Abstract
Long-term antigen expression is believed to play an important role in modulation of T-cell responses to chronic virus infections. However, recent studies suggest that immune responses may occur late after apparently acute infections. We have now analyzed the CD8 T-cell response to vesicular stomatitis virus (VSV), which is thought to cause to an infection characterized by rapid virus clearance by innate and adaptive immune system components. Unexpectedly, virus-encoded antigen was detectable more than 6 weeks after intranasal VSV infection in both draining and nondraining lymph nodes by adoptively transferred CD8 T cells. Infection with Listeria monocytogenes expressing the same antigen did not result in prolonged antigen presentation. Weeks after VSV infection, discrete T-cell clustering with dendritic cells within the lymph node was observed after transfer of antigen-specific CD8 T cells. Moreover, memory CD8 T cells as defined by phenotype and function were generated from naïve CD8 T cells entering the response late after infection. These findings suggested that protracted antigen presentation after an apparently acute virus infection may contribute to an ongoing antiviral immune response.
CD8 T cells play an important role in providing immunity to viruses and other intracellular pathogens (28). After encounter with viral or bacterial antigens, presented by a sufficiently activated antigen-presenting cell (APC), naïve CD8 T cells divide rapidly and acquire effector functions, including the ability to produce cytokines such as gamma interferon (IFN-γ) and tumor necrosis factor alpha (11). Initiation of a productive CD8 T-cell response, i.e., leading to memory development, appears to require only a brief initial encounter with antigen (16, 34, 41, 51). However, more-sustained T-cell-APC interactions are needed to mount a maximal proliferative response and generate optimal numbers of memory cells (7, 26, 41). The large population of antigen-specific effector cells produced by proliferation following strong T-cell-APC interactions undergoes a contraction phase resulting in the death of approximately 90 to 95% of the total number of cells present at the peak of the response. This phase appears to be antigen independent and is programmed during the initiation of the response (2). The level of inflammation early during priming is also a key determinant of the overall expansion and contraction of the response, with inflammatory mediators exerting both positive and negative effects on the response depending on the context in which they are perceived (3, 8, 9, 25, 49). The surviving cells eventually form the memory population that is maintained at a relatively constant level by homeostatic turnover. The cytokines interleukin-7 (IL-7) and IL-15 play important roles in maintaining survival and inducing proliferation, respectively, of memory CD8 T cells. Importantly, long-term survival of memory T cells is believed to be antigen independent (36, 48), although there appears to be a role for major histocompatibility complex and T-cell receptor (TCR) signaling in memory T-cell function and maintenance (20, 21, 39, 43).
Although antigen availability during the first few hours after infection or immunization may be sufficient to trigger initial T-cell activation and proliferation in vivo, there may be an ongoing role for antigen in development of the primary response and in memory T-cell generation (46). For example, a recent report demonstrated that adoptively transferred TCR-transgenic CD4 T cells can respond to antigen in the local draining lymph nodes (LN) several days after subcutaneous immunization with soluble protein and adjuvant (5). These responding cells can also contribute to the central memory population. Similarly, following intravenous (i.v.) infection with recombinant vesicular stomatitis virus encoding ovalbumin (VSV-Ova), adoptively transferred Ova-specific TCR-transgenic CD8 T cells respond to antigen up to 4 days after infection (10). The responding “late-comers” did generate memory cells but were not preferentially recruited into the memory pool. In similar experiments performed following Listeria monocytogenes infection, Ova-specific TCR-transgenic CD8 T cells transferred 4 days after infection expanded ∼10-fold, underwent little contraction, and went on to form memory cells (50). Thus, naïve T cells may enter into the primary immune response over several days after infection and contribute to the formation of the memory population.
In the case of chronic or latent virus infections, the presence of antigen long term is thought to play a significant role in shaping the ongoing T-cell response. For example, chronic lymphocytic choriomeningitis virus infection results in clonal exhaustion of CD8 T cells, likely due, at least in part, to continuous T-cell encounter with high levels of antigen (54). In other situations where antigen load may be more limited or localized to specific organs, such as in latent herpesvirus infections, memory CD8 T cells remain functional but exhibit characteristics distinct from memory cells raised by acute infections (19, 23, 35, 37, 38, 44). In addition, a recent study demonstrated that naïve CD8 T cells are continuously recruited into the response to persistent infection with either polyoma virus or lymphocytic choriomeningitis virus (53). However, there may also exist infections previously categorized as acute in which viral antigen and perhaps viral genetic material are present for protracted time periods. Recently, such a scenario has been described following intranasal infection of mice with the segmented negative-stranded RNA influenza virus, a member of the Orthomyxoviridae family (14, 55). Thus, influenza virus nucleoprotein (NP)-derived antigenic peptides are present in the lung-draining LN for at least 60 days postinfection. Although mRNA encoding NP is not detected at 30 days postinfection by PCR (55), it remains possible that viral genomic RNA is present and that low-level transcription and translation of viral proteins occurs. In order to determine whether the presence of antigen following “acute” virus infection applied to other viruses, we assessed the presence of virally encoded antigen following VSV infection. Unexpectedly, VSV-encoded antigen was detectable in LN several weeks postinfection. Moreover, naïve CD8 T cells activated well after resolution of the initial infection progressed to memory T-cell development. Our results indicated that long-term antigen expression following viral infection may be a more common event than previously appreciated that could affect overall immunity to certain viral infections or vaccinations.
MATERIALS AND METHODS
Mice and reagents.
C57BL/6 (CD45.2) mice, 5 to 6 weeks of age, were purchased from Jackson Laboratories (Bar Harbor, ME). TCR-transgenic OT-I-RAG−/− (CD45.1) mice (12) were bred in-house and used between the ages of 3 and 6 months old.
Infections.
Mice were infected with recombinant Indiana serotype VSV-Ova (24) or wild-type VSV-Ind. For intranasal infections, C57BL/6 mice were lightly anesthetized by an intraperitoneal injection with avertin (2,2,2-tribromoethanol). A dose of 5 × 104 PFU of VSV-Ova or VSV-Ind suspended in 50 μl Hanks' balanced saline solution (HBSS) was delivered intranasally using a 200-μl pipette. In some experiments, 1 × 103 CFU of Listeria monocytogenes encoding ovalbumin (40) was injected via the tail vein, while 1 × 104 CFU was used as the intranasal dose. For antibiotic treatment to clear L. monocytogenes infection, ampicillin (2 mg/ml) was administered in the drinking water for 7 days.
Adoptive transfer of TCR-transgenic T cells.
Carboxy fluorescein succinimidyl ester (CFSE) (30)-labeled OT-I cells (2.5 × 105 to 2 × 106) were transferred by i.v. injection at various times following infection.
Cell isolation and flow cytometry.
Mice were given a lethal dose of avertin intraperitoneally, and the inguinal and cervical LN were removed. Animals were then perfused with phosphate-buffered saline (PBS; with heparin), and the lungs and spleen were removed. The LN and spleen were crushed between frosted glass slides and passed through nylon mesh. The lung was cut into small pieces and collagenase digested at 37°C for 1 h. The lung fragments were then crushed through 40-μm nylon cell strainers and then washed and resuspended in Hanks' balanced saline solution. Donor CFSE-labeled cells were detected using their CD45 allele status. Ova-specific cells were detected using H-2Kb tetramers containing the Ova-derived SIINFEKL peptide as previously described (1, 31). For staining, lymphocytes were suspended in PBS-0.2% bovine serum albumin (BSA)-0.01% NaN3 at a concentration of ∼1 × 107/ml. For tetramer staining, cells were incubated at room temperature for 1 h with tetramer-APC plus the appropriate dilution of anti-CD8 peridinin-chlorophyll-protein complex (clone 53.6-72) along with antibodies specific for CD45.1, CD11a, and CD62L (all monoclonal antibodies [MAbs] were from BD Pharmingen or EBioscience) at 4°C for 20 min, washed, and then fixed in PBS with 3% paraformaldehyde. Relative fluorescence intensities were measured with a FACSCalibur (BD Biosciences, San Jose, CA). Data were analyzed using FlowJo software (Tree Star, San Carlos, CA).
Confocal microscopy.
LN were harvested and immediately fixed with 2% paraformaldehyde for 2 h at 4°C. The LN were then washed four times with cold PBS for 20 min each at 4°C. The tissues were then incubated with Cy5-conjugated anti-CD45.1 MAb and biotin-conjugated anti-CD11c MAb diluted in 2% normal goat serum and 2% fetal calf serum in PBS in 48-well plates at 4°C overnight. The next day the tissues were washed four times as described above. The tissues were then incubated with Cy3-conjugated streptavidin diluted in PBS at 4°C overnight. The stained LN were washed as before and then mounted on glass slides using Immu-Mount (Thermo Shandon, Pittsburgh, PA). The stained LN were analyzed by confocal microscopy using a Zeiss LSM 510 Meta (Carl Zeiss MicroImaging Inc., Thornwood, NY) using a 10× water immersion objective. Images were analyzed using Imaris 3D software (Bitplane, Inc.).
Intracellular detection of IFN-γ.
Lymphocytes were isolated from the spleen and were cultured for 5 h with 1 μg/ml Golgistop (BD PharMingen), with or without 1 μg/ml of the Ova-derived peptide SIINFEKL. After culture, cells were stained for surface molecules and then fixed, and cell membranes were permeabilized in cytofix/cytoperm solution (BD PharMingen) and stained with anti-IFN-γ-phycoerythrin or control rat immunoglobulin G1-phycoerythrin. Cells were then washed, and the fluorescence intensity was measured on a FACSCalibur.
RESULTS
VSV infection results in long-term antigen persistence.
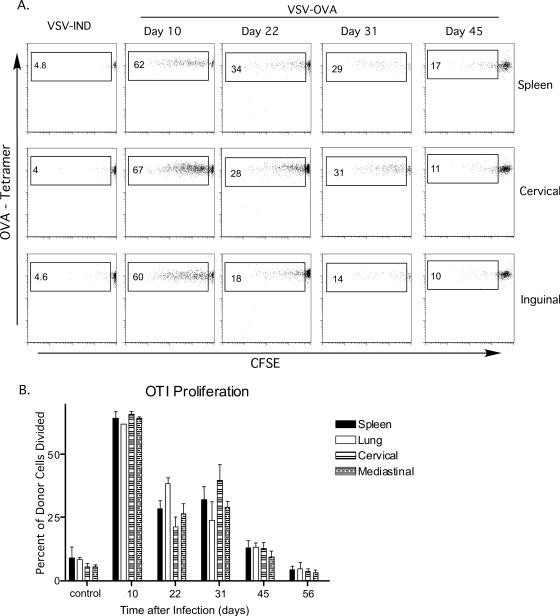
Previous work from our group indicated that there is residual antigen presentation as late as 2 months after an acute influenza virus infection in the lungs (55). There is also evidence that peptide antigens introduced into the lung by aerosolization are retained and presented to naïve cells adoptively transferred into animals as late as 3 weeks after cessation of aerosolization (unpublished data) (15). These data suggest the possibility that the lung environment is in some way involved in prolonged antigen retention and presentation. To test this possibility and to determine whether infection with a virus other than influenza virus would result in long-term antigen expression, we employed intranasal infection with a recombinant strain of VSV that encodes ovalbumin (24). To detect the presence of antigen in vivo, naïve CFSE-labeled OT-I cells were adoptively transferred at various intervals after infection. Five days later, the CFSE profiles of cells from the spleen, lung, and lung-draining and nondraining LN were examined. Robust proliferation of OT-I cells, as signified by CFSE dilution, was evident in the spleen, lungs, and lung-draining (cervical and mediastinal) and nondraining (inguinal) LN 10 days after infection (Fig. 1A and B). Thus, virus-encoded antigen remained several days after VSV has been reported to be cleared from infected mice (6). Even more surprising was the demonstration that antigen could be detected by adoptively transferred CD8 T cells transferred 22, 31, or 45 days after VSV infection. The levels of proliferation observed at the various time points were similar in all the tissues examined (Fig. 1B), suggesting the presence of similar amounts of antigen. Control values are essentially based on a small number of events due to background, since OT-I division does not occur in the absence of antigen. Thus, the values from day 45, although perhaps not dramatically different than control (Fig. 1B), are based on actual proliferative events, as shown in Fig. 1A. It was clear, however, that antigen levels as signified by OT-I proliferation decreased gradually over time. Thus, the ability to detect antigen became inconsistent at ∼8 weeks after infection, such that a small amount of OT-I proliferation was detected in only a subset of the mice tested (data not shown).
FIG. 1.
Antigen long-term persistence following VSV infection. Mice were infected intranasally with 5 × 104 PFU VSV-Ova or VSV-Ind (control), and 2 × 106 CFSE-labeled OT-I cells were transferred intravenously at the indicated times after infection. (A) CFSE profiles of donor CD8+ CD45.1+ cells harvested from different tissues 5 days after transfer. Numbers indicate the percentages of the donor population that had divided. (B) Percentages of donor cells from the indicated tissues which responded to antigen after being transferred into mice at the indicated times postinfection. This experiment was performed three times with groups of two to four mice/experiment.
Antigen is localized to secondary lymphoid tissues.
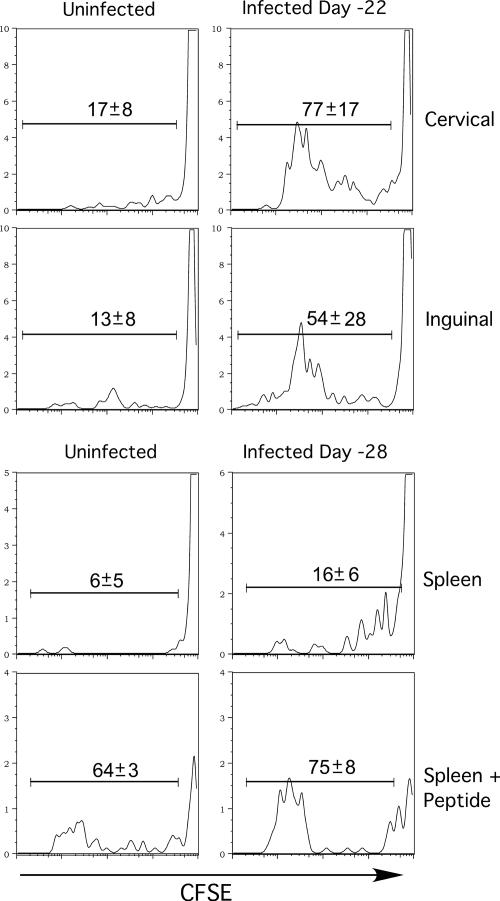
The presence of proliferating OT-I cells in different tissues suggested that residual viral antigens were present in several compartments. However, the OT-I cells could have encountered antigen elsewhere, since activation results in widespread dissemination of CD8 T cells (27, 32, 33). To determine the site(s) where antigen was localized, we adoptively transferred CFSE-labeled OT-I cells into animals infected 3 to 4 weeks earlier and removed the tissues 22 to 24 h later, prior to the initiation of cell division. The isolated lymphocytes were then cultured in vitro for 3 days in the presence of IL-2 (55). Interestingly, OT-I cells harvested from both the draining (cervical and mediastinal) and the nondraining (inguinal) LN divided in culture and had therefore encountered antigen in vivo (Fig. 2). The data also indicated that transferred OT-I cells had encountered antigen in the spleens of animals that had been infected with VSV 28 days prior to transfer (Fig. 2). No proliferation was detected when lung lymphocytes were cultured (data not shown), implying that antigen was preferentially presented in the lymphoid tissues and that the appearance of activated cells in this site was the result of migration.
FIG. 2.
Persistent antigen is present in the spleen and lymph nodes. CFSE-labeled OT-I cells were transferred into mice 22 or 28 days postinfection, and lymphocytes were harvested 22 to 24 h later and placed in culture for 3 days in IL-2-supplemented medium. Histograms show the degree of CFSE dilution by CD8+ CD45.1+ cells harvested from the inguinal or cervical LN or spleens of previously infected mice. The SIINFEKL peptide (1 μg/ml) was added to spleen cultures as a positive control.
Lack of antigen persistence following Listeria monocytogenes infection.
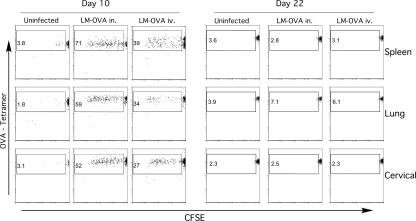
The phenomenon of antigen persistence and long-term antigen presentation following acute infection has previously been shown with influenza virus and now with VSV. To determine if the lung environment is somehow responsible for retention of antigen, we performed experiments with L. monocytogenes, an intracellular bacterial pathogen that is cleared several days after infection (21). Mice were infected intravenously or intranasally with recombinant L. monocytogenes expressing Ova (LM-Ova). In order to ensure that no residual bacteria were present at the time of analysis which could complicate data interpretation, we treated mice with ampicillin starting on day 7 after infection. Ten days postinfection, naïve CFSE-labeled OT-I cells were transferred into these animals. Five days later the spleen, lung, cervical, mediastinal, and inguinal LN were harvested and analyzed for OT-I proliferation. At this time point, OT-I cells that had divided were present in all the tissues we examined (Fig. 3). There was a lower percentage of responding cells in mice that were infected intravenously, but this may be a result of lower antigen dose. When OT-I cells were transferred to mice either intranasally or intravenously infected 22 days earlier, no CFSE dilution occurred. In addition, antigen persistence also occurred following i.v. VSV infection (data not shown). These results indicated that long-term antigen presentation was not a hallmark of infection in general and was not related to the route of infection.
FIG. 3.
Antigen persistence does not occur following Listeria monocytogenes infection. CD45.2 B6 mice were inoculated with 1 × 104 CFU LM-Ova intranasally or 1 × 103 CFU LM-Ova intravenously, placed on ampicillin at day 7 after infection, and received naive CFSE-labeled CD45.1 OT-I cells at 10 or 22 days postinfection. Plots show the CFSE profiles of donor CD8+ CD45.1+ cells harvested 5 days after transfer. The numbers represent the percentages of Ova-specific donor cells that have divided.
Transferred cells responding to viral antigen acquire an activated phenotype.
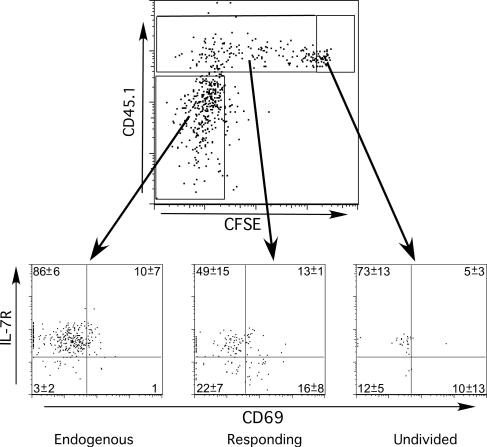
Following encounter with antigen in the primary response to infection, naive T cells undergo certain phenotypic changes indicative of activation. Further changes in expression of surface markers occur as CD8 T cells transit toward the memory cell lineage. We therefore examined the expression of two molecules whose expression is regulated during T-cell activation and memory development and compared their expression with division history based on CFSE dilution. CFSE-labeled CD45.1 OT-I cells were transferred to CD45.2 mice infected 4 weeks earlier with VSV-Ova. Six days later the CD45.1+ OT-I cells in the mediastinal LN and the endogenous tetramer+ CD8 memory T cells were analyzed for expression of CD69 and the IL-7 receptor (IL-7R). CD69 expression is transiently upregulated early after activation (56), while IL-7R is initially downregulated followed by reexpression as effector CD8 T cells progress toward memory development (17, 42). Endogenous Ova-specific memory CD8 T cells were IL-7R+ and lacked CD69. Naïve OT-I cells (i.e., CFSEhigh) exhibited a similar phenotype. However, those OT-I cells that had undergone a variable number of divisions contained populations of CD69- IL-7R+, CD69+ IL-7R−, CD69− IL-7R−, and CD69+ IL-7R+ cells. CD69 expression in general was low, in keeping with the rapid loss of this molecule after initial activation. The largest population (CD69− IL-7R+) was primarily comprised of those cells that had undergone the most divisions and were largely CFSE− (data not shown). These data indicated that T-cell activation in response to persistent antigen resembled a primary response, albeit substantially less robust.
T-cell activation by residual antigen occurs in the DC-rich regions of lymph nodes.
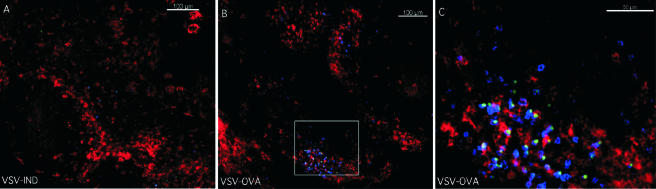
Our data indicated that residual antigen was present in the LN and spleen of mice for at least 6 weeks after initial VSV infection. Although the precise source of antigen is unknown at present, we wished to determine the anatomical characteristics of persistent antigen presentation to CD8 T cells. To this end, mice were infected intranasally with VSV-Ova followed by transfer of CFSE-labeled OT-I cells 3 weeks later. To visualize the localization of dendritic cells (DC) and transferred OT-I cells using confocal microscopy, we labeled LN with MAb specific for CD11c and CD45.1 (donor). We observed that a greater accumulation of OT-I cells occurred in LN derived from VSV-Ova-infected versus VSV-Ind-infected mice, even prior to the initiation of cell division (data not shown). In addition, CFSE-labeled OT-I cells colocalized with CD11c+ cells with DC morphology 48 h posttransfer (data not shown). By 4 days after transfer, a substantial increase in CD45.1+ OT-I cells was observed in LN from mice previously infected with VSV-Ova but not in those infected with VSV-Ind (Fig. 5A and B). Discrete clusters of OT-I cells were noted in regions rich with CD11c+ cells (Fig. 5B and C). Many of these cells were larger, blasting cells that were CD45.1+, had lost CFSE, and colocalized with CD11c+ cells. These data suggested that residual VSV-encoded antigen was presented by DC to naïve CD8 T cells, resulting in induction of T-cell blastogenesis and expansion.
FIG. 5.
Residual antigen present in the DC-rich regions of lymph nodes drives proliferation of naïve cells. CFSE-labeled CD45.1 OT-I cells (1.25 × 106) were transferred i.v. into CD45.2 B6 mice 21 days after intranasal infection with VSV-Ova or VSV-Ind. Cervical LN were harvested from animals 4 days after transfer, reacted with MAb specific for CD11c (red) and CD45.1 (blue), and then analyzed by confocal microscopy using a 10× water objective. Cells with CFSE remaining appear green.
Memory phenotype CD8 T cells are generated by naïve T-cell encounters with residual VSV-derived antigen.
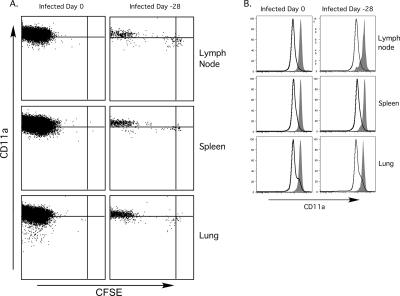
Naive CD8 T cells that encounter antigen in the absence of proper costimulatory signaling undergo an initial phase of proliferation but are subsequently deleted or anergized (22, 52). In our experiments naïve OT-I cells were encountering antigen long after the acute phase of VSV infection, when it might be predicted that costimulation is limited. We therefore undertook a series of experiments to determine whether memory cells could be generated from naïve CD8 T cells responding to persistent Ova peptide. CFSE-labeled OT-I cells were transferred to mice infected intranasally (i.n.) with VSV-Ova 4 weeks earlier, and 4 weeks after transfer the tissues were analyzed for proliferation, persistence, phenotype, and effector function of the transferred cells. Most of the donor cells recovered from the LN, spleen, and lung showed complete dilution of CFSE, although small populations of undivided cells or cells that had partially diluted CFSE were present (Fig. 6A). In contrast, virtually all the cells transferred into recently infected animals had completely diluted their CFSE content. Thus, although many cells responding late after infection had lost CFSE, the retention of a population of CFSE+ cells suggested that antigen levels were limiting late after infection. We also measured CD11a expression, since this integrin is upregulated upon T-cell activation and remains at high levels on memory CD8 T cells (31). Bona-fide memory OT-I cells generated by primary infection were uniformly CD11ahigh, and most OT-I cells derived from transfer late after infection expressed similarly high levels of CD11a (Fig. 6B). The level of induction of CD11a also correlated with division history (Fig. 6A). Those cells that had largely lost CFSE expressed higher CD11a levels compared to CFSEhigh cells. The observation that there were lower levels of the activation marker CD11a on undivided cells and cells that had undergone limited division supported the theory that these cells had undergone limited stimulation. In any case, the persistence of CFSElow CD11ahigh antigen-experienced cells a month after antigen encounter suggested that the signals needed to drive memory cell differentiation were available for a protracted period following initial VSV infection.
FIG. 6.
Memory phenotype cells are produced by OT-I cells responding to residual VSV antigen. A total of 2.5 × 105 naive CFSE-labeled OT-I cells were transferred into mice 28 days after i.n. infection with 5 × 104 PFU VSV-Ova or on the same day as infection (control) and then harvested 28 days later. (A) CD11a expression and the CFSE content of donor CD8+ CD45.1+ cells as assessed by flow cytometry. (B) CD11a expression by the donor CD8+ T-cell population (filled) and the endogenous CD8+ T-cell population (open) from animals that were infected 0 or 28 days prior to transfer.
Induction of functional memory CD8 T cells in response to persistent antigen.
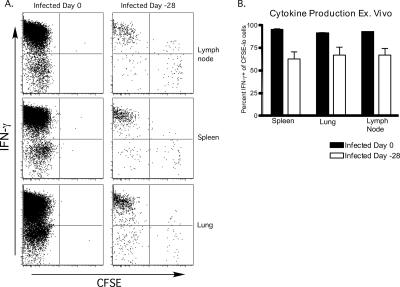
The phenotype of cells that encountered antigen a month after infection was similar to cells that encountered antigen during the acute phase of VSV infection. However, it remained to be seen whether such cells exhibited functional properties of memory CD8 T cells. To determine this, the ability of the long-lived OT-I cells to produce IFN-γ was assessed. We transferred naïve CFSE-labeled OT-I cells into mice 28 days after infection, or into mice infected the same day (control), and harvested cells from the spleen, lung, and LN 28 days later. The cells were stimulated in vitro with SIINFEKL peptide in the presence of brefeldin A and then stained for the presence of intracellular IFN-γ. As expected, most of the memory cells generated in newly infected mice produced IFN-γ (Fig. 7A). Similarly, the majority of memory cells generated from OT-I cells transferred to mice 28 days postinfection produced IFN-γ (Fig. 7A). OT-I cells that had undergone the greatest number of divisions were the best producers of IFN-γ (Fig. 7A). However, on average, the percentage of IFN-γ producers among CFSElow cells derived from “late” transferred OT-I cells was somewhat lower than the fraction derived from OT-I cells activated at the time of infection (Fig. 7B). Naïve OT-I cells stimulated in an identical fashion did not produce IFN-γ (data not shown). These data indicated that activation of naïve CD8 T cells following recognition of residual VSV-encoded antigen resulted in generation of functional memory T cells.
FIG. 7.
Functional memory cells are generated in response to persistent VSV antigen. A total of 2.5 × 105 naive CFSE-labeled OT-I cells were transferred into mice 0 or 28 days after i.n. infection with 5 × 104 PFU VSV-Ova, and lymphocytes from the indicated tissues were harvested 28 days later and cultured in vitro with the SIINFEKL peptide (1 μg/ml) in the presence of brefeldin A. Cells were then analyzed for intracellular IFN-γ levels by flow cytometry. (A) CFSE profiles and IFN-γ expression of donor CD8+ CD45.1+ cells after in vitro restimulation. (B) The percentage of CFSElow cells that produced IFN-γ after restimulation. (n = 2 for group infected on day 0; n = 6 for group infected on day −28).
DISCUSSION
In this report we show that acute VSV infection is followed by continual antigen presentation for a period of at least 6 weeks. This residual antigen was presented in a form that is recognizable by naïve CD8 T cells and was capable of driving proliferation of these cells. Evidence suggested that residual antigen was retained in the LN and the spleen but not in the lung, the initial site of infection. Intranasal inoculation of VSV resulted in a systemic infection, and this was reflected by the fact that similar amounts of residual antigen were detected by adoptively transferred naïve cells in the spleen as well as LN that do or do not drain the lung. These observations indicated that residual viral antigens persisted in the secondary lymphoid organs.
The source of the chronically presented VSV antigen is unknown at present, although several possibilities exist. Perhaps antigen produced in the initial infection was retained long-term by APC, although this seems unlikely given the known life span of the major DC populations (18). In addition, infection with L. monocytogenes-Ova did not result in protracted antigen presentation despite abundant presentation of Ova peptide early after infection, indicating that properties of the virus, and not simply antigen production, were essential to long-term presentation. Alternatively, residual VSV antigen could be derived from persistently infected peripheral tissues (e.g., nervous tissue) with antigen being carried to the lymphoid tissues by migrating DC (13). It is also possible that nonmigratory cells in the lymphoid tissues harbor viral genetic material. This possibility is supported by the recent demonstration that VSV genomic RNA (gRNA) is detectable in the LN of mice for at least 2 months following i.n. VSV infection (45). Moreover, previous studies showed that VSV gRNA is present in cattle and hamsters for protracted times after VSV infection (4, 29). Whether VSV proteins were produced in these scenarios was not determined. Our data favor the hypothesis that in the apparent absence of infectious virus (6, 45), low-level translation of VSV mRNA occurs, resulting in production of protein and subsequent antigen expression.
Antigen presentation late after acute viral infection presumably occurs in an environment with reduced inflammation compared to an active primary infection. However, at least a subset of naïve cells stimulated in this context was neither deleted nor rendered anergic but instead developed into long-lived memory cells with robust IFN-γ-producing capability. In fact the memory cells generated late after infection had a similar phenotype to the memory cells generated during acute VSV infection in terms of CD11a and CD62L expression. Examination of the LN of animals 21 days after infection by confocal microscopy showed distinct regions of proliferation of adoptively transferred CFSE-labeled cells. These regions of proliferation were located in areas that were rich in CD11c+ cells, suggesting that DC had acquired viral protein and were able to present antigen to naïve cells long after initial viral infection. These results suggested a previously unknown mechanism whereby there is continual generation of memory cells after an acute infection. Thus, naïve cells that were not drawn into the initial response to acute infection, or naïve cells emigrating from the thymus, could encounter antigen in this context and be stimulated to proliferate and develop into memory cells. Such a scenario has been described in the context of chronic viral infection (53).
Recent reports from us and others show that antigen is present in LN of mice for at least 2 months after intranasal influenza virus infection (14, 55). Interestingly, in this case, antigen was detectable only in the LN draining the respiratory tract, namely, the cervical and mediastinal LN. This result suggests the possibility that, since productive influenza virus infection preferentially occurs in the lung epithelium (47), antigen was being acquired from persistently infected tissue. Although mRNA for influenza virus nucleoprotein was not detected 30 days after infection, an analysis of gRNA was not performed (55). For several months after influenza virus infection, a subset of CD8 T cells present in the lung and the draining LN are CD69+ IL-7Rlow, resembling recently activated cells. These data suggest that the presence of persistent antigen after influenza virus infection may have functional consequences in terms of maintaining “activated” CD8 T cells that could be involved in protection against secondary infection. In addition, memory CD8 T cells presumably encountering influenza virus antigen in the mediastinal LN do not recirculate in the blood, as opposed to memory cells in other tissues (55). Thus, long-term antigen presentation may have profound effects on the immune response to virus infection. Whether the activated influenza virus-specific cells are continually derived from naïve CD8 T cells is unknown, but TCR-transgenic CD4 T cells transferred to previously infected mice generate memory cells (14). VSV-specific memory CD8 T cells do not generally exhibit a recently activated phenotype (Fig. 4), but our findings suggested that small numbers of naïve T cells could be recruited into the response while antigen was available. It will be interesting to determine whether our findings apply to other viruses and to determine the functional consequences of persistent antigen presentation following “acute” infections.
FIG. 4.
Transferred cells acquire an activated phenotype. Naïve CFSE-labeled CD45.1+ OT-I cells were transferred into CD45.2 B6 mice 28 days after infection, and 6 days after transfer lymphocytes were harvested and reacted with Ova tetramer and MAb specific for CD45.1, CD8, CD69, and IL-7R. The upper plot shows the total population of Ova-specific CD8+ cells, from cervical LN, subdivided into endogenous cells, responding donor cells, and undivided donor cells. The lower plots show the expression of CD69 and IL-7R by these populations. Shown is a representative sample of a group of three animals. Numbers represent the means and standard deviations of the group.
Acknowledgments
This work was supported by National Institutes of Health grants AI41576 and DK45260 (L.L.) and R21 AI65895 (L.S.C.) and a Damon-Runyon Cancer Research fellowship (K.M.K.).
Footnotes
Published ahead of print on 6 December 2006.
REFERENCES
- 1.Altman, J. D., P. A. H. Moss, P. J. R. Goulder, D. H. Barouch, M. G. McHeyzer-Williams, J. I. Bell, A. J. McMichael, and M. M. Davis. 1996. Phenotypic analysis of antigen-specific T lymphocytes. Science 274:94-96. [DOI] [PubMed] [Google Scholar]
- 2.Badovinac, V. P., B. B. Porter, and J. T. Harty. 2002. Programmed contraction of CD8+ T cells after infection. Nat. Immunol. 3:619-626. [DOI] [PubMed] [Google Scholar]
- 3.Badovinac, V. P., B. B. Porter, and J. T. Harty. 2004. CD8+ T cell contraction is controlled by early inflammation. Nat. Immunol. 5:809-817. [DOI] [PubMed] [Google Scholar]
- 4.Barrera, J. C., and G. J. Letchworth. 1996. Persistence of vesicular stomatitis virus New Jersey RNA in convalescent hamsters. Virology 219:453-464. [DOI] [PubMed] [Google Scholar]
- 5.Catron, D. M., L. K. Rusch, J. Hataye, A. A. Itano, and M. K. Jenkins. 2006. CD4+ T cells that enter the draining lymph nodes after antigen injection participate in the primary response and become central-memory cells. J. Exp. Med. 203:1045-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ciavarra, R. P., A. Stephens, S. Nagy, M. Sekellick, and C. Steel. 2006. Evaluation of immunological paradigms in a virus model: are dendritic cells critical for antiviral immunity and viral clearance? J. Immunol. 177:492-500. [DOI] [PubMed] [Google Scholar]
- 7.Curtsinger, J. M., C. M. Johnson, and M. F. Mescher. 2003. CD8 T cell clonal expansion and development of effector function require prolonged exposure to antigen, costimulation, and signal 3 cytokine. J. Immunol. 171:5165-5171. [DOI] [PubMed] [Google Scholar]
- 8.Curtsinger, J. M., C. S. Schmidt, A. Mondino, D. C. Lins, R. M. Kedl, M. K. Jenkins, and M. F. Mescher. 1999. Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. J. Immunol. 162:3256-3262. [PubMed] [Google Scholar]
- 9.Curtsinger, J. M., J. O. Valenzuela, P. Agarwal, D. Lins, and M. F. Mescher. 2005. Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J. Immunol. 174:4465-4469. [DOI] [PubMed] [Google Scholar]
- 10.D'Souza, W. N., and S. M. Hedrick. 2006. Cutting edge: latecomer CD8 T cells are imprinted with a unique differentiation program. J. Immunol. 177:777-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haring, J. S., V. P. Badovinac, and J. T. Harty. 2006. Inflaming the CD8+ T cell response. Immunity 25:19-29. [DOI] [PubMed] [Google Scholar]
- 12.Hogquist, K. A., S. C. Jameson, W. R. Heath, J. L. Howard, M. J. Bevan, and F. R. Carbone. 1994. T cell receptor antagonistic peptides induce positive selection. Cell 76:17-27. [DOI] [PubMed] [Google Scholar]
- 13.Itano, A. A., S. J. McSorley, R. L. Reinhardt, B. D. Ehst, E. Ingulli, A. Y. Rudensky, and M. K. Jenkins. 2003. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity 19:47-57. [DOI] [PubMed] [Google Scholar]
- 14.Jelley-Gibbs, D. M., D. M. Brown, J. P. Dibble, L. Haynes, S. M. Eaton, and S. L. Swain. 2005. Unexpected prolonged presentation of influenza antigens promotes CD4 T cell memory generation. J. Exp. Med. 202:697-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Julia, V., E. M. Hessel, L. Malherbe, N. Glaichenhaus, A. O'Garra, and R. L. Coffman. 2002. A restricted subset of dendritic cells captures airborne antigens and remains able to activate specific T cells long after antigen exposure. Immunity 16:271-283. [DOI] [PubMed] [Google Scholar]
- 16.Kaech, S. M., and R. Ahmed. 2001. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat. Immunol. 2:415-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaech, S. M., J. T. Tan, E. J. Wherry, B. T. Konieczny, C. D. Surh, and R. Ahmed. 2003. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 4:1191-1198. [DOI] [PubMed] [Google Scholar]
- 18.Kamath, A. T., S. Henri, F. Battye, D. F. Tough, and K. Shortman. 2002. Developmental kinetics and lifespan of dendritic cells in mouse lymphoid organs. Blood 100:1734-1741. [PubMed] [Google Scholar]
- 19.Karrer, U., S. Sierro, M. Wagner, A. Oxenius, H. Hengel, U. H. Koszinowski, R. E. Phillips, and P. Klenerman. 2003. Memory inflation: continuous accumulation of antiviral CD8+ T cells over time. J. Immunol. 170:2022-2029. [DOI] [PubMed] [Google Scholar]
- 20.Kassiotis, G., S. Garcia, E. Simpson, and B. Stockinger. 2002. Impairment of immunological memory in the absence of MHC despite survival of memory T cells. Nat. Immunol. 3:244-250. [DOI] [PubMed] [Google Scholar]
- 21.Kassiotis, G., R. Zamoyska, and B. Stockinger. 2003. Involvement of avidity for major histocompatibility complex in homeostasis of naive and memory T cells. J. Exp. Med. 197:1007-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kearney, E. R., K. A. Pape, D. Y. Loh, and M. K. Jenkins. 1994. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity 1:327-339. [DOI] [PubMed] [Google Scholar]
- 23.Khanna, K. M., R. H. Bonneau, P. R. Kinchington, and R. L. Hendricks. 2003. Herpes simplex virus-specific memory CD8+ T cells are selectively activated and retained in latently infected sensory ganglia. Immunity 18:593-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim, S. K., D. S. Reed, S. Olson, M. J. Schnell, J. K. Rose, P. A. Morton, and L. Lefrançois. 1998. Generation of mucosal cytotoxic T cells against soluble protein by tissue-specific environmental and costimulatory signals. Proc. Natl. Acad. Sci. USA 95:10814-10819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolumam, G. A., S. Thomas, L. J. Thompson, J. Sprent, and K. Murali-Krishna. 2005. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J. Exp. Med. 202:637-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lefrancois, L., A. Marzo, and K. Williams. 2003. Sustained response initiation is required for T cell clonal expansion but not for effector or memory development in vivo. J. Immunol. 171:2832-2839. [DOI] [PubMed] [Google Scholar]
- 27.Lefrançois, L., A. L. Marzo, D. Masopust, K. S. Schluns, and V. Vezy. 2002. Migration of primary and memory CD8 T cells. Adv. Exp. Med. Biol. 512:141-146. [DOI] [PubMed] [Google Scholar]
- 28.Lefrancois, L. 2006. Development, trafficking, and function of memory T-cell subsets. Immunol. Rev. 211:93-103. [DOI] [PubMed] [Google Scholar]
- 29.Letchworth, G. J., J. C. Barrera, J. R. Fishel, and L. Rodriguez. 1996. Vesicular stomatitis New Jersey virus RNA persists in cattle following convalescence. Virology 219:480-484. [DOI] [PubMed] [Google Scholar]
- 30.Lyons, A. B., and C. R. Parish. 1994. Determination of lymphocyte division by flow cytometry. J. Immunol. Methods 171:131-137. [DOI] [PubMed] [Google Scholar]
- 31.Masopust, D., J. Jiang, H. Shen, and L. Lefrançois. 2001. Direct analysis of the dynamics of the intestinal mucosa CD8 T cell response to systemic virus infection. J. Immunol. 166:2348-2356. [DOI] [PubMed] [Google Scholar]
- 32.Masopust, D., V. Vezys, A. L. Marzo, and L. Lefrançois. 2001. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291:2413-2417. [DOI] [PubMed] [Google Scholar]
- 33.Masopust, D., V. Vezys, E. J. Usherwood, L. S. Cauley, S. Olson, A. L. Marzo, R. L. Ward, D. L. Woodland, and L. Lefrancois. 2004. Activated primary and memory CD8 T cells migrate to nonlymphoid tissues regardless of site of activation or tissue of origin. J. Immunol. 172:4875-4882. [DOI] [PubMed] [Google Scholar]
- 34.Mercado, R., S. Vijh, S. E. Allen, K. Kerksiek, I. M. Pilip, and E. G. Pamer. 2000. Early programming of T cell populations responding to bacterial infection. J. Immunol. 165:6833-6839. [DOI] [PubMed] [Google Scholar]
- 35.Munks, M. W., K. S. Cho, A. K. Pinto, S. Sierro, P. Klenerman, and A. B. Hill. 2006. Four distinct patterns of memory CD8 T cell responses to chronic murine cytomegalovirus infection. J. Immunol. 177:450-458. [DOI] [PubMed] [Google Scholar]
- 36.Murali-Krishna, K., L. L. Lau, S. Sambhara, F. Lemonnier, J. Altman, and R. Ahmed. 1999. Persistence of memory CD8 T cells in MHC class I-deficient mice. Science 286:1377-1381. [DOI] [PubMed] [Google Scholar]
- 37.Obar, J. J., S. G. Crist, E. K. Leung, and E. J. Usherwood. 2004. IL-15-independent proliferative renewal of memory CD8+ T cells in latent gammaherpesvirus infection. J. Immunol. 173:2705-2714. [DOI] [PubMed] [Google Scholar]
- 38.Obar, J. J., S. Fuse, E. K. Leung, S. C. Bellfy, and E. J. Usherwood. 2006. Gammaherpesvirus persistence alters key CD8 T-cell memory characteristics and enhances antiviral protection. J. Virol. 80:8303-8315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Polic, B., D. Kunkel, A. Scheffold, and K. Rajewsky. 2001. How alpha beta T cells deal with induced TCR alpha ablation. Proc. Natl. Acad. Sci. USA 98:8744-8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pope, C., S.-K. Kim, A. Marzo, D. Masopust, K. Williams, J. Jiang, H. Shen, and L. Lefrançois. 2001. Organ-specific regulation of the CD8 T cell response to Listeria monocytogenes infection. J. Immunol. 166:3402-3409. [DOI] [PubMed] [Google Scholar]
- 41.Prlic, M., G. Hernandez-Hoyos, and M. J. Bevan. 2006. Duration of the initial TCR stimulus controls the magnitude but not functionality of the CD8+ T cell response. J. Exp. Med. 203:2135-2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schluns, K. S., W. C. Kieper, S. C. Jameson, and L. Lefrançois. 2000. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat. Immunol. 1:426-432. [DOI] [PubMed] [Google Scholar]
- 43.Seddon, B., P. Tomlinson, and R. Zamoyska. 2003. Interleukin 7 and T cell receptor signals regulate homeostasis of CD4 memory cells. Nat. Immunol. 4:680-686. [DOI] [PubMed] [Google Scholar]
- 44.Sierro, S., R. Rothkopf, and P. Klenerman. 2005. Evolution of diverse antiviral CD8+ T cell populations after murine cytomegalovirus infection. Eur. J. Immunol. 35:1113-1123. [DOI] [PubMed] [Google Scholar]
- 45.Simon, I. D., J. Publicover, and J. K. Rose. 2007. Replication and propagation of attenuated vesicular stomatitis virus vectors in vivo: vector spread correlates with induction of immune responses and persistence of genomic RNA. J. Virol. 81:2078-2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sprent, J., and D. F. Tough. 1994. Lymphocyte life-span and memory. Science 265:1395-1400. [DOI] [PubMed] [Google Scholar]
- 47.Steinhauer, D. A. 1999. Role of hemagglutinin cleavage for the pathogenicity of influenza virus. Virology 258:1-20. [DOI] [PubMed] [Google Scholar]
- 48.Swain, S. L., H. Hu, and G. Huston. 1999. Class II-independent generation of CD4 memory T cells from effectors. Science 286:1381-1383. [DOI] [PubMed] [Google Scholar]
- 49.Thompson, L. J., G. A. Kolumam, S. Thomas, and K. Murali-Krishna. 2006. Innate inflammatory signals induced by various pathogens differentially dictate the IFN-I dependence of CD8 T cells for clonal expansion and memory formation. J. Immunol. 177:1746-1754. [DOI] [PubMed] [Google Scholar]
- 50.van Faassen, H., M. Saldanha, D. Gilbertson, R. Dudani, L. Krishnan, and S. Sad. 2005. Reducing the stimulation of CD8+ T cells during infection with intracellular bacteria promotes differentiation primarily into a central (CD62Lhigh CD44high) subset. J. Immunol. 174:5341-5350. [DOI] [PubMed] [Google Scholar]
- 51.Van Stipdonk, M. J., E. E. Lemmens, and S. P. Schoenberger. 2001. Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat. Immunol. 2:423-429. [DOI] [PubMed] [Google Scholar]
- 52.Vezys, V., and L. Lefrançois. 2002. Inflammatory signals drive organ-specific autoimmunity to normally cross-tolerizing endogenous antigen. J. Immunol. 169:6677-6680. [DOI] [PubMed] [Google Scholar]
- 53.Vezys, V., D. Masopust, C. C. Kemball, D. L. Barber, L. A. O'mara, C. P. Larsen, T. C. Pearson, R. Ahmed, and A. E. Lukacher. 2006. Continuous recruitment of naive T cells contributes to heterogeneity of antiviral CD8 T cells during persistent infection. J. Exp. Med. 203:2263-2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wherry, E. J., D. L. Barber, S. M. Kaech, J. N. Blattman, and R. Ahmed. 2004. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc. Natl. Acad. Sci. USA 101:16004-16009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zammit, D. J., D. L. Turner, K. D. Klonowski, L. Lefrançois, and L. S. Cauley. 2006. Residual antigen presentation after influenza virus infection affects CD8 T cell activation and migration. Immunity 24:439-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ziegler, S. F., F. Ramsdell, and M. R. Alderson. 1994. The activation antigen CD69. Stem Cells 12:456-465. [DOI] [PubMed] [Google Scholar]