMV-Hsp70-transfected and infected Caco-2 cells. Caco-2 cells were treated as described for panel a (1 PFU/cell, 18 h). Supernatants were titrated by a standard plaque assay with MA104 cells as described in Materials and Methods (22). Results are expressed as PFU/ml and represent means ± SEM for three experiments. Note that the overexpression of Hsp70 in Caco-2 cells was not followed by any change in viral protein level or virus production.
Increased amounts of viral proteins correlate with their decreased ubiquitination.
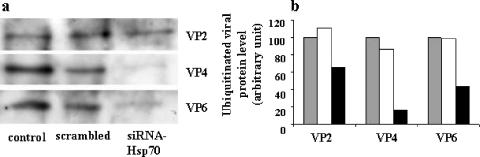
It has been established that Hsp70, together with Hsp60 and Hsp40, is directly involved in the transfer of misfolded proteins to the proteasome machinery through a ubiquitination-dependent process (13). Thus, Hsp70 depletion of Caco-2 cells should result in increased levels of viral proteins due to the lack of an efficient system to transport proteins to sites of degradation. To check this hypothesis, experiments were performed in which Hsp70 siRNA-transfected Caco-2 cells were infected (1 PFU/cell, 6 h) and the ubiquitination of viral proteins was measured.
Cell homogenates were subjected to immunoprecipitation with an anti-ubiquitin antibody, and viral proteins were separated by electrophoresis and revealed by immunoblotting using antibodies against these structural viral proteins. As illustrated in Fig. 7a and b, depletion of cellular Hsp70 was associated with a decrease of ubiquitinated structural viral proteins VP2, VP4, and VP6 in infected Caco-2 cells at 6 h postinfection. This result is in good agreement with the idea that Hsp70 may direct viral proteins to a ubiquitin-dependent degradation process.
FIG. 7.
siRNA-Hsp70 effect on virus protein ubiquitination in infected Caco-2 cells. (a) A cell suspension of 5 × 105 Caco-2 cells was supplied with 2 μg of siRNA-Hsp70 and then electroporated. Transfected cells were infected with rotavirus strain RF (1 PFU/cell) for 18 h as described in Materials and Methods. Infected lysates were subjected to immunoprecipitation using anti-ubiquitin antibodies, as described in Materials and Methods. Immunopurified ubiquitinated proteins were subjected to immunoblot analysis using anti-VP2, -VP4, and -VP6 antibodies as described in Materials and Methods. (b) Quantitative analysis of ubiquitin-labeled VP2, VP4, and VP6 levels in siRNA-transfected and rotavirus-infected Caco-2 cells. Caco-2 cells were treated as described for panel a, and the amounts of ubiquitinated VP2, VP4, and VP6 were quantified using immunoblot scanning. Control (gray histograms), scrambled siRNA-treated (white histograms), and siRNA-Hsp70-treated (black histograms) cells were studied. Note that the decrease in expression level of Hsp70 in Caco-2 cells results in a significant decrease of the ubiquitination of virus structural proteins.
Proteasome inhibitors increase the VP4 level in Caco-2 cells.
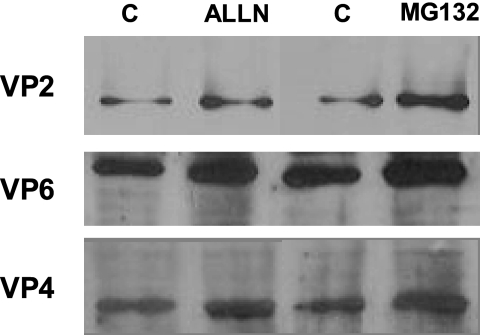
To support the hypothesis discussed above, we tested whether two proteasome inhibitors, namely, ALLN and MG132, might interfere with the level of one of the structural viral proteins, i.e., VP4, in infected Caco-2 cells. These cells were treated with the inhibitors for the last hours of infection at 12 h p.i., i.e., when VP4 synthesis was efficient. As shown in the blots (Fig. 8), the use of these inhibitors induced an increase in the spike protein VP4 (3-fold with ALLN and 2.3-fold with MG132 compared to the dimethyl sulfoxide vehicle), indicating that VP4 is a regular and natural substrate of the proteasome pathway.
FIG. 8.
