Abstract
Sapoviruses are one of the major agents of acute gastroenteritis in childhood. They form a tight genetic cluster (genus) in the Caliciviridae family that regroups both animal and human pathogenic strains. No permissive tissue culture has been developed for human sapovirus, limiting its characterization to surrogate systems. We report here on the first extensive characterization of the key enzyme of replication, the RNA-dependent RNA polymerase (RdRp) associated with the 3Dpol-like protein. Enzymatically active sapovirus 3Dpol and its defective mutant were expressed in Escherichia coli and purified. The overall structure of the sapovirus 3Dpol was determined by X-ray crystallography to 2.32-Å resolution. It revealed a right hand fold typical for template-dependent polynucleotide polymerases. The carboxyl terminus is located within the active site cleft, as observed in the RdRp of some (norovirus) but not other (lagovirus) caliciviruses. Sapovirus 3Dpol prefers Mn2+ over Mg2+ but may utilize either as a cofactor in vitro. In a synthetic RNA template-dependent reaction, sapovirus 3Dpol synthesizes a double-stranded RNA or labels the template 3′ terminus by terminal transferase activity. Initiation of RNA synthesis occurs de novo on heteropolymeric templates or in a primer-dependent manner on polyadenylated templates. Strikingly, this mode of initiation of RNA synthesis was also described for norovirus, but not for lagovirus, suggesting structural and functional homologies in the RNA-dependent RNA polymerase of human pathogenic caliciviruses. This first experimental evidence makes sapovirus 3Dpol an attractive target for developing drugs to control calicivirus infection in humans.
Sapovirus (SV) is a global agent of viral gastroenteritis, mainly infecting children under 5 years of age and being one of the most common agents of viral gastroenteritis among infants in Japan (9). Sapovirus infections have also been reported in all age groups (18, 23, 24, 33, 41, 52), and outbreaks of sapovirus gastroenteritis have been described in child day care centers (38), elderly persons' homes (23), and hospitals (24, 53).
Sapovirus belongs to the Caliciviridae family, which includes four genera (norovirus, sapovirus, lagovirus, and vesivirus). The human pathogenic caliciviruses have been so far exclusively limited to the norovirus and sapovirus genera. Both genera also include animal pathogenic strains, although animal pathogenic caliciviruses are mainly represented in the vesivirus and lagovirus genera. Phylogenetic analysis of the Caliciviridae based on the complete capsid gene shows that sapovirus strains cluster separately within the Caliciviridae (19). This genus seems to be more distantly related to the human norovirus than to the lagovirus (19). The sapovirus genus is divided in five genogroups (15). Strains belonging to genogroups 1, 2, 4, and 5 infect humans, with the genogroup 1 strains being predominantly detected over the years (18). Animal sapovirus strains, i.e., porcine strains, cluster within genogroup 3 (15). The porcine enteric calicivirus remains so far the only strain of the sapovirus species that can be propagated in cultured cells (7, 8).
Sapovirus is a nonenveloped RNA virus with a single-stranded positive-sense genome of approximately 7.4 kb (7,320 to 7,431 nucleotides [nt]) (20). The viral genome consists of two open reading frames encoding the nonstructural proteins and the capsid protein or virion protein 1 (VP1) encoded in open reading frame 1 (ORF-1) and a protein termed VP2 which is encoded in ORF-2 that is located at the 3′ end of the genome (Fig. 1). ORF-1 is predicted to encode a single polyprotein that is cotranslationally processed by the viral protease, resulting in the appearance of nonstructural proteins required for replication of the viral genome. Among those, the RNA-dependent RNA polymerase (3Dpol-like, further referred to as 3Dpol) is predicted to play a key role in the replication of the genome, as well as in the synthesis and amplification of a subgenomic RNA. The subgenomic RNA corresponds to the region of the genome that encodes VP1 and VP2 as well as the 3′ end (Fig. 1). Downstream from ORF-2, a 55- to 82-nt untranslated region is present (20), which is followed by a poly(A) tail of variable length.
FIG. 1.
Genome organization of sapovirus clone pJG-SapI (GenBank accession number AY694184) and expression of sapovirus 3D-like polymerase (3Dpol). The complete sapovirus clone pJG-Sap01 (7,429 bp in length) and the ORF-1 (6,842 bp in length) and ORF-2 (497 bp in length) are shown. The putative cleavage sites at the interface 3Cpro/3Dpol (Q1207/D) and 3Dpol/VP1 (E1721/G) are indicated. Both the 3Dpol wild type and 3Dpol active site mutant were expressed. The domain VZ10SV-AY694184-11-3DL-RdRp predicted to encompass the active RNA-dependent RNA polymerase of sapovirus (aa position 1207 to 1721 in the ORF-1 polyprotein of clone pJG-Sap01) is shown. The recombinant proteins display a His6 tag at the C terminus or N terminus. For generation of the synthetic subgenomic RNA, a template DNA encompassing the complete VP1 and VP2 genes was used. Synthetic subgenomic RNA was generated by T7-mediated in vitro transcription followed by poly(A)-tailing yielding a polyadenylated product of about 2,259 nucleotides in length.
So far, the structure and function of the viral enzymes involved in replication of the sapovirus genome remain uncharacterized. Recently, Oka et al. have described the processing of sapovirus polyprotein precursor in a cell-free system (46). In that study, the 3Cpro3Dpol protein precursor was recovered after processing of the ORF-1-encoded polyprotein, suggesting that the 3Cpro3Dpol protein precursor is one of the active forms of the viral polymerase.
In our study, we have examined the structural and functional features of sapovirus 3Dpol polymerase. The three-dimensional structure of the sapovirus 3Dpol was solved using X-ray crystallography and displays a “right hand” shape, with the C terminus of the enzyme entering the channel containing the active site. Furthermore, we present the first experimental evidence for the RNA-dependent RNA polymerase (RdRp) and terminal transferase activities associated with sapovirus 3Dpol, showing that initiation of RNA synthesis by sapovirus 3Dpol on heteropolymeric RNA template occurs de novo. We conclude that sapovirus 3Dpol displays structural and functional features similar to the RNA-dependent RNA polymerase of norovirus (3Dpol), a related human pathogenic calicivirus.
MATERIALS AND METHODS
Bioinformatics analysis.
Full-genome sequences of a representative set of sapoviruses and other caliciviruses were retrieved from the GenBank database and put into the Viralis database (A. E. Gorbalenya, unpublished data). They were aligned using the MUSCLE (13) and HMMER2.3.2 (12) programs, taking into consideration also the phylogenic structure of the Caliciviridae family and cleavage site annotations in the calicivirus genome files. A manually adjusted alignment of the 3Cpro3Dpol area was used for predicting the location of 3Dpol domain.
Generation of synthetic RNA templates.
Sapovirus clone pJG-Sap01 (GenBank accession number AY694184), which was characterized by phylogenetic analysis based on the complete sequence of its capsid gene as belonging to sapovirus GGI (Manchester-like), was used for generation of a DNA fragment of 2,259 bp in length corresponding to the complete subgenomic RNA of sapovirus (nt 5170 to 7429). The DNA fragment was generated by PCR amplification, using a T7 promoter sequence fused at its 3′ end to a gene-specific sequence as a forward primer and a gene-specific primer complementary to the 3′ end of the sequence as a reverse primer. For amplification of DNA, primers 86-Sap-T7-for, consisting of the T7 promoter sequence fused at its 3′ end to a gene-specific sequence (5′-CAGAGATGCATAATACGACTCACTATAGGGAGAGCCACCATGGAGGGCAATGGCTCCAACCCAGAGC-3′), and 48-Sap-ORF-2-rev (5′-AGGGACGGCGACAATCGCTTAATTGTC-3′) were used. Reaction conditions, amplification cycles, and identification of the products were performed as previously described for generation of norovirus synthetic subgenomic RNA (50, 51).
For in vitro transcription, the Megascript T7 Kit (Ambion) was used according to the manufacturer's instructions. The reaction mix consisted of 1 μg purified cDNA, 2 μl of 10× T7 reaction buffer, 20 U of RNase inhibitor (RNAsin, 40 U/μl; Promega), 7.5 mM (each) ATP, CTP, GTP, and UTP, 10 U T7 polymerase, and RNase-DNase-free water to a final volume of 20 μl. The reaction was run for 2 h at 37°C, then 20 U DNase I (Ambion) was added, and the reaction mixture was incubated at 37°C for 30 min. poly(A)-tailing of synthesized RNA was performed with the poly(A)-tailing kit (Ambion) according to manufacturer's instructions. The reaction products were then purified with the MEGAclear kit (Ambion) according to the manufacturer's instructions. The RNA concentration in the sample was determined by measuring the UV absorbance at 280 nm.
Expression and purification of sapovirus 3Dpol.
The DNA corresponding to the sapovirus 3Dpol coding sequence was generated by PCR from sapovirus full-length cDNA clone pJG-Sap01 (GenBank accession number AY694184) as described above. The PCR product was molecularly cloned into the pDEST14 vector (Gateway) according to the manufacturer's instructions and using primers VZ7-Sap-3D-H6C-for (5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTTCGAAGGAGATGCCACCATGAAAGATGAATTTCAATGGAAGGGTTTGCCCGTGG-3) and VZ8-Sap-3D-H6C-rev (5′-GGGGACCACTTTGTACAAGAAAGCTGGGTCTTATTAGTGATGGTGATGGTGATGCTCCATCTCAAACACTATTTTGTGGGTTCC-3′), yielding clone pVZ-Sap-3D-H6C. The 3Dpol active site mutant was generated from the pVZ-Sap-3D-H6C clone by site-directed mutagenesis using the QuikChange site-directed mutagenesis kit (Stratagene) and primers 91-Sap-μpol-for (5′-GTCCACACGTACGGTGGTGGTTGCATGTATAGT-3′) and 92-Sap-μpol-rev (5′-ACTATACATGCAACCACCACCGTACGTGTGGAC-3′) according to the manufacturer's instructions, yielding the pVZ-Sap-3D-H6C-D347GD348G clone. The 3Dpol active site mutant bears substitutions in the GDD motif of both aspartates with glycine (GD347GD348G). Sapovirus 3Dpol and 3Dpol active site mutants were expressed in Escherichia coli. For this purpose, E. coli Rosetta2 (DE3)pLysS cells (Novagen) were transformed with the pVZ-Sap-3D-H6C and pVZ-Sap-3D-H6C-D343GD344G clones. Cells were grown at 37°C in 2YT medium with ampicillin (100 mg/liter) and chloramphenicol (34 mg/liter). Upon reaching an optical density at 600 nm of 0.6, protein expression was induced by adding isopropyl-β-d-thiogalactopyranoside (IPTG) to a final concentration of 1 mM. Cultures were then incubated at 25°C overnight. Cell pellets obtained from 500-ml cultures were washed once in 4 ml phosphate-buffered saline and 1% Triton X-100 (Merck). Cells were treated with DNase (10 U/ml) for 15 min at 37°C, then sonicated on ice, and resuspended in binding buffer (20 mM Tris-HCl [pH 7.9], 500 mM NaCl, 5 mM imidazole). After centrifugation at 4,300 rpm at 4°C for 40 min, the cleared lysate was obtained. The His6-tagged protein was bound on a Ni-nitrilotriacetic acid (NTA)-Sepharose resin (Novagen) preequilibrated with the binding buffer. The bound protein was washed with the binding buffer containing 60 mM imidazole and eluted with the binding buffer containing 500 mM imidazole. The eluted protein was then dialyzed against buffer A (25 mM Tris-HCl [pH 8.0], 1 mM β-mercaptoethanol, 100 mM NaCl, 5 mM MgCl2, 10% glycerol, and 0.1% Triton X-100). The protein concentration was determined with the BCA protein assay kit (Pierce) based on the Biuret reaction. The fraction containing the recombinant protein was diluted in glycerol to a final volume of 50% and stored at −20°C.
Crystallization of sapovirus 3Dpol.
The DNA corresponding to sapovirus 3Dpol coding sequence was generated by PCR from sapovirus full-length cDNA clone pJG-Sap01 (GenBank accession number AY694184) as described above. The PCR product was molecularly cloned into the pDEST14 vector (Gateway) according to the manufacturer's instructions and using primers VZ5-Sap-3D-H6N-for (5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTTCGAAGGAGATGCCACCATGAAACATCACCACACCATCACGATGAATTTCAATGGAAGGGTTTGCCCGTGG-3′) and VZ6-Sap-3D-H6N-rev (5′-GGGGACCACTTTGTACAAGAAAGCTGGGTCTTATTACTCCATCTCAAACACTATTTTGTGGGTTCC-3′), yielding clone pVZ-Sap-3D-H6N. A sequence corresponding to the ribosome binding site as well that for a hexahistidine tag were included at the 5′ end of the gene. A multiparameter expression screening in Escherichia coli was performed using a sparse matrix (1), followed by a dot blot procedure (61). The best expression condition [Rosetta2 (DE3)pLysS (Novagen) strain using 2YT medium at 37°C for 3 h after induction with 0.5 mM IPTG] was determined for protein production. The cell pellet was harvested by centrifugation and then resuspended in lysis buffer (5 g cell pellet per 30 ml) containing 50 mM Tris, pH 7.8, 300 mM NaCl, and 5 mM imidazole. The lysis solution was sonicated on ice for 2 min with 3-s pulses. The lysate was clarified by centrifugation at 22,000 rpm for 1 h. The supernatant was retained and filtered through a 0.4-μm filter, and the N-terminal His6 tag was utilized in nickel-affinity chromatography (Ni-NTA) with the sapovirus RdRp eluting over a 0 to 300 mM imidazole gradient. The purified protein was further polished on a Sephadex 200 gel filtration column with a buffer containing 50 mM bicine, pH 8.8, 300 mM NaCl before concentration to 7 mg/ml, as measured by using the theoretical extinction coefficient determined at 280 nm. The protein sample was centrifuged at 100,000 × g for 40 min to remove any aggregate arising from the concentration procedure. Subsequent crystallization trials were carried out at the EMBL high-throughput crystallization facility Hamburg (Hamburg, Germany), with the initial trials leading to optimized conditions of 20% polyethylene glycol (PEG) 4k, 0.25 M ammonium sulfate, 0.1 M citrate, pH 5.5, and a protein concentration of 5 mg/ml.
The diffraction data (Table 1) were collected on beam line BW7B at the EMBL Hamburg Outstation at DESY (Hamburg, Germany). The diffraction data were measured to 2.32-Å resolution, and the data were processed and scaled using the MOSFLM and SCALA programs of the CCP4 program package (14, 31). The phases were obtained by molecular replacement using the program AMORE (42) and the rabbit hemorrhagic disease virus (RHDV) RdRp (PDB identification no. 1KHV) as the model (43). The crystals were primitive orthorhombic in space group P212121, with unit cell dimensions of a = 58.43, b = 93.88, and c = 94.77 Å, and contain one molecule in the asymmetric unit. The model was built using consecutive rounds of ARP/wARP (29), manual building, and refinement with REFMAC5 (39). The coordinates and structure factors have been deposited with the PDB with code 2CKW. A summary of the model quality is given in Table 1.
TABLE 1.
Crystallographic data for the sapovirus RNA-dependent RNA polymerase
| Parameter | Resulta |
|---|---|
| Wavelength (Å) | 0.961 |
| No. of total reflections | 105,410 |
| No. of unique reflections | 22,387 |
| Rmergeb (%) | 11.5 (36.4) |
| I/σI | 5.2 (2) |
| Highest resolution shell (Å) | 2.46-2.32 |
| Rworkc (%) | 17.3 |
| Rfreec (%) | 22.9 |
| a (Å) | 58.43 |
| b (Å) | 93.88 |
| c (Å) | 94.77 |
| Space group | P212121 |
| Resolution limits (Å) | 43.93-2.32 |
| Completeness (%) | 99.5 (99.4) |
| Mean multiplicity | 3.9 |
| No. of residues in most favored regions | 377 (90.4%) |
| No. of residues in additional allowed | |
| regions | 36 (8.6%) |
| No. of residues in generously allowed | |
| regions | 3 (0.7%) |
| No. of residues in disallowed regions | 1 (0.2%) ASN 288 |
| Average B values | 20 |
| Bond length RMS deviation (Å) | 0.022 |
| Bond angle RMS deviation (degrees) | 1.95 |
Value in parentheses refers to the high-resolution shell.
Rmerge = Σh Σi (Ii(h) − <I(h)>)/Σh Σi Ii(h), where Ii(h) is the ith intensity measurement and <I(h)> is the mean of all measurements of I(h).
Rwork and Rfree = Σh(F(h)obs − F(h)calc)/ΣhF(h)obs.
Micrococcal nuclease treatment of purified sapovirus 3Dpol.
Purified sapovirus 3Dpol was treated with micrococcal nuclease, as described previously for norovirus 3Dpol (50), to remove RNA or DNA fragments that might act as primers in the 3Dpol assay. The activity of the polymerase was subsequently assessed in the RNA-dependent RNA polymerase assay.
RNA-dependent RNA polymerase assay.
The RNA-dependent RNA polymerase activity of sapovirus 3Dpol was assessed in vitro. The reaction mixture consisted of 1 μg synthetic RNA template (2,259 nt in length, yielding a final concentration of 0.024 μM), 50 mM HEPES (pH 7.0), 3 mM magnesium acetate, 4 mM DTT, and 6 μM ZnCl2, 50 U of RNase inhibitor (RNAsin; Promega), 0.4 mM (each) ATP, CTP, and GTP, 0.1 mM UTP as well as 0.07 μM [α-32P]UTP (3,000 Ci/mmol; Hartmann Analytic) when [α-32P]UMP incorporation was assessed, and RNase-DNase free water to a final volume of 50 μl. In each reaction, 3 μM purified SV 3Dpol was added, and the reaction was carried out at 37°C for 2 h. It was stopped by adding 50 μl of stop solution (4 M ammonium acetate, 100 mM EDTA) and purified with the MEGAclear kit (Ambion) according to the manufacturer's instructions.
Reaction products were separated on agarose gels under nondenaturing or denaturing conditions, as described previously (50). They were visualized using either UV transillumination or autoradiography (50), and incorporation of [α-32P]UMP was determined by precipitation of the SV 3Dpol products as described previously (50).
For primer-dependent initiation of RNA synthesis by norovirus 3Dpol on homopolymeric RNA templates, 1 μg homopolymeric poly(U) RNA, poly(G) RNA, poly(C) RNA or poly(A) RNA was used as template, together with 3 μM oligo(A)20, oligo(C)20, oligo(G)20, or oligo(U)20 RNA primer under the conditions described above. Similarly, primer-dependent initiation of RNA synthesis by sapovirus 3Dpol on polyadenylated subgenomic RNA (0.024 μM) was investigated in the presence of 3 μM oligo(U)20 RNA primer. In parallel, initiation of RNA synthesis by sapovirus 3Dpol was investigated under the same conditions as described except that anti-subgenomic RNA (0.024 μM) was used in the absence (primer-independent) of an RNA primer.
Nuclease treatment of sapovirus 3Dpol reaction product.
The 3Dpol reaction products of 1 μg RNA template (final concentration of 0.024 μM) were incubated with S1 nuclease (Promega) and 1 μl 10× S1 nuclease buffer (500 mM sodium acetate [pH 4.5], 45 mM ZnSO4) with low (50 mM) or high (250 mM) NaCl concentrations and RNase-DNase free water to a final volume of 10 μl. The reaction mixture was incubated at 37°C for 60 min and then stopped by the addition of 1 μl of 1 M EDTA. The reaction mixture was resuspended in denaturing loading buffer, heated at 65°C (or boiled when appropriate) for 5 min, then flash chilled on ice, loaded onto agarose gels, and autoradiographed and/or visualized under UV-light after ethidium bromide staining.
Assessing the terminal transferase activity of sapovirus 3Dpol.
The same conditions described for assessment of sapovirus 3Dpol activity were used with in vitro-transcribed subgenomic RNA as a template, except that instead of a nucleoside triphosphate (NTP) mixture, only [α-32P]ATP, [α-32P]GTP, [α-32P]CTP, or [α-32P]UTP was added separately (each at a final concentration of 0.07 μM, 3,000 Ci/mmol) to the reaction mixture. Incorporation of [α-32P]AMP, [α-32P]GMP, [α-32P]CMP, or [α-32P]UMP radioactivity as well as visualization of the products was performed as described above.
RESULTS
Domain design.
Prior analysis of polyprotein processing showed that 3Dpol is proteolytically released as part of a larger and stable 3Cpro3Dpol protein in sapovirus (46). To predict the 3Cpro/3Dpol domain border in sapovirus, a calicivirus-wide amino acid alignment of 3Cpro3Dpol was produced, as described in Materials and Methods. It was used to align the 3Cpro/3Dpol cleavage site in those caliciviruses, where 3Cpro3Dpol is processed to 3Cpro and 3Dpol, and identify its tentative counterpart in the 3Cpro/3Dpol junction area of sapoviruses. Using this approach, the N terminus of 3Dpol was predicted to start with Asp1207 (Fig. 1).
Heterologous expression and purification of recombinant sapovirus 3Dpol.
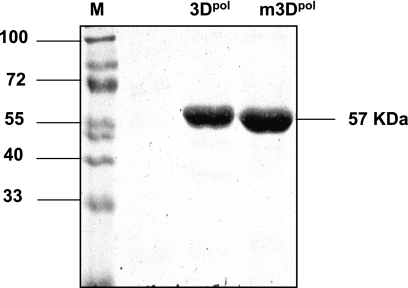
A fusion protein bearing a His6 tag at its C terminus or at its N terminus was overexpressed in E. coli and purified by Ni-NTA affinity chromatography. A soluble sapovirus protein of about 57 kDa was obtained (Fig. 2). Analogously, an active site mutant of sapovirus 3Dpol (m3Dpol) was expressed and purified. It displayed the same characteristics in terms of solubility and migration during sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis (Fig. 2).
FIG. 2.
Expression and purification of sapovirus 3Dpol in E. coli. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of purified recombinant sapovirus 3Dpol expressed in E. coli and carrying a C-terminal His6 tag. 3Dpol, wild-type sapovirus 3D-like polymerase; m3Dpol, active site sapovirus 3Dpol mutant (YGD343GD344G); M, molecular mass marker (in kDa).
Structure of sapovirus 3Dpol.
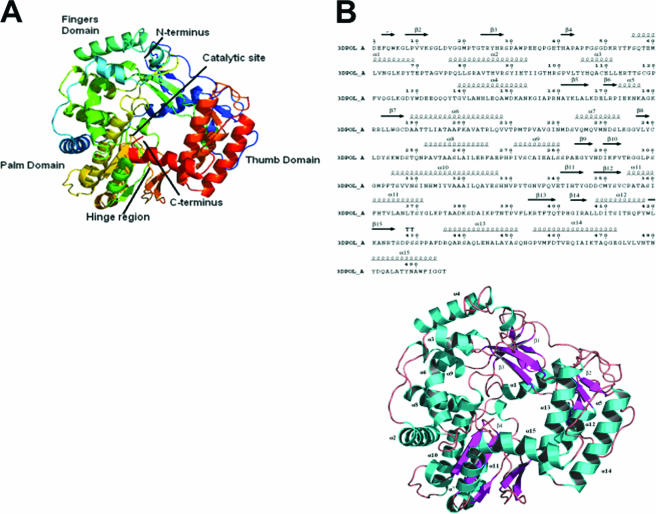
The structure of the 3Dpol from sapovirus has been determined to 2.32 Å resolution (PDB identification no. 2CKW). The model was refined to an R factor of 17.3% and free R factor of 22.9% and contains 488 amino acids (amino acids [aa] 1 to 375 and 385 to 496) and 271 water molecules. The N-terminal His6 tag, 19 C-terminal amino acids (aa 497 to 515), and 8 amino acids between pro375 and lys385 are missing from the structure. These residues cannot be modeled because of poor experimental electron density. The domain structure of sapovirus 3Dpol resembles the right hand configuration that was used to first describe the canonical structure of DNA polymerase (47) and has since been seen in the published structures of many DNA and RNA polymerases (6, 10, 21, 30, 43, 44, 58). The fingers and palm of the right hand form comparatively rigid domains, with the more flexible thumb domain positioned in the enclosed conformation, making contacts with the finger domain (6, 43). The fingers, thumb, and palm domains combine to form a semicylinder-like shape with a diameter of 58.4 Å around a central channel with a diameter of 25 Å, giving the overall structure 2,051 surface atoms and an accessible surface area of 21,752 Å2. The sapovirus 3Dpol adopts an enclosed conformation where the extended N-terminal region, common in all RdRp structures, bridges across the thumb and finger domains to form the right-hand-shaped semicylinder that encloses the active site, as first seen for the hepatitis C virus RdRp (30) (Fig. 3A). The N-terminal domain (residues 1 to 57) comprises two small anti-parallel β-strands (β1 and β2), which form a twisted anti-parallel β-sheet with β strands from the finger domain. The rest of the N-terminal domain consists of an extended interwoven loop which bridges between the fingers and the thumb domains, forming the closed right hand. The N-terminal region (residues 12 to 54) has been identified as a region which can undergo significant conformational variation, depending on whether the RdRp has the open (inactive) or closed (active) conformation, as seen previously in other viral RNA-dependent RNA polymerases (6, 10, 21, 43).
FIG. 3.
Structure of sapovirus 3Dpol. (A) The structure is colored from the N terminus (blue) to the C terminus (red). The three major domains of thumb, fingers, and palm are clearly visible with the N-terminal extension (blue) reaching across from the top of the fingers to the thumb domain (red). (B) A ribbon diagram of the sapovirus 3Dpol. The α-helices are represented by α 1 to 15 (aqua) and the β-sheets are represented by β 1 to 4 (lilac).
The sapovirus 3Dpol finger domain contains a five-stranded β-sheet and the eight α-helices (Fig. 3B), as also observed for both the norovirus and RHDV 3Dpol. The finger domain is inserted between the N-terminal extension and the highly conserved palm domain. The interface between the N-terminal extension consists of the β-sheet region, containing four strands from the finger domain and one from the N-terminal extension, a long loop region (residues 173 to 188), and α-helix 1. The finger domain contains the RdRp helix-loop-helix region (residues 252 to 280), which is situated on the outer face of the domain. The remainder of the finger domain is made up of five α-helices (α2 to 6). The sapovirus 3Dpol structure displays the same short loop segment between the third and fourth α-helices reported to be essential for the use of an RNA-primer duplex as a polymerization template (22).
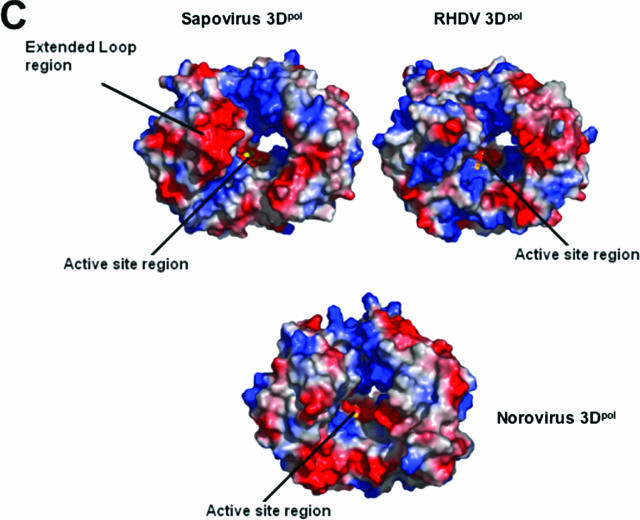
The α-helices 2, 6, and 8 form the finger-palm interface with α-helix 10 in the palm domain. The most striking difference between the finger domains in the sapovirus 3Dpol and the RHDV 3Dpol is an extended loop region 130 to 138 which is positioned on the opposite side of the central cavity adjacent to the C-terminal residues. This extended loop region contains three additional residues compared with the norovirus and RHDV 3Dpol models and is positioned further toward the opening of the central cavity, forming a negative patch on one side of the opening to the active site (Fig. 4A).
FIG. 4.
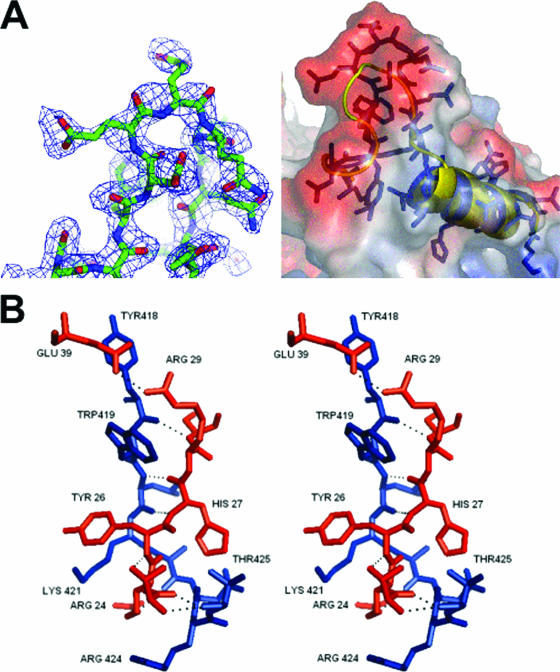
Structural comparison of sapovirus, norovirus, and RHDV 3D-like polymerases (3Dpol). (A) A two-dimensional representation of the super-positioning of the RDHV and sapovirus 3Dpol loop region in the palm domain of sapovirus 3Dpol. The loop region (residues 130 to 138) in the sapovirus 3Dpol is clearly extended in comparison to its structural homologue RHDV 3Dpol, and the negative (red section) charged patch of residues faces into the opening of the central core, with the positive region facing out from the 3Dpol into the solvent-exposed area. (B) A stereo representation of the interdigitation between the N-terminal extension (residues 24 to 39) and the thumb domain counterparts (residues 418 to 425) of sapovirus 3Dpol. (C) A comparison of electrostatic surfaces between the sapovirus 3Dpol and its close structural homologues, the RDHV 3Dpol (PDB identification no. 1KHV) and the norovirus 3Dpol (PDB identification no. 2B43). The sapovirus 3Dpol contains two major differences on the surface at the opening of the binding site core. The first is the extended loop region (residues 130 to 138) on the upper left of the diagram in red. The second is the C-terminal tail which folds back into the core, producing a second negative patch at the entrance to the core of the palm domain (lower right, in red). In comparison, the RHDV 3Dpol has very little negative charge surrounding the entrance to the core palm domain. In contrast, the C-terminal tail of norovirus 3Dpol displays a conformation similar to that of sapovirus 3Dpol.
The palm domain has the highest structural conservation, with an α/β fold containing a three-stranded anti-parallel β-sheet, consisting of a single β strand and a β hairpin locked between α-helix 10 and α-helix 11 on the internal side of the polymerase and α-helix 7 on the outer side. The palm domain contains the catalytically important GDD sequence, which is present in all known RdRps of positive-stranded RNA viruses. The GDD motif (Gly346, Asp347, and Asp348) is thought to be involved in metal ion interactions linked to nucleotidyl transfers during RNA elongation (44). However, no metals are observed in this model. Soaking crystals with either Mn2+ or Mg2+ did not result in observable density for the metals in difference maps. The structure of a sapovirus 3Dpol active site mutant (GD347GD348G) at 3-Å resolution (data to be published) shows the Cα trace to be approximately the same as the native structure, indicating that the loss of activity is the result of the loss of the enzymatically active aspartate side chains and is not the result of a conformational change. The interface between the palm region and the thumb is supplied via an extended loop (residues 388 to 403) and a loosely arranged β-hairpin structure (residues 404 to 409) on which the thumb domain is believed to hinge.
The thumb domain contains four α-helices interconnected by two extended loops, one linking α-helix 12 with 13 and the other linking α-helix 14 with 15. The thumb domain interacts with the N-terminal extension via α-helix 12 and the loop containing residues 418 to 435 which link helix 12 and 13. The part of the loop containing residues 418 to 425 forms a small two-stranded β-sheet with residues 24 to 28 from the N-terminal extension. This interaction involves seven hydrogen bonds between the two domains (Fig. 4B), resulting in the formation of a complex interdigitation of the side chains, which locks the right hand into the enclosed conformation (Fig. 4B). The side chains of Arg25, Tyr26, and Arg28 interdigitate with the side chains of Arg424, Lys421, and Trp419. Glu39 forms an additional hydrogen bond with Arg29, the side chains of which are located between the Tyr418 and Trp419 side chains (Fig. 4B). These interactions form an interlocked bridge between the thumb and N-terminal extension that resembles the fingers of joined hands. The C terminus of sapovirus 3Dpol points toward the channel containing the active site and is situated opposite to the extended loop region (residues 130 to 138), forming two negatively charged patches on either side of the mouth of the active site channel. These negatively charged patches may help regulate the RNA binding to the positively charged central core (Fig. 4C).
Biochemical characterization of sapovirus 3Dpol activity.
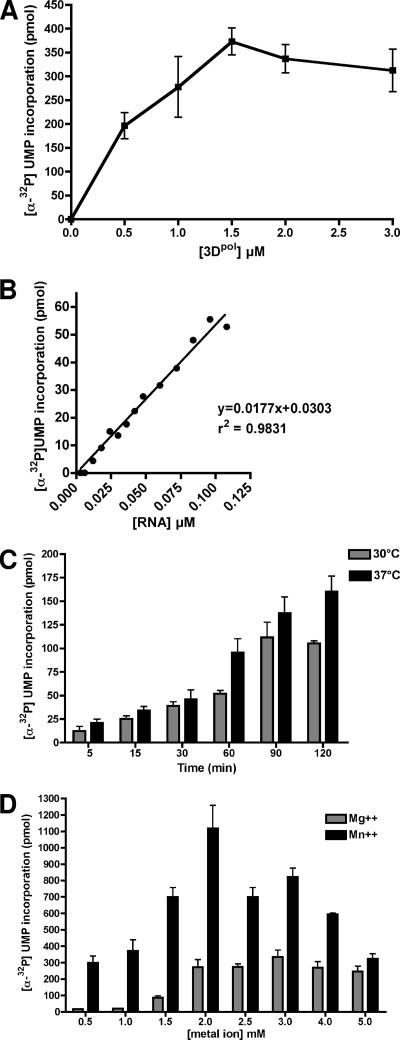
RNA synthesis by SV 3Dpol was characterized by measuring the relationship between incorporation of [α-32P]UMP and enzyme concentration in the reaction mixture. As shown in Fig. 5A, the amount of UMP incorporated increased with the concentration of sapovirus 3Dpol up to 1.5 μM, where the reaction reached its saturation phase. Sapovirus 3Dpol activity displayed a clear correlation to the template's concentration over a broad linear range (0.000 to 0.100 μM; r2 = 0.9831; 95% confidence intervals, 0.9726 to 0.9974; P < 0.0001) (Fig. 5B). The temperature dependence of 3Dpol activity was also determined. Sapovirus 3Dpol was found to display a higher activity at 37°C compared to 30°C (Fig. 5C). To address the metal ion dependence of sapovirus 3Dpol, the enzyme's activity was measured in the presence of increasing concentrations of Mg2+ or Mn2+, using subgenomic RNA as a template. Over a concentration range from 0.5 to 5 mM, sapovirus 3Dpol displayed a clear preference for Mn2+ over Mg2+ (Fig. 5D). However, sapovirus 3Dpol was also active in the presence of Mg2+, indicating that sapovirus 3Dpol displays flexibility with respect to the use of Mg2+ or Mn2+ as a cofactor.
FIG. 5.
Concentration dependence, substrate dependence, temperature dependence, and metal ion dependence of sapovirus 3Dpol activity. In all reaction mixtures, subgenomic RNA was used as a template. (A) Concentration-dependent activity of sapovirus 3Dpol. RNA synthesis analysis was performed in the presence of 0.024 μM RNA and increasing concentrations of sapovirus 3Dpol (0.0 to 3.0 μM). Incorporation of [α-32P]UMP was measured as indicated. The reaction was run at 37°C for 2 h. The means and standard errors of the means of results from three independent experiments are shown. (B) Correlation of sapovirus 3Dpol activity with the template's concentration. RNA synthesis analysis was performed in the presence 3 μM sapovirus 3Dpol for 2 h at 37°C, and increasing concentrations of subgenomic RNA were used as templates (0.00 to 0.100 μM). Incorporation of [α-32P]UMP was measured as indicated. (C) Temperature-dependent activity of sapovirus 3Dpol. The reaction was run at 30°C or 37°C and stopped at the indicated time. In all reaction mixtures, 3 μM sapovirus 3Dpol was used. Incorporation of [α-32P]UMP was measured as indicated. The means and standard errors of the means of results from three independent experiments are shown. (D) Metal ion dependence of sapovirus 3Dpol activity. RNA synthesis was performed in the presence of 3 μM sapovirus 3Dpol for 2 h at 37°C. Subgenomic RNA was used as a template (0.024 μM), with increasing concentrations (0.5 to 5 mM) of Mg acetate or MnCl2. Incorporation of [α-32P]UMP was measured as indicated. The means and standard errors of the means of results from three independent experiments are shown.
Sapovirus 3Dpol synthesizes RNA from a subgenomic polyadenylated RNA template only in the presence of an oligo(U)20 primer.
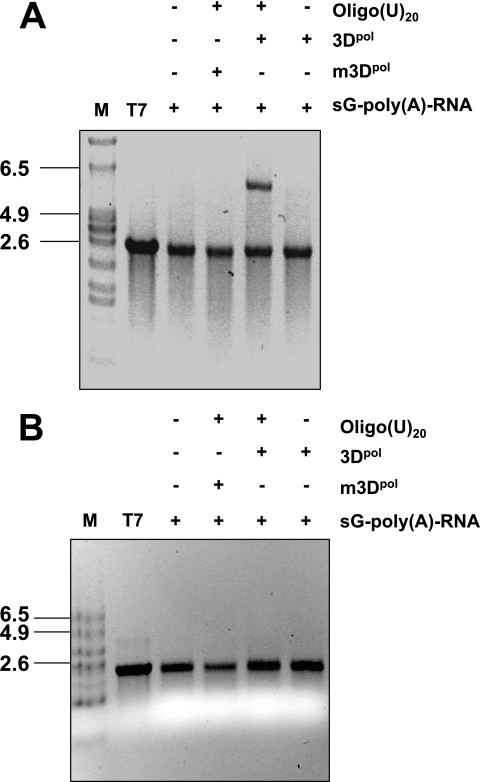
To characterize the mode of RNA synthesis by sapovirus 3Dpol, an enzymatic assay was developed using a synthetic subgenomic polyadenylated RNA as template. This subgenomic RNA is predicted to be a naturally occurring transcript during the replication of the sapovirus genome. Importantly, possible DNA or RNA contaminants in the recombinant protein preparation, which could prime RNA synthesis, were eliminated by treatment with micrococcal nuclease. Sapovirus 3Dpol was able to synthesize the full-length complement of the subgenomic RNA template (2,259 nt) in a primer-dependent manner. On nondenaturing agarose gels, the product of the 3Dpol reaction migrated more slowly than the template (Fig. 6A), being about twice the size of the template. In contrast, the replication product was not generated by mutated 3Dpol or when the oligo(U)20 RNA primer was omitted from the reaction (Fig. 6A), indicating the strict primer dependency of sapovirus 3Dpol for initiation of RNA synthesis on polyadenylated subgenomic RNA template. Upon denaturation, the reaction product migrated similarly to the template, indicating that the product resulting from sapovirus 3Dpol RNA synthesis was not covalently linked to template RNA (Fig. 6B).
FIG. 6.
RNA synthesis by sapovirus 3Dpol. (A) RNA synthesis was examined in the presence of a synthetic subgenomic polyadenylated RNA [sG-poly(A)-RNA] used as a template in the presence (+) or absence (−) of 3 μM oligo(U)20 RNA-primer and in the presence of wild-type sapovirus 3Dpol (3Dpol) or an active site 3Dpol mutant (m3Dpol, YGD343GD344G), as indicated. Reaction products were analyzed on a nondenaturing agarose gel and visualized by UV transillumination after ethidium bromide staining. In the reaction using m3Dpol instead of wild-type 3Dpol, or omitting the addition of oligo(U)20 RNA-primer, the residual band observed corresponds to the synthetic subgenomic RNA template used in the reaction. T7, synthetic subgenomic RNA generated by T7-mediated in vitro transcription; M, RNA molecular mass marker. (B) Strand separation analysis of the reaction product of in vitro RNA synthesis by sapovirus 3Dpol. The reaction product was generated from RNA synthesis by sapovirus 3Dpol (3Dpol) from a synthetic subgenomic polyadenylated RNA [sG-poly(A)-RNA] used as a template, as indicated. Reaction products were visualized on denaturing formaldehyde-agarose gels. T7, synthetic subgenomic polyadenylated RNA generated by T7-mediated in vitro transcription; M, RNA molecular mass marker.
Sapovirus 3Dpol initiates RNA synthesis de novo on anti-subgenomic heteropolymeric RNA templates.
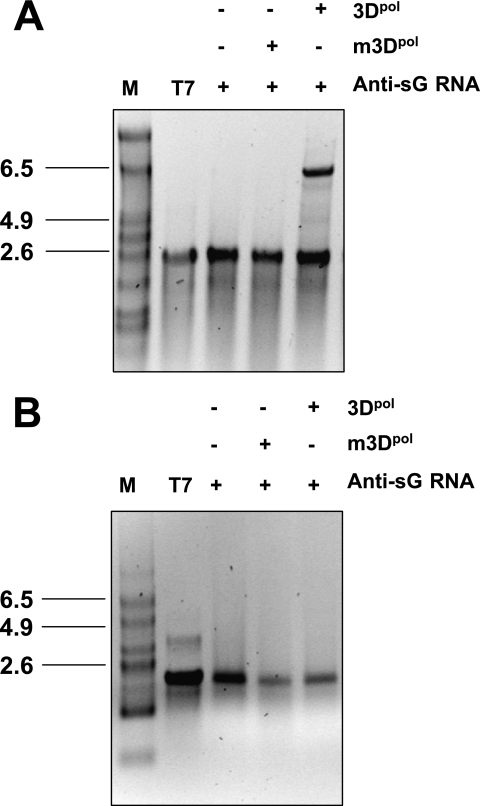
To assess the ability of sapovirus 3Dpol to initiate RNA synthesis on anti-subgenomic RNA, sapovirus anti-subgenomic RNA was synthesized by in vitro transcription. Sapovirus 3Dpol was able to replicate anti-subgenomic RNA in the absence of an RNA primer (Fig. 7A). Strand separation analysis of the sapovirus 3Dpol replication product allowed complete resolution of the double-stranded RNA on denaturing gels, indicating that the replication product did not consist of two RNA strands covalently linked, as would had been expected in the case of a back-priming initiation of sapovirus 3Dpol RNA synthesis (Fig. 7B).
FIG. 7.
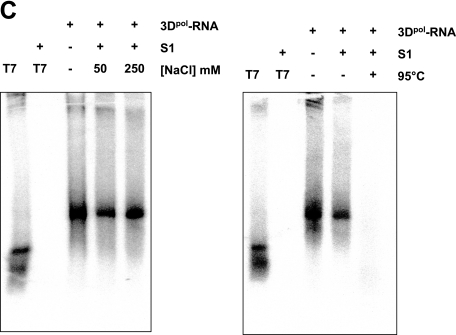
De novo initiation of RNA synthesis on sapovirus anti-subgenomic RNA. (A) RNA synthesis was examined in the presence of a synthetic anti-subgenomic RNA (Anti-sG RNA) used as a template and in the presence wild-type sapovirus 3Dpol (3Dpol) or an active site 3Dpol mutant (m3Dpol, YGD343GD344G), as indicated. Reaction products were analyzed on nondenaturing agarose gel and visualized by UV transillumination after ethidium bromide staining. In the reaction using m3Dpol instead of wild-type 3Dpol, the residual band observed corresponds to the synthetic anti-subgenomic RNA template used in the reaction. T7, synthetic anti-subgenomic RNA generated by T7-mediated in vitro transcription; M, RNA molecular mass marker; +, present; −, absent. (B) Strand separation analysis of the reaction product of in vitro RNA synthesis by sapovirus 3Dpol. The reaction product was generated from RNA synthesis by sapovirus 3Dpol from a synthetic anti-subgenomic RNA (Anti-sG RNA) used as a template, as indicated. Reaction products were visualized on denaturing formaldehyde-agarose gels. T7, synthetic anti-subgenomic RNA generated by T7-mediated in vitro transcription; M, RNA molecular mass marker. (C) The sapovirus 3Dpol synthesis product (3Dpol-RNA) was generated from synthetic anti-subgenomic RNA (T7) used as a template. The RNA synthesis product was then incubated in the presence (+) or absence (−) of S1 nuclease (S1), in the presence of low-salt (50 mM NaCl) or high-salt (250 mM NaCl) concentrations, or after heating (95°C, 5 min) and rapid chilling on ice for 5 min, as indicated. Reactions products were analyzed on nondenaturing agarose gels and visualized by autoradiography. 3Dpol-RNA, sapovirus 3Dpol synthesis product using anti-subgenomic RNA as a template. T7, synthetic anti-subgenomic RNA generated by T7-mediated in vitro transcription.
To further characterize the nature of the product of sapovirus 3Dpol synthesis, treatment with nuclease S1 was performed. In contrast to the exogenous single-stranded RNA template, the sapovirus 3Dpol product was not susceptible to S1 nuclease treatment, indicating that it is double-stranded RNA (Fig. 7C). However, decreasing NaCl concentrations (50 mM) in the S1 nuclease reaction allowed partial digestion of the synthesized RNA, as expected in the case of low-ionic-strength buffer (Fig. 7C). Denaturation of SV 3Dpol products by heating at 95°C followed by incubation with S1 nuclease allowed their complete digestion, indicating that sapovirus 3Dpol synthesis product consists of the cRNA strands (Fig. 7D).
Sapovirus 3Dpol initiates RNA synthesis on homopolymeric RNA templates.
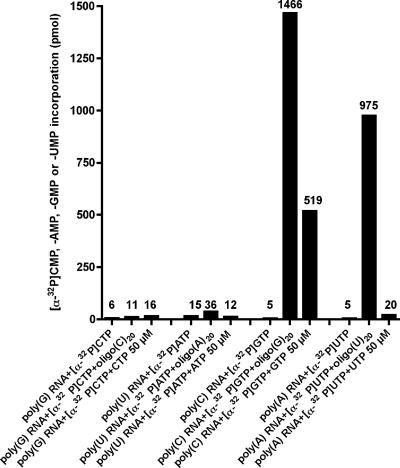
Initiation of RNA synthesis by sapovirus 3Dpol was examined on homopolymeric RNA templates. In the absence of an oligonucleotide primer, no incorporation of [α-32P]AMP, [α-32P]CMP, [α-32P]GMP, or [α-32P]UMP was seen (Fig. 8). In contrast, addition of a complementary oligonucleotide RNA-primer to the reaction led to incorporation of [α-32P]GMP and [α-32P]UMP on homopolymeric poly(C)-RNA and poly(A) RNA templates, respectively. Interestingly, addition of cold GTP to the reaction mixture (50 μM) allowed incorporation of [α-32P]GMP even in the absence of an oligonucleotide RNA-primer. This was only observed on poly(C) templates, indicating that synthesis of RNA from poly(C) homopolymeric templates can take place in a primer-independent manner. For poly(G), poly(U), and poly(A) RNA templates, no incorporation of [α-32P]CMP, [α-32P]AMP, or [α-32P]UMP was seen in the absence of a primer, even after addition of high concentrations of cold CTP, ATP, or UTP, respectively.
FIG. 8.
De novo initiation of RNA synthesis by sapovirus 3Dpol on homopolymeric templates. RNA synthesis was performed by incubating poly(G) RNA, poly(U) RNA, poly(C) RNA, and poly(A) RNA templates with [α-32P]CTP, [α-32P]ATP, [α-32P]GTP, or [α-32P]UTP, respectively, and with or without 3 μM oligo(C)20, oligo(A)20, oligo(G)20, or oligo(U)20 RNA-primer, respectively. In parallel, RNA synthesis was performed in the absence of an oligo(RNA)-primer but in the presence of 50 μM cold CTP, ATP, GTP, or UTP for poly(G)-RNA, poly(U) RNA, poly(C) RNA, and poly(A) RNA templates, respectively. Incorporation of [α-32P]CMP, [α-32P]AMP, [α-32P]GMP, or [α-32P]UMP was measured as indicated.
Our results indicate that sapovirus 3Dpol initiates RNA synthesis on poly(C) or poly(A) homopolymeric RNA templates in a primer-dependent manner. In addition, de novo initiation is possible on poly(C)-RNA templates in the presence of high concentrations of GTP.
Sapovirus 3Dpol exhibits a terminal transferase activity in vitro.
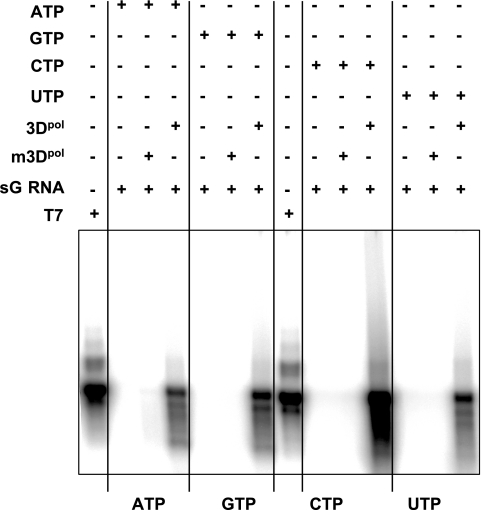
Terminal transferase activity of RNA polymerase has already been reported for RNA viruses, e.g., hepatitis C virus (49) and poliovirus (2), adding nucleotides to the 3′ ends of newly synthesized RNAs. This activity could potentially be used by RNA viruses as a mechanism to restore the 3′ initiation site of RNA synthesis (49) and is one of the prerequisites for initiation of RNA synthesis by back priming. To characterize the terminal transferase activity of sapovirus 3Dpol, the enzyme was incubated with subgenomic RNA template in a reaction mixture containing the α-32P-labeled version of only one of the NTPs. Sapovirus 3Dpol displayed terminal transferase activity that was higher in the presence of CTP than in that of GTP, ATP, or UTP (Fig. 9).
FIG. 9.
Terminal transferase activity of sapovirus 3Dpol. Terminal transferase activity was examined in a reaction mixture consisting of 3 μM sapovirus 3Dpol or 3Dpol mutated in its active site (m3Dpol) incubated for 2 h at 37°C in the presence of subgenomic RNA (sG RNA) used as a template (0.024 μM) and [α-32P]ATP, [α-32P]GTP, [α-32P]CTP, or [α-32P]UTP, as indicated. Reactions products were analyzed on nondenaturing agarose gels and visualized by autoradiography. T7, synthetic subgenomic RNA generated by T7-mediated in vitro transcription; +, present; −, absent.
DISCUSSION
Sapovirus is a member of the Caliciviridae, a virus family regrouping animal (mainly vesivirus and lagovirus but also norovirus and sapovirus) and human pathogenic (exclusively norovirus [NV] and SV) strains. Classification of caliciviruses has been so far based on the sequence of the complete capsid gene, mainly because of the lack of a cell system to isolate and type norovirus, sapovirus, and lagovirus strains, with the exception of vesivirus, i.e., the feline calicivirus (FCV). This limitation in the cultivation of calicivirus has hampered studies on the replication strategies of these important human and animal pathogens. An alternative approach to cell-based studies has relied on structural and functional characterization in vitro of the viral enzymes putatively implicated in replication, such as the viral polymerase (4, 17, 21, 43, 44, 50, 51, 59, 60, 62), the viral protease (5, 40, 45, 54-56, 65), or the viral NTPase (36, 48). Furthermore, reverse genetic systems for Norwalk virus (norovirus) (3, 28) and porcine enteric calcivirus (sapovirus) have been developed (7, 8). Those systems may allow us, in the near future, to address the replication strategy of norovirus and sapovirus in cell-based systems.
Recently, Oka et al. brought new insights in the translation strategy of sapovirus (46), showing in a cell-free system that the processing of the polyprotein precursor of sapovirus occurs cotranslationally, subsequently leading to generation of at least 7 viral proteins. Among those, proteins carrying the putative NTPase, the putative VPg (virion protein genome-linked), the putative protease-polymerase precursor (3Cpro3Dpol), and the VP1 were identified. This processing is similar to the one observed in human (NV) and animal (FCV and RHDV) caliciviruses, with one exception relative to the processing of the protease-polymerase (3Cpro3Dpol) precursor. Indeed, in NV, FCV, and RHDV, the polymerase 3Dpol rather than the fusion protein 3Cpro3Dpol is released from polyprotein, and it has been identified as a stable product in translation systems in vitro (5, 37, 57, 64). Similar observations were made in prokaryotic expression systems for NV, FCV, and RHDV (32, 55-57, 62-64). However, expression of FCV ORF-1 in mammalian cells yielded only a stable protease-polymerase precursor, indicating that the cleavage of the protease-polymerase precursor possibly depends on the expression system, as suggested by others (46).
In this study, the structure and functional properties of sapovirus 3Dpol have been determined for the first time. According to our data, sapovirus 3Dpol displays the typical right hand shape of viral RNA polymerases, with its C terminus located near the active site cleft. Our results also show that, in contrast to its active site mutant, sapovirus 3Dpol is able to synthesize RNA in vitro. Its activity is concentration, temperature, and metal ion dependent. Initiation of RNA synthesis is primer dependent on polyadenylated templates or occurs de novo on heteropolymeric templates, whereas labeling of the template's 3′ terminus depends on the terminal transferase activity of the enzyme.
To investigate the polymerase activity of sapovirus in vitro, a 3Dpol domain encompassing the predicted sapovirus RNA polymerase motifs A to E was delineated in the polyprotein precursor. This domain displayed homologies to the other RNA-dependent RNA polymerases of calicivirus, i.e., the norovirus, vesivirus, and lagovirus. Amplification of the encoding DNA and subsequent cloning in a prokaryotic expression vector yielded the soluble recombinant protein that was purified by Ni-NTA affinity chromatography. As a negative control, an active site mutant of sapovirus 3Dpol was generated.
In the next step, the structure of sapovirus RdRp was determined at 2.3-Å resolution. For this purpose, an N-terminal His-tagged recombinant sapovirus 3Dpol was expressed and purified. The choice to place the His tag at the N terminus of the protein is explained by the fact that, in norovirus, the C terminus of the protein is located within the active site cleft (21, 44). To exclude a possible interaction of a C-terminal His tag with the active site cleft that may influence folding of the protein's C terminus, the His tag was placed at the N terminus of the recombinant sapovirus 3Dpol. Sapovirus displayed a right hand structure typical for RNA/DNA polymerases (6, 10, 16, 21, 43, 44). The sapovirus 3Dpol structure has the greatest similarity with the previously submitted RNA-dependent RNA polymerase structure from the RHDV (43) and contains important structural (21, 44) and functional (17, 50, 51) similarities with the norovirus RNA-dependent RNA polymerase. The overall structure of the finger, palm, and thumb domains are most similar to the RHDV 3Dpol and NV 3Dpol with 35% and 34% sequence identity, respectively. A superimposition of the sapovirus 3Dpol structure with the RdRps from RHDV and norovirus produced a root mean square (RMS) deviation over 418 Cαs of 5.44 Å for RHDV RdRp and over 423 Cαs of 13.77 Å for norovirus RdRp. The fingers and palm of the right hand form comparatively rigid domains in relation to each other, with the thumb domain presumed to flex on the hinge region (residues 388 to 403) between the palm and thumb domains, as seen in RHDV and norovirus 3Dpol (21, 43). The sapovirus 3Dpol structure exhibits an enclosed conformation, with the thumb making contacts with the N-terminal extension between the finger and thumb domains. The tip of the thumb domain forms an extended loop (residues 419 to 336) that displays significant conformational variation in other calicivirus 3Dpol structures, depending on whether the RdRp has the open (inactive) (21, 42) or closed (active) conformation. This variability in the conformation of the loop region of the thumb domain has been previously seen in structures of other viral RNA-dependent RNA polymerases, such as hepatitis C virus NS5B (6, 21, 44). This would suggest that the sapovirus 3Dpol thumb domain could display similar conformational variation. The structural similarities that exist in the shortened loop section of sapovirus, norovirus, and RHDV 3Dpol (21, 43, 44) suggest that calicivirus RdRps have similar mechanisms for activation of RNA elongation, allowing both single-stranded and double-stranded RNA activation templates. The interdigitation within the sapovirus 3Dpol structure provides a locking mechanism due to hydrogen bonding interactions and buried hydrophobic surfaces, holding the 3Dpol in the enclosed conformation. An examination of the closed conformations of norovirus and RHDV polymerase structures shows similar mechanisms of interdigitation holding the thumb and finger domains in the closed conformation (43, 44), with a conserved C-terminal binding domain and conserved counterparts on the N-terminal extensions. The N and C-terminal sequences of the interdigitated regions are also conserved in the porcine enteric calicivirus (sapovirus) and feline calicivirus (vesivirus), both animal pathogens. In poliovirus (1TQL), human rhinovirus (1XR5), and foot-and-mouth disease virus 3Dpol structures (1UO9), all of which exhibit the enclosed form of RNA-dependent RNA polymerases structures, there is little sequence conservation of these residues making specific contacts between thumb and finger in sapovirus 3Dpol (16, 34, 58), and a visual examination of these structures revealed a number of contacts between the thumb and N-terminal extension domains. However, there was no indication of interlocking of the residue side chains. The C-terminal end of the sapovirus RdRp points back toward the active site channel (21, 44), an observation of some relevance because there has been some discussion as to whether this feature is important in the regulation of initiation of RNA synthesis (44).
In a further step, initiation of RNA synthesis by sapovirus 3Dpol was examined in vitro. For this purpose, a C-terminally His-tagged recombinant sapovirus 3Dpol was expressed and purified. The choice to place the His tag at the C terminus of the protein is explained by the fact that, in norovirus, an N-terminally His-tagged norovirus 3Dpol seems to inhibit the activity of the enzyme (4). Furthermore, to be able to compare the mechanisms of initiation of RNA synthesis in both norovirus and sapovirus 3Dpol, the His tag was designed to be located at the C terminus of the sapovirus 3Dpol, similar to previously characterized norovirus 3Dpol (50, 51).
On synthetic polyadenylated RNA templates, sapovirus 3Dpol was able to initiate RNA synthesis in a strict primer-dependent manner. Sapovirus 3Dpol activity was found to depend on the presence of Mg2+ or Mn2+, with a clear preference for Mn2+. This flexibility for metal ion usage has already been described for the 3Dpol of norovirus (4, 17, 50, 51) but also for various viral RNA-dependent RNA polymerases (11, 59), i.e., poliovirus and brome mosaic virus, where the RNA polymerase displays a preference for Mn2+ as well (25).
Our data also indicate that sapovirus 3Dpol synthesizes RNA from heteropolymeric subgenomic RNA by a de novo initiation mechanism rather than by back priming. This observation is supported by strand separation of the synthesis product of sapovirus 3Dpol, yielding two single RNA strands, as well as by its resistance to digestion by S1 nuclease, suggesting that it does not consist of a hairpin dimer. Furthermore, primer-independent initiation was possible on poly(C)-RNA templates in the presence of high concentrations of GTP, indicating a de novo initiation of RNA synthesis on those templates, as observed in norovirus (50) and hepatitis C virus (35). De novo initiation on single-stranded templates is common in RNA viruses and has been also reported in norovirus (50, 51) but also in various RNA-dependent RNA polymerases (26), like brome mosaic virus replicase (27), bovine viral diarrhea virus NS5B (25), and hepatitis C NS5B (35). In norovirus, the 3Dpol initiates RNA synthesis in a primer-dependent manner on polyadenylated templates, whereas initiation of RNA synthesis on heteropolymeric templates occurs de novo (50, 51).
In conclusion and according to our data, sapovirus 3Dpol displays functional and structural features similar to norovirus 3Dpol. Both sapovirus 3Dpol and norovirus (44) display the characteristic right hand shape, with the C terminus being located within the active site channel. In contrast and according to Ng et al., the C terminus of the lagovirus (RHDV) is not located within the active site cleft (43), and initiation of RNA synthesis by RHDV polymerase was reported to occur by back priming (60). Both sapovirus and norovirus 3Dpol are able to initiate RNA synthesis de novo on heteropolymeric templates, whereas initiation of RNA synthesis on homopolymeric templates but also on polyadenylated subgenomic RNA is primer dependent (50). Both sapovirus and norovirus 3Dpol display a terminal transferase activity with a preference for CTP (50), and both are able to initiate RNA synthesis on homopolymeric poly(C) templates in the presence of high concentrations of GTP (51). These findings identify important structural and functional properties that separate calicivirus RNA-dependent RNA polymerases of human, i.e., norovirus and sapovirus, from nonhuman, i.e., lagovirus, origin. This clustering between the two representatives of human pathogenic calciviruses raises the question of the relevance of this homology in terms of pathogenicity and/or virulence. In this context, further structural and functional characterization of the other viral enzymes involved in replication of the calicivirus genome, such as the 3Cprotease and the 2CNTPase, may be of prime interest.
Acknowledgments
We are grateful to Katrin Jäger and Ivonne Robel for excellent technical assistance during the course of the study. A.G. thanks Alexander Kravchenko and Dmitry Samborskiy for support of the Viralis platform.
This work was supported by the European project “VIZIER” (“Comparative Structural Genomics of Viral Enzymes Involved in Replication”) funded by the 6th Framework Programme of the European Commission under the reference LSHG-CT-2004-511960.
Footnotes
Published ahead of print on 22 November 2006.
REFERENCES
- 1.Abergel, C., B. Coutard, D. Byrne, S. Chenivesse, J. B. Claude, C. Deregnaucourt, T. Fricaux, C. Gianesini-Boutreux, S. Jeudy, R. Lebrun, C. Maza, C. Notredame, O. Poirot, K. Suhre, M. Varagnol, and J. M. Claverie. 2003. Structural genomics of highly conserved microbial genes of unknown function in search of new antibacterial targets. J. Struct. Funct. Genomics 4:141-157. [DOI] [PubMed] [Google Scholar]
- 2.Arnold, J. J., S. K. Ghosh, and C. E. Cameron. 1999. Poliovirus RNA-dependent RNA polymerase (3D(pol)). Divalent cation modulation of primer, template, and nucleotide selection. J. Biol. Chem. 274:37060-37069. [DOI] [PubMed] [Google Scholar]
- 3.Asanaka, M., R. L. Atmar, V. Ruvolo, S. E. Crawford, F. H. Neill, and M. K. Estes. 2005. Replication and packaging of Norwalk virus RNA in cultured mammalian cells. Proc. Natl. Acad. Sci. USA 102:10327-10332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belliot, G., S. V. Sosnovtsev, K. O. Chang, V. Babu, U. Uche, J. J. Arnold, C. E. Cameron, and K. Y. Green. 2005. Norovirus proteinase-polymerase and polymerase are both active forms of RNA-dependent RNA polymerase. J. Virol. 79:2393-2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belliot, G., S. V. Sosnovtsev, T. Mitra, C. Hammer, M. Garfield, and K. Y. Green. 2003. In vitro proteolytic processing of the MD145 norovirus ORF1 nonstructural polyprotein yields stable precursors and products similar to those detected in calicivirus-infected cells. J. Virol. 77:10957-10974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biswal, B. K., M. M. Cherney, M. Wang, L. Chan, C. G. Yannopoulos, D. Bilimoria, O. Nicolas, J. Bedard, and M. N. James. 2005. Crystal structures of the RNA-dependent RNA polymerase genotype 2a of hepatitis C virus reveal two conformations and suggest mechanisms of inhibition by non-nucleoside inhibitors. J. Biol. Chem. 280:18202-18210. [DOI] [PubMed] [Google Scholar]
- 7.Chang, K. O., S. S. Sosnovtsev, G. Belliot, Q. Wang, L. J. Saif, and K. Y. Green. 2005. Reverse genetics system for porcine enteric calicivirus, a prototype sapovirus in the Caliciviridae. J. Virol. 79:1409-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang, K. O., S. V. Sosnovtsev, G. Belliot, Y. Kim, L. J. Saif, and K. Y. Green. 2004. Bile acids are essential for porcine enteric calicivirus replication in association with down-regulation of signal transducer and activator of transcription 1. Proc. Natl. Acad. Sci. USA 101:8733-8738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiba, S., S. Nakata, K. Numata-Kinoshita, and S. Honma. 2000. Sapporo virus: history and recent findings. J. Infect. Dis. 181(Suppl. 2):S303-S308. [DOI] [PubMed] [Google Scholar]
- 10.Choi, K. H., J. M. Groarke, D. C. Young, R. J. Kuhn, J. L. Smith, D. C. Pevear, and M. G. Rossmann. 2004. The structure of the RNA-dependent RNA polymerase from bovine viral diarrhea virus establishes the role of GTP in de novo initiation. Proc. Natl. Acad. Sci. USA 101:4425-4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crotty, S., D. Gohara, D. K. Gilligan, S. Karelsky, C. E. Cameron, and R. Andino. 2003. Manganese-dependent polioviruses caused by mutations within the viral polymerase. J. Virol. 77:5378-5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eddy, S. R. 1998. Profile hidden Markov models. Bioinformatics 14:755-763. [DOI] [PubMed] [Google Scholar]
- 13.Edgar, R. C. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32:1792-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans, P. R. 1993. Data collection and processing, p. 114-122. In Proceedings of the CCP4 study weekend, 29 to 30 January 1993. Science and Engineering Research Council Daresbury Laboratory, Daresbury, Warrington, Cheshire, United Kingdom.
- 15.Farkas, T., W. M. Zhong, Y. Jing, P. W. Huang, S. M. Espinosa, N. Martinez, A. L. Morrow, G. M. Ruiz-Palacios, L. K. Pickering, and X. Jiang. 2004. Genetic diversity among sapoviruses. Arch. Virol. 149:1309-1323. [DOI] [PubMed] [Google Scholar]
- 16.Ferrer-Orta, C., A. Arias, R. Perez-Luque, C. Escarmis, E. Domingo, and N. Verdaguer. 2004. Structure of foot-and-mouth disease virus RNA-dependent RNA polymerase and its complex with a template-primer RNA. J. Biol. Chem. 279:47212-47221. [DOI] [PubMed] [Google Scholar]
- 17.Fukushi, S., S. Kojima, R. Takai, F. B. Hoshino, T. Oka, N. Takeda, K. Katayama, and T. Kageyama. 2004. Poly(A)- and primer-independent RNA polymerase of norovirus. J. Virol. 78:3889-3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallimore, C. I., M. Iturriza-Gomara, D. Lewis, D. Cubitt, H. Cotterill, and J. J. Gray. 2006. Characterization of sapoviruses collected in the United Kingdom from 1989 to 2004. J. Med. Virol. 78:673-682. [DOI] [PubMed] [Google Scholar]
- 19.Green, K. Y., T. Ando, M. S. Balayan, T. Berke, I. N. Clarke, M. K. Estes, D. O. Matson, S. Nakata, J. D. Neill, M. J. Studdert, and H. J. Thiel. 2000. Taxonomy of the caliciviruses. J. Infect. Dis. 181(Suppl. 2):S322-S330. [DOI] [PubMed] [Google Scholar]
- 20.Green, K. Y., R. M. Chanock, and A. Z. Kapikian. 2001. Human caliciviruses, p. 841-874. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams and Wilkins, Philadelphia, PA.
- 21.Högbom, M., J. Rohayem, T. Unge, and A. Jones. 22 September 2005, posting date. Crystal structure of the Norwalk virus RNA dependent RNA polymerase from strain Hu/NLV/Dresden174/1997/GE. PDB ID: 2B43. http://www.pdb.org/.
- 22.Hong, Z., C. E. Cameron, M. P. Walker, C. Castro, N. Yao, J. Y. N. Lau, and W. Zhong. 2001. A novel mechanism to ensure terminal initiation by hepatitis C virus NS5B polymerase. Virology 285:6-11. [DOI] [PubMed] [Google Scholar]
- 23.Humphrey, T. J., J. G. Cruickshank, and W. D. Cubitt. 1984. An outbreak of calicivirus associated gastroenteritis in an elderly persons home. A possible zoonosis? J. Hyg. (London) 93:293-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johansson, P. J., K. Bergentoft, P. A. Larsson, G. Magnusson, A. Widell, M. Thorhagen, and K. O. Hedlund. 2005. A nosocomial sapovirus-associated outbreak of gastroenteritis in adults. Scand. J. Infect. Dis. 37:200-204. [DOI] [PubMed] [Google Scholar]
- 25.Kao, C. C., A. M. Del Vecchio, and W. Zhong. 1999. De novo initiation of RNA synthesis by a recombinant flaviviridae RNA-dependent RNA polymerase. Virology 253:1-7. [DOI] [PubMed] [Google Scholar]
- 26.Kao, C. C., P. Singh, and D. J. Ecker. 2001. De novo initiation of viral RNA-dependent RNA synthesis. Virology 287:251-260. [DOI] [PubMed] [Google Scholar]
- 27.Kao, C. C., and J. H. Sun. 1996. Initiation of minus-strand RNA synthesis by the brome mosaicvirus RNA-dependent RNA polymerase: use of oligoribonucleotide primers. J. Virol. 70:6826-6830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katayama, K., G. S. Hansman, T. Oka, S. Ogawa, and N. Takeda. 2006. Investigation of norovirus replication in a human cell line. Arch. Virol. 151:1291-1308. [DOI] [PubMed] [Google Scholar]
- 29.Lamzin, V. S., A. Perrakis, and K. S. Wilson. 2001. The ARP/WARP suite for automated construction and refinement of protein models. In M. G. Rossmann and E. Arnold (ed.), Int. tables for crystallography, vol. F. Crystallography of biological macromolecules Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 30.Lesburg, C. A., M. B. Cable, E. Ferrari, Z. Hong, A. F. Mannarino, and P. C. Weber. 1999. Crystal structure of the RNA-dependent RNA polymerase from hepatitis C virus reveals a fully encircled active site. Nat. Struct. Biol. 6:937-943. [DOI] [PubMed] [Google Scholar]
- 31.Leslie, A. G. W. 1992. Joint ccp4 and ESF-EACMB newsletter on protein crystallography. Daresbury Laboratory, Warrington, United Kingdom.
- 32.Liu, B. L., G. J. Viljoen, I. N. Clarke, and P. R. Lambden. 1999. Identification of further proteolytic cleavage sites in the Southampton calicivirus polyprotein by expression of the viral protease in E. coli. J. Gen. Virol. 80(Pt 2):291-296. [DOI] [PubMed] [Google Scholar]
- 33.Lopman, B. A., D. W. Brown, and M. Koopmans. 2002. Human caliciviruses in Europe. J. Clin. Virol. 24:137-160. [DOI] [PubMed] [Google Scholar]
- 34.Love, R. A., K. A. Maegley, X. Yu, R. A. Ferre, L. K. Lingardo, W. Diehl, H. E. Parge, P. S. Dragovich, and S. A. Fuhrman. 2004. The crystal structure of the RNA-dependent RNA polymerase from human rhinovirus: a dual function target for common cold antiviral therapy. Structure 12:1533-1544. [DOI] [PubMed] [Google Scholar]
- 35.Luo, G., R. K. Hamatake, D. M. Mathis, J. Racela, K. L. Rigat, J. Lemm, and R. J. Colonno. 2000. De novo initiation of RNA synthesis by the RNA-dependent RNA polymerase (NS5B) of hepatitis C virus. J. Virol. 74:851-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marin, M. S., R. Casais, J. M. Alonso, and F. Parra. 2000. ATP binding and ATPase activities associated with recombinant rabbit hemorrhagic disease virus 2C-like polypeptide. J. Virol. 74:10846-10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin Alonso, J. M., R. Casais, J. A. Boga, and F. Parra. 1996. Processing of rabbit hemorrhagic disease virus polyprotein. J. Virol. 70:1261-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matson, D. O., M. K. Estes, R. I. Glass, A. V. Bartlett, M. Penaranda, E. Calomeni, T. Tanaka, S. Nakata, and S. Chiba. 1989. Human calicivirus-associated diarrhea in children attending day care centers. J. Infect. Dis. 159:71-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murshudov, G. N., A. A. Vagin, and E. J. Dodson. 1997. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D 53:240-255. [DOI] [PubMed] [Google Scholar]
- 40.Nakamura, K., Y. Someya, T. Kumasaka, G. Ueno, M. Yamamoto, T. Sato, N. Takeda, T. Miyamura, and N. Tanaka. 2005. A norovirus protease structure provides insights into active and substrate binding site integrity. J. Virol. 79:13685-13693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakata, S., S. Chiba, H. Terashima, and T. Nakao. 1985. Prevalence of antibody to human calicivirus in Japan and Southeast Asia determined by radioimmunoassay. J. Clin. Microbiol. 22:519-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Navazza, J. 1994. AMoRe: an automated package for molecular replacement. N. Acta Crystallogr. A 50:157-163. [Google Scholar]
- 43.Ng, K. K., M. M. Cherney, A. L. Vazquez, A. Machin, J. M. Alonso, F. Parra, and M. N. James. 2002. Crystal structures of active and inactive conformations of a caliciviral RNA-dependent RNA polymerase. J. Biol. Chem. 277:1381-1387. [DOI] [PubMed] [Google Scholar]
- 44.Ng, K. K., N. Pendas-Franco, J. Rojo, J. A. Boga, A. Machin, J. M. Alonso, and F. Parra. 2004. Crystal structure of norwalk virus polymerase reveals the carboxyl terminus in the active site cleft. J. Biol. Chem. 279:16638-16645. [DOI] [PubMed] [Google Scholar]
- 45.Oka, T., K. Katayama, S. Ogawa, G. S. Hansman, T. Kageyama, T. Miyamura, and N. Takeda. 2005. Cleavage activity of the sapovirus 3C-like protease in Escherichia coli. Arch. Virol. 150:2539-2548. [DOI] [PubMed] [Google Scholar]
- 46.Oka, T., K. Katayama, S. Ogawa, G. S. Hansman, T. Kageyama, H. Ushijima, T. Miyamura, and N. Takeda. 2005. Proteolytic processing of sapovirus ORF1 polyprotein. J. Virol. 79:7283-7290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ollis, D. L., P. Brick, R. Hamlin, N. G. Xuong, and T. A. Steitz. 1985. Structure of large fragment of Escherichia coli DNA polymerase I complexed with dTMP. Nature 313:762-766. [DOI] [PubMed] [Google Scholar]
- 48.Pfister, T., and E. Wimmer. 2001. Polypeptide p41 of a Norwalk-like virus is a nucleic acid-independent nucleoside triphosphatase. J. Virol. 75:1611-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ranjith-Kumar, C. T., J. Gajewski, L. Gutshall, D. Maley, R. T. Sarisky, and C. C. Kao. 2001. Terminal nucleotidyl transferase activity of recombinant Flaviviridae RNA-dependent RNA polymerases: implication for viral RNA synthesis. J. Virol. 75:8615-8623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rohayem, J., K. Jager, I. Robel, U. Scheffler, A. Temme, and W. Rudolph. 2006. Characterization of norovirus 3Dpol RNA-dependent RNA polymerase activity and initiation of RNA synthesis. J. Gen. Virol. 87:2621-2630. [DOI] [PubMed] [Google Scholar]
- 51.Rohayem, J., I. Robel, K. Jager, U. Scheffler, and W. Rudolph. 2006. Protein-primed and de novo initiation of RNA synthesis by norovirus 3Dpol. J. Virol. 80:7060-7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sakuma, Y., S. Chiba, R. Kogasaka, H. Terashima, S. Nakamura, K. Horino, and T. Nakao. 1981. Prevalence of antibody to human calicivirus in general population of northern Japan. J. Med. Virol. 7:221-225. [DOI] [PubMed] [Google Scholar]
- 53.Simor, A. E., M. McArthur, A. McGeer, S. Shurtleff, and M. Jabar. 1990. Calicivirus gastroenteritis in a geriatric long-term care facility-Ontario. Can. Dis. Wkly. Rep. 16:239-240, 243. [PubMed] [Google Scholar]
- 54.Someya, Y., N. Takeda, and T. Miyamura. 2005. Characterization of the norovirus 3C-like protease. Virus Res. 110:91-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Someya, Y., N. Takeda, and T. Miyamura. 2000. Complete nucleotide sequence of the chiba virus genome and functional expression of the 3C-like protease in Escherichia coli. Virology 278:490-500. [DOI] [PubMed] [Google Scholar]
- 56.Someya, Y., N. Takeda, and T. Miyamura. 2002. Identification of active-site amino acid residues in the Chiba virus 3C-like protease. J. Virol. 76:5949-5958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sosnovtseva, S. A., S. V. Sosnovtsev, and K. Y. Green. 1999. Mapping of the feline calicivirus proteinase responsible for autocatalytic processing of the nonstructural polyprotein and identification of a stable proteinase-polymerase precursor protein. J. Virol. 73:6626-6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thompson, A. A., and O. B. Peersen. 2004. Structural basis for proteolysis-dependent activation of the poliovirus RNA-dependent RNA polymerase. EMBO J. 23:3462-3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vazquez, A. L., J. M. Alonso, and F. Parra. 2000. Mutation analysis of the GDD sequence motif of a calicivirus RNA-dependent RNA polymerase. J. Virol. 74:3888-3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vazquez, A. L., J. M. Martin Alonso, R. Casais, J. A. Boga, and F. Parra. 1998. Expression of enzymatically active rabbit hemorrhagic disease virus RNA-dependent RNA polymerase in Escherichia coli. J. Virol. 72:2999-3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vincentelli, R., S. Canaan, J. Offant, C. Cambillau, and C. Bignon. 2005. Automated expression and solubility screening of His-tagged proteins in 96-well format. Anal. Biochem. 346:77-84. [DOI] [PubMed] [Google Scholar]
- 62.Wei, L., J. S. Huhn, A. Mory, H. B. Pathak, S. V. Sosnovtsev, K. Y. Green, and C. E. Cameron. 2001. Proteinase-polymerase precursor as the active form of feline calicivirus RNA-dependent RNA polymerase. J. Virol. 75:1211-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wirblich, C., M. Sibilia, M. B. Boniotti, C. Rossi, H. J. Thiel, and G. Meyers. 1995. 3C-like protease of rabbit hemorrhagic disease virus: identification of cleavage sites in the ORF1 polyprotein and analysis of cleavage specificity. J. Virol. 69:7159-7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wirblich, C., H. J. Thiel, and G. Meyers. 1996. Genetic map of the calicivirus rabbit hemorrhagic disease virus as deduced from in vitro translation studies. J. Virol. 70:7974-7983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zeitler, C. E., M. K. Estes, and B. V. Venkataram Prasad. 2006. X-ray crystallographic structure of the Norwalk virus protease at 1.5-A resolution. J. Virol. 80:5050-5058. [DOI] [PMC free article] [PubMed] [Google Scholar]