Abstract
The Kaposi's sarcoma-associated herpesvirus latent protein LANA2 has been suggested to have an important role in the transforming activity of the virus based on its capacity to inhibit p53 and PKR-dependent apoptosis as well as the interferon-dependent response. Here, we describe a novel interaction between LANA2 and both the phosphoserine/phosphothreonine-binding 14-3-3 proteins and the transcription factor FOXO3a. In addition, our results indicate that LANA2 inhibits the transcriptional activity of FOXO3a and blocks the G2/M arrest induced by 14-3-3 protein overexpression. These results suggest a novel mechanism by which LANA2 may promote tumorigenesis.
Kaposi's sarcoma-associated herpesvirus (KSHV; also called human herpesvirus 8) is a gammaherpesvirus associated with all clinical forms of Kaposi's sarcoma, primary effusion lymphomas, and a subset of Castleman's disease (6, 23). The genome of KSHV contains cellular homologues that permit the manipulation of the local environment for efficient viral replication and evasion of the immune response and that also may contribute to host proliferation and cell transformation. One of these genes is the ORFK10.5 encoding a latent protein called LANA2 (latency associated nuclear antigen 2; also called viral interferon regulatory factor 3, or vIRF3), detected exclusively in KSHV-infected B cells (16, 22). LANA2 functions as a dominant-negative mutant of both IRF-3 and IRF-7 and inhibits virus-mediated transcriptional activity of the IFNA promoter (16). In addition, LANA2 is able to inactivate the apoptosis and transcriptional activation mediated by the tumor suppressor gene product p53 (22) as well as the apoptosis induced by activation of the serine/threonine kinase PKR (9). These findings together suggest that LANA2 may have a role in KSHV-mediated oncogenesis in hematopoietic tissues.
The 14-3-3 proteins are a family of highly conserved dimeric regulatory proteins involved in many biologically important processes, such as cell cycle control, apoptosis, and oncogenesis (10). Thus, 14-3-3 proteins promote the cytoplasmic localization of the FOXO class of the forkhead family of winged-helix transcription factors, FOXO1, FOXO3a, and FOXO4, resulting in the inhibition of their ability to bind DNA and to activate the transcription of target genes (3). Activation of genes by FOXOs results in the arrest of the cells at G1 (17, 21) and the induction of apoptosis in many cancer cell lines (5, 11, 18).
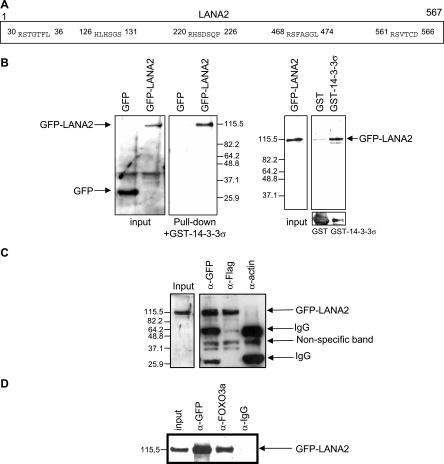
Binding of 14-3-3 proteins to target proteins is generally mediated through the sequences R[SFYW]XpSXP (interaction motif 1), RX[SYFWTQAD]Xp(S/T)X[PLM] (interaction motif 2), or through consensus sequences which do not exactly match the mode 1 and mode 2 ligands [RHK][STALV]X[p- (S/T)]X[PESRDIF]. By using the Eukaryotic Linear Motif server at http://elm.eu.org/, we found that LANA2 contains five putative 14-3-3 binding sites, as shown in Fig. 1A. Based on this finding, we carried out interaction assay using glutathione S-transferase (GST)-14-3-3 sigma (σ) protein and extracts from MCF-7 cells transfected with the enhanced green fluorescent protein (EGFP)-LANA2 plasmid (19). EGFP-LANA2 but not EGFP was detected after incubation of the cell extracts with GST-14-3-3σ (Fig. 1B, left). In addition, EGFP-LANA2 was detected only after incubation with GST-14-3-3σ but not with GST alone (Fig. 1B, right). In order to determine whether LANA2 also interacts with 14-3-3 protein in vivo, we carried out immunoprecipitation assays in cells cotransfected with EGFP-LANA2 and Flag-14-3-3. Our results show that EGFP-LANA2 is detected after incubation of the cell extracts with anti-GFP (JL-8; Clontech) or anti-Flag antibody (Invitrogen) but not in the extracts incubated with the anti-actin antibody (MP Biomedicals) (Fig. 1C). In addition, a band corresponding to LANA2 was detected after incubation of the KSHV-infected B-cell extracts with anti-14-3-3 antibody (data not shown). These results demonstrate that LANA2 also interacts with 14-3-3 proteins in vivo. Only four other viral proteins, the middle tumor antigen of murine polyomavirus, the hepatitis C virus core protein, the nonstructural proteins NS2 of minute virus of mice, and the Vpr protein of human immunodeficiency virus type 1, have previously been demonstrated to interact with 14-3-3 proteins (2, 4, 14, 20). One of the cellular 14-3-3 binding proteins is the transcription factor FOXO3a. In the presence of survival factors, Akt phosphorylates FOXO3a, leading to association of FOXO3a with 14-3-3 proteins and translocation of the transcription factor from the nucleus to the cytoplasm where FOXO3a is inactive (3, 5, 15, 24). In order to determine if LANA2 may interact also with this transcription factor, immunoprecipitation assays using extracts obtained from EGFP-LANA2-transfected MCF-7 cells were carried out. Again, EGFP-LANA2 was precipitated after incubation of the extracts with anti-GFP or anti-FOXO3a antibody, indicating an interaction between LANA2 and FOXO3a (Fig. 1D). However, when we carried out interaction assays using extracts from MCF-7 cells transfected with LANA2 deletion constructs fused to EGFP, we detected three out of four LANA2 fragments interacting with GST-14-3-3 and only one of the LANA2 fragments interacting with GST-FOXO (data not shown). These results suggest that the interaction between LANA2 and 14-3-3 protein is mediated by several LANA2 domains, in contrast to a more restricted LANA2-FOXO interaction.
FIG. 1.
LANA2 interacts with 14-3-3 and FOXO3a proteins. (A) Schematic representation of the 14-3-3 binding motifs in LANA2. (B) LANA2 binds to 14-3-3 in vitro. Cell extracts from EGFP- or EGFP-LANA2-transfected cells were incubated with GST-14-3-3σ, and after centrifugation with glutathione beads and transfer to a nitrocellulose membrane, LANA2 was detected with anti-GFP antibody (Pull-down). The level of EGFP or EGFP-LANA2 in the cell extracts is also shown in the left panel (Input). Cell extracts obtained from EGFP-LANA2-transfected cells were incubated with GST or GST-14-3-3σ (far right, bottom), and LANA2 was detected using anti-GFP antibody (far right, top). (C) LANA2 binds to 14-3-3 protein in vivo. MCF-7 cells were cotransfected with GFP-LANA2 and Flag-14-3-3σ, and 48 h after transfection, the cells were lysed. LANA2 or 14-3-3 protein was immunoprecipitated with anti-GFP or anti-Flag antibodies, respectively. Immunoprecipitated proteins were immunoblotted with anti-GFP antibodies. (D) LANA2 binds to FOXO3a in vivo. MCF-7 cells were transfected with GFP-LANA2, and cell lysates were subjected to immunoprecipitation with anti-GFP or anti-FOXO3a antibody. Immunoprecipitated proteins were immunoblotted with anti-GFP antibody.