Effects of proteasome inhibitors on viral structural protein levels. Caco-2 cells infected with rotavirus strain RF (10 PFU/cell) for 12 h were subjected to proteasome inhibitor treatment with either ALLN (10 μM), MG132 (10 μM), or vehicle (lanes C) for the last 2 h of infection. Cells were harvested, and cell lysates were analyzed by immunoblotting using monoclonal anti-VP2, -VP6, and -VP4 as described in Materials and Methods. Note that treatment with proteasome inhibitors resulted in significant increases of viral structural protein levels, as follows: for VP2, 2.1-fold with ALLN and 2.8-fold with MG132; for VP6, 2.2-fold with ALLN and 2.4-fold with MG132; and for VP4, 2.3-fold with ALLN and 3-fold with MG132.
DISCUSSION
This work demonstrates that the level of the major inducible heat shock protein, Hsp70, rapidly and transiently increases upon rotavirus infection of intestinal cells. The present results also suggest an active role for this protein in the control of the level of viral structural proteins and rotavirus morphogenesis through a ubiquitin-dependent process. Other heat shock proteins have already been shown to be involved in rotavirus infection. The 70-kDa heat shock cognate protein (Hsc70), whose level is not modified upon rotavirus infection, has been shown to play a role in the entry process of the virus (15, 41) thanks to its well-known involvement in the clathrin-coated vesicle formation process (18). Previous works have shown that the levels of other members of the Hsp superfamily, namely, Grp78 and Grp94, also increased upon rotavirus infection of MA104 cells (8, 39), but the exact function of this phenomenon has not been documented further.
Interestingly, other groups have also analyzed the level of Hsp70 in rotavirus-infected cells, using either transcriptomic (8) or biochemical (39) approaches, and they did not find significant changes in the Hsp70 level. However, both sets of results may be reconciled by considering two important factors, namely, the kinetics of the Hsp70 level after rotavirus infection and the cell lines used to evaluate the Hsp level. In our hands, the rapid and transient increase of the Hsp70 level in rotavirus-infected Caco-2 cells was an early event (detected as soon as 2 h p.i.). In a previous study using a microarray approach and rotavirus-infected Caco-2 cells, it was shown that the Hsp70 mRNA level does not change at either 1 h or 16 h p.i. (8). It is interesting that our present results show that Hsp70 increased only transiently between 2 and 7 h, at the protein level, which is compatible with the microarray data. In another work, the levels of two other Hsps, namely, Grp78 and Grp94, were shown to increase at 5 h p.i. in MA104 cells (a nonpolarized, nondifferentiated monkey kidney cell line), using bidimensional gel electrophoresis (39). The authors indicated that the Hsp70 level remained essentially unchanged. However, it has to be pointed out that these two cell lines differ strongly regarding the rotavirus cell cycle, since efficient rotavirus production followed by cell lysis occurs after 6 to 10 h in MA104 cells (21), whereas progeny virions are released at the apical membranes of Caco-2 cells through an atypical trafficking process without cell lysis only at 15 to 18 h p.i. (21). Thus, we favor the idea that in Caco-2 cells, the rapid and transient induction of Hsp70 synthesis is the consequence of rotavirus entry into Caco-2 cells and likely reflects the generation of an adaptive response of the host cell to resist the viral attack. This conclusion is strongly supported here by the observation that silencing the Hsp70 level resulted in increased rotavirus morphogenesis.
A surprising observation was that forced overexpression of Hsp70 following pCMV-Hsp70 transfection does not result in a significant decrease of viral protein synthesis or virus morphogenesis, in contrast to what can be expected from the above conclusion. Overexpression of Hsp70 primarily resulted in a deleterious effect on Caco-2 cell survival. This is likely due to the fact that Caco-2 cells already express a rather high basal level of Hsp70 (200 ng/mg of proteins). This is expected from an intestinal cell line whose function is to serve as a first line of defense against a large panel of “physiological” stresses (23). Overexpression of Hsp70 in this context will increase interactions of this molecular chaperone with a number of signaling molecules, including cell cycle regulators (36) and cell death regulators (1), and may explain why this is toxic for these particular cells. We think that Hsp70-overexpressing Caco-2 cells are no more capable of constructing an adaptive response to rotavirus infection, thus indicating that there must be a relatively small concentration window in which Hsp70 levels may be confined.
It is well established that Hsp70 exerts a chaperone function that drives newly synthesized proteins either to a stabilized conformation or to the cellular degradation machineries. While the possibility that Hsp70 could be beneficial for the host has been suggested (2, 34), a proviral role for Hsp70 and other related chaperones has also been described in different models. Evidence that Hsp70 may be involved in virus replication and the assembly of progeny virions has been provided for vaccinia virus (20), adenovirus, poliovirus, and coxsackievirus B (14, 26, 27). Host Hsp70 is also involved in the assembly of preinitiation complexes at the origin of DNA replication in human papillomavirus (25). The 70-kDa Hsp family from both eukaryotes and prokaryotes was suggested to promote viral capsid assembly of polyomavirus (6). Note that most of these studies reflect a relatively rapid effect of Hsp70 to preferentially bind nascent viral proteins, mainly from the outer layer, namely, external capsids or spikes, and to be involved in early steps of the viral replication cycle.
