Abstract
Biliary atresia is a devastating disorder of the newborn in which afflicted infants develop inflammation and fibrosis of the extrahepatic biliary tract, resulting in cirrhosis and end-stage liver disease. Infection with a virus is thought to be a contributing factor in the etiology of biliary atresia. In the murine model of biliary atresia, perinatal exposure to rhesus rotavirus (RRV) results in biliary epithelial cell infection causing bile duct obstruction. The purpose of this study was to determine if tropism for the biliary epithelial cell was unique to RRV. Newborn mice underwent intraperitoneal injection with five strains of rotavirus: RRV (simian), SA11-FM (simian/bovine), SA11-SM (simian), EDIM (murine), and Wa (human). RRV and SA11-FM caused clinical manifestations of bile duct obstruction and high mortality. SA11-SM caused clinical signs of hepatobiliary injury but the mortality was markedly reduced. EDIM and Wa caused no sign of hepatobiliary disease. The systemic and temporal distribution of viral protein and live virus varied according to the injected strain. Immunohistochemistry revealed that RRV and SA11-FM targeted the biliary epithelial cells. In contrast, SA11-SM was found in the liver but in not in the biliary epithelium. These results indicate that strain-specific characteristics dictate tropism for cells of hepatobiliary origin which in turn impact the ability to induce the murine model of biliary atresia.
Extrahepatic biliary atresia (EHBA) is a unique disease of infancy in which there is inflammation and fibrosis of the extrahepatic biliary tract, leading to obstructive jaundice. Afflicted infants appear healthy at birth but develop cholestatic jaundice in the first few months of life. Upon diagnosis, surgical reconstruction of the extrahepatic biliary tree can restore biliary drainage in some patients; however, most affected children develop biliary cirrhosis and manifestations of end-stage liver disease (2). Consequently, EHBA is the most common indication for liver transplantation in the pediatric population (16). Currently, the 10-year survival for infants who have EHBA is only 70% (1, 5).
Perinatal infection by a virus triggering a host inflammatory response is a proposed mechanism by which EHBA occurs (31). Two types of evidence support a viral role in the pathogenesis of biliary atresia. The first type is patient-based studies in which viruses, including reovirus (11, 19, 20), cytomegalovirus (7, 9), human papillomavirus (8), Epstein-Barr virus (10), and rotavirus (27), were found in the livers of infants with biliary atresia. The second type of evidence is the murine model of inflammatory cholangiopathy in which newborn mice injected with rhesus rotavirus (RRV) develop extrahepatic biliary obstruction and death (28). This model shares many similarities to the disease process found in children affected with EHBA, including a histologic pattern of inflammatory cell infiltrate found in the portal tract associated with bile duct injury, a gross morphological pattern of extrahepatic bile duct destruction (25), and a temporal dependence with respect to the timing of the inoculation and the resulting induction of biliary obstruction (6). Although this evidence suggests a viral role in the etiology of biliary atresia, the mechanism by which a specific virus causes biliary atresia has not been established.
The purpose of the present study was to begin to determine how infection with a virus contributes to the pathogenesis of biliary atresia. We based our studies on the recently reported observation that, in the murine model of biliary atresia, the biliary epithelium is the specific target of perinatal RRV infection (30). To determine if the biliary epithelial tropism was unique to RRV, we performed intraperitoneal injections of newborn mice with simian (i.e., RRV, SA11-FM, and SA11-SM), mouse (i.e., EDIM), and human (i.e., Wa) strains of rotavirus and found differing patterns of temporal and systemic viral spread. In addition to RRV, the two simian strains SA11-FM and SA11-SM were found within the liver after inoculation, but the pattern of hepatobiliary injury varied according to the injected rotavirus strain. These results indicate that within the rotavirus family, viral determinants specific to each strain dictate tropism for cells of hepatobiliary origin, which in turn impact the ability to induce the murine model of biliary atresia.
MATERIALS AND METHODS
Rotavirus strains.
We used five strains of rotavirus in these studies—the simian strains RRV (kindly provided by H. Greenberg, Stanford University, Palo Alto, CA), SA11-fast moving (SA11-FM, as previously described by Gorziglia et al.) (12), SA11-slow moving (SA11-SM, kindly provided by Y. Hoshino, National Institutes of Health, Bethesda, MD), the mouse strain epizootic diarrhea of infant mice (EDIM, kindly provided by M. Collins, Microbiological Associates, Bethesda, MD), and the human strain Wa (kindly provided by R. Wyatt, National Institutes of Health, Bethesda, MD). Confirmation of strains and their gene segment variations was preformed using electropherograms. SA11-FM is a reassortant that contains a VP4 protein gene segment believed to be derived from a bovine rotavirus, while SA11-SM represents the original simian rotavirus strain (12). SA11-FM and SA11-SM are differentiated by the migration rate during electrophoretic profiling of gene segment 4. The five strains of rotavirus were maintained in the monkey kidney epithelial cell line MA104 (ATCC, Manassas, VA). All five strains of virus were plaque purified. Freon was used to concentrate the virus, followed by centrifugation to remove the cellular debris. Fluorescent focus-forming viral titration assays in MA104 cells were used to determine the concentration of each strain (FFU/ml) (14).
Experimental model of biliary atresia.
Breeding pairs of BALB/c mice (Harlan Labs, Indianapolis, IN) were kept in microisolator cages in a virus-free environment. They had free access to sterilized chow and water. The mice were bred, and pregnant female mice were separated approximately 1 week before their expected delivery. Only litters of greater than 4 pups were used. Intraperitoneal injection of newborn pups with the different strains of rotavirus at a dose of 1.5 × 106 FFU per mouse (antigen concentrations per strain: RRV, 1,150.7 ± 23.7 ng/ml; SA11-FM, 1,187.7 ± 36.4 ng/ml; SA11-SM, 1,266.4 ± 90.2 ng/ml; EDIM, 708.8 ± 9.7 ng/ml; WA, 963.6 ± 63.1 ng/ml) was performed within 24 h of birth. Newborn pups injected with normal saline served as controls. Injected pups were monitored for 21 days. Weight gain, clinical signs of hepatobiliary injury (i.e., jaundice in non-fur-covered skin, acholic stools, and bilirubinuria), and survival were recorded. The presence of bilirubin in the urine was detected quantitatively using commercially available urine dipsticks (Bayer Co., Elkhart, IN).
