Abstract
The development of versatile vaccine platforms is a priority that is recognized by health authorities worldwide; such platforms should induce both arms of the immune system, the humoral and cytotoxic-T-lymphocyte responses. In this study, we have established that a vaccine platform based on the coat protein of papaya mosaic virus (PapMV CP), previously shown to induce a humoral response, can induce major histocompatibility complex (MHC) class I cross-presentation of HLA-A*0201 epitopes from gp100, a melanoma antigen, and from influenza virus M1 matrix protein. PapMV proteins were able to assemble into stable virus-like particles (VLPs) in a crystalline and repetitive structure. When we pulsed HLA-A*0201+ antigen-presenting cells (APCs) with the recombinant PapMV FLU or gp100, we noted that antigen-specific CD8+ T cells were highly reactive to these APCs, demonstrating that the epitope from the VLPs were processed and loaded on the MHC class I complex. APCs were preincubated with two different proteasome inhibitors, which did not affect the efficiency of peptide presentation on MHC class I. Classical presentation from an endogenous antigen was abolished in the same conditions. Clearly, antigen presentation mediated by the PapMV system was proteasome independent. Finally, PapMV-pulsed APCs had the capacity to expand highly avid antigen-specific T cells against the influenza virus M1 HLA-A*0201 epitope when cocultured with autologous peripheral blood mononuclear cells. This study demonstrates the potential of PapMV for MHC class I cross-presentation and for the expansion of human antigen-specific T cells. It makes VLPs from PapMV CP a very attractive platform to trigger cellular responses for vaccine development against chronic infectious diseases and cancers.
The need for the development of versatile and efficient vaccine platforms has become a priority (7). The resistance of bacteria to antibiotics, the aging of the population in developed countries, which weakens the capacity of the immune system to respond to pathogens and cancers, the emergence of new pandemic strains of viruses (avian influenza virus, Ebola virus, etc.), the increasing number of deaths in developed countries from cancers, and the weak immunogenicity of currently available vaccines are major problems that justify this need (7).
Ideally, an adjuvant should trigger both arms of the immune system, the humoral and cytotoxic-T-lymphocyte (CTL) responses, to face the threats of chronic diseases (those caused by hepatitis C virus [HCV] and human immunodeficiency virus type 1 [HIV-1] and many others) and increasing cancer cases. However, vaccine development usually focuses on the induction of humoral immune responses, which has led to the development of adjuvants with the ability to enhance antibody responses. Specifically, aluminum salts (20) and oil-in-water emulsion MF59 (28), known to stimulate only humoral responses, are the most important available licensed adjuvants for humans. Consequently, most current vaccines failed to elicit significant Th1 responses or CTLs (19), which may be required to neutralize escape mechanisms from microbes and cancer cells.
Several viruses (HIV-1, influenza virus, and HCV, for example) have acquired immune escape mechanisms by constantly modifying their surface epitopes, resulting in resistance to preexisting antibodies. Therefore, an efficient approach is to develop a vaccine platform able to trigger cellular responses towards highly conserved antigens, such as viral nucleocapsids, structural proteins located inside the virus, or highly conserved viral proteins involved in the replication process. A major obstacle to this development is that exogenous antigens, as they are normally presented in a vaccine preparation, rarely lead to CTL responses due to their inability to present major histocompatibility complex (MHC) class I epitopes, which are usually restricted to endogenously synthesized proteins (12). In some circumstances, exogenous antigens have been shown to be presented by MHC class I molecules, a process named cross-presentation (31). A vaccine with the ability to induce the loading of MHC class I molecules by cross-presentation from an exogenous particle therefore could be used to induce a cellular response that is deficient in currently available vaccines.
Virus-like particles (VLPs) have recently demonstrated promising attributes for vaccination (26). VLPs made from the major capsid protein L1 of human papillomavirus were recently shown to provide 100% protection in women against the development of cervical cancers (2, 11), and this is probably the most efficient vaccine currently available. Protection appears to be provided by the neutralization of antibodies, from which titers can be improved by using aluminum-based salt adjuvants (9). Other platforms, such as the bacteriophage Qβ (23), hepatitis B virus (HBV) VLPs made of viral core protein (25, 30), parvovirus VLPs (1, 27), and plant virus VLPs (5), have the capacity to carry epitopes and evoke strong antibody responses. HBV VLPs supplemented with CpG (33) and parvovirus VLPs (21) have also shown their capacity to induce CTL responses. Vaccine platforms derived from plant viruses were previously reported to be biased toward a Th1 response, as suggested by antibody isotyping (22, 24) and cytokine secretion (29). The icosahedral VLPs of HBV were demonstrated to mediate cross-presentation on MHC class I molecules (40). In this study, HBV VLPs alone failed to induce gamma interferon (IFN-γ) secretion by CD8+-specific T cells in the absence of CpGs, which acted as a costimulatory signal and activated antigen-presenting cells (APCs) (40). However, cross-presentation induced by VLPs in human cells has never been properly demonstrated and characterized previously.
We have developed a vaccine platform based on the coat protein (CP) of papaya mosaic virus (PapMV). We have shown that the PapMV CP expression in Escherichia coli leads to the formation of VLPs that appear to be similar to the wild-type (WT) virus (43). Furthermore, we recently established that PapMV VLPs can be used as epitope carriers, induce memory response, and trigger a balanced Th1/Th2 response in the absence of adjuvant (J. Denis, N. Majeau, E. Acosta-Ramirez, C. Savard, M.-C. Bédard, S. Simard, K. Lecours, M. Bolduc, C. Paré, P. Tessier, A. Lamarre, R. Lapointe, C. Lopes Macias, and D. Leclerc, submitted for publication). The repetitive and crystalline structure of VLPs is important for the induction of immune responses, since a monomeric form of the protein structurally identical to the WT protein (17) is unable to induce an immune response (J. Denis et al.; submitted). The balanced immunoglobulin G1/immunoglobulin G2a profile suggests that the PapMV platform is able to induce CTL responses. However, it is unclear if engineered PapMV VLPs can mediate cross-presentation in human cells.
We have engineered PapMV CP by the fusion of two different MHC class I epitopes at its carboxy terminus with an influenza virus M1 epitope or with one derived from gp100 protein, a protein found in melanoma. We have demonstrated that the PapMV platform mediates the cross-presentation of the inserted peptides. Processing appears to be proteasome independent, and APCs pulsed with modified PapMV VLPs are efficient in inducing the expansion of avid antigen-specific T cells. This is the first demonstration that plant VLPs can mediate MHC class I cross-presentation and generate avid T cells in human cells.
