Abstract
Herpes simplex virus type 1 (HSV-1) and HSV-2 cause very similar acute infections but differ in their abilities to reactivate from trigeminal and dorsal root ganglia. To investigate differences in patterns of viral infection, we colabeled murine sensory ganglia for evidence of HSV infection and for the sensory neuron marker A5 or KH10. During acute infection, 7 to 10% of HSV-1 or HSV-2 antigen-positive neurons were A5 positive and 13 to 16% were KH10 positive, suggesting that both viruses reach each type of neuron in a manner proportional to their representation in uninfected ganglia. In murine trigeminal ganglia harvested during HSV latency, 25% of HSV-1 latency-associated transcript (LAT)- and 4% of HSV-2 LAT-expressing neurons were A5 positive, while 12% of HSV-1 LAT- and 42% of HSV-2 LAT-expressing neurons were KH10 positive. A similar difference was observed in murine dorsal root ganglia. These differences could not be attributed to differences in LAT expression levels in A5- versus KH10-positive neurons. Thus, HSV-1 demonstrated a preference for the establishment of latency in A5-positive neurons, while HSV-2 demonstrated a preference for the establishment of latency in KH10-positive neurons. A chimeric HSV-2 mutant that expresses the HSV-1 LAT exhibited an HSV-1 phenotype, preferentially establishing latency in A5-positive neurons. These data imply that the HSV-1 and HSV-2 LAT regions influence the ability of virus to establish latency in different neuronal subtypes. That the same chimeric virus has a characteristic HSV-1 reactivation phenotype further suggests that LAT-influenced establishment of latency in specific neuronal subtypes could be an important part of the mechanism by which LAT influences viral reactivation phenotypes.
Primary infection of mice with herpes simplex virus type 1 (HSV-1) and HSV-2 is characterized by viral replication at the site of inoculation, followed by retrograde axonal transport of the virus to corresponding sensory ganglia where infection follows two very different pathways (14, 19, 28, 33). In some neurons, the virus expresses productive cycle genes, replicates, and causes host cell death, whereas in other neurons, the virus establishes a latent infection characterized by limited viral transcription except for the latency-associated transcripts (LATs), which accumulate to high copy number in the nuclei of latently infected cells (29). The LATs code from the long repeat region of the viral genome and run antisense to the immediate-early transactivator ICP0, the protein kinase R inhibitor ICP34.5, the 3′ end of the immediate-early transactivator ICP4 and the AL gene (24). A unique feature of the major 2-kb LAT is that it is a stable intron, spliced from a much less stable primary transcript (5). Studies from multiple labs suggest that the LAT region of the viral genome plays an important role in both the establishment (25, 31) and the reactivation of latent infection (13). The mechanisms responsible for this are poorly defined. Hypotheses (reviewed in reference 3) include that the HSV-1 LATs mediate latent infection by the antisense regulation of expression of the key viral immediate-early gene ICP0 (5) or by the inhibition of apoptosis (1, 23), an action that appears to at least in part be mediated by a LAT region micro-RNA (7), and also might be mediated by the interferons (21). To date, no published studies address the mechanism of HSV-2 LAT action.
The clinical diseases caused by HSV-1 and HSV-2 are very similar except for marked differences in site-specific reactivation. Whereas HSV-1 reactivates most efficiently from trigeminal ganglia (TG), giving rise to recurrent disease of the face, eyes, and oropharynx, HSV-2 reactivates more efficiently from the lumbar-sacral ganglia, giving rise to recurrent disease below the waist, including genital disease (16). Studies of HSV-1 and HSV-2 chimeric viruses in rabbit and guinea pig models indicate that a 2.8-kb region of the viral genome that codes for LAT plays an important role in determining the efficiency of this site-specific reactivation. Specifically, an HSV-2 virus engineered to express HSV-1 LAT instead of the native LAT (HSV-2 333/LAT1) reactivated more efficiently than did HSV-2 from the trigeminal ganglion (35), and an HSV-1 virus engineered to express HSV-2 LAT instead of the native LAT (HSV-1 17syn+/LAT2) reactivated less efficiently from the trigeminal ganglion (9).
Primary sensory neurons are a diverse population of cells that can be classified according to cellular morphology, physiological response properties, and patterns of gene expression, and in a recent study, we demonstrated that the distribution of productive and latent HSV-1 infection among populations of ganglionic neurons is not random. Specifically, we showed that although all neuronal populations are capable of supporting a productive HSV-1 infection, some neuronal populations of the trigeminal ganglion are more likely than others to be associated with latent infection. Using a battery of antisera capable of recognizing different populations of ganglionic neurons, we found that neurons identified by monoclonal antibody (MAb) A5 (specific for a population of neurons expressing Galβ1-4GlcNAc-R epitopes) were the principal reservoir of latent infection, whereas latent infection was rarely found in neurons identified by MAb KH10 (a different population of ganglionic neurons expressing Galα1-3Galβ1-4NAc-R epitopes) (6, 34). These findings highlight not only the key role that the host neuron may play in regulating the repertoire of viral gene expression during establishment of HSV latent infection but also the importance of considering the complex neuronal composition of primary sensory ganglia in interpreting the results of in vivo studies of HSV infection.
In the current study, we tested the hypothesis that HSV-1 and HSV-2 establish latent infection in different populations of ganglionic neurons. This hypothesis derives from the role that LAT plays in the establishment of HSV latency, the LAT-dependent, site-specific reactivation phenotypes of HSV-1 and HSV-2, and differences in the HSV-1 and HSV-2 LAT coding regions. In the course of this study, we found significant differences in the latent distributions of HSV-1 and HSV-2, with KH10-positive neurons serving as the principal reservoir of HSV-2 latent infection. We also found that these differences are attributable to the same 2.8-kb portion of the LAT coding region that controls the site-specific reactivation phenotype (9, 35).
MATERIALS AND METHODS
Virus and animal preparation.
The recombinant viruses HSV-2 333/Lat1 and HSV-2 333/Lat1R have been described in detail previously (35). HSV-1 strains KOS and 17+ and HSV-2 strain 333 and the recombinant viruses HSV-2 333/Lat1 and HSV-2 333/Lat1R were propagated as previously described (34). Viral titers of 1 × 108 PFU/ml were typically obtained. Protocols were approved by the UCSF Committee on Animal Research. Six-week-old female outbred Swiss Webster mice (Simonsen Laboratories, Gilroy, CA) were anesthetized and inoculated as previously described (11, 19). In brief, mice were anesthetized by intraperitoneal injection of pentobarbital. For ocular inoculations, this was followed by topical proparacaine (1%). Once adequate anesthesia was obtained, the corneal epithelium was abraded with a sterile 27-gauge needle, and the ocular surface was inoculated with about 106 PFU of viral stock. For rear footpad inoculations, the keratinized surface of the footpad was lightly abraded with an emery board, followed by the application of about 3 × 106 PFU of viral stock to the abraded skin. Mice were then allowed to recover from anesthesia (about 45 to 60 min) on their backs. In those experiments requiring the use of an antiviral drug, acyclovir (1.2 mg/ml) was placed in the drinking water 40 h after viral inoculation. Uninfected trigeminal ganglia from LAT 3098 transgenic mice (8) were a generous gift of David Bloom (Gainesville, FL).
Tissue preparation.
Three or 21 days postinoculation, mice were euthanized by carbon dioxide inhalation and thoracotomy. Cardiac perfusion with 0.1 M phosphate-buffered saline (PBS, pH 7.4) was performed immediately, followed by perfusion with 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (PB, pH 7.4). Dissected ganglia were immersion fixed in 4% PFA at 4°C for 30 to 60 min, equilibrated with 30% sucrose in 0.1 M PBS at 4°C, pooled in groups of 10 (TG) or 50 dorsal root ganglia (DRG), embedded in Tissue-Tek O.C.T. compound (Sakura Finetechnical, Tokyo, Japan), and snap-frozen in liquid nitrogen. Serial sections (7 μm) of these pooled blocks of infected ganglia were then collected as four alternate sets onto SuperFrost Plus microscope slides (Fisher Scientific, Pittsburgh, PA) and stored at −80°C. In this way, we generated four equivalent sets of 28-μm step sections through each block of tissue.
Dual fluorescent staining.
Dual fluorescent staining of frozen tissue sections was carried out as previously described (18, 34). The following antisera were used: mouse MAbs A5 and KH10 (Developmental Studies Hybridoma Bank, Iowa City, IA), anti-TrkA (a gift from Louis Reichardt, UCSF), anti-c-ret (Amgen, Inc., Thousand Oaks, CA), anti-pan Trk (Promega, Madison, WI), and anti-CGRP (Amersham, Arlington Heights, IL). Fluorescein isothiocyanate-conjugated rabbit anti-HSV-1 and anti-HSV-2 antisera were from DAKO, Glostrup, Denmark. Bandeiraea Simplicifolia isolectin B4 (BSL-IB4) was obtained from Vector Labs (Burlingame, CA).
Preparation of DIG-labeled RNA probes for fluorescence in situ hybridization (FISH).
Labeled riboprobes for the HSV-1 stable LAT intron were prepared using plasmid pATD-19 as a template (29). This plasmid contains a 347-bp fragment of HSV-1 DNA (genomic nucleotides 119629 to 119975). Labeled riboprobes for the HSV-2 stable LAT intron were prepared using a 782-bp (StuI-XhoI) fragment of pBam/Xho4Z as a template. Plasmid Bam/Xho4Z contains a 1.8-kb BamHI-XhoI fragment of HSV-2 DNA (genomic nucleotides 120489 to 122276) cloned into the multiple cloning site of pGEM-4Z (Promega). Plasmids were linearized with either HindIII (for HSV-1) or StuI (for HSV-2), purified by phenol-chloroform extraction and ethanol precipitation, and then incubated with digoxigenin (DIG) RNA labeling mix (Roche, Mannheim, Germany) and either T3 (for HSV-1) or SP6 (for HSV-2) polymerase for 3 h at 37°C. After treatment with DNase I, probes were purified using G-50 Sephadex columns (Roche) and dissolved into 50% formamide in diethylpyrocarbonate-treated water. Probe concentrations were determined by spotting each probe onto a nylon membrane; visualizing with anti-DIG-AP Fab fragments (Roche), nitroblue tetrazolium (Roche), and 5-bromo-4-chloro-3-indolylphosphate toluidinium (Roche); and comparing the intensity of the generated signal to that of the labeled control RNA (Roche).
Combined staining by fluorescent in situ hybridization and immunohistochemistry.
Tissue sections were postfixed with 4% PFA in 0.1 M PB for 10 min and then sequentially washed in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), diethyl pyrocarbonate (DEPC)-treated water, and 0.1 M triethanolamine hydrochloride (TEA). Tissue sections were then treated with 0.25% acetic anhydride in 0.1 M TEA (3 min), washed in 2× SSC, and incubated with prehybridization buffer (50% formamide, 40 μg/ml salmon sperm DNA, 5× SSC) at 45°C for 2 h. Each riboprobe was heated to 80°C for 10 min, chilled on ice, and then diluted to 1 μg/ml in preheated (55°C) hybridization buffer (50% formamide, 1× Denhardt's solution, 10 mM EDTA, 10% dextran sulfate, 0.5 mg/ml yeast tRNA, 0.5 mg/ml salmon sperm DNA, 3× SSC). Prehybridized tissue was incubated with each probe at 55°C overnight. After hybridization, tissue was washed with 2× SSC (10 min) and treated with 20 μg/ml of RNase A in 2× SSC at 37°C for 30 min, followed by serial washing with graduated dilutions of SSC at 55°C. Tissue was then equilibrated with 0.1 M PBS and incubated with anti-DIG-rhodamine Fab fragments (Roche) diluted in 0.1 M PBS with 1× blocking solution (Roche) for 30 min. After washing with 0.1 M PBS for 10 min, tissue was blocked with 3% normal goat serum in 0.1 M PBS for 30 min and then incubated with either MAb A5 or KH10 at 4°C for 2 nights. Tissue was then washed with 0.1 M PBS, blocked with 3% normal goat serum in 0.1 M PBS and incubated for 1 h with FITC-labeled goat anti-mouse immunoglobulin M (Biosource, Camarillo, CA) diluted 1:100 in 0.1 M PBS with 1% normal goat serum. Stained tissue sections were then washed with 0.1 M PBS and cover slipped with Vectashield mounting medium (Vector Labs).
Evaluation of LAT probe signal intensity by confocal microscopy.
Staining was visualized with a Zeiss Axiovert 200 M inverted microscope equipped with an LSM 5 Pa laser module (Zeiss, Inc., Jena, Germany). LAT FISH signal intensity over neuronal nuclei was assayed using the histogram function of LSM 3.0 image acquisition and analysis software (Zeiss, Inc.), generating a mean signal intensity/pixel in arbitrary 8-bit grayscale units (0 to 256 units).
Statistical analysis.
To determine whether there were significant differences in the pattern of ganglionic latent infection, pair-wise comparisons were made using the χ2 test. Since we performed multiple comparisons, a Bonferroni-adjusted P value of <0.00625 was used to assign significance. The two-tailed Student's t test and Kolmogorov-Smirnov test were used to determine whether there were significant differences in LAT signal intensities among different neuronal cell types.
RESULTS
Following ocular inoculation with HSV-1 or HSV-2, we assayed latently infected murine TG by FISH for viral LAT expression in order to determine the proportion of the latent viral load present in populations of neurons identifiable by MAbs A5 and KH10. In the mouse trigeminal ganglion, MAbs A5 and KH10 recognize neuronal populations with very little overlap (Table 1). Most A5-positive neurons are immunoreactive for the calcitonin gene-related peptide and the high-affinity nerve growth factor receptor (TrkA), whereas KH10-positive neurons colabel with the lectin BSL-IB4, identifying them as a population of small-diameter, glial cell line-derived neurotrophic factor-responsive neurons (2, 4, 20, 26). These neurochemical characteristics imply that both KH10- and A5-positive neurons transmit nociceptive stimuli but represent functionally distinct sensory neuronal populations (27, 30).
TABLE 1.
Percentage of A5+ and KH10+ neurons that colabel with other neuronal markersa
| Neuron | Neurons (%) colabeling with:
|
||||
|---|---|---|---|---|---|
| TrkA | PanTrk | CGRP | RET | BSL-IB4 | |
| A5+ | 78 | 94 | 72 | 8 | <1 |
| KH10+ | <1 | <1 | 6 | 88 | 100 |
Data reflect analysis of more than 7,000 A5- and KH10-positive trigeminal ganglion neurons and suggest that MAbs A5 and KH10 recognize subsets of ganglionic neurons with very little overlap. Direct demonstration of this possibility is problematic, however, since MAbs A5 and KH10 are both immunoglobulins M.
Consistent with our previous report (34), 21 days after ocular inoculation with HSV-1 (KOS strain), 50% of the LAT-expressing TG neurons were A5 positive, while only 7% expressed the KH10 marker (Table 2 and Fig. 1). This distribution cannot be explained by the neuronal composition of the ganglion (Table 2), and our previous studies showed that the expression of these markers does not change following infection (34). This distribution also cannot be explained by differential access of peripherally inoculated virus to the two cell types since these neurons become productively infected in proportion to their representation in the ganglion (Table 3 and see reference 34). Thus, these data suggest that a greater proportion of A5-positive neurons survive acute ganglionic infection with HSV-1 to become latently infected than do KH10-positive neurons.
TABLE 2.
Distribution of latently infected neurons 21 days following peripheral viral inoculationa
| Virus | Treatment | % of colabeling neuronsa (no. of colabeling neurons/total no. of neurons) in:
|
|||
|---|---|---|---|---|---|
| Trigeminal ganglia (cornea)
|
Lumbar-sacral dorsal root ganglia (footpad)
|
||||
| A5+ | KH10+ | A5+ | KH10+ | ||
| Uninfected | 9 (197/2,155) | 16 (324/2,063) | 11 (335/2,980) | 26 (459/1,782) | |
| HSV-1 | None | 50 (478/949) | 7 (39/568) | 38 (175/458) | 3 (13/429) |
| HSV-1 | Acyclovir | 25 (59/239) | 12 (47/379) | 25 (134/528) | 7 (28/369) |
| HSV-2 | Acyclovir | 4 (18/468) | 42 (161/394) | 2 (14/646) | 38 (108/288) |
Total neurons (uninfected) or LAT-positive neurons (HSV infected) that colabel with MAb A5 (A5+) or KH10 (KH10+). Each data point reflects an analysis of step sections taken through at least two blocks (20 trigeminal ganglia or 100 dorsal root ganglia) of latently infected tissue.
FIG. 1.
Identification of primary sensory neurons latently infected with HSV-1. Sections of infected murine trigeminal ganglia were assayed by both in situ hybridization for HSV-1 LAT (red-labeled nuclei) and immunofluorescent staining with MAbs A5 and KH10 (green-labeled cell bodies) in order to identify the neuronal subpopulations latently infected with HSV-1. In these representative pairs of images, HSV-1 LAT expression is present in A5-positive neurons but not in KH10-positive neurons. Bar = 100 μm.
TABLE 3.
Distribution of productively infected neurons 3 days following ocular inoculation
| Virus | Treatment | % of colabeling neurons (no. of colabeling neurons/total no. of neurons)a
|
|
|---|---|---|---|
| A5+ | KH10+ | ||
| Uninfected | 9 (197/2,155) | 16 (324/2,063) | |
| HSV-1 | None | 7b | 16b |
| HSV-2 | Acyclovir | 10 (38/397) | 13 (56/432) |
TG neurons (uninfected) or TG neurons with productive viral gene expression (HSV infected) that colabel with MAb A5 (A5+) or KH10 (KH10+). Each data point reflects an analysis of step sections taken through one block of tissue (10 ganglia).
Previously published data (34) are presented for purposes of comparison.
When administered under the same experimental conditions as those of HSV-1, HSV-2 (333 strain) caused fatal encephalitis in 100% of infected mice. Decreasing the viral inoculum by 5 logs prevented encephalitis but also largely prevented the establishment of latent infection. Adding the viral DNA polymerase inhibitor acyclovir to the drinking water at 40 h postinoculation prevented fatal encephalitis without preventing ganglionic infection. In mice infected with HSV-2 and treated with acyclovir in this manner, 42% of LAT-expressing TG neurons at 21 days postinoculation were KH10 positive and only 4% were A5 positive (Table 2 and Fig. 2). These data imply that HSV-2 latent infection of the TG is preferentially established in KH10-positive neurons, with a pattern of infection significantly different from that observed with HSV-1 (P < 0.000001).
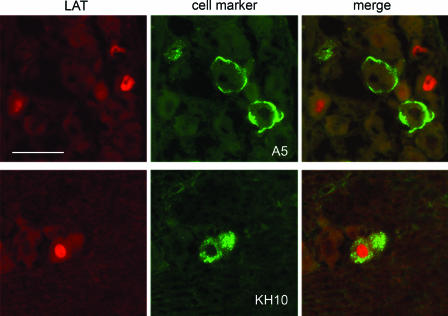
FIG. 2.
Identification of primary sensory neurons latently infected with HSV-2. Sections of infected murine trigeminal ganglia were assayed by both in situ hybridization for HSV-2 LAT (red-labeled nuclei) and immunofluorescent staining with MAbs A5 and KH10 (green-labeled cell bodies) in order to identify the neuronal subpopulations latently infected with HSV-2. In these representative pairs of images, HSV-2 LAT expression is present in KH10-positive neurons but not in A5-positive neurons. Bar = 100 μm.
To determine whether the two different patterns of latent infection were attributable to our use of acyclovir during HSV-2 infection, we examined the effect of acyclovir on the distribution of HSV-1 latent infection. As summarized in Table 2, the treatment of mice with acyclovir during acute ganglionic infection reduced the proportion of HSV-1 LAT-expressing neurons that were A5 positive (to 25%) and increased the proportion that were KH10 positive (to 12%), in effect shifting the distribution of LAT-expressing neurons closer to the representation of A5- and KH10-positive neurons in uninfected ganglia and to the distribution of productive HSV-1 infection among these two neuronal populations. However, the pattern of HSV-1 LAT expression in the TG of acyclovir-treated mice was still significantly different from that observed with HSV-2 (P < 0.000001), and from the distribution of neurons in the uninfected ganglion. Thus, treatment with acyclovir blunts but does not eliminate the preferential establishment of HSV-1 latency in A5-positive neurons.
The effect of acyclovir administration on the neuronal distribution of latent HSV-1 infection argues strongly that the tropism of HSV-1 latent infection for A5-positive neurons is dependent upon viral replication and is not simply an artifact of differential accumulation of LAT in A5- and KH10-positive neurons. However, to examine this issue more closely we performed two experiments specifically addressing the question of whether the differential distribution of HSV-1 and HSV-2 in A5- and KH10-positive neurons in latently infected ganglia was due to differential accumulation of LAT in these cells.
First, we carried out digital analysis of confocal microscopic images of latently infected ganglionic tissue probed by immunofluorescence and FISH. As seen in Fig. 3, we found no significant difference in LAT signal intensity (HSV-1 or HSV-2) among A5- and KH10-positive neurons. Although there are limits to this type of data acquisition and analysis, this observation is consistent with the hypothesis that A5- and KH10-positive neurons are equally permissive for the accumulation of HSV-1 and HSV-2 LAT.
FIG. 3.

Cumulative distribution of LAT in situ hybridization signal intensity in latently infected neurons. Each point represents LAT signal intensity in either A5-positive (red circles) or KH10-positive (blue triangles) trigeminal ganglion neurons 21 days after ocular inoculation with either HSV-1 (A) or HSV-2 (B). Following infection with either HSV-1 or HSV-2 there were no significant differences in LAT signal strength in A5- and KH10-positive neurons. This was true whether the data was analyzed by a two-tailed Student's t test (for HSV-1, P was 0.6; for HSV-2, P was 0.28) or by the Kolmogorov-Smirnov test for equality of distribution (for HSV-1, P was 0.98; for HSV-2, P was 0.19).
Second, we examined the trigeminal ganglia of transgenic mice that contain the HSV-1 LAT-expressing region (8). In these mice, 7.7% (79/1,025) of the LAT-expressing neurons were A5 positive and 15.4% (154/1,000) were KH10 positive, which was very similar to the proportion of all ganglionic neurons that were A5 (8.4%; 86/1,026) and KH10 positive (14.9%; 150/1,007) in these tissues. Thus, these data are also consistent with the hypothesis that A5- and KH10-positive neurons are equally permissive for the accumulation of HSV-1 and HSV-2 LAT.
To determine whether different patterns of HSV-1 and HSV-2 latent infection were unique to the TG, we studied the distribution of latent HSV infection in murine DRG following footpad inoculation. As summarized in Table 2, the results were very similar to those observed with the TG. Following footpad inoculation, HSV-1 preferentially established latent infection in A5-positive DRG neurons, whereas HSV-2 preferentially established latent infection in KH10-positive DRG neurons. These differences were statistically significant (P < 0.000001). We also noted that KH10-positive neurons are more prevalent in DRG than in TG (26 versus 16%, respectively; P < 0.000001).
We hypothesized that differences in the genotypes and phenotypes of LATs expressed by HSV-1 and HSV-2 may be responsible for the different patterns of latent infection with these two viruses. To explore a possible role for the viral LATs in the differential establishment of HSV-1 and HSV-2 latent infection, we next examined the neuronal distribution of latent viral infection following ocular inoculation with HSV-2 333/LAT1. Mice infected with either the viral rescuant (HSV-2 333/LAT1R) or the 17+ strain of HSV-1 (source of the donor LAT) served as controls. As summarized in Table 4 HSV-2 333/LAT1 established latent infection in the TG with an HSV-1 phenotype; 40% of the neurons latently infected with HSV-2 333/LAT1 were A5 positive, while only 2% expressed the KH10 marker. In contrast, HSV-2 333/LAT1R established latent infection with an HSV-2 phenotype, with a preference for KH10-positive neurons. These data strongly suggest that the different patterns of latent infection observed with HSV-1 and HSV-2 are due to a viral function associated with a 2.8-kb portion of the LAT coding region.
TABLE 4.
Distribution of latently infected trigeminal ganglion neurons following ocular inoculationa
| Virus | % of colabeling neurons (no. of colabeling neurons/total no. of neurons)b
|
|
|---|---|---|
| A5+ | KH10+ | |
| HSV-2 333/LAT1 | 40 (237/589) | 2 (13/685) |
| HSV-2 333/LAT1R | 7 (29/411) | 44 (109/249) |
| HSV-1 (17+) | 54 (80/147) | 4 (10/164) |
All mice were treated with acyclovir starting at 40 h postinoculation. Each data point for the recombinant viruses reflects analysis of step sections taken through two blocks of infected tissue (20 ganglia). Each data point for the HSV-1 (17+) reflects analysis of step sections taken through one block of infected tissue (10 ganglia).
Expressed as percentage of neurons with LAT expression (21 days postinoculation) that colabel with MAb A5 (A5+) or KH10 (KH10+).
DISCUSSION
Primary ganglionic infection with HSV-1 is characterized by two very different patterns of viral gene expression. In some neurons, there is abundant expression of lytic cycle genes, resulting in the production of infectious virus, whereas in other neurons, a latent infection is established (14, 19, 28, 33). This pattern is not random; primary sensory neurons identified by MAb KH10 are very permissive for productive infection with HSV-1, whereas neurons identified by MAb A5 serve as the principal reservoir of latent infection (34). In the current study, we demonstrate that HSV-2 follows a very different pattern of ganglionic infection, with KH10-positive neurons serving as the principal reservoir of latent infection in both the TG and lumbar-sacral DRG. These data suggest that HSV-1 and HSV-2 take advantage of two different biological niches within the peripheral nervous system: niches that go beyond the simple boundaries of “above versus below” the waist.
It is almost certain that the different lactoseries glycoconjugates recognized by MAbs A5 and KH10 (4, 6, 12) have little to do with the differences in permissiveness of the neurons recognized by these markers. It is more likely that the expression of these markers simply correlates with differences in cellular protein expression that enable different neuronal populations to differentially support or suppress the expression of key viral genes, thus leading to different outcomes of infection. As suggested by the immunostaining patterns in Table 1, A5- and KH10-positive neurons largely represent two different classes of intensively studied nociceptors, small-diameter BSL-IB4-positive and BSL-IB4-negative ganglionic neurons; neurons with differential responsiveness to nerve growth factor and glial cell line-derived neurotrophic factor (20, 27). It is therefore tempting to speculate that neurotrophic factor-responsive signaling pathways may play a role in regulating the establishment and maintenance of HSV latent infection. These considerations highlight the importance of studying the interaction of HSV with different sensory neuronal subtypes, and not just ganglia as whole, in understanding viral latency and reactivation.
The ability of acyclovir to reduce the tropism of HSV-1 latent infection for A5-positive neurons implies that the preferential establishment of latency in these cells is dependent upon viral replication. One possibility is that type-specific sequences enhance viral spread to A5-positive neurons during establishment of latency and that acyclovir inhibits this spread. Alternatively, by inhibiting viral DNA replication, acyclovir might tip the course of infection in replication-permissive neurons toward latency, thus reducing the relative latent viral load in A5-positive neurons.
The HSV LAT is the only available reliable marker of latently infected neurons. While neurons may contain viral DNA as detected by PCR-based techniques, it is unclear whether viral DNA detected in this manner represents reactivation-competent latent virus or simply fragments of the viral genome. While it is not known whether viral reactivation is restricted to LAT-producing neurons, animal data showing the altered recurrence phenotypes of viruses with impaired capacity to produce LAT supports the conclusion that LAT production is a biologically relevant marker of viral latency (10, 13, 15, 17, 22, 32).
The different distributions of latent HSV-1 and HSV-2 in murine sensory ganglia cannot simply be a consequence of differential LAT accumulation in A5-positive and KH10-positive neurons. First, we found no significant difference in HSV-1 or HSV-2 LAT signal intensity among A5- and KH10-positive neurons. Second, studies of HSV-1 LAT transgene expression in murine trigeminal ganglia revealed no evidence of differential LAT accumulation in A5- and KH10-positive neurons. Third, the treatment of mice with acyclovir, a drug that inhibits productive viral infection but should not affect the accumulation of LAT, shifted the distribution of LAT-positive, latently infected neurons closer to the distribution of A5- and KH10-positive neurons in uninfected ganglia. Fourth, we previously demonstrated that the ratio of LAT-expressing A5-positive and KH10-positive neurons was independent of the sensitivity of the assay used to measure LAT (34), an observation that would not be expected if there were different levels of LAT expression in these neuronal populations. And fifth, following ocular inoculation with a TK deletion virus where latency is the only possible outcome of infection, the distribution of viral latency in A5-positive and KH10-positive neurons, as assayed by LAT expression, closely matches the relative distribution of these neurons in the TG; a result that demonstrates similar accumulation of LAT in A5- and KH10-positive neurons if the virus has equal opportunity to establish latency in these two neuronal populations (Y. Imai et al., personal communication).
In the course of these studies, we found that a chimeric HSV-2 mutant that expresses the HSV-1 LAT (HSV-2 333/LAT1) establishes latency primarily in A5-positive neurons, similar to HSV-1. This indicates that the difference in the latency phenotype of the two viruses can be mapped to a small portion of the long repeat region of the viral genome, the same region that codes for the stable LAT intron and which conveys the phenotype of virus type-associated, site-specific recurrence (35). This viral function might be associated with any or all of the substituted sequences, including the LAT promoter, the primary 5′ end sequences or the sequences coding for the LAT intron. It might also be associated with sequences coding in an antisense direction relative to LAT, such as those recently described for AL (24) or with the overlapping 3′ end of the ICP0 coding region, although the close homology of the HSV-1 and HSV-2 ICP0 3′ amino acid sequences makes this unlikely.
Our findings are particularly interesting in light of our previous report of KOS 62, an HSV-1 LAT deletion virus that expresses the lacZ gene under control of the HSV-1 LAT promoter. Although containing a 1.6-kb LAT region deletion, this virus preferentially established latency (as assayed by β-galactosidase expression) in A5 neurons, similar to that with the wild-type virus. Together with the current findings, this suggests that critical sequences for neuron subtype-specific establishment of latency may reside in regions not shared by the 1.6-kb deletion in KOS 62 and the 2.8-kb substitution in HSV-2 333/LAT1. Thus, sequences in the HSV-1 LAT promoter, the LAT 5′ exon, or in the 3′ half of the LAT intron seem likely to be important for this phenotype. It also is possible that these findings are influenced by other differences between the HSV-1 backbone of KOS 62 and the HSV-2 backbone of HSV-2 333/LAT1.
Implication of the LAT region of the viral genome in neuronal type-specific establishment of latency is intriguing since it appears to play an important role in both the establishment (25, 31) and reactivation of latent infection (13), and more recent studies suggest that LAT influences neuronal survival, possibly via an effect on apoptosis (1, 3, 7, 23). None of the leading hypotheses of LAT function serve to explain the type-specific role of LAT sequences either in viral reactivation phenotype or in establishment of latency. Thus, further investigation into the mechanism of LAT action is clearly warranted. Our findings are consistent with a potential role for LAT sequences in selectively promoting latent infection in specific types of sensory neurons via an as-yet-undefined mechanism.
The ability of LAT region sequences to direct latent infection to specific sensory neuronal subtypes and the corresponding differences in the abilities of each virus to recur from different anatomical locations (16) imply that HSV derives an advantage in recurrence frequency from establishing latency in different neurons in each anatomical location, A5-positive neurons in trigeminal ganglia and KH10-positive neurons in lumbar-sacral ganglia. The increased prevalence of KH10-positive neurons in lumbar-sacral dorsal root ganglia suggests one mechanism by which this phenotype could influence type-specific recurrence patterns.
Acknowledgments
We thank Kathleen Apakupakul for expert technical assistance and Anita Edgecombe for assistance in the preparing the manuscript. We also thank David Bloom for the trigeminal ganglia from his LAT 3098 transgenic mice.
This research was supported by National Institutes of Health grants EY10008 and EY02162, a grant from That Man May See (San Francisco, CA), a Senior Scientific Investigator Award from Research to Prevent Blindness (New York, NY), and the Littlefield Foundation (El Sobrante, CA).
Footnotes
Published ahead of print on 6 December 2006.
REFERENCES
- 1.Ahmed, M., M. Lock, C. G. Miller, and N. W. Fraser. 2002. Regions of the herpes simplex virus type 1 latency-associated transcript that protect cells from apoptosis in vitro and protect neuronal cells in vivo. J. Virol. 76:717-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett, D. L., G. J. Michael, N. Ramachandran, J. B. Munson, S. Averill, Q. Yan, S. B. McMahon, and J. V. Priestly. 1998. A distinct subgroup of small DRG cells express GDNF receptor components and GDNF is protective for these neurons after nerve injury. J. Neurosci. 18:3059-3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bloom, D. C. 2004. HSV LAT and neuronal survival. Int. Rev. Immunol. 23:187-198. [DOI] [PubMed] [Google Scholar]
- 4.Dodd, J., and T. M. Jessell. 1985. Lactoseries carbohydrates specify subsets of dorsal root ganglion neurons projecting to the superficial dorsal horn of rat spinal cord. J. Neurosci. 5:3278-3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farrell, M. J., A. T. Dobson, and L. T. Feldman. 1991. Herpes simplex virus latency-associated transcript is a stable intron. Proc. Natl. Acad. Sci. USA 88:790-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fenderson, B. A., A. C. Hahnel, and E. M. Eddy. 1983. Immunohistochemical localization of two monoclonal antibody-defined carbohydrate antigens during early murine embryogenesis. Dev. Biol. 100:318-327. [DOI] [PubMed] [Google Scholar]
- 7.Gupta, A., J. J. Gartner, P. Sethupathy, A. G. Hatzigeorgiou, and N. W. Fraser. 2006. Anti-apoptotic function of a microRNA encoded by the HSV-1 latency-associated transcript. Nature 442:82-85. [DOI] [PubMed] [Google Scholar]
- 8.Gussow, A. M., N. V. Giordani, R. K. Tran, Y. Imai, D. L. Kwiatkowski, G. F. Rall, T. P. Margolis, and D. C. Bloom. 2006. Tissue-specific splicing of the herpes simplex virus type 1 latency-associated transcript (LAT) intron in LAT transgenic mice. J. Virol. 81:9414-9423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill, J., A. Patel, P. Bhattacharjee, and P. Krause. 2003. An HSV-1 chimeric containing HSV-2 latency associated transcript (LAT) sequences has significantly reduced adrenergic reactivation in the rabbit eye model. Curr. Eye Res. 26:219-224. [DOI] [PubMed] [Google Scholar]
- 10.Hill, J. M., F. Sedarati, R. T. Javier, E. K. Wagner, and J. G. Stevens. 1990. Herpes simplex virus latent phase transcription facilitates in vivo reactivation. Virology 174:117-125. [DOI] [PubMed] [Google Scholar]
- 11.Javier, R. T., J. G. Stevens, V. B. Dissette, and E. K. Wagner. 1988. A herpes simplex virus transcript abundant in latently infected neurons is dispensable for establishment of the latent state. Virology 166:254-257. [DOI] [PubMed] [Google Scholar]
- 12.Jessell, T. M., and J. Dodd. 1985. Structure and expression of differentiation antigens on functional subclasses of primary sensory neurons. Philos. Trans. R. Soc. Lond. B 308:271-281. [DOI] [PubMed] [Google Scholar]
- 13.Kent, J. R., W. Kang, C. Miller, and N. W. Fraser. 2003. Herpes simplex virus latency-associated transcript gene function. J. Neurovirol. 9:285-290. [DOI] [PubMed] [Google Scholar]
- 14.Kosz-Vnenchak, M., D. M. Coen, and D. M. Knipe. 1990. Restricted expression of herpes simplex virus lytic genes during establishment of latent infection by thymidine kinase-negative mutant viruses. J. Virol. 64:5396-5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krause, P. R., L. R. Stanberry, N. Bourne, B. Connelly, J. F. Kurawadwala, A. Patel, and S. E. Straus. 1995. Expression of the herpes simplex virus type 2 latency associated transcript enhances spontaneous reactivation of genital herpes in latently infected guinea pigs. J. Exp. Med. 181:297-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lafferty, W. E., R. W. Coombs, J. Benedetti, C. Critchlow, and L. Corey. 1987. Recurrences after oral and genital herpes simplex virus infection. Influence of site of infection and viral type. N. Engl. J. Med. 316:1444-1449. [DOI] [PubMed] [Google Scholar]
- 17.Leib, D. A., C. L. Bogard, M. Kosz-Vnenchak, K. A. Hicks, D. M. Coen, D. M. Knipe, and P. A. Shaffer. 1989. A deletion mutant of the latency-associated transcript of herpes simplex virus type 1 reactivates from the latent state with reduced frequency. J. Virol. 63:2893-2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Margolis, T. P., C. R. Dawson, and J. H. LaVail. 1992. Herpes simplex viral infection of the mouse trigeminal ganglion. Immunohistochemical analysis of cell populations. Investig. Ophthalmol. Vis. Sci. 33:259-267. [PubMed] [Google Scholar]
- 19.Margolis, T. P., F. Sedarati, A. T. Dobson, L. T. Feldman, and J. G. Stevens. 1992. Pathways of viral gene expression during acute neuronal infection with HSV-1. Virology 189:150-160. [DOI] [PubMed] [Google Scholar]
- 20.Molliver, D. C., D. E. Wright, M. L. Leitner, M. S. Parsadanian, K. Doster, D. Wen, Q. Yan, and W. D. Snider. 1997. IB4-binding DRG neurons switch from NGF to GDNF dependence in early postnatal life. Neuron 19:849-861. [DOI] [PubMed] [Google Scholar]
- 21.Peng, W., G. Henderson, M. Inman, L. BenMohamed, G. C. Perng, S. W. Wechsler, and C. Jones. 2005. The locus encompassing the latency-associated transcript of herpes simplex virus type 1 interferes with and delays interferon expression in productively infected neuroblastoma cells and trigeminal ganglia of acutely infected mice. J. Virol. 79:6162-6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perng, G. C., E. C. Dunkel, P. A. Geary, S. M. Slaina, H. Ghiashi, R. Kaiwar, A. B. Nesburn, and S. L. Wechsler. 1994. The latency-associated transcript gene of herpes simplex virus type 1 (HSV-1) is required for efficient in vivo spontaneous reactivation of HSV-1 from latency. J. Virol. 68:8045-8055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perng, G. C., C. J. Jones, M. Ciacci-Zanella, G. Stone, G. Henderson, A. Yukht, S. M. Slanina, F. M. Hofman, H. Ghiashi, A. B. Nesburn, and S. L. Wechsler. 2000. Virus-induced neuronal apoptosis blocked by the herpes simplex virus latency-associated transcript. Science 287:1500-1503. [DOI] [PubMed] [Google Scholar]
- 24.Perng, G. C., B. Maguen, L. Jin, J. Mott, K. R. Kurylo, L. BenMohammed, A. Yukht, N. Osario, A. B. Nesburn, G. Henderson, M. Inman, C. Jones, and S. L. Wechsler. 2002. A novel herpes simplex virus type 1 transcript (AL-RNA) antisense to the 5′ end of the latency-associated transcript produces a protein in infected rabbits. J. Virol. 76:8003-8010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sawtell, N. M. 1997. Comprehensive quantification of herpes simplex virus latency at the single-cell level. J. Virol. 71:5423-5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silverman, J. D., and L. Kruger. 1990. Selective neuronal glycoconjugate expression in sensory and autonomic ganglia: relation of lectin reactivity to peptide and enzyme markers. J. Neurocytol. 19:789-801. [DOI] [PubMed] [Google Scholar]
- 27.Snider, W. D., and S. B. McMahon. 1998. Tackling pain at the source: new ideas about nociceptors. Neuron 20:629-632. [DOI] [PubMed] [Google Scholar]
- 28.Speck, P. G., and A. Simmons. 1991. Divergent molecular pathways of productive and latent infection with a virulent strain of herpes simplex virus type 1. J. Virol. 65:4001-4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stevens, J. G., E. K. Wagner, G. B. Devi-Rao, M. L. Cook, and L. T. Feldman. 1987. RNA complementary to a herpesvirus alpha gene mRNA is prominent in latently infected neurons. Science 235:1056-1059. [DOI] [PubMed] [Google Scholar]
- 30.Stucky, C. L., and G. R. Lewin. 1999. Isolectin B4-positive and -negative nociceptors are functionally distinct. J. Neurosci. 19:6497-6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson, R. L., and N. M. Sawtell. 1997. The herpes simplex virus type 1 latency-associated transcript gene regulates the establishment of latency. J. Virol. 71:5432-5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trousdale, M. D., I. Steiner, J. G. Spivack, S. L. Deshmane, S. M. Brown, A. R. MacLean, J. H. Subak-Sharpe, and N. W. Fraser. 1991. In vivo and in vitro reactivation impairment of a herpes simplex virus type 1 latency-associated transcript variant in a rabbit eye model. J. Virol. 65:6989-6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valyi-Nagy, T., S. L. Deshmane, J. G. Spivack, I. Steiner, C. I. Ace, C. M. Preston, and N. W. Fraser. 1991. Investigation of herpes simplex virus type 1 (HSV-1) gene expression and DNA synthesis during the establishment of latent infection by an HSV-1 mutant, in1814, that does not replicate in mouse trigeminal ganglia. J. Gen. Virol. 72:641-649. [DOI] [PubMed] [Google Scholar]
- 34.Yang, L., C. C. Voytek, and T. P. Margolis. 2000. Immunohistochemical analysis of primary sensory neurons latently infected with herpes simplex virus type 1. J. Virol. 74:209-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshikawa, T., J. Hill, L. R. Stanberry, N. Bourne, J. F. Kurawadwala, and P. R. Krause. 1996. The characteristic site-specific reactivation phenotypes of HSV-1 and HSV-2 depend upon the latency-associated transcript region. J. Exp. Med. 184:659-664. [DOI] [PMC free article] [PubMed] [Google Scholar]