A subset of BALB/c pups injected with the different strains of rotavirus was sacrificed at 2, 5, 7, 10, or 14 days postinoculation. The gross morphology of the liver and the hepatobiliary system was documented. The patency of the extrahepatic biliary tract was determined by cholangiography in which methylene blue was injected into the gallbladder of mice sacrificed at day 14 postinoculation. Samples of the liver, bowel, spleen, kidney, and brain were collected. The tissues were weighed (wet weight), homogenized in Earle's balance salt solution, and frozen in liquid nitrogen. Samples were stored at −80°C until analyzed.
A subset of injected mice had a portion of the liver and the extrahepatic biliary tract harvested and preserved in formalin. Serial sections underwent hematoxylin and eosin or Masson's trichrome staining. For the extrahepatic biliary tract, a scoring system designed to assess the degree of injury was developed. Longitudinal sections were graded according to location and severity of injury. Evaluations included intraepithelial inflammation, epithelial damage, edema, submucosal inflammation, and stromal proliferation. An ordinal scoring system was employed which was scaled from 0 to 4: 0 indicated an absence of any injury or change, 2 indicated moderate injury, and 4 indicated severe injury or complete loss of structure. Bile ducts harvested from mice in each experimental group were assessed, and a total injury score representing a culmination of all five categories was generated. The maximum total injury score possible was 20. Other mice had a portion of liver and the extrahepatic biliary tract harvested and snap-frozen in liquid nitrogen to allow for immunohistochemical studies.
Quantification of rotavirus protein and live infectious virus.
The quantity of rotavirus protein in the tissue suspensions was determined by a sandwich enzyme-linked immunosorbent assay (ELISA) (18). A specimen was considered positive for rotavirus if the average absorbance of the positive wells was ≥2 times that of the negative wells and ≥0.15 (18). A standard curve using purified virus was generated, and the amount of rotavirus protein was quantified according to that curve and expressed as nanograms per milligram (wet weight) of tissue.
Tissue samples were analyzed for the presence of infectious rotavirus by fluorescent focus-forming viral titration assays (17). Briefly, 96-well plates were seeded with MA104 cells 4 days prior to use. Once confluent, the MA104 cells were exposed to serially diluted samples for 1.5 h. Wells were washed and covered with Dulbecco's modified Eagle's medium containing 4 μg of trypsin/ml and incubated at 37°C for 14 to 16 h. Diluent was aspirated, and cold 80% acetone was added and incubated for 15 min at 20°C. The acetone was aspirated and the wells dried. Guinea pig anti-rotavirus immunoglobulin G (IgG) primary antibody diluted 1:1,000 was added and incubated for 30 min. Wells were aspirated and washed with phosphate-buffered saline (PBS). Fluorescein isothiocyanate (FITC)-tagged goat anti-guinea pig IgG secondary antibody diluted 1:500 was added for 30 min at 37°C. Wells were washed twice and allowed to dry completely. Plates were scored using a UV microscope (10× objective), and quantities of infectious virus were reported as FFU per milligram (wet weight) of tissue.
Immunohistochemical analysis for the presence of rotavirus.
Tissue harvested from mice at the various time points described above was embedded in Histo Prep (Fisher Scientific, Pittsburgh, PA) over dry ice. Samples were stored at −80°C until they were sectioned for histological analysis. Frozen samples were cut in 7-μm sections and placed on polylysine microscope slides (Erie Scientific Co., Portsmouth, NH) that were fixed in cold acetone and air dried. For single-staining immunohistochemistry, sections were rehydrated in PBS, and endogenous peroxidase activity was blocked with 0.8% hydrogen peroxide in methanol. Slides were incubated with guinea pig anti-rotavirus IgG primary antibody diluted 1:500 in PBS for 1 h and then washed and incubated with horseradish peroxidase-conjugated goat anti-guinea pig IgG (PK-4007; Vector Laboratories) secondary antibody diluted 1:200 for 1 h. Sections were rinsed in PBS, covered with ABC solution, followed by 3,3′-diaminobenzidine substrate (Vector Laboratories). Hematoxylin was used to counterstain slides, followed by rinses in graded ethanol and xylene solutions.
For dual-staining immunohistochemistry, sections were rehydrated and blocked with 2% normal goat serum and mouse-on-mouse (MOM) blocking reagent (Vector Laboratories) for 60 min at room temperature. Slides were washed in PBS and then incubated in working solution of MOM diluent for 5 min, followed by incubation with cytokeratin-7 (CK-7) primary antibody (BD Biosciences, San Diego, CA) diluted 1:100 in MOM for 30 min. The slides were washed and incubated with guinea pig anti-rotavirus IgG primary antibody diluted 1:1,000 for 60 min. FITC-conjugated goat anti-mouse IgG secondary antibody diluted 1:400 and Texas Red-conjugated goat anti-guinea pig IgG secondary antibody diluted 1:100 were added and incubated for 30 min. Sections were rinsed, and coverslips were applied with anti-quench mounting medium (Ted Pella, Inc., Citifluor Ltd., London, United Kingdom).
Statistical analysis.
Results of morbidity and mortality from rotavirus inoculation were based on at least 10 pups per experimental group. Findings were expressed as percentages of pups expressing symptoms (at least two findings) and percent survival. Analysis of these noncontinuous variables was done using chi-square and Fisher exact testing. Each subset utilized for the ELISA and FFU assays consisted of at least 10 pups. Results of these continuous variables were expressed as means ± standard errors of the means and analyzed using analysis of variance, with post hoc testing where appropriate. Biliary injury, in each category, was represented as a median score followed by its range. Comparisons were made using analysis of variance on ranks with Dunn's method for post hoc testing. A P value of less than 0.05 was considered significant.
RESULTS
Effect of rotavirus strain on induction of the murine model of biliary atresia.
Pups injected with RRV developed the clinical signs of biliary obstruction—jaundice, bilirubinuria, and acholic stools (Fig. 1A). Additionally, they experienced significant growth retardation (Fig. 1B). The greatest difference in weight between RRV-injected pups versus saline controls was noted at day 14 postinoculation (mean weight of RRV injected pups [5.8 ± 0.3 g, n = 21] versus that of saline-injected controls [9.1 ± 0.8 g, n = 6], P < 0.05). Mortality was 81.8% (Fig. 1C) for pups injected with RRV. These findings were consistent with previous reports in which the murine model of biliary atresia was established (24-26, 28).
FIG. 1.