MATERIALS AND METHODS
Cloning and engineering of PapMV gp100 and PapMV FLU constructs.
The PapMV CP construct (CPΔN5) used for this study has been described previously (43). To generate the PapMV gp100 construct, sense (5′ CTAGTTCTTCTGCGTTCACCATCATGGACCAGGTTCCGTTCTCTGTTTCTGTTTCTCAGCTGA 3′) and antisense (5′ CTAGTCAGCTGAGAAACAGAAACAGAGAACGGAACCTGGTCCATGATGGTGAACGCAGAAGAA 3′) oligonucleotides were annealed and cloned into the SpeI and MluI sites of the PapMV CP clone linearized with the same enzymes. To generate the PapMV FLU construct, sense (5′ CTAGTTCTCCGCTGACCAAAGGTATCCTGGGTTTCGTTTTCACCCTGACCGTTCCGTCTGAAA 3′) and antisense (5′ CTAGTTTCAGACGGAACGGTCAGGGTGAAAACGAAACCCAGGATACCTTTGGTCAGCGGAGAA 3′) oligonucleotides were annealed and cloned as before at the C terminus of PapMV CP. The resulting clones, PapMV gp100 and PapMV FLU, were made of the PapMV CP gene with fusion of the peptide at their C termini, followed by a six-histidine tag for the purification process. We kept five amino acids on each side of the HLA-A*0201 epitopes to ensure efficient processing, as in native gp100 and influenza virus M1 proteins. The sequences of the PapMV clones were confirmed by DNA sequencing.
Expression of PapMV, PapMV FLU, and PapMV gp100 in E. coli.
The expression and purification steps were performed as described elsewhere (43). Three modifications were made to this protocol: the bacteria were lysed by one passage through the French press, and two washing steps to remove lipopolysaccharide contaminants from our preparations, one with 10 mM Tris-HCl, 50 mM imidazole, 0.5% Triton X-100, pH 8, and another with 10 mM Tris-HCl, 50 mM imidazole, 1% Zwittergent, pH 8, were added before the elution step. For the PapMV gp100, PapMV FLU, and PapMV CP proteins, the eluted proteins were subjected to a high-speed ultracentrifugation (100,000 × g) for 120 min in a Beckman 50.2 Ti rotor. VLP pellets were resuspended in endotoxin-free phosphate-buffered saline (PBS; Sigma, St. Louis, MO). Finally, the protein solutions were filtered with 0.45-μm filters. Protein concentrations were evaluated by use of a bicinchoninic acid protein kit (Pierce, Rockford, IL). The lipopolysaccharide level in the purified proteins was evaluated with the Limulus test according to the manufacturer's instructions (Cambrex, Walkersville, MD) and was below 0.005 endotoxin units/μg of protein. This procedure yielded more than 20 mg of purified VLPs per liter of bacterial culture. The gp100 (ITD QVP FSV and IMD QVP FSV) and FLU (GIL GFV FTL) peptides (underlining indicates a modified site at position 210) were synthesized by Macromolecular Resources (Fort Collins, CO) and resuspended in dimethyl sulfoxide (Sigma).
Media and cell culture.
T lymphocytes, dendritic cells (DCs), and CD40-stimulated B lymphocytes (CD40-B) were cultured as previously described (13) in complete medium, which is Iscove's modified Dulbecco's medium (Invitrogen, Carlsbad, CA, and Wisent, St-Bruno, Quebec, Canada) supplemented with 7.5% human serum (heat inactivated, prepared from normal donors), 2 mM l-glutamine, 100 U/ml penicillin-streptomycin, and 10 μg/ml gentamicin (the last three from Invitrogen and Wisent).
CD40-B were expanded and cultured from peripheral blood mononuclear cells (PBMCs) (13, 18) by the addition of 500 ng/ml of soluble trimeric CD40L (Immunex Corporation, Seattle, WA) and 500 U/ml recombinant human interleukin 4 (IL-4) (Peprotech, Rocky Hill, NJ).
DCs were generated from PBMCs collected by apheresis preparation from normal donors (16) by modifying the original protocol described by Sallusto and Lanzavecchia (34). Briefly, PBMCs were enriched from blood by centrifugation on a lymphocyte separation medium (Wisent). The monocytes were enriched by 2 h of adherence in tissue culture flasks or plates at 37°C (3 × 107 cells in T-25, 1.5 × 107 cells/well in 6-well flat bottom plates, or 5 × 106 cells/well in 24-well flat bottom plates, all from Costar, Corning, NY). Adherent cells were washed once with PBS (Wisent) and then cultured in complete medium supplemented with 100 ng/ml of granulocyte-macrophage colony-stimulating factor (1,000 U/ml) and 500 ng/ml of IL-4 (1,000 U/ml) (both from Peprotech). Granulocyte-macrophage colony-stimulating factor and IL-4 were added again on days 3 and 5. PapMV was added on day 6 and harvested on day 7 for recognition and expansion experiments.
The melanoma cell line 1088mel was established at the Surgery Branch (National Cancer Institute, NIH, Bethesda, MD). SK23, T2, and the breast tumor cell line MDA231 were obtained from the American Type Culture Collection (Manassas, VA). All tumor cell lines were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 2 mM l-glutamine, 100 U/ml penicillin-streptomycin, and 10 μg/ml gentamicin.
Electron microscopy and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Proteins were diluted in PBS and absorbed for 3 min on carbon-coated Formvar grids. The grids were washed twice with deionized water and stained with uranyl acetate 0.1% for 10 min at room temperature. The grids were then observed on a Jeol JEM220FS transmission electron microscope. The average length of 100 VLPs was evaluated with Adobe Photoshop software (San Jose, CA).
SDS-PAGE analyses were performed (18) using the mini-protean system from Bio-Rad (Hercules, CA). Proteins were revealed by Coomassie blue staining (Bio-Rad). In some experiments, proteinase K (Invitrogen) was added at a final concentration of 13 μg/ml.
Cross-presentation from PapMV-pulsed APCs.
Different target cells were analyzed for MHC class I presentation of defined epitopes with gp100- or influenza virus M1-specific T cells. gp100-specific CD8+ T-cell clones, kindly provided by Mark Dudley (National Cancer Institute; NIH, Bethesda, MD), are specific to both the native HLA-A*0201-restricted epitope at positions 209 to 217 (ITD QVP FSV) and to the modified version with an M at position 210, which enhances the stability of the peptide-MHC complex. gp100-specific T cells were expanded under the rapid expansion protocol described by Dudley et al. (8).