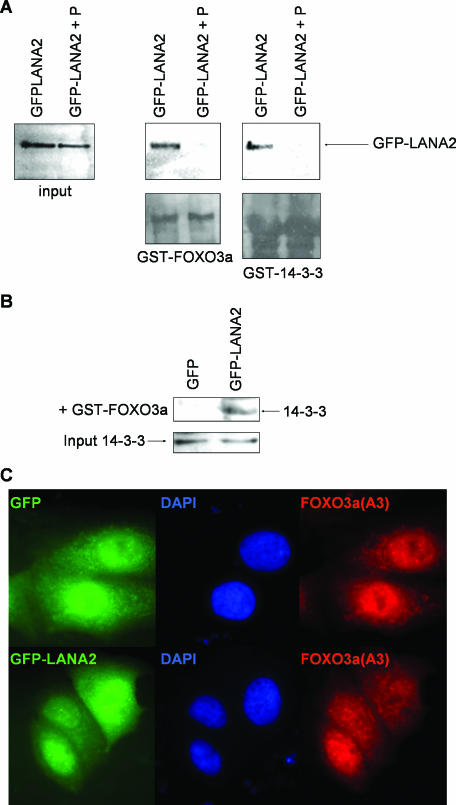
The interaction between 14-3-3 protein and most of its targets depends on the phosphorylation of the protein target. In vitro binding assays were then carried out using extracts fromcells transfected with EGFP-LANA2 and treated with phosphatase. LANA2 was not detected after incubation with GST-14-3-3 when the cell extracts were treated with phosphatase, demonstrating the dependence on LANA2 phosphorylation (Fig. 2A), as reported for most of the 14-3-3 binding partners. In addition, the GST-FOXO3a protein obtained from Escherichia coli was incubated with extracts obtained from cells transfected with EGFP-LANA2 and left untreated or treated with 20 units of calf intestinal phosphatase (Roche) at 37°C for 15 min. LANA2 was detected after incubation with GST-FOXO3a only when the cell extracts were not treated with phosphatase, indicating that the interaction between LANA2 and FOXO3a depends on the phosphorylation of LANA2 (Fig. 2A). In addition, the use of nonphosphorylated GST-FOXO3a indicates that, in contrast to 14-3-3 that interacts exclusively with phosphorylated FOXO3a, LANA2 was able to bind to nonphosphorylated FOXO3a. We then postulated that the LANA2 protein may permit the interaction between 14-3-3 and the unphosphorylated FOXO3a. Cell extracts obtained after transfection of EGFP or EGFP-LANA2 in MCF-7 cells were incubated with GST-FOXO3a extracted from E. coli, and a 14-3-3-FOXO3a interaction was determined by Western blot analysis using anti-14-3-3 antibody (Santa Cruz Biotechnology). As expected, the unphosphorylated GST-FOXO3a did not coprecipitate with 14-3-3 after incubation of the cell extracts obtained from the EGFP-transfected cells with the GST-FOXO3a protein (Fig. 2B). However, 14-3-3 protein was detected when the cell extracts were transfected with EGFP-LANA2 (Fig. 2B), indicating that LANA2 facilitates the binding between 14-3-3 and unphosphorylated FOXO3a. This hypothesis was also supported after analysis of the localization of the unphosphorylatable FOXO3a, FOXO3a(A3), in the presence or absence of LANA2. MCF-7 cells were cotransfected with pcDNA-FOXO3a(A3) (8) and GFP or GFP-LANA2. Forty-eight hours after transfection, analysis of the subcellular localization of the mutant FOXO3a(A3) was determined after immunostaining with anti-polyhistidine antibody (Sigma). FOXO3a(A3) was detected mainly in the nucleus of the cells expressing GFP. In contrast, some cytoplasmic expression was also detected in the GFP-LANA2-expressing cells (Fig. 2C).
FIG. 2.
LANA2 interaction with 14-3-3 protein requires the phosphorylation of LANA2 and allows the binding between 14-3-3 and nonphosphorylated FOXO3a. (A) MCF-7 cells were transfected with GFP-LANA2, and untreated cell lysates or cell lysates treated with phosphatase were incubated with GST-FOXO3a or GST-14-3-3σ. Western blot analysis against 14-3-3 protein or FOXO3a (lower blots) proves that equal amounts of the protein were present in each reaction. (B) 14-3-3 protein binds to GST-FOXO3a when LANA2 is present. MCF-7 cells were transfected with GFP or GFP-LANA2, and cell extracts were incubated with GST-FOXO3a. The presence of 14-3-3 protein was then detected using anti-14-3-3 antibody. The level of 14-3-3 protein present in both cell extracts is shown (Input). (C) FOXO3A(A3) is detected in the cytoplasm when LANA2 is expressed. MCF-7 cells were transfected with pcDNA-FOXO3a(A3) and GFP or GFP-LANA2, and at 48 h, localization of FOXO3a(A3) after immunostaining of cells using anti-polyhistidine antibody was determined.
