Abstract
Only a few monoclonal antibodies (MAbs) have been isolated that recognize conserved sites in human immunodeficiency virus type 1 (HIV-1) Env proteins and possess broad neutralizing activities. Other MAbs directed against targets in various domains of Env have been described that are strongly neutralizing, but they possess limited breadth. One such MAb, 2909, possesses a uniquely potent neutralizing activity specific for a quaternary epitope on SF162 Env that requires the presence of both the V2 and the V3 domains. We now show that replacement of the SF162 V3 sequence with consensus V3 sequences of multiple subtypes led to attenuated but still potent neutralization by 2909 and that the main determinants for the type specificity of 2909 reside in the V2 domain. A substitution at position 160 completely eliminated 2909 reactivity, and mutations at position 167 either attenuated or potentiated neutralization by this antibody. Different substitutions at the same positions in V2 were previously shown to introduce epitopes recognized by MAbs 10/76b and C108g and to allow potent neutralization by these MAbs. Two substitutions at key positions in the V2 domain of JR-FL Env also allowed potent expression of the 2909 epitope, and single substitutions in YU2 V2 were sufficient for expression of the 2909, C108g, and 10/76b epitopes. These results demonstrate that the minimal epitopes for 2909, C108g, and 10/76b differed from that of the clade B consensus sequence only at single positions and suggest that all three MAbs recognize distinct variants of a relatively conserved sequence in V2 that is a particularly sensitive mediator of HIV-1 neutralization.
A major factor thwarting the development of a successful human immunodeficiency virus type 1 (HIV-1) vaccine is the resistance of primary isolates to neutralization by classes of antibodies commonly induced after infection or immunization (1, 45). Sequence variability at major neutralization sites contributes to this effect, but recent evidence argues that the major factor in this resistance is conformational shielding of susceptible epitopes in the native oligomeric complex (18, 28). N-linked glycans located in various regions of Env play a general role in epitope masking (6, 7, 22, 39), and increasing evidence documents a dominant role for the V1/V2 domain in such masking (6, 12, 18, 28, 34, 44). One approach being investigated to overcome the effects of this masking is to delete the V2 domain from Env-based immunogens. Oligomeric V2-deleted forms of gp140 have been reported to possess enhanced immunogenicity over the wild-type molecule and to produce increased titers of neutralizing antibodies (8, 21, 33, 43). However, these effects are only modest, and recent studies indicate that this approach involves the induction of type-specific neutralizing antibodies directed mostly toward highly variable epitopes in V1 that possess limited neutralizing activities for heterologous isolates (10, 42).
The critical role of conformational masking in neutralization resistance poses a major conundrum for HIV vaccine development. The limited number of known neutralization targets that are insensitive to masking, such as those seen by broadly neutralizing monoclonal antibodies (MAbs) b12, 2G12, and 2F5, are poorly immunogenic (4, 26, 31), and available antibodies against these epitopes possess unusual immunoglobulin structures that are quite distant from germ line configurations and thus are difficult to elicit (3, 5, 29, 46). Thus, it is important to identify additional immunogenic targets that can mediate potent neutralization and that are either reasonably well conserved or present in a limited number of variants suitable for formulation into a multivalent vaccine.
One potential target for neutralizing antibodies that has not been sufficiently exploited is the V1/V2 domain itself. In addition to their roles in epitope masking, the V1 and V2 domains contain neutralization epitopes (11, 13, 15, 16, 23, 24, 32, 38). The general interest in such MAbs has been limited due to their restricted specificities and, in most cases, relatively weak neutralizing activities. However, several anti-V2 MAbs possess unusually potent type-specific neutralizing activities. These include C108g, directed against a complex epitope localized in the V2 domain (36, 40), and 2909, the first anti-HIV MAb that reacts specifically with a quaternary epitope restricted to native Env oligomers present on the surface of intact virion particles (14). The epitopes recognized by these MAbs have not been well characterized, and thus, the potential utility of these and related epitopes as vaccine targets is unclear.
C108g was isolated from a chimpanzee that was infected with the IIIB virus isolate and then immunized with soluble MN gp120 (38). This MAb reacts in a type-specific manner with IIIB and BaL isolates, and it possesses potent neutralizing activity for viruses with these Envs (37). C108g binds to both soluble gp120 and isolated IIIB V1/V2 fusion protein, and its reactivity with these antigens is sensitive to both deglycosylation and reduction of disulfide bonds (27, 38, 40). Determinants of the epitope were mapped to a specific N-linked glycan present at position 160 in the N-terminal portion of the V2 domain and to adjacent residues at positions 162 to 169. The 162-169 peptide sequence required for C108g reactivity is also recognized by 10/76b, a MAb isolated from a rat immunized with soluble IIIB gp120 (23, 32). In contrast to C108g, 10/76b reacts strongly with denatured gp120 and linear peptides that include residues 162 to 169, and while 10/76b strongly neutralizes an SF162 variant containing the required peptide sequence, its neutralizing potency is highly attenuated by the addition of the glycan at position 160 (27). The reactivities of both C108g and 10/76b are strictly dependent on the presence of G at position 167.
2909 was isolated from an asymptomatic HIV-infected patient by screening directly for neutralizing activity against SF162 pseudotypes (14). This MAb possesses remarkably potent neutralizing activity for SF162, with a 50% inhibitory concentration (IC50) value reported to be significantly lower than that of any previously described MAb, including the broadly cross-reactive MAbs 2F5, b12, and 2G12. 2909 is highly type specific for native SF162 virions; it does not bind to soluble SF162 Env proteins or to virions containing SF162 Env from which the V2 or V3 domain has been deleted. In addition, its binding to SF162 virions is competed by MAbs to sites in V2 and V3 and by ligands to the CD4-binding domain, b12 and sCD4 (14). These data suggest that 2909 is directed against a quaternary epitope that is specifically expressed on native viral oligomers and is composed of, or closely associated with, sites in the V2 and V3 and the CD4-binding domains.
The exceptional neutralizing activity of C108g and 2909 for viruses bearing the corresponding epitopes suggests the potential clinical utility of HIV-1 vaccines that elicit antibodies against more representative forms of these epitopes. Exploring this possibility requires more information regarding the structures of these epitopes and how they differ from more widely distributed sequences in this region of Env. The present study defined the determinants of the specificity of the 2909 epitope and demonstrated a relationship between the V2 sequences recognized by 2909, those recognized by C108g and 10/76b, and the clade B consensus sequence in this region.
MATERIALS AND METHODS
Monoclonal antibodies.
