Abstract
Innate inflammatory events promoting antiviral defense in the liver against murine cytomegalovirus (MCMV) infection have been characterized. However, the mechanisms that regulate the selective recruitment of inflammatory T lymphocytes to the liver during MCMV infection have not been defined. The studies presented here demonstrate the expression of monokine induced by gamma interferon (IFN-γ; Mig/CXCL9) and IFN-γ-inducible protein 10 (IP-10/CXCL10) in liver leukocytes and correlate their production with the infiltration of MCMV-specific CD8 T cells into the liver. Antibody-mediated neutralization of CXCL9 and CXCL10 and studies using mice deficient in CXCR3, the primary known receptor for these chemokines, revealed that CXCR3-dependent mechanisms promote the infiltration of virus-specific CD8 T cells into the liver during acute infection with MCMV. Furthermore, CXCR3 functions augmented the hepatic accumulation of CD8 T-cell IFN-γ responses to MCMV. Evaluation of protective functions demonstrated enhanced pathology that overlapped with transient increases in virus titers in CXCR3-deficient mice. However, ultimate viral clearance and survival were not compromised. Thus, CXCR3-mediated signals support the accumulation of MCMV-specific CD8 T cells that contribute to, but are not exclusively required for, protective responses in a virus-infected tissue site.
Host responses to viral infections involve complex interactions between cytokines and chemokines that provide key communication signals resulting in the effective development of innate and adaptive immunity. Murine cytomegalovirus (MCMV) is a herpesvirus with pathogenic potential that can induce high levels of viral replication in the spleen and liver with associated loss of liver function during acute infection. Thus, innate immune responses are critical in limiting viral spread and averting virus-induced disease (1, 2, 57). These responses have been well characterized and are centered on the early activation of natural killer (NK) cells and the delivery of NK cell-derived gamma interferon (IFN-γ) through a MIP-1α/CCL3-dependent inflammatory response in the liver (1, 2, 4, 35, 37, 40, 41, 43, 50). Nonetheless, restriction of virus-associated tissue damage and promotion of viral clearance from MCMV-infected tissues requires T-lymphocyte activation (reviewed in references 19 and 20).
The protective function of T lymphocytes, particularly the CD8+ T-cell subset, against MCMV infection has been well documented and includes the production of IFN-γ and tumor necrosis factor alpha during late acute infection, or between 4 and 9 days following MCMV challenge (5, 8, 11-13, 15, 17-20, 24, 36, 39, 48, 51). In the liver, adoptive transfer of MCMV-primed CD8 T cells into γ-irradiated hosts leads to effective control of viral replication and specifically limits the extent of liver pathology (38, 39). Additionally, T-cell depletion studies demonstrated a role for these cells in controlling hepatic damage and promoting host survival (34, 51), suggesting that the MCMV-infected liver is sensitive to immune control by CD8 T cells. Furthermore, studies have demonstrated the accumulation and development of an effective CD8 T-cell response in various tissues that becomes maximally apparent 7 days after infection with MCMV (12, 20). Thus, it is clear that CD8 T cells support hepatic immunity; however, the mechanisms promoting the recruitment of activated CD8 T lymphocytes into the liver during MCMV infection have not been evaluated.
Chemokines are a superfamily of inflammatory cytokines that route the migration and localization of immune effector cells in tissues by binding and signaling them through seven-transmembrane G-protein-coupled receptors (26, 27, 44, 59). Tissue-infiltrating T cells and Th1-polarized T cells have been shown to express the chemokine receptor CXCR3 and respond to the IFN-γ-inducible CXCR3 ligands monokine induced by IFN-γ (Mig/CXCL9) and IFN-γ-inducible protein 10 (IP-10/CXCL10) (23, 25, 26, 31, 44, 53). CXCR3 and its ligands have been shown to function in host resistance to virus infection in a number of different murine models by regulating the trafficking of activated inflammatory T cells (7, 22, 26, 30, 59). Moreover, liver production of CXCL9 and CXCL10 has been associated with the infiltration of CXCR3-expressing T lymphocytes in individuals with chronic hepatitis C virus infections (47). Studies have also shown that CXCR3 activation promotes the transendothelial migration of effector T cells across hepatic endothelium in response to CXCR3 ligands (6). Together, these observations suggest a functional role for CXCR3 and its ligands in promoting T-lymphocyte migration into the liver.
Previous studies have shown that NK cell inflammation can support the expression of CXCL9 in the liver and promote antiviral effects (41). Nevertheless, a role for CXCL9 in the recruitment of protective hepatic T lymphocytes has not been shown. Furthermore, production of CXCL9 and CXCL10 during late acute MCMV infection remains to be determined. Likewise, the biological responses on the recruitment of activated T lymphocytes resulting from CXCR3 expression have not been evaluated during MCMV infection in the liver.
The studies presented here were undertaken to determine the functional significance of CXCL9 and CXCL10 production and CXCR3 expression during late acute MCMV infection in the liver. The results demonstrate a correlation between CXCL9 and CXCL10 production and the hepatic accumulation of MCMV-specific CD8 T cells that express CXCR3 during late acute infection. Antibody neutralization of these CXCR3-binding chemokines and studies using mice deficient in the CXCR3 receptor establish that CXCR3-dependent mechanisms enhance the effective recruitment of inflammatory and virus-specific IFN-γ-secreting CD8 T cells. The results suggest that the inefficient infiltration of activated virus-specific CD8 T cells provides a brief window of opportunity for enhanced virus replication and liver pathology. Yet, susceptibility and viral clearance are not hindered. Collectively, these results identify chemokine and chemokine receptor responses that support the localization of selected T lymphocytes that contribute in part to the effectiveness of antiviral responses in infected tissue sites.
MATERIALS AND METHODS
Mice.
Specific-pathogen-free C57BL/6 (H-2b) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). CXCR3-deficient (CXCR3−/−) mice (Deltagen, San Carlos, CA) backcrossed for at least 10 generations to C57BL/6 (Jackson Laboratory) were used to establish a colony at Brown University. The genotypes were verified by PCR using genomic tail DNA. All experiments used male mice between 6 and 8 weeks of age. Mouse handling and experimental procedures were conducted in accordance with institutional guidelines.
Virus infections and in vivo treatment protocols.
Infections were initiated on day 0 (uninfected) by intraperitoneal injections of 5 × 104 PFU of Smith strain MCMV prepared from salivary gland extracts (40-42). For CXCL9 and CXCL10 neutralization, mice were treated with rabbit polyclonal antiserum against CXCL9 or CXCL10 (22, 49) or with control rabbit serum (Jackson Immunoresearch Laboratories, Inc., West Grove, PA) on days 0, 1, 3, and 5 after infection.
Isolation of hepatic leukocytes.
Hepatic leukocytes were prepared using published methods (14, 41, 42) with modifications. Briefly, livers were passed through a 70-μm-pore-size nylon cell strainer (BD Falcon, Franklin Lakes, NJ), washed three times in 3% phosphate-buffered saline (PBS)-serum, and treated with ammonium chloride to lyse red blood cells. Cell suspensions were then layered onto two-step discontinuous Percoll gradients (Pharmacia Fine Chemicals, Piscataway, NJ) for density separation. Hepatic leukocytes were collected after centrifugation for 30 min at 900 × g. Cell yields and viabilities were determined by trypan blue exclusion (Invitrogen Life Technologies, Gaithersburg, MD).
Generation of conditioned media and cytokine analysis.
Liver leukocytes were plated in flat-bottom microtiter plates at 106 cells/well in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum (HyClone Laboratories, Logan, UT). After 24 h of incubation at 37°C, microtiter plates were centrifuged and cell supernatants were collected. Levels of soluble cytokine proteins in conditioned media were measured using commercial sandwich enzyme-linked immunosorbent assay (ELISA) kits or Duoset ELISA development systems (R&D Systems Inc., Minneapolis, MN) for IFN-γ, CXCL9, or CXCL10, as recommended by the manufacturer.