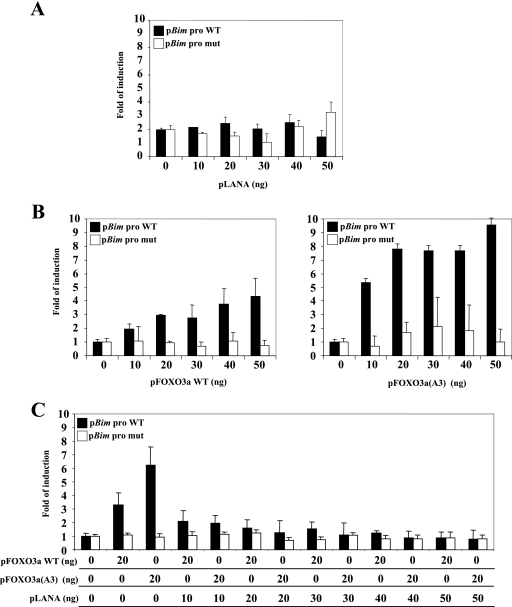
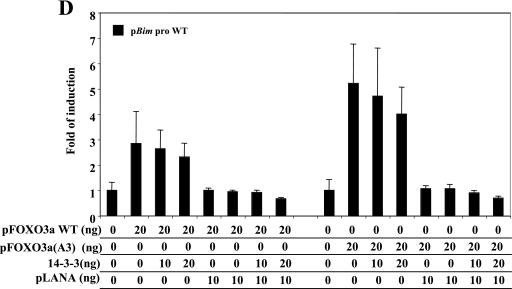
The interaction between 14-3-3 proteins and FOXO3a retains the transcriptional factor in the cytoplasm, inhibiting the transactivation of FOXO3a targets, such as Bim (3). Using reporter assays, determined by dual-luciferase assay (Promega), we investigated whether LANA2 modulated the FOXO3a-mediated transcription of the Bim promoter. Using the pGL3-Bim-Luc reporter construct (8), we showed that transfection of the pcDNA-LANA2 plasmid (19) does not have any effect on the Bim promoter (Fig. 3A). We also show that transfection with both the wild-type (WT) pcDNA-FOXO3a and the pcDNA-FOXO3a(A3) mutant plasmids (8) induces transcriptional activation of the Bim promoter in a dose-dependent manner (Fig. 3B). However, cotransfection of LANA2 inhibited the transactivation of the wild-type Bim promoter mediated by FOXO3a WT or the mutant FOXO3a(A3) in a dose-response manner (Fig. 3C). We then analyzed if this inhibition could be increased after cotransfection of 14-3-3 proteins. Analysis of the effect of 14-3-3 expression on the Bim transactivation shows a small decrease, due probably to the low levels of phosphorylated FOXO3a. However, cotransfection of LANA2 and 14-3-3 proteins clearly reduces this transactivation (around 30% of decrease over the LANA2 effect can be observed by 14-3-3 expression) (Fig. 3D). FOXO family members play a pivotal role in the inhibition of cell transformation and tumorigenesis, suggesting that the inhibition of FOXO3a by LANA2 may represent a new pathway by which KSHV promotes tumorigenesis. Furthermore, 14-3-3 proteins have been connected to cell cycle regulation and signaling, suggesting that the association between LANA2 and 14-3-3 proteins may also deregulate proliferation control and contribute to the development of cell proliferation and neoplasia.
FIG.3.
Repression of FOXO3a-mediated transcription by LANA2 and 14-3-3 proteins. (A) LANA2 expression does not have any effect on the Bim promoter. The wild-type Bim promoter luciferase report construct or a mutant lacking the FOXO binding site was transfected into MCF-7 cells together with increasing amounts of pcDNA-LANA2 plasmid and then analyzed for luciferase activity 48 h later. (B) Transactivation of the reporter plasmid containing the Bim promoter in response to FOXO3a WT or FOXO 3a(A3) mutant. Cells were transfected with the wild-type or the mutant Bim promoter luciferase report construct together with increasing amounts of pcDNA-FOXO3a WT or pcDNA-FOXO3a(A3) mutant and then analyzed for luciferase activity. (C) FOXO3a WT- or FOXO3a(A3) mutant-stimulated expression of the Bim promoter reporter is inhibited by LANA2. MCF-7 cells were cotransfected with the Bim promoter luciferase report plasmid, pcDNA-FOXO3a WT or pcDNA-FOXO3a(A3) mutant, and increasing amounts of pcDNA-LANA2. (D) FOXO3a WT- or FOXO3a(A3) mutant-stimulated expression of the Bim promoter reporter is inhibited by 14-3-3 plus LANA2. MCF-7 cells were cotransfected with the Bim promoter luciferase reporter plasmid, pcDNA-FOXO3a WT, or pcDNA-FOXO3a(A3) mutant and increasing amounts of Flag-14-3-3 alone or in combination with pcDNA-LANA2. After 48 h transfected cells were recovered, and luciferase activity was measured. The total amount of transfected DNA in each experiment was kept constant by the addition of empty vector. The data shown are representative of three independent experiments; error bars represent the standard deviations of the data.