The characteristics of chimpanzee MAb C108g and its epitope were previously described (27, 36, 38, 40). 10/76b was isolated from a rat immunized with soluble HXB10 gp120 (23), and characteristics of its epitope have also been described previously (32). MAb 2909 was isolated from an HIV-1-infected individual by screening for the neutralization of HIV-1 pseudotyped with SF162 Env (14). The following control MAbs were obtained from the NIH AIDS Research and Reference Reagent Program. IgG1b12 (2), directed against an epitope that overlaps the CD4-binding site, was contributed by Dennis Burton and Paul Parren; 2G12 (35), directed against a conformational epitope involving high-mannose glycans, and 2F5 (25), directed against an epitope in the ectodomain of gp41, were contributed by Hermann Katinger. A V3 MAb pool consisting of equal concentrations of nine human MAbs (447-52D, 4117C, 4148, 2182, 2191, 2219, 2412, 2442, and 2456) highly reactive with clade B V3 sequences was previously described (18).
Generation of chimeric and variant forms of Env.
Variant Envs containing modified residues in the V1, V2, and/or V3 domain were generated by sequentially introducing the necessary modifications by site-directed mutagenesis using the QuikChange kit (Stratagene, Inc.). SF162 variants containing modified V3 sequences were previously described (18). SF162 V2 variants used to analyze the 2909 epitope contained modifications at residues 160 and 161 (KV to NI), 167 (N to D or G), and 169 (M to V), in various combinations, while JR-FL variants contained modifications at residues 160 (N to K), 167 (D to G), and 168 (E to K). For isolate YU2, modifications were made at residue 160 (N to K), to introduce the 2909 epitope, and at residue 167 (D to G), to introduce the C108g and 10/76b epitopes. In addition, two N-linked glycosylation sites in V1 that were present in YU2 but absent from SF162 were also removed. One site was located at N131, the asparagine residue immediately N terminal to the first cysteine of the V2 loop, and the second site was at position 140. These sites were mutated individually and in tandem by converting the T of the glycosylation motif (NXT) to A. The wild-type and variant sequences of the relevant regions are listed in Tables 1, 2, and 3. The replacement of the SF162 V1/V2 domain with that of the JR-FL N160K/E168K mutant was made by exchanging restriction fragments generated by cleavage at unique DraIII and StuI sites located immediately N terminal and C terminal, respectively, to the V1/V2 domains of both Envs.
TABLE 1.
Effect of V3 sequence variation on neutralization sensitivity of 2909 for chimeric SF162 Envs
| V3 origina | V3 sequence | IC50 (μg/ml)b | IC50 ratioc |
|---|---|---|---|
| SF162 | CTRPNNNTRKSITIGPGRAFYATGDIIGDIRQAHC | 0.00019 | |
| Clade C | ------------R----QT---------------- | 0.0079 | 40 |
| Clade A1 | ------------R----Q----------------- | 0.029 | 150 |
| CRF02_AG | -----------VR----QT---------------- | 0.24 | 1,300 |
| Clade F | ------------H----Q------E------K--- | 0.38 | 2,000 |
| Clade B | ------------H--------T--E---------- | 0.54 | 2,900 |
| Clade H | ------------HL---Q----------------- | 0.96 | 5,000 |
| CRF01_AE | ----S----T-------QV--R---------K-Y- | >20 | >100,000 |
V3 domains corresponded to SF162 or to the consensus sequence of the indicated subtype.
IC50 values reported are averages of at least three independent measurements.
Indicates the n-fold increase in IC50 values for the pseudotypes with SF162 Envs with the variant V3 compared to those for SF162 Env.
TABLE 2.
Neutralization endpoints for 2909 against Envs with variant sequences in the V1/V2 domain
| Clade B consensusb | V2 sequence at positiona | IC50 (μg/ml) | IC90 (μg/ml) |
|---|---|---|---|
| 160167 | |||
| GEIKNCSFNITTSIRDKVQKEYALFY | |||
| SF162 | --------KV-----N-M-------- | 0.00019 | 1.1 |
| SF-NI (K160N/V161I) | ---------------N-M-------- | >20 | >20 |
| SF-GKV (N167G/M169V) | --------KV-----G---------- | 1.3 | >20 |
| SF-NKV (M169V) | --------KV-----N---------- | 0.0088 | 5.5 |
| SF-DKV (N167D/M169V) | --------KV---------------- | 0.000078 | 0.010 |
| SF-DKM (N167D) | --------KV-------M-------- | 0.000080 | 0.0031 |
| JR-FL | ----------------E--------- | >100 | >100 |
| JR-FL (N160K) | --------K-------E--------- | 25 | >100 |
| JR-FL (E168K) | -------------------------- | >20 | >20 |
| JR-FL (N160S/E168K) | --------S----------------- | 0.70 | 6.3 |
| JR-FL (N160K/E168K) | --------K----------------- | 0.00034 | 0.0093 |
| SF162 (JR-FL V1V2 N160K/E168K) | --------K----------------- | 0.00012 | 0.0030 |
| YU2 | -------------------------- | >20 | >20 |
| YU2 (N160K) | --------K----------------- | 0.079 | 6.8 |
| YU2 (T133A/N160K)c | --------K----------------- | 0.0053 | 2.9 |
V2 residues 152-177 (based on the HXB2 numbering system) are compared to the clade B consensus sequence. Dashes indicate residues identical to those of the consensus sequence. N-linked glycosylation signals [NX(S/T)] are underlined.
Env variants are described by listing in parentheses the original residue, the position, and the modified residue. For SF162 variants, a shorter name corresponding to that used in Fig. 1 is also provided in which the residues at key positions 160 to 161 or 167 to 169 are indicated. IC50 and IC90 values are averages of at least three independent assays.
See Table 3 for the YU2 V1 region sequence.
TABLE 3.
50% neutralization endpoints for V2-specific MAbs C108g and 10/76b against YU2 Envs with modified V1 and V2 sequences
| Envelope | V1 region | V2 region | IC50 (μg/ml)
|
|
|---|---|---|---|---|
| C108g | 10/76b | |||
| 131140 | 160167 | |||
| YU2 | LNCTDLRNATNTT | GEIKNCSFNITTSIRDKVQKEYALFY | >200 | >200 |
| YU2 (D167G) | ------------- | ---------------G---------- | 0.24 | 11 |
| YU2 (T133A/D167G) | ---A--------- | ---------------G---------- | 0.057 | 2.6 |
| YU2 (T133A/T142A/D167G) | ---A--------A | ---------------G---------- | 0.0095 | 1.7 |
| YU2 (T133A/T142A/T162A/D167G) | ---A--------A | ----------A----G---------- | 7.7 | 0.033 |
Viral neutralization assays.
