Abstract
Several lines of evidence suggest that microglia have important roles in the pathogenesis of prion diseases. Here, we establish a novel microglial cell line (MG20) from neonatal tga20 mice that overexpress murine prion protein. After exposure to Chandler scrapie, we observed the replication and accumulation of disease-associated forms of the prion protein in MG20 cells up to the 15th passage. Furthermore, MG20 cells were susceptible to ME7, Obihiro scrapie, and bovine spongiform encephalopathy agents. Thus, MG20 cell lines persistently infected with various murine prion strains provide a useful model for the study of the pathogenesis of prion diseases.
Prion diseases (transmissible spongiform encephalopathies) are fatal, neurodegenerative disorders and include mainly Creutzfeldt-Jakob disease (CJD), bovine spongiform encephalopathy (BSE), scrapie, and chronic wasting disease. The pathological features of prion diseases are brain vacuolation, neuronal death, astrocytosis, and microgliosis (7, 29). The key event in the pathogenesis of prion diseases is the conformational change from the normal host prion protein (PrPC) into the abnormal, disease-associated form (PrPSc). PrPC is rich in α-helical structures and is protease sensitive, whereas PrPSc is largely composed of β-sheets (9, 22) and is partially protease resistant. PrPSc is thought to be the main, if not the only, constituent of the agent of prion diseases (24, 25), and the detection of PrPSc correlates with the presence of infectivity (18, 26).
Several studies have indicated that microglia are related to the pathogenetic events in the loss of neurons in prion diseases (14, 19, 35). Cell culture models have provided valuable tools for investigating the roles of microglia in the pathogenesis of prion diseases (4, 5). The neuronal toxicity of an amyloid, fibril-forming, PrP-derived peptide (PrP106-126) and PrPSc has been documented in in vitro studies (12, 13), and this toxicity is greatly enhanced in the presence of microglia (5, 8), possibly due to the secretion of cytokines and neurotoxic reactive oxygen species from activated microglia (8, 10, 23, 34). A recent study of the rodent CJD model revealed that the presence of infectivity is detected in microglial cells isolated from the brain (3). Therefore, a better understanding of the roles of microglia in neurodegeneration could be obtained from the physiological and biochemical characterization of prion-infected microglial cells. However, to date, persistent prion replication has been reported in only a limited number of cell lines (1, 20, 27, 28, 33) and none of them were of microglial origin.
It has been demonstrated that a large amount of PrP in cultured cells improves the susceptibility to in vitro challenges with various murine prion strains (20). We therefore have generated a microglial cell line from brains of transgenic mice (tga20) overexpressing murine PrP (11) and examined whether these cells are susceptible to persistent infection to various prion strains.
Establishment of microglial cell lines from tga20 and PrP-deficient mice.
Primary microglial cells were cultured from the brains of newborn murine PrP overexpressing mice (tga20) (11) and PrP-deficient mice (PrP0/0 mice) (36), as previously described (31). These cells were immortalized by infection with a c-myc-containing replication-deficient retroviral vector. Wild-type microglial cells (MG6 cells) were similarly established from C57BL/6 mice (32). The immortalized cell clones derived from tga20 mice (MG20 cells) and PrP0/0 mice (MG0 cells) were characterized by immunolabeling with the following cell type-specific antibodies: anti-Mac-1 and F4/80 for microglia, anti-glial fibrillary acidic protein for astrocytes, and anti-microtubule-associated protein 2 for neuronal cells. In a manner similar to that of the wild-type MG6 cells, MG20 and MG0 cells expressed Mac-1 (Fig. 1A) and F4/80, but did not express glial fibrillary acidic protein and microtubule-associated protein 2 (data not shown). MG20 and MG0 cells were mostly rounded or flattened in shape, but occasionally had a spindle or ramified morphology in culture. These morphological characteristics were similar to those of primary microglia (3) and microglial cell lines (21), including MG6 cells (32). In addition, they actively phagocytosed latex beads and produced inflammatory cytokines, such as tumor necrosis factor alpha, interleukin-1α, and interleukin-6, when stimulated by lipopolysaccharide (data not shown), indicating that established cell lines possess morphological, physiological, and immunological characteristics of microglia in the mouse brain.