Flow cytometric analysis.
Cell surface staining was performed as previously described (14, 41, 42), using the following fluorochrome-conjugated monoclonal antibodies: CD8α-fluorescein isothiocyanate (FITC) (clone 53-6.7), CD4-FITC (clone GK1.5), T-cell receptor β-chain (TCRβ)-phycoerythrin (PE) (clone H57-597), and CXCR3-PE (clone 220803). CD8 T cells were phenotypically identified as CD8α+ CD4− TCRβ+ populations. All antibodies were diluted in the presence of anti-CD16/CD32 monoclonal antibody (clone 2.4G2) to block the nonspecific binding of antibodies to the receptor of the Fc portion of the immunoglobulin (Ig). Isotype control antibodies were used to correct for background fluorescence and set analysis gates. At least 20,000 to 40,000 events were acquired per sample using a FACSCalibur and CellQuest software (BD Biosciences, San Jose, CA). All monoclonal antibodies were obtained from BD Biosciences, except for anti-CXCR3, which was purchased from R&D Systems Inc.
Major histocompatibility complex class I tetramer staining of hepatic leukocytes.
In order to quantitate CD8 T-cell responses specific to MCMV, flow cytometric analysis was used to determine the direct binding of the TCR with a class I tetrameric major histocompatibility complex of H-2Db and the immunodominant peptide from the M45 viral protein (11, 12). To enumerate T cells, hepatic leukocytes were stained with CD8α-FITC monoclonal antibody as described above and with the M45-HGIRNASFI-Db tetramer (12), followed by streptavidin-allophycocyanin or streptavidin-PE (BD Biosciences). Approximately 100,000 events were collected within a leukocyte acquisition gate using a FACSCalibur and CellQuest-Pro software.
Peptide-specific in vitro stimulations and intracellular IFN-γ staining.
To evaluate IFN-γ-producing T cells in the liver upon MCMV-specific activation, liver leukocytes from uninfected or MCMV-infected mice were plated in triplicate at 106 cells/well with 10% fetal calf serum-RPMI 1640 or medium supplemented with 100 ng/ml of peptide in the presence of brefeldin A (Sigma-Aldrich). After 5 h of incubation, leukocytes were stained with FITC-labeled CD8α (clone 53-6.7), fixed with 4% formaldehyde in PBS, and then treated with permeabilization buffer before being stained with PE-conjugated rat anti-mouse IFN-γ (clone XMG1.2) or isotype control rat IgG1 (BD Biosciences). Isotype control antibodies were used to correct for background fluorescence and set analysis gates. Approximately 100,000 events were collected within a leukocyte acquisition gate using a FACSCalibur and CellQuest-Pro software.
Immunofluorescence.
After removal, liver samples were embedded and frozen in liquid nitrogen. Sections were cut at a thickness of 5 μm using a Leica CM 3050S cryostat (Leica Microsystems, Deerfield, IL), fixed in cold acetone, rehydrated in PBS, and blocked for nonspecific staining with 10% normal fetal bovine serum in PBS. For dual fluorescent detection of cellular antigens, the following monoclonal antibodies were used: rat anti-mouse CD4 (clone GK1.5), rat anti-mouse CD8 (clone 53-6.7), and rabbit anti-mouse CXCR3 (clone 220803), all from R&D Systems, Inc. For detection of CD4 and CD8 primary antibodies, a tetramethyl rhodamine-conjugated F(ab′)2 fragment against rat IgG was used. For detection of CXCR3 primary antibodies, a FITC-conjugated F(ab′)2 fragment against rabbit IgG was used. Both secondary antibodies were from Jackson ImmunoResearch (West Grove, PA). Equivalent concentrations of rat or rabbit IgG were used as control antibodies (Sigma Chemical Co., St. Louis, MO). Staining specificity was documented by lack of reaction with control antibodies in the absence of primary antibody. Dual-stained slides were then subjected to fluorescence microscopy using an Olympus CX41RF microscope equipped with a fluorescence illuminator (Optical Analysis Corporation, Nashua, NH). Images were photographed with a DP70 digital camera and software (Optical Analysis Corporation).
Enzyme analyses.
Liver damage was determined by measuring alanine aminotransferase (ALT) levels in serum samples using an ALT colorimetric kit according to the manufacturer's instructions (Biotron Diagnostics, Hemet, CA).
Viral titers.
Viral titers were assessed with plaque assays on NIH 3T3 fibroblasts (ATCC CRL 1658; Manassas, VA) by methods previously described (14, 40-42). To enumerate plaques, cells were fixed with 10% buffered formalin and stained with 0.1% crystal violet. Titers were quantitated as log10 PFU/gram of tissue.
Statistical analyses.
The statistical significance of the experimental results was analyzed by the two-tailed Student t test where indicated.
RESULTS
Kinetics of CD8 T-cell responses in the liver during acute MCMV infection.
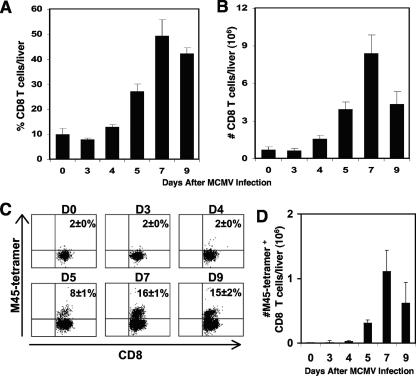
Several studies have demonstrated the accumulation and development of effective CD8 T-cell responses in various tissues during acute infections with MCMV that become maximally apparent by day 7 postinfection (12, 20). To evaluate the kinetics of CD8 T-cell accumulation in the liver, flow cytometric analyses were used to document the expression of cell surface markers for CD8 T cells (CD8α+ CD4− TCRβ+) in liver leukocytes from uninfected and MCMV-infected C57BL/6 mice. Concurrent with previous observations, CD8 T cells were not readily evident on days 3 and 4 postinfection, but the frequencies and absolute numbers were prominently elevated by day 5, with maximal responses peaking on day 7 of infection (Fig. 1A and B). By day 9 of infection, there was a significant 50% reduction in the total number of CD8 T cells compared to the results on day 7, although the proportions were marginally reduced.
FIG. 1.
Kinetics of CD8 T-cell responses in the liver following MCMV infection. Samples were prepared from C57BL/6 mice that were uninfected (day 0) or infected with MCMV after the indicated numbers of days. Total liver leukocytes were harvested and analyzed by flow cytometry. The percentages (A) and numbers (B) of CD8 T cells (CD8α+ CD4− TCRβ+) per liver are shown. To characterize the accumulation of virus-specific CD8 T cells, liver leukocytes were labeled with CD8α and the M45-H-2Db tetramer and examined by flow cytometry. The percentages (C) and total numbers (D) of virus-specific CD8 T cells were identified by analysis of M45 tetramer-positive expression after gating on the CD8α-positive cells. Data shown are the means ± standard errors for five or six mice. Results shown are representative of two experiments.
To assess the accumulation of CD8 T cells responding specifically to virus infection, liver leukocytes isolated from uninfected or MCMV-infected C57BL/6 mice were subjected to flow cytometric analysis using H-2Db tetramers binding an immunodominant MCMV peptide, M45, and cell surface markers for CD8 T cells. The results show that 8% ± 1% of the T cells accumulating in the liver on day 5 of infection were recognizing M45 on H-2Db (Fig. 1C). By day 7 of infection, there was an additional twofold increase in the frequency of M45-specific CD8 T cells. The response was still present but marginally diminished on day 9 of infection. In contrast, the CD8 T-cell responses to M45 on days 3 and 4 remained comparable to the basal levels observed in uninfected mice. As shown in Fig. 1D, the absolute number of virus-specific CD8 T cells was also profoundly increased from 0.01 × 106 ± 0 × 106 cells in uninfected mice to 0.3 × 106 ± 0.05 × 106 and 1.1 × 106 ± 0.3 × 106 cells on days 5 and 7 following infection, respectively. Thus, there was a 30-fold increase in the absolute number of CD8 T cells recognizing M45 on H-2Db on day 5 and a 110-fold increase in this population at the maximal peak of CD8 T-cell accumulation on day 7 following MCMV infection. By day 9, the absolute number of virus-specific CD8 T cells declined by 56% compared to the value at day 7 after infection. The M45-specific CD8 T-cell numbers on days 3 and 4 of infection remained relatively unchanged compared to those observed in uninfected mice. Taken together, the results demonstrate that CD8 T cells, and a subset of infiltrating CD8 T cells responding specifically to MCMV, accumulate in the liver during late acute infection.