Clinical results of inoculation of BALB/c pups with five strains of rotavirus. Newborn BALB/c pups were inoculated intraperitoneally with 1.5 × 106 FFU of the different strains of rotavirus on day 1 of life and monitored for 21 days. (A) Percentage of mice that developed clinical symptoms of hepatobiliary injury; (B) average daily weight of pups injected with the different strains of rotavirus; (C) survival of mice infected with the different strains of rotavirus. The mortality rate of pups inoculated with SA11-SM was 21%, which was significantly higher than that of mice injected with saline, EDIM, or Wa (*, P < 0.05). The mortality rates of mice injected with RRV and SA11-FM were 82% and 90%, respectively, which was significantly higher than that of mice injected with saline, EDIM, Wa, or SA 11-SM (+, P < 0.05).
To determine whether the strain of rotavirus impacted the ability to induce the murine model of biliary atresia, we determined the effect of inoculation with four additional rotavirus strains including SA11-FM, SA11-SM, EDIM, and Wa. Pups injected with SA11-FM developed clinical manifestations of biliary obstruction, including acholic stools, jaundice, and bilirubinuria (Fig. 1A). There was significant growth retardation (Fig. 1B) (SA11-FM mean weight [4.2 ± 0.5 g, n = 15] versus that of saline controls, P < 0.05), and the mortality rate, 91%, was similar to that of those pups injected with RRV (Fig. 1C).
While some pups injected with SA11-SM developed clinical manifestations of liver injury, the percentage affected was lower than that of pups injected with RRV or SA11-FM (Fig. 1A). These pups experienced significant growth retardation (Fig. 1B) (SA11-SM mean weight [6.6 ± 1.3 g, n = 23] versus that of saline controls, P < 0.05). The mortality rate of pups injected with SA11-SM was 21%, which was significantly lower than that of mice injected with RRV and SA11-FM (P < 0.05) (Fig. 1C). A dose-response study was performed by injecting newborn pups with increasing amounts of SA11-SM. Despite increasing the infecting dose of SA11-SM up to 100-fold, the percentage of mice that developed hepatobiliary injury remained the same (data not shown).
Newborn pups injected with the EDIM and Wa showed no signs of hepatobiliary injury (Fig. 1A). Almost all of the pups injected with EDIM developed diarrhea. These symptoms were associated with growth retardation, most clearly seen at day 14 (Fig. 1B) (EDIM mean weight [6.4 ± 1.7 g, n = 16] versus that of saline controls, P < 0.05). The mortality rate was 0% (Fig. 1C). A dose-response study was performed by injecting newborn pups with increasing amounts of EDIM. Despite increasing the dose 20-fold, mice injected with EDIM did not develop any clinical signs of hepatobiliary injury (data not shown). Most of the mice developed diarrhea and growth retardation (high-dose EDIM mean weight [7.8 ± 0.15 g, n = 13] versus that of saline controls, P < 0.05), and all mice survived. A few pups injected with Wa had diarrhea, and these pups also experienced growth retardation (Fig. 1B) (Wa mean weight [7.4 ± 0.6 g, n = 23] versus that of saline controls, P < 0.05). The mortality rate in this experimental group was 0%. No mice inoculated with RRV, SA11-FM, and SA11-SM developed diarrhea.
Systemic and temporal distribution of rotavirus protein and live virus following inoculation. (i) Viral protein.
Inoculation with the different strains of rotavirus caused marked differences in the subsequent clinical course and outcome. To examine the systemic and temporal distribution of rotavirus following intraperitoneal inoculation with the different strains of rotavirus, we performed ELISA on tissue extracts to detect the presence of the rotavirus protein. In mice injected with RRV, rotavirus protein could be detected in the liver within 2 days of inoculation (Table 1 ). The peak amount was detected at day 10, indicating that there was accumulation of rotavirus protein within the liver over time. Rotavirus protein was also found in the intestine, spleen, kidney, and brain but at levels significantly lower that that detected within the liver (Table 1). The distribution of viral protein among the tissues tested suggested that RRV had tropism for the hepatobiliary system, which was consistent with its ability to cause the model of biliary atresia.
TABLE 1.
Rotavirus proteins in tissue extracts from mice inoculated with the five different strainsa
| Organ | Day postinjection | Amtb of rotavirus protein from strain:
|
||||
|---|---|---|---|---|---|---|
| RRV | SA11-FM | SA11-SM | EDIM | Wa | ||
| Liver | 2 | 1.48 ± 0.43 | 3.31 ± 0.14 | 0.86 ± 0.39 | 2.14 ± 0.64 | 6.17 ± 1.25 |
| 5 | 7.72 ± 2.04 | 2.16 ± 0.94 | — | 5.00 ± 1.32 | 0.45 ± 0.20 | |
| 7 | 4.47 ± 0.46 | 6.53 ± 0.93 | 5.84 ± 1.02 | 6.76 ± 1.86 | — | |
| 10 | 56.04 ± 16.55 | 4.31 ± 0.71 | 0.47 ± 0.20 | 0.82 ± 0.29 | 0.02 ± 0.02 | |
| 14 | 0.64 ± 0.11 | 1.55 ± 0.89 | 0.21 ± 0.13 | — | — | |
| Intestine | 2 | 1.19 ± 0.08 | 1.66 ± 0.09 | 0.11 ± 0.11 | 1.10 ± 0.16 | 4.15 ± 0.78 |
| 5 | 1.00 ± 0.38 | 0.14 ± 0.06 | 1.35 ± 0.83 | 3.94 ± 0.68 | 0.22 ± 0.08 | |
| 7 | 1.09 ± 0.20 | 0.30 ± 0.11 | 0.04 ± 0.02 | 3.59 ± 1.13 | 0.08 ± 0.08 | |
| 10 | 0.16 ± 0.04 | 0.30 ± 0.20 | — | 10.59 ± 2.98 | — | |
| 14 | —c | 0.03 ± 0.03 | — | 0.52 ± 0.15 | — | |
| Spleen | 2 | — | — | — | 13.39 ± 2.46 | 14.57 ± 6.72 |
| 5 | 1.88 ± 0.95 | 0.53 ± 0.33 | 0.36 ± 0.36 | 3.58 ± 0.56 | 0.81 ± 0.54 | |
| 7 | 1.93 ± 0.42 | 1.96 ± 0.92 | — | 9.18 ± 2.19 | — | |
| 10 | 3.93 ± 0.98 | — | — | — | — | |
| 14 | — | — | — | — | — | |
| Kidney | 2 | — | 0.08 ± 0.12 | — | 11.22 ± 2.41 | 7.83 ± 2.59 |
| 5 | 0.24 ± 0.12 | 0.25 ± 0.25 | 0.34 ± 0.22 | 1.18 ± 0.18 | 0.50 ± 0.31 | |
| 7 | 0.97 ± 0.20 | 0.20 ± 0.06 | — | 6.17 ± 2.52 | — | |
| 10 | 1.13 ± 0.27 | 0.08 ± 0.08 | — | 0.53 ± 0.26 | — | |
| 14 | — | — | — | — | — | |
| Brain | 2 | — | — | — | 2.41 ± 0.46 | — |
| 5 | — | — | — | 0.01 ± 0.01 | — | |
| 7 | 0.02 ± 0.01 | 0.02 ± 0.01 | — | — | — | |
| 10 | 0.03 ± 0.03 | — | — | — | — | |
| 14 | — | — | — | — | — | |
Groups of ≥10 pups per time point were injected with 1.5 × 106 FFU of the five strains of rotavirus. Organs were harvested on the day postinfection indicated and homogenized, and the presence of rotavirus protein was determined by ELISA.