FIG. 1.
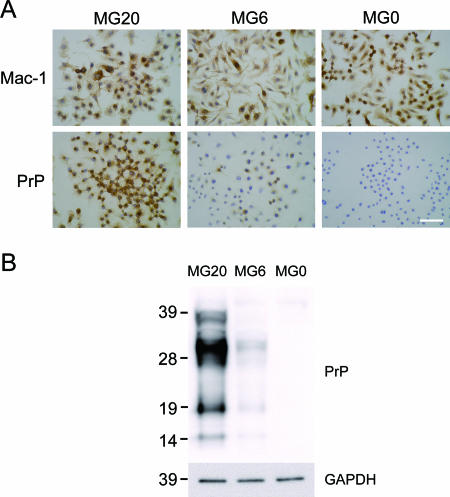
Immunostaining and PrP expression of microglial cell lines derived from PrP-overexpressing (MG20), C57BL/6 (MG6), and PrP-deficient (MG0) mice. (A) Each cell line was immunolabeled with anti-Mac-1 and PrP antibodies and stained with 3,3-diaminobenzidine substrate as chromogen (brown). Nuclei were lightly counterstained with hematoxylin (blue). Scale bar, 50 μm. (B) The levels of PrP were compared in microglial cell lines by semiquantitative immunoblotting with MAb T2. Proteins in postnuclear cell extracts prepared without proteinase K treatment were precipitated with acetone prior to electrophoresis. Molecular mass standards (kDa) are indicated on the left. GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was used as a control to confirm protein content of cell extracts.
Expression of PrP in microglial cell lines.
The levels of PrP expression in MG20, MG6, and MG0 cells were analyzed by immunocytochemistry with monoclonal antibody (MAb) T2 (15). Wild-type MG6 cells showed moderate staining with MAb T2, whereas PrP-overexpressing MG20 cells were more densely stained than MG6 cells (Fig. 1A). No immunoreactivity was found in PrP-deficient MG0 cells. PrP expression levels were further evaluated by immunoblotting. Both MG6 and MG20 but not MG0 cells expressed PrP, which was in agreement with the immunocytochemistry results. MG20 cells expressed a PrP level approximately nine times that of MG6 cells as shown by densitometry. These data indicate that MG20 cells overexpressed PrP at levels similar to that of the brain tissue in tga20 mice from which they were derived (11). Thus, the MG20 cell line could be a potential candidate for persistent infection with prion strains in vitro.
Infection of MG20 cells with murine scrapie prions and screening of PrPSc-positive subclones.
First, we examined the susceptibility of MG20 cells to murine scrapie infection. MG20 cells were incubated with 1% (wt/vol) brain homogenates from terminally ill mice infected with Chandler scrapie (16). PrPSc-positive subclones were screened by the enzyme-linked immunosorbent assay (ELISA) method using the Seprion ligand system (Microsens Biotechnologies, United Kingdom) (17). The proportion of PrPSc-positive subclones was around 20% (22 out of 103 tested subclones) in MG20 cells. Several subclones with strong signals in the ELISA were transferred into 100-mm petri dishes at the second passage and then passaged at a 1:5 split ratio every 4 to 5 days.
Production of PrPSc in scrapie-exposed MG20 cell culture.
We examined seven representative PrPSc-positive MG20 subclones by immunoblotting after treatment with proteinase K (PK) at the fifth passage. Mock-infected MG20 subclones showed no accumulation of PrPSc, whereas all of the infected MG20 subclones had PrPSc detectable by immunoblotting (data not shown). One of the subclones (termed ScMG20/Chan) with a significantly strong PrPSc signal was selected, and the production of PrPSc was examined after continuous passages. The PrPSc signals were detected for at least up to 15 passages without any loss of signal intensity during passages (Fig. 2A). In contrast to MG20 cells, MG6 cells expressing PrP levels nine times lower than those of MG20 cells did not support PrPSc production upon exposure to Chandler and ME7 scrapie (data not shown). This result is consistent with a previous study reporting that the poor efficiency of prion infection in wild-type N2a is improved by increasing the level of PrP (20). It is likely that the overexpression of PrP increases the susceptibility of microglial cells to prion infection.
FIG. 2.