Kinetics of IFN-γ, CXCL9, and CXCL10 responses to MCMV infection in the liver.
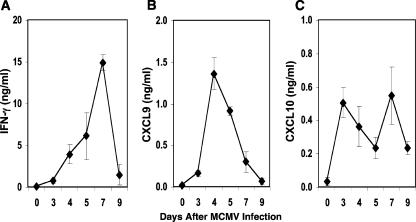
As activated CD8 T cells are known to secrete high levels of IFN-γ, the levels of cytokine production were measured in liver leukocyte-conditioned media prepared from uninfected or MCMV-infected mice at the indicated time points. As shown in Fig. 2A, IFN-γ production was detected at low levels on day 3 and increased steadily thereafter, reaching maximal levels on day 7 following infection. By day 9 postinfection, IFN-γ production was significantly reduced by 90%. These studies demonstrate the kinetics of IFN-γ during late acute MCMV infection in the liver and establish a correlation between cytokine production and CD8 T-cell responses.
FIG. 2.
Kinetics of IFN-γ, CXCL9, and CXCL10 in the liver during MCMV infection. Liver leukocyte-conditioned media were prepared from C57BL/6 mice that were uninfected (day 0) or infected with MCMV after the indicated numbers of days. IFN-γ (A), CXCL9 (B), and CXCL10 (C) proteins were measured by ELISA. Data shown are the means ± standard errors for four to six mice tested individually. Results shown are representative of at least two experiments.
To evaluate whether CD8 T-cell infiltration was associated with the chemokines CXCL9 and CXCL10, potent chemoattractants for activated T cells (23, 31, 44, 59), their levels of production were examined. CXCL9 protein was profoundly elevated and peaked on day 4 in response to MCMV infection (Fig. 2B). These levels declined by 33% and 78% on days 5 and 7 of infection, respectively. On day 9 following infection, CXCL9 levels were comparable to levels measured in uninfected mice. By contrast, CXCL10 exhibited a biphasic response, with maximal levels observed 3 and 7 days after MCMV challenge (Fig. 2C). Both peaks were followed by 55% declines in CXCL10 protein levels, but production was maintained and remained above the levels detected in uninfected mice. Collectively, these results demonstrate that MCMV induces significant levels of CXCL9 and CXCL10 in the liver. Furthermore, the maximal expression of these chemokines precedes peak IFN-γ and CD8 T-cell responses to infection.
Effects of CXCL9 and CXCL10 on MCMV-specific CD8 T-cell responses in the liver.
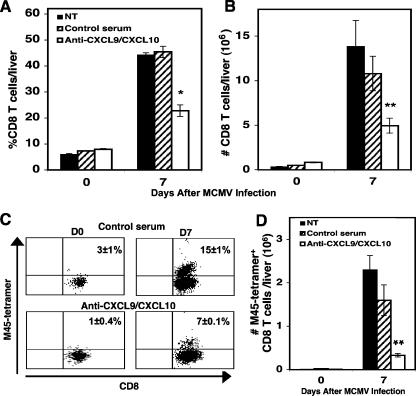
To ascertain whether CXCL9 and CXCL10 are essential participants in the recruitment of CD8 T cells into infected livers, uninfected or day 7 MCMV-infected mice were left untreated, treated with control serum, or treated with neutralizing polyclonal antibodies against CXCL9 and CXCL10. Flow cytometric analysis revealed that administration of anti-CXCL9 and anti-CXCL10 to MCMV-infected mice resulted in a 50% reduction in the frequency (Fig. 3A) and total number (Fig. 3B) of CD8 T cells accumulating in the liver compared with the levels observed in infected mice treated with control serum. Furthermore, neutralization of CXCL9 and CXCL10 resulted in a 50% decrease in the frequency (Fig. 3C) and a 79% decrease in the total number (Fig. 3D) of M45-specific CD8 T cells, compared with the levels observed in infected mice administered control serum (Fig. 3C and D). Also, treatment of mice with anti-CXCL9 or anti-CXCL10 individually resulted in a 36% or 42% decrease in the frequency of accumulating CD8 T cells, respectively (data not shown). Hence, these studies identify CXCL9 and CXCL10 as key factors in promoting the recruitment of CD8 T cells responding to the presence of MCMV in the liver.
FIG. 3.
Effects of CXCL9 and CXCL10 on MCMV-specific CD8 T-cell responses. Samples were prepared from C57BL/6 mice that were not treated (NT), treated with control serum, or treated with immune serum containing antibodies against CXCL9 and CXCL10 and that were either uninfected or infected with MCMV for 7 days, the maximal peak for CD8 T-cell infiltration. Total liver leukocytes were harvested and analyzed by flow cytometry. The percentages (A) and numbers (B) of CD8 T cells (CD8α+ CD4− TCRβ+) per liver are shown. As described in Materials and Methods and in the legend to Fig. 2, the percentages (C) and total numbers (D) of virus-specific CD8 T cells were identified by analysis of M45 tetramer-positive expression after gating on the CD8α-positive cells. Data shown are the means ± standard errors for three mice per treatment. Differences between values for infected control and anti-CXCL9/CXCL10-treated mice are significant at P values of ≤0.03 (*) and P values of ≤0.001 (**).
Characterization of CXCR3 expression on CD8 T cells during MCMV infection in the liver.
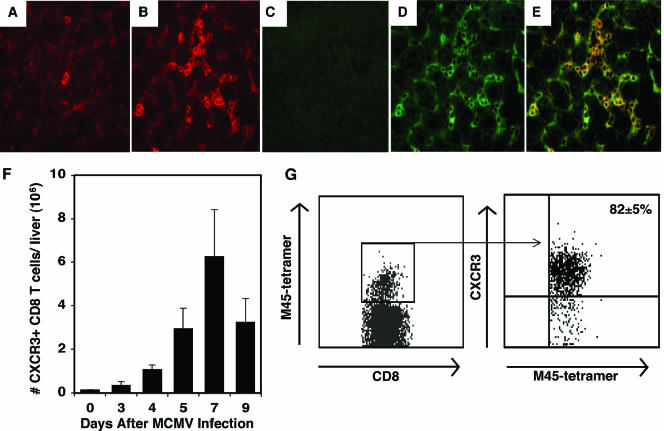
Because CXCL9 and CXCL10 were found to be produced in the liver and exhibited an effect on CD8 T-cell accumulation at this site, experiments were conducted to determine whether CXCR3, the common receptor for CXCL9 and CXCL10, was detectable on CD8 T cells infiltrating the livers of mice infected with MCMV. Liver sections prepared from mice that were uninfected or infected with MCMV for 7 days were examined for CD8 and CXCR3 expression by immunohistochemistry. In uninfected mice, few cells within the liver parenchyma stained positive for CD8 (Fig. 4A), whereas CD8 expression was readily detected throughout the liver following infection (Fig. 4B). The presence of CXCR3 was not detected in liver sections prepared from uninfected mice (Fig. 4C). However, cells expressing CXCR3 were easily observed in MCMV-infected tissues (Fig. 4D), and the results show that a significant majority of the localized CD8 T cells expressed CXCR3 (Fig. 4E). Flow cytometric analysis demonstrated an increase in the accumulation of the total number of CXCR3-expressing CD8 T cells on day 5, with maximal expression observed on day 7 following infection (Fig. 4F). Furthermore, the results show that at least 82% of CD8 T cells recognizing M45 tetramers display CXCR3 on day 7 postinfection, when maximal CD8 T-cell accumulation is detected (Fig. 4G). Collectively, these findings indicate that CXCR3 is expressed on the surfaces of CD8 T cells and a subset of MCMV-specific CD8 T cells accumulating in the liver during infection. Moreover, the results suggest that the infiltrating CD8 T cells are responding to the CXCR3 ligands CXCL9 and CXCL10.