T-cell lines specific to the influenza virus M1-derived HLA-A*0201-restricted epitope (59-67; GIL GFV FTL) were generated as follows. PBMCs from HLA-A*02+ normal donors were identified by flow cytometry (FACSCalibur; BD Biosciences, Mississauga, Ontario, Canada) with a specific antibody (OneLambda, Canoga Park, CA). PBMCs prepared as described above were stimulated in multiple wells of 48-well plates with 1 μM of synthetic FLU peptide in the medium described above. Cultures were restimulated 7 days later with either peptide-pulsed (1 μM for 3 h followed by three washes in PBS) autologous PBMCs or CD40-B cultures. IL-2 (Chiron, Emeryville, CA) was then added every 2 or 3 days at 150 IU/ml, and the cultures were kept between 0.5 × 106 and 2 × 106 cells/ml. The specificities of the individual cultures were assessed by IFN-γ secretion assays by enzyme-linked immunosorbent assay (ELISA) with coupled antibodies (Endogen, Woburn, MA) after coculturing with peptide-pulsed T2 cells as described previously (15).
To evaluate cross-presentation mediated by PapMV CP, CD40-B or DCs were pulsed with various versions of modified PapMV CP at 10 to 50 μg/ml for 20 h. The cells were harvested, washed twice with PBS, and seeded in complete media (at 4 × 104 to 10 × 104 cells/well) in 96-well plates. gp100- or influenza virus M1-specific T cells were added at 2 × 104 to 10 × 104 cells/well in complete media for 20 h. Culture supernatants were harvested, and IFN-γ was evaluated by ELISA (18). In some experiments, APCs were pretreated for 1 h with 50 to 70 μM chloroquine (Sigma), 20 to 25 μg/ml lactacystin, or 1.3 to 3.3 μM MG-132 (the last two from Calbiochem, San Diego, CA). The cells were washed with PBS and resuspended in media containing 1/20 of the original inhibitor concentration. Treated cells were pulsed with the different PapMV variants, and MHC class I-mediated presentation was analyzed as described above.
T-cell expansion.
CD40-B or DCs were pulsed with various versions of PapMV CP for 20 h. The cells were harvested and washed twice with PBS. Pulsed APCs (2 × 105 to 5 × 105) were cocultured with autologous PBMCs at 2 × 106 cells in 500 μl of complete media, in single wells of a 48-well plate. When the medium turned yellow, 200 μl of it was removed, and 400 μl fresh complete medium was added. On days 7 to 10, freshly PapMV-pulsed APCs (5 × 105; pulsed for 20 h and washed twice in PBS) were added to individual cultures. IL-2 was then added at 100 IU/ml every 3 days.
T-cell specificity was assessed on days 15 to 20. Briefly, expanded cells were cocultured with peptide-pulsed T2 cells as described previously (15) or with PapMV-pulsed APCs. IFN-γ secretion was evaluated by ELISA. Alternatively, the frequency of antigen-specific T cells was assessed by enzyme-linked immunospot assay (ELISPOT assay), using coupled antibodies (MABTECH, Stockholm, Sweden) according to the manufacturer's instructions. Spots were enumerated with an automated counter (CTL Technologies, Cleveland, OH).
RESULTS
Production, purification, and characterization of PapMV CP, PapMV gp100, and PapMV FLU VLPs.
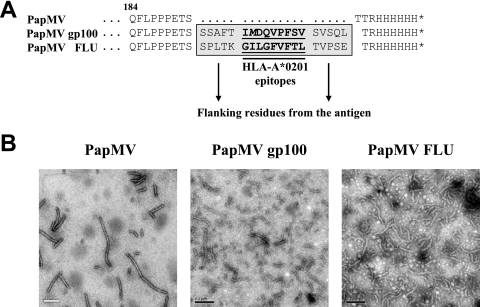
PapMV VLPs form stable and repetitive structures that are excellent platforms for the induction of humoral responses. In this study, we evaluated the potential of PapMV VLPs as a tool for cross-presenting MHC class I epitopes on human-derived APCs. Specifically, we inserted, at the C terminus of PapMV CP, HLA-A*0201 epitopes from a well-defined tumor antigen gp100 and from influenza virus M1 protein (Fig. 1A). The 9-mer HLA-A*0201 epitopes were flanked by five native residues on the N and C termini to favor natural processing by the proteasome as in whole WT proteins. Electron microscopy of the different PapMV VLPs produced in E. coli (Fig. 1B) revealed the typically long, rod-shaped structure, ranging from 80 to 200 nm in length and being 15 nm in diameter for PapMV VLPs and 16 nm for engineered PapMV gp100 and FLU VLPs. PapMV CP was able to spontaneously assemble in E. coli into VLPs that were similar in size and shape to PapMV VLPs.
FIG. 1.
Preparation of chimerical PapMV CP fused with MHC class I epitopes from influenza virus M1 and gp100 proteins. (A) C-terminal sequences of PapMV CP (PapMV) and modified PapMV with HLA-A*0201-restricted epitopes from influenza virus M1 protein (positions 57 to 65; designated PapMV FLU) or from gp100 (positions 209 to 217 with an M in position 210; designated PapMV gp100) constructs. (B) Electronic microscopy of each recombinant autoassembled PapMV preparation.
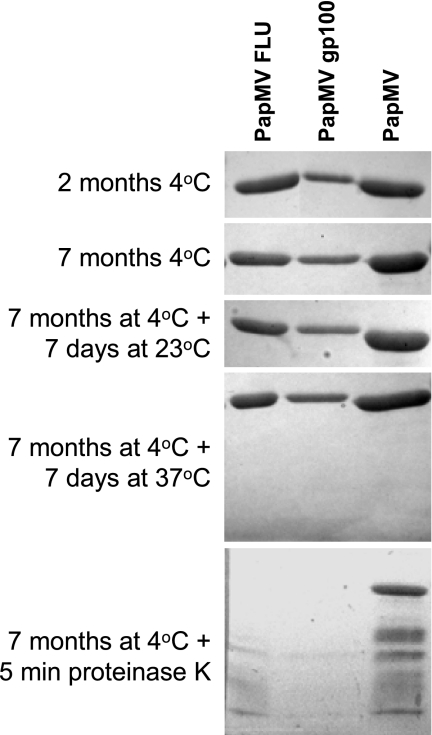
Stability is an important attribute for a vaccine. We performed SDS-PAGE analysis of fresh and 7-month-old VLP preparations that were kept in PBS at 4°C (Fig. 2). No evidence of degradation was found on the gel. Furthermore, we incubated the preparations for an additional 7 days at room temperature or at 37°C, without any noticeable degradation. Finally, we incubated the PapMV preparations with proteinase K as a positive control for degradation, which resulted in the rapid breakdown of the engineered PapMV VLPs. PapMV VLPs without fusions were more resistant to proteinase K, indicating that fusion at the C terminus probably destabilizes this region locally and increases susceptibility to the enzyme. In summary, we have prepared stable PapMV VLPs with MHC class I epitopes inserted in the C terminus.