Neutralization activity was determined as previously described (19) with a single-cycle infectivity assay using virions generated from the Env-defective luciferase-expressing pNL4-3.Luc.R−E− genome (9) pseudotyped with the molecularly cloned HIV Env of interest. In brief, pseudotyped virions were incubated with serial dilutions of MAbs for 1 h at 37°C and were then added to U87-T4-CCR5 target cells plated out in 96-well plates in the presence of Polybrene (10 μg/ml). The amount of virus used in each neutralization assay was normalized to produce a standard level of luciferase activity (generally 25,000 to 50,000 units). After 24 h, cells were refed with RPMI medium containing 10% fetal bovine serum and Polybrene and incubated for an additional 24 to 48 h. Luciferase activity was determined 48 to 72 h postinfection with a microtiter plate luminometer (HARTA, Inc.), using assay reagents from Promega, Inc. IC50 and IC90 values were determined by interpolation from neutralization curves and are averages of at least three independent assays.
RESULTS
Mapping V3 determinants of the 2909 epitope.
The various domain determinants required for 2909 reactivity were mapped by examining the neutralizing activity of this MAb against a series of SF162 mutants bearing changes in both the V1/V2 and V3 domains. As previously reported, 2909 possessed extremely potent neutralizing activity for SF162, with an IC50 value in the picomolar range (0.00019 μg/ml). Replacing the V3 domain of SF162 with V3 consensus sequences of various subtypes resulted in significant reductions in sensitivity to neutralization by 2909, although in many cases the neutralization potency remained quite high (Table 1). The most sensitive of these variants was the clade C consensus sequence (IC50, 0.0079 μg/ml); this V3 sequence differed from the SF162 sequence at three positions (T310R, R315Q, and A316T). The clade A1 variant possessed only the first two of these changes and was approximately fourfold less sensitive than the clade C variant, suggesting that the A316T change may in some contexts enhance 2909 reactivity. Several other variants that possessed three or four modifications corresponding to consensus sequences of clades CRF02_AG, clade F, clade B, and clade H were more resistant, but they were still neutralized, with IC50 values below 1 μg/ml. The only variant resistant to 2909 at the highest concentration tested corresponded to the CRF01_AE consensus sequence and contained seven modified residues.
These results confirmed a role for the V3 domain in expression of the 2909 epitope and the ultrasensitivity of SF162 Env to this MAb but also demonstrated the considerable tolerance of 2909 for V3 sequence variation. The greater reactivity of 2909 for clade A and C sequences over that for the clade B sequence indicated that the clade B signature residue R315 was not a major determinant for 2909 reactivity, despite its isolation from a clade B-infected patient (14). The 2909 IC50s were in many cases similar to or lower than those of broadly neutralizing antibodies b12 and 2G12 for the same viruses (18). Thus, while specific features of the SF162 V3 sequence were required for the optimal recognition of viruses by 2909, this MAb retained sufficient affinity for many SF162 Env chimeras expressing variant V3 sequences corresponding to consensus sequences of multiple viral subtypes to mediate relatively potent neutralization.
Determinants in the V2 domain required for 2909 reactivity.
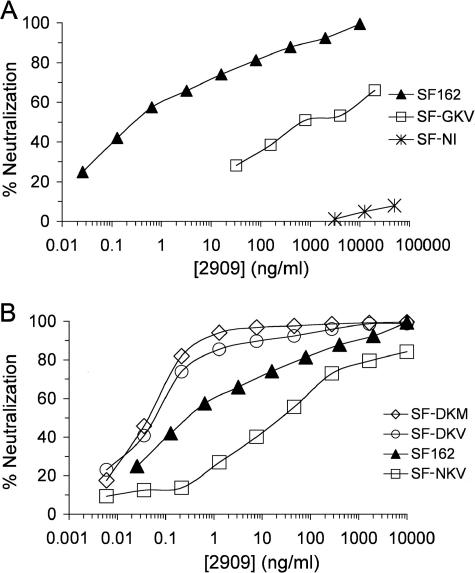
To map determinants in the V2 domain required for the 2909 epitope, the neutralizing activity of 2909 for several V1/V2 variants was examined. The initial mutants analyzed were two SF162 variants that had been modified to express determinants of the C108g and 10/76b epitopes (27): K160N/V161I (the SF-NI mutant), in which the 160 glycosylation site was inserted by replacing residues KV at positions 160 and 161 with NI, and N167G/M169V (the SF-GKV mutant), in which residues N167 and M169 were replaced with G and V, respectively (Fig. 1A and Table 2). The SF-NI mutant containing the N-linked glycosylation site at position 160 had no detectable reactivity to 2909 (Fig. 1A), suggesting a critical role for the SF162-specific K160 and/or V161 residue for the 2909 epitope. 2909 possessed highly attenuated activity with the SF-GKV mutant, with an IC50 value increase of >6,800 (Fig. 1A and Table 2), indicating that one or both of the residues at positions 167 and 169 were also important contributing factors to the epitope.
FIG. 1.
Representative neutralization titers for 2909 against pseudovirions containing Env proteins corresponding to (panel A) wild-type SF162, SF162 mutants N167G/M169V (GKV), and K160N/V161I (NI) and (panel B) wild-type SF162 and SF162 mutants N167D (DKM), N167D/M169V (DKV), and M169V (NKV). Note that the antibody concentration units are ng/ml.
The contributions of individual residues at positions 167 and 169 to the 2909 epitope were probed by introducing single changes at these positions (Fig. 1B). Converting only M169 to V (NKM to NKV) resulted in a 46-fold increase in the IC50 value and a 5-fold increase in the IC90 value compared to that from the wild-type sequence (Table 2), indicating that M at position 169 was preferred by 2909 over V, the consensus residue at this position. On the other hand, when N167 was replaced by the consensus residue (D) at this position (NKM to DKM), a considerable enhancement in reactivity with 2909 was obtained over that for the wild-type SF162 Env. This enhancement was particularly impressive at higher levels of neutralization. Whereas the 2909 neutralization curves for wild-type SF162 typically exhibited gradual slopes that never plateaud, the neutralization curve for the N167D mutant exhibited a sharper slope and reached a plateau of >99% neutralization at relatively low 2909 concentrations. This resulted in a 2- to 3-fold decrease in the IC50 value over that of the parental Env but a decrease of >350-fold in the IC90 value. Consistent with the decreased activity of 2909 with the single mutant in which M169 was changed to V, the DKV mutant in which the N167D and M169V changes were combined was neutralized slightly less well than the DKM mutant (threefold higher IC90 value), although still considerably better than the wild-type SF162 Env. These results indicated a dominant effect for the D167 residue on the 2909 epitope.
Expression of the 2909 epitope in JR-FL Env upon introduction of key V2 residues.
The results described above indicated that key determinants for the 2909 epitope resided at positions 160-161 and 167 of the SF162 V2 domain. Since SF162 Env is unusually sensitive to neutralization by many MAbs and polyclonal antisera, it was of interest to examine the effect of inserting the key residues required for expression of the 2909 epitope into Envs that possessed more resistant neutralization phenotypes typical of primary isolates. This was tested with two neutralization-resistant Envs, JR-FL and YU2 (18, 28).