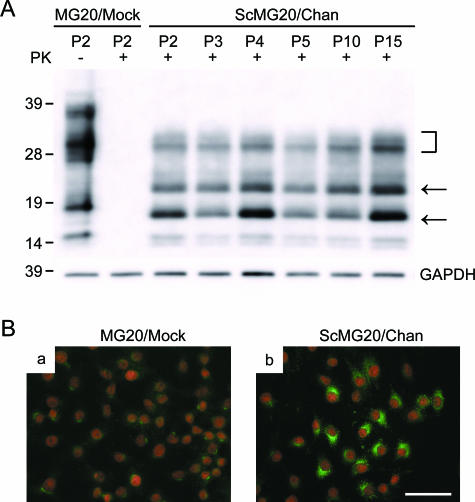
Immunoblotting and immunofluorescence detection of PrPSc in MG20 subclone. (A) A mock-infected subclone (MG20/mock) was obtained from MG20 cells exposed to normal brain homogenates. The PrPSc-positive subclone (ScMG20/Chan) was chosen after screening by ELISA using the Seprion ligand system. MG20/mock cells and ScMG20/Chan cells were passaged every 4 to 5 days. Cell extracts from cultures at the indicated passage number (P2 to P15), either with (+) or without (−) PK treatment, were analyzed by immunoblotting with T2 monoclonal antibody (2.5 × 105 cells equivalent per lane). The arrows and bracket indicate un-, mono-, and diglycosylated PrPSc molecules, respectively. Molecular mass standards (kDa) are indicated on the left. GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was used as a control to confirm the protein content of cell extracts. (B) MG20/mock cells (a) and ScMG20/Chan cells (b) growing on slide glasses were fixed with 4% paraformaldehyde for 30 min and were permeabilized with 0.1% Triton X-100. Following denaturation with 5 M GdnScn in 8.5% polyethylene glycol 8000, cells were immunolabeled with T2 antibody, followed by Alexa Fluor 488-conjugated secondary antibody (green). Nuclei were counterstained with propidium iodide (red). Scale bar, 50 μm.
By using immunofluorescence detection, we next assessed the proportion of cells accumulating with PrPSc. After fixation with 4% paraformaldehyde, infected and mock-infected MG20 cells were treated with 5 M GdnSCN in 8.5% polyethylene glycol 8000 to enhance the binding of PrP antibodies (33). Around 60% of the ScMG20/Chan cells showed intracellularly bright and punctate guanidine-dependent fluorescent signals, which were assumed to reflect PrPSc accumulation, whereas these were not seen in mock-infected cells (Fig. 2B). No major morphological alterations were identified between mock-infected MG20 cells and ScMG20/Chan cells.
Infectivity in scrapie-exposed MG20 cell culture.
The infectivity in ScMG20/Chan cells at the 12th passage was assayed using tga20 and CD-1 mice. Freeze-thawed cell lysates were inoculated intracranially into tga20 mice and CD-1 mice (5 × 105 cells/20 μl per head). As a positive control, 20 μl of 10% (wt/vol) mouse brain homogenates were inoculated. All of the ScMG20/Chan cell-infected mice developed typical signs of Chandler scrapie and the times of death were 60 ± 0.8 days (mean ± standard deviation) for tga20 mice (n = 8) and 144 ± 7.9 days for CD-1 mice (n = 5), respectively. These mean incubation periods were similar to those of positive control mice, whose times of death were 59 ± 1.3 days for tga20 mice (n = 6) and 147 ± 4.4 days for CD-1 mice (n = 7), respectively. The accumulation of PrPSc in their brains was confirmed by immunoblotting Any mice inoculated with mock-infected MG20 cells or normal mouse brain homogenates did not exhibit scrapie symptoms at all. In addition to incubation periods, the lesion profiles and the immunoblot profiles of PK-treated PrPSc from CD-1 mouse brains infected with ScMG20/Chan cells were similar to those infected with Chandler scrapie mouse brain homogenates (data not shown). These results indicate that the biological characteristics of the prion strain were well maintained in ScMG20/Chan cells.
Biochemical features of PrPSc propagated in MG20 cell culture.