FIG. 4.
Characterization of CXCR3 expression on CD8 T cells in the liver during MCMV infection. Liver sections were prepared from uninfected (A and C) or MCMV-infected (B, D, and E) mice for 7 days and immunostained with CD8 or CXCR3 primary antibodies followed by tetramethyl rhodamine- or FITC-conjugated secondary antibody, respectively, as described in Materials and Methods. Stain results shown are representative of the livers of mice stained for CD8 alone (A and B) (red cells), stained for CXCR3 alone (C and D) (green cells), or dual stained with CD8 and CXCR3 (E) (yellow cells). Magnification, ×40. Results are representative of one of three experiments with similar results. To quantitate the total number of CD8 T cells expressing CXCR3, samples were prepared from mice that were uninfected or infected with MCMV for the indicated numbers of days following infection. Total liver leukocytes were harvested and analyzed by flow cytometry. (F) The numbers of CD8α+ TCRβ+ CXCR3+ cells per liver are shown. Data are the means ± standard errors for six mice. To characterize the accumulation of virus-specific CD8 T cells expressing CXCR3, liver leukocytes were labeled with CD8α, CXCR3, and the M45-H-2Db tetramer and examined by flow cytometry. (G) The frequency of virus-specific CD8 T cells expressing CXCR3 was identified by analysis of M45 tetramer-positive expression after gating on the CD8α-positive cells, followed by gating of CXCR3-positive cells. Data shown are the means ± standard errors for three mice.
CXCR3-dependent effects on MCMV-specific CD8 T-cell responses in the liver.
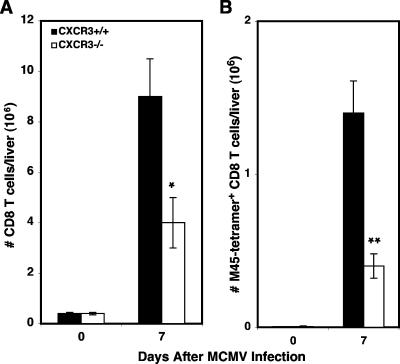
For an independent and more complete evaluation of the role of CXCR3 in CD8 T-cell migration into the liver during MCMV infection, liver leukocytes were isolated from C57BL/6 immunocompetent (CXCR3+/+) or C57BL/6 CXCR3-deficient (CXCR3−/−) mice that were uninfected or infected with MCMV for 7 days. As shown in Fig. 5A, the total number of CD8 T cells accumulating in the livers of infected CXCR3−/− mice was decreased by 56% (4 × 106 ± 1 × 106 cells) compared with that in CXCR3+/+ mice (9 × 106 ± 1.4 × 106 cells). In addition, CXCR3−/− mice had a significant 70% decrease (0.4 × 106 ± 0.8 × 106 cells) in the total number of CD8 T cells binding the M45 tetramer compared with CXCR3+/+ mice (1.4 × 106 ± 0.2 × 106 cells) (Fig. 5B). Thus, CXCR3 promotes the accumulation of CD8 T cells responding to MCMV infection in the liver.
FIG. 5.
Effects of CXCR3 on virus-specific CD8 T-cell responses. Total liver leukocytes were prepared from C57BL/6 immunocompetent (CXCR3+/+) or C57BL/6 CXCR3-deficient (CXCR3−/−) mice that were uninfected or infected with MCMV for 7 days. The numbers of CD8 T cells (A) and virus-specific CD8 T cells (B) were determined as described in Materials and Methods and in the legend to Fig. 4. Data shown are the means ± standard errors for three mice. Differences between values for infected CXCR3+/+ and CXCR3−/− mice are significant at P values of ≤0.05 (*) and P values of ≤0.01 (**).
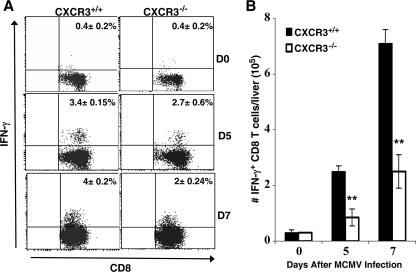
To directly address the effects of CXCR3 in the recruitment of functional effector CD8 T cells, liver leukocytes were prepared from CXCR3+/+ and CXCR3−/− mice that were uninfected or infected with MCMV for 5 or 7 days. The frequency and total number of antigen-specific, IFN-γ-producing CD8 T cells were determined by intracellular expression of IFN-γ after restimulation with the H-2Db M45 viral peptide. The results show expression of IFN-γ in both groups of mice during infection (Fig. 6A and B). However, CXCR3−/− mice had 21% and 50% reductions in the frequency of CD8 T cells responding to the M45 viral peptide compared with CXCR3+/+ mice on days 5 and 7 of infection, respectively (Fig. 6A). In terms of total leukocyte numbers, CXCR3−/− mice had a 65% decline in the accumulation of activated CD8 T cells 5 and 7 days after viral challenge (Fig. 6B). Both groups demonstrated <0.4% IFN-γ expression in uninfected mice. Taken together, these results define a role for CXCR3 in promoting the recruitment of activated virus-specific CD8 T cells into the liver.
FIG. 6.
Effects of CXCR3 on intracellular IFN-γ expression in MCMV infection-primed CD8 T cells. Liver leukocytes were prepared from CXCR3+/+ or CXCR3−/− mice that were uninfected or infected with MCMV for 5 or 7 days. As described in Materials and Methods, cells were cultured for 5 h with 100 ng/ml of M45 viral peptide. Leukocytes were then harvested and stained for surface expression of CD8, fixed, permeabilized, and stained for intracellular IFN-γ. Results show the percentages (A) and total numbers (B) of CD8 T cells expressing IFN-γ after infection as indicated. Data shown are the means ± standard errors for three mice. Similar results were obtained in an additional experiment. Differences between values for infected CXCR3+/+ and CXCR3−/− mice are significant at P values of ≤0.01 (**).
Effects of CXCR3 on virus-induced liver disease and host survival.
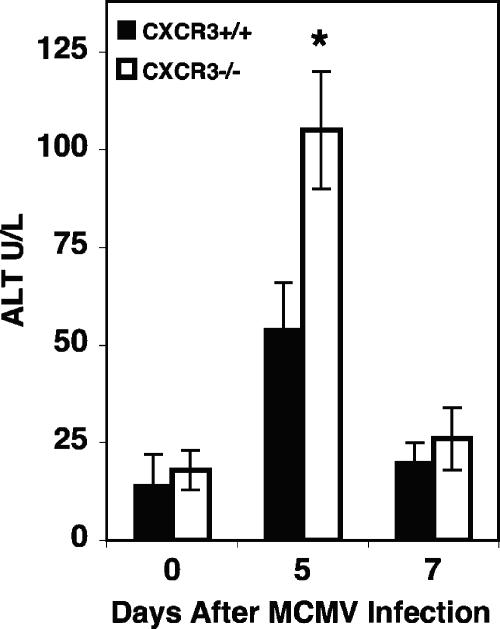
Previous studies have demonstrated that immunocompetent C57BL/6 mice establish viral clearance and resolution of virus-induced liver disease between 5 and 7 days of MCMV infection (12, 14, 41, 42, 51). To specifically evaluate the effects of CXCR3 responses on overall liver function, expression of the liver enzyme ALT was measured in serum samples from CXCR3+/+ and CXCR3−/− mice that were uninfected or infected with MCMV for 5 or 7 days. Uninfected mice had comparable baseline levels of the circulating enzyme (Fig. 7). By day 5 after infection, significant elevations in ALT levels above baseline levels were detected in both groups of mice (Fig. 7). However, the levels observed in CXCR3−/− mice reached values of 105 ± 15 U/liter and were twofold higher than the ALT levels evident in CXCR3+/+ mice (54 ± 12 U/liter), indicating augmented liver pathology in the absence of the CXCR3 receptor. On day 7 of infection, ALT levels declined to values of 26 ± 8 U/liter and 20 ± 5 U/liter in CXCR3−/− and CXCR3+/+ mice, respectively. Thus, both groups of mice had ALT levels comparable to uninfected baseline levels by day 7 of MCMV infection.