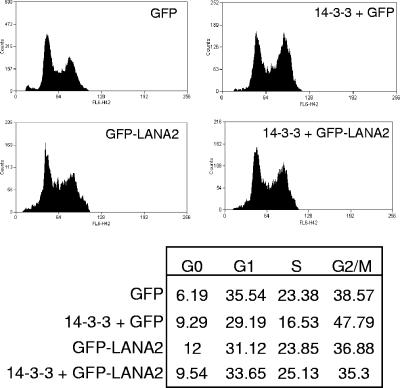
Cell cycle deregulation caused by changes in 14-3-3 expression has been implicated in cancer formation. In this sense, ectopic expression of 14-3-3σ in cycling cells results in a G2 arrest (13). An implication of FOXO proteins in the control of G2/M transition has also been described. Cells with active phosphatidylinositol 3-kinase are delayed in mitotic exit because of decreased in expression of cyclin B and Polo-like kinase (1), which may be mediated by association of forkhead factors with 14-3-3 proteins (12). In order to determine if the expression of LANA2 is able to alter the control of cell cycle mediated by these proteins, MCF-7 cells were transfected with pLXSN-14-3-3σ (7) in the presence or absence of GFP-LANA2, and at 48 h cells were fixed with 1% paraformaldehyde, stained by using propidium iodine, and analyzed by flow cytometry using cyan (DAKO Cytomation) as previously described (19). MCF-7 cells transfected with 14-3-3 protein were arrested in G2/M phase as previously described (Fig. 4). However, cotransfection of LANA2 inhibited the G2/M cell cycle arrest mediated by 14-3-3, suggesting again that LANA2 may play an important role in regulating KSHV-infected B cell growth through its interaction with both proteins.
FIG. 4.
LANA2 inhibits cell cycle arrest mediated by 14-3-3σ overexpression. MCF-7 cells were transfected with pLXSN-14-3-3σ and EGFP or EGFP-LANA2, and 48 h after treatment the cell cycle profile of EGFP-positive cells was analyzed. The percentages of cells in each phase of the cell cycle are given in the table. The data shown are representative of three independent experiments producing similar results.
Acknowledgments
We thank Mark Bedford for the kind gift of the GST-14-3-3 and the Flag-14-3-3 plasmids.
This work was supported by a grant from the Ministerio de Educación y Ciencia de España (BIO2005-00599) and Fundación de Investigación Médica Mutua Madrileña. E.W.-F.L. was supported by Cancer Research-UK, AICR, and LRF.
Footnotes
Published ahead of print on 15 November 2006.
REFERENCES
- 1.Alvarez, B., A. C. Martinez, B. M. Burgering, and A. C. Carrera. 2001. Forkhead transcription factors contribute to execution of the mitotic programme in mammals. Nature 413:744-747. [DOI] [PubMed] [Google Scholar]
- 2.Aoki, H., J. Hayashi, M. Moriyama, Y. Arakawa, and O. Hino. 2000. Hepatitis C virus core protein interacts with 14-3-3 protein and activates the kinase Raf-1. J. Virol. 74:1736-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biggs, W. H., III, J. Meisenhelder, T. Hunter, W. K. Cavenee, and K. C. Arden. 1999. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc. Natl. Acad. Sci. USA 96:7421-7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brockhaus, K., S. Plaza, D. J. Pintel, J. Rommelaere, and N. Salome. 1996. Nonstructural proteins NS2 of minute virus of mice associate in vivo with 14-3-3 protein family members. J. Virol. 70:7527-7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunet, A., A. Bonni, M. J. Zigmond, M. Z. Lin, P. Juo, L. S. Hu, M. J. Anderson, K. C. Arden, J. Blenis, and M. E. Greenberg. 1999. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96:857-868. [DOI] [PubMed] [Google Scholar]
- 6.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865-1869. [DOI] [PubMed] [Google Scholar]
- 7.Dellambra, E., O. Golisano, S. Bondanza, E. Siviero, P. Lacal, M. Molinari, S. D'Atri, and M. De Luca. 2000. Downregulation of 14-3-3sigma prevents clonal evolution and leads to immortalization of primary human keratinocytes. J. Cell Biol. 149:1117-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Essafi, A., S. Fernandez de Mattos, Y. A. Hassen, I. Soeiro, G. J. Mufti, N. S. Thomas, R. H. Medema, and E. W. Lam. 2005. Direct transcriptional regulation of Bim by FoxO3a mediates STI571-induced apoptosis in Bcr-Abl-expressing cells. Oncogene 24:2317-2329. [DOI] [PubMed] [Google Scholar]