FIG. 2.
Recombinant PapMV CPs are stable. The integrity of the three PapMV-purified proteins was analyzed by SDS-PAGE 2 and 7 months after their synthesis. Also, 7-month-old proteins were incubated for 7 additional days at 23 or 37°C. Finally, proteinase K was added for 5 min prior to the addition of loading buffer and incubation in boiling water. The loading of PapMV gp100 was lower due to low protein concentration compared to those of the two other PapMVs.
Cross-presentation of PapMV VLP MHC class I epitopes to specific CD8+ T cells.
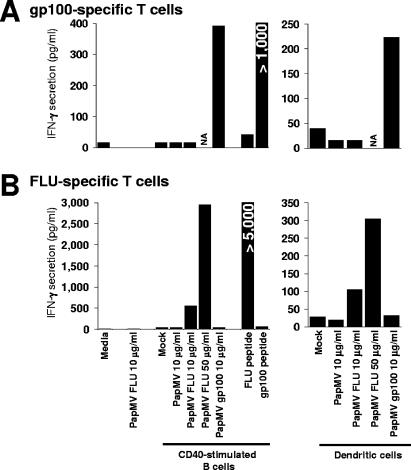
We next assessed the capacity of cross-presentation of the inserted MHC class I epitopes within PapMV. DCs and CD40-activated B lymphocytes were prepared from HLA-A*02 donors as sources of APCs. DCs are defined as optimal APCs (3); we and others have demonstrated that B lymphocytes expanded after CD40 stimulation are efficient APCs (4, 13, 14, 36, 44, 45). These APCs were pulsed with the different versions of PapMV VLPs and cocultured with defined T cells specific to MHC class I epitopes from gp100 and influenza virus M1 proteins. As seen in Fig. 3A, only APCs pulsed with PapMV gp100 were recognized by the gp100-specific T-cell clone. Conversely, only PapMV FLU-pulsed APCs were recognized by influenza virus M1-specific T lymphocytes (Fig. 3B). The specificity of each T-cell culture was confirmed by pulsing CD40-B with the synthetic peptides corresponding to each epitope. Also, the addition of PapMV FLU to specific T cells failed to stimulate IFN-γ secretion, meaning that APCs are essential for peptide recognition.
FIG. 3.
MHC class I epitopes from both antigens can be cross-presented when pulsed on two different APC sources. CD40-activated B lymphocytes and DCs were prepared from an HLA-A*0201 donor and pulsed for 18 h with the indicated preparations at various concentrations. Pulsed cells were washed, and gp100-specific (A) or FLU-specific (B) T cells were added for an additional 18 h. Evidence of T-cell activation was revealed by IFN-γ secretion determined by ELISA. NA, not available.
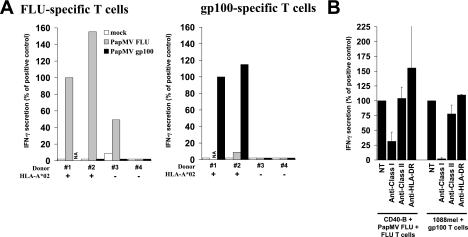
To ensure that HLA-A*02 was the restriction element involved in peptide presentation, we performed similar experiments with APCs prepared from two additional HLA-A*02+ donors and from two others negative for this allele. As shown in Fig. 4A, FLU-specific T cells were mostly reactive with PapMV FLU pulsed on HLA-A*02+ donors (left panel). The weak reactivity with donor 3 may be explained by the fact that the FLU T-cell line was heterogeneous, and T cells specific to the peptide, potentially presented by another MHC class I allele, may have occurred in culture. Moreover, the gp100-specific T-cell clone was reactive only with PapMV gp100-pulsed HLA-A*02+ APCs (Fig. 4A; right panel), further confirming that APCs expressing the relevant restriction element are necessary for antigenic presentation. Finally, MHC class I presentation was controlled by using antibodies blocking either MHC class I, MHC class II, or HLA-DR presentation, as described elsewhere (13, 15). As a control, a melanoma line expressing both HLA-A*0201 and gp100 was cocultured with the gp100-specific T-cell clone, and only antibody blocking MHC class I presentation abrogated recognition, as expected (Fig. 4B, right section). Coculturing of gp100-specific T-cell clones with either HLA-A*02−/gp100+, HLA-A*02+/gp100−, or HLA-A*02−/gp100− tumor cell lines failed to provoke IFN-γ secretion (data not included; demonstrated elsewhere [8, 15]). When the panel of blocking antibodies was applied to PapMV FLU-pulsed APCs, only anti-MHC class I decreased recognition by the specific T-cell line (Fig. 4B, left section). The latter data showed that the FLU peptide derived from PapMV FLU was presented by MHC class I, specifically HLA-A*02.
FIG. 4.
Epitope presentation mediated by PapMV pulsing was HLA-A*02 restricted. (A) PapMV FLU- or PapMV gp100-pulsed CD40-activated B lymphocytes prepared from HLA-A*02-positive or -negative donors were cocultured with FLU-specific (left panel) or gp100-specific (right panel) T cells. (B) An HLA-A*0201+ and gp100+ melanoma line or PapMV FLU-pulsed HLA-A*02+ CD40-activated B cells were incubated with antibodies blocking MHC class I, MHC class II, or HLA-DR presentation. FLU- or gp100-specific T cells were then added. Results are represented as percentages of recognition based on IFN-γ secretion assay, with 100% corresponding to the amount secreted by positive controls (NT). NA, not available.
MHC class I cross-presentation mediated by PapMV VLPs is proteasome independent.