The V2 sequence of JR-FL Env resembles the clade B consensus sequence in that it contains the N-linked glycosylation signal at position 160 and D at position 167 but differs from the consensus sequence by the presence of an acidic E at position 168 in place of the basic residue K generally found at that position. Wild-type JR-FL Env was completely resistant to 2909, but changing residue N160 to K resulted in weak reactivity with 2909 (IC50 of 25 μg/ml) (Fig. 2 and Table 2). The effect of the E168 residue on expression of this epitope was examined by replacing it with the consensus K residue. The E168K mutation by itself did not lead to reactivity with 2909 (Table 2); however, combining the E168K and N160K mutations resulted in ultrapotent neutralization by 2909. The neutralization curve for the N160K/E168K mutant resembled those of the SF162 mutants containing D167, both in the sharper slopes of the curves and in the attainment of plateaus at ∼100% neutralization. This modified slope was reflected in the fact that whereas the 2909 IC50 value for this mutant was similar to that for SF162 Env, its IC90 value was lower by more than 100-fold.
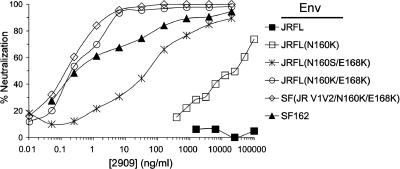
FIG. 2.
Representative neutralization curves of 2909 against pseudovirions containing wild-type SF162 and JR-FL Envs and JR-FL variants with mutations N160K, N160S/E168K, and N160K/E168K. Also shown is the neutralization curve obtained for chimeric SF162 Env containing the V1/V2 domain from the JR-FL N160K/E168K mutant [SF(JR V1V2 N160K/E168K)].
The effective neutralization of the JR-FL N160K/E168K V2 mutant was particularly impressive in view of the fact that this Env contains the clade B consensus V3 sequence, which as shown above, was recognized relatively poorly by 2909 (Table 1). To examine the effect of combining the N160K/E168K mutations in the JR-FL V1/V2 sequence with the optimal SF162 V3 sequence, the JR-FL N160K/E168K V1/V2 domain was introduced into SF162 to produce the chimeric SF(JR-FL V1/V2 N160K/E168K) Env. This Env possessed a sensitivity to 2909 that was similar to that of the SF162 (N167D) variant and was about threefold more sensitive than the JR-FL variant with the same V1/V2 sequence (Fig. 2 and Table 2). This indicated that the large reduction in 2909 activity that resulted from substitutions in the V3 domain of SF162 Env was not seen in Env variants that expressed the optimized V2 sequence for the epitope.
The requirement for K at position 160 could indicate a strict dependence on this residue or could be due to a masking effect of the 160 glycan on 2909 reactivity. To examine these possibilities, an S was substituted at position 160 in place of K, together with the E168K modification (Fig. 2). The S160 substitution also resulted in the loss of the N-linked glycosylation signal at position 160 but placed a neutral serine residue at this position instead of a basic lysine. The resulting mutant was recognized by 2909, although with considerably lower potency than the mutant with K160. The change from K to S at position 160 resulted in a 200-fold increase in the IC50 value and an increase of >600-fold in the IC90 value. This result indicated that the removal of the 160 glycan by itself was not sufficient for full 2909 activity and that while the presence of K at position 160 was not an absolute requirement for 2909 reactivity, this residue contributed strongly to the high-affinity recognition by this MAb.
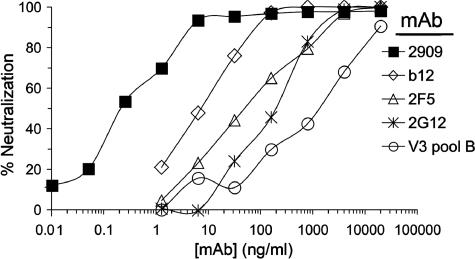
The potency of the neutralizing activity of 2909 for the JR-FL Env variant with the N160K/E160K mutations was highlighted by comparing this neutralization to that of several MAbs directed against highly conserved epitopes that are considered to be highly sensitive neutralization targets (3). 2909 neutralized virus pseudotyped with this Env more potently than MAb b12, 2F5, or 2G12 (Fig. 3). Based on the neutralization curves shown in Fig. 3, the IC50 value for 2909 was >20-fold lower than that of b12, >150-fold lower than that of 2F5, and >580-fold lower than that of 2G12. In addition, although the N160K/E168K JR-FL mutant was somewhat more sensitive (∼sevenfold lower IC50) to a pool of anti-V3 MAbs than wild-type JR-FL Env, the IC50 value of 2909 was >4,000-fold lower than that of the anti-V3 pool. This indicated that the potent activity of 2909 for this variant occurred despite the effective masking of standard V3 epitopes and the relative resistance of this Env to MAbs directed against conserved neutralization targets.
FIG. 3.
Neutralization curves obtained for various MAbs against virus pseudotyped with the JR-FL N160K/E168K Env. The 2909 neutralization curve is compared to those of MAbs IgG-b12, 2F5, and 2G12 and a pool of clade B-derived anti-V3 MAbs.
Expression of the 2909 epitope in YU2 Env.
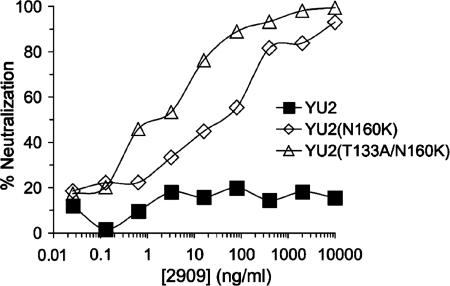
The YU2 Env V2 sequence is identical to the consensus clade B sequence in the region of residues 152 to 177 and includes the glycosylation site at position 160 and DKV at positions 167 to 169. Although the wild-type YU2 Env was completely resistant to 2909, the introduction of just the N160K mutation allowed significant 2909 reactivity, with an IC50 of 0.079 μg/ml (Fig. 4 and Table 2). Although neutralization of this mutant by 2909 was quite potent compared to other MAbs against this Env, this Env was considerably less sensitive to this MAb than the SF162 and JR-FL variants containing the same residues at the key positions shown above to define the 2909 epitope. YU2 differed from both the SF162 and the JR-FL Envs by the presence of an N-linked glycosylation signal adjacent to C131, one of the cysteine residues involved in forming the V1 loop (see Table 3). A glycan at this position was previously shown to be an effective masking determinant for V3 epitopes (18). To examine the possible masking effect of this glycan on the 2909 epitope, this glycosylation signal was mutated by converting T133 to A. This mutation resulted in an additional 15-fold reduction in the IC50 of 2909, to 0.0053 μg/ml (Table 2). Despite this increased potency, the sensitivity of this YU2 variant remained ∼60-fold lower than that of the optimized SF162 and JR-FL variants; this decreased sensitivity might reflect additional masking effects by other residues, or it might reflect changes in the V3 region or elsewhere that reduced the affinity of the epitope.
FIG. 4.
Representative neutralization curves for 2909 against virus pseudotyped with wild-type and variant forms of YU2 Env containing mutations N160K and T133A/N160K.
Requirements for expression of C108g and 10/76b epitopes in YU2.
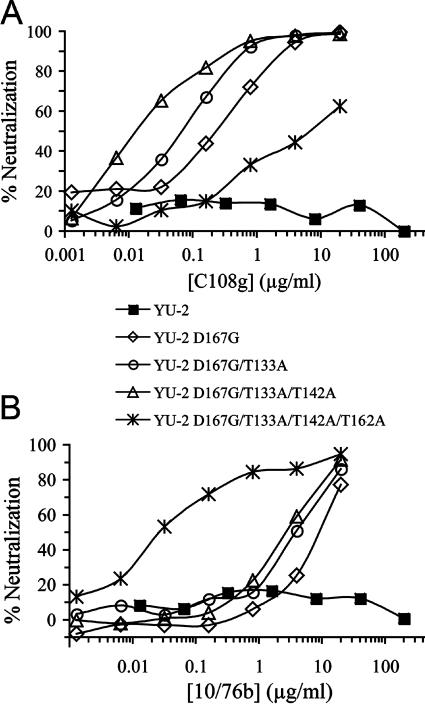
Previous studies showed that the presence of residues G167 and V169 in the V2 domain allows expression of the C108g and 10/76b epitopes in several different Env backbones (36, 40). YU2 Env contains D and V at these positions, suggesting that the only element of the C108g and 10/76b epitopes missing from this Env was G167. To test this, and to examine the potencies of these epitopes when expressed in a highly neutralization-resistant Env, the D167G mutation was introduced into YU2 Env (Table 3). This mutation did in fact result in significant neutralization by C108g (IC50, 0.24 μg/ml) and also allowed weak neutralization by 10/76b (IC50, 11 μg/ml). Since the glycan at position N131 present in YU2 Env partially masked the 2909 epitope (Fig. 4), the effect of removing this glycan on sensitivities to C108g and 10/76b was examined. As shown in Fig. 5, the T133A mutation resulted in an approximate fourfold decrease in the IC50 values of both C108g and 10/76b. Removing a second V1 glycosylation site at position 140 that was present in YU2 but not in SF162 Env resulted in an additional sixfold increase in sensitivity to C108g (IC50, 0.0095 μg/ml) but had a minimal effect on sensitivity to 10/76b. On the other hand, eliminating the glycan at position 160 in V2 by mutating T162 to A in conjunction with the two V1 glycan and D167G mutations resulted in a large decrease in sensitivity to C108g (Fig. 5A) but a large increase in sensitivity to 10/76b (IC50, 0.033 μg/ml) (Fig. 5B). This was consistent with previous data showing that while the glycan at position 160 is an important component of the C108g epitope, it acted as a potent masking element for the 10/76b epitope (27).
FIG. 5.
Representative neutralization curves for MAbs C108g (panel A) and 10/76b (panel B) against virus pseudotyped with wild-type YU2 Env and YU2 variants containing the mutation D167G by itself or with additional mutations at N-linked glycosylation sites located in V1 (T133A and T142A) and V2 (T162A).
DISCUSSION
The data described in this study define the variable region determinants of the quaternary epitope identified by MAb 2909 and demonstrate that the main factor for the limited reactivity of this MAb is its requirement for a relatively rare polymorphism at position 160 in the V2 domain. The positions in V2 that affected the 2909 epitope were also determinants for two unrelated MAbs, C108g and 10/76b, that also possessed potent neutralizing activities for certain isolates. Reciprocal relationships were observed between these epitopes. While 2909 preferred a K at position 160 and 10/76b was inhibited by the glycan normally present at this position, C108g reactivity was enhanced by the presence of this glycan; and whereas 2909 strongly preferred N or D at position 167, both C108g and 10/76b required a G at this position. Any additional components that may be required for expression of these epitopes were present in all Envs tested and thus appeared to be relatively conserved.
A major limitation of the utility of the 2909, C108g, and 10/76b epitopes as vaccine targets is their limited distribution in clinical isolates. A new and encouraging finding of the present study is that the critical determinants of each of these epitopes differed from the clade B consensus sequence only at single positions: residue 160 for 2909 and residue 167 for C108g and 10/76b. This leads to the conclusion that these MAbs recognize variant forms of a generally conserved structure in V2. The unusually potent neutralizing activities of these MAbs for Envs that express appropriate forms of these epitopes further indicate that this region in the V2 domain is highly accessible on the surface of infectious virions and functions as an ultrasensitive neutralization target.
An interesting finding was that, although 2909 was isolated by screening for the neutralization of SF162 pseudotypes, SF162 Env expressed a suboptimal form of the 2909 epitope. This was reflected by the relatively flat slope of the SF162 neutralization curve and by incomplete neutralization even at very high antibody concentrations (Fig. 1A). Replacement of the N present at position 167 of SF162 Env with D resulted in more robust neutralization, with a sharper slope for the neutralization curve, somewhat lower IC50s, dramatically lower IC90s, and 100% neutralization plateaus at relatively low MAb concentrations. D167 is the consensus residue at this position and is present in 70% of clade B sequences and >97% of clade A and C sequences, while N167 is the second most common residue at this position, present in 16% of clade B sequences. Thus, the 2909 requirements at position 167 are widely distributed and are not limiting factors for 2909 reactivity.
Residue 168 also strongly influenced the potency of neutralization by 2909, with K being the preferred substituent at this position. JR-FL Env has an acidic E at position 168, and the JR-FL N160K mutant was neutralized only weakly by 2909 (Fig. 2 and Table 2). Adding the E168K mutation to the N160K variant resulted in a >60,000-fold decrease in IC50 values for 2909 over that for the single point mutants. K168 is the clade B consensus residue, found in 89% of clade B sequences, while E is present at this position in only 2/176 clade B sequences. The reactivity of 2909 is influenced to a lesser extent by the residue at position 169, and both of the common residues found at this position, V and M, were efficiently recognized by the MAb. In the SF162 backbone, 2909 preferred M169 over V169, although this preference was less apparent when the sequence at position 167 was optimized by replacing N with D (Fig. 1B). V is the clade B consensus residue at position 169 (found in 61% of sequences), while M, the residue present in SF162, is the second most common residue (present in 20% of sequences).
The highly limited breadth of reactivity of 2909 is due to the rarity of the requisite substituents at position 160. The K160 required for optimal 2909 reactivity occurs in only 5% of clade B sequences in a recently reported HIV-1 sequence database (20). S at position 160 allowed for weak 2909 reactivity, and this substituent is found in only ∼1% of clade B sequences. On the other hand, 2909 did not recognize variants that possessed the N-linked glycosylation site at position 160, a highly conserved feature that is present in 88% of clade B sequences, 94% of clade C sequences, and 98% of clade A sequences (20).
The V3 loop is also an essential component of the 2909 epitope and an important determinant of its reactivity. However, whereas 2909 reacted preferentially with the SF162-specific V3 sequence, it also recognized viruses possessing a number of divergent V3 domains (Table 1). The contribution of the V3 region to the strength of reactivity with 2909 appeared to be diminished for variants possessing more optimal forms of the V2 component of the epitope, as indicated by the observation that fairly similar neutralization profiles were obtained when the enhanced form of the epitope (containing D167) was introduced into either the SF162 or the JR-FL backbone (Fig. 2), despite the suboptimal V3 sequence of the JR-FL Env.
Recognition by both C108g and 10/76b was strictly dependent on the presence of Gly at position 167 (38, 40), and a single D167G substitution was sufficient to introduce both epitopes into YU2 Env (Fig. 5 and Table 3), although potent neutralization by 10/76b was also dependent on the loss of the N-linked glycosylation site at position 160. G167 is present in the IIIB-related viruses and in the BaL primary isolate, both of which are highly sensitive to C108g (37). Otherwise, G167 occurs infrequently, found in only 8% of clade B sequences. C108g and 10/76b do not recognize Envs that contain the more common D or N residue at position 167 (27), thus accounting for the narrow distribution of these epitopes.
The potency levels of 2909, C108g, and 10/76b for YU2 variants expressing their respective epitopes were increased upon mutation of the glycosylation site at position 131, at the base of the V1 loop. C108g potency was further increased when this mutation was combined with the mutation of a second glycosylation site at position 140 in V1 (Table 3). These two V1 glycans were recently shown to contribute to the strong masking activity of the YU2 V1/V2 domain for V3 epitopes (18), and the present results indicate that they also function as masking elements for epitopes in V2. The presence of an N-linked glycosylation site at the base of the V1 loop occurs fairly frequently in subtype B sequences and is present in 72% of the sequences in the 2003 database. However, a glycan at this position is found less frequently for other subtypes, occurring in 51% of clade A viruses (30/59) and only 39% (47/122) of clade C viruses. This suggests that whereas masking of V2 epitopes by a glycan at this position might occur in the majority of clade B isolates, it would be less common for non-clade B viruses.
A key question arising from the description of these highly sensitive type-specific V2 neutralization epitopes is whether more conserved forms of these targets are present in isolates that possess more representative sequences at the key positions that define epitope specificity. The frequency in infected subjects of antibodies against other quaternary epitopes that have different and perhaps broader distributions than the 2909 epitope is unknown, since the quaternary nature of the epitope has precluded the identification of such specificities by standard binding assays that use soluble antigens. The fact that 2909 was detected by screening against a heterologous virus and was the first HIV-1 antibody isolated in this functional screening assay raises the possibility that antibodies specific for additional quaternary epitopes may be produced at reasonable frequencies. The frequency of antibodies against more representative forms of C108g-like epitopes is also unknown. Most of the available V2-specific MAbs were generated by immunization with, or screening against, IIIB-derived Env proteins, and these MAbs recognize sequences containing G167 and are often dependent on this residue (17). Additional attempts to isolate antibodies against proteins with consensus sequences at the critical positions in this region are needed to determine the frequency and properties of such antibodies.
It is interesting to note the unusual properties of the C108g and 2909 epitopes that potentiate the neutralizing activities of these antibodies. C108g recognizes a conformation-dependent structure that incorporates components of the highly conserved glycan at position 160 into the epitope, while 2909 recognizes a quaternary epitope that includes regions of both the V2 and V3 domains. Antibodies capable of interacting with epitopes that combine the conserved segments of the 2909 and C108g epitopes, i.e., the consensus residue D at position 167 preferred by 2909 together with the glycan at position 160 required for potent C108g activity, might possess broad and potent neutralizing activity and would be attractive products for HIV vaccines. Additional insights into the structural requirements of these epitopes may facilitate the design of immunogens that efficiently induce antibodies against more representative forms of these epitopes that also possess potent neutralizing activities but greater cross-reactivities. Of particular importance, the identification of a single locus in V2 that is a critical determinant of three discrete epitopes capable of mediating potent neutralization suggests the merit of continued efforts toward understanding the structure and immunogenic properties of this region and clarifying its role in viral infection.
Acknowledgments
These studies were supported by U.S. Public Health Service grants AI46283 and AI50452 to A.P. and HL59725, AI36085, and AI47053 to S.Z.-P., by the Immunology Core of the NYU Center for AIDS Research (NIH grant AI27742), and by research funds from Department of Veterans Affairs.
We thank Karl Drlica for helpful comments on the manuscript.
Footnotes
Published ahead of print on 22 November 2006.
REFERENCES
- 1.Burton, D. R., R. C. Desrosiers, R. W. Doms, W. C. Koff, P. D. Kwong, J. P. Moore, G. J. Nabel, J. Sodroski, I. A. Wilson, and R. T. Wyatt. 2004. HIV vaccine design and the neutralizing antibody problem. Nat. Immunol. 5:233-236. [DOI] [PubMed] [Google Scholar]
- 2.Burton, D. R., J. Pyati, R. Koduri, S. J. Sharp, G. B. Thornton, P. W. Parren, L. S. Sawyer, R. M. Hendry, N. Dunlop, P. L. Nara, et al. 1994. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science 266:1024-1027. [DOI] [PubMed] [Google Scholar]
- 3.Burton, D. R., R. L. Stanfield, and I. A. Wilson. 2005. Antibody vs. HIV in a clash of evolutionary titans. Proc. Natl. Acad. Sci. USA 102:14943-14948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calarese, D. A., H. K. Lee, C. Y. Huang, M. D. Best, R. D. Astronomo, R. L. Stanfield, H. Katinger, D. R. Burton, C. H. Wong, and I. A. Wilson. 2005. Dissection of the carbohydrate specificity of the broadly neutralizing anti-HIV-1 antibody 2G12. Proc. Natl. Acad. Sci. USA 102:13372-13377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calarese, D. A., C. N. Scanlan, M. B. Zwick, S. Deechongkit, Y. Mimura, R. Kunert, P. Zhu, M. R. Wormald, R. L. Stanfield, K. H. Roux, J. W. Kelly, P. M. Rudd, R. A. Dwek, H. Katinger, D. R. Burton, and I. A. Wilson. 2003. Antibody domain exchange is an immunological solution to carbohydrate cluster recognition. Science 300:2065-2071. [DOI] [PubMed] [Google Scholar]
- 6.Cao, J., N. Sullivan, E. Desjardin, C. Parolin, J. Robinson, R. Wyatt, and J. Sodroski. 1997. Replication and neutralization of human immunodeficiency virus type 1 lacking the V1 and V2 variable loops of the gp120 envelope glycoprotein. J. Virol. 71:9808-9812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng-Mayer, C., A. Brown, J. Harouse, P. A. Luciw, and A. J. Mayer. 1999. Selection for neutralization resistance of the simian/human immunodeficiency virus SHIVSF33A variant in vivo by virtue of sequence changes in the extracellular envelope glycoprotein that modify N-linked glycosylation. J. Virol. 73:5294-5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cherpelis, S., I. Shrivastava, A. Gettie, X. Jin, D. D. Ho, S. W. Barnett, and L. Stamatatos. 2001. DNA vaccination with the human immunodeficiency virus type 1 SF162ΔV2 envelope elicits immune responses that offer partial protection from simian/human immunodeficiency virus infection to CD8+ T-cell-depleted rhesus macaques. J. Virol. 75:1547-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Connor, R., B. K. Chen, S. Choe, and N. R. Landau. 1995. Vpr is required for efficient replication of human immunodeficiency virus type 1 in mononuclear phagocytes. Virology 206:935-944. [DOI] [PubMed] [Google Scholar]
- 10.Derby, N. R., Z. Kraft, E. Kan, E. T. Crooks, S. W. Barnett, I. K. Srivastava, J. M. Binley, and L. Stamatatos. 2006. Antibody responses elicited in macaques immunized with human immunodeficiency virus type 1 (HIV-1) SF162-derived gp140 envelope immunogens: comparison with those elicited during homologous simian/human immunodeficiency virus SHIVSF162P4 and heterologous HIV-1 infection. J. Virol. 80:8745-8762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ditzel, H. J., J. M. Binley, J. P. Moore, J. Sodroski, N. Sullivan, L. S. Sawyer, R. M. Hendry, W. P. Yang, C. F. Barbas III, and D. R. Burton. 1995. Neutralizing recombinant human antibodies to a conformational V2- and CD4-binding site-sensitive epitope of HIV-1 gp120 isolated by using an epitope-masking procedure. J. Immunol. 154:893-906. [PubMed] [Google Scholar]
- 12.Fox, D. G., P. Balfe, C. P. Palmer, J. C. May, C. Arnold, and J. A. McKeating. 1997. Length polymorphism within the second variable region of the human immunodeficiency virus type 1 envelope glycoprotein affects accessibility of the receptor binding site. J. Virol. 71:759-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorny, M. K., J. P. Moore, A. J. Conley, S. Karwowska, J. Sodroski, C. Williams, S. Burda, L. J. Boots, and S. Zolla-Pazner. 1994. Human anti-V2 monoclonal antibody that neutralizes primary but not laboratory isolates of human immunodeficiency virus type 1. J. Virol. 68:8312-8320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorny, M. K., L. Stamatatos, B. Volsky, K. Revesz, C. Williams, X.-H. Wang, S. Cohen, R. Staudinger, and S. Zolla-Pazner. 2005. Identification of a new quaternary neutralizing epitope on human immunodeficiency virus type 1 virus particles. J. Virol. 79:5232-5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He, Y., W. J. Honnen, C. P. Krachmarov, M. Burkhart, S. C. Kayman, J. Corvalan, and A. Pinter. 2002. Efficient isolation of novel human monoclonal antibodies with neutralizing activity against HIV-1 from transgenic mice expressing human Ig loci. J. Immunol. 169:595-605. [DOI] [PubMed] [Google Scholar]
- 16.Ho, D. D., M. S. C. Fung, Y. Cao, X. L. Li, C. Sun, T. W. Chang, and N. C. Sun. 1991. Another discontinuous epitope on glycoprotein gp120 that is important in human immunodeficiency virus type 1 neutralization is identified by a monoclonal antibody. Proc. Natl. Acad. Sci. USA 88:8949-8952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korber, B. T. M., C. Brander, B. F. Haynes, R. Koup, J. P. Moore, B. D. Walker, and D. I. Watkins (ed.). 2005. HIV molecular immunology, vol. 2 (LA-UR 06-0036). Los Alamos National Laboratory, Theoretical Biology and Biophysics, Los Alamos, NM.
- 18.Krachmarov, C. P., W. J. Honnen, S. C. Kayman, M. K. Gorny, S. Zolla-Pazner, and A. Pinter. 2006. Factors determining the breadth and potency of neutralization by V3-specific human monoclonal antibodies derived from subjects infected with clade A or clade B strains of human immunodeficiency virus type 1. J. Virol. 80:7127-7135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krachmarov, C. P., S. C. Kayman, W. J. Honnen, O. Trochev, and A. Pinter. 2001. V3-specific polyclonal antibodies affinity purified from sera of infected humans effectively neutralize primary isolates of human immunodeficiency virus type 1. AIDS Res. Hum. Retrovir. 17:1737-1748. [DOI] [PubMed] [Google Scholar]
- 20.Leitner, T., B. Foley, B. H. Hahn, P. A. Marx, F. MCutchan, J. Mellors, S. M. Wolinsky, and B. Korber. 2004. HIV sequence compendium 2003. Theoretical Biology and Biophysics Group, Los Alamos National Laboratory, Los Alamos, NM.
- 21.Lian, Y., I. Srivastava, V. R. Gómez-Román, J. zur Megede, Y. Sun, E. Kan, S. Hilt, S. Engelbrecht, S. Himathongkham, P. A. Luciw, G. Otten, J. B. Ulmer, J. J. Donnelly, D. Rabussay, D. Montefiori, E. J. van Rensburg, and S. W. Barnett. 2005. Evaluation of envelope vaccines derived from the South African subtype C human immunodeficiency virus type 1 TV1 strain. J. Virol. 79:13338-13349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ly, A., and L. Stamatatos. 2000. V2 loop glycosylation of the human immunodeficiency virus type1 SF162 envelope facilitates interaction of this protein with CD4 and CCR5 receptors and protects the virus from neutralization by anti-V3 loop and anti-CD4 binding site antibodies. J. Virol. 74:6769-6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKeating, J. A., C. Shotton, J. Cordell, S. Graham, P. Balfe, N. Sullivan, M. Charles, M. Page, A. Bolmstedt, S. Olofsson, et al. 1993. Characterization of neutralizing monoclonal antibodies to linear and conformation-dependent epitopes within the first and second variable domains of human immunodeficiency virus type 1 gp120. J. Virol. 67:4932-4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore, J. P., Q. J. Sattentau, H. Yoshiyama, M. Thali, M. Charles, N. Sullivan, S.-W. Poon, M. S. Fung, F. Traincard, M. Pincus, G. Robey, J. E. Robinson, D. D. Ho, and J. Sodroski. 1993. Probing the structure of the V2 domain of human immunodeficiency virus type 1 surface glycoprotein gp120 with a panel of eight monoclonal antibodies: human immune response to the V1 and V2 domains. J. Virol. 67:6136-6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muster, T., F. Steindl, M. Purtscher, A. Trkola, A. Klima, G. Himmler, F. Rüker, and H. Katinger. 1993. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J. Virol. 67:6642-6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pantophlet, R., I. A. Wilson, and D. R. Burton. 2004. Improved design of an antigen with enhanced specificity for the broadly HIV-neutralizing antibody b12. Protein Eng. Des. Sel. 17:749-758. [DOI] [PubMed] [Google Scholar]
- 27.Pinter, A., W. J. Honnen, P. D'Agostino, M. K. Gorny, S. Zolla-Pazner, and S. C. Kayman. 2005. The C108g epitope in the V2 domain of gp120 functions as a potent neutralization target when introduced into envelope proteins derived from human immunodeficiency virus type 1 primary isolates. J. Virol. 79:6909-6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinter, A., W. J. Honnen, Y. He, M. K. Gorny, S. Zolla-Pazner, and S. C. Kayman. 2004. The V1/V2 domain of gp120 is a global regulator of the sensitivity of primary human immunodeficiency virus type 1 isolates to neutralization by antibodies commonly induced upon infection. J. Virol. 78:5205-5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saphire, E. O., P. W. Parren, R. Pantophlet, M. B. Zwick, G. M. Morris, P. M. Rudd, R. A. Dwek, R. L. Stanfield, D. R. Burton, and I. A. Wilson. 2001. Crystal structure of a neutralizing human IGG against HIV-1: a template for vaccine design. Science 293:1155-1159. [DOI] [PubMed] [Google Scholar]
- 30.Saunders, C. J., R. A. McCaffrey, I. Zharkikh, Z. Kraft, S. E. Malenbaum, B. Burke, C. Cheng-Mayer, and L. Stamatatos. 2005. The V1, V2, and V3 regions of the human immunodeficiency virus type 1 envelope differentially affect the viral phenotype in an isolate-dependent manner. J. Virol. 79:9069-9080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Selvarajah, S., B. Puffer, R. Pantophlet, M. Law, R. W. Doms, and D. R. Burton. 2005. Comparing antigenicity and immunogenicity of engineered gp120. J. Virol. 79:12148-12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shotton, C., C. Arnold, Q. Sattentau, J. Sodroski, and J. A. McKeating. 1995. Identification and characterization of monoclonal antibodies specific for polymorphic antigenic determinants within the V2 region of the human immunodeficiency virus type 1 envelope glycoprotein. J. Virol. 69:222-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Srivastava, I. K., K. VanDorsten, L. Vojtech, S. W. Barnett, and L. Stamatatos. 2003. Changes in the immunogenic properties of soluble gp140 human immunodeficiency virus envelope constructs upon partial deletion of the second hypervariable region. J. Virol. 77:2310-2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stamatatos, L., and C. Cheng-Mayer. 1998. An envelope modification that renders a primary, neutralization-resistant clade B human immunodeficiency virus type 1 isolate highly susceptible to neutralization by sera from other clades. J. Virol. 72:7840-7845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trkola, A., M. Purtscher, T. Muster, C. Ballaun, A. Buchacher, N. Sullivan, K. Srinivasan, J. Sodroski, J. P. Moore, and H. Katinger. 1996. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J. Virol. 70:1100-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vijh-Warrier, S., E. Murphy, I. Yokoyama, and S. A. Tilley. 1995. Characterization of the variable regions of a chimpanzee monoclonal antibody with potent neutralizing activity against HIV-1. Mol. Immunol. 32:1081-1092. [DOI] [PubMed] [Google Scholar]
- 37.Vijh-Warrier, S., A. Pinter, W. J. Honnen, and S. A. Tilley. 1996. Synergistic neutralization of human immunodeficiency virus type 1 by a chimpanzee monoclonal antibody against the V2 domain of gp120 in combination with monoclonal antibodies against the V3 loop and the CD4-binding site. J. Virol. 70:4466-4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Warrier, S. V., A. Pinter, W. J. Honnen, M. Girard, E. Muchmore, and S. A. Tilley. 1994. A novel, glycan-dependent epitope in the V2 domain of human immunodeficiency virus type 1 gp120 is recognized by a highly potent, neutralizing chimpanzee monoclonal antibody. J. Virol. 68:4636-4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei, X., J. M. Decker, S. Wang, H. Hui, J. C. Kappes, X. Wu, J. F. Salazar-Gonzalez, M. G. Salazar, J. M. Kilby, M. S. Saag, N. L. Komarova, M. A. Nowak, B. H. Hahn, P. D. Kwong, and G. M. Shaw. 2003. Antibody neutralization and escape by HIV-1. Nature 422:307-312. [DOI] [PubMed] [Google Scholar]
- 40.Wu, Z., S. C. Kayman, W. Honnen, K. Revesz, H. Chen, S. Vijh-Warrier, S. A. Tilley, J. McKeating, C. Shotton, and A. Pinter. 1995. Characterization of neutralization epitopes in the V2 region of human immunodeficiency virus type 1 gp120: role of glycosylation in the correct folding of the V1/V2 domain. J. Virol. 69:2271-2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reference deleted.
- 42.Xu, R., I. K. Srivastava, L. Kuller, I. Zarkikh, Z. Kraft, Z. Fagrouch, N. L. Letvin, J. L. Heeney, S. W. Barnett, and L. Stamatatos. 2006. Immunization with HIV-1 SF162-derived envelope gp140 proteins does not protect macaques from heterologous simian-human immunodeficiency virus SHIV89.6P infection. Virology 349:276-289. [DOI] [PubMed] [Google Scholar]
- 43.Yang, Z.-Y., B. K. Chakrabarti, L. Xu, B. Welcher, W.-P. Kong, K. Leung, A. Panet, J. R. Mascola, and G. J. Nabel. 2004. Selective modification of variable loops alters tropism and enhances immunogenicity of human immunodeficiency virus type 1 envelope. J. Virol. 78:4029-4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu, C. B., L. Zhu, S. Holz-Smith, T. J. Matthews, and C. H. Chen. 2001. The role of the third beta strand in gp120 conformation and neutralization sensitivity of the HIV-1 primary isolate DH012. Proc. Natl. Acad. Sci. USA 98:15227-15232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zolla-Pazner, S. 2004. Identifying epitopes of HIV-1 that induce protective antibodies. Nat. Rev. Immunol. 4:199-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zwick, M. B., H. K. Komori, R. L. Stanfield, S. Church, M. Wang, P. W. H. I. Parren, R. Kunert, H. Katinger, I. A. Wilson, and D. R. Burton. 2004. The long third complementarity-determining region of the heavy chain is important in the activity of the broadly neutralizing antihuman immunodeficiency virus type 1 antibody 2F5. J. Virol. 78:3155-3161. [DOI] [PMC free article] [PubMed] [Google Scholar]