Our results favor the idea that Hsp70 may serve as a host factor which is able to control the amount of viral protein available for virus morphogenesis. Indeed, Hsp70 depletion results in increased viral protein amounts and increased virus morphogenesis, which correlate with decreased ubiquitination.
It was shown previously that several molecular chaperones, particularly Hsp70, are directly involved during the protein ubiquitin tagging process leading to the proteasome-dependent protein degradation pathway. Indeed, through its C-terminal EEVD motif, Hsp70 can interact with the protein CHIP, which is known to favor interactions of protein substrates with ubiquitin ligase enzymes (28). This correlates well with the present results showing that during rotavirus infection, the major viral structural proteins are ubiquitinated, and that this is strongly dependent on the level of cellular Hsp70. As a consequence of rotavirus protein ubiquitination, degradation of these proteins is observed.
Indeed, polyubiquitination of proteins is known to be associated with their degradation through the proteasome (16). However, the exact ubiquitination state of viral proteins and their precise destination remain to be explored, since ubiquitination is a multifunctional process that may be involved in various protein degradation pathways (38) as well as intracellular targeting pathways. More recently, it was proposed that the monoubiquitination of proteins may result in their targeting to the endosomal/lysosomal compartment, where either degradation or further processing may occur (19). We show here that proteasome inhibitors, under conditions where the Hsp70 level remains unaffected, induce an increase in the VP4 level. This is in good agreement with the idea that Hsp70 may be involved in a ubiquitin-dependent degradation pathway. Whether Hsp70-dependent ubiquitination of rotavirus structural proteins is involved only in this degradation pathway or may also play an additional role in targeting or assembly of progeny virions remains to be clarified. Further studies will try to delineate how Hsp70 may be involved in effective control of the bioavailability of rotaviral proteins.
Acknowledgments
This work is dedicated to Jean Cohen, who left us too early.
We are indebted to Jean Cohen and Annie Charpilienne (INRA, Gif sur Yvette, France) for providing us with the rotavirus RF strain, related antibodies, and purified VP4.
A.H.B. and A.G. were supported by a grant from the research ministry of France. This work was supported by grants from INSERM, UPMC, ACI Microbiology, ACI Nanosciences, and the French Ministry of Research.
Footnotes
Published ahead of print on 1 November 2006.
REFERENCES
- 1.Beere, H. M., B. B. Wolf, K. Cain, D. D. Mosser, A. Mahboubi, T. Kuwana, P. Tailor, R. I. Morimoto, G. M. Cohen, and D. R. Green. 2000. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat. Cell Biol. 2:469-475. [DOI] [PubMed] [Google Scholar]
- 2.Brenner, B. G., and M. A. Wainberg. 1999. Heat shock protein-based therapeutic strategies against human immunodeficiency virus type 1 infection. Infect. Dis. Obstet. Gynecol. 7:80-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broquet, A. H., G. Thomas, J. Masliah, G. Trugnan, and M. Bachelet. 2003. Expression of the molecular chaperone Hsp70 in detergent-resistant microdomains correlates with its membrane delivery and release. J. Biol. Chem. 278:21601-21606. [DOI] [PubMed] [Google Scholar]
- 4.Bukau, B., and A. L. Horwich. 1998. The Hsp70 and Hsp60 chaperone machines. Cell 92:351-366. [DOI] [PubMed] [Google Scholar]
- 5.Burns, J. W., H. B. Greenberg, R. D. Shaw, and M. K. Estes. 1988. Functional and topographical analyses of epitopes on the hemagglutinin (VP4) of the simian rotavirus SA11. J. Virol. 62:2164-2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chromy, L. R., J. M. Pipas, and R. L. Garcea. 2003. Chaperone-mediated in vitro assembly of polyomavirus capsids. Proc. Natl. Acad. Sci. USA 100:10477-10482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciarlet, M., and M. K. Estes. 2001. Interactions between rotavirus and gastrointestinal cells. Curr. Opin. Microbiol. 4:435-441. [DOI] [PubMed] [Google Scholar]