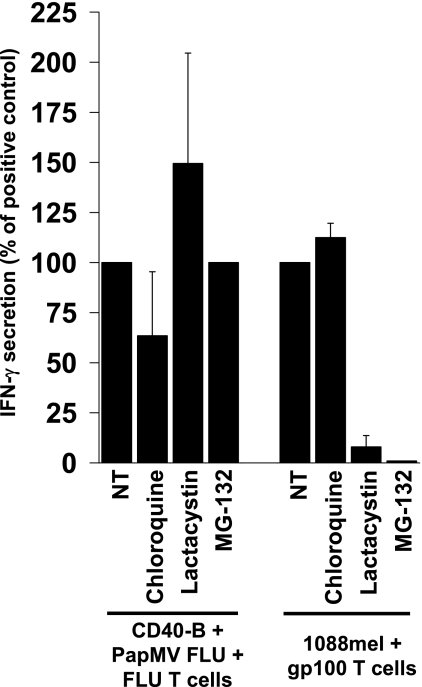
Antigens have to be processed by proteases to generate epitopes recognized by T cells, and for MHC class I epitopes, classical processing is mediated by proteasomes. Furthermore, the cross-presentation of exogenous antigen by MHC class I can also be mediated by cathepsins and other residents of the endosomal pathway (31). Interestingly, we know that the VLPs from PapMV can get internalized in vesicles when pulsed on APCs (J. Denis et al., submitted). We determined if the processing of PapMV VLPs was mediated by proteasomes. We exploited two different proteasome inhibitors, lactacystin and MG-132, and controlled their activities by blocking classical MHC class I presentation by melanoma cells of the HLA-A*0201 epitope from gp100 (Fig. 5, right section). When using PapMV FLU-pulsed APCs, presentation of the M1 peptide was unaffected by both inhibitors (Fig. 5, left section). Pretreatment with chloroquine, which neutralizes the pH of endosomes, had a weak, negative effect on the cross-presentation of the FLU epitope, whereas similar treatment did not change the classical MHC class I presentation of melanoma cells, as expected.
FIG. 5.
MHC class I cross-presentation mediated by PapMV is proteasome independent. An HLA-A*0201+ and gp100+ melanoma line or PapMV FLU-pulsed HLA-A*02+ CD40-activated B cells were incubated with chloroquine, which neutralizes the pH of endosomes/lysosomes, or with lactacystin or MG-132, two proteasome inhibitors. Cells were washed and pulsed with PapMV FLU, and FLU- or gp100-specific T cells were then added as described in Materials and Methods. The results are presented as percentages of recognition based on an IFN-γ secretion assay, with 100% corresponding to the amount secreted by positive controls (NT).
Overall, these data suggest that the MHC class I cross-presentation mediated by the PapMV VLPs is proteasome independent.
Expansion of FLU-specific T cells with PapMV FLU VLP.
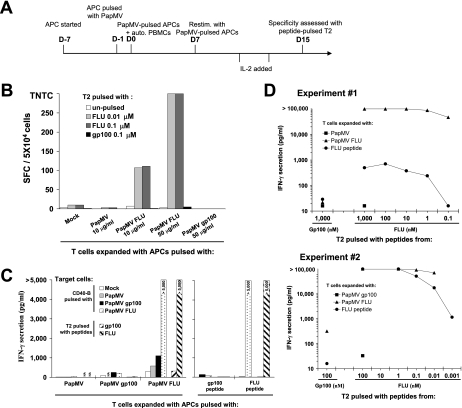
We have demonstrated that PapMV VLPs pulsed on APCs can efficiently mediate cross-presentation when exposed to defined specific T cells. We have also evaluated if APCs pulsed with PapMV VLPs would have the capacity to expand antigen-specific T lymphocytes from a heterogeneous blood T-cell population prepared from PBMCs. DCs and CD40-activated B lymphocytes were pulsed with PapMV VLPs and cocultured with autologous PBMCs according to the protocol outlined in Fig. 6A. The frequency of specific expanded T cells was first evaluated by ELISPOT assay. As shown in Fig. 6B, cells specific to HLA-A*0201 influenza virus M1 peptide were generated when PapMV FLU-pulsed APCs were used with PBMCs. Unpulsed APCs or those pulsed with PapMV or PapMV gp100 failed to generate FLU-specific T cells, as expected. No gp100-specific T cells were generated, as expected for a healthy donor with no melanoma. Expanded T cells were next evaluated for reactivity against various pulsed APCs, and IFN-γ secretion seemed equally high when T cells expanded with APCs pulsed either with the FLU peptide or PapMV FLU were deployed to expand T cells (>5,000) (Fig. 6C). APCs pulsed with either PapMV, PapMV gp100, or PapMV FLU failed to generate T cells specific to PapMV CP, indicating that cellular preimmunity to PapMV CP was marginal. Finally, expanded T cells were cocultured with T2 cells pulsed with different amounts of HLA-A*0201 influenza virus M1 peptide (Fig. 6D). In two independent experiments, highly reactive T cells with high avidity were generated, since T cells had the capacity to recognize T2 cells pulsed with 100 or 10 pM of the peptide. Secretion was mostly >100,000 pg/ml, which is very high, and T cells raised with APCs pulsed with the influenza virus M1 peptide were generally less avid.
FIG. 6.
Expansion of T lymphocytes specific to the HLA-A*0201 epitope from influenza virus M1 protein with PapMV FLU-pulsed APCs. (A) Schematic representation of the T-cell expansion protocol. auto., autologous; Restim., restimulated; D-7, day −7; D0, day 0. (B) PBMCs from an HLA-A2+ normal donor were cocultured according to the protocol for panel A with autologous CD40-activated B cells unpulsed (mock) or pulsed with either PapMV, PapMV FLU, or PapMV gp100. Cultured cells were restimulated on day 7, and IL-2 was added at different times. The specificity of the expanded cells was assessed on day 15 by coculturing with T2 cells pulsed with HLA-A*0201 peptides from FLU or gp100. The frequency of IFN-γ-secreting cells was determined by ELISPOT assay. SFC, spot-forming cells; TNTC, too numerous to count. (C) Expanded T cells were cocultured with CD40-activated B lymphocytes pulsed with either the indicated PapMV CP or synthetic peptides. (D) Expanded T cells were cocultured with T2 cells pulsed with the indicated concentrations of synthetic peptides. For panels C and D, culture supernatants were harvested, and evidence of T-cell activation was revealed by IFN-γ secretion determined by ELISA.