Values are expressed as mean ng/mg of tissue weight ± standard errors.
—, value below the limit of detection.
In mice injected with SA11-FM, rotavirus protein could be detected in the liver within 2 days after inoculation, with levels increasing to the maximum at day 7 (Table 1). The peak amount detected was markedly lower than that found in RRV-injected mice. Similar to RRV, rotavirus protein could be detected in intestine, spleen, kidney, and brain in mice injected with SA11-FM but at lower levels than found in the liver. In SA11-SM-injected mice, rotavirus protein could be detected in the liver 2 days following inoculation with the maximal amount found at day 7. Rotavirus protein was detected in the intestine at lower amounts, with small amounts found at day 5 in the spleen and kidney. In contrast to mice injected with RRV and SA11-FM, no rotavirus protein was found within the brain in mice injected with SA11-SM. In EDIM-injected mice, large amounts of rotavirus protein were found in the intestines, spleen, and kidney, with moderate levels within the liver and small amounts detected in the brain. Rotavirus protein was detected in the liver despite the fact that no clinical symptoms of hepatobiliary injury were observed. In Wa-injected mice, large amounts were detected in the spleen and kidney, with moderate amounts found within the liver and intestines.
(ii) Live virus.
The detection of viral protein within a tissue reflects the presence of either live virus or processed viral protein. To determine whether there was live virus in the different tissues, viral focus-forming assays were performed on the tissue extracts from the liver and the intestine. Focus-forming assays revealed the presence of RRV, SA11-FM, and SA11-SM within the liver (Table 2). The largest amount of infectious virus was found in the livers of pups injected with RRV. Levels of RRV peaked at 7 days, suggesting that the virus was replicating within the liver after inoculation and then fell to undetectable levels by day 14.
TABLE 2.
Live virus in liver and intestine extracts following inoculation with the five strains of rotavirusa
| Organ | Day postinjection | Amtb of virus of strain:
|
||||
|---|---|---|---|---|---|---|
| RRV | SA11-FM | SA11-SM | EDIM | Wa | ||
| Liver | 2 | 508.3 ± 541.6 | 10,230.5 ± 5,851 | 319.40 ± 217.6 | — | — |
| 5 | 1,504.0 ± 343.9 | 2,976.0 ± 724.6 | 1,786.0 ± 853.9 | — | — | |
| 7 | 41,914.0 ± 54,173.0 | 1,262.4 ± 769.0 | 2,830.0 ± 769.2 | — | — | |
| 10 | 3,678.7 ± 1,938.3 | — | — | — | — | |
| 14 | —c | — | — | — | — | |
| Intestine | 2 | 111.2 ± 77.2 | 4,763.3 ± 6,786.0 | — | 15,611.4 ± 5,995 | — |
| 5 | — | — | — | 718.50 ± 143.3 | — | |
| 7 | — | 124.1 ± 113.2 | — | 3,049.3 ± 2,162.3 | — | |
| 10 | 38.1 ± 19.4 | — | — | 13,469.0 ± 9,452 | — | |
| 14 | — | — | — | 26.3 ± 16.6 | — | |
Groups of ≥10 pups per time point were injected with 1.5 × 106 FFU of the five strains of rotavirus. Organs were harvested on the day postinfection indicated and homogenized, and the amount of virus was determined by focus-forming assay in MA104 cells.
Values are expressed as mean FFU/mg tissue weight ± standard errors.
—, value below the limit of detection.
A different temporal pattern of live virus was observed in pups injected with SA11-FM. The peak amount of virus was detected in the liver at day 2 following inoculation. SA11-FM was still detectable at day 5 and 7 and disappeared by day 10. Although SA11-FM was detectable in the liver, the levels of virus were markedly lower than those found in RRV-injected mice. The temporal distribution pattern of SA11-SM was similar to that found in RRV-injected pups where there was little detected at day 2, the maximum was detected at day 7, and none was detectable by day 10. The maximal amount detected, however, was markedly lower than found in RRV-injected mice. In contrast to RRV, SA11-FM, and SA11-SM, neither live EDIM nor Wa was detectable in the liver at the time points tested. This indicated that the rotavirus protein levels detected by ELISA reflected processed viral antigen. Infectious EDIM was found within the intestine, which is the likely explanation for the development of diarrhea.
Gross morphology and histology of the hepatobiliary system following inoculation with the different strains of rotavirus.
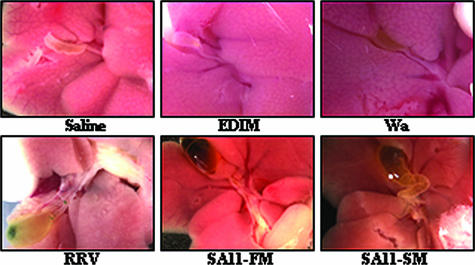
We next examined the effect of viral inoculation on the gross morphology and histologic appearance of the hepatobiliary system. The gross morphology of the liver was similar in mice inoculated with the different strains of rotavirus. In contrast, the gross morphology of the extrahepatic biliary tree following inoculation with the different strains of rotavirus revealed differing patterns of injury. At 14 days following inoculation, the gallbladders of pups injected with RRV and SA11-FM were enlarged and contained thick, green inspissated bile (Fig. 2). In SA11-SM-injected mice, the appearance of the extrahepatic tract varied. In some pups, the gallbladder was enlarged, containing sludge, while others had no extrahepatic biliary abnormalities (Fig. 2). The extrahepatic biliary systems of pups injected with EDIM and Wa were indistinguishable from those of mice injected with saline. Cholangiography in which methylene blue was injected into the gallbladder performed at 14 days following inoculation revealed obstruction of the extrahepatic biliary tract in 85% of RRV-injected mice (23 of 27 mice in which cholangiography was performed) and 89% of SA11-FM injected pups (31 of 35) at day 14 postinoculation (Fig. 3). On occasion, newborn mice injected with SA11-SM had obstructed biliary tracts (5 of 42), but most had patent extrahepatic biliary tracts, even if there was sludge within the gallbladder (Fig. 3). The extrahepatic biliary tract was patent in 100% of newborn mice injected with EDIM and Wa, consistent with the absence of the clinical manifestations of hepatobiliary injury.