- 8.Cuadras, M. A., D. A. Feigelstock, S. An, and H. B. Greenberg. 2002. Gene expression pattern in Caco-2 cells following rotavirus infection. J. Virol. 76:4467-4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dormitzer, P. R., E. B. Nason, B. V. Prasad, and S. C. Harrison. 2004. Structural rearrangements in the membrane penetration protein of a non-enveloped virus. Nature 430:1053-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dormitzer, P. R., Z. Y. Sun, G. Wagner, and S. C. Harrison. 2002. The rhesus rotavirus VP4 sialic acid binding domain has a galectin fold with a novel carbohydrate binding site. EMBO J. 21:885-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elbashir, S. M., J. Harborth, K. Weber, and T. Tuschl. 2002. Analysis of gene function in somatic mammalian cells using small interfering RNAs. Methods 26:199-213. [DOI] [PubMed] [Google Scholar]
- 12.Elbert, M., D. Cohen, and A. Musch. 2006. PAR1b promotes cell-cell adhesion and inhibits dishevelled-mediated transformation of Madin-Darby canine kidney cells. Mol. Biol. Cell 17:3345-3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esser, C., S. Alberti, and J. Hohfeld. 2004. Cooperation of molecular chaperones with the ubiquitin/proteasome system. Biochim. Biophys. Acta 1695:171-188. [DOI] [PubMed] [Google Scholar]
- 14.Glotzer, J. B., M. Saltik, S. Chiocca, A. I. Michou, P. Moseley, and M. Cotten. 2000. Activation of heat-shock response by an adenovirus is essential for virus replication. Nature 407:207-211. [DOI] [PubMed] [Google Scholar]
- 15.Guerrero, C. A., D. Bouyssounade, S. Zarate, P. Isa, T. Lopez, R. Espinosa, P. Romero, E. Mendez, S. Lopez, and C. F. Arias. 2002. Heat shock cognate protein 70 is involved in rotavirus cell entry. J. Virol. 76:4096-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haglund, K., and I. Dikic. 2005. Ubiquitylation and cell signaling. EMBO J. 24:3353-3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartl, F. U., and M. Hayer-Hartl. 2002. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science 295:1852-1858. [DOI] [PubMed] [Google Scholar]
- 18.Heymann, J. B., K. Iwasaki, Y. I. Yim, N. Cheng, D. M. Belnap, L. E. Greene, E. Eisenberg, and A. C. Steven. 2005. Visualization of the binding of Hsc70 ATPase to clathrin baskets: implications for an uncoating mechanism. J. Biol. Chem. 280:7156-7161. [DOI] [PubMed] [Google Scholar]
- 19.Hoeller, D., N. Crosetto, B. Blagoev, C. Raiborg, R. Tikkanen, S. Wagner, K. Kowanetz, R. Breitling, M. Mann, H. Stenmark, and I. Dikic. 2006. Regulation of ubiquitin-binding proteins by monoubiquitination. Nat. Cell Biol. 8:163-169. [DOI] [PubMed] [Google Scholar]
- 20.Jindal, S., and R. A. Young. 1992. Vaccinia virus infection induces a stress response that leads to association of Hsp70 with viral proteins. J. Virol. 66:5357-5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jourdan, N., M. Maurice, D. Delautier, A. M. Quero, A. L. Servin, and G. Trugnan. 1997. Rotavirus is released from the apical surface of cultured human intestinal cells through nonconventional vesicular transport that bypasses the Golgi apparatus. J. Virol. 71:8268-8278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kohli, E., L. Maurice, J. F. Vautherot, C. Bourgeois, J. B. Bour, J. Cohen, and P. Pothier. 1992. Localization of group-specific epitopes on the major capsid protein of group A rotavirus. J. Gen. Virol. 73:907-914. [DOI] [PubMed] [Google Scholar]
- 23.Kojima, K., M. W. Musch, H. Ren, D. L. Boone, B. A. Hendrickson, A. Ma, and E. B. Chang. 2003. Enteric flora and lymphocyte-derived cytokines determine expression of heat shock proteins in mouse colonic epithelial cells. Gastroenterology 124:1395-1407. [DOI] [PubMed] [Google Scholar]
- 24.L'Haridon, R., and R. Scherrer. 1976. In vitro culture of a rotavirus associated with neonatal calf diarrhea. Ann. Rech. Vet. 7:373-381. [PubMed] [Google Scholar]
- 25.Liu, J. S., S. R. Kuo, A. M. Makhov, D. M. Cyr, J. D. Griffith, T. R. Broker, and L. T. Chow. 1998. Human Hsp70 and Hsp40 chaperone proteins facilitate human papillomavirus-11 E1 protein binding to the origin and stimulate cell-free DNA replication. J. Biol. Chem. 273:30704-30712. [DOI] [PubMed] [Google Scholar]