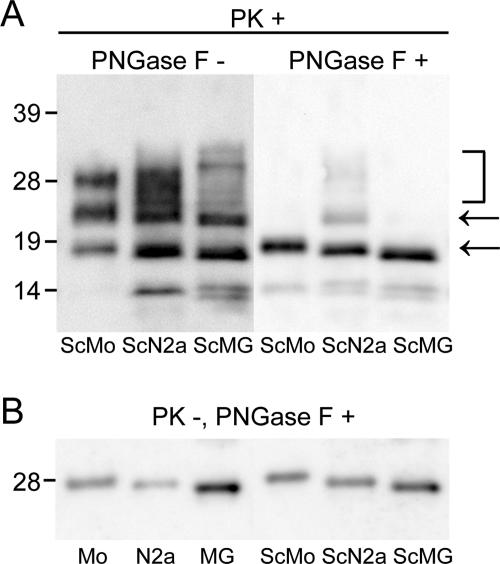
Birkett et al. (6) have reported that the biological characteristics of the scrapie strains were maintained in infected cells, although the biochemical features of PrPSc (e.g., glycoform profiles and molecular mass) differed from those of PrPSc propagated in the brains. Therefore, the immunoblot profiles of the PK-treated PrPSc from ScMG20/Chan cells, ScN2a58 cells (20), and Chandler scrapie-infected CD-1 mouse brains were compared. As shown in Fig. 3A, un- and monoglycosylated PrPSc derived from ScN2a58 and ScMG20/Chan cells migrated faster, by around 0.5 kDa and 1 kDa, respectively, than did the brain-derived isoform. The difference in molecular masses was further confirmed after the removal of carbohydrates with N-glycosidase F (PNGase F). To examine whether the difference in molecular masses comes from the PK cleavage site heterogeneities of PrPSc, we analyzed infected or uninfected brain homogenates and cell lysates without PK treatment by immunoblotting after the removal of carbohydrates. Consistent with PK-treated PrPSc, cell-derived unglycosylated PrP without PK treatment migrated faster than did the brain-derived isoform (Fig. 3B). These results suggest that the differences in the molecular masses of PrPC among brain tissues and cell cultures were probably responsible for those of PrPSc. Our observations are consistent with a recent study on scrapie- and CJD-infected GT1-7 cells, suggesting that heterogeneity in the constitution of glycosylphosphatidylinositol (GPI) moieties is involved in the size differences of unglycosylated PrP between brain tissues and cells (2).
FIG. 3.
Comparison of PrPSc glycosylation patterns and molecular size of unglycosylated PrPSc derived from scrapie-infected brain and ScN2a58 and ScMG20/Chan cells with (+) or without (−) PK treatment. (A) Immunoblot analysis of PK-treated samples from Chandler scrapie-infected mouse brain (ScMo), ScN2a58 cells (ScN2a), and ScMG20/Chan cells (ScMG) with or without deglycosylation by PNGase F treatment. PrPSc was demonstrated by immunoblotting with MAb T2. The arrows and brackets indicate un-, mono-, and diglycosylated PrPSc molecules, respectively. (B) Immunoblot analysis of PK-untreated samples from normal mouse brain (Mo), N2a58 cells (N2a), MG20 cells (MG), Chandler scrapie-infected mouse brain (ScMo), ScN2a58 cells (ScN2a), and ScMG20/Chan cells (ScMG) with deglycosylation by PNGase F. PrP was demonstrated by immunoblotting with MAb 3H2, which recognizes the epitope in N-terminal PrP. Molecular mass standards (kDa) are indicated on the left.
Exposure of MG20 cells to various prion strains.
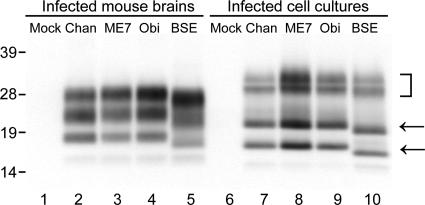
To assess the susceptibility of MG20 cells to other murine prion strains, we exposed MG20 cells to 1% (wt/vol) brain homogenates from terminally ill mice infected with ME7, Obihiro scrapie (30), and BSE agents (15). As in the case of ScMG20/Chan cells, several subclones positive for PrPSc by ELISA were selected and expanded. The accumulation of PrPSc in these infected MG20 cells was examined by immunoblotting at the fifth passage. All the subclones infected with each prion strain demonstrated substantial PrPSc accumulation. (Fig. 4). In BSE-infected MG20 cells, un- and monoglycosylated PrPSc migrated faster, by around 1 kDa, than did scrapie-infected, cell-derived isoforms, possibly due to the difference in the N-terminal cleavage site after protease treatment (15). This unique characteristic of PrPSc from the mouse-propagated BSE agent was also preserved in the cell-propagated BSE agent.