FIG. 7.
Effects of CXCR3 on susceptibility to liver disease. CXCR3+/+ and CXCR3−/− mice were infected with 5 × 104 PFU of MCMV for 5 or 7 days. Serum samples were collected and used to measure ALT levels as described in Materials and Methods. Means ± standard errors for three mice are shown. Differences between values for CXCR3+/+ and CXCR3−/− mice are significant at P values of ≤0.05 (*). Data are representative of at least two experiments.
To assess the contribution of CXCR3 responses to antiviral defense, liver and spleen samples were prepared from CXCR3+/+ and CXCR3−/− mice. Mice were infected with 5 × 104 PFU of MCMV for 5 or 7 days, and viral burdens were evaluated. Compared with those in CXCR3+/+ mice, viral titers in the liver and spleen were increased by 1 log10 on day 5 after infection in CXCR3−/− mice (Table 1) . However, the viral burden on day 7 of MCMV infection was below the limit of detection in both compartments of CXCR3+/+ and CXCR3−/− mice (Table 1). These results show a significant but transient increase in viral burden in CXCR3−/− mice, although viral clearance was not compromised. Moreover, in host survival studies wherein mice were challenged with 105 PFU of MCMV, both groups of mice survived beyond 30 days of infection at this virus dose. Altogether, these studies suggest that CXCR3 functions promote resolution of hepatic damage and contribute in part to the control of virus replication and MCMV resistance during acute infection.
TABLE 1.
Effect of CXCR3 on MCMV replication and susceptibility
| Strain | Viral titer (log10 PFU/g) for indicated site and daya
|
Mortality rateb | |||
|---|---|---|---|---|---|
| Day 5
|
Day 7
|
||||
| Liver | Spleen | Liver | Spleen | ||
| CXCR3+/+ | 2.23 ± 0.04 | 2.63 ± 0.01 | BLD | BLD | 0/6 |
| CXCR3−/− | 3.04 ± 0.29c | 3.42 ± 0.2 | BLD | BLD | 0/6 |
Data shown represent the means ± standard errors for three mice. BLD, below the level of detection, or ≤2.0 log10 PFU/g.
The average survival time for all mice was >30 days.
This number compared with that for the control is significant (P ≤ 0.05).
DISCUSSION
In this study, the functional importance of CXCR3-dependent mechanisms in the liver during late acute MCMV infection was investigated. The results show that the production of the chemokines CXCL9 and CXCL10, two known CXCR3 ligands, correlated with the migration of CD8 T cells and a subset of CD8 T cells recognizing the M45 MCMV peptide on H-2Db. Infiltration of these virus-specific CD8 T cells was dramatically reduced in infected mice treated with neutralizing antibodies to CXCL9 and CXCL10. The studies also demonstrate the expression of CXCR3 on trafficking CD8 T cells and clearly establish a function for CXCR3 in promoting the accumulation of virus-specific and IFN-γ-secreting CD8 T cells into the liver. Furthermore, as CXCR3-deficient mice exhibited a temporary elevation in viral burden and enhanced liver pathology, the effects mediated by CXCR3-dependent signaling contribute to the development of effective hepatic immunity.
Additional studies have demonstrated that CD8 T cells accumulate in the liver following infection with MCMV (12, 19, 20, 48, 51); however, this is the first report documenting the mechanisms required for promoting their recruitment and that of MCMV-specific CD8 T cells. Our results show a clear induction of CXCL9 and CXCL10 protein in the liver during MCMV infection. CXCL9 protein rose sharply and was detected at higher levels than CXCL10, reaching utmost expression immediately before the initial influx of CD8 T cells. Interestingly, the peak accumulation of virus-specific CD8 T cells and maximal levels of IFN-γ production were accompanied by profound reductions in the levels of CXCL9 detected in the liver. One plausible proposal for this observation is that CD8 T cells were consuming CXCL9 in the multistep process of migration. In support of this suggestion, studies have provided evidence demonstrating that trafficking cells downregulate local chemokine levels in an effort to control the magnitude of an inflammatory response and promote tissue homeostasis (29, 52, 58). In a recent report, endotoxin-induced alveolar monocyte recruitment was shown to be associated with consumption of MCP-1/CCL2 chemokine levels in the lung (29). The main determinant of this response was the expression of the CCL2 binding chemokine receptor CCR2 on the alveolar monocytes. In another study, inflammatory T cells responding to allogeneic implants were regulated by the utilization of CCL2 at the inflammatory site (52). Ongoing studies are determining whether trafficking CD8 T cells contribute to the resolution of inflammation during MCMV infection by disrupting the local chemokine environment.
In contrast to CXCL9 production, CXCL10 production remained sustained in the liver. It is speculated that CXCL9 induction reflects the innate NK cell-mediated IFN-γ effects (37, 40, 41, 43, 50), as this chemokine is highly dependent on IFN-γ for production (53, 56). However, CXCL10 can be induced by IFN-γ in addition to other molecules, including tumor necrosis factor alpha, which is found in the liver during MCMV infection (20, 33, 36, 51). In addition, as antibody neutralization of these CXCR3 ligands reduced the proportions and total numbers of hepatic MCMV-specific CD8 T cells, it is probable that the combined effects of these chemokines initiate the CD8 T-cell inflammatory response but CXCL10 may exert distinct functions during late acute MCMV infection in the liver that remain to be determined.
The studies presented here demonstrate the expression of CXCR3 on CD8 T cells and on the majority of CD8 T cells responding to M45 in the liver, suggesting that CXCR3 significantly contributes to the recruitment of virus-specific CD8 T cells. In concurrence with this conclusion, mice genetically deficient in CXCR3 exhibited markedly reduced abilities to accumulate M45-specific CD8 T cells. Collectively, our results directly indicate that the CD8 T-cell inflammatory response mounted against MCMV is largely dependent on the biological function of CXCR3 and its ligands CXCL9 and CXCL10. Although not measured in the studies presented here, the similar decreases in CD8 T-cell infiltration observed in CXCR3−/− mice and in our neutralization studies suggest that IFN-inducible T-cell α-chemoattractant (ITAC/CXCL11), which also acts through CXCR3 binding (59), is not involved under the conditions of MCMV infection. These results are consistent with reports indicating that the expression of CXCR3 on activated T lymphocytes infiltrating hepatitis C virus-infected livers is a response to the localized production of CXCR3 ligands at this site (47). Furthermore, the accumulation of NK cells, dendritic cells, and CD4+ T-lymphocyte subsets is not affected in CXCR3-deficient mice during MCMV infection in the liver (data not shown). Thus, it appears that the effects of CXCR3 are predominately on CD8 T cells, perhaps reflecting the sensitivity of the liver to immune responses mediated by this selective subset of T-lymphocyte effector cells during late acute MCMV infection (48).
