Abstract
Adenoviruses (Ads) are responsible for respiratory, ocular, and gastrointestinal illnesses in humans. While the majority of serotypes utilize coxsackievirus-adenovirus receptor (CAR) as their primary attachment receptor, subgroup B and subgroup D Ad37 serotypes use CD46. Given the propensity of Ad vectors to activate host immune responses, we sought to investigate their potential for type I interferon induction. We found that CD46 Ads were capable of alpha interferon (IFN-α) induction by peripheral blood mononuclear cells and that plasmacytoid dendritic cells (pDCs) were the principal producers of this cytokine. IFN-α induction correlated with the permissivity of pDCs to CD46- but not CAR-utilizing Ad serotypes. A role for Toll-like receptor 9 (TLR9) recognition of Ad was supported by the requirement for viral DNA and efficient endosomal acidification and by the ability of a TLR9-inhibitory oligonucleotide to attenuate IFN-α induction. Cell lines expressing TLR9 that are permissive to infection by both CAR- and CD46-utilizing serotypes showed a preferential induction of TLR9-mediated events by CD46-utilizing Ads. Specifically, the latter virus types induced higher levels of cytokine expression and NF-κB activation in HeLa cells than CAR-dependent Ad types, despite equivalent infection rates. Therefore, infectivity alone is not sufficient for TLR9 activation, but this activation instead is regulated by a specific receptor entry pathway. These data reveal a novel mode of host immune recognition of Ad with implications for Ad pathogenesis and for the use of unconventional Ad vectors for gene delivery and vaccine development.
A major impediment that hampers the use of adenovirus (Ad) vectors in the clinic is their propensity to activate both innate and adaptive immune responses that are often accompanied by immediate toxicity and the eventual elimination of transduced cells (36). Previous studies have indicated that the activation of innate immunity does not require infectious virus particles and is dose dependent (33, 55). Interactions of adenovirus with cell integrins have been implicated in the production of proinflammatory cytokines and increased cell survival (40, 41). However, the precise mechanisms involved in the activation of the innate immune system have not yet been defined. Therefore, we sought to uncover the molecular interactions responsible for activation of innate immune responses to adenoviruses.
Human Adenoviridae are comprised of 51 serotypes that were originally subdivided into 6 groups based on their ability to agglutinate red blood cells, a process that occurs via multimeric associations of the Ad homotrimeric fiber protein with host cell surface receptors (52). The majority of Ad subgroups, A, C, D, E, and F, recognize coxsackievirus-adenovirus receptor (CAR) (4, 42), a member of the immunoglobulin superfamily, whereas Ads belonging to subgroup B and subgroup D Ad37 serotypes use CD46, a member of the family of complement regulatory proteins, as their primary attachment receptor (15, 43, 47, 54). Of particular note, association of CD46 with subgroup B serotypes and Ad37 expands host cell tropism. Thus, hematopoetic cells that are refractory to infection by CAR-utilizing Ads are susceptible to subgroup B and Ad37 virus types due to the expression of CD46 on these cells (20, 21, 43, 45). Consequently, replication-defective Ad vectors based on CD46-utilizing serotypes, such as Ad35, are currently being evaluated as vaccines and for treatment of hematopoietic disorders (35, 51). These in vivo studies have suggested that CD46-utilizing vectors administered locally (i.e., intramuscularly) have a higher safety profile due to lower levels of immune activation (14, 37). However, these investigations have not examined the consequences of vector association with human peripheral blood mononuclear cell (PBMC) populations, a situation that could enhance innate immune responses detrimental to the host. Therefore, we have sought to gain further knowledge of the interactions of CD46-utilizing Ad vectors with specific blood-derived components of the innate immune response.
Our previous studies showed that infection of human PBMC by the CD46-utilizing Ads Ad37, Ad16, and Ad35 leads to an impairment in cytokine production (22). CD46 Ads, but not the CAR-utilizing Ad5, interfere with gamma interferon signaling events in monocytes that are necessary for expression of the C/EBPβ transcription factor, which in turn is required for subsequent proinflammatory cytokine gene transcription. Interestingly, recent studies showed that certain innate immune responses can differentially regulate proinflammatory cytokine expression and antiviral activity (18). Therefore, we sought to determine whether CD46-mediated adenovirus entry into cells influences the antiviral immune response that is characterized by type 1 interferon production.
Type l interferons are encoded by 12 alpha genes and 1 beta gene that express proteins with antiviral, antiproliferative, and immunomodulatory effects (50). Leukocytes have been shown to produce alpha/beta interferon (IFN-α/β) in response to a variety of bacterial and viral pathogens through recognition of pathogen-associated molecular patterns by Toll-like receptors (TLRs). The family of TLRs includes many members, each with a unique specificity. For instance, TLR2/6, TLR4, and TLR5 recognize bacterial products such as peptidoglycan, lipopolysaccharides, and flagellin, while the endosomal TLR3, TLR7/8, and TLR9 recognize double-stranded RNA, single-stranded RNA, and unmethylated CpG dinucleotides, respectively. TLR engagement leads to signaling events that ultimately induce IFN-α/β expression via activation of NF-κB and the IRF family of transcription factors.
In these studies we analyzed IFN-α expression by PBMC upon exposure to both CAR- and CD46-utilizing Ad vectors, and we provide evidence for the involvement of TLR9 in immune activation. Infectivity and cell entry are not sufficient for TLR9 recognition of Ads; rather, this recognition stems from the utilization of a specific receptor-mediated entry pathway.
MATERIALS AND METHODS
Viruses.
Adenovirus vectors were propagated in 293 cells as previously described (22). Ad5.F16 empty capsids (EC), produced during normal viral propagation, were readily purified from mature particles on CsCl gradients due to an approximate 1.5-cm distance separating the two viral species. Virtually no green fluorescent protein (GFP) expression was detected in EC-infected cell cultures, indicating that the maximum amount of contaminating mature virions (mature capsids [MC]) was low and probably less than 5%. The protein composition of EC and MC particles was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Two micrograms of each virus was separated on a 4 to 20% Novex acrylamide gel (Invitrogen, Carlsbad, CA) under denaturing conditions and stained with SYPRO Ruby (Invitrogen-Molecular Probes, Eugene, OR) according to the manufacturer's suggestions. Virions were fluorescently labeled with Cy3 dye (Amersham Biosciences, Piscataway, NJ) by incubating 100 μg virus in a 5-ml 0.1 M sodium carbonate (pH 9.3) solution with dissolved Cy3 dye for 30 min at room temperature. Unconjugated Cy3 was separated from the labeled virions by rebanding on CsCl gradients and dialysis as described above. Infectivity by the GFP-encoding virus was not affected by dye conjugation, as determined by fluorescence-activated cell sorting (FACS) comparing transduction of HeLa cells with Cy3-labeled Ad5.F16 MC versus that with unlabeled Ad5.F16 MC using Cell Quest software (BD Biosciences, San Jose, CA). Herpes simplex virus type 1 (HSV-1) (F strain) was provided by Pietro P. Sanna (The Scripps Research Institute, La Jolla, CA). Viruses were UV inactivated with a 20-min exposure to 254-nm radiation at a 5-cm distance from the UV source.
Lymphocyte isolation and cytokine expression.
PBMC were isolated from human blood by Ficoll density gradient centrifugation and cultured as previously described (22). Plasmacytoid dendritic cells (pDC) were purified from PBMC by positive selection using the MACS BDCA-4 cell isolation kit (Miltenyi Biotec, Gladback, Germany) according to the manufacturer's suggestions and cultured at 1 × 106 cells/ml.
To measure cytokine expression, 2 × 105 PBMC or pDC (in a final volume of 200 μl) were cultured with 104 Ad particles/cell, unless otherwise indicated, for 1 to 2 days. The endosomal pH inhibitors bafilomycin A1, chloroquine, and ammonium chloride were added to the cultures at the concentrations indicated 30 min prior to the addition of virus. PBMC were cultured with 100 Ad5.F16 particles/cell or 1 PFU HSV-1/cell in the presence of phosphorothioate-modified control (TCCTGCAGGTTAAGT) or the TLR9-inhibitory oligonucleotide IRS869 (TCCTGGAGGGGTTGT) at various concentrations. No significant cell death or decrease in infectivity was seen in cultures treated with the inhibitors or oligonucleotides. Culture supernatants were analyzed for the presence of IFN-α by enzyme-linked immunosorbent assay (ELISA) using the Human IFN-α ELISA kit (Biosource, Camarillo, CA). HeLa cells were cultured at 2 × 105 cells/well and incubated with the indicated concentration of Ad for 2 days in 12-well plates. The culture supernatants were assayed for IFN-β using the Human IFN-β ELISA kit (Biomedical Laboratories, Piscataway, NJ). Infectivity was assessed by FACS.
Fluorescence microscopy.
To assess Ad cell entry, HeLa cells grown on glass coverslips were incubated with 104 Cy3-labeled Ad5.F16 MC or Ad5.F16 EC for 1 h at 4°C to allow attachment to cells and subsequently transferred to 37°C for 2 h to permit virus internalization. Cells were washed in PBS, fixed in 4% paraformaldehyde for 20 min, and washed again with PBS before the nuclei were stained with 300 nM 4′,6′-diamidino-2-phenylindole (DAPI) for 5 min. Excess DAPI was removed with three washes of PBS prior to mounting the coverslips onto glass slides using the ProLong Gold antifade reagent (Invitrogen-Molecular Probes, Eugene, OR). Fluorescent images were obtained on a DeltaVision deconvolution microscope using softWoRx 2.5 software (Applied Precision, LLC, Issaquah, WA). After obtaining deconvoluted volume images, the fluorescence emissions from Ad5.F16 MC and Ad5.F16 EC were analyzed from the central stacks within each cell (1.2-μM thickness, total) in order to optimize evaluation of internalized virus particles. Each cell interior is shown at a 0o and 90o rotation around the y axis.
Protein and reverse transcriptase PCR (RT-PCR) analyses.
NF-κB activation in HeLa cells was assessed by measuring the degradation of its cytoplasmic binding partner, IkBα, using Western blot analysis as previously described. Samples containing 5 × 105 HeLa cells were incubated with 104 viral particles/cell at 4°C for 45 min to allow virus binding and then transferred to 37°C for 45 min to allow virus entry.
TLR7 and TLR9 expression in HeLa was determined by RT-PCR. HeLa RNA was purified using the Total RNA purification kit from Gentra Systems (Minneapolis, MN). One microgram of isolated RNA was used to reverse transcribe and amplify TLR7 and TLR9 mRNA using the SuperScript One-Step RT-PCR kit from Invitrogen according to the manufacturer's protocol. For amplification, 35 cycles (94°C for 15 s, 55°C for 30 s, and 72 C for 1 min) were carried out with the following primer sequences: TLR7 (5′ TGATCGTGGACTGCACAGAC and 3′ TAGTTCTGTTAAAGTAGATG) generates a 551-bp fragment; TLR9 (5′ GGCTGTTCCGAAGTCTGTG and 3′ TAGGACAACAGCAGATACTC) generates a 547-bp fragment.
Genetic analysis.
Genomic sequences of 10 serotypes were identified and sorted into 2 groups with respect to CD46 or CAR usage. Given the small sample sizes and the data type (discrete), the Chi square (χ2) test was deemed most appropriate for the analysis. In the model we tested, the number of rrCpGyy motifs is randomly and equally distributed by length of genome in both classes of serotype (CD46 or CAR). The critical value for χ2 (α = 0.05; ν = 1) is 3.84. Our computed χ2 value (Yates' correction for degrees of freedom = 1) is 21.812, and the associated P value is 0.0000030103. A more detailed description of the statistical calculations is available (data not shown).
RESULTS
CD46-utilizing adenoviruses induce IFN-α expression.
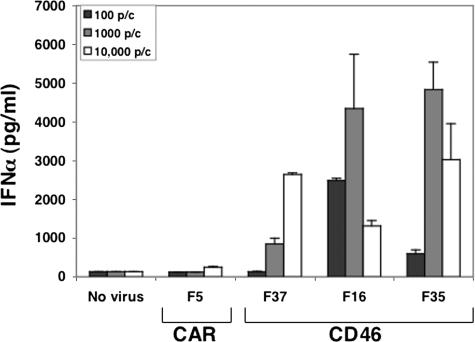
In order to reduce the complexities involved in analyzing Ad-immune cell interactions, including the impact of postentry processes (e.g., virus capsid disassembly), we utilized fiber-pseudotyped Ad5 vectors that vary only in a single protein, known as fiber. Ad5.F5 is the prototypical CAR-utilizing vector that expresses the Ad5 fiber, whereas the Ad5.F37, Ad5.F16, and Ad5.F35 vectors display CD46-binding fibers derived from serotypes 37, 16, and 35, respectively. Various amounts of each vector were cultured with primary human PBMC, and then antiviral cytokines were assayed by ELISA. Ad vectors equipped with CD46-binding fibers but not with a CAR-binding fiber stimulated expression of IFN-α (Fig. 1). The induction of IFN-α was dose dependent, with as few as 100 Ad5.F16 particles causing a significant rise in cytokine production. However, very large doses of Ad5.F16 and Ad5.F35 induced somewhat lower levels of IFN-α, a finding that was replicated in multiple experiments.
FIG. 1.
CD46-utilizing Ads induce IFN-α expression. PBMC were incubated for 48 h with fiber-pseudotyped Ad5 vectors at increasing concentrations (particles/cell) as indicated. Culture supernatants were then analyzed for IFN-α by ELISA.
Plasmacytoid dendritic cells are the principal source of adenovirus-induced IFN-α.
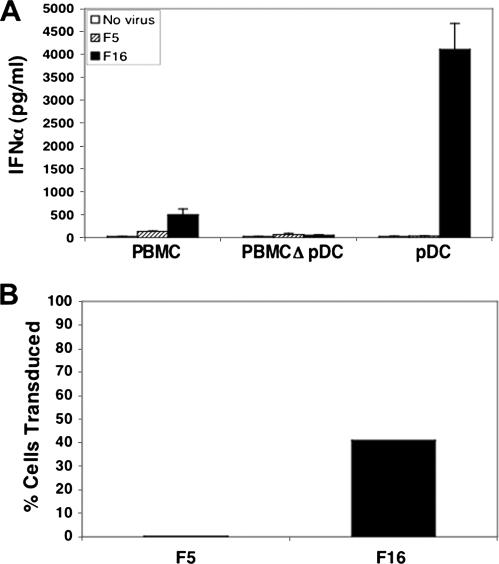
An important question arising from these findings is which cell population(s) was responsible for virus-induced cytokine production. Since pDC have been identified as the principal IFN-α-producing cell type in response to infection (7, 31, 46), we exposed PBMC, PBMC depleted of pDC, or purified pDC to either Ad5.F5 or Ad5.F16 particles (Fig. 2A). PBMC cultures depleted of pDC did not express IFN-α in response to Ad5.F16, whereas purified pDC expressed large amounts of this cytokine, providing strong evidence that pDC are the major IFN-α-producing cell type in blood that responds to Ad infection. In addition, pDC were refractory to Ad5.F5 infection, while Ad5.F16 transduced 41% of these cells (Fig. 2B), indicating that the ability of Ad vectors to induce IFN-α production in pDC correlates with their infectivity.
FIG. 2.
Plasmacytoid dendritic cells produce IFN-α in response to CD46-utilizing Ads. The culture supernates from PBMC, PBMC depleted of pDC (PBMCΔpDC), and purified pDC were analyzed for IFN-α production by ELISA (A). Purified human pDC were cultured with 104 Ad5.F5 (F5) or Ad5.F16 (F16) viral particles/cell for 48 h and then analyzed by FACS for Ad-encoded GFP expression (B).
IFN-α expression by CD46-utilizing Ads requires TLR activation.
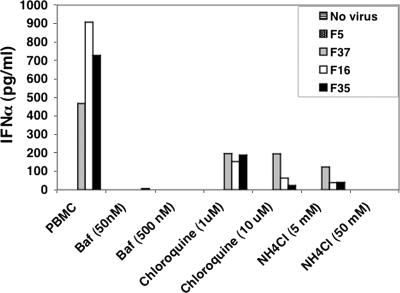
Since a major route of type l IFN induction by microbial pathogens involves recognition of pathogen-associated molecular patterns by TLRs, we sought to determine whether a specific TLR might be associated with Ad-induced IFN-α production. While there are 10 members of the human TLR family, we focused our analyses on the endosomally localized TLR7 and TLR9 in human pDC. Since intracellular compartments are major sites of Ad entry, TLR7 and TLR9 could potentially recognize Ad single-stranded RNA transcripts or Ad genomic DNA, respectively. To investigate the potential role of these innate immune receptors, we treated cells with bafilomycin A1, chloroquine, and ammonium chloride to block endosomal acidification, a prerequisite for TLR7 and TLR9 activation (Fig. 3) (31, 32). Treatment of cells with these pH inhibitors caused a drastic reduction in IFN-α production by Ad5.F37, Ad5.F16, and Ad5.F35, consistent with a role for TLR7 or TLR9 activation in cytokine induction.
FIG. 3.
IFN-α induction by CD46 Ads is pH dependent. PBMC were exposed to 104 viral particles/cell in the absence or presence of various concentrations of the endosomal pH inhibitors bafilomycin A1 (Baf), chloroquine, and ammonium chloride (NH4Cl) for 48 h. Culture supernatants were analyzed for IFN-α production by ELISA.
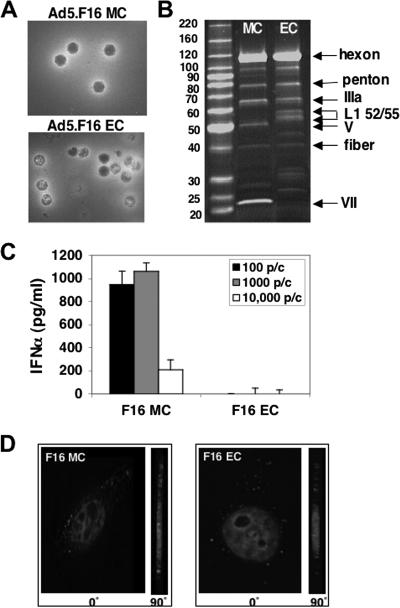
To investigate further the potential role of endosome-associated TLRs in IFN-α production, we evaluated cytokine induction by immature Ad5.F16 particles (EC) that lack their genome as a consequence of incomplete viral particle maturation. As expected, Ad5.F16 EC appeared structurally similar to MC with respect to their outer capsids but lacked an electron-dense core, indicative of an absence of viral DNA (Fig. 4A). Moreover, SDS-PAGE analysis of MC and EC (Fig. 4B) revealed that these particles had very similar levels of outer capsid proteins (i.e., hexon, penton base, and fiber), whereas EC particles selectively lacked the DNA-associated capsid proteins V and VII. EC but not MC also contained the scaffold proteins L1 52/55 that are normally found in immature particles prior to viral capsid maturation and assembly (17, 49). Unlike mature Ad particles, Ad5.F16 EC were incapable of inducing IFN-α expression (Fig. 4C). The lack of IFN-α induction by Ad5.F16 EC was not due to an inability of these particles to infect cells, because, as noted by others (17, 48), Ad5.F16 EC were fully capable of binding and entering cells, as assessed by deconvolution fluorescence microscopy (Fig. 4D). Together these findings suggest a requirement for Ad viral DNA for IFN-α induction.
FIG. 4.
Mature but not immature/empty Ad particles induce IFN-α production. Negative-stain electron microscopic (A) and SDS-PAGE (B) analyses were performed with Ad5.F16 MC and EC devoid of viral DNA. PBMC were incubated with 100, 1,000, or 10,000 particles/cell of Ad5.F16 MC or EC for 48 h, and then culture supernatants were analyzed for IFN-α production by ELISA (C). Internalization of Cy3-labeled Ad5.F16 MC and Ad5.F16 EC into HeLa cells following incubation at 37°C for 45 min was determined by fluorescence deconvolution microscopy. Cell interiors are shown at 0o and 90o angles around the y axis with nuclei stained with DAPI (D).
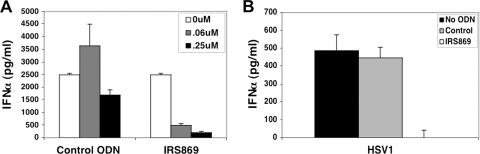
Further analyses were performed to discern whether TLR7 or TLR9 was responsible for Ad-induced IFN-α production using an oligonucleotide antagonist that specifically interferes with TLR9 but not TLR7 signaling (2). The inhibitory oligonucleotide, designated IRS869, caused a dose-dependent decrease in IFN-α expression, with a 0.25 μM concentration of this compound decreasing IFN-α production by 92% (Fig. 5A). The IRS869 oligonucleotide also blocked IFN-α induction by HSV-1 (Fig. 5B), a known activator of IFN-α expression via TLR9 engagement (27). Therefore, these findings indicate that CD46-utilizing adenovirus likely induce IFN-α expression via TLR9 activation.
FIG. 5.
A TLR9-inhibitory oligonucleotide abolishes induction of IFN-α by a CD46-utilizing Ad. (A) PBMC were infected with Ad5.F16 (100 viral particles/cell) in the absence or presence of various concentrations of a control oligodinucleotide (ODN) or the TLR9-selective IRS869 oligonucleotide, and the culture supernatants were assayed for IFN-α by ELISA after 24 h. PBMC were infected with HSV-1 (MOI = 1) in the presence of 0.25 μM control or IRS869 oligonucleotides, and IFN-α production was quantitated 24 h after infection by ELISA (B).
CD46-utilizing Ads preferentially induce TLR9 signaling.
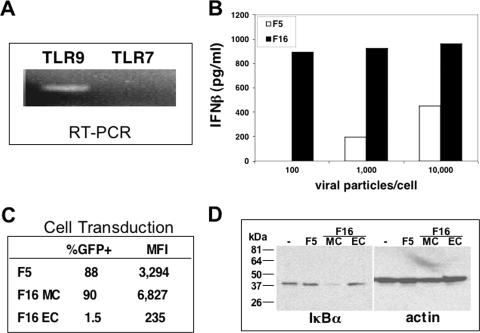
In the studies described above, Ad-induced IFN production was analyzed in cells that were permissive only for CD46-utilizing Ad serotypes, and therefore it was not possible to determine whether distinct receptor-mediated entry pathways differed in their capacity to trigger cytokine responses. Thus, we next examined cytokine production in cells that were equally permissive to both CAR- and CD46-dependent viruses. We chose HeLa cells for these studies because they express IFN-β in response to viral infection (39) and, unlike pDC, they can be transduced with equal efficiencies by CD46- and CAR-utilizing Ads. HeLa cells also express TLR9, as detected by RT-PCR analysis (Fig. 6A), thus providing an appropriate cell culture model for studying Ad recognition by this receptor. The Ad vector displaying the Ad16 fiber induced significantly higher levels of IFN-β than the CAR-utilizing Ad5.F5 vector at each concentration examined (Fig. 6B). Specifically, no IFN-β expression was detected in cell cultures infected with 100 Ad5.F5 particles/cell, while maximum cytokine levels were induced with the same amount of Ad5.F16. The difference in the abilities of Ad5.F5 and Ad5.F16 for cytokine activation was not due to differences in infectivity, since the two vectors had similar infection rates, 88% and 90%, respectively, as determined by Ad-mediated transgene delivery (Fig. 6C). To analyze additional TLR signaling events triggered by Ad entry into HeLa cells, we examined the activation of the transcription factor NF-κB, since this key transcription factor is universally activated upon stimulation of TLR family members (24). NF-κB activation was evaluated by quantifying the levels of the IκBα protein following exposure of HeLa cells to CAR- and CD46-utilizing Ads. IκB proteins retain NF-κB in the cytoplasm until IκB becomes phosphorylated and degraded in response to stimuli, resulting in the nuclear translocation of NF-κB. Therefore, a reduction in IκB levels is generally considered to be indicative of NF-κB activation. As can be seen in Fig. 6D, IκBα levels remained unchanged in Ad5.F5 and Ad5F16 EC-treated cells, whereas a significant decrease in this protein occurred upon infection with Ad5.F15 MC. These data confirm and extend the interferon studies demonstrating marked differences in NF-κB activation induced by CAR- and CD46-utilizing Ad serotypes. Thus, we concluded that Ad entry into cells is necessary but not sufficient for TLR9 activation and that specific receptor-mediated cell entry pathways largely govern this immune event.
FIG. 6.
The CD46-utilizing Ads preferentially induce IFN-β in HeLa. RT-PCR was performed to detect expression of TLR9 and TLR7 in HeLa cells (A). HeLa cells were infected with various concentrations of CAR-utilizing Ad5.F5 or CD46-utilizing Ad5.F16, and 48 h later, IFN-β production was assessed by ELISA (B). The infection of HeLa cells by different Ad vectors was determined by FACS analyses of GFP expression. Both the extent (%GFP+) and mean fluorescence intensity values (MFI) were determined for each sample (C). Ad-induced NF-κB activation in infected cells was determined by Western blot analysis of IκBα degradation. Cell lysates were generated from uninfected HeLa cells (−) or from cells treated with Ad5.F5 or Ad5.F16 MC or EC for 45 min (D).
CD46-utilizing adenoviruses contain fewer TLR9-stimulatory sequences than CAR-utilizing Ads.
TLR9 is a DNA sensor that specifically recognizes nonmethylated CpG sequences that are disproportionately present in the genomes of bacteria and large eukaryotic viruses (5, 8, 19, 23). In an attempt to learn whether CAR- and CD46-utilizing Ads contain similar numbers of TLR9-activating sequences, we searched the entire genome sequences of 10 different virus serotypes available in a public database to determine the number of CpG dinucleotides present in each. We found that, on average, subgroup B serotypes have 586 fewer CpG sequences than CAR-utilizing Ads, which translates to a 26% overall decrease (Table 1). Because the flanking nucleotides of a CpG sequence contribute to TLR9 activation, analysis of a consensus hexamer known to stimulate TLR9 was also done. While the motif consisting of two purines (rr) preceding the CpG dinucleotide followed by two pyrimidines (yy) was determined to be optimal for stimulation of murine TLR9, it is also capable of activating human cells (3, 26). We therefore extended our analysis to determine the number and frequency of TLR9 (rrCGyy) motifs present and found 100 to 163 potential TLR9-stimulatory hexamer motifs per genome (Table 1). As in the previous analysis, the CD46-utilizing Ad genomes contained 26% fewer (P = 3.01 × 10−6) TLR9 motifs than those of CAR-utilizing Ads. This suppression in CpG motifs may indicate that the genomes of CD46-utilizing Ads have evolved under selective pressure exerted by the innate immune system to evade recognition.
TABLE 1.
Genomic analyses of adenovirusesa
| Receptor usage | Serotype | Subgroup | Amt of CpG (count/ genome) | Amt of rrCpGyy (count/genome) | Frequency of rrCGyy (per 1,000) |
|---|---|---|---|---|---|
| CD46 | Ad7 | B | 1,701 | 100 | 2.82 |
| CD46 | Ad11 | B | 1,639 | 117 | 3.36 |
| CD46 | Ad35 | B | 1,638 | 117 | 3.36 |
| CAR | Ad12 | A | 1,500 | 136 | 3.99 |
| CAR | Ad1 | C | 2,436 | 153 | 4.25 |
| CAR | Ad2 | C | 2,428 | 154 | 4.29 |
| CAR | Ad5 | C | 2,414 | 151 | 4.20 |
| CAR | Ad17 | D | 2,410 | 142 | 4.05 |
| CAR | Ad4 | E | 2,539 | 163 | 4.53 |
| CAR | AdF | F | 1,989 | 155 | 4.53 |
The overall numbers of CpG islands and rrCpGyy motifs were determined for each serotype by sequential searching for the string in the five different frameshifts of each sequence. The relative frequency of the number of motifs was computed by dividing the observed number of motifs by the corrected genome size (sum of the number of linear 6-mer strings of nucleotides). Note that the range of counts of rrCpGyy for serotypes that use CD46 (110 to 117) does not overlap the range of counts for CAR-utilizing Ads (136 to 163).
DISCUSSION
Adenovirus engagement of its primary attachment receptor initiates the infectious entry pathway into host cells. The specific receptor utilized influences cellular tropism of the virus and, as the findings presented here demonstrate, has a profound affect on activation of TLR9-dependent innate immune responses. These studies place adenovirus on a short but growing list of viral pathogens recognized by TLR9, including murine cytomegalovirus, HSV-1, and herpes simplex virus type 2, which has important implications in viral pathogenesis (6, 13, 25, 31).
Our investigations uncovered the first evidence for a connection between TLR activation and specific receptor-mediated cell entry pathways. Thus, Ad5.F16 was more efficient than a CAR-utilizing Ad (Ad5.F5) at stimulating TLR9-mediated events, such as NF-κB activation and IFN-β expression in HeLa cells. This difference was not due to unequal infection rates, since Ad5.F5 and Ad5.F16 transduced HeLa cells with similar efficiencies. We have also observed preferential NF-κB activation by CD46-utilizing Ads in TLR9-positive human B lymphoblastoid cells (data not shown). Therefore, we conclude that cell entry per se is not sufficient for TLR9 activation by Ad but that processes dependent on fiber-receptor interactions are also crucial.
It is not entirely clear why distinct cell entry pathways influence innate immune responses by Ads; however, differences in the intracellular localization of subgroup B and C Ad serotypes are known to occur, as originally observed by using electron microscopy (11). These observations were further supported by the work of Defer et al., who found preferential association of subgroup B Ad3 with endosomes and cytoskeletal fractions shortly after infection compared to that of subgroup C Ad2 (12). In addition, recent confocal microscopy studies confirm colocalization of subgroup B Ads with late endosomes and lysosomes, while subgroup C virons rarely visit these compartments due to their ability to rapidly escape early endosomes (34, 44). Interestingly, TLR9 is found in endosomes and lysosomes upon stimulation but appears to require lysosomal function for proper signaling (1, 29, 30). Therefore, the ability of subgroup B Ads to activate TLR9 appears to correlate with their localization to lysosomes. Our data indicate that only at relatively high multiplicities of infection might CAR-utilizing Ads trigger similar innate immune responses.
While there is a clear difference between CAR- and CD46-utilizing Ad postentry destinations, how receptor engagement controls these events is unknown. CD46 is internalized by one of two pathways. Pathway 1 has been proposed for unligated CD46, which enters cells constitutively and is recycled to the cell's surface. Upon crosslinking, however, CD46 travels down an alternative pathway that leads to its degradation (10). Therefore, engagement of CD46 by Ad may also result in virion trafficking to a degradative pathway, consistent with subgroup B Ad colocalization with late endosomes and lysosomes. Our observations may also be consistent with recent live cell imaging studies demonstrating the existence of two distinct clathrin-mediated endocytic pathways comprised of dynamic and static endosomes (28). The dynamic early endosomes were reported to quickly mature to late endosomes in a microtubule-dependent manner, resulting in the degradation of their cargo, whereas static endosomes that contain recycling receptors are slow to mature and do not require the microtubule network. In line with this proposed model and other published work, it seems reasonable to predict that Ad-CAR association results in their movement into static early endosomes. At high multiplicities of infection, CAR-dependent Ads likely saturate the recycling pathway and a minor fraction enter the degradative pathway. CD46 engagement by distinct Ad types, on the other hand, shunts Ads to dynamic early endosomes that traffic to TLR9-enriched lysosomes. Consistent with this model, the microtubule-destabilizing drug nocodazole completely prevents PBMC IFN-α induction by Ad5.F16, as well as by HSV-1, while not affecting infectivity (data not shown).
A question raised by our studies is how DNA viruses, and Ad in particular, establish a successful infection in the face of a vigorous innate immune response. Our genomic analysis reveals one possible explanation in that a lower frequency of potential stimulatory CpG motifs are present in Ad viral genomes of the subgroup B serotypes. Thus, the 26% (average) reduction in stimulatory CpG motifs in subgroup B genomes may be a compensatory adaptation that counteracts TLR9-dependent interferon responses that would likely be detrimental for virus survival in the host (16). Because CAR-utilizing Ads are inefficient at transducing pDC and are not potent TLR9 activators in other cell types, they would not be expected to be under the same selective pressure to decrease their CpG motifs.
Ad5-based vectors can target tissues that express TLR9, such as epithelium, skeletal muscle, and hepatocytes, so the potent innate immune response induced upon high-dose Ad administration in humans (∼1013 particles) may be, in part, a consequence of TLR9 activation. Indeed, proinflammatory cytokines induced, such as IL-6, TNF-α, and IL-12, following Ad5 vector exposure are classic downstream targets of TLR signaling events (9, 24). The importance of the viral genome in activating cellular responses has been demonstrated by gene chip analysis of Ad5-infected lung fibroblasts, where a >3-fold increase in host mRNA induction over that in cells exposed to empty capsids was observed (48). The recent success in achieving long-term transgene expression with mice, rats, and nonhuman primate models by using helper-dependent Ad vectors that are devoid of all viral genes also appears to be consistent with our findings. The viral genomes of helper-dependent Ad vectors are replaced with transgenes and stuffer DNA derived from humans and mice, which generally contain fewer CpG motifs than pathogens, and this results in prolonged expression of therapeutic genes with less toxicity than traditional E1A-deleted Ad vectors (38).
In contrast to Ad-mediated gene transfer for chronic diseases, it may be advantageous to achieve a certain level of innate immune responses during Ad-mediated vaccination. In this regard, Ad5-based vaccine vectors pseudotyped with subgroup B fibers, such as those used in our studies, may be more effective than vectors based solely on a subgroup B virus (i.e., Ad35). Consistent with this concept, the quality and magnitude of anti-human immunodeficiency virus Gag immune responses elicited by coadministration of a vaccine vector with a TLR9 agonist were increased over those achieved with vector alone (53). Thus, further knowledge of the precise mechanisms involved in the activation of innate immune responses by Ad may help improve approaches for in vivo gene transfer and vaccine development.
Acknowledgments
This work was supported by NIH grants R01 EY011431-09 and R24 EY014174-03 to G.R.N. and NINDS grant 5T32NS41219 to M.I.-M.
We thank Cathy Hsu for her technical assistance in obtaining electron micrographs of Ad samples, Pietro P. Sanna for providing us with HSV-1, Caroline Lanigan for statistical analysis, and Joan Gausepohl for her help in the preparation of the manuscript.
This is manuscript no. 18456 from The Scripps Research Institute.
Footnotes
Published ahead of print on 15 November 2006.
REFERENCES
- 1.Ahmad, N. P., H. Hacker, M. Rutz, S. Bauer, R. M. Vabulas, and H. Wagner. 2002. Bacterial CpG-DNA and lipopolysaccharides activate Toll-like receptors at distinct cellular compartments. Eur. J. Immunol. 32:1958-1968. [DOI] [PubMed] [Google Scholar]
- 2.Barrat, F. J., T. Meeker, J. Gregorio, J. H. Chan, S. Uematsu, S. Akira, B. Chang, O. Duramad, and R. L. Coffman. 2006. Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J. Exp. Med. 202:1131-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauer, S., C. J. Kirschning, H. Häcker, V. Redecke, S. Hausmann, S. Akira, H. Wagner, and G. B. Lipford. 2001. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc. Natl. Acad. Sci. USA 98:9237-9242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergelson, J. M., J. A. Cunningham, G. Droguett, E. A. Kurt-Jones, A. Krithivas, J. S. Hong, M. S. Horwitz, R. L. Crowell, and R. W. Finberg. 1997. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science 275:1320-1323. [DOI] [PubMed] [Google Scholar]
- 5.Bird, A. P. 1987. CpG islands as gene markers in the vertebrate nucleus. Trends Genet. 3:342-347. [Google Scholar]
- 6.Casrouge, A., S.-Y. Zhang, C. Eidenschenk, E. Jouanguy, A. Puel, K. Yang, A. Alcais, C. Picard, N. Mahfoufi, N. Nicolas, L. Lorenzo, S. Plancoulaine, B. Sénèchal, F. Geissmann, K. Tabeta, K. Hoebe, X. Du, R. L. Miller, B. Hèron, C. Mignot, T. B. de Villemeur, P. Lebon, O. Dulac, F. Rozenberg, B. Beutler, M. Tardieu, L. Abel, and J.-L. Casanova. 2006. Herpes simplex virus encephalitis in human UNC-93B deficiency. Science 314:308-312. [DOI] [PubMed] [Google Scholar]
- 7.Cella, M., D. Jarrossay, F. Facchetti, O. Alebardi, H. Nakajima, A. Lanzavecchia, and M. Colonna. 1999. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat. Med. 5:919-923. [DOI] [PubMed] [Google Scholar]
- 8.Cornelie, S., J. Hoebeke, A. M. Schacht, B. Bertin, J. Vicogne, M. Capron, and G. Riveau. 2004. Direct evidence that toll-like receptor 9 (TLR9) functionally binds plasmid DNA by specific cytosine-phosphate-guanine motif recognition. J. Biol. Chem. 279:15124-15129. [DOI] [PubMed] [Google Scholar]
- 9.Cotter, M. J., and D. A. Muruve. 2005. The induction of inflammation by adenovirus vectors used for gene therapy. Front. Biosci. 10:1098-1105. [DOI] [PubMed] [Google Scholar]
- 10.Crimeen-Irwin, B., S. Ellis, D. Christiansen, M. J. Lundford-Menting, J. Milland, M. Lanteri, B. E. Loveland, D. Gerlier, and S. M. Russell. 2003. Ligand binding determines whether CD46 is internalized by clathrin-coated pits or macropinocytosis. J. Biol. Chem. 278:46927-46937. [DOI] [PubMed] [Google Scholar]
- 11.Dales, S., and Y. Chardonnet. 1970. Early events in the interaction of adenoviruses with HeLa cells: association with microtubules and the nuclear pore complex during vectorial movement of the inoculum. Virology 56:465-483. [DOI] [PubMed] [Google Scholar]
- 12.Defer, C., M. T. Belin, M. L. Caillet-Boudin, and P. Boulanger. 1990. Human adenovirus-host cell interactions: comparative study with members of subgroups B and C. J. Virol. 9:3661-3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delale, T., A. Paquin, C. Asselin-Paturel, M. Dalod, G. Brizard, E. E. Bates, P. Kastner, S. Chan, S. Akira, A. Vicari, C. A. Biron, G. Trinchieri, and F. Briere. 2005. MyD88-dependent and -independent murine cytomegalovirus sensing for IFN-alpha release and initiation of immune responses in vivo. J. Immunol. 175:6723-6732. [DOI] [PubMed] [Google Scholar]
- 14.DiPaolo, N., S. Ni, A. Gagger, R. Strauss, S. Tuve, Z. Y. Yi, D. Stone, D. Shayakhmetov, N. Kiviat, P. Touré, S. Sow, B. Horvat, and A. Lieber. 2005. Evaluation of adenovirus vectors containing serotype 35 fibers for vaccination. Mol. Ther. 13:756-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaggar, A., D. M. Shayakhmetov, and A. Lieber. 2003. CD46 is a cellular receptor for group B adenoviruses. Nat. Med. 9:1408-1412. [DOI] [PubMed] [Google Scholar]
- 16.Gallagher, J. G., and N. Khoobyarian. 1972. Adenovirus susceptibility to human interferon during one-step replication. Infect. Immun. 5:905-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gustin, K. E., and M. J. Imperiale. 1998. Encapsidation of viral DNA required the adenovirus L1 52/55-kilodalton protein. J. Virol. 72:7860-7870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Häcker, H., V. Redecke, G. Blagoev, I. Kratchmarova, L.-C. Hsu, G. G. Wang, M. P. Kamps, E. Raz, H. Wagner, G. Häcker, M. Mann, and M. Karin. 2006. Specificity in Toll-like receptor signalling through distinct effector functions of TRAF3 and TRAF6. Nature 439:204-207. [DOI] [PubMed] [Google Scholar]
- 19.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Tadeda, and S. Akira. 2000. A Toll-like receptor recognized bacterial DNA. Nature 408:740-745. [DOI] [PubMed] [Google Scholar]
- 20.Horvath, J., and J. M. Weber. 1988. Nonpermissivity of human peripheral blood lymphocytes to adenovirus type 2 infection. J. Virol. 62:341-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang, S., R. I. Endo, and G. R. Nemerow. 1995. Upregulation of integrins αvβ3 and αvβ5 on human monocytes and T lymphocytes facilitates adenovirus-mediated gene delivery. J. Virol. 69:2257-2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iacobelli-Martinez, M., R. R. Nepomuceno, J. Connolly, and G. R. Nemerow. 2005. CD46-utilizing adenoviruses inhibit C/EBPβ-dependent expression of proinflammatory cytokines. J. Virol. 79:11259-11268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karlin, S., W. Doerfler, and L. R. Cardon. 1994. Why is CpG suppressed in the genomes of virtually all small eukaryotic viruses but not in those of large eukaryotic viruses? J. Virol. 68:2889-2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawai, T., and S. Akira. 2006. TLR signaling. Cell Death Differ. 13:816-825. [DOI] [PubMed] [Google Scholar]
- 25.Krug, A., G. D. Luker, W. Barchet, D. A. Leib, S. Akira, and M. Colonna. 2004. Herpes simplex virus type 1 activates murine natural interferon-producing cells through toll-like receptor 9. Blood 103:1433-1437. [DOI] [PubMed] [Google Scholar]
- 26.Krug, A., S. Rothenfusser, V. Hornung, B. Jahrsdorfer, S. Blackwell, Z. K. Ballas, S. Endres, A. M. Krieg, and G. Hartmann. 2001. Identification of CpG oligonucleotide sequences with high induction of IFN-alpha/beta in plasmacytoid dendritic cells. Eur. J. Immunol. 31:2154-2163. [DOI] [PubMed] [Google Scholar]
- 27.Krug, A., R. Veeraswamy, A. Pekosz, O. Kanagawa, E. R. Unamue, M. Colonna, and M. Cella. 2003. Interferon-producing cells fail to induce proliferation of naive T cells but can promote expansion and T helper 1 differentiation of antigen-experienced unpolarized T cells. J. Exp. Med. 197:899-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lakadamyali, M., M. J. Rust, and X. Zhuang. 2006. Ligands for clathrin-mediated endocytosis are differentially sorted into distinct populations of early endosomes. Cell 124:997-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Latz, E., A. Schoenemeyer, A. Visintin, K. A. Fitzgerald, B. G. Monks, C. F. Knetter, E. Lien, N. J. Nilsen, T. Espevik, and D. T. Golenbock. 2004. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat. Immunol. 5:190-198. [DOI] [PubMed] [Google Scholar]
- 30.Leifer, C. A., M. N. Kennedy, A. Mazzoni, C. Lee, M. J. Kruhiak, and D. M. Segal. 2004. TLR9 is localized in the endoplasmic reticulum prior to stimulation. J. Immunol. 173:1179-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lund, J., A. Sato, S. Akira, R. Medzhitov, and A. Iwasaki. 2003. Toll-like receptor 9-mediated recognition of herpes simplex virus-2 by plasmacytoid dendritic cells. J. Exp. Med. 198:513-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lund, J. M., L. Alexopoulou, A. Sato, M. Karow, N. C. Adams, N. W. Gale, A. Iwasaki, and R. A. Flavell. 2004. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc. Natl. Acad. Sci. USA 101:5598-5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCoy, R. D., B. L. Davidson, B. J. Roessler, G. B. Huffnagle, S. L. Janich, T. J. Laing, and R. H. Simon. 1995. Pulmonary inflammation induced by incomplete or inactivated adenoviral particles. Hum. Gene Ther. 6:1553-1560. [DOI] [PubMed] [Google Scholar]
- 34.Miyazawa, N., R. G. Crystal, and P. L. Leopold. 2001. Adenovirus serotype 7 retention in a late endosomal compartment prior to cytosol escape is modulated by fiber protein. J. Virol. 75:1387-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mizuguchi, H., and T. Hayakawa. 2002. Adenovirus vectors containing chimeric type 5 and type 35 fiber proteins exhibit altered and expanded tropism and increase the size limit of foreign genes. Gene 285:69-77. [DOI] [PubMed] [Google Scholar]
- 36.Muruve, D. A. 2004. The innate immune response to adenovirus vectors. Hum. Gene Ther. 15:1157-1166. [DOI] [PubMed] [Google Scholar]
- 37.Nanda, A., D. M. Lynch, J. Goudsmit, A. A. Lemckert, B. A. Ewald, S. M. Sumida, D. M. Truitt, P. Abbink, M. G. Kishko, D. A. Gorgone, M. A. Lifton, L. Shen, A. Carville, K. G. Mansfield, M. J. Havenga, and D. H. Barouch. 2005. Immunogenicity of recombinant fiber-chimeric adenovirus serotype 35 vector-based vaccines in mice and rhesus monkeys. J. Virol. 79:14161-14168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palmer, D. J., and P. Ng. 2005. Helper-dependent adenoviral vectors for gene therapy. Hum. Gene Ther. 16:1-16. [DOI] [PubMed] [Google Scholar]
- 39.Parekh, B. S., and T. Maniatis. 1999. Virus infection leads to localized hyperacetylation of histones H3 and H4 at the IFN-β promoter. Mol. Cell 3:125-129. [DOI] [PubMed] [Google Scholar]
- 40.Philpott, N. J., M. Nociari, K. B. Eldon, and E. Falck-Pedersen. 2004. Adenovirus-induced maturation of dendritic cells through a P13 kinase-mediated TNF-alpha induction pathway. Proc. Natl. Acad. Sci. USA 101:6200-6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rajala, M. S., R. V. Rajala, R. A. Astley, A. L. Butt, and J. Chodosh. 2005. Corneal cell survival in adenovirus type 19 infection requires phosphoinositide 3-kinase/Akt activation. J. Virol. 79:12332-12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roelvink, P. W., A. Lizonova, J. G. M. Lee, Y. Li, J. M. Bergelson, R. W. Finberg, D. E. Brough, I. Kovesdi, and T. J. Wickham. 1998. The coxsackievirus-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J. Virol. 72:7909-7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Segerman, A., Y.-F. Mei, and G. Wadell. 2000. Adenovirus types 11p and 35p show high binding efficiencies for committed hematopoietic cell lines and are infective to these cell lines. J. Virol. 74:1457-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shayakhmetov, D. M., Z. Y. Li, V. Ternovoi, A. Gaggar, H. Gharwan, and A. Lieber. 2003. The interaction between the fiber knob domain and the cellular attachment receptor determines the intracellular trafficking route of adenoviruses. J. Virol. 77:3712-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shayakhmetov, D. M., T. Papayannopoulou, G. Stamatoyannopoulos, and A. Lieber. 2000. Efficient gene transfer into human CD34+ cells by a retargeted adenovirus vector. J. Virol. 74:2567-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siegal, F. P., N. Kadowaki, M. Shodell, P. A. Fitzgerald-Bocarsly, K. Shah, S. Ho, S. Antonenko, and Y.-J. Liu. 1999. The nature of the principal Type 1 interferon-producing cells in human blood. Science 284:1835-1837. [DOI] [PubMed] [Google Scholar]
- 47.Sirena, D., B. Lilienfeld, M. Eisenhut, S. Kälin, K. Boucke, R. R. Beerli, L. Vogt, C. Ruedl, M. F. Bachmann, U. F. Greber, and S. Hemmi. 2004. The human membrane cofactor CD46 is a receptor for species B adenovirus serotype 3. J. Virol. 78:4454-4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stilwell, J. L., and R. J. Samulski. 2004. Role of viral vectors and virion shells in cellular gene expression. Mol. Ther. 9:337-346. [DOI] [PubMed] [Google Scholar]
- 49.Takahashi, E., S. L. Cohen, P. K. Tsai, and J. A. Sweeney. 2006. Quantitation of adenovirus type 5 empty capsids. Anal. Biochem. 349:208-217. [DOI] [PubMed] [Google Scholar]
- 50.Theofilopoulos, A. N., R. Baccala, B. Beutler, and D. H. Kono. 2005. Type I interferons α/β in immunity and autoimmunity. Annu. Rev. Biochem. 23:307-336. [DOI] [PubMed] [Google Scholar]
- 51.Vogels, R., D. Zuijdgeest, R. van Rijnsoever, E. Hartkoorn, I. Damen, M. P. De Bethune, S. Kostense, G. Penders, N. Helmus, W. Koudstaal, M. Cecchini, A. Wetterwald, M. Sprangers, A. Lemckert, O. Ophorst, B. Koel, M. van Meerendonk, P. Quax, L. Panitti, J. Grimbergen, A. Bout, J. Goudsmit, and M. Havenga. 2003. Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: efficient human cell infection and bypass of preexisting adenovirus immunity. J. Virol. 77:8263-8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wadell, G. 1969. Hemagglutination with adenovirus serotypes belonging to Rosen's subgroups II and 3. Proc. Soc. Exp. Biol. Med. 132:413-421. [DOI] [PubMed] [Google Scholar]
- 53.Wille-Reece, U., B. J. Flynn, K. Loré, R. A. Koup, A. P. Miles, A. Saul, R. M. Kedl, J. J. Mattapallil, W. R. Weiss, M. Roederer, and R. A. Seder. 2006. Toll-like receptor agonists influence the magnitude and quality of memory T cell responses after prime-boost immunization in nonhuman primates. J. Exp. Med. 203:1249-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu, E., S. A. Trauger, L. Pache, T.-M. Mullen, D. J. Von Seggern, G. Siuzdak, and G. R. Nemerow. 2004. Membrane cofactor protein is a receptor for adenoviruses associated with epidemic keratoconjunctivitis. J. Virol. 78:3897-3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yei, S., N. Mittereder, S. Wert, J. A. Whitsett, R. W. WIlmott, and B. C. Trapnell. 1994. In vivo evaluation of the safety of adenovirus-mediated transfer of the human cystic fibrosis transmembrane conductance regulator cDNA to the lung. Human Gene Ther. 5:731-744. [DOI] [PubMed] [Google Scholar]