FIG. 4.
Immunoblot analysis of PrPSc derived from mouse brains and MG20 cells infected with various prion strains. Lanes 1 to 5, infected mouse brains; lanes 6 to 10, infected MG20 cells; lanes 1 and 6, mock infected; lanes 2 and 4, Chandler (Chan) infected; lanes 3 and 8, ME7 infected; lanes 4 and 9, Obihiro (Obi) infected; lanes 5 and 10, BSE infected. The arrows and bracket indicate un-, mono-, and diglycosylated PrPSc molecules, respectively. Molecular mass standards (kDa) are indicated on the left.
In conclusion, we demonstrated that prions could replicate and conserve their biological characteristics in the microglial cell line derived from PrP-overexpressing mice. Although the involvement of microglia in the induction of neuropathogenesis has been considered elsewhere, the precise roles of microglia still remain largely obscure. Therefore, cell culture models using infected microglial cell lines offer a potentially powerful route to investigate the possible roles of microglia in prion diseases.
Acknowledgments
We are grateful to N. Nishida for the donation of the N2a58 cell line, M. Horiuchi for the donation of Chandler scrapie brain, and S. Wilson for technical advice. We thank N. Yamaguchi for her technical help.
This work was supported by a grant-in-aid for the strategic cooperation to control emerging and reemerging infections from the Ministry of Education, Culture, Sports, and Technology of Japan and the Bovine Spongiform Encephalopathy Control Project from the Ministry of Agriculture, Forestry and Fisheries of Japan.
Footnotes
Published ahead of print on 22 November 2006.
REFERENCES
- 1.Archer, F., C. Bachelin, O. Andreoletti, N. Besnard, G. Perrot, C. Langevin, A. Le Dur, D. Vilette, A. Baron-Van Evercooren, J. L. Vilotte, and H. Laude. 2004. Cultured peripheral neuroglial cells are highly permissive to sheep prion infection. J. Virol. 78:482-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arima, K., N. Nishida, S. Sakaguchi, K. Shigematsu, R. Atarashi, N. Yamaguchi, D. Yoshikawa, J. Yoon, K. Watanabe, N. Kobayashi, S. Mouillet-Richard, S. Lehmann, and S. Katamine. 2005. Biological and biochemical characteristics of prion strains conserved in persistently infected cell cultures. J. Virol. 79:7104-7112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker, C. A., D. Martin, and L. Manuelidis. 2002. Microglia from Creutzfeldt-Jakob disease-infected brains are infectious and show specific mRNA activation profiles. J. Virol. 76:10905-10913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bate, C., R. S. Boshuizen, J. P. Langeveld, and A. Williams. 2002. Temporal and spatial relationship between the death of PrP-damaged neurones and microglial activation. Neuroreport 13:1695-1700. [DOI] [PubMed] [Google Scholar]
- 5.Bate, C., S. Reid, and A. Williams. 2001. Killing of prion-damaged neurones by microglia. Neuroreport 12:2589-2594. [DOI] [PubMed] [Google Scholar]
- 6.Birkett, C. R., R. M. Hennion, D. A. Bembridge, M. C. Clarke, A. Chree, M. E. Bruce, and C. J. Bostock. 2001. Scrapie strains maintain biological phenotypes on propagation in a cell line in culture. EMBO J. 20:3351-3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown, A. R., J. Webb, S. Rebus, R. Walker, A. Williams, and J. K. Fazakerley. 2003. Inducible cytokine gene expression in the brain in the ME7/CV mouse model of scrapie is highly restricted, is at a strikingly low level relative to the degree of gliosis and occurs only late in disease. J. Gen. Virol. 84:2605-2611. [DOI] [PubMed] [Google Scholar]