In concurrence with other reports (11, 12, 48, 51), our results show that an effective CD8 T-cell response begins to develop at day 5 and peaks at day 7 following infection with MCMV. The studies presented here also demonstrate a significant reduction in the recruitment of activated antigen-specific CD8 T cells, as measured by the release of IFN-γ upon restimulation with the MCMV M45 peptide, in CXCR3-deficient mice infected with MCMV for 5 or 7 days. The observed reduction in activated antigen-specific CD8 T cells on day 5 postinfection was associated with significant increases in viral replication and elevations in the levels of the circulating liver enzyme ALT, indicative of generalized liver cell damage. However, it was unforeseen that despite these outcomes, by day 7 of infection, viral titers were below the level of detection and ALT values were profoundly reduced, comparable to the effects observed in CXCR3+/+ mice. Furthermore, the rates of survival of CXCR3+/+ and CXCR3−/− mice were similar at the virus dose used in the studies.
In contrast to the results observed in CXCR3+/+ mice, our results show that only a small fraction of the antigen-specific CD8 T cells accumulated in the livers of CXCR3−/− mice, suggesting that the liver can indeed be sensitive to immune control by a limited number of activated CD8 T cells. This is in contrast to reports for mice that are more susceptible to MCMV infection and therefore highly dependent on a more pronounced CD8 T-cell response for viral clearance from tissues (19, 20, 34, 51). It has been clearly established that resistance to MCMV is strongly correlated with the ability of the host genotype to elicit an effective innate NK cell response that provides immune defense by killing infected cells and producing cytokines (1, 2, 4, 21, 35, 40, 41, 43, 45, 46, 57). The studies presented here utilized CXCR3-deficient mice with a C57BL/6 background, an MCMV-resistant strain. These mice exhibited NK cell inflammation and the production of systemic and hepatic IFN-γ during early (40 to 72 h) infection with MCMV (K. L. Hokeness and T. P. Salazar-Mather, unpublished results). Therefore, the observed transient elevation in viral titers and delay in liver disease resolution may reflect the lingering protective abilities of innate immune responses in the presence of compromised CD8 T-cell function, highlighting the important interplay and/or compensatory functions between innate and adaptive immunity against a cytopathic virus.
In addition, the reduced accumulation of activated virus-specific CD8 T cells may provide a brief window of opportunity for elevated virus replication and enhanced liver pathology as the host responds to the virus through activation of alternative chemokine pathways. Hence, while CXCR3 critically affects the accumulation of CD8 T cells in the MCMV-infected liver, other signaling receptors that could serve a compensatory function in the recruitment of activated T cells during a virus infection include CCR5 and CCR2 (10, 32). Ongoing studies have identified a role for CCR2, but not CCR5, in the additional promotion of CD8 T-cell recruitment into the liver during infection (K. L. Hokeness and T. P. Salazar-Mather, unpublished results). Therefore, it is likely that in the absence of CXCR3, CD8 T cells respond to other chemokines produced in the liver as a consequence of MCMV infection by activation of functionally redundant chemokine receptors.
In contrast to the results observed in CXCR3-deficient mice, previous studies have demonstrated profound increases in both viral titers and mortality in the presence of neutralizing antibodies against CXCL9 during MCMV infection of immunocompetent mice (41). Moreover, unlike that of CXCR3-deficient mice, antibody neutralization of CXCL9 and/or CXCL10 resulted in increased and sustained ALT levels at days 5 and 7 of MCMV infection (data not shown). Collectively, these results suggest that mice genetically deficient in the CXCR3 receptor are conditioned to upregulate alternative chemokine pathways to compensate for the loss of this integral signaling component. In contrast, antibody neutralization of chemokines in immunocompetent mice may not result in the upregulation of these compensatory mechanisms.
In addition, our studies do not eliminate a potential role for CXCR3, CXCL9, and/or CXCL10 in promoting ancillary functions independent of their relevance in recruiting lymphocytes to inflammatory sites. It is still plausible that CXCL9 and/or CXCL10 could affect the activation of other cell subsets, including NK cells, by enhancing cytolytic activity as a result of augmented IFN-γ production or enhanced proliferation, ultimately leading to increased antiviral activity (28, 54, 55). Conversely, CXCL9 and/or CXCL10 could play a role in limiting cytokine-mediated liver immunopathology.
These hypotheses are supported by an expanding list of biological activities being determined for chemokines. Recent studies have demonstrated that elevations in circulating CXCL9 levels as a result of induced IFN-γ production can incite the mobilization of NK cells from the bone marrow into the circulation (54), suggesting a function for CXCL9 in the augmentation of innate and adaptive immune responses. Additional findings describe a role for CXCL9 (55) and CXCL10 (7) in stimulating the effector functions of CXCR3-expressing leukocytes, cytokine production, and promotion of T-cell proliferation, suggesting that chemokines can function in a manner similar to that of T-lymphocyte costimulatory molecules. Furthermore, CXCL10 has been shown to modulate cytokine release from peripheral blood mononuclear cell cultures following stimulation with environmental antigens (9). In the liver, elevated levels of CXCL10 have been associated with reductions in liver injury and tissue regeneration (3, 16). Further functions of CXCL9 and/or CXCL10 independent of CD8 T-lymphocyte recruitment during MCMV infection in the liver have yet to be defined. Hence, numerous questions regarding the methods employed by IFN-γ-inducible chemokines to promote effective antiviral immunity in infected tissue sites remain, and further investigations are aimed at dissecting these mechanisms.
In summary, this work characterizes a key molecular mechanism governing the recruitment of cells for adaptive immune responses against a virus infection in the liver. Our results show that CXCR3-dependent functions are involved in promoting the accumulation of activated virus-specific CD8 T lymphocytes that contribute to effective hepatic immunity. The studies presented here emphasize the relative importance of chemokine/chemokine receptor functions in the selective recruitment of lymphocytes responding to a virus infection in tissue sites.
Acknowledgments
We thank Thomas A. Hamilton of the Department of Immunology, Lerner Research Institute, Cleveland Clinic Foundation, for his gift of rabbit antiserum neutralizing Mig/CXCL9 and IP-10/CXCL10. We also acknowledge the generosity of Ann B. Hill of the Department of Molecular Microbiology and Immunology, Oregon Health and Science University, for providing the M45 peptide and tetramer and for helpful technical discussions.
This work was supported by Public Health Service grant R01 CA-102708 (to T.P.S.-M.) from the National Institutes of Health.
Footnotes
Published ahead of print on 15 November 2006.
REFERENCES
- 1.Biron, C. A., K. B. Nguyen, G. C. Pien, L. P. Cousens, and T. P. Salazar-Mather. 1999. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu. Rev. Immunol. 17:189-220. [DOI] [PubMed] [Google Scholar]
- 2.Biron, C. A., M. Dalod, and T. P. Salazar-Mather. 2002. Innate immunity and viral infections, p. 139-160. In S. H. E. Kaufmann, A. Sher, and R. Ahmed (ed.), Immunology of infectious diseases. American Society for Microbiology, Washington, DC.