From these experiments, we conclude that PapMV-pulsed APCs had the capacity to expand specific T cells with high avidity. Interestingly, we found no evidence of preexisting cellular preimmunity to PapMV CP.
DISCUSSION
Vaccine platforms allowing MHC class I presentation would be of substantial advantage to elicit potentially long-lasting cellular responses against highly conserved viral or tumor antigens. In this report, we demonstrated that a plant virus-derived VLP can mediate MHC class I cross-presentation in human APCs, a process that is not affected by treatment with two different proteasome inhibitors. Importantly, APCs pulsed with PapMV FLU efficiently expanded influenza virus M1 HLA-A*0201 epitope-specific T cells. Expanded T cells seem to be highly reactive and avid considering their high IFN-γ secretion at very low peptide concentration.
Stability is an important prerequisite for a vaccine. By using a plant virus as a vaccine platform, we take advantage of a genuine characteristic of plant viruses that have been selected to be stable in adverse conditions in their natural environment. This was reflected by stability for more than 7 months in PBS and during incubation for a week at 37°C. Engineered PapMV VLPs showed the same behavior as WT constructs. This result is consistent with previous data disclosing that PapMV VLPs remain stable to temperatures reaching 60°C (43).
In this study, we demonstrated that activated B lymphocytes and DCs can efficiently cross-present antigens. Basically, exogenous antigens get internalized in APCs, and, according to most accepted models, reach the cytoplasmic compartment, thereby gaining access to the proteasome (6, 10, 31, 32, 39). Cross-presentation mechanisms were reviewed recently by Cresswell et al. (6) and by Rock and Shen (31, 37). Interestingly, we found that PapMV VLPs were not processed by proteasomes prior to MHC class I cross-presentation. An alternative proteasome-independent cross-presentation pathway has been revealed with ovalbumin incorporated into microsphere polymers and has been defined as the vacuolar pathway (31). In this pathway, the antigen is processed by lysosomal proteases, such as cathepsin S (38), and peptides are loaded on recycling MHC class I molecules in the endosomal/lysosomal compartments (31). Cross-presentation mediated by the HBV VLP for delivering a MHC class I epitope from lymphocytic choriomeningitis virus (33) has also been shown to be transporter associated with antigen processing (TAP) independent. Other antigens coupled with different carriers have been found to get cross-presented by a proteasome-independent pathway (35, 41, 42). PapMV VLPs, with their complex repetitive structures, may act similarly, and we are currently investigating the exact processing mechanism involved in epitope release.
Interestingly, PapMV FLU had the capacity to expand highly reactive specific T cells when pulsed on APCs. APCs pulsed with the synthetic peptide also raised specific T cells, but generally less effectively, when pulsed at 1 μg/ml as opposed to an estimated 2 μg/ml of equivalent FLU peptide with PapMV FLU (total 50 μg/ml). These differences are marginal, and hazardous to compare, considering the difference in loading mechanisms and the fact that PapMV FLU needed to be processed to release the peptide. The results presented in Fig. 3 suppose that loading is more efficient when the synthetic peptide is directly added, but the T-cell expansion experiment results shown in Fig. 6D revealed that pulsing with PapMV VLPs is superior to peptide pulsing. The apparent high efficiency in T-cell expansion with PapMV FLU could be explained by possible costimulation from PapMV CP on APCs through Toll-like receptors or other pathogen-associated molecular pattern receptors. Actually, a VLP derived from human papillomavirus has been demonstrated to stimulate DCs to produce IFN-α and Th1 immune responses via MyD88, a key signaling molecule in several Toll-like receptors (46). The potential stimulatory effects of PapMV CP on DC functions are under investigation.
In conclusion, we show for the first time that a plant virus VLP is able to trigger cross-presentation of MHC class I epitopes and in vitro CD8+ T-cell proliferation in human cells. These results are now opening new avenues in the development of candidate vaccines with PapMV VLPs. The induction of cross-presentation implies that engineered PapMV VLPs could be used for triggering a specific CTL response in humans. Therefore, the PapMV platform could be exploited in cancer immunotherapy and for vaccine development against chronic infectious diseases such as those caused by HIV-1 and HCV. In addition, the PapMV platform has the advantage of triggering both arms of the immune responses, namely, the humoral and CTL responses, a characteristic that is unique for a vaccine platform. Finally, PapMV VLPs are highly purified, stable proteins devoid of chemicals, adjuvants (such as alum), or potentially toxic agents (such as thimerosal) often added to vaccine preparations as stabilizing agents. This is a major advantage considering the projected increase in the practice of vaccination in the future to fight chronic infectious diseases and cancers.
Acknowledgments
We thank Ovid Da Silva from the Research Support Office, Research Centre, CHUM, for text editing.
R.L. is the recipient of a Fonds de la recherché en santé du Québec (FRSQ) scholarship. This work was supported by grants from the Canadian Institutes of Health Research (CIHR) and from La fondation du CHUM.
Footnotes
Published ahead of print on 22 November 2006.
