Abstract
Some individuals infected with dengue virus develop dengue hemorrhagic fever (DHF), a viral hemorrhagic disease characterized by a transient period of localized plasma leakage. To determine the importance of vascular endothelial growth factor A (VEGF-A) in this syndrome, we compared plasma levels of VEGF-A and the soluble forms of its receptors in patients with DHF to patients with dengue fever (DF), a milder form of dengue virus infection without plasma leakage. We observed a rise in the plasma levels of free, but not total VEGF-A in DHF patients at the time of plasma leakage. This was associated with a decline in the soluble form of VEGF receptor 2 (VEGFR2) and VEGF-soluble VEGFR2 complexes, but not the soluble form of VEGFR1. The severity of plasma leakage in patients inversely correlated with plasma levels of soluble VEGFR2. In vitro, dengue virus suppressed soluble VEGFR2 production by endothelial cells but up-regulated surface VEGFR2 expression and promoted response to VEGF stimulation. In vivo, plasma viral load correlated with the degree of decline in plasma soluble VEGFR2. These results suggest that VEGF regulates vascular permeability and its activity is controlled by binding to soluble VEGFR2. Dengue virus-induced changes in surface and soluble VEGFR2 expression may be an important mechanism of plasma leakage in DHF.
Plasma leakage is a hallmark of a number of severe viral diseases, including dengue hemorrhagic fever (DHF), hantavirus pulmonary syndrome, and hemorrhagic fever with renal syndrome (17, 33, 34). Infection with any of the four serotypes of dengue virus can lead to DHF, a syndrome characterized by a sudden and transient period of localized plasma leakage manifesting as fluid accumulation in the pleural and/or abdominal cavity at the time of defervescence (4). Only 3 to 5% of dengue virus-infected individuals develop DHF, and the risk of developing this condition increases significantly in those previously infected with another serotype of dengue virus (secondary infection) (8). Plasma leakage in DHF generally lasts no more than 48 h and is usually followed by rapid, complete recovery. Consistent with the clinical course, plasma leakage in DHF occurs with a relative lack of tissue inflammation, suggesting that a transient change in factors that regulate vascular permeability in the physiological state may be the mechanism of plasma leakage in this disease (7).
Although elevated levels of cytokines with a permeability-enhancing effect, such as interferons (IFNs), interleukin-2 (IL-2), IL-8, and tumor necrosis factor alpha, have been reported in DHF, the relative role of these cytokines in plasma leakage is not known (20, 36). A recent study has demonstrated elevated levels of vascular endothelial growth factor A (VEGF-A), the most potent permeability-enhancing cytokine, in DHF (44). At least two VEGF receptors are expressed on endothelial cells; both are transmembrane receptor tyrosine kinases, namely, VEGFR1 or Fms-like tyrosine kinase 1 (Flt-1) and VEGFR2 or KDR (kinase insert domain receptor) (40). VEGFR1 is expressed on cell types other than endothelial cells, notably macrophages, while VEGFR2 is expressed primarily on endothelial cells and their progenitors (35, 38). VEGF binding to VEGFR2 on endothelial cells results in phosphorylation of the receptors, changes in endothelial cell morphology, increased permeability, and cell proliferation (15). The role of VEGFR1 in enhancing permeability is still controversial. A well-characterized soluble form of VEGFR1 (sVEGFR1), a product of alternatively spliced mRNA, has been shown to bind VEGF and inhibit its activity in vivo (26, 31). In contrast, little is known about the biosynthetic pathway of the recently reported soluble form of VEGR2 (sVEGFR2) and about its functions (11).
In this study, we compared plasma levels of VEGF and its soluble receptors throughout the course of the illness in patients with DHF to those of patients with dengue fever (DF), a milder form of dengue virus infection without plasma leakage. We found evidence suggesting that VEGF participates in the regulation of vascular permeability and its activity is controlled by a soluble form of its receptor, VEGFR2. Our in vitro results also support a novel mechanism by which dengue virus might modify vascular permeability by altering the production of sVEGFR2 and the expression of surface VEGFR2 on endothelial cells.
MATERIALS AND METHODS
Collection of specimens.
Plasma samples were collected between 1994 and 2001 from children with acute febrile illnesses recruited for a prospective study at the Queen Sirikit National Institute of Child Health, Bangkok, Thailand, that was previously described (25). Children with febrile illness without an obvious source of infection were enrolled during the first 3 days of illness and observed in the hospital until 1 day after defervescence. Blood samples were collected daily during hospitalization and at a follow-up visit during convalescence (approximately 4 to 6 days after discharge) for hematocrit and platelet count determinations and serologic and virologic testing. On the day after defervescence, a right lateral decubitus chest radiograph was obtained to detect pleural fluid. Subjects were categorized as having DF or DHF on the basis of the World Health Organization criteria (4). For the present study, archived frozen plasma samples from 22 DF and 23 DHF patients whose duration of stay was at least 5 days were analyzed. There were 17, 16, 10, and 2 cases of dengue 1, 2, 3, and 4 virus infections, respectively. These were classified serologically into 9 cases of primary dengue virus infection and 36 cases of secondary dengue virus infection. This study was approved by the Institutional Review Boards of the Thai Ministry of Public Health, the U.S. Army Surgeon General's Office, and the University of Massachusetts Medical School. Written consent was obtained from the parents or legal guardian of each participant.
ELISA.
Levels of VEGF, sVEGFR1, and sVEGFR2 in plasma were measured with sandwich enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems). The antibodies used in these ELISAs do not cross-react with molecules related to the assayed ligand within the range of concentrations reported in this study (manufacturer's [R&D Systems] product information and unpublished data). To dissociate VEGF-receptor complexes, plasma samples were incubated with an equal volume of 1.5 M glycine for 1 h at 37°C. Tris-HCl (1.5 M, pH 9.7) was then added to the mixture, and it was incubated at room temperature for 15 min. The samples were then used in the VEGF sandwich ELISA with the modification that the recombinant VEGF used to generate the standard curve was treated in a fashion similar to that used for the samples. Phosphorylated VEGFR2 was measured with an ELISA kit (R&D Systems). Dengue viral antigen was measured in a sandwich ELISA with a monoclonal antibody (3H5) specific for dengue 2 virus envelope protein as the capture antibody and a horseradish peroxidase-conjugated polyclonal human anti-flavivirus antibody as the detecting antibody as previously described (28).
Detection of VEGF bound to sVEGFR2.
sVEGFR2 in plasma samples was captured by incubating plasma samples overnight at 4°C in wells coated with antibody specific to VEGFR2. Samples were removed, and the wells were washed once with phosphate-buffered saline-Tween. To dissociate the captured sVEGFR2 from the associated VEGF molecules, 50 μl of assay diluent and 50 μl of 1.5 M glycine were added to each well and incubated at 37°C for 1 h. Fifty microliters of Tris HCl (1.5 M, pH 9.7) was then added to each well. The amounts of dissociated VEGF and sVEGFR2 in the mixtures were then measured by the corresponding sandwich ELISA with similarly treated recombinant antigens to generate standard curves. In pilot experiments with mixtures of recombinant VEGFR2 and VEGF at various molar ratios, we found that VEGF alone did not give a signal in this assay (unpublished data). The amount of VEGF bound to sVEGFR2 in plasma samples was calculated by the following formula: plasma VEGF that bound to sVEGFR2 (picograms per milliliter) = plate-bound VEGF (picograms per milliliter) × plasma sVEGFR2 (picograms per milliliter)/plate-bound sVEGFR2 (picograms per milliliter).
Infection of endothelial cells.
Human umbilical vein endothelial cells (HUVEC) were grown and maintained as instructed by the manufacturer (Cambrex). Cells were used up to passage 10 for these studies. Dengue type 2 virus strain 16681 was grown in the C6/36 mosquito cell line, and titers were determined on LLC-MK2 cells as previously described (37). Where indicated, the virus was inactivated by UV irradiation with a germicidal lamp (60 min); absence of viable virus was confirmed by plaque assay. HUVEC were infected with live or inactivated dengue virus at a multiplicity of infection (MOI) of 5 unless otherwise indicated or were exposed to supernatant of uninfected C6/36 cells as a control. In some experiments, HUVEC were infected with live or inactivated dengue virus in the presence of a 1:25,000 dilution of immune plasma (neutralizing titer of 1:486 against dengue 2 virus). HUVEC supernatants were collected at the indicated time points and assayed for sVEGFR2 by ELISA. Cells were collected and stained with antibody to VEGFR2 (R&D Systems) and analyzed with a FACScalibur flow cytometer (Becton Dickinson). Solubilized HUVEC membrane fractions were prepared in 20 mM Tris Cl (pH 8.0) with 1% NP-40 and protease inhibitor cocktail (Sigma) and analyzed for VEGFR2 by ELISA. Where indicated, HUVEC were treated with recombinant VEGF (R&D Systems) at 1 ng/ml for 5 min; cells were then lysed, and the amounts of phosphorylated VEGFR2 were analyzed by ELISA (R&D Systems).
Measurement of plasma viral load.
RNA was extracted from 100 μl of plasma, and viral RNA genome content was measured with a fluorogenic reverse transcription-PCR as previously described (22). cDNA of the dengue virus 3′ noncoding region was cloned into a pUC vector, and serial dilutions of the plasmid were used to construct a standard curve for each viral serotype. Plasma dengue virus RNA levels were expressed as genome equivalent cDNA copies per milliliter. All unknown samples and controls were assayed in triplicate. The ABI gene detection system 7700 (Perkin-Elmer Applied Biosystems) was used for PCR cycling amplification, data collection, and analysis.
Statistical analysis.
For statistical analysis, the day of defervescence, corresponding to the usual period of plasma leakage in DHF, was defined as fever day 0 and the days before (fever days −1, −2, etc.) and after (fever days +1, +2, etc.) were numbered sequentially. Continuous variables were analyzed by Kolmogorov-Smirnov's test for normality of distribution. Normally distributed variables were compared between groups with Student's t test. A paired t test was used to analyze differences in variables of individuals at different time points or related variables within individual samples. A P value of less than 0.05 was considered to indicate statistical significance. All tests were two sided. Associations between continuous variables were calculated by either parametric Pearson correlation or nonparametric Spearman correlation.
RESULTS
Plasma VEGF in patients with DF and DHF.
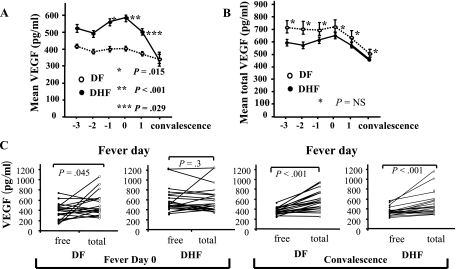
We first measured levels of VEGF in serial plasma samples from subjects with acute dengue illness enrolled in a prospective cohort study, including a group with DHF and a comparable group with DF. The mean levels of plasma VEGF in DHF patients increased during illness, with the highest mean values observed on the day of defervescence (fever day 0; Fig. 1A). The levels of VEGF were significantly higher in subjects with DHF than in those with DF on fever days −1 (1 day prior to defervescence), 0, and +1 (1 day after defervescence).
FIG. 1.
VEGF levels in serial plasma samples from DHF and DF patients. (A) Mean levels of VEGF in plasma samples from DHF (n = 23, filled symbols) and DF (n = 22, open symbols) patients over the course of the illness and at convalescence (4 to 6 days after discharge). Error bars represent standard errors of each group of samples at the indicated time points. P values are for comparisons between DF and DHF patients. (B) Mean total VEGF in plasma samples from DF (open symbols) and DHF (filled symbols) patients. NS, no statistically significant difference. (C) Comparisons between free and total VEGF levels within individual plasma samples from DF and DHF patients at fever day 0 and during convalescence.
Previous studies have shown that VEGF bound to a soluble form of its receptor, VEGFR1, cannot be detected with this ELISA system (23). Therefore, the differences in free VEGF levels might reflect differences in total (bound plus free) VEGF or alternatively might reflect changes in relative binding. To measure total VEGF, we dissociated protein complexes by incubating plasma samples with 1.5 M glycine prior to ELISA. In contrast to free VEGF levels, total VGEF levels were not different between DHF and DF patients (Fig. 1B). At the follow-up visit, free VEGF levels had declined significantly in subjects with DHF, and neither free nor total VEGF levels were significantly different between DF and DHF subjects (P = 0.93 for free VEGF, P = 0.48 for total VEGF, Fig. 1A and B). The levels of free VEGF on fever day 0 were significantly lower than the levels of total VEGF in the same plasma samples from DF patients but not in those from DHF patients (P = 0.045, by paired t test, Fig. 1C), indicating the presence of the bound form of VEGF in DF, but not DHF, patients. During convalescence, free VEGF levels were lower than total VEGF in both DF and DHF patients, suggesting reemergence of the bound form of VEGF in DHF patients at this time point (Fig. 1C).
Different profiles of soluble VEGF receptors in DF and DHF patients.
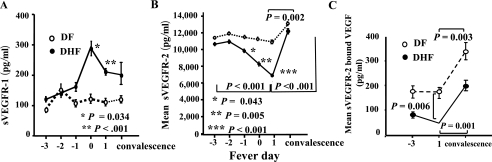
A potential candidate for a VEGF-binding molecule is its soluble receptor. A soluble form of VEGFR1 (sVEGFR1 or sFlt-1) is generated by differential splicing of VEGFR1 mRNA (26). We measured plasma levels of soluble VEGFR1 and observed a sharp rise in sVEGFR1 levels in DHF patients on fever day 0 when plasma leakage occurred (Fig. 2A), followed by a decline during convalescence.
FIG. 2.
sVEGFR1 and sVEGFR2 in DF and DHF patients. (A) Levels of sVEGFR1 in plasma samples from DHF (n = 23, filled symbols) and DF (n = 22, open symbols) patients over the course of illness. Error bars represents means ± standard errors. P values are for comparisons between DF and DHF patients. (B) Levels of sVEGFR2 in plasma samples from DHF and DF patients. P values are for comparisons between DF and DHF patients. Horizontal brackets indicate P values derived from paired Student t tests comparing levels in the same individuals on different days. (C) Levels of VEGF bound to VEGFR2 in plasma of DF and DHF patients on fever days −3 and +1 and during convalescence. The vertical bracket indicates P values derived from a Student t test comparing DF and DHF patients. Horizontal brackets indicate P values derived from paired Student t tests comparing levels on different days.
Since the increase in sVEGFR1 did not provide an explanation for the increase in free VEGF in DHF cases, we next determined the plasma levels of sVEGFR2 (11). The levels of plasma sVEGFR2 were more than 20-fold higher than sVEGFR1 levels (Fig. 2B). In contrast to the changes in sVEGFR1 levels, sVEGFR2 levels in DHF patients declined dramatically toward the time of plasma leakage while the levels in DF patients remained relatively unchanged (Fig. 2B). The mean level of sVEGFR2 in DHF patients became significantly lower than in DF patients 1 day prior to defervescence and later (Fig. 2B). During convalescence, the levels of sVEGFR2 in DHF patients increased and approached the levels in DF patients (Fig. 2B).
The progressive decline of sVEGFR2 levels and the concomitant increase in unbound VEGF in DHF patients suggested that sVEGFR2 may be the molecule that bound VEGF in vivo. To directly measure VEGF bound to sVEGFR2, we established a capture assay in which plasma sVEGFR2 was captured with a plate-bound antibody and the amount of captured sVEGFR2 and associated VEGF was quantified by ELISA. The mean level of VEGF bound to sVEGFR2 in DHF patients was significantly lower than that in DF patients 1 day after defervescence (46 ± 13.7 versus 173 ± 39.6 pg/ml, P = 0.006, Fig. 2C). The levels of sVEGFR2-bound VEGF increased during convalescence in both DF and DHF patients.
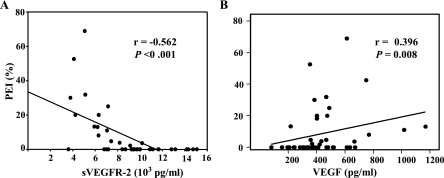
Plasma leakage in DHF patients often leads to the formation of pleural effusion. To evaluate the biological relevance of sVEGFR2 in the regulation of vascular permeability, we analyzed the correlation between the levels of plasma sVEGFR2 on fever day +1 and the amount of pleural fluid measured as a pleural effusion index, an indicator of the severity of plasma leakage. There was an inverse correlation between plasma sVEGFR2 levels and the amount of pleural fluid measured at the time of plasma leakage (r = −0.562, P < 0.001, by Spearman's analysis, Fig. 3A). In addition, the amount of pleural fluid showed a direct correlation with plasma levels of VEGF (r = 0.396, P = 0.008, by Spearman's analysis, Fig. 3B). Taken together, these findings support the role of sVEGFR2 in the regulation of VEGF activity and vascular permeability in vivo.
FIG. 3.
Correlations between the severity of plasma leakage and plasma sVEGFR2 and free VEGF levels. (A) Linear regression analysis revealed an inverse correlation between the levels of sVEGFR2 on fever day +1 and the pleural effusion index (PEI), a semiquantitative measurement of the severity of pleural effusion. A right lateral decubitus chest radiograph was obtained on fever day +1, and the index was calculated as follows: 100 × (maximum width of right pleural effusion)/(maximum width of right hemithorax). (B) Correlations between the levels of free VEGF and the amount of pleural fluid.
Dengue virus alters the production of soluble and surface VEGFR2 by endothelial cells.
Several studies have demonstrated greater viral loads in patients with DHF compared to those with DF, suggesting that direct viral effects, or the magnitude of the host response to the virus, may be critical in determining disease severity (29, 45). Dengue virus has been shown to infect endothelial cells in vitro (5). A recent study has demonstrated dengue virus antigen in endothelial cells in vivo although the dengue virus genome was not detected in these cells (24). We postulated that exposure to dengue virus may alter the expression of soluble and surface VEGFR2 by endothelial cells, resulting in changes in vascular permeability.
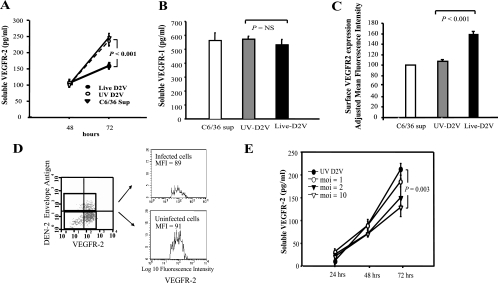
We exposed HUVEC to dengue 2 virus and measured the production of sVEGFR2 and VEGFR2 surface expression. Infection with dengue virus resulted in suppression of sVEGFR2 levels after 72 h of infection but not at earlier time points (Fig. 4A). This was not due to cell death since no cytopathic effects were observed in these cells. Furthermore, this effect was specific to sVEGFR2 since the levels of sVEGFR1 in the culture supernatants were not altered by dengue virus infection (Fig. 4B). The down-regulation of sVEGFR2 required live virus and was associated with an increase in cell surface VEGFR2 expression. (Fig. 4A and C). Exposure to live virus resulted in increased surface VEGFR2 expression in both infected and uninfected cells in the same culture, indicating that viral or host-derived factors, rather than intracellular viral replication, mediate this effect (Fig. 4D). Consistent with the flow cytometry findings, the levels of VEGFR2 in the membrane fraction of an infected HUVEC monolayer were higher than those of uninfected cells (353 ± 8 pg/ml [infected cells] versus 213 ± 24 pg/ml [uninfected cells], P = 0.006). The suppression of sVEGFR2 production appeared to be viral dose dependent, as infection at a high MOI of 10 but not at a low MOI of 2 resulted in a significant decrease in sVEGFR2 levels (Fig. 4E).
FIG. 4.
Effects of dengue virus on soluble and cell surface VEGFR2 expression by HUVEC. (A) HUVEC were exposed to live dengue 2 virus (DEN 2V, filled circles), UV-inactivated virus (UV DEN 2V, empty circles), or C6/36 cell supernatant as a control (C6/36 Sup, triangles). At the time points indicated, supernatants were collected and analyzed for sVEGFR2. Data represent means (n = 4) ± standard deviations. P < 0.001 for the comparison between cells exposed to live virus and UV-inactivated virus or C6/36 cell supernatant. (B) Levels of sVEGFR1 in supernatants from HUVEC after a 72-h exposure to live dengue 2 virus (black bar), UV-inactivated virus (gray bar), or C6/36 cell supernatant as a control (white bar). (C). Surface expression of HUVEC exposed to live dengue 2 virus (black bar), UV-inactivated virus (gray bar), or C6/36 cell supernatant as a control (white bar). Staining intensity of UV-inactivated virus- and live dengue virus-exposed cells was compared against that of C6/36 supernatant-exposed cells, which is expressed as 100%. Data represent means (n = 4) ± standard deviations. (D) Cells exposed to live virus were stained for dengue virus envelope antigen and cell surface VEGFR2, cells were gated on the basis of the expression of viral envelope antigen, and the surface VEGFR2 expression of virus-infected and uninfected cells was analyzed. MFI, mean fluorescence intensity. (E) HUVEC were infected with live dengue 2 virus at an MOI of 1 (empty circles), 2 (filled triangles), or 10 (empty triangles) or with inactivated virus (filled circles). The levels of sVEGFR2 in the supernatants at the indicated time points were analyzed. Data represent means ± standard deviations of triplicate samples. P = 0.003 for the comparison between cells infected with an MOI of 10 and cells exposed to inactivated virus.
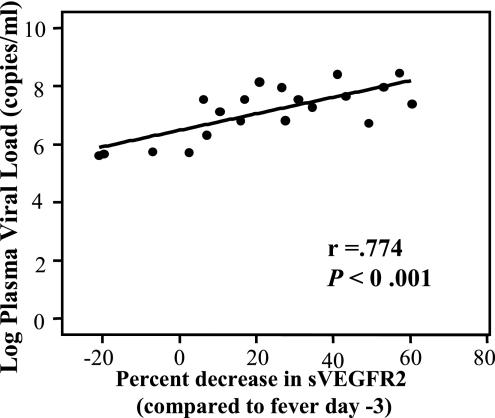
To evaluate the relationship between virus and sVEGFR2 in vivo, we analyzed the correlation between the plasma viral load and sVEGFR2 levels. The plasma viral load was measured in samples collected 2 days prior to defervescence because circulating virus rapidly declined after this time point. The plasma viral load of samples from fever day −2 showed no correlation with sVEGFR2 levels in the same samples, calculated as percent decline from the levels on fever day −3 (r = 0.022, P = 0.978, by Pearson's analysis). However, it showed a significant correlation with the decline in sVEGFR2 levels 3 days later, during the period of plasma leakage (r = 0.774, P < 0.001 by Pearson's analysis, Fig. 5). This result suggests that a greater viral load is associated with a more severe decline in sVEGFR2 levels, with a lag period between the two events.
FIG. 5.
Correlations between plasma viral load and percent decline in sVEGFR2. Linear regression analysis revealed the correlation between the percent decline in levels of sVEGFR2 on fever day +1 (compared to the levels on fever day −3) and the plasma viral RNA from fever day −2.
Dengue virus immune plasma enhances viral infection, suppresses sVEGFR2 production, and promotes VEGFR2 signaling in HUVEC.
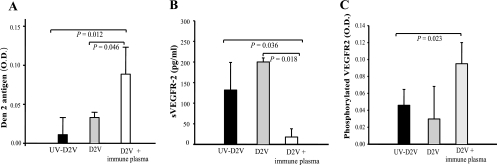
Prior exposure to dengue virus is a major risk factor for the development of plasma leakage in subsequent dengue virus infections (8). One of the proposed mechanisms is the presence of nonneutralizing antibodies, which facilitate viral uptake and replication (27). To determine whether dengue virus-specific antibodies can enhance infection of endothelial cells and the subsequent suppression of sVEGFR2 production, we inoculated HUVEC with virus at a low MOI of 2 in the presence of immune plasma, which was diluted beyond its neutralizing titer of 1:486. We observed more viral antigen but less sVEGFR2 in culture supernatants (collected at 48 and 72 h postinfection for viral antigen and sVEGFR2 assays, respectively) of these cells than in cells infected with virus in the absence of immune plasma (Fig. 6A and B). We then examined the responsiveness of these cells to VEGF stimulation by measuring the levels of VEGR2 that underwent receptor phosphorylation in response to VEGF treatment (1 ng/ml). More phosphorylated VEGFR2 was detected in HUVEC that were inoculated with dengue virus immune complexes than in cells treated otherwise, suggesting an enhanced response to cytokine stimulation (Fig. 6C).
FIG. 6.
Effects of dengue virus immune plasma on viral replication, sVEGFR2 production, and VEGF signaling in HUVEC. HUVEC were exposed to live dengue 2 virus with or without diluted immune plasma or to inactivated virus in the presence of immune plasma. Viral envelope protein (A) and sVEGFR2 (B) in the supernatants were measured 48 and 72 h after viral exposure, respectively. Cells were then treated for 5 min with recombinant VEGF (1 ng/ml), and the amount of phosphorylated VEGFR2 was measured (C). Data represent means ± standard deviations. n = 4 per group. O.D., optical density.
DISCUSSION
Plasma leakage is the central event in the pathogenesis of DHF. Elevated levels of cytokines with permeability-enhancing effects have been reported in DHF, although their relative importance is not known. Elevated levels of VEGF, a potent vascular permeability-enhancing cytokine, have been previously reported in DHF patients (44). Here we demonstrated that the increase in plasma free VEGF levels was associated with a decrease in its soluble receptor, VEGFR2. We also present the first evidence of VEGF binding to naturally occurring sVEGFR2 in vivo, suggesting its role as a regulator of VEGF function.
Studies of patients with preeclampsia and studies with animal models have demonstrated that VEGF activity in vivo could be inhibited by binding to the soluble form of another VEGF receptor, VEGFR1 (31). Several pieces of evidence in the present study, however, implicate sVEGFR2 as the more important regulator of VEGF effects in vivo. First, sVEGFR2 is present at much higher levels in plasma than is sVEGFR1. The levels of sVEGFR1 observed in our patients were comparable to the levels reported in normal adults but were 10-fold lower than the levels found in pregnant women, whose sVEGFR1 was produced by the placenta (11, 31). The binding capacity of sVEGFR1, even at peak levels in our study subjects, was too low to neutralize a significant fraction of plasma VEGF. Second, the decline in plasma sVEGFR2 and sVEGFR2-VEGF complexes was temporally associated with an increase in free VEGF and the onset of plasma leakage. Third, the correlation between the amount of pleural effusion and the decline in sVEGFR2 supports its role in modulating VEGF effects. These results are consistent with animal studies demonstrating that blocking of VEGF functions with an sVEGFR2-Fc chimeric molecule reduced malignant ascitic fluid formation (9, 21). Although VEGF forms complexes with sVEGFR2 in vivo, it is notable that plasma free VEGF still exists in the presence of a molar excess of sVEGFR2. This may be due to the relatively low affinity of VEGF for binding to VEGFR2 (35, 39). Collectively, these observations suggest that under non-pregnancy-related conditions, sVEGFR2 binds VEGF and regulates its activities.
The structure and the biosynthetic pathway of sVEGFR2 are not known. No alternatively spliced form of VEGFR2 mRNA encoding a soluble receptor has been reported. The temporal association between the decline in sVEGFR2 levels and an increase in surface VEGFR2 expression on endothelial cells suggests that sVEGFR2 is a proteolytic product of membrane VEGFR2. Inhibition of cleavage of surface receptors may increase ligand sensitivity by increasing receptor density, as well as decreasing inhibitory soluble receptors (3). Although the association between the decline in sVEGFR2 and plasma leakage, which suggests enhanced VEGF activity in vivo, is consistent with this view, it remains to be demonstrated that there is an up-regulation of surface VEGFR2 and enhanced signaling through VEGFR2 in vivo during plasma leakage.
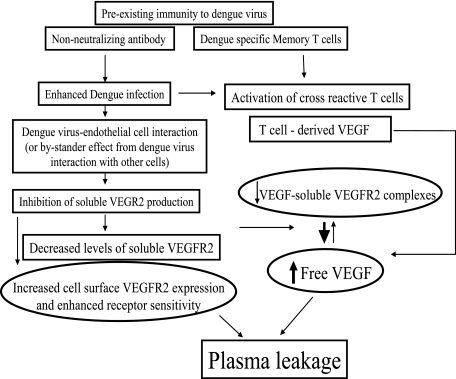
We have shown in this study that exposure of endothelial cells to live dengue virus resulted in decreased production of the soluble form of VEGFR2 and enhanced surface VEGFR2 expression. The correlation between plasma viral load and the subsequent decline in sVEGFR2 levels lend support to this in vitro finding. Previous studies have shown increased sensitivity of endothelial cells exposed to live dengue virus to inflammatory cytokine-induced changes in permeability (42). Our observations suggest a novel mechanism by which viral factors (or host factors triggered by virus) may regulate the cleavage of surface VEGFR2, resulting in changes in soluble and surface VEGF receptors and, consequently, changes in vascular permeability. Although our findings suggest a model in which viral infection of endothelial cells leads to changes in membrane and sVEGFR2 (Fig. 7), definitive in vivo evidence of dengue virus infection of endothelial cells has been lacking (24). The lack of in vivo evidence of endothelial cell infection in dengue cases is in contrast to the well-documented infection of endothelial cells in hantavirus pulmonary syndrome, in which pulmonary capillary infection is associated with increased vascular permeability (46). Thus, other mechanisms controlling sVEGFR2 levels may be more important in vivo than the direct viral mechanism suggested by our in vitro data. It is possible that the effects on endothelial cells in vivo may be an indirect consequence of the interaction between virus and other cell types, as reported in other viral hemorrhagic diseases. In a rhesus model of Ebola virus infection, it has been demonstrated that factors released from infected macrophages enhanced vascular permeability (13, 14). Unlike sVEGFR2, exposure to live virus has no effects on the production of sVEGFR1 by endothelial cells. This suggests that the elevated sVEGFR1 levels in DHF patients may reflect indirect effects of virus on endothelial cells or may be secondary to host immune responses. Alternatively, the elevated sVEGFR1 level may reflect activation of other cell types known to express VEGFR1, particularly macrophages, during viral infection (38). These findings underscore the need for additional studies to identify target cells that are infected with dengue virus in vivo. Such studies are challenging, since the mortality rate of DHF is significantly lower than those of other viral hemorrhagic diseases and deaths typically occur late in infection (4).
FIG. 7.
Model for regulation of VEGF activity by dengue virus and dengue virus-specific immunity. Preexisting nonneutralizing antibody may enhance dengue virus uptake, resulting in a greater viral load. Dengue virus down-regulates the production of sVEGFR2 either directly by interaction with endothelial cells or indirectly via interaction with other cell types. The reduction in sVEGFR2 production results in decreased plasma sVEGFR2 and increased free, biologically active VEGF. VEGF may also be produced by activated dengue virus-specific T cells. A concurrent increase in surface expression of VEGFR2 may enhance endothelial cell responsiveness to VEGF stimulation. The combination of increased biologically active VEGF and enhanced receptor responsiveness results in increased vascular permeability and clinical plasma leakage.
The lack of a relevant animal model that mimics the pattern of plasma leakage in DHF has not allowed an in vivo experiment to precisely delineate the role of VEGF relative to other cytokines reported to be elevated in DHF, such as IL-2, IFN-γ, tumor necrosis factor alpha, and IFNs, in plasma leakage (6, 19, 41). However, the temporal pattern of VEGF and its known biological effects argue for its role as a mediator involved in plasma leakage in DHF. Unlike these proinflammatory cytokines with peak levels occurring early in the course of the disease prior to plasma leakage, the peak levels of plasma VEGF occurred simultaneously with plasma leakage. Furthermore, increased vascular permeability induced by VEGF can occur in the absence of overt inflammation, as evidenced by its role during embryogenesis (10, 12). This effect is consistent with the unique characteristics of plasma leakage in DHF, which is transient and occurs without significant tissue inflammation (7). The levels of VEGF in DHF cases were comparable to those reported in conditions with clinically significant plasma leakage and were much higher than the elevated levels reported in conditions associated with localized vasculogenesis (1, 2, 43). The association between plasma leakage and the elevation of free VEGF, together with minimal tissue pathology in DHF, supports a role for VEGF in the regulation of vascular permeability in this condition.
DHF occurs mostly in individuals previously infected with dengue virus of another serotype. Preexisting dengue virus-specific antibody may enhance the uptake of dengue virus during secondary infection, resulting in a greater viral load, which according to our model would lead to decreased sVEGFR2 levels and enhanced VEGF activity (Fig. 7) (16, 18, 29). A greater viral load may also lead to the exaggerated activation of both innate and adaptive immunity. In particular, T cells have been shown to produce VEGF, and VEGF has been reported to induce IFN-γ production by T lymphocytes (30, 32). Activation of dengue virus-reactive memory T cells that cross-recognize another dengue virus serotype may therefore contribute to the elevated VEGF and IFN-γ levels observed in DHF patients.
Acknowledgments
We thank Ananda Nisalak and the Arbovirology Section and the Molecular Section of the Armed Forces Research Institute of Medical Sciences, for serological and molecular diagnosis; Mammen P. Mammen, Jr., for support; Suchitra Nimmannitya for reviewing clinical diagnoses; and doctors and nurses of the Queen Sirikit National Institute of Child Health and the staff of the Armed Forces Research Institute of Medical Sciences for patient care and sample collection.
This work was supported by National Institutes of Health grant NIH-P01AI34533 and the Military Infectious Disease Research Program.
The opinions and assertions contained herein are ours and are not to be construed as official or reflecting the view of the U.S. Government.
Footnotes
Published ahead of print on 6 December 2006.
REFERENCES
- 1.Abrahamov, D., E. Erez, M. Tamariz, O. Dagan, E. Pearl, Y. Abrahamov, B. Gendel, N. Desai, J. Kats, B. Vidne, and V. Barak. 2002. Plasma vascular endothelial growth factor level is a predictor of the severity of postoperative capillary leak syndrome in neonates undergoing cardiopulmonary bypass. Pediatr. Surg. Int. 18:54-59. [DOI] [PubMed] [Google Scholar]
- 2.Adams, J., P. J. Carder, S. Downey, M. A. Forbes, K. MacLennan, V. Allgar, S. Kaufman, S. Hallam, R. Bicknell, J. J. Walker, F. Cairnduff, P. J. Selby, T. J. Perren, M. Lansdown, and R. E. Banks. 2000. Vascular endothelial growth factor (VEGF) in breast cancer: comparison of plasma, serum, and tissue VEGF and microvessel density and effects of tamoxifen. Cancer Res. 60:2898-2905. [PubMed] [Google Scholar]
- 3.Ahonen, M., M. Poukkula, A. H. Baker, M. Kashiwagi, H. Nagase, J. E. Eriksson, and V. M. Kahari. 2003. Tissue inhibitor of metalloproteinases-3 induces apoptosis in melanoma cells by stabilization of death receptors. Oncogene 22:2121-2134. [DOI] [PubMed] [Google Scholar]
- 4.Anonymous. 1986. Dengue hemorrhagic fever: diagnosis, treatment and control. World Health Organization, Geneva, Switzerland.
- 5.Avirutnan, P., P. Malasit, B. Seliger, S. Bhakdi, and M. Husmann. 1998. Dengue virus infection of human endothelial cells leads to chemokine production, complement activation, and apoptosis. J. Immunol. 161:6338-6346. [PubMed] [Google Scholar]
- 6.Bente, D. A., M. W. Melkus, J. V. Garcia, and R. Rico-Hesse. 2005. Dengue fever in humanized NOD/SCID mice. J. Virol. 79:13797-13799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhamarapravati, N., P. Tuchinda, and V. Boonyapaknavik. 1967. Pathology of Thailand haemorrhagic fever: a study of 100 autopsy cases. Ann. Trop. Med. Parasitol. 61:500-510. [DOI] [PubMed] [Google Scholar]
- 8.Burke, D. S., A. Nisalak, D. E. Johnson, and R. M. Scott. 1988. A prospective study of dengue infections in Bangkok. Am. J. Trop. Med. Hyg. 38:172-180. [DOI] [PubMed] [Google Scholar]
- 9.Byrne, A. T., L. Ross, J. Holash, M. Nakanishi, L. Hu, J. I. Hofmann, G. D. Yancopoulos, and R. B. Jaffe. 2003. Vascular endothelial growth factor-trap decreases tumor burden, inhibits ascites, and causes dramatic vascular remodeling in an ovarian cancer model. Clin. Cancer Res. 9:5721-5728. [PubMed] [Google Scholar]
- 10.Carmeliet, P., and D. Collen. 1997. Molecular analysis of blood vessel formation and disease. Am. J. Physiol. 273:H2091-H2104. [DOI] [PubMed] [Google Scholar]
- 11.Ebos, J. M., G. Bocci, S. Man, P. E. Thorpe, D. J. Hicklin, D. Zhou, X. Jia, and R. S. Kerbel. 2004. A naturally occurring soluble form of vascular endothelial growth factor receptor 2 detected in mouse and human plasma. Mol. Cancer Res. 2:315-326. [PubMed] [Google Scholar]
- 12.Ferrara, N., K. Carver-Moore, H. Chen, M. Dowd, L. Lu, K. S. O'Shea, L. Powell-Braxton, K. J. Hillan, and M. W. Moore. 1996. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 380:439-442. [DOI] [PubMed] [Google Scholar]
- 13.Geisbert, T. W., H. A. Young, P. B. Jahrling, K. J. Davis, E. Kagan, and L. E. Hensley. 2003. Mechanisms underlying coagulation abnormalities in Ebola hemorrhagic fever: overexpression of tissue factor in primate monocytes/macrophages is a key event. J. Infect. Dis. 188:1618-1629. [DOI] [PubMed] [Google Scholar]
- 14.Geisbert, T. W., H. A. Young, P. B. Jahrling, K. J. Davis, T. Larsen, E. Kagan, and L. E. Hensley. 2003. Pathogenesis of Ebola hemorrhagic fever in primate models: evidence that hemorrhage is not a direct effect of virus-induced cytolysis of endothelial cells. Am. J. Pathol. 163:2371-2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gille, H., J. Kowalski, B. Li, J. LeCouter, B. Moffat, T. F. Zioncheck, N. Pelletier, and N. Ferrara. 2001. Analysis of biological effects and signaling properties of Flt-1 (VEGFR-1) and KDR (VEGFR-2). A reassessment using novel receptor-specific vascular endothelial growth factor mutants. J. Biol. Chem. 276:3222-3230. [DOI] [PubMed] [Google Scholar]
- 16.Halstead, S. B. 1979. In vivo enhancement of dengue virus infection in rhesus monkeys by passively transferred antibody. J. Infect. Dis. 140:527-533. [DOI] [PubMed] [Google Scholar]
- 17.Halstead, S. B. 1988. Pathogenesis of dengue: challenges to molecular biology. Science 239:476-481. [DOI] [PubMed] [Google Scholar]
- 18.Halstead, S. B., and E. J. O'Rourke. 1977. Dengue viruses and mononuclear phagocytes. I. Infection enhancement by non-neutralizing antibody. J. Exp. Med. 146:201-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halstead, S. B., H. Shotwell, and J. Casals. 1973. Studies on the pathogenesis of dengue infection in monkeys. II. Clinical laboratory responses to heterologous infection. J. Infect. Dis. 128:15-22. [DOI] [PubMed] [Google Scholar]
- 20.Hober, D., L. Poli, B. Roblin, P. Gestas, E. Chungue, G. Granic, P. Imbert, J. L. Pecarere, R. Vergez-Pascal, P. Wattre, et al. 1993. Serum levels of tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and interleukin-1β (IL-1β) in dengue-infected patients. Am. J. Trop. Med. Hyg. 48:324-331. [DOI] [PubMed] [Google Scholar]
- 21.Holash, J., S. Davis, N. Papadopoulos, S. D. Croll, L. Ho, M. Russell, P. Boland, R. Leidich, D. Hylton, E. Burova, E. Ioffe, T. Huang, C. Radziejewski, K. Bailey, J. P. Fandl, T. Daly, S. J. Wiegand, G. D. Yancopoulos, and J. S. Rudge. 2002. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc. Natl. Acad. Sci. USA 99:11393-11398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Houng, H. S., R. Chung-Ming Chen, D. W. Vaughn, and N. Kanesa-thasan. 2001. Development of a fluorogenic RT-PCR system for quantitative identification of dengue virus serotypes 1-4 using conserved and serotype-specific 3′ noncoding sequences. J. Virol. Methods 95:19-32. [DOI] [PubMed] [Google Scholar]
- 23.Jelkmann, W. 2001. Pitfalls in the measurement of circulating vascular endothelial growth factor. Clin. Chem. 47:617-623. [PubMed] [Google Scholar]
- 24.Jessie, K., M. Y. Fong, S. Devi, S. K. Lam, and K. T. Wong. 2004. Localization of dengue virus in naturally infected human tissues, by immunohistochemistry and in situ hybridization. J. Infect. Dis. 189:1411-1418. [DOI] [PubMed] [Google Scholar]
- 25.Kalayanarooj, S., D. W. Vaughn, S. Nimmannitya, S. Green, S. Suntayakorn, N. Kunentrasai, W. Viramitrachai, S. Ratanachu-eke, S. Kiatpolpoj, B. L. Innis, A. L. Rothman, A. Nisalak, and F. A. Ennis. 1997. Early clinical and laboratory indicators of acute dengue illness. J. Infect. Dis. 176:313-321. [DOI] [PubMed] [Google Scholar]
- 26.Kendall, R. L., G. Wang, and K. A. Thomas. 1996. Identification of a natural soluble form of the vascular endothelial growth factor receptor, FLT-1, and its heterodimerization with KDR. Biochem. Biophys. Res. Commun. 226:324-328. [DOI] [PubMed] [Google Scholar]
- 27.Kliks, S. C., A. Nisalak, W. E. Brandt, L. Wahl, and D. S. Burke. 1989. Antibody-dependent enhancement of dengue virus growth in human monocytes as a risk factor for dengue hemorrhagic fever. Am. J. Trop. Med. Hyg. 40:444-451. [DOI] [PubMed] [Google Scholar]
- 28.Kuno, G., D. J. Gubler, and N. S. Santiago de Weil. 1985. Antigen capture ELISA for the identification of dengue viruses. J. Virol. Methods 12:93-103. [DOI] [PubMed] [Google Scholar]
- 29.Libraty, D. H., T. P. Endy, H. S. Houng, S. Green, S. Kalayanarooj, S. Suntayakorn, W. Chansiriwongs, D. W. Vaughn, A. Nisalak, F. A. Ennis, and A. L. Rothman. 2002. Differing influences of virus burden and immune activation on disease severity in secondary dengue-3 virus infections. J. Infect. Dis. 185:1213-1221. [DOI] [PubMed] [Google Scholar]
- 30.Matsuyama, W., R. Kubota, T. Hashiguchi, H. Momi, M. Kawabata, M. Nakagawa, K. Arimura, and M. Osame. 2002. Purified protein derivative of tuberculin upregulates the expression of vascular endothelial growth factor in T lymphocytes in vitro. Immunology 106:96-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maynard, S. E., J. Y. Min, J. Merchan, K. H. Lim, J. Li, S. Mondal, T. A. Libermann, J. P. Morgan, F. W. Sellke, I. E. Stillman, F. H. Epstein, V. P. Sukhatme, and S. A. Karumanchi. 2003. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Investig. 111:649-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mor, F., F. J. Quintana, and I. R. Cohen. 2004. Angiogenesis-inflammation cross-talk: vascular endothelial growth factor is secreted by activated T cells and induces Th1 polarization. J. Immunol. 172:4618-4623. [DOI] [PubMed] [Google Scholar]
- 33.Peters, C. J., G. L. Simpson, and H. Levy. 1999. Spectrum of hantavirus infection: hemorrhagic fever with renal syndrome and hantavirus pulmonary syndrome. Annu. Rev. Med. 50:531-545. [DOI] [PubMed] [Google Scholar]
- 34.Peters, C. J., and S. R. Zaki. 2002. Role of the endothelium in viral hemorrhagic fevers. Crit. Care Med. 30:S268-S273. [DOI] [PubMed] [Google Scholar]
- 35.Quinn, T. P., K. G. Peters, C. De Vries, N. Ferrara, and L. T. Williams. 1993. Fetal liver kinase 1 is a receptor for vascular endothelial growth factor and is selectively expressed in vascular endothelium. Proc. Natl. Acad. Sci. USA 90:7533-7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raghupathy, R., U. C. Chaturvedi, H. Al-Sayer, E. A. Elbishbishi, R. Agarwal, R. Nagar, S. Kapoor, A. Misra, A. Mathur, H. Nusrat, F. Azizieh, M. A. Khan, and A. S. Mustafa. 1998. Elevated levels of IL-8 in dengue hemorrhagic fever. J. Med. Virol. 56:280-285. [DOI] [PubMed] [Google Scholar]
- 37.Russell, P. K., A. Nisalak, P. Sukhavachana, and S. Vivona. 1967. A plaque reduction test for dengue virus neutralizing antibodies. J. Immunol. 99:285-290. [PubMed] [Google Scholar]
- 38.Sawano, A., S. Iwai, Y. Sakurai, M. Ito, K. Shitara, T. Nakahata, and M. Shibuya. 2001. Flt-1, vascular endothelial growth factor receptor 1, is a novel cell surface marker for the lineage of monocyte-macrophages in humans. Blood 97:785-791. [DOI] [PubMed] [Google Scholar]
- 39.Shibuya, M. 2003. Vascular endothelial growth factor receptor-2: its unique signaling and specific ligand, VEGF-E. Cancer Sci. 94:751-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shibuya, M., N. Ito, and L. Claesson-Welsh. 1999. Structure and function of vascular endothelial growth factor receptor-1 and -2. Curr. Top. Microbiol. Immunol. 237:59-83. [DOI] [PubMed] [Google Scholar]
- 41.Shresta, S., J. L. Kyle, H. M. Snider, M. Basavapatna, P. R. Beatty, and E. Harris. 2004. Interferon-dependent immunity is essential for resistance to primary dengue virus infection in mice, whereas T- and B-cell-dependent immunity are less critical. J. Virol. 78:2701-2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Talavera, D., A. M. Castillo, M. C. Dominguez, A. E. Gutierrez, and I. Meza. 2004. IL8 release, tight junction and cytoskeleton dynamic reorganization conducive to permeability increase are induced by dengue virus infection of microvascular endothelial monolayers. J. Gen. Virol. 85:1801-1813. [DOI] [PubMed] [Google Scholar]
- 43.Thickett, D. R., L. Armstrong, S. J. Christie, and A. B. Millar. 2001. Vascular endothelial growth factor may contribute to increased vascular permeability in acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 164:1601-1605. [DOI] [PubMed] [Google Scholar]
- 44.Tseng, C. S., H. W. Lo, H. C. Teng, W. C. Lo, and C. G. Ker. 2005. Elevated levels of plasma VEGF in patients with dengue hemorrhagic fever. FEMS Immunol. Med. Microbiol. 43:99-102. [DOI] [PubMed] [Google Scholar]
- 45.Vaughn, D. W., S. Green, S. Kalayanarooj, B. L. Innis, S. Nimmannitya, S. Suntayakorn, T. P. Endy, B. Raengsakulrach, A. L. Rothman, F. A. Ennis, and A. Nisalak. 2000. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J. Infect. Dis. 181:2-9. [DOI] [PubMed] [Google Scholar]
- 46.Zaki, S. R., P. W. Greer, L. M. Coffield, C. S. Goldsmith, K. B. Nolte, K. Foucar, R. M. Feddersen, R. E. Zumwalt, G. L. Miller, A. S. Khan, et al. 1995. Hantavirus pulmonary syndrome. Pathogenesis of an emerging infectious disease. Am. J. Pathol. 146:552-579. [PMC free article] [PubMed] [Google Scholar]