- 3.Bone-Larson, C. L., C. M. Hogaboam, H. Evanhoff, R. M. Strieter, and S. L. Kunkel. 2001. IFN-γ-inducible protein-10 (CXCL10) is hepatoprotective during acute liver injury through the induction of CXCR2 on hepatocytes. J. Immunol. 167:7077-7083. [DOI] [PubMed] [Google Scholar]
- 4.Brown, M. G., A. O. Dokun, J. W. Heusel, H. R. Smith, D. L. Beckman, E. A. Blattenberger, C. E. Dubbelde, L. R. Stone, A. A. Scalzo, and W. M. Yokoyama. 2001. Vital involvement of natural killer cell activation receptor in resistance to viral infection. Science 292:934-937. [DOI] [PubMed] [Google Scholar]
- 5.Cavanaugh, V. J., Y. Deng, M. P. Birkenbach, J. S. Slater, and A. E. Campbell. 2003. Vigorous innate and virus-specific cytotoxic T-lymphocyte responses to murine cytomegalovirus in the submaxillary salivary gland. J. Virol. 77:1703-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curbishley, S. M., B. Eksteen, R. P. Gladue, P. Lalor, and D. H. Adams. 2005. CXCR 3 activation promotes lymphocyte transendothelial migration across human hepatic endothelium under fluid flow. Am. J. Pathol. 167:887-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dufour, J. H., M. Dziejman, M. T. Liu, J. H. Leung, T. E. Lane, and A. D. Luster. 2002. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J. Immunol. 168:3195-3204. [DOI] [PubMed] [Google Scholar]
- 8.Folker-Marcus, P. S., J. Markus, J. Podlech, D. Thomas, P. Deegan, M. Reddehase, and R. Holtappels. 2005. Highly protective in vivo function of cytomegalovirus IE1 epitope-specific memory CD8 T cells purified by T-cell receptor-based cell sorting. J. Virol. 79:5400-5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gangur, V., F. E. R. Simons, and K. T. Hayglass. 1998. Human IP-10 selectively promotes dominance of polyclonally activated and environmental antigen-driven IFN-γ over IL-4 production. FASEB J. 12:705-713. [DOI] [PubMed] [Google Scholar]
- 10.Glass, W. G., and T. E. Lane. 2003. Functional analysis of the CC chemokine receptor 5 (CCR5) on virus-specific CD8+ T cells following coronavirus infection of the central nervous system. Virology 312:407-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gold, M. C., M. W. Munks, M. Wagner, U. H. Koszinowski, A. B. Hill, and S. P. Fling. 2002. The murine cytomegalovirus immunomodulatory gene m152 prevents recognition of infected cells by M45-specific CTL but does not alter the immunodominance of the M45-specific CD8 T cell response in vivo. J. Immunol. 169:359-365. [DOI] [PubMed] [Google Scholar]
- 12.Gold, M. C., M. W. Munks, M. Wagner, C. W. McMahon, A. Kelly, D. G. Kavanagh, M. K. Slifka, U. H. Koszinowski, D. H. Raulet, and A. B. Hill. 2004. Murine cytomegalovirus interference with antigen presentation has little effect on the size or the effector memory phenotype of the CD8 T cell response. J. Immunol. 172:6944-6953. [DOI] [PubMed] [Google Scholar]
- 13.Hengel, H., P. Lucin, S. Jonjic, T. Ruppert, and U. H. Koszinowski. 1994. Restoration of cytomegalovirus antigen presentation by gamma interferon combats viral escape. J. Virol. 68:289-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hokeness, K. L., W. A. Kuziel, C. A. Biron, and T. P. Salazar-Mather. 2005. Monocyte chemoattractant protein-1 and CCR2 interactions are required for IFN-α/β-induced inflammatory responses and antiviral defense in liver. J. Immunol. 174:1549-1556. [DOI] [PubMed] [Google Scholar]
- 15.Jonjic, S., I. Pavic, P. Lucin, D. Rukavina, and U. H. Koszinowski. 1990. Efficacious control of cytomegalovirus infection after long-term depletion of CD8+ T lymphocytes. J. Virol. 64:5457-5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koniaris, L. G., T. Zimmers-Koniaris, E. C. Hsiao, K. Chavin, J. V. Sitzmann, and J. M. Farber. 2001. Cytokine-responsive gene-2/IFN-inducible protein-10 expression in multiple models of liver and bile duct injury suggests a role in tissue regeneration. J. Immunol. 167:399-406. [DOI] [PubMed] [Google Scholar]
- 17.Koszinowski, U. H., G. M. Keil, H. Schwartz, J. Shickedanz, and M. J. Reddehase. 1987. A nonstructural polypeptide encoded by immediate-early transcription unit 1 of murine cytomegalovirus is recognized by cytolytic T lymphocytes. J. Exp. Med. 166:289-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koszinowski, U. H., M. J. Reddehase, G. M. Keil, and J. Schickedanz. 1987. Host immune response to cytomegalovirus: products of transfected viral immediate-early genes are recognized by cloned cytolytic T lymphocytes. J. Virol. 61:2054-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koszinowski, U. H., M. Del Val, and M. J. Reddehase. 1990. Cellular and molecular basis of the protective immune response to cytomegalovirus infection. Curr. Top. Microbiol. Immunol. 154:3317-3325. [DOI] [PubMed] [Google Scholar]
- 20.Krmpotic, A., I. Bubic, B. Polic, P. Lucin, and S. Jonjic. 2003. Pathogenesis of murine cytomegalovirus infection. Microbes Infect. 5:1263-1277. [DOI] [PubMed] [Google Scholar]
- 21.Lathbury, L. J., J. E. Allen, G. R. Shellam, and A. A. Scalzo. 1996. Effect of host genotype in determining the relative roles of natural killer cells and T cells in mediating protection against murine cytomegalovirus infection. J. Gen. Virol. 10:2605-2613. [DOI] [PubMed] [Google Scholar]
- 22.Liu, M. T., B. P. Chen, P. Oertel, M. J. Buchmeier, D. Armstrong, T. A. Hamilton, and T. E. Lane. 2000. Cutting edge: the T cell chemoattractant IFN-inducible protein 10 is essential in host defense against viral-induced neurologic disease. J. Immunol. 165:2327-2330. [DOI] [PubMed] [Google Scholar]
- 23.Loetscher, M., B. Gerber, P. Loetscher, S. A. Jones, L. Piali, I. Clark-Lewis, M. Baggiolini, and B. Moser. 1996. Chemokine receptor specific for IP10 and Mig: structure, function, and expression in activated T-lymphocytes. J. Exp. Med. 184:963-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lucin, P., I. Pavic, B. Polic, S. Jonjic, and U. H. Koszinowski. 1992. Gamma interferon-dependent clearance of cytomegalovirus infection in salivary glands. J. Virol. 66:1977-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luster, A. D., J. C. Unkeless, and J. V. Ravetch. 1985. Gamma-interferon transcriptionally regulates an early-response gene containing homology to platelet proteins. Nature 315:672-676. [DOI] [PubMed] [Google Scholar]
- 26.Luster, A. D. 2002. The role of chemokines in linking innate and adaptive immunity. Curr. Opin. Immunol. 14:129-135. [DOI] [PubMed] [Google Scholar]
- 27.Luster A. D., R. Alon, and U. H. von Andrian. 2005. Immune cell migration in inflammation: present and future therapeutic targets. Nat. Immunol. 6:1182-1190. [DOI] [PubMed] [Google Scholar]
- 28.Mahalingam, T. R., J. M. Farber, and G. Karupiah. 1999. The interferon-inducible chemokines MuMig and Crg-2 exhibit antiviral activity in vivo. J. Virol. 73:1479-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maus, U. A., S. Wellmann, C. Hampl, W. A. Kuziel, M. Srivastasta, M. Mack, M. B. Everhart, T. S. Blackwell, J. W. Christman, D. Schlöndorff, R. M. Bohle, W. Seeger, and J. Lohmeyer. 2005. CCR2-positive monocytes recruited to inflamed lungs downregulate local CCL2 levels. Am. J. Physiol. Lung Cell. Mol. Physiol. 288:L350-L358. [DOI] [PubMed] [Google Scholar]
- 30.Molesworth-Kenyon, S., A. Mates, R. Yin, R. Strieter, J. Oakes, and R. Lausch. 2005. CXCR3, IP-10, and Mig are required for CD4+ T cell recruitment during the DTH response to HSV-1 yet are independent of the mechanism for viral clearance. Virology 333:1-9. [DOI] [PubMed] [Google Scholar]