- 9.Esteban, M., M. A. Garcia, E. Domingo-Gil, J. Arroyo, C. Nombela, and C. Rivas. 2003. The latency protein LANA2 from Kaposi's sarcoma-associated herpesvirus inhibits apoptosis induced by dsRNA-activated protein kinase but not RNase L activation. J. Gen. Virol. 84:1463-1470. [DOI] [PubMed] [Google Scholar]
- 10.Fu, H., R. R. Subramanian, and S. C. Masters. 2000. 14-3-3 proteins: structure, function, and regulation. Annu. Rev. Pharmacol. Toxicol. 40:617-647. [DOI] [PubMed] [Google Scholar]
- 11.Gilley, J., P. J. Coffer, and J. Ham. 2003. FOXO transcription factors directly activate bim gene expression and promote apoptosis in sympathetic neurons. J. Cell Biol. 162:613-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hermeking, H., and A. Benzinger. 2006. 14-3-3 proteins in cell cycle regulation. Semin. Cancer Biol. 16:183-192. [DOI] [PubMed] [Google Scholar]
- 13.Hermeking, H., C. Lengauer, K. Polyak, T. C. He, L. Zhang, S. Thiagalingam, K. W. Kinzler, and B. Vogelstein. 1997. 14-3-3 sigma is a p53-regulated inhibitor of G2/M progression. Mol. Cell 1:3-11. [DOI] [PubMed] [Google Scholar]
- 14.Kino, T., A. Gragerov, A. Valentin, M. Tsopanomihalou, G. Ilyina-Gragerova, R. Erwin-Cohen, G. P. Chrousos, and G. N. Pavlakis. 2005. Vpr protein of human immunodeficiency virus type 1 binds to 14-3-3 proteins and facilitates complex formation with Cdc25C: implications for cell cycle arrest. J. Virol. 79:2780-2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kops, G. J., N. D. de Ruiter, A. M. De Vries-Smits, D. R. Powell, J. L. Bos, and B. M. Burgering. 1999. Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature 398:630-634. [DOI] [PubMed] [Google Scholar]
- 16.Lubyova, B., and P. M. Pitha. 2000. Characterization of a novel human herpesvirus 8-encoded protein, vIRF-3, that shows homology to viral and cellular interferon regulatory factors. J. Virol. 74:8194-8201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medema, R. H., G. J. Kops, J. L. Bos, and B. M. Burgering. 2000. AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature 404:782-787. [DOI] [PubMed] [Google Scholar]
- 18.Modur, V., R. Nagarajan, B. M. Evers, and J. Milbrandt. 2002. FOXO proteins regulate tumor necrosis factor-related apoptosis inducing ligand expression. Implications for PTEN mutation in prostate cancer. J. Biol. Chem. 277:47928-47937. [DOI] [PubMed] [Google Scholar]
- 19.Munoz-Fontela, C., E. Rodriguez, C. Nombela, J. Arroyo, and C. Rivas. 2003. Characterization of the bipartite nuclear localization signal of protein LANA2 from Kaposi's sarcoma-associated herpesvirus. Biochem. J. 374:545-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pallas, D. C., H. Fu, L. C. Haehnel, W. Weller, R. J. Collier, and T. M. Roberts. 1994. Association of polyomavirus middle tumor antigen with 14-3-3 proteins. Science 265:535-537. [DOI] [PubMed] [Google Scholar]
- 21.Ramaswamy, S., N. Nakamura, I. Sansal, L. Bergeron, and W. R. Sellers. 2002. A novel mechanism of gene regulation and tumor suppression by the transcription factor FKHR. Cancer Cell 2:81-91. [DOI] [PubMed] [Google Scholar]
- 22.Rivas, C., A. E. Thlick, C. Parravicini, P. S. Moore, and Y. Chang. 2001. Kaposi's sarcoma-associated herpesvirus LANA2 is a B-cell-specific latent viral protein that inhibits p53. J. Virol. 75:429-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soulier, J., L. Grollet, E. Oksenhendler, P. Cacoub, D. Cazals-Hatem, P. Babinet, M. F. d'Agay, J. P. Clauvel, M. Raphael, L. Degos, et al. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood 86:1276-1280. [PubMed] [Google Scholar]
- 24.Tang, E. D., G. Nunez, F. G. Barr, and K. L. Guan. 1999. Negative regulation of the forkhead transcription factor FKHR by Akt. J. Biol. Chem. 274:16741-16746. [DOI] [PubMed] [Google Scholar]