FIG. 2.
Gross morphology of the liver and extrahepatic biliary tract. Appearances of livers and gallbladders harvested from mice sacrificed 14 days after inoculation with the five strains of rotavirus or saline are shown. Magnification, ×10. The gallbladders of mice inoculated with RRV and SA11-FM were enlarged and contained thick inspissated bile. The gallbladders of mice injected with SA-11SM were enlarged.
FIG. 3.
Methylene blue cholangiography of the extrahepatic biliary tracts of mice inoculated with the five strains of rotavirus. Methylene blue dye was injected into the gallbladders of mice using a 27-gauge needle 14 days post-viral inoculation. In mice injected with RRV and SA-11FM, there is blockage of the main bile duct (white arrows indicate sites of obstruction). In contrast, dye filled the complete extrahepatic biliary system in mice injected with SA11-SM, EDIM, Wa, or saline.
Histologic evaluation of the liver of newborn mice injected with RRV or SA11-FM revealed an infiltrate of inflammatory cells within the area of the portal tract (Fig. 4). The histology of the livers of SA11-SM-, EDIM-, and Wa-injected mice were similar to that of saline controls. The histology of the extrahepatic biliary tract of injected mice revealed notable findings (Fig. 5). At 7 days after inoculation, there was intraepithelial inflammation, epithelial damage, edema, submucosal inflammation, and stromal proliferation in the biliary tracts harvested from mice injected with RRV, SA11-FM, or SA11-SM, with median total scores of 10 (range, 6 to 16), 7 (range, 5 to 9), and 6 (range, 4 to 8), respectively (Fig. 5). Inflammatory cells were often found within the lumen of the bile duct in mice inoculated with RRV or SA11-FM (Fig. 5). In contrast, the inflammation rarely extended into the lumen of the bile duct in mice injected with SA11-SM. Histologic evaluation of the hepatobiliary tree of pups injected with EDIM and Wa revealed minimal changes, consistent with the findings observed by gross morphology (Fig. 5). The histologic total score of injury was statistically higher in RRV-, SA11-FM-, or SA11-SM-injected mice than in EDIM-, Wa-, and saline-injected animals (Table 3).
FIG. 4.
Histology of the liver following viral inoculation. The histologic appearance of portal triads in liver sections harvested from mice 7 days after inoculation with the five strains of rotavirus or saline is shown. The black arrows indicate intrahepatic bile ductules. In mice inoculated with RRV and SA11-FM, there was infiltration of the periportal area with inflammatory cells (black arrowheads). In contrast, there were minimal inflammatory cells in the region of the portal tract of mice injected with SA11-SM, EDIM, Wa, or saline. Sections were stained with hematoxylin and eosin. Magnification, ×40.
FIG. 5.
Histology of the extrahepatic biliary tract. Longitudinal sections of the extrahepatic biliary ducts were harvested at day 7 following injection. The sections were stained with Masson's trichrome to best delineate the histologic changes that took place following infection. Biliary epithelial cells stained pink. Inflammatory cells stained blue. The black arrows indicate sites of severe biliary epithelial cell injury duct. The black arrowheads indicate periductal inflammatory infiltrate. In mice injected with RRV or SA-11FM, the inflammatory cells extend into the lumen of the bile.
TABLE 3.
Severity of injury as indicated by histologic assessment to the extrahepatic biliary duct following injection with five strains of rotavirusa
| Damage type | Injury scoreb after inoculation with:
|
|||||
|---|---|---|---|---|---|---|
| Rotavirus strain:
|
Saline | |||||
| RRV | SA11-FM | SA11-SM | EDIM | Wa | ||
| Intraepithelial inflammation | 2.0 (1-4) | 1.0 (1-2) | 1.0 (1-2) | 0 (0) | 0.5 (0-1) | 0 (0) |
| Epithelial damage | 2.5 (2-4) | 2.0 (1-2) | 1.0 (0-2) | 0 (0) | 0 (0-1) | 0 (0) |
| Edema | 2.0 (1-3) | 2.0 (1-2) | 1.5 (1-2) | 1.0 (0-1) | 1.0 (0-2) | 0 (0) |
| Submucosal inflammation | 2.0 (1-3) | 2.0 (2-3) | 2.0 (2-2) | 1.0 (0-2) | 1.0 (0-2) | 0 (0) |
| Stromal proliferation | 1.0 (1-2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Total injury score | 10.0 (6-16)*c | 7.0 (5-9)* | 6.0 (4-8)* | 1.0 (1-3) | 2.0 (1-5) | 0 (0) |
Severity of bile duct injury 7 days after inoculation with the five different strains of rotavirus, as determined by histologic appearance of the extrahepatic biliary tract. All sections were graded by a pathologist.
Injury scoring is based on an ordinal scale from 0 to 4. Values are expressed as median scores, with ranges indicated in parentheses. The maximum total injury score possible was 20.
*, P < 0.05 compared with saline controls.
Immunohistochemical analysis for rotavirus following inoculation.
To determine the site of tropism within the hepatobiliary system following inoculation, immunohistochemical analysis using a polyclonal guinea pig anti-rotavirus IgG antibody was performed. Immunohistochemistry of liver samples harvested at day 5 following inoculation with RRV, SA11-FM, or SA11-SM revealed rotavirus in the region of the portal triad (Fig. 6). No immunohistochemical evidence of rotavirus was detected in the livers of mice injected with EDIM or Wa (Fig. 6).
FIG. 6.
Immunohistochemistry for rotavirus in liver sections harvested from mice infected with the different strains of rotavirus. Single-staining immunohistochemistry of liver sections using peroxidase-labeled guinea pig IgG antirotavirus antibody. Rotavirus was detected around the portal triads (white arrows). Sections were counterstained with hematoxylin.