- 8.Brown, D. R., B. Schmidt, and H. A. Kretzschmar. 1996. Role of microglia and host prion protein in neurotoxicity of a prion protein fragment. Nature 380:345-347. [DOI] [PubMed] [Google Scholar]
- 9.Caughey, B. W., A. Dong, K. S. Bhat, D. Ernst, S. F. Hayes, and W. S. Caughey. 1991. Secondary structure analysis of the scrapie-associated protein PrP 27-30 in water by infrared spectroscopy. Biochemistry 30:7672-7680. [DOI] [PubMed] [Google Scholar]
- 10.Eikelenboom, P., C. Bate, W. A. Van Gool, J. J. Hoozemans, J. M. Rozemuller, R. Veerhuis, and A. Williams. 2002. Neuroinflammation in Alzheimer's disease and prion disease. Glia 40:232-239. [DOI] [PubMed] [Google Scholar]
- 11.Fischer, M., T. Rulicke, A. Raeber, A. Sailer, M. Moser, B. Oesch, S. Brandner, A. Aguzzi, and C. Weissmann. 1996. Prion protein (PrP) with amino-proximal deletions restoring susceptibility of PrP knockout mice to scrapie. EMBO J. 15:1255-1264. [PMC free article] [PubMed] [Google Scholar]
- 12.Forloni, G., N. Angeretti, R. Chiesa, E. Monzani, M. Salmona, O. Bugiani, and F. Tagliavini. 1993. Neurotoxicity of a prion protein fragment. Nature 362:543-546. [DOI] [PubMed] [Google Scholar]
- 13.Giese, A., D. R. Brown, M. H. Groschup, C. Feldmann, I. Haist, and H. A. Kretzschmar. 1998. Role of microglia in neuronal cell death in prion disease. Brain Pathol. 8:449-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guiroy, D. C., I. Wakayama, P. P. Liberski, and D. C. Gajdusek. 1994. Relationship of microglia and scrapie amyloid-immunoreactive plaques in kuru, Creutzfeldt-Jakob disease and Gerstmann-Straussler syndrome. Acta Neuropathol. 87:526-530. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi, H. K., T. Yokoyama, M. Takata, Y. Iwamaru, M. Imamura, Y. K. Ushiki, and M. Shinagawa. 2005. The N-terminal cleavage site of PrPSc from BSE differs from that of PrPSc from scrapie. Biochem. Biophys. Res. Commun. 328:1024-1027. [DOI] [PubMed] [Google Scholar]
- 16.Kimberlin, R. H., and R. F. Marsh. 1975. Comparison of scrapie and transmissible mink encephalopathy in hamsters. I. Biochemical studies of brain during development of disease. J. Infect. Dis. 131:97-103. [DOI] [PubMed] [Google Scholar]
- 17.Lane, A., C. J. Stanley, S. Dealler, and S. M. Wilson. 2003. Polymeric ligands with specificity for aggregated prion proteins. Clin. Chem. 49:1774-1775. [Google Scholar]
- 18.McKinley, M. P., D. C. Bolton, and S. B. Prusiner. 1983. A protease-resistant protein is a structural component of the scrapie prion. Cell 35:57-62. [DOI] [PubMed] [Google Scholar]
- 19.Mühleisen, H., J. Gehrmann, and R. Meyermann. 1995. Reactive microglia in Creutzfeldt-Jakob disease. Neuropathol. Appl. Neurobiol. 21:505-517. [DOI] [PubMed] [Google Scholar]
- 20.Nishida, N., D. A. Harris, D. Vilette, H. Laude, Y. Frobert, J. Grassi, D. Casanova, O. Milhavet, and S. Lehmann. 2000. Successful transmission of three mouse-adapted scrapie strains to murine neuroblastoma cell lines overexpressing wild-type mouse prion protein. J. Virol. 74:320-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohsawa, K., Y. Imai, K. Nakajima, and S. Kohsaka. 1997. Generation and characterization of a microglial cell line, MG5, derived from a p53-deficient mouse. Glia 21:285-298. [PubMed] [Google Scholar]
- 22.Pan, K. M., M. Baldwin, J. Nguyen, M. Gasset, A. Serban, D. Groth, I. Mehlhorn, Z. Huang, R. J. Fletterick, F. E. Cohen, and S. B. Prusiner. 1993. Conversion of alpha-helices into beta-sheets features in the formation of the scrapie prion proteins. Proc. Natl. Acad. Sci. USA 90:10962-10966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peyrin, J. M., C. I. Lasmezas, S. Haik, F. Tagliavini, M. Salmona, A. Williams, D. Richie, J. P. Deslys, and D. Dormont. 1999. Microglial cells respond to amyloidogenic PrP peptide by the production of inflammatory cytokines. Neuroreport 10:723-729. [DOI] [PubMed] [Google Scholar]
- 24.Prusiner, S. B. 1993. Genetic and infectious prion diseases. Arch. Neurol. 50:1129-1153. [DOI] [PubMed] [Google Scholar]
- 25.Prusiner, S. B. 1982. Novel proteinaceous infectious particles cause scrapie. Science 216:136-144. [DOI] [PubMed] [Google Scholar]
- 26.Race, R., A. Jenny, and D. Sutton. 1998. Scrapie infectivity and proteinase K-resistant prion protein in sheep placenta, brain, spleen, and lymph node: implications for transmission and antemortem diagnosis. J. Infect. Dis. 178:949-953. [DOI] [PubMed] [Google Scholar]
- 27.Race, R. E., L. H. Fadness, and B. Chesebro. 1987. Characterization of scrapie infection in mouse neuroblastoma cells. J. Gen. Virol. 68:1391-1399. [DOI] [PubMed] [Google Scholar]
- 28.Schätzl, H. M., L. Laszlo, D. M. Holtzman, J. Tatzelt, S. J. DeArmond, R. I. Weiner, W. C. Mobley, and S. B. Prusiner. 1997. A hypothalamic neuronal cell line persistently infected with scrapie prions exhibits apoptosis. J. Virol. 71:8821-8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scott, J. R. 1993. Scrapie pathogenesis. Br. Med. Bull. 49:778-791. [DOI] [PubMed] [Google Scholar]
- 30.Shinagawa, M., K. Takahashi, S. Sasaki, S. Doi, H. Goto, and G. Sato. 1985. Characterization of scrapie agent isolated from sheep in Japan. Microbiol. Immunol. 29:543-551. [DOI] [PubMed] [Google Scholar]
- 31.Suzumura, A., S. G. Mezitis, N. K. Gonatas, and D. H. Silberberg. 1987. MHC antigen expression on bulk isolated macrophage-microglia from newborn mouse brain: induction of Ia antigen expression by gamma-interferon. J. Neuroimmunol. 15:263-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takenouchi, T., K. Ogihara, M. Sato, and H. Kitani. 2005. Inhibitory effects of U73122 and U73343 on Ca2+ influx and pore formation induced by the activation of P2X7 nucleotide receptors in mouse microglial cell line. Biochim. Biophys. Acta 1726:177-186. [DOI] [PubMed] [Google Scholar]
- 33.Taraboulos, A., D. Serban, and S. B. Prusiner. 1990. Scrapie prion proteins accumulate in the cytoplasm of persistently infected cultured cells. J. Cell Biol. 110:2117-2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams, A., A. M. Van Dam, D. Ritchie, P. Eikelenboom, and H. Fraser. 1997. Immunocytochemical appearance of cytokines, prostaglandin E2 and lipocortin-1 in the CNS during the incubation period of murine scrapie correlates with progressive PrP accumulations. Brain Res. 754:171-180. [DOI] [PubMed] [Google Scholar]
- 35.Williams, A. E., L. J. Lawson, V. H. Perry, and H. Fraser. 1994. Characterization of the microglial response in murine scrapie. Neuropathol. Appl. Neurobiol. 20:47-55. [DOI] [PubMed] [Google Scholar]
- 36.Yokoyama, T., K. M. Kimura, Y. Ushiki, S. Yamada, A. Morooka, T. Nakashiba, T. Sassa, and S. Itohara. 2001. In vivo conversion of cellular prion protein to pathogenic isoforms, as monitored by conformation-specific antibodies. J. Biol. Chem. 276:11265-11271. [DOI] [PubMed] [Google Scholar]