- 26.Macejak, D. G., and R. B. Luftig. 1991. Association of HSP70 with the adenovirus type 5 fiber protein in infected HEp-2 cells. Virology 180:120-125. [DOI] [PubMed] [Google Scholar]
- 27.Macejak, D. G., and P. Sarnow. 1992. Association of heat shock protein 70 with enterovirus capsid precursor P1 in infected human cells. J. Virol. 66:1520-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McDonough, H., and C. Patterson. 2003. CHIP: a link between the chaperone and proteasome systems. Cell Stress Chaperones 8:303-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monnier, N., K. Higo-Moriguchi, Z. Y. Sun, B. V. Prasad, K. Taniguchi, and P. R. Dormitzer. 2006. High-resolution molecular and antigen structure of the VP8* core of a sialic acid-independent human rotavirus strain. J. Virol. 80:1513-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nejmeddine, M., G. Trugnan, C. Sapin, E. Kohli, L. Svensson, S. Lopez, and J. Cohen. 2000. Rotavirus spike protein VP4 is present at the plasma membrane and is associated with microtubules in infected cells. J. Virol. 74:3313-3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phillips, B., K. Abravaya, and R. I. Morimoto. 1991. Analysis of the specificity and mechanism of transcriptional activation of the human hsp70 gene during infection by DNA viruses. J. Virol. 65:5680-5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piron, M., P. Vende, J. Cohen, and D. Poncet. 1998. Rotavirus RNA-binding protein NSP3 interacts with eIF4GI and evicts the poly(A) binding protein from eIF4F. EMBO J. 17:5811-5821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roseto, A., R. Scherrer, J. Cohen, M. C. Guillemin, A. Charpilienne, C. Feynerol, and J. Peries. 1983. Isolation and characterization of anti-rotavirus immunoglobulins secreted by cloned hybridoma cell lines. J. Gen. Virol. 64:237-240. [DOI] [PubMed] [Google Scholar]
- 34.Santoro, M. G. 1994. Heat shock proteins and virus replication: hsp70s as mediators of the antiviral effects of prostaglandins. Experientia 50:1039-1047. [DOI] [PubMed] [Google Scholar]
- 35.Sapin, C., O. Colard, O. Delmas, C. Tessier, M. Breton, V. Enouf, S. Chwetzoff, J. Ouanich, J. Cohen, C. Wolf, and G. Trugnan. 2002. Rafts promote assembly and atypical targeting of a nonenveloped virus, rotavirus, in Caco-2 cells. J. Virol. 76:4591-4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang, H. G., S. Takayama, U. R. Rapp, and J. C. Reed. 1996. Bcl-2 interacting protein, BAG-1, binds to and activates the kinase Raf-1. Proc. Natl. Acad. Sci. USA 93:7063-7068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Welch, W. J. 1992. Mammalian stress response: cell physiology, structure/function of stress proteins, and implications for medicine and disease. Physiol. Rev. 72:1063-1081. [DOI] [PubMed] [Google Scholar]
- 38.Welchman, R. L., C. Gordon, and R. J. Mayer. 2005. Ubiquitin and ubiquitin-like proteins as multifunctional signals. Nat. Rev. Mol. Cell. Biol. 6:599-609. [DOI] [PubMed] [Google Scholar]
- 39.Xu, A., A. R. Bellamy, and J. A. Taylor. 1998. BiP (GRP78) and endoplasmin (GRP94) are induced following rotavirus infection and bind transiently to an endoplasmic reticulum-localized virion component. J. Virol. 72:9865-9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoder, J. D., and P. R. Dormitzer. 2006. Alternative intermolecular contacts underlie the rotavirus VP5* two- to three-fold rearrangement. EMBO J. 25:1559-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zarate, S., M. A. Cuadras, R. Espinosa, P. Romero, K. O. Juarez, M. Camacho-Nuez, C. F. Arias, and S. Lopez. 2003. Interaction of rotaviruses with Hsc70 during cell entry is mediated by VP5. J. Virol. 77:7254-7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zweibaum, A., H. P. Hauri, E. Sterchi, I. Chantret, K. Haffen, J. Bamat, and B. Sordat. 1984. Immunohistological evidence, obtained with monoclonal antibodies, of small intestinal brush border hydrolases in human colon cancers and foetal colons. Int. J. Cancer 34:591-598. [DOI] [PubMed] [Google Scholar]