A portal triad consists of a bile duct, portal vein, and hepatic artery. Within the periportal region can be found hepatocytes, biliary epithelial cells, and endothelial cells lining the hepatic artery and the portal vein. To determine the cellular target of viral inoculation within the liver and extrahepatic biliary tree, dual-staining techniques were used. Because CK-7 is found on biliary epithelial cells, we used a mouse anti-CK-7 antibody to determine if the target of rotavirus infection was the biliary epithelium (13, 29). The polyclonal guinea pig anti-rotavirus IgG antibody was used to stain for rotavirus. In RRV-injected mice, colocalization was found beginning at day 5 postinoculation. A temporal analysis of RRV-injected mice showed that colocalization to the biliary epithelium occurred in both the liver (Fig. 7) and the extrahepatic biliary tract (Fig. 8) beginning at day 5 and 7 postinoculation. At 10 days postinoculation, rotavirus remained present; however, the biliary architecture was disturbed and intact bile ducts could not be identified (data not shown). At day 14, neither RRV nor intact biliary epithelium could be identified (data not shown).
FIG. 7.
Rotavirus and cytokeratin 7 immunohistochemistry of liver sections harvested from mice injected with the different strains of rotavirus. Dual-staining immunohistochemistry was performed on liver sections harvested after inoculation with the five strains of rotavirus or saline. The sections were harvested at day 5 following injection in all mice, except those injected with SA-11FM, which were harvested at day 2. The sections underwent indirect immunofluorescence for cytokeratin 7 and rotavirus as described in Materials and Methods. Cytokeratin 7 is found in the biliary epithelial cells. Column 1, FITC-cytokeratin 7 (green); column 2, Texas red-rotavirus (red); column 3, merged image of columns 1 and 2. Colocalization of virus to the biliary epithelium appears as orange staining. Magnification, ×20.
FIG. 8.
Rotavirus and cytokeratin 7 immunohistochemistry of the extrahepatic biliary ducts harvested from mice injected with the different strains of rotavirus. Dual-staining immunohistochemistry was performed on the extrahepatic biliary ducts harvested after inoculation with the five strains of rotavirus. The sections were harvested at day 5 following injection in all mice, except those injected with SA-11FM, which were harvested at day 2. The sections underwent indirect immunofluorescence for cytokeratin 7 and rotavirus as described in Materials and Methods. Cytokeratin 7 is found in the biliary epithelial cells. Column 1, FITC-cytokeratin 7 (green); column 2, Texas Red-rotavirus (red); column 3, merged image column 1 and 2. Colocalization of virus to biliary epithelium appears as orange staining. Magnification, ×20.
SA11-FM colocalized to the biliary epithelium at 2 days postinjection in the liver and the extrahepatic biliary tract (Fig. 7 and 8) and became undetectable by day 5. SA11-SM was detected in the periportal area within the liver but could not be colocalized to the biliary epithelium at any time point tested (Fig. 7). Despite extensive screening, SA11-SM was not detected in the extrahepatic biliary tract (Fig. 8). Neither EDIM nor Wa was identified in the liver or the biliary tract at any time point tested, consistent with their inability to cause hepatobiliary injury (Fig. 7 and 8).
DISCUSSION
The pathogenesis of biliary atresia remains unknown. Viruses are thought to be a cause of biliary atresia, but the specific mechanisms by which they contribute has not been elucidated. In the current study, we show that, in the murine model of biliary atresia, RRV specifically targets the biliary epithelium for infection. To determine whether RRV tropism for the biliary epithelium was unique, we tested the effect of four other strains of rotavirus and found that the simian strain SA11-FM could also target the biliary epithelium for infection, but its temporal and systemic distribution following inoculation was different from RRV. SA11-SM could also be found in the hepatobiliary system but in a different location, in smaller quantities, and with less frequency than RRV. Inoculation with the mouse strain EDIM and the human strain Wa had no effect on the liver. Although the results of the current study are necessarily descriptive, they suggest that specific characteristics of a rotavirus strain determine its ability to target a cell of hepatobiliary origin for infection. These results provide a framework for future studies designed to identify the molecular basis for this strain specificity.
Consistent with reports in which the model was established (24-26, 28), newborn mice injected with RRV developed the murine model of biliary atresia with the clinical manifestations of jaundice, acholic stools, and bilirubinuria. Cholangiography revealed bile duct obstruction. Because previous studies had not established the targets of RRV infection, we characterized the temporal and systemic spread of RRV following intraperitoneal injection and found that, among the tissues surveyed, the liver was the primary site of tropism. The temporal pattern of expression in which the amount of infectious virus increased in the liver over time suggested that there was ongoing RRV replication within the liver. To determine the cellular target within the liver, immunohistochemistry was performed which identified the biliary epithelium as the primary site of RRV infection. These novel findings indicate that infection and replication within the biliary epithelium by RRV appear to be the initial insult that triggers the subsequent development of extrahepatic biliary obstruction. It is interesting to note that the paradigm of rotavirus infection of the intestinal epithelium resulting in a noninflammatory diarrhea (21) differs from the profound inflammatory response seen at the level of the hepatobiliary epithelium (30). The cause of this difference is currently unknown and warrants further study.
Inoculation of newborn mice with the related simian strains SA11-FM and SA11-SM revealed interesting results. Mice injected with SA11-FM developed manifestations of hepatobiliary injury that were very similar to those of RRV-injected mice; however, the temporal and systemic distribution of viral protein and live virus was markedly different. The highest amount of infectious SA11-FM detected within the liver and the only time point at which colocalization in the biliary epithelium could be demonstrated occurred at 2 days after inoculation. In contrast to RRV, where the amount of live virus in the liver increased over time, the amount of SA11-FM within the liver decreased. In addition, the overall amount of virus found within the liver was markedly lower than that found in RRV-injected mice. In mice injected with SA11-SM, we expected that if mice showed clinical signs of hepatobiliary disease, the pattern of injury would be similar to that found in mice injected with RRV or SA11-FM. Instead, we found that mice developed clinical symptoms of hepatic injury, but the extrahepatic biliary system was patent and the mice recovered. Live SA11-SM could be found within the liver, and immunohistochemistry showed that protein could be detected within the hepatobiliary system but its pattern of distribution was different than RRV and SA11-FM. Dual-staining immunohistochemical analysis localized SA11-SM to periportal tissue outside the biliary epithelium, indicating that SA11-SM targeted a different cell within the hepatobiliary system for infection.