- 31.Moser, B., M. Wolf, A. Walz, and P. Loetscher. 2004. Chemokines: multiple levels of leukocyte migration control. Trends Immunol. 25:75-84. [DOI] [PubMed] [Google Scholar]
- 32.Nansen, A., O. Marker, C. Bartholdy, and A. R. Thomsen. 2000. CCR2+ and CCR5+ CD8+ T cells increase during viral infection and migrate to sites of infection. Eur. J. Immunol. 30:1797-1806. [DOI] [PubMed] [Google Scholar]
- 33.Ohmori, Y., L. Wyner, S. Narumi, D. Armstrong, M. Stoler, and T. A. Hamilton. 1993. Tumor necrosis factor-alpha induces cell type and tissue-specific expression of chemoattractant cytokines in vivo. Am. J. Pathol. 142:861-870. [PMC free article] [PubMed] [Google Scholar]
- 34.Olver, S. D., P. Price, and G. R. Shellam. 1994. Cytomegalovirus hepatitis: characterization of the inflammatory infiltrate in resistant and susceptible mice. Clin. Exp. Immunol. 3:375-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Orange, J. S., B. Wang, C. Terhorst, and C. A. Biron. 1995. Requirement for natural killer cell-produced interferon-γ in defense against murine cytomegalovirus infection and enhancement of this defense pathway by interleukin 12 administration. J. Exp. Med. 182:1045-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orange, J. S., and C. A. Biron. 1996. Characterization of early IL-12, IFN-alpha/beta, and TNF effects on antiviral state and NK cell responses during murine cytomegalovirus infection. J. Immunol. 156:4746-4756. [PubMed] [Google Scholar]
- 37.Pien, G. C., A. R. Satoskar, K. Takeda, S. Akira, and C. A. Biron. 2000. Cutting edge: selective IL-18 requirements for induction of compartmental IFN-gamma responses during viral infection. J. Immunol. 165:4787-4791. [DOI] [PubMed] [Google Scholar]
- 38.Reddehase, M. J., F. Weiland, K. Munch, S. Jonjic, A. Luske, and U. H. Koszinowski. 1985. Interstitial murine cytomegalovirus pneumonia after irradiation: characterization of cells that limit viral replication during established infection of the lungs. J. Virol. 55:264-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reddehase, M. J., S. Jonjic, F. Weiland, W. Mutter, and U. H. Koszinowski. 1988. Adoptive immunotherapy of murine cytomegalovirus adrenalitis in the immunocompromised host: CD4-helper-independent antiviral function of CD8-positive memory T lymphocytes derived from latently infected donors. J. Virol. 62:1061-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salazar-Mather, T. P., J. S. Orange, and C. A. Biron. 1998. Early murine cytomegalovirus (MCMV) infection induces liver natural killer (NK) cell inflammation and protection through macrophage inflammatory protein 1α (MIP-1α)-dependent pathways. J. Exp. Med. 187:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salazar-Mather, T. P., T. A. Hamilton, and C. A. Biron. 2000. A chemokine-to-cytokine-to-chemokine cascade critical in antiviral defense. J. Clin. Investig. 105:985-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salazar-Mather, T. P., C. A. Lewis, and C. A. Biron. 2002. Type I interferons regulate inflammatory cell trafficking and macrophage inflammatory protein 1α delivery to the liver. J. Clin. Investig. 110:321-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salazar-Mather. T. P., and K. L. Hokeness. 2006. Cytokine and chemokine networks: pathways to antiviral defense. Curr. Top. Microbiol. Immunol. 303:29-46. [DOI] [PubMed] [Google Scholar]
- 44.Sallusto, F., D. Lenig, C. R. McKay, and A. Lanzavecchia. 1998. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J. Exp. Med. 187:875-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scalzo, A. A. 2002. Successful control of viruses by NK cells—a balance of opposing forces. Trends Microbiol. 10:470-474. [DOI] [PubMed] [Google Scholar]
- 46.Scalzo, A. A., N. A. Fitzgerald, C. R. Wallace, A. E. Gibbons, Y. C. Smart, R. C. Burton, and G. R. Shellam. 1992. The effect of the Cmv-1 resistance gene, which is linked to the natural killer cell gene complex, is mediated by natural killer cells. J. Immunol. 149:581-589. [PubMed] [Google Scholar]
- 47.Shields, P. L., C. M. Morland, M. Salmon, S. Qin, S. G. Hubscher, and D. H. Adams. 1999. Chemokine and chemokine receptor interactions provide a mechanism for selective T cell recruitment to specific liver compartments within hepatitis C-infected liver. J. Immunol. 163:6236-6243. [PubMed] [Google Scholar]
- 48.Sierro, S., R. Rothkopf, and P. Klenermann. 2005. Evolution of diverse antiviral CD8+ T cell populations after murine cytomegalovirus infection. Eur. J. Immunol. 35:1113-1123. [DOI] [PubMed] [Google Scholar]
- 49.Tannenbaum, C. S., R. Tubbs, D. Armstrong, J. H. Finke, R. M. Bukowski, and T. A. Hamilton. 1998. The CXC chemokines IP-10 and Mig are necessary for IL-12-mediated regression of the mouse RENCA tumor. J. Immunol. 161:927-932. [PubMed] [Google Scholar]
- 50.Tay, C. H., and R. M. Welsh. 1997. Distinct organ-dependent mechanisms for the control of murine cytomegalovirus infection by natural killer cells. J. Virol. 71:267-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trgovich, J., D. Stimac, B. Polic, A. Krmpotic, E. Pernjak-Pugel, J. Tomac, M. Hasan, B. Wraber, and S. Jonjic. 2000. Immune responses and cytokine induction in the development of severe hepatitis during acute infections with murine cytomegalovirus. Arch. Virol. 45:2601-2618. [DOI] [PubMed] [Google Scholar]
- 52.Tylaska, L. A., L. Boring, W. Weng, R. Aiello, I. F. Charo, B. J. Rollins, and R. P. Gladue. 2002. Ccr2 regulates the level of MCP-1/CCL2 in vitro and at inflammatory sites and controls T cell activation in response to alloantigen. Cytokine 18:184-190. [DOI] [PubMed] [Google Scholar]
- 53.Vanguri, P., and J. M. Farber. 1990. Identification of CRG-2: an interferon-inducible mRNA predicted to encode a murine monokine. J. Biol. Chem. 265:15049-15057. [PubMed] [Google Scholar]
- 54.Wald, O., I. D. Weiss, H. Wald, H. Shoham, Y. Bar-Shavit, K. Beider, E. Galun, L. Weiss, L. Flaishon, I. Shachar, A. Nagler, B. Lu, C. Gerard, J. L. Gao, E. Mishani, J. Farber, and A. Peled. 2006. IFN-γ acts on T cells to induce NK cell mobilization and accumulation in target organs. J. Immunol. 176:4716-4729. [DOI] [PubMed] [Google Scholar]
- 55.Whitting, D., G. Hsieh, J. J. Yun, A. Banerji, W. Yao, M. C. Fishbein, J. Belperio, R. M. Strieter, B. Bonavida, and A. Ardehali. 2004. Chemokine monokine induced by IFN-γ/CXC chemokine ligand 9 stimulates T lymphocyte proliferation and effector cytokine production. J. Immunol. 172:7417-7424. [DOI] [PubMed] [Google Scholar]
- 56.Wong, P., C. W. Severns, N. B. Guyer, and T. M. Wright. 1994. A unique palindromic element mediates gamma interferon induction of mig gene expression. Mol. Cell. Biol. 14:914-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yokoyama, W. M., S. Kim, and A. R. French. 2004. The dynamic life of natural killer cells. Annu. Rev. Immunol. 22:405-429. [DOI] [PubMed] [Google Scholar]
- 58.Zhang, P., G. J. Bagby, J. K. Knolls, D. A. Welsh, W. R. Summer, J. Andresen, and S. Nelson. 2001. The effects of granulocyte colony-stimulating factor and neutrophil recruitment on the pulmonary chemokine response to intratracheal endotoxin. J. Immunol. 166:458-465. [DOI] [PubMed] [Google Scholar]
- 59.Zlotnik, A., and O. Yoshie. 2000. Chemokines: a new classification system and their role in immunity. Immunity 12:121-127. [DOI] [PubMed] [Google Scholar]