REFERENCES
- 1.Antonis, A. F., C. J. Bruschke, P. Rueda, L. Maranga, J. I. Casal, C. Vela, L. A. Hilgers, P. B. Belt, K. Weerdmeester, M. J. Carrondo, and J. P. Langeveld. 2006. A novel recombinant virus-like particle vaccine for prevention of porcine parvovirus-induced reproductive failure. Vaccine 24:5481-5490. [DOI] [PubMed] [Google Scholar]
- 2.Ault, K. A. 2006. Vaccines for the prevention of human papillomavirus and associated gynecologic diseases: a review. Obstet. Gynecol. Surv. 61:S26-S31. [DOI] [PubMed] [Google Scholar]
- 3.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 4.Bergwelt-Baildon, M., J. L. Schultze, B. Maecker, I. Menezes, and L. M. Nadler. 2004. Correspondence re R. Lapointe et al., CD40-stimulated B lymphocytes pulsed with tumor antigens are effective antigen-presenting cells that can generate specific T cells. Cancer Res. 2003;63:2836-43. Cancer Res. 64:4055-4056. [DOI] [PubMed] [Google Scholar]
- 5.Canizares, M. C., L. Nicholson, and G. P. Lomonossoff. 2005. Use of viral vectors for vaccine production in plants. Immunol. Cell Biol. 83:263-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cresswell, P., A. L. Ackerman, A. Giodini, D. R. Peaper, and P. A. Wearsch. 2005. Mechanisms of MHC class I-restricted antigen processing and cross-presentation. Immunol. Rev. 207:145-157. [DOI] [PubMed] [Google Scholar]
- 7.Daar, A. S., H. Thorsteinsdottir, D. K. Martin, A. C. Smith, S. Nast, and P. A. Singer. 2002. Top ten biotechnologies for improving health in developing countries. Nat. Genet. 32:229-232. [DOI] [PubMed] [Google Scholar]
- 8.Dudley, M. E., M. I. Nishimura, A. K. Holt, and S. A. Rosenberg. 1999. Antitumor immunization with a minimal peptide epitope (G9-209-2M) leads to a functionally heterogeneous CTL response. J. Immunother. 22:288-298. [DOI] [PubMed] [Google Scholar]
- 9.Giannini, S. L., E. Hanon, P. Moris, M. Van Mechelen, S. Morel, F. Dessy, M. A. Fourneau, B. Colau, J. Suzich, G. Losonksy, M. T. Martin, G. Dubin, and M. A. Wettendorff. 2006. Enhanced humoral and memory B cellular immunity using HPV16/18 L1 VLP vaccine formulated with the MPL/aluminium salt combination (AS04) compared to aluminium salt only. Vaccine 14:5937-5949. [DOI] [PubMed] [Google Scholar]
- 10.Groothuis, T. A., and J. Neefjes. 2005. The many roads to cross-presentation. J. Exp. Med. 202:1313-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harper, D. M., E. L. Franco, C. Wheeler, D. G. Ferris, D. Jenkins, A. Schuind, T. Zahaf, B. Innis, P. Naud, N. S. De Carvalho, C. M. Roteli-Martins, J. Teixeira, M. M. Blatter, A. P. Korn, W. Quint, and G. Dubin. 2004. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet 364:1757-1765. [DOI] [PubMed] [Google Scholar]
- 12.Koch, J., and R. Tampe. 2006. The macromolecular peptide-loading complex in MHC class I-dependent antigen presentation. Cell. Mol. Life Sci. 63:653-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lapointe, R., A. Bellemare-Pelletier, F. Housseau, J. Thibodeau, and P. Hwu. 2003. CD40-stimulated B lymphocytes pulsed with tumor antigens are effective antigen-presenting cells that can generate specific T cells. Cancer Res. 63:2836-2843. [PubMed] [Google Scholar]
- 14.Lapointe, R., J. Thibodeau, and P. Hwu. 2004. Correspondence re R. Lapointe et al., CD40-stimulated B lymphocytes pulsed with tumor antigens are effective antigen-presenting cells that can generate specific T cells. Cancer Res. 2003;63:2836-43. Reply. Cancer Res. 64:4056-4057. [PubMed] [Google Scholar]
- 15.Lapointe, R., R. E. Royal, M. E. Reeves, I. Altomare, P. F. Robbins, and P. Hwu. 2001. Retrovirally-transduced human dendritic cells can generate T cells recognizing multiple MHC class I and class II epitopes from the melanoma antigen gp100. J. Immunol. 167:4758-4764. [DOI] [PubMed] [Google Scholar]
- 16.Lapointe, R., J. F. Toso, C. Butts, H. A. Young, and P. Hwu. 2000. Human dendritic cells require multiple activation signals for the efficient generation of tumor antigen-specific T lymphocytes. Eur. J. Immunol. 30:3291-3298. [DOI] [PubMed] [Google Scholar]
- 17.Lecours, K., M. H. Tremblay, M. E. Gagne, S. M. Gagne, and D. Leclerc. 2006. Purification and biochemical characterization of a monomeric form of papaya mosaic potexvirus coat protein. Protein Expr. Purif. 47:273-280. [DOI] [PubMed] [Google Scholar]
- 18.Lepage, S., and R. Lapointe. 2006. Melanosomal targeting sequences from gp100 are essential for MHC class II-restricted endogenous epitope presentation and mobilization to endosomal compartments. Cancer Res. 66:2423-2432. [DOI] [PubMed] [Google Scholar]
- 19.Lindblad, E. B. 2004. Aluminium adjuvants—in retrospect and prospect. Vaccine 22:3658-3668. [DOI] [PubMed] [Google Scholar]
- 20.Lindblad, E. B. 2004. Aluminium compounds for use in vaccines. Immunol. Cell Biol. 82:497-505. [DOI] [PubMed] [Google Scholar]
- 21.Martinez, X., M. Regner, J. Kovarik, S. Zarei, C. Hauser, P. H. Lambert, C. Leclerc, and C. A. Siegrist. 2003. CD4-independent protective cytotoxic T cells induced in early life by a non-replicative delivery system based on virus-like particles. Virology 305:428-435. [DOI] [PubMed] [Google Scholar]
- 22.Marusic, C., P. Rizza, L. Lattanzi, C. Mancini, M. Spada, F. Belardelli, E. Benvenuto, and I. Capone. 2001. Chimeric plant virus particles as immunogens for inducing murine and human immune responses against human immunodeficiency virus type 1. J. Virol. 75:8434-8439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maurer, P., G. T. Jennings, J. Willers, F. Rohner, Y. Lindman, K. Roubicek, W. A. Renner, P. Muller, and M. F. Bachmann. 2005. A therapeutic vaccine for nicotine dependence: preclinical efficacy, and phase I safety and immunogenicity. Eur. J. Immunol. 35:2031-2040. [DOI] [PubMed] [Google Scholar]
- 24.McInerney, T. L., F. R. Brennan, T. D. Jones, and N. J. Dimmock. 1999. Analysis of the ability of five adjuvants to enhance immune responses to a chimeric plant virus displaying an HIV-1 peptide. Vaccine 17:1359-1368. [DOI] [PubMed] [Google Scholar]
- 25.Mihailova, M., M. Boos, I. Petrovskis, V. Ose, D. Skrastina, M. Fiedler, I. Sominskaya, S. Ross, P. Pumpens, M. Roggendorf, and S. Viazov. 2006. Recombinant virus-like particles as a carrier of B- and T-cell epitopes of hepatitis C virus (HCV). Vaccine 24:4369-4377. [DOI] [PubMed] [Google Scholar]
- 26.Noad, R., and P. Roy. 2003. Virus-like particles as immunogens. Trends Microbiol. 11:438-444. [DOI] [PubMed] [Google Scholar]
- 27.Ogasawara, Y., G. Amexis, H. Yamaguchi, S. Kajigaya, S. H. Leppla, and N. S. Young. 2006. Recombinant viral-like particles of parvovirus B19 as antigen carriers of anthrax protective antigen. In Vivo 20:319-324. [PubMed] [Google Scholar]
- 28.Ott, G., R. Radhakrishnan, J.-H. Fang, and M. Hora. 2000. The adjuvant MF59: a 10-year perspective, p. 211-228. In D. T. O'Hagan (ed.), Vaccine adjuvants: preparation methods and research protocols. Humana Press, Totowa, NJ.
- 29.Piazzolla, G., M. Nuzzaci, C. Tortorella, E. Panella, A. Natilla, D. Boscia, S. A. De, P. Piazzolla, and S. Antonaci. 2005. Immunogenic properties of a chimeric plant virus expressing a hepatitis C virus (HCV)-derived epitope: new prospects for an HCV vaccine. J. Clin. Immunol. 25:142-152. [DOI] [PubMed] [Google Scholar]
- 30.Pumpens, P., R. Razanskas, P. Pushko, R. Renhof, I. Gusars, D. Skrastina, V. Ose, G. Borisova, I. Sominskaya, I. Petrovskis, J. Jansons, and K. Sasnauskas. 2002. Evaluation of HBs, HBc, and frCP virus-like particles for expression of human papillomavirus 16 E7 oncoprotein epitopes. Intervirology 45:24-32. [DOI] [PubMed] [Google Scholar]
- 31.Rock, K. L., and L. Shen. 2005. Cross-presentation: underlying mechanisms and role in immune surveillance. Immunol. Rev. 207:166-183. [DOI] [PubMed] [Google Scholar]
- 32.Rock, K. L., I. A. York, and A. L. Goldberg. 2004. Post-proteasomal antigen processing for major histocompatibility complex class I presentation. Nat. Immunol. 5:670-677. [DOI] [PubMed] [Google Scholar]
- 33.Ruedl, C., T. Storni, F. Lechner, T. Bachi, and M. F. Bachmann. 2002. Cross-presentation of virus-like particles by skin-derived CD8(−) dendritic cells: a dispensable role for TAP. Eur. J. Immunol. 32:818-825. [DOI] [PubMed] [Google Scholar]
- 34.Sallusto, F., and A. Lanzavecchia. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 179:1109-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schnurr, M., Q. Chen, A. Shin, W. Chen, T. Toy, C. Jenderek, S. Green, L. Miloradovic, D. Drane, I. D. Davis, J. Villadangos, K. Shortman, E. Maraskovsky, and J. Cebon. 2005. Tumor antigen processing and presentation depend critically on dendritic cell type and the mode of antigen delivery. Blood 105:2465-2472. [DOI] [PubMed] [Google Scholar]
- 36.Schultze, J. L., S. Michalak, M. J. Seamon, G. Dranoff, K. Jung, J. Daley, J. C. Delgado, J. G. Gribben, and L. M. Nadler. 1997. CD40-activated human B cells: an alternative source of highly efficient antigen presenting cells to generate autologous antigen-specific T cells for adoptive immunotherapy. J. Clin. Investig. 100:2757-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shen, L., and K. L. Rock. 2006. Priming of T cells by exogenous antigen cross-presented on MHC class I molecules. Curr. Opin. Immunol. 18:85-91. [DOI] [PubMed] [Google Scholar]
- 38.Shen, L., L. J. Sigal, M. Boes, and K. L. Rock. 2004. Important role of cathepsin S in generating peptides for TAP-independent MHC class I crosspresentation in vivo. Immunity 21:155-165. [DOI] [PubMed] [Google Scholar]
- 39.Shen, L. J., and K. L. Rock. 2004. Cellular protein is the source of cross-priming antigen in vivo. Proc. Natl. Acad. Sci. USA 101:3035-3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Storni, T., and M. F. Bachmann. 2004. Loading of MHC class I and II presentation pathways by exogenous antigens: a quantitative in vivo comparison. J. Immunol. 172:6129-6135. [DOI] [PubMed] [Google Scholar]
- 41.Tobian, A. A., D. H. Canaday, W. H. Boom, and C. V. Harding. 2004. Bacterial heat shock proteins promote CD91-dependent class I MHC cross-presentation of chaperoned peptide to CD8+ T cells by cytosolic mechanisms in dendritic cells versus vacuolar mechanisms in macrophages. J. Immunol. 172:5277-5286. [DOI] [PubMed] [Google Scholar]
- 42.Tobian, A. A., C. V. Harding, and D. H. Canaday. 2005. Mycobacterium tuberculosis heat shock fusion protein enhances class I MHC cross-processing and -presentation by B lymphocytes. J. Immunol. 174:5209-5214. [DOI] [PubMed] [Google Scholar]
- 43.Tremblay, M. H., N. Majeau, M. E. Gagne, K. Lecours, H. Morin, J. B. Duvignaud, M. Bolduc, N. Chouinard, C. Pare, S. Gagne, and D. Leclerc. 2006. Effect of mutations K97A and E128A on RNA binding and self assembly of papaya mosaic potexvirus coat protein. FEBS J. 273:14-25. [DOI] [PubMed] [Google Scholar]
- 44.von Bergwelt-Baildon, M. S., R. H. Vonderheide, B. Maecker, N. Hirano, K. S. Anderson, M. O. Butler, Z. N. Xia, W. Y. Zeng, K. W. Wucherpfennig, L. M. Nadler, and J. L. Schultze. 2002. Human primary and memory cytotoxic T lymphocyte responses are efficiently induced by means of CD40-activated B cells as antigen-presenting cells: potential for clinical application. Blood 99:3319-3325. [DOI] [PubMed] [Google Scholar]
- 45.Vonderheide, R. H., W. C. Hahn, J. L. Schultze, and L. M. Nadler. 1999. The telomerase catalytic subunit is a widely expressed tumor-associated antigen recognized by cytotoxic T lymphocytes. Immunity 10:673-679. [DOI] [PubMed] [Google Scholar]
- 46.Yang, R., F. M. Murillo, H. Cui, R. Blosser, S. Uematsu, K. Takeda, S. Akira, R. P. Viscidi, and R. B. Roden. 2004. Papillomavirus-like particles stimulate murine bone marrow-derived dendritic cells to produce alpha interferon and Th1 immune responses via MyD88. J. Virol. 78:11152-11160. [DOI] [PMC free article] [PubMed] [Google Scholar]