Inoculation with the mouse strain EDIM and the human strain Wa caused no clinical signs of hepatobiliary injury, and consistent with the lack of injury, we found no detectable infectious virus within the liver. Previous studies have shown that the extraintestinal spread of rotavirus is strain dependent (22, 23, 32). In those studies, the presence of virus within the liver was used as a marker of spread (22, 23). The results of the current study expand on these observations showing that not only is the extraintestinal spread of rotavirus strain dependent but the specific targets of inoculation within the liver vary, which in turn, impacts the subsequent development of clinical manifestations of disease and the survival of the injected mouse.
The molecular basis for rotavirus tropism for cells of hepatobiliary origin has not been defined. Rotaviruses contain 11 double-stranded RNA gene segments. Among the 11 gene segments, 6 encode viral structural proteins (VP1 to 4, VP6, and VP7) and are found in an intact infectious virus. The remaining genes encode nonstructural proteins (NSP1 to 6). Reverse genetics is difficult to perform in rotavirus (15); however, the rotavirus genome can be manipulated using the property of gene reassortment. When two strains of rotavirus are simultaneously coinjected into a host cell, progeny will be generated that contain different combinations of the parental genes. Single-gene reassortants (i.e., all but one gene derived from one parent) allow for the determination of the function of that gene. Using such techniques, the extraintestinal spread of rotavirus has been linked to the NSP3 gene derived from RRV (23). The results of the current studies provide the framework for further manipulation. If RRV, SA11-FM, or SA11-SM was coinjected with either Wa or EDIM, progeny may be obtained that would allow the identification of the specific genes that confer tropism for the biliary epithelium. In so doing, studies such as these may allow the identification of rotavirus genes responsible for hepatobiliary tropism. Similarities between the genes within RRV and SA11-FM that target the biliary epithelium for infection may allow for the determination of how rotavirus targets the biliary epithelium for injury and infection. Alternatively, sequencing of these genes may provide insight into the varied responses seen in our experiments. These genes may also be compared with the genes within SA11-SM which confer tropism for a different hepatobiliary cell. Likely candidate genes are those encoding the VP4 and VP7 proteins which are found on the outermost layer of the rotavirus capsid. These proteins are thought to regulate viral host cell attachment and entry. It is worth noting that SA11-FM and SA11-SM are closely related strains differing in only the 2 gene segments encoding the viral proteins VP4 and NSP5/6, which are believed to be obtained by reassortment between SA11 and a bovine rotavirus (12; unpublished observations); thus, VP4 elements emerge as a prime focus for future investigations.
It is important to note that the route of rotavirus administration used in these studies was intraperitoneal injection. The intraperitoneal route was chosen because it most consistently induces the murine model of biliary atresia. Though this may bypass the normal barriers of virus spread, the goal of this study was to address strain-specific differences in rotaviruses and their ability to produce the model of biliary atresia. Oral inoculation of RRV to newborn pups can result in biliary obstruction but less often and less reliably.
The results of the current study were observed in the mouse. It remains to be determined if these results contribute to the understanding of the disease process that occurs in infants. It is noteworthy that recent reports have shown that infection with rotavirus in humans causes systemic viremia (3). Because rotavirus has been found in the blood of infected infants, it is possible that a perinatal rotavirus inoculation could result in biliary epithelial injury in a young infant. Detection of rotavirus within the livers of children affected with biliary atresia has been reported but not in a consistent fashion (4, 27). Further study will be necessary to determine whether rotavirus has a causal effect in the disease process that occurs in humans.
Acknowledgments
G.M.T. was supported in part by NIH K08 DK0728858-01.
Footnotes
Published ahead of print on 22 November 2006.
REFERENCES
- 1.Altman, R. P., J. R. Lilly, J. Greenfield, A. Weinberg, K. Van Leeuwen, and L. Flanigan. 1997. A multivariable risk factor analysis of the portoenterostomy (Kasai) procedure for biliary atresia. Ann. Surg. 226:348-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balistreri, W. F., R. Grand, J. H. Hoofnagle, F. J. Suchy, F. C. Ryckman, D. H. Perlmutter, and R. J. Sokol. 1996. Biliary atresia: current concepts and research directions. Summary of a symposium. Hepatology 23:1682-1692. [DOI] [PubMed] [Google Scholar]
- 3.Blutt, S. E., C. D. Kirkwood, V. Parreno, K. L. Warfield, M. Ciarlet, M. K. Estes, K. Bok, R. F. Bishop, and M. E. Conner. 2003. Rotavirus antigenaemia and viraemia: a common event? Lancet 362:1445-1449. [DOI] [PubMed] [Google Scholar]
- 4.Bobo, L., C. Ojeh, D. Chiu, A. Machado, P. Colombani, and K. Schwarz. 1997. Lack of evidence for rotavirus by polymerase chain reaction/enzyme immunoassay of hepatobiliary samples from children with biliary atresia. Pediatr. Res. 41:229-234. [DOI] [PubMed] [Google Scholar]
- 5.Chardot, C., M. Carton, N. Spire-Bendelac, C. Le Pommelet, J.-L. Golmard, and B. Auvert. 1999. Prognosis of biliary atresia in the era of liver transplantation: French national study from 1986 to 1996. Hepatology 30:606-611. [DOI] [PubMed] [Google Scholar]
- 6.Czech-Schmidt, G., W. Verhagen, P. Szavay, J. Leonhardt, and C. Petersen. 2001. Immunological gap in the infectious animal model for biliary atresia. J. Surg. Res. 101:62-67. [DOI] [PubMed] [Google Scholar]
- 7.Domiati-Saad, R., D. B. Dawson, L. R. Margraf, M. J. Finegold, A. G. Weinberg, and B. B. Rogers. 2000. Cytomegalovirus and human herpesvirus 6, but not human papillomavirus, are present in neonatal giant cell hepatitis and extrahepatic biliary atresia. Pediatr. Dev. Pathol. 3:367-373. [DOI] [PubMed] [Google Scholar]
- 8.Drut, R., R. M. Drut, M. A. Gomez, E. Cueto Rua, and M. M. Lojo. 1998. Presence of human papillomavirus in extrahepatic biliary atresia. J. Pediatr. Gastroenterol. Nutr. 27:530-535. [DOI] [PubMed] [Google Scholar]
- 9.Fischler, B., A. Ehrnst, M. Forsgren, C. Orvell, and A. Nemeth. 1998. The viral association of neonatal cholestasis in Sweden: a possible link between cytomegalovirus infection and extrahepatic biliary atresia. J. Pediatr. Gastroenterol. Nutr. 27:57-64. [DOI] [PubMed] [Google Scholar]
- 10.Fjaer, R. B., A. L. Bruu, and S. A. Nordbo. 2005. Extrahepatic bile duct atresia and viral involvement. Pediatr. Transplant. 9:68-73. [DOI] [PubMed] [Google Scholar]
- 11.Glaser, J. H., W. F. Balistreri, and R. Morecki. 1984. Role of reovirus type 3 in persistent infantile cholestasis. J. Pediatr. 105:912-915. [DOI] [PubMed] [Google Scholar]
- 12.Gorziglia, M., G. Larralde, and R. L. Ward. 1990. Neutralization epitopes on rotavirus SA11 4fM outer capsid proteins. J. Virol. 64:4534-4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kinugasa, Y., Y. Nakashima, S. Matsuo, K. Shono, S. Suita, and K. Sueishi. 1999. Bile ductular proliferation as a prognostic factor in biliary atresia: an immunohistochemical assessment. J. Pediatr. Surg. 34:1715-1720. [DOI] [PubMed] [Google Scholar]
- 14.Knowlton, D. R., D. M. Spector, and R. L. Ward. 1991. Development of an improved method for measuring neutralizing antibody to rotavirus. J. Virol. Methods 33:127-134. [DOI] [PubMed] [Google Scholar]
- 15.Komoto, S., J. Sasaki, and K. Taniguchi. 2006. Reverse genetics system for introduction of site-specific mutations into the double-stranded RNA genome of infectious rotavirus. Proc. Natl. Acad. Sci. USA 103:4646-4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDiarmid, S. 2000. Liver transplantation. The pediatric challenge. Clin. Liver Dis. 4:879-925. [DOI] [PubMed] [Google Scholar]
- 17.McNeal, M. M., R. L. Broome, and R. L. Ward. 1994. Active immunity against rotavirus infection in mice is correlated with viral replication and titers of serum rotavirus IgA following vaccination. Virology 204:642-650. [DOI] [PubMed] [Google Scholar]
- 18.McNeal, M. M., M. N. Rae, J. A. Bean, and R. L. Ward. 1999. Antibody-dependent and -independent protection following intranasal immunization of mice with rotavirus particles. J. Virol. 73:7565-7573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morecki, R., J. H. Glaser, S. Cho, W. F. Balistreri, and M. S. Horwitz. 1984. Biliary atresia and reovirus type 3 infection. N. Engl. J. Med. 310:1610. [DOI] [PubMed] [Google Scholar]
- 20.Morecki, R., J. H. Glaser, A. B. Johnson, and Y. Kress. 1984. Detection of reovirus type 3 in the porta hepatis of an infant with extrahepatic biliary atresia: ultrastructural and immunocytochemical study. Hepatology 4:1137-1142. [DOI] [PubMed] [Google Scholar]
- 21.Morris, A. P., and M. K. Estes. 2001. Microbes and microbial toxins: paradigms for microbial-mucosal interactions. VIII. Pathological consequences of rotavirus infection and its enterotoxin. Am. J. Physiol. Gastrointest. Liver Physiol. 281:G303-G310. [DOI] [PubMed] [Google Scholar]
- 22.Mossel, E. C., and R. F. Ramig. 2003. A lymphatic mechanism of rotavirus extraintestinal spread in the neonatal mouse. J. Virol. 77:12352-12356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mossel, E. C., and R. F. Ramig. 2002. Rotavirus genome segment 7 (NSP3) is a determinant of extraintestinal spread in the neonatal mouse. J. Virol. 76:6502-6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petersen, C., D. Biermanns, M. Kuske, L. Meyer-Junghanel, and H. Mildenberger. 1997. New aspects in a murine model for extrahepatic biliary atresia. J. Pediatr. Surg. 32:1190-1195. [DOI] [PubMed] [Google Scholar]
- 25.Petersen, C., S. Grasshoff, and L. Luciano. 1998. Diverse morphology of biliary atresia in an animal model. J. Hepatol. 28:603-607. [DOI] [PubMed] [Google Scholar]
- 26.Petersen, C., M. Kuske, E. Bruns, D. Biermanns, P. V. Wussow, and H. Mildenberger. 1998. Progress in developing animal models for biliary atresia. Eur. J. Pediatr. Surg. 8:137-141. [DOI] [PubMed] [Google Scholar]
- 27.Riepenhoff-Talty, M., V. Gouvea, M. J. Evans, L. Svensson, E. Hoffenberg, R. J. Sokol, I. Uhnoo, S. J. Greenberg, K. Schakel, G. Zhaori, J. Fitzgerald, S. Chong, M. el-Yousef, A. Nemeth, M. Brown, D. Piccoli, J. Hyams, D. Ruffin, and T. Rossi. 1996. Detection of group C rotavirus in infants with extrahepatic biliary atresia. J. Infect. Dis. 174:8-15. [DOI] [PubMed] [Google Scholar]
- 28.Riepenhoff-Talty, M., K. Schaekel, H. F. Clark, W. Mueller, I. Uhnoo, T. Rossi, J. Fisher, and P. L. Ogra. 1993. Group A rotaviruses produce extrahepatic biliary obstruction in orally inoculated newborn mice. Pediatr. Res. 33:394-399. [DOI] [PubMed] [Google Scholar]
- 29.Sasaki, H., M. Nio, D. Iwami, N. Funaki, R. Ohi, and H. Sasano. 2001. Cytokeratin subtypes in biliary atresia: immunohistochemical study. Pathol. Int. 51:511-518. [DOI] [PubMed] [Google Scholar]
- 30.Shivakumar, P., K. M. Campbell, G. E. Sabla, A. Miethke, G. Tiao, M. M. McNeal, R. L. Ward, and J. A. Bezerra. 2004. Obstruction of extrahepatic bile ducts by lymphocytes is regulated by IFN-gamma in experimental biliary atresia. J. Clin. Investig. 114:322-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sokol, R. J., and C. Mack. 2001. Etiopathogenesis of biliary atresia. Semin. Liver Dis. 21:517-524. [DOI] [PubMed] [Google Scholar]
- 32.Uhnoo, I., M. Riepenhoff-Talty, T. Dharakul, P. Chegas, J. E. Fisher, H. B. Greenberg, and P. L. Ogra. 1990. Extramucosal spread and development of hepatitis in immunodeficient and normal mice infected with rhesus rotavirus. J. Virol. 64:361-368. [DOI] [PMC free article] [PubMed] [Google Scholar]