Abstract
Type I interferon (IFN), which includes the IFN-α and -β subtypes, plays an essential role in host defense against influenza A virus. However, the relative contribution of IFN-β remains unresolved. In mice, type I IFN is effective against influenza viruses only if the IFN-induced resistance factor Mx1 is present, though most inbred mouse strains, including the recently developed IFN-β-deficient mice, bear only defective Mx1 alleles. We therefore generated IFN-β-deficient mice carrying functional Mx1 alleles (designated Mx-BKO) and compared them to either wild-type mice bearing functional copies of both IFN-β and Mx1 (designated Mx-wt) or mice carrying functional Mx1 alleles but lacking functional type I IFN receptors (designated Mx-IFNAR). Influenza A virus strain SC35M (H7N7) grew to high titers and readily formed plaques in monolayers of Mx-BKO and Mx-IFNAR embryo fibroblasts which showed no spontaneous expression of Mx1. In contrast, Mx-wt embryo fibroblasts were found to constitutively express Mx1, most likely explaining why SC35M did not grow to high titers and formed no visible plaques in such cells. In vivo challenge experiments in which SC35M was applied via the intranasal route showed that the 50% lethal dose was about 20-fold lower in Mx-BKO mice than in Mx-wt mice and that virus titers in the lungs were increased in Mx-BKO mice. The resistance of Mx-BKO mice to influenza A virus strain PR/8/34 (H1N1) was also substantially reduced, demonstrating that IFN-β plays an important role in the defense against influenza A virus that cannot be compensated for by IFN-α.
Type I interferon (IFN) plays a key role in the innate immune response against many viruses (12). In addition, it further shapes the adaptive immune response against viral and nonviral pathogens (3). In the mouse, type I IFN comprises 14 IFN-α isoforms and a single form of IFN-β (28). Virus-induced synthesis of type I IFN in fibroblasts is hierarchical, with IFN-β appearing first, followed by IFN-α in a second wave (7, 17). Studies with primary fibroblasts of mice with a targeted deletion of the IFN-β gene largely confirmed the view that IFN-β serves as immediate-early IFN (21).
If the model of hierarchical organization of type I IFN expression observed in fibroblasts is maintained in the critical cell types of the intact organism, IFN-β-deficient mice should exhibit enhanced susceptibility to virus infections. As predicted from this model, IFN-β-deficient mice exhibited greatly enhanced susceptibility to intranasal challenge with vaccinia virus (4). IFN-β-deficient mice were also more susceptible to coxsackievirus B3 (5) and Friend retrovirus (11) infections. However, IFN-β-deficient and wild-type mice were equally susceptible to intravenous challenge with vesicular stomatitis virus (1), which is highly lethal for mice lacking functional type I IFN receptors (25). Furthermore, wild-type and IFN-β-deficient mice did not differ significantly in susceptibility to La Crosse virus (G. Blakqori, S. Delhaye, S. Haid, M. Habjan, U. Kalinke, S. Weiss, T. Michiels, P. Staeheli, and F. Weber, submitted for publication), a member of the Orthobunyaviridae. Available information thus suggests that IFN-β has a beneficial role during some but not all virus infections.
Resistance of mice to influenza and influenza-like viruses is largely dependent on restriction factor Mx1, the synthesis of which is under stringent control of type I IFN (22). Since most inbred mouse strains carry defective Mx1 genes (23), the protective potential of IFN against influenza viruses cannot be fully appreciated in regular inbred mice and a beneficial role of IFN-β might be missed.
By conventional breeding of available strains, we generated IFN-β-deficient mice that carry intact Mx1 alleles. We observed that cultured embryo fibroblasts from such animals were far more susceptible to influenza A virus infections than cells from mice with intact Mx1 and IFN-β genes. In addition, challenge experiments with two different strains of influenza A virus revealed significantly reduced survival rates and enhanced virus titers in the lungs of IFN-β-deficient mice, demonstrating that IFN-β contributes to innate immunity to influenza A virus.
MATERIALS AND METHODS
Mice.
The intact Mx1 allele of strain A2G was introduced into C57BL/6 mice by backcrossing for 20 generations, yielding strain B6.A2G-Mx1 (14). Here, this strain is designated Mx-wt. IFN-β0/0 mice (7) were backcrossed for 12 generations onto a C57BL/6 background. The resulting animals were subsequently crossed with B6·A2G-Mx1 mice, and offspring with intact Mx1 and defective IFN-β alleles were selected. Here, this strain is designated Mx-BKO. IFNAR10/0 mice (27) were backcrossed for 10 generations onto a C57BL/6 background before breeding with B6 A2G-Mx1 mice. Offspring with intact Mx1 and defective IFNAR1 alleles were selected. Here, this strain is designated Mx-IFNAR. Six- to 8-week-old animals were used for the challenge experiments, which were performed in accordance with the guidelines of the local animal care committee. Animals were euthanized if severe symptoms developed. Fifty percent lethal doses (LD50s) were calculated as described previously (20).
Cells.
Mouse embryo fibroblasts (MEF) were prepared from 13-day-old embryos by trypsin digestion. MEF were maintained in Dulbecco's modified Eagle-high-glucose medium containing 10% fetal bovine serum and passaged twice weekly at a splitting ratio of 1:3. Cultures were used between passages 3 and 10. MDCK and Vero cells were maintained in Dulbecco's modified Eagle-high-glucose medium containing 10% fetal bovine serum.
Viruses.
Stocks of the mouse-adapted influenza A virus strain SC35M (H7N7) (9) and the Mount Sinai strain of PR/8/34 (H1N1) (19) were prepared in embryonated chicken eggs. Stocks of PR8ΔNS1 with a targeted deletion of the NS1 coding region (10) were prepared in Vero cells.
Virus infections.
Animals were anesthetized by intraperitoneal injection of a mixture of ketamine (100 μg per gram body weight) and xylazine (5 μg per gram) and infected intranasally with the doses of virus indicated below in 50 μl of phosphate-buffered saline-0.3% bovine serum albumin.
Preparation of lung extracts and virus titrations.
Lungs were removed, immediately frozen in liquid nitrogen, and stored at −80°C until use. Lung homogenates were prepared by grinding the tissue in a mortar with sterile quartz sand. The material was suspended in 1 ml of phosphate-buffered saline, tissue debris was removed by low-speed centrifugation, and samples were frozen at −80°C. Titers of SC35M were determined by performing plaque assays with MDCK cells. Since our PR8 strain does not readily form visible plaques, titers were determined by staining the monolayers with a virus-specific antiserum. Briefly, at 16 h postinfection with 10-fold dilutions of samples, the cell monolayers were fixed, permeabilized, and stained with a rabbit antiserum that detects the viral nucleoprotein, followed by a Cy3-labeled secondary antibody. Foci of infected cells were counted using a fluorescence microscope.
Analysis of Mx protein expression.
Western blot and immunofluorescence analyses were done using a monoclonal antibody with high specificity for mouse Mx1 (8).
IFN treatment of cells.
Cells were treated with 1,000 units/ml of human hybrid IFN-α B/D which was shown to be active on mouse cells (13, 18).
RESULTS
Enhanced growth of SC35M in MEF lacking IFN-β.
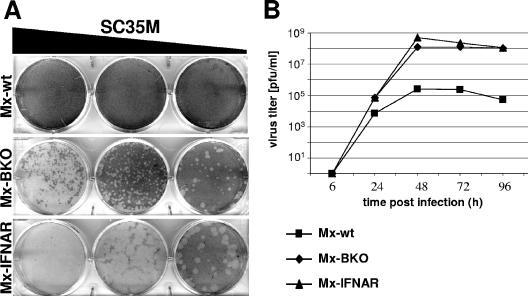
To evaluate the roles of virus-induced IFN-α and -β in resistance to influenza A virus, we first compared levels of viral plaque formation in primary MEF cultures derived from mice with functional Mx1 alleles and either a defective type I IFN receptor (Mx-IFNAR) or defective IFN-β alleles (Mx-BKO) and those derived from normal controls (Mx-wt). Influenza A virus strain SC35M readily formed plaques (Fig. 1A) and replicated to high titers (Fig. 1B) in MEF cultures from Mx-IFNAR mice. In infected MEF cultures from Mx-wt mice, no plaques were visible (Fig. 1A) and virus titers in MEF supernatants were reduced more than 500-fold (Fig. 1B). Interestingly, MEF cultures from Mx-BKO mice were almost as susceptible to SC35M as cells from Mx-IFNAR mice, which are nonresponsive to all type I IFNs (Fig. 1), indicating that IFN-β is very important for influenza virus restriction in fibroblasts.
FIG. 1.
Enhanced replication of influenza A virus in MEF lacking IFN-β. (A) Viral plaque assays. MEF from Mx-wt, Mx-BKO, or Mx-IFNAR mice were infected with 10-fold dilutions of SC35M and stained for plaques 3 days later. (B) Viral growth curves. MEF were infected with a low dose (multiplicity of infection, 0.01) of SC35M. At the indicated times postinfection, culture supernatants were harvested and analyzed for infectious virus.
Tight regulation of Mx1 gene expression in MEF lacking IFN-β.
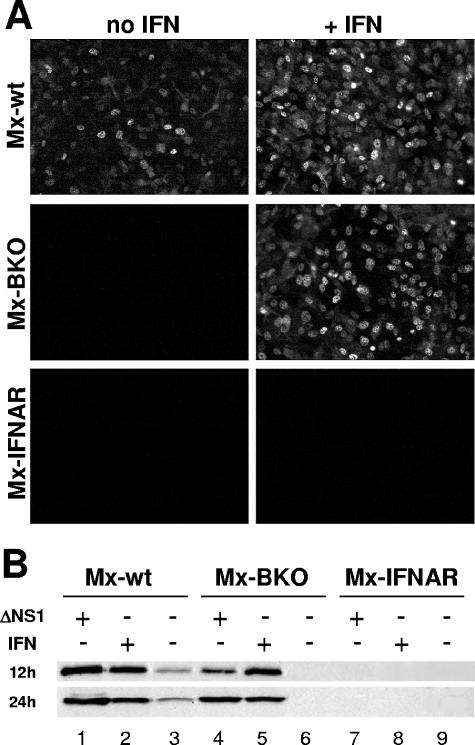
Since Mx1 is a major resistance factor for influenza virus in mice (15), we compared spontaneous and IFN-induced expression levels of Mx1 in the various MEF cultures by using a Mx-specific antibody. Prominent nuclear staining of Mx1 was observed after IFN treatment of MEF from Mx-wt and Mx-BKO mice (Fig. 2A). As expected, IFN treatment was not effective in MEF from Mx-IFNAR animals.
FIG. 2.
Tight regulation of Mx1 gene expression in MEF lacking IFN-β. (A) MEF from Mx-wt, Mx-BKO, or Mx-IFNAR mice were treated for 24 h with either plain medium or medium containing 1,000 units/ml of human IFN-α B/D and subsequently stained for nuclear Mx1 protein by using a specific antibody. (B) MEF from the various mouse strains were treated either for 16 h with human IFN-α B/D (1,000 units/ml) or for 12 and 24 h with influenza A virus PR8ΔNS1 (multiplicity of infection, 2) as indicated. The cells were analyzed for Mx1 and actin protein levels by Western blotting. The Mx1 signals were quantified and corrected for loading differences by normalization to the corresponding actin signals. The signal of Mx1 in wild-type cells at 12 h post-virus infection was set at 100%. Values shown in the figure are percentages of this reference value. +, present, −, absent.
Spontaneous expression of the Mx1 gene was readily detectable in untreated MEF from Mx-wt animals (Fig. 2A), whereas no spontaneous Mx1 expression was observed in MEF from Mx-BKO or Mx-IFNAR mice. These results were confirmed by Western blot analysis (Fig. 2B) which showed that Mx1 levels in untreated Mx-wt cells reached roughly 20% of the levels in cells induced with IFN. In agreement with the immunofluorescence data, Western blot analysis indicated that spontaneous expression of Mx1 is extremely low in Mx-BKO and Mx-IFNAR cells (Fig. 2B).
The various MEF cultures might differ in responsiveness to viral stimuli. We therefore performed infection experiments with the influenza A virus mutant strain PR8ΔNS1 that lacks the IFN-antagonistic factor NS1 and thus activates IFN genes very strongly (26). Mx-IFNAR MEF completely failed to respond to this stimulus (Fig. 2B), confirming earlier observations that Mx gene activation requires signaling through the type I IFN receptor complex (2). In contrast to Mx-IFNAR cells, cells from both Mx-wt and Mx-BKO mice showed strong responses to PR8ΔNS1 (Fig. 2B). However, we noted a distinct difference between Mx-wt and Mx-BKO cells in the kinetics but not in the overall extent of the responses. At 12 h postinfection with PR8ΔNS1, the Mx1 signal in Mx-BKO cells was about fivefold less intense than that in Mx-wt cells. This difference was no longer observed at 24 h postinfection (Fig. 2B).
Enhanced influenza virus susceptibility of IFN-β-deficient mice.
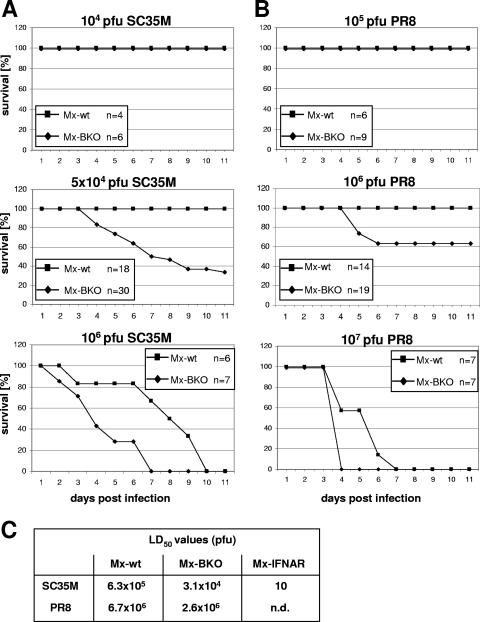
In a first series of experiments, various doses of SC35M were applied intranasally to groups of Mx-wt and Mx-BKO mice, and the infected animals were monitored for at least 11 days. When 104 PFU of SC35M were used, no symptoms were recorded at any time point, and all animals survived (Fig. 3A, top panel). However, when the virus dose was 5 × 104 PFU, severe disease in 20 of 30 infected Mx-BKO mice but in none of 18 Mx-wt mice was noted (Fig. 3A, middle panel). Severe disease in Mx-BKO mice was accompanied by the loss of 25 to 30% of total body weight, ruffled fur, hypothermia, and apathy. At this stage, the animals were euthanized in accordance with the local animal care committee guidelines. The remaining 10 Mx-BKO mice showed mild to moderate symptoms between days 4 and 9 postinfection, and maximal weight losses amounted to less than 30% of body weight. Most Mx-wt mice infected with 5 × 104 PFU of SC35M also displayed typical disease symptoms which, however, were much milder than those in Mx-BKO mice. Maximal weight losses were less than 30% in all 18 Mx-wt animals used for this experiment, and all animals survived (Fig. 3A, middle panel). When the virus dose was raised to 106 PFU, all Mx-wt and Mx-BKO mice developed severe symptoms (Fig. 3A, bottom panel). However, disease progression was significantly faster in Mx-BKO mice than in Mx-wt mice. From the data shown in Fig. 3A, the LD50s of SC35M were determined to be 6.3 × 105 PFU for Mx-wt mice and 3.1 × 104 PFU for Mx-BKO mice (Fig. 3C). Thus, Mx-BKO mice were substantially more susceptible to SC35M than Mx-wt mice. It should be noted, however, that the LD50 of SC35M for Mx-IFNAR mice was only 10 PFU (Fig. 3C), demonstrating that the lack of IFN-β is compensated for to a large extent by IFN-α.
FIG. 3.
Enhanced influenza A virus susceptibility of IFN-β-deficient mice. (A and B) Survival curves of Mx-wt and Mx-BKO mice infected with the indicated doses of influenza A virus strain SC35M (A) or PR8 (B). Physical statuses and weights of the animals were determined daily. Mice were killed in the case of strong symptoms or the loss of more than 30% of total body weight. n, number of animals per group. (C) Calculated LD50s of SC35M and PR8. n.d., not determined.
In a second series of experiments, we determined whether Mx-BKO mice might also show enhanced susceptibility to strain PR8, which is derived from a human influenza A virus isolate. At a dose of 105 PFU per animal, neither Mx-wt nor Mx-BKO mice developed symptoms (Fig. 3B, top panel). At 106 PFU per animal, 7 of 19 Mx-BKO mice developed severe disease. In contrast, none of the 14 Mx-wt animals became seriously ill under these conditions (Fig. 3B, middle panel). At 107 PFU per animal, all mice of both groups became severely ill. Again, disease progression was faster in Mx-BKO mice than in Mx-wt mice (Fig. 3B, bottom panel). From the data shown in Fig. 3B, the LD50s of PR8 were determined to be 6.7 × 106 PFU for Mx-wt mice and 2.6 × 106 PFU for Mx-BKO mice (Fig. 3C).
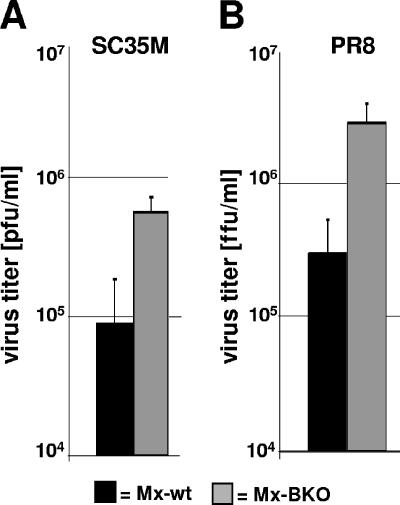
To determine whether the enhanced susceptibility of Mx-BKO mice could be correlated with enhanced virus replication in the lungs, we infected new groups of animals with 5 × 104 PFU of SC35M or 106 FFU of PR8 and harvested the lungs at 72 h postinfection. The average virus titers were about fivefold higher in the lungs of Mx-BKO mice infected with either SC35M (Fig. 4A) or PR8 (Fig. 4B). Thus, experiments with two different strains of influenza A virus yielded essentially identical results, clearly demonstrating that IFN-β is essential for influenza virus resistance.
FIG. 4.
Enhanced influenza A virus replication in the lungs of IFN-β-deficient mice. Mx-wt and Mx-BKO mice were infected with either SC35M (5 × 104 PFU) (A) or PR8 (106 FFU) (B). At 72 h postinfection, the lungs were removed and the virus titers in lung homogenates were determined. Groups of six animals were analyzed. Mean values and standard deviations are displayed.
DISCUSSION
Determining the role of type I IFN in host defense against influenza virus with the help of the mouse model system is complicated by the fact that most laboratory mouse strains carry defective Mx1 resistance genes (23). Consequently, these strains fail to make efficient use of virus-induced IFN for protection against this important human pathogen (24). Therefore, the contribution of IFN-β to protection against influenza A virus cannot be appreciated adequately by using common inbred mouse strains. In this study, we generated IFN-β-deficient mice that carry functional Mx1 alleles and demonstrated that such mice exhibit substantially reduced resistance to two different strains of influenza A virus.
The strongest effect of the IFN-β deficiency was observed in primary MEF cultures. Influenza virus strain SC35M was severely restricted in cells of Mx-wt mice even in the absence of exogenous IFN. In contrast, high virus titers were obtained with cells from both Mx-BKO and Mx-IFNAR mice (Fig. 1). A far less dramatic effect of the IFN-β gene deletion was observed during in vivo challenge with influenza viruses. Deficiency of IFN-β lowered the LD50 of SC35M by only a factor of 20. In contrast, the lack of a functional IFN receptor decreased the LD50 more than 10,000-fold. A simple explanation for the apparent discrepancy between the cell culture and in vivo data are that spontaneous expression of biologically relevant levels of Mx1 is not found in all cell types. In fact, depending on the cell culture conditions (6), primary MEF cultures (Fig. 2) and peritoneal macrophages (16) from Mx-wt mice do express fairly high levels of Mx1. Similarly, spleen cells from Mx-wt mice express substantial levels of Mx1, whereas the vast majority of cells in infected lungs do not (unpublished data). The fact that Mx-wt and Mx-BKO mice differed in susceptibility to influenza virus by a factor of about 20 may be explained by the assumption that early synthesis of IFN-β promotes virus-triggered expression of IFN-α in lung epithelial cells. Since IFN-β presumably accounts for a substantial fraction of the type I IFN activity in infected organs, we cannot exclude the alternative view that the reduced survival of Mx-BKO mice is due simply to reduced overall levels of type I IFN in the absence of IFN-β.
An important aspect of our study was that spontaneous expression of Mx1 in MEF cultures was no longer detected when the IFN-β gene was deleted (Fig. 2). This observation suggests that low-level expression of Mx1 in the absence of exogenous inducers is not due to intrinsic promoter leakiness or contamination of the primary MEF cultures with an undefined cell type constitutively synthesizing IFN-α. Rather, in agreement with a recent report (21), it appears more likely that the IFN-β gene is expressed at low levels in MEF.
Our results are compatible with current models (3) demonstrating that expression of type I IFN genes is stringently orchestrated and that the IFN-β gene occupies a position at the top of the hierarchy of type I IFN. When IFN-β is missing, IFN-α synthesis is delayed, thus enhancing the probability that the invading virus can overrun the innate immune response of the host. However, since Mx-IFNAR mice that lack a functional type I IFN receptor were dramatically more susceptible to influenza A virus than Mx-BKO mice, it is evident that the lack of IFN-β is compensated for to a large extent by IFN-α in vivo. In addition, PR8ΔNS1, a virus strain that has lost the ability to inhibit the induction of IFN, was a surprisingly potent inducer of Mx1 in MEF of IFN-β-deficient mice (Fig. 2B). This result agrees well with the findings of other investigators (1) demonstrating that IFN-β greatly facilitates virus induction of IFN-α genes, although there is no absolute requirement for priming by IFN-β.
Acknowledgments
We thank Jürgen Stech (Marburg) and Andrej Egorov (Vienna) for providing seeds of SC35M and PR8ΔNS1, respectively. Purified hybrid IFN-α B/D was kindly provided by Heinz-Kurt Hochkeppel (Novartis, Basel, Switzerland).
This work was supported in part by grants of the Deutsche Forschungsgemeinschaft to P.S., G.K., and S.W.
Footnotes
Published ahead of print on 6 December 2006.
REFERENCES
- 1.Barchet, W., M. Cella, B. Odermatt, C. Asselin-Paturel, M. Colonna, and U. Kalinke. 2002. Virus-induced interferon alpha production by a dendritic cell subset in the absence of feedback signaling in vivo. J. Exp. Med. 195:507-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bazzigher, L., J. Pavlovic, O. Haller, and P. Staeheli. 1992. Mx genes show weaker primary response to virus than other interferon-regulated genes. Virology 186:154-160. [DOI] [PubMed] [Google Scholar]
- 3.Decker, T., M. Muller, and S. Stockinger. 2005. The yin and yang of type I interferon activity in bacterial infection. Nat. Rev. Immunol. 5:675-687. [DOI] [PubMed] [Google Scholar]
- 4.Deonarain, R., A. Alcami, M. Alexiou, M. J. Dallman, D. R. Gewert, and A. C. Porter. 2000. Impaired antiviral response and alpha/beta interferon induction in mice lacking beta interferon. J. Virol. 74:3404-3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deonarain, R., D. Cerullo, K. Fuse, P. P. Liu, and E. N. Fish. 2004. Protective role for interferon-beta in coxsackievirus B3 infection. Circulation 110:3540-3543. [DOI] [PubMed] [Google Scholar]
- 6.Dreiding, P., P. Staeheli, and O. Haller. 1985. Interferon-induced protein Mx accumulates in nuclei of mouse cells expressing resistance to influenza viruses. Virology 140:192-196. [DOI] [PubMed] [Google Scholar]
- 7.Erlandsson, L., R. Blumenthal, M. L. Eloranta, H. Engel, G. Alm, S. Weiss, and T. Leanderson. 1998. Interferon-beta is required for interferon-alpha production in mouse fibroblasts. Curr. Biol. 8:223-226. [DOI] [PubMed] [Google Scholar]
- 8.Flohr, F., S. Schneider-Schaulies, O. Haller, and G. Kochs. 1999. The central interactive region of human MxA GTPase is involved in GTPase activation and interaction with viral target structures. FEBS Lett. 463:24-28. [DOI] [PubMed] [Google Scholar]
- 9.Gabriel, G., B. Dauber, T. Wolff, O. Planz, H. D. Klenk, and J. Stech. 2005. The viral polymerase mediates adaptation of an avian influenza virus to a mammalian host. Proc. Natl. Acad. Sci. USA 102:18590-18595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia-Sastre, A., A. Egorov, D. Matassov, S. Brandt, D. E. Levy, J. E. Durbin, P. Palese, and T. Muster. 1998. Influenza A virus lacking the NS1 gene replicates in interferon-deficient systems. Virology 252:324-330. [DOI] [PubMed] [Google Scholar]
- 11.Gerlach, N., S. Schimmer, S. Weiss, U. Kalinke, and U. Dittmer. 2006. Effects of type I interferons on Friend retrovirus infection. J. Virol. 80:3438-3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haller, O., G. Kochs, and F. Weber. 2006. The interferon response circuit: induction and suppression by pathogenic viruses. Virology 344:119-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horisberger, M. A., and K. de Staritzky. 1987. A recombinant human interferon-alpha B/D hybrid with a broad host-range. J. Gen. Virol. 68:945-948. [DOI] [PubMed] [Google Scholar]
- 14.Horisberger, M. A., P. Staeheli, and O. Haller. 1983. Interferon induces a unique protein in mouse cells bearing a gene for resistance to influenza virus. Proc. Natl. Acad. Sci. USA 80:1910-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kolb, E., E. Laine, D. Strehler, and P. Staeheli. 1992. Resistance to influenza virus infection of Mx transgenic mice expressing Mx protein under the control of two constitutive promoters. J. Virol. 66:1709-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindenmann, J., E. Deuel, S. Fanconi, and O. Haller. 1978. Inborn resistance of mice to myxoviruses: macrophages express phenotype in vitro. J. Exp. Med. 147:531-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marie, I., J. E. Durbin, and D. E. Levy. 1998. Differential viral induction of distinct interferon-alpha genes by positive feedback through interferon regulatory factor-7. EMBO J. 17:6660-6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meister, A., G. Uze, K. E. Mogensen, I. Gresser, M. G. Tovey, M. Grutter, and F. Meyer. 1986. Biological activities and receptor binding of two human recombinant interferons and their hybrids. J. Gen. Virol. 67:1633-1643. [DOI] [PubMed] [Google Scholar]
- 19.Palese, P., and J. L. Schulman. 1976. Mapping of the influenza virus genome: identification of the hemagglutinin and the neuraminidase genes. Proc. Natl. Acad. Sci. USA 73:2142-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reed, L. J., and H. Münch. 1938. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 21.Samuelsson, C. V., S. Lienenklaus, P. P. Muller, R. Zawatzky, H. Hauser, and S. Weiss. 2005. Transformation of mouse fibroblasts alters the induction pattern of type I IFNs after virus infection. Biochem. Biophys. Res. Commun. 335:584-589. [DOI] [PubMed] [Google Scholar]
- 22.Staeheli, P., P. Danielson, O. Haller, and J. G. Sutcliffe. 1986. Transcriptional activation of the mouse Mx gene by type I interferon. Mol. Cell. Biol. 6:4770-4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Staeheli, P., R. Grob, E. Meier, J. G. Sutcliffe, and O. Haller. 1988. Influenza virus-susceptible mice carry Mx genes with a large deletion or a nonsense mutation. Mol. Cell. Biol. 8:4518-4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Staeheli, P., O. Haller, W. Boll, J. Lindenmann, and C. Weissmann. 1986. Mx protein: constitutive expression in 3T3 cells transformed with cloned Mx cDNA confers selective resistance to influenza virus. Cell 44:147-158. [DOI] [PubMed] [Google Scholar]
- 25.Steinhoff, U., U. Muller, A. Schertler, H. Hengartner, M. Aguet, and R. M. Zinkernagel. 1995. Antiviral protection by vesicular stomatitis virus-specific antibodies in alpha/beta interferon receptor-deficient mice. J. Virol. 69:2153-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Talon, J., C. M. Horvath, R. Polley, C. F. Basler, T. Muster, P. Palese, and A. Garcia-Sastre. 2000. Activation of interferon regulatory factor 3 is inhibited by the influenza A virus NS1 protein. J. Virol. 74:7989-7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van den Broek, M. F., U. Muller, S. Huang, M. Aguet, and R. M. Zinkernagel. 1995. Antiviral defense in mice lacking both alpha/beta and gamma interferon receptors. J. Virol. 69:4792-4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Pesch, V., H. Lanaya, J. C. Renauld, and T. Michiels. 2004. Characterization of the murine alpha interferon gene family. J. Virol. 78:8219-8228. [DOI] [PMC free article] [PubMed